Abstract
Objective
Reversible splenial lesion syndrome (RESLES) is a clinical radiological syndrome characterized by a reversible lesion of the splenium of the corpus callosum with a decreased apparent diffusion coefficient (ADC) value. The clinical manifestations of RESLES are diverse.
Methods
Fifteen cases of adult RESLES patients (10 males and 5 females) were retrospectively selected from the radiology system using the key word “corpus callosum” at a university-affiliated tertiary care hospital between May 1, 2015 and December 31, 2019. The possible precipitating factors, clinicoradiological findings and modified Rankin Scale (mRS) on follow-up were then analyzed.
Results
The patient ages ranged from 22 to 53 years old. The mean age was 34 years old. The most common neurological symptoms included headache (3/15), dizziness (3/15), first onset of seizure (3/15), paroxysmal blurred vision (2/15), vertigo (2/15), amnesia (2/15), and confused consciousness without seizure (2/15), followed by drowsiness (1/15), paresthesia (1/15), dysmetria (1/15) and dysarthria (1/15). The precipitating factors included infection, seizure, anti-epileptic treatment with levetiracetam, carbamazepine, valproate, hyperglycemia, hypoglycemia, cerebral venous sinus thrombosis, and rabies vaccine injection prior to the onset of RESLES. All cases were carefully followed up and had excellent prognoses.
Conclusion
RESLES manifests as variety of symptoms with less specificity and precipitating factors. Paroxysmal blurred vision may be a relatively specific symptom of RESLES. Levetiracetam, carbamazepine or valproate could be the cause of RESLES, exposure to the rabies vaccine could be another predisposing factors for RESLES as well. RESLES type 1 was therefore found to be highly “reversible” with an excellent prognosis.
Keywords: reversible splenial lesion syndrome (RESLES), corpus callosum, magnetic resonance imaging, levetiracetam, carbamazepine, valproate
Introduction
Reversible splenial lesion syndrome (RESLES) is a clinical radiological syndrome characterized by the presence of a reversible lesion involving the splenium of the corpus callosum (SCC) with a decreased apparent diffusion coefficient (ADC) value of the lesion on ADC maps. RESLES often resolves spontaneously with a favorable clinical outcome. In 1999, RESLES was first reported by Kim and colleagues (1). They reported a group of epileptic patients with concurrent lesions of the corpus callosum (CC), and speculated that these lesions were caused by the use of antiepileptic drugs. Garcia-Monco et al. (2) proposed RESLES with a diversity of etiologies in 2011, Since that time, an increasing number of RESLES cases have been reported, with most such studies being in the case report format. Besides seizures and withdraw from antiepileptic drugs (3-5), reports have revealed a variety of etiologies, including the use of other pharmacological agents such as metronidazole (6), olanzapine (7), infections like influenza A (8, 9), rotavirus infection (10), streptococcus pneumoniae (11), meningococcal meningitis (12), metabolic conditions such as hypoglycemia or hypernatremia (2, 13, 14), glufosinate ammonium poisoning (15) and miscellaneous conditions including malnutrition, vitamin B12 deficiency (16), malnutrition (17), high-altitude cerebral edema (18), systemic lupus erythematosus (SLE) (19), Kawasaki disease (20), anti-voltage-gated potassium channel (VGKC) autoantibody syndrome (21), malignancy (13), cerebral venous sinus thrombosis (22), and preeclampsia (23). The heterogeneity in the clinical manifestations makes RESLES hard to predict before magnatic resonance imaging (MRI) and the precise pathophysiological mechanism of RESLES thus remains unclear. The subsequent cases are offered to provide the medical community with more evidence to stimulate research into the nature of these lesions. RESLES is reported to be a rare clinico-radiological disorder; nevertheless, in this cases series fifteen cases from different clinical settings are described, with our findings suggesting that it could be less rare than previously reported.
Materials and Methods
Criteria for RESLES
RESLES was diagnosed mainly based on the revised inclusion criteria of Garcia-Monco et al. (2011) (2); we included lesions involving the SCC as shown by MRI. Patients in whom lesions were persistent (or follow-up was not available) were not included. Other exclusion criteria included: the presence of extracallosal lesions, asymmetrical lesions, lesions without restricted diffusion on the MRI, or patients with acute disseminated encephalopathy and other common demyelinating disorders involving the CC.
Patient selection
The following is a retrospective observational study. Patients were treated at a tertiary hospital affiliated with Zhejiang University between May 1, 2015 and December 31, 2019. Two board-certified, fellowship-trained neurologists with more than 10 years of experience made an independent review and read the radiological images to confirm a diagnosis of RESLES. We selected patients by searching through our Picture Archiving and Communication Systems (PACS) using the keywords “corpus callosum.” Reports of brain MR+diffusion-weighted images (DWI) with the words “corpus callosum” were found for 613 records and 485 cases of patients in our MRI record system. Each report was read, and those with “splenium of corpus callosum” were selected, thus resulting in a total of 197 cases. Each MRI was read, patients were selected according to inclusion criteria and exclusion criteria listed above. Finally, a total of fifteen patients were selected.
Imaging Evaluation
MRI was the main technique used for evaluation. Brain MR+DWI was conducted in each patient using a Siemens Skyra 3.0 Tesla MRI or GE Signa HDx 3.0 Tesla MRI scanner with 5-mm thick sections, including T1, T2, fluid attenuation inversion recovery (FLAIR), DWI and ADC.
Data collection
The medical history of each patient was reviewed. Information was collected including possible precipitating factors, demographics, clinical characteristics, biochemical results, pathogenic assessments, drugs, imaging findings, the treatments offered at baseline and during hospital stay, and the follow-up results. The prognosis of each patient was evaluated using a modified Rankin Scale (mRS): 0, No symptoms; 1, No significant disability. Able to carry out all usual activities despite some symptoms; 2, Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities; 3, Moderate disability. Requires some help, but able to walk unassisted; 4, Moderately severe disability. Unable to attend to own body needs without assistance and unable to walk unassisted; 5, Severe disability. Requires constant nursing care and attention, bedridden, incontinent.
Results
Two board-certified, fellowship-trained neurologists with more than 10 years of experience made an independent review and read the radiological images to confirm the diagnosis of RESLES. The consistency of the diagnosis was deemed to be 100%.
Our series of fifteen cases included ten males and five females. The patient ages ranged from 22 to 53 years old. The mean age was 34 years old. The clinical characteristics of the patients are shown in Tables 1 and 2. The most common neurological symptoms before MRI were headache (3/15), dizziness (3/15), first onset of seizure (3/15), paroxysmal blurred vision (2/15), vertigo (2/15), amnesia (2/15), and confused consciousness without seizure (2/15), followed by drowsiness (1/15), paresthesia (1/15), dysmetria (1/15) and dysarthria (1/15). One patient had recurrent seizures before and around the time of the discovery of the CC lesion. Most of the patients recovered completely with an mRS score of 0 except for case 3, in which the patient complained of slight amnesia after 3 months, which finally recovered without any sequelae a year later.
Table 1.
Clinical Characters of the Fifteen Patients with RESLES.
| Patient no. | Sex/ age |
CNS manifestations | Presumed etiology | Associated drugs | Therapy | Inpatient/ outpatient |
Hospital stays | mRS (up to 3m) |
|---|---|---|---|---|---|---|---|---|
| 1 | m/48 | Sluggish in consciousness | Pneumonia | Piperacillin and tazobactam | Ceftriaxon, arithromycin (moxifloxacin) | Inpatient | 11 | 0 |
| 2 | m/43 | Disarthria | Suspected hyponatremia | None | Clopidogrel, | Outpatient | 0 | |
| 3 | m/44 | Seizure, drowsiness and amnesia | Hypoglycemia | Gliclazide, dimethyldiguanide 250 mg.bid | 50% dextrose, potassium chloride, aspirin, atorvastatin | Inpatient | 8 | 1 |
| 4 | m/35 | Headache | Infection | acyclovir, acetaminophen | saline | Inpatient | 9 | 0 |
| 5 | f/25 | Seizure, confused consciousness | Hyporexia hyponatremia | None | Saline, acyclovir, levetiracetam | Outpatient | 0 | |
| 6 | f/40 | Headache | postpartum, Pulmonary embolism | None | Warfarin | Inpatient | 11 | 0 |
| 7 | f/24 | Recurrent seizure | levetiracetam | Levetiracetam | Levetiracetam | Outpatient | 0 | |
| 8 | m/23 | Paroxysmal blurred vision | bacterial respiratory infection | Levofloxacin, acetaminophen | Levofloxacin, Aspirin, atorvastatin | Inpatient | 10 | 0 |
| 9 | m/23 | Dizziness with nausea and vomiting | Rabies vaccine injection | rabies vaccine, Amoxicillin and paracetamol | Ofloxacin, betahistine | Outpatient | 0 | |
| 10 | m/25 | Dizziness, paresthesia, dysmetria, memory problem, proxysmal vertigo, motor fine skills deficit | Hyperglycemia | None | Aspirin, atorvastatin, insulin, metformin | Inpatient | 5 | 0 |
| 11 | f/43 | None | Carbamazepine | Cabamazepine | Outpatient | 0 | 0 | |
| 12 | f/22 | Hedache, loss of consciousness | Valproate/seizure | Valproate | Valproate | Inpatient | 4 | 0 |
| 13 | m/25 | Dizzy, Paroxysmal blurred vision, unstable walk | Unknown | None | Aspirin | Outpatient | 0 | 0 |
| 14 | m/30 | None | Presumed virus infection | None | Amoxicillin, ceftriaxone, levofloxacin, piperacillin and tazobactam, linezolid, compound paracetamol, indomethacin | Inpatient | 8 | 0 |
| 15 | m/53 | Convulsion | Seizure | None | None | Outpatient | 0 | 0 |
RESLES: reversible splenial lesion syndrome, Patient no.: patient number, m: male, f:female, CNS: central nervous system, mRS: modified Rankin Scale
Table 2.
Biochemical and Virus Results of Fifteen Patients with RESLES.
| Patient no. | WBC (/μL) Normal range: 4,000-10,000 |
CRP (mg/L) Normal range: 0-5 |
Glu (mmol/L) Normal range: 4.16-5.8 |
Na+ (mmol/L) Normal range: 137-147 |
Cl- (mmol/L) Normal range: 99-110 |
ALT (U/L) Normal range: 9-50 |
Osmotic pressure (mmol/L) Normal range: 280-320 | CSF: pressure (mmH2O), WBC (/μL), protein (mg/L) | Virus workups |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5,600 | 218.5↑↑ | 10.27 | 137 | 106 | 144↑ | 291 | 120, 6, 353 | CMV-IgG(+), IgM(-), HSV1-IgG(+), IgM(-), HSV2-IgG(-), IgM(-), EBVCA-IgG(+), EBVCA-IgM(-), EHF Ab-IgM(-) |
| 2 | 6,900 | 8.1↑ | 8.39 | 135↓ | 100 | 76↑ | 283.59 | NE | HBsAb(+), HBcAb(+) |
| 3 | 1,1000↑ | 1.41 | <2↓ | 141 | 106 | 12 | 289.73 | NE | HBsAb(+), HBcAb(+) |
| 4 | 3,100 | 22↑ | 5.19 | 134↓ | 102 | 34 | 276.63↓ | 210, 4, 265.5 | of Measle, EBVCA, rubella, CMV, HSV-1 virus IgG Abs(+), HSV-2 IgG(-), IgM(-), ADVAb(-) |
| 5 | 8,300 | 4.8 | 6.54 | 124↓ | 91↓ | 24 | 257.44↓ | NE | HBV(-) |
| 6 | 4,200 | 0.6 | 7.76 | 145 | 107 | 23 | 305.06 | NE | HBV(-) |
| 7 | 7,600 | 14.2↑ | 5.63 | 142 | 105 | 53↑ | 293.83 | NE | NE |
| 8 | 9,700 | 61.9↑ | 5.03 | 141 | 100 | 27 | 291.93 | 120, 4, 176 | HSV-Ab(-), HBs Ab(+), second generation sequencing(-) |
| 9 | 10,300↑ | 4.4 | NE | NE | NE | NE | NE | NE | NE |
| 10 | 10,800 | 3 | 35.2↑ | 127↓ | 94↓ | 96↑ | 291.02 | NE | NE |
| 11 | NE | NE | NE | NE | NE | NE | NE | NE | NE |
| 12 | 11,100 | 75.1 | 7.27 | 136↓ | 101 | 10 | 282 | 80, 4, 205 | HSV-I IgG-, HSV-I IgM-, HSV-II IgG-, HSV-II IgM-, Abs of influenza A,B- |
| 13 | 7,300 | 1.5 | 5.79 | 139 | 106 | NE | 287 | NE | NE |
| 14 | 19,300 | 199.2 | 7.14 | 133↓ | 96 | 72 | 279↓ | NE | Abs of influenza A, B-, CMV-DNA-, CMV-IgG+, IgM-, EBV Ab-, EBV-DNA-, HSV Ab-, |
| 15 | NE | NE | NE | NE | NE | NE | NE | NE | NE |
RESLES: reversible splenial lesion syndrome, CSF: cerebrospinal fluid, BA: basic activity, NE: not examined, HBV: hepatitis B virus, CMV: cytomegalovirus, HSV: herpes simplex virus, EBV: Epstein-Barr virus, EHF epidemic hemorrhagic fever, IgG: immunoglobulin G, IgM: immunoglobulinM, Ab: antibody, NE: not examined
During the prodromal period, fever was the most common symptom (6/15; cases 1, 4, 8, 9, 12, 14); three out of six cases had a respiratory infection (cases 1, 6, 14), one patient had an intestinal infection, while causes of fever in the other two remain unknown. There was one case of pulmonary embolism (PE, case 6) and rabies vaccine injection (case 9) as shown in Fig. 1. Medications during the prodromal period included: antibiotics, such as piperacillin and tazobactam, amoxicillin, ceftriaxone, and levofloxacin; compound paracetamol; antiepileptic medications like levetiracetam, carbamazepine, valproate; antidiabetic drugs like gliclazide and dimethyldiguanide. There were six cases of hyponatremia (cases 2, 4, 5, 10, 12, 14, in cases 5 and 10, the sodium concentrations were under 130 mmol/L), one case of hyperglycemia (case 10) and one case of hypoglycemia (case 3). There were three cases of a decreased osmotic pressure (cases 4, 5, 14). Eight out of fifteen (8/15) were admitted to hospital for 4-12 days, while the other seven cases were followed up in an outpatient clinic. One underwent second generation sequencing (case 8), no virus or bacteria was found. 4/15 of the patients (4/15, case 1, 4, 8, 12, 14) underwent multiple virus antibody workups, which were all negative for IgM antibodies including cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein-Barr virus (EBV) and measle virus, adenovirus, rubella virus and an influenza virus in a single patient.
Figure 1.
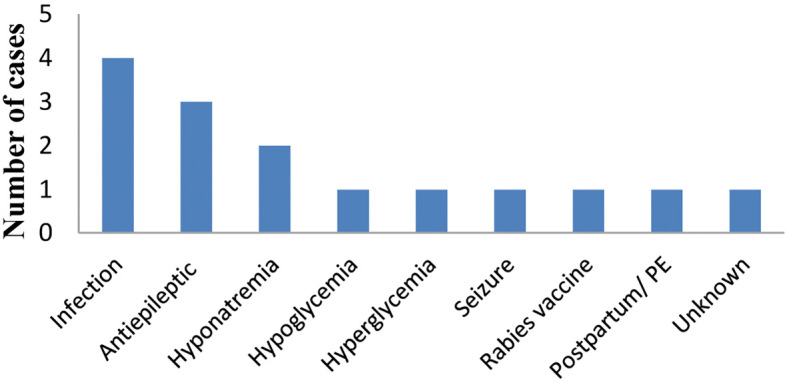
Number of cases with different etiologies of RESLES. RESLES: reversible splenial lesion syndrome
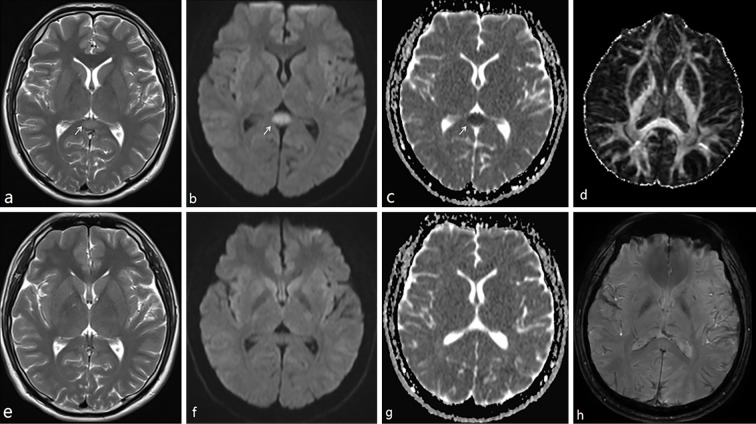
All lesions were symmetrical and oval-shaped, and they were located in the midline of the splenium of the corpus callosum with DWI hyperintensity, T1 hypointensity, and T2 hyperintensity. Susceptibility weighted imaging (SWI) and diffusion tensor imaging (DTI) were conducted in case 8 as shown in Fig. 2; DTI showed no obvious abnormalities on the fractional anisotropy (FA) map. SWI showed no microbleeding. The mean duration between the presumed onset of RESLES and MRI 10 days (3-34 days).
Figure 2.
The cerebral MRI features of case 8. a, b, and c were conducted seven days after the onset of his symptoms. d and h were taken 15 days after the onset of symptoms. e, f, and g were taken 33 days after the onset of his symptoms. MR demonstrated an isolated oval lesion in the splenium of the corpus callosum (arrows) with hypointensity on ADC (c), hyperintensity on T2 (a), and DWI (b). All abnormal signals disappeared upon a repeat of MR imaging (e: T2, f: DWI, g: ADC). No FA change was observed on DTI; no microbleeding was seen on SWI. ADC: apparent diffusion coefficient, DWI: diffusion-weighted images, FA: fractionary anisotropy, DTI: diffusion tensor imaging, SWI: susceptibility weighted imaging
Discussion
Most of the previous descriptions of RESLES have been in the case report format; thus, the incidence of RESLES remains unknown. Fifteen cases of RESLES were found among 197 cases of “splenium of corpus callosum” and 485 cases of “corpus callosum” in reports of brain MR+DWI, suggesting that it is not so rare.
This study is one of the biggest case series with a diversity of clinical manifestations and inducing factors, some of which are first time reported.
The patient ages ranged from 22 to 53 years old with a mean age of 34 years old, and this was similar to a previous report, the age of onset varied from 13 to 32 years (average 25.6±7.98) (24). Our series of fifteen cases included ten males and five females. However, a large number of cases is needed in order to claim that this condition has a male predominance.
Over the years, there have been various terms used to describe splenial lesions, including “mild encephalitis with a reversible lesion in the splenium (MERS),” “reversible splenial lesion syndrome (RESLES),” “cytotoxic lesions of the corpus callosum (CLOCCs).” MERS is an acute clinico-neuroradiological syndrome characterized by mild encephalitis or encephalopathy presenting as a reversible solitary mass in the central portion of the splenium of the corpus callosum (28). However, encephalitis/encephalopathy is not always mild. The spectrum of RESLES includes MERS. MERS cases were not excluded from our cases series of RESLES. In our case series, there were 2/15 cases of MERS (case 1, 12), besides, RESLES contains more heterogenic causes and symptoms such as Marchiafava-Bignami disease (MBD) (26). CLOCCs are various entities associated with a variety of causes with restricted diffusion, and some of these lesions are not reversible.
The splenial lesion of MERS and RESLES contains two different patterns according to the lesion location (27, 28): type 1, an isolated lesion located in the center of the splenium of CC, mostly are round or oval, some of the lesion extended along splenium; type 2, a lesion centered in splenium and extended into other brain areas (29). Our cases of RESLES were all isolated symmetrical lesions in SCC without any extracallosal lesions (type 1).
A broad spectrum of symptoms was reported in previously published papers of MERS and RESLES (Table 3). A disturbed consciousness and headache were common symptoms in RESLES. In our report, patients presented with significant clinical heterogeneity, ranging from headache, seizure, confusion and alterations in consciousness, paresthesia, dysmetria, memory deficits, paroxysmal vertigo, slurred speech, to paroxysmal blurred vision. Studying these cases helps to understand the functions of the SCC and the symptoms associated with its damage, thus making it easier to identify and diagnose RESLES in clinical practice.
Table 3.
Symptoms of MERS and RESLES Type 1 and 2.
| RESLES type 1 | RESLES type 2 | |||
|---|---|---|---|---|
| MERS type 1 | MERS type 2 | |||
| Disturbed consciousness (24, 40), somnolence (40-42), confusion (15, 21, 40, 43), memory problems (15, 21, 42), disorientated (41, 43, 44), apathy (40, 45), mental abnormalities (46), delirious behavior (24), delirium (47), agraphia (48), ideomotor apraxia (49), alien hand syndrome (49), autotopagnosia (49) | Disturbed consciousness (7, 9, 12, 18, 56-58), lethargy (25), somnolence (25, 26, 59), confusion (26), cognitive impairment (26), behative impairment (26), behavioral disorders (26), hallucination (25), delirium (25, 57), disorientated (44), apathy (45) | Drowsiness (64-66), confusion (67), stupor (39), delirium (64) | Disturbed consciousness (70), stupor (26, 39), somnolence (26, 41), cognitive impairment (26), coma (26), interhemispheric disconnection (26), cognitive impairment (26) | |
| Headache (22, 24, 41, 43, 47, 50-53), status migrainosus (54), vertigo (24, 51), dizziness (43) | Headache (18, 25, 26, 38, 45, 57, 60), vertigo (25), dizziness (26, 60), phonophobia (60), photophobia (60), | Headache (64, 65, 68), dizziness (67) | Headache (41) | |
| Seizure (24, 26, 40) | Seizure (25, 61) | Seizure (67) | Seizure (26, 67) | |
| Slurred language (24), dysarthria (40, 43), gait difficulty(49), tremor (24), tremulousness (40), dysmetria (21), ataxia (21, 26, 47), fatigue (21), | Alalia (62), monoparesis (63), gait difficulty (37, 57), tremor (57) | Dysarthria (26, 66), monoparesis (59), ataxia (67) | Dysarthria (70), ataxia (26), limb hypertonia (26) | |
| Visual disturbance(26, 41, 50, 52), kaleidoscopic visual illusion (55) | Olfactory disturbance (9) | Visual hallucination (26) | ||
| Limb numbness (24) paresthesias (43) | Paresthesias (57) | Paresthesia (69), facial numbness (59), | ||
| Urinary retention (43), diaphoresis (40) | Urinary retention (27, 57, 60) | Urinary retention (18, 68) |
All contents in MERS type 1 column also be contents of RESLES type 1. All contents in MERS type 2 column also be contents of RESLES type 2. MERS: mild encephalitis with a reversible splenial lesion syndrome, RESLES: reversible splenial lesion syndrome
Typical MRI features of RESLES are reversible, nonenhanced rounded or oval lesions located in the splenium of the corpus callosum; isointensity to slight hypointensity on T1WI without contrast enhancement, hyperintensity on T2-weighted images (T2WI), FLAIR, restricted diffusion on DWI, and a decreased ADC value of the lesion on ADC maps. The SCC abnormalities had disappeared upon the completion of follow-up MRI studies performed 10 to 32 days after the first MRI study by Zhu et al. (24), the DTI imaging value of the SCC lesion was reported to be mildly decreased in the FA map, but with a normal projecting direction of white matter fibers (24). In case 8 of our series (Fig. 2), no FA change was found, most likely due to the reversible character of the clinico-radiological change. The possible mechanisms for the restricted diffusion of the SCC include intramyelinic edema, reversible demyelination, damage to the blood-brain barrier, arginine vasopressin release, and inflammatory cell-induced cytotoxic edema. Follow-up assessment with DTI may help to distinguish the “real” reversibility of splenial lesions in future research.
RESLES and MERS were previously reported to have a diverse number of etiologies (Table 4). Though an increasing number of publications have focused on RESLES, its exact pathophysiology remains unknown. Because of the characteristic reversible restricted diffusion on DWI and low ADC values, transient intramyelinic cytotoxic edema has often been thought to be a cause rather than persistent ischemia. Antiepileptic drug toxicity and associated changes in salt homeostasis and transhemispheric seizure propagation are other suspected mechanisms (30, 31). Cell-cytokine interactions lead to massively elevated extracellular glutamate levels, and this phenomenon is thought to be important in development of SCC lesions. Because of the heterogeneous nature of the etiologies, no definite common mechanism has yet been identified.
Table 4.
Etiologies of MERS and RESLES Type 1 and 2.
| RESLES type 1 | RESLES type 2 | ||||
|---|---|---|---|---|---|
| MERS type 1 | MERS type 2 | ||||
| Infection | Infuenza B (24), rotavirus, herpes virus-6 (24), Epstein-Barr virus (24), puumala hantavirus (50), mumps virus (26) | Infuenza A (9, 59), VZV (25), adenovirus (63), nonfulminant hepatitis A (62), klebsiella pneumoniae (46), meningococcal meningitis (12), Mycoplasma pneumoniae (26, 27, 41, 46, 56), legionella pneumophila (37), tick-bites (38) | Epstein-Barr virus (64, 67) | ||
| Seizures | Seizure (71) | ||||
| Drug | Ipilimumba (57), sympathomimetic (55), minocycline (51) | ||||
| Use of AEDs | Phenytoin (1, 52), carbamazepine (3), vigabatrin (52) | Olanzapine (7) | |||
| withdrawal of AEDs | Valproate (26), oxcarbazepine (5, 33), topiramate (30), levetiracetam (34), phenytoin (3, 42), carbamazepine (72) | Oxcarbazepine (33) | |||
| Autoimmune disease | Anti-Yo rhombencehhalitis (39) | ||||
| Metabolic disturbances | Hypoglycemia (40, 48), methyl bromide poisoning (43), glufosinate ammonium poisoning (15), anorexia nervosa (73) | Amanita phalloides intoxication (61), hemolytic uremic syndrome (44) | Carbon monoxide poisoning (26), hypoglycemia (26, 70), MBD (26), osmotic myelinolysis (13) | ||
| Miscellaneous conditions | cerebral venous thrombus (22), Anti-VGKC autoantibody syndrome (21), Charcot-Marie-Tooth disease (74), blood transfusion (49), migraine with aura (75) |
Notes: All etiologies in MERS column also be etiologies of RESLES but are not listed in RESLES column now. MERS: mild encephalitis with a reversible splenial lesion syndrome, AEDs: antiepileptic drugs, VZV: varicella zoster virus, VGKC: voltage-gated potassium channel, MBD: Marchiafava-Bignami disease
Two injections of the rabies vaccine preceded the onset of symptoms by 11 and 4 days in case 9. Hara et al. described an eight-year-old boy who appeared to have clinically-mild encephalitis with a reversible splenial lesion following a mumps vaccination (32). No other cases of this nature have been reported. Rabies vaccine may be one of the precipitating factors of RESLES. RESLES in case 9 manifested as fever and tinnitus five days after the second injection of rabies vaccine. The pathological mechanism is unknown, however, a vaccine may induce an inflammatory reaction and thus cause a transient influx of inflammatory cells. The effects of the rabies virus on RESLES has yet to be determined and thus requires further research.
Certain medications also induce RESLES. Besides the use and withdrawal of antiepileptic medications, such as oxcarbazepine (5, 33), levetiracetam (34), and phenytoin (35), psychiatric medication use, including olanzapine (7), and glufosinate ammonium poisoning (15) can also purportedly induce RESLES. It remains unclear, however, whether suspected seizures or valproate, which was used after a transient loss of conscious, may have induced RESLES in case 12. Valproate was not reported to induce RESLES previously.
Levetiracetam was suspected to play a role in the onset of RESLES in case 7, to the best of our knowledge, this is the first report of levetiracetam to be associated with the etiology of RESLES, while the withdrawal of levetiracetam has been previously reported to cause RESLES (34). After carbamazepine treatment was given because of vestibular paroxysmia in case 12, an SCC lesion was discovered 34 days later after carbamazepine treatment. The antiepileptic treatment without epilepsy attack followed by a subsequent SCC lesion strongly supports to the role of antiepileptic medications in the onset of RESLES.
Treatment with antiepileptic drugs like carbamazepine and the drugs' effects on rapid concentration changes of antiepileptic drugs (AEDs) can influence fluid balance systems through arginine vasopressin release. These drugs can also increase the number of proinflammatory and proconvulsive cytokines. Öztoprak et al. proposed a possible mechanism of onset in RESLES for patients experiencing seizures (36). The authors suggest that the discharge of the corpus callosum disseminated in seizures caused a decrease in free water dispersion in the corpus callosum. Further studies are needed to investigate this mechanism.
A marked elevation of urinary β2-microglobulin was reported in patients with RESLES. We found normal levels of blood and urinary β2-microglobulin in case 8. More research is needed to elucidate the relationship between β2-microglobulin and RESLES.
Hyponatremia has also been observed in RESLES. In our cohort, six of fifteen cases (cases 2, 4, 5, 10, 12, 14) had hyponatremia; in cases 5 and 10, sodium concentrations were under 130 mmol/L. There were no antiepileptic treatments before the onset of hyponatremia. Therefore, hyponatremia, in these cases, was not induced by antiepileptic medicine. Case 5 had been trying to lose weight and thus had a seriously restricted intake, and thus developed serious diarrhea, nausea and vomiting, which may explain her markedly decreased plasma sodium levels.
Hyponatremia has also been noticed in different reports (14, 18, 27, 37-39). Six out of sixteen MERS adult patients showed hyponatremia upon admission in a review by Yuan et al. (27), The etiologies of these cases with hyponatremia includes tick bites, anti-Yo rhombencehhalitis and Legionnaires' disease.
Hyponatremia may be secondary to these etiologies or predisposing factor in these cases. Takanashi et al. reported that there were significant differences between the sodium levels of patients with MERS and those with upper respiratory infection, other types of encephalopathy and febrile seizures, respectively (14). Hyponatremia itself may act as an important precipitating factor of RESLES in these cases.
Syndrome of inappropriate secretion of antidiuretic hormone (SIADH), AED administration or withdrawal, high altitude cerebral edema (HACE) seem to cause hyponatremia, water/electrolyte imbalance, cerebral edema as the underlying pathophysiological mechanism in RESLES (14).
Infections and metabolic abnormalities were the most common etiologies in our series (three cases had respiratory infection, one patient had intestinal infection, one with hypoglycemia, one with hyperglycemia and hyponatremia, and another with hyponatremia). In addition, One case of RESLES were induced by the use of levetiracetam, carbamazepine, valproate respectively in our series, thus supporting the fact that seizures and antiepileptic drugs were some of the most common etiologies in previous report (2).
In our series of fifteen cases, there was no obvious relationship between the clinical manifestations and the etiology, except in case 3 and case 10, both of which were presumably caused by an abnormality in their glucose metabolism - hypoglycemia in case 3 and hyperglycemia in case 10. These two patients both had memory loss, possibly related to dysregulations in the energy utilization accompanying hyperglycemia and hypoglycemia.
The diverse pathologies reported in various manuscripts and neuroimaging studies cause significant uncertainty about the precise mechanisms of reversible splenial lesions in RESLES. It has been proposed that viral or induced antibodies show selected affinity for the splenial axonal receptors. SCC is specifically vulnerable to excitotoxic injury in metabolic causes - one possible mechanism of splenial involvement in various pathological events induced by viruses or certain medicines. However, no common pathophysiological mechanism has so far been able to explain the splenial predilection or diversity of heterogeneous etiologies in this disease. A large number of patients demonstrated such etiological problems as taking antiepileptic drugs, but only a small number of such patients developed RESLES. Further investigation is needed to determine whether a genetic susceptibility exists in RESLES patients.
Generally, RESLES has a highly benign prognosis and it is usually associated with a complete recovery without any obvious neurological sequelae shortly after the acute course, as illustrated by our cases. However, some cases have been previously reported with poor prognoses, such as those who entered a comatose or vegetative state, even after the abnormal lesions had disappeared (24). The results of previous studies indicate that severe disturbances in consciousness at the onset of the disease, diffuse slow waves on electroencephalogram (EEG) findings, and extracallosal lesions are indicative of a relatively poor prognosis in RESLES. Our case series excluded cases with extracallosal lesions, and thus it seemed that RESLES type 1 without any extracallosal lesions had a better prognosis.
There are several limitations associated with our study. First, this was a retrospective study. Functional MRI, such as the use of DTI, was only provided in one case of RESLES. Furthermore, β2-microglobulin was only tested in a single case. These initial findings, however, provide many clues about the factors contributing to the onset of RESLES and provide guidance for the future research into this clinico-radiological disease.
Conclusion
In this series of fifteen cases of RESLES, we reported a variety of clinical manifestations with identical radiological lesions located in SCC. These cases are groundbreaking in their contribution to medical knowledge about the functions of SCC. This report is also the first to show the involvement of levetiracetam, valproate and rabies vaccination in the etiology of RESLES. RESLES type 1 without extracallosal lesions are therefore deemed to be highly “reversible” with an excellent prognosis.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by by grant No. 81201722 from the National Natural Science Foundation of China, by grant No. LY20H090001 from the Natural Science Foundation of Zhejiang Province, by grant No. 2018ZD025 from the Health Commission of Zhejiang Province, and by grant No. 2017ZYC-A18 from the clinical research foundation of Zhejiang Medical Association.
Acknowledgement
Thanks to Yating Lv from The Affiliated Hospital of Hangzhou Normal University for helping to process the DTI.
References
- 1. Kim SS, Chang KH, Kim ST, et al. Focal lesion in the splenium of the corpus callosum in epileptic patients: antiepileptic drug toxicity? AJNR Am J Neuroradiol 20: 125-129, 1999. [PubMed] [Google Scholar]
- 2. Garcia-Monco JC, Cortina IE, Ferreira E, et al. Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging 21: e1-e14, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Polster T, Hoppe M, Ebner A. Transient lesion in the splenium of the corpus callosum: three further cases in epileptic patients and a pathophysiological hypothesis. J Neurol Neurosurg Psychiatry 70: 459-463, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mirsattari SM, Lee DH, Jones MW, Blume WT. Transient lesion in the splenium of the corpus callosum in an epileptic patient. Neurology 60: 1838-1841, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Jing C, Sun L, Wang Z, Chu C, Lin W. Reversible splenial lesion syndrome due to oxcarbazepine withdrawal: case report and literature review. J Int Med Res 46: 1277-1281, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neshige S, Kanaya Y, Takeshima S, Yoshimoto T, Tanaka A, Kuriyama M. [Reversible changes on MR images in a patient with metronidazole-induced encephalopathy]. Rinsho Shinkeigaku (Clin Neurol) 55: 174-177, 2015. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 7. Kaino K, Kumagai R, Furukawa S, et al. Reversible splenial lesion syndrome with a hyperosmolar hyperglycemic state and neuroleptic malignant syndrome caused by olanzapine. J Diabetes Investig 8: 392-394, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takayanagi M, Okabe S, Yamamoto K, et al. Kleine-Levin syndrome elicited by encephalopathy with reversible splenial lesion. Pediatr Int 59: 929-931, 2017. [DOI] [PubMed] [Google Scholar]
- 9. Takatsu H, Ishimaru N, Ito M, Kinami S. Mild encephalitis/encephalopathy with a reversible splenial lesion in an adult patient with influenza. Intern Med 56: 3093-3095, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karampatsas K, Spyridou C, Morrison IR, Tong CY, Prendergast AJ. Rotavirus-associated mild encephalopathy with a reversible splenial lesion (MERS)-case report and review of the literature. BMC Infect Dis 15: 446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avcu G, Kilinc MA, Eraslan C, Karapinar B, Vardar F. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) associated with Streptococcus pneumoniae Bacteraemia. J Infect Public Health 10: 479-482, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi Y, Yasunishi M, Hayashi M, Asano T, Kimura A, Inuzuka T. Reversible splenial lesion of the corpus callosum associated with meningococcal meningitis. J Neurol Sci 373: 81-82, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Maeda M, Tsukahara H, Terada H, et al. Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol 33: 229-236, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Takanashi J, Tada H, Maeda M, Suzuki M, Terada H, Barkovich AJ. Encephalopathy with a reversible splenial lesion is associated with hyponatremia. Brain Dev 31: 217-220, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Jeong TO, Yoon JC, Lee JB, Jin YH, Hwang SB. Reversible splenial lesion syndrome (RESLES) following glufosinate ammonium poisoning. J Neuroimaging 25: 1050-1052, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Theeler BJ, Wilson DJ, Crawford CM, Grazko M. Optic neuropathy and a reversible splenial lesion after gastric bypass: shared pathophysiology? J Neurol Sci 291: 92-94, 2010. [DOI] [PubMed] [Google Scholar]
- 17. Kosugi T, Isoda H, Imai M, Sakahara H. Reversible focal splenial lesion of the corpus callosum on MR images in a patient with malnutrition. Magn Reson Med Sci 3: 211-214, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Pan JJ, Zhao YY, Lu C, Hu YH, Yang Y. Mild encephalitis/encephalopathy with a reversible splenial lesion: five cases and a literature review. Neurol Sci 36: 2043-2051, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Soon GS, Rodan LH, Laughlin S, Laxer RM, Benseler S, Silverman ED. Reversible splenial lesion syndrome in pediatric systemic lupus erythematosus. J Rheumatol 39: 1698-1699, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Itamura S, Kamada M, Nakagawa N. Kawasaki disease complicated with reversible splenial lesion and acute myocarditis. Pediatr Cardiol 32: 696-699, 2011. [DOI] [PubMed] [Google Scholar]
- 21. Gilder TR, Hawley JS, Theeler BJ. Association of reversible splenial lesion syndrome (RESLES) with anti-VGKC autoantibody syndrome: a case report. Neurol Sci 37: 817-819, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Liu D, Yang B, et al. Reversible splenial lesion syndrome (RESLES) coinciding with cerebral venous thrombosis: a report of two cases. Ther Adv Neurol Disord 10: 375-379, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Z, Xu M, Shang D, Luo B. A case of reversible splenial lesions in late postpartum preeclampsia. Intern Med 51: 787-790, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Zhu Y, Zheng J, Zhang L, et al. Reversible splenial lesion syndrome associated with encephalitis/encephalopathy presenting with great clinical heterogeneity. BMC Neurol 16: 49, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 63: 1854-1858, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Zhang S, Ma Y, Feng J. Clinicoradiological spectrum of reversible splenial lesion syndrome (RESLES) in adults: a retrospective study of a rare entity. Medicine (Baltimore) 94: e512, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan J, Yang S, Wang S, Qin W, Yang L, Hu W. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults-a case report and literature review. BMC Neurol 17: 103, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Notebaert A, Willems J, Coucke L, Van Coster R, Verhelst H. Expanding the spectrum of MERS type 2 lesions, a particular form of encephalitis. Pediatr Neurol 48: 135-138, 2013. [DOI] [PubMed] [Google Scholar]
- 29. Dong K, Zhang Q, Ding J, et al. Mild encephalopathy with a reversible splenial lesion mimicking transient ischemic attack: a case report. Medicine (Baltimore) 95: e5258, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prilipko O, Delavelle J, Lazeyras F, Seeck M. Reversible cytotoxic edema in the splenium of the corpus callosum related to antiepileptic treatment: report of two cases and literature review. Epilepsia 46: 1633-1636, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Doherty MJ, Jayadev S, Watson NF, Konchada RS, Hallam DK. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol 62: 433-437, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Hara M, Mizuochi T, Kawano G, et al. A case of clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. Brain Dev 33: 842-844, 2011. [DOI] [PubMed] [Google Scholar]
- 33. Merizalde M, Navalon P, Gonzalez MF, Dominguez A, Livianos L, Martinez JC. Manic espisode, confusional syndrome and reversible splenial lesion after abrupt withdrawal of oxcarbazepine. J Affect Disord 210: 122-124, 2017. [DOI] [PubMed] [Google Scholar]
- 34. Sawagashira R, Narita H, Hashimoto N, et al. Transient lesions of the splenium of the corpus callosum following rapid withdrawal of levetiracetam. Epileptic Disord 19: 379-382, 2017. [DOI] [PubMed] [Google Scholar]
- 35. Duberkar D, Jawale R. Transient lesion in the splenium of corpus callosum due to abrupt phenytoin withdrawal. Neurol India 65(Suppl): S104, 2017. [DOI] [PubMed] [Google Scholar]
- 36. Öztoprak Î, Engin A, Gümüs C, Egilmez H, Öztoprak B. Transient splenial lesions of the corpus callosum in different stages of evolution. Clin Radiol 62: 907-913, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Tomizawa Y, Hoshino Y, Sasaki F, et al. Diagnostic utility of splenial lesions in a case of Legionnaires' disease due to Legionella pneumophila serogroup 2. Intern Med 54: 3079-3082, 2015. [DOI] [PubMed] [Google Scholar]
- 38. Vollmann H, Hagemann G, Mentzel HJ, Witte OW, Redecker C. Isolated reversible splenial lesion in tick-borne encephalitis: a case report and literature review. Clin Neurol Neurosurg 113: 430-433, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Renard D, Taieb G, Briere C, Bengler C, Castelnovo G. Mild encephalitis/encephalopathy with a reversible splenial, white matter, putaminal, and thalamic lesions following anti-Yo rhombencephalitis. Acta Neurol Belg 112: 405-407, 2012. [DOI] [PubMed] [Google Scholar]
- 40. Kim JH, Choi JY, Koh SB, Lee Y. Reversible splenial abnormality in hypoglycemic encephalopathy. Neuroradiology 49: 217-222, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Dong X, Cong S. Reversible splenial lesion syndrome associated with acute Mycoplasma pneumoniae-associated encephalitis: A report of four cases and literature review. Exp Ther Med 16: 2152-2159, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Wang X, Shi X, Qiu W, Miao A. Reversible lesion involving the splenium of the corpus callosum caused by phenytoin sodium withdrawal. Neurol Sci 38: 689-691, 2017. [DOI] [PubMed] [Google Scholar]
- 43. Kang K, Song YM, Jo KD, Roh JK. Diffuse lesion in the splenium of the corpus callosum in patients with methyl bromide poisoning. J Neurol Neurosurg Psychiatry 77: 703-704, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gawlitza M, Hoffmann KT, Lobsien D. Mild encephalitis/encephalopathy with reversible splenial and cerebellar lesions (MERS type II) in a patient with hemolytic uremic syndrome (HUS). J Neuroimaging 25: 145-146, 2015. [DOI] [PubMed] [Google Scholar]
- 45. Degirmenci E, Degirmenci T, Cetin EN, Kiroglu Y. Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) in a patient presenting with papilledema. Acta Neurol Belg 115: 153-155, 2015. [DOI] [PubMed] [Google Scholar]
- 46. Li C, Wu X, Qi H, et al. Reversible splenial lesion syndrome associated with lobar pneumonia: case report and review of literature. Medicine (Baltimore) 95: e4798, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oaklander AL, Buchbinder BR. Pregabalin-withdrawal encephalopathy and splenial edema: a link to high-altitude illness? Ann Neurol 58: 309-312, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Miyakawa Y, Fuchigami T, Aoki M, et al. Agraphia with reversible splenial corpus callosum lesion caused by hypoglycemia. Brain Dev 40: 592-595, 2018. [DOI] [PubMed] [Google Scholar]
- 49. Ma X, Su W, Chen H. Reversible splenial lesion syndrome after blood transfusion presents callosal disconnection syndrome: a case report. Medicine (Baltimore) 97: e11127, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lebecque O, Mulquin N, Dupont M. Cytotoxic lesion of the corpus callosum caused by puumala hantavirus infection. J Belg Soc Radiol 103: 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gruhbaum B, Salzer H, Nasel C, Lernbass I. Reversible cytotoxic oedema in the splenium of the corpus callosum related to tetracycline therapy. Pediatr Radiol 40: 1693-1695, 2010. [DOI] [PubMed] [Google Scholar]
- 52. Rocha AJ da, Reis F, Gama HP, et al. Focal transient lesion in the splenium of the corpus callosum in three non-epileptic patients. Neuroradiology 48: 731-735, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Steiner T, Ettinger J, Peng Z, et al. Hyperintense lesion in the corpus callosum associated with Puumala hantavirus infection. J Neurol 259: 1742-1745, 2012. [DOI] [PubMed] [Google Scholar]
- 54. Samanta D. Transient lesion in the splenium of the corpus callosum in status migrainosus. Acta Neurol Belg 115: 397-398, 2015. [DOI] [PubMed] [Google Scholar]
- 55. Winslow H, Mickey B, Frohman EM. Sympathomimetic-induced kaleidoscopic visual illusion associated with a reversible splenium lesion. Arch Neurol 63: 135-137, 2006. [DOI] [PubMed] [Google Scholar]
- 56. Shibuya H, Osamura K, Hara K, Hisada T. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion due to Mycoplasma pneumoniae infection. Intern Med 51: 1647-1648, 2012. [DOI] [PubMed] [Google Scholar]
- 57. Conry RM, Sullivan JC, Nabors LB 3rd. Ipilimumab-induced encephalopathy with a reversible splenial lesion. Cancer Immunol Res 3: 598-601, 2015. [DOI] [PubMed] [Google Scholar]
- 58. Mitaki S, Onoda K, Ishihara M, Nabika Y, Yamaguchi S. Dysfunction of default-mode network in encephalopathy with a reversible corpus callosum lesion. J Neurol Sci 317: 154-156, 2012. [DOI] [PubMed] [Google Scholar]
- 59. Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T. Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. AJNR Am J Neuroradiol 27: 1983-1986, 2006. [PMC free article] [PubMed] [Google Scholar]
- 60. Tascilar N, Aydemir H, Emre U, Unal A, Atasoy HT, Ekem S. Unusual combination of reversible splenial lesion and meningitis-retention syndrome in aseptic meningomyelitis. Clinics (Sao Paulo) 64: 932-937, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alakbarova N, Eraslan C, Celebisoy N, Karasoy H, Gonul AS. Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) development after Amanita phalloides intoxication. Acta Neurol Belg 116: 211-213, 2016. [DOI] [PubMed] [Google Scholar]
- 62. Ko SY, Kim BK, Kim DW, et al. Reversible splenial lesion on the corpus callosum in nonfulminant hepatitis A presenting as encephalopathy. Clin Mol Hepatol 20: 398-401, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hibino M, Horiuchi S, Okubo Y, Kakutani T, Ohe M, Kondo T. Transient hemiparesis and hemianesthesia in an atypical case of adult-onset clinically mild encephalitis/encephalopathy with a reversible splenial lesion associated with adenovirus infection. Intern Med 53: 1183-1185, 2014. [DOI] [PubMed] [Google Scholar]
- 64. Takanashi J, Barkovich AJ, Shiihara T, et al. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am J Neuroradiol 27: 836-838, 2006. [PMC free article] [PubMed] [Google Scholar]
- 65. Takanashi J, Hirasawa K, Tada H. Reversible restricted diffusion of entire corpus callosum. J Neurol Sci 247: 101-104, 2006. [DOI] [PubMed] [Google Scholar]
- 66. Cho JS, Ha SW, Han YS, et al. Mild encephalopathy with reversible lesion in the splenium of the corpus callosum and bilateral frontal white matter. J Clin Neurol 3: 53-56, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hagemann G, Mentzel HJ, Weisser H, Kunze A, Terborg C. Multiple reversible MR signal changes caused by Epstein-Barr virus encephalitis. AJNR Am J Neuroradiol 27: 1447-1449, 2006. [PMC free article] [PubMed] [Google Scholar]
- 68. Kitami M, Kubo S, Nakamura S, Shiozawa S, Isobe H, Furukawa Y. Acute urinary retention in a 23-year-old woman with mild encephalopathy with a reversible splenial lesion: a case report. J Med Case Rep 5: 159, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shankar B, Narayanan R, Muralitharan P, Ulaganathan B. Evaluation of mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) by diffusion-weighted and diffusion tensor imaging. BMJ Case Rep 2014: bcr2014204078, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bottcher J, Kunze A, Kurrat C, et al. Localized reversible reduction of apparent diffusion coefficient in transient hypoglycemia-induced hemiparesis. Stroke 36: e20-e22, 2005. [DOI] [PubMed] [Google Scholar]
- 71. Oster J, Doherty C, Grant PE, Simon M, Cole AJ. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia 44: 852-854, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Narita H, Odawara T, Kawanishi C, Kishida I, Iseki E, Kosaka K. Transient lesion in the splenium of the corpus callosum, possibly due to carbamazepine. Psychiatry Clin Neurosci 57: 550-551, 2003. [DOI] [PubMed] [Google Scholar]
- 73. Nishimura K, Takei N, Suzuki K, et al. A transient lesion in splenium of the corpus callosum in a patient with childhood-onset anorexia nervosa. Int J Eat Disord 39: 527-529, 2006. [DOI] [PubMed] [Google Scholar]
- 74. Okada K, Fujiwara H, Tsuji S. X-linked Charcot-Marie-Tooth disease with transient splenium lesion on MRI. Intern Med 45: 33-34, 2006. [DOI] [PubMed] [Google Scholar]
- 75. Agarwal A, Kanupriya V, Maller V. Transient restricted diffusion in the splenium of the corpus callosum in migraine with aura. Wien Klin Wochenschr 124: 146-147, 2012. [DOI] [PubMed] [Google Scholar]