Abstract
A 51-year-old man developed a sudden headache during golf practice, followed by a high fever. He was admitted with suspected neutrophilic meningitis and was diagnosed with chemical meningitis caused by a dermoid cyst rupture based on the characteristic magnetic resonance imaging (MRI) findings, which showed multiple lipid droplets in his ventricle and cistern. His repetitive golf-swing motion was suggested to be the cause of his dermoid cyst rupture. On MRI, the lipid droplets appeared to have migrated by gravity because of the body position. Therefore, the body position should be considered to prevent obstructive hydrocephalus by lipid droplets after a dermoid cyst rupture.
Keywords: dermoid cyst rupture, chemical meningitis, lipid droplet, gravity, golf swing
Introduction
Dermoid cysts are rare, benign, intracranial tumors. They seldom rupture; however, dermoid cyst ruptures often result in complications, including chemical meningitis and obstructive hydrocephalus (1). A dermoid cyst rupture is diagnosed by the presence of multiple lipid droplets, which flow from the ruptured cyst into the subarachnoid space and ventricles, on magnetic resonance imaging (MRI). Spontaneous ruptures and trauma are common causes of a dermoid cyst rupture (2), but sport activities have not been reported as a probable cause to date. We herein describe a case of chemical meningitis after a dermoid cyst rupture, which we speculate was induced by his repetitive golf-swing motion, and discuss the implications of golf-swinging in association with a dermoid cyst rupture. In addition, we demonstrate the movement of lipid droplets with changes in body positions and discuss the effect of gravity on the migration of lipid droplets, which might cause obstructive hydrocephalus.
Case Report
A 51-year-old man presented at our department with a severe headache and a high fever. His medical history included herpes zoster and sudden deafness without sequelae. He had no allergies or family history of any significant medical condition. Five days before presentation, he went to a driving range for golf practice. On hitting the 70th golf ball, he suddenly experienced an uncomfortable feeling in his head, followed by nausea and dizziness. After returning home, he had a headache and also vomited. The next day, he developed a fever of 38.4°C, which subsided after the oral administration of acetaminophen. His fever and headache gradually resolved over the next three days. A day before presentation, his headache and fever relapsed, and could not be ameliorated with oral acetaminophen. His symptoms thereafter deteriorated, and the patient presented at our hospital on foot.
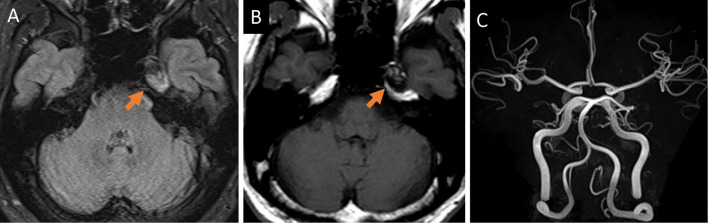
On physical examination, his body temperature was 38.6℃ and he was awake and alert. He had nuchal rigidity and a worsening or jolt accentuation of headache. No skin rash or other neurological focal symptoms were observed. Laboratory test results revealed an elevated C-reactive protein level of 0.51 mg/dL and a high white blood cell (WBC) count of 9.3×103/μL. A cerebrospinal fluid (CSF) analysis revealed a white and turbid appearance with a high opening pressure of 270 mm H2O, elevated WBC count of 556/mm3 with neutrophilic pleocytosis of 96% neutrophils, elevated protein levels of 59 mg/dL, and low glucose levels of 38 mg/dL with low CSF/blood glucose ratio of 0.32. Gram staining of CSF was negative for microorganisms. Brain computed tomography showed multiple low-density particles in the ventricles and cisterns (Fig. 1A, B) with a high signal intensity on MRI T1-weighted images (T1-WI) (Fig. 1C, D). These particles had a high signal intensity on fluid-attenuated inversion recovery (FLAIR) images (Fig. 1E), which disappeared on fat-suppressed FLAIR sequences (Fig. 1F), with no enhancement on gadolinium-enhanced T1-WI (Fig. 1G). Susceptibility-weighted imaging (SWI) revealed more low-intensity particles in the cerebral sulcus and cisterns than those on T1-WI (Fig. 1H). A residual dermoid cyst was observed near the left cavernous sinus (Fig. 2A, B). MR angiography showed normal findings, including a normal vertebral artery and basilar artery (Fig. 2C).
Figure 1.

Low-density particles were visible in the cerebral ventricles and cisterns on brain computed tomography (A and B, arrows); they showed high signal intensities on T1-weighted image (T1-WI) (C and D, arrows). These particles showed high intensities on fluid-attenuated inversion recovery (FLAIR) image (E), but disappeared on fat-suppressed FLAIR imaging (F) and showed no enhancement on gadolinium-enhanced T1-WI (G). Susceptibility-weighted imaging showed more low-intensity particles in the cisterns than those on T1-WI (H, arrows).
Figure 2.
Residual dermoid cyst, which showed high intensities with small iso-intensity on fluid-attenuated inversion recovery image (A, arrow) and mosaic pattern with high and low intensity on T1-WI (B, arrow), can be seen near the left cavernous sinus. Magnetic resonance angiography showed normal findings, including a normally appearing vertebral and basilar artery (C).
We diagnosed the patient to have chemical meningitis due to a golf swing-induced dermoid cyst rupture and admitted him to our hospital. Because the possibility of bacterial meningitis could not be ruled out from the CSF findings, intravenous meropenem and vancomycin were administered until a negative CSF culture result was obtained on the 5th day after admission.
The patient's symptoms gradually improved with rest. He spent most of his time in the hospital (including sleep time) in the sitting position, as his headache was least intense when he was seated upright. T1-WI on day 10 after admission revealed the migration of lipid droplets from the cephalad toward the cerebral sulcus (Fig. 3A, B), due to the effects of gravity. Furthermore, the lipid droplets in the anterior horn of the lateral ventricle migrated to the posterior horn against gravity when the patient changed his position from supine to prone (Fig. 3C, D). On the 18th day after admission, we confirmed symptom relief and an improvement of the CSF examination results. The patient was discharged and instructed to avoid handstands and diving to prevent the occurrence of obstructive hydrocephalus.
Figure 3.
T1-WI at admission reveal multiple lipid droplets in the cisterns and cerebral sulcus (A, arrows), and that on T1-WI acquired at day 10 since admission, some lipid droplets moved from the cephalad into the cerebral sulcus (arrowheads) against gravity (B). T1-WI showed migration of one lipid droplet from the anterior horn of the lateral ventricle in the supine position (C, arrowhead) to the posterior horn in the prone position (D, arrowhead) against gravity, which was different from a droplet in the Sylvian fissure that did not move (arrow).
Discussion
It has been reported that 8.2% cases of dermoid cyst ruptures present with chemical meningitis (1). The CSF examination findings of high protein levels, low glucose level, and neutrophilic pleocytosis in patients with chemical meningitis resemble those of patients with bacterial meningitis (3,4); however, the presence of multiple lipid droplets in the subarachnoid space, visualized on MRI scanning, helped in making a differential diagnosis. High-intensity particles visible on both T1-WI and T2-WI/FLAIR, but absent on fat-suppressed MRI, is a characteristic radiological pattern (5). Lipid particles presenting as artifacts, known as “chemical shift artifacts” on SWI, were also the main indicators of diagnosis (5). Steroid therapy has been reported as a potential treatment strategy for chemical meningitis caused by a dermoid cyst rupture (4). We did not administer steroids in this case because we could not initially exclude the possibility of bacterial meningitis, and moreover, his symptoms subsided when the antibiotics were stopped.
Based on the patient's medical history, we speculate that the dermoid cyst may possibly have ruptured during his golf-swing motion. Head movements and even brain pulsations, such as the hammer-like actions, have been suggested as probable actions that cause a spontaneous dermoid cyst rupture (6). Golf-swings are high-speed, forceful, rotational movements of the head and body, with a prominent shift of body-weight (7). However, amateur golfers tend to show a vertical head movement, such as the head-down movement, during the downward swing, and an early head-up movement while hitting the ball (8). Moreover, players swing dozens of times over a short duration when practicing golf at a driving range. In our case, we believe that the repeated golf-swings, accompanied by the strained, hammer-like vertical head movements, had thus induced the dermoid cyst rupture.
Obstructive hydrocephalus is a complication of a dermoid cyst rupture (5). Obstruction of the intraventricular pathways at the aqueduct, fourth ventricle, and foramen of Monro by lipid droplets is a possible cause (9,10). Our case demonstrated the migration of lipid droplets in the cerebral sulcus and ventricle due to the effects of gravity. To prevent obstructive hydrocephalus after a dermoid cyst rupture, the body posture of patients should be considered owing to the effect of gravity on the lipid droplets.
In conclusion, we herein described a case of chemical meningitis caused by a dermoid cyst rupture that was presumed to have been induced by a repetitive golf-swinging motion. This case report, along with characteristic MRI findings, might help clinicians to identify a repetitive golf-swinging motion as a probable cause of dermoid cyst rupture and thus establish a diagnosis. This report also informs clinicians that the effect of gravity and body position on the migration of lipid droplets should be considered to prevent obstructive hydrocephalus after a dermoid cyst rupture.
Informed consent was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr. Nakauchi for his comments on the diagnosis and treatment and Mr. Amano for his cooperation in performing the MRI scans.
References
- 1.El-Bahy K, Kotb A, Galal A, El-Hakim A. Ruptured intracranial dermoid cysts. Acta Neurochir (Wien) 148: 457-462, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Ramlakhan R, Candy S. Traumatic rupture of an intracranial dermoid cyst. Radiol Case Rep 10: 1053, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro S, Castelnovo G, Lebayon A, Fuentes S, Bouly S, Labauge P. Chemical meningitis in reaction to subarachnoid fatty droplets. Neurology 65: 937, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Yamagami K, Kakuta N, Seki K, et al. Acute urinary retention induced by chemical meningitis which occurred due to a ruptured dermoid cyst. Intern Med 57: 729-731, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shashidhar A, Sadashiva N, Prabhuraj AR, et al. Ruptured intracranial dermoid cysts: a retrospective institutional review. J Clin Neurosci 67: 172-177, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Lunardi P, Missori P. Supratentorial dermoid cysts. J Neurosurg 75: 262-266, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Choi MH, Hong JM, Lee JS, Shin DH, Choi HA, Lee K. Preferential location for arterial dissection presenting as golf-related stroke. Am J Neuroradiol 35: 323-326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong JM, Kim TJ, Lee JS, Lee JS. Repetitive internal carotid artery compression of the hyoid: a new mechanism of golfer's stroke? J Neurol Neurosurg Psychiatry 82: 233-234, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Karabulut N, Oguzkurt L. Tetraventricular hydrocephalus due to ruptured intracranial dermoid cyst. Eur Radiol 10: 1810-1811, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Orakcioglu B, Halatsch ME, Fortunati M, Unterberg A, Yonekawa Y. Intracranial dermoid cysts: variations of radiological and clinical features. Acta Neurochir (Wien) 150: 1227-1234, 2008. [DOI] [PubMed] [Google Scholar]