Abstract
Anti-transcriptional intermediary factor 1γ (anti-TIF1γ) antibody-positive dermatomyositis (DM) is strongly associated with cancer, although the mechanism of action is still unclear. We herein describe the first known case of an 80-year-old woman diagnosed with TIF1γ-positive primary pulmonary lymphoepithelioma-like carcinoma (LELC) coexisting with anti-TIF1γ antibody-positive DM. The diagnosis of LELC can only be made by a surgical lung biopsy, and not by a computed tomography-guided biopsy, because of heavy lymphocytic infiltration. This instructive case reaffirmed the importance of active screening for malignancy in patients with anti-TIF1γ antibody-positive DM. Interestingly, the results also suggested that the strong relationship which exists between anti-TIF1γ antibody-positive DM and cancer is potentially caused by tumor-derived TIF1γ.
Keywords: primary lung lymphoepithelial carcinoma, dermatomyositis, anti-transcriptional intermediary factor 1γ, Epstein-Barr virus
Introduction
Lymphoepithelioma-like carcinoma (LELC) is an Epstein-Barr virus (EBV)-associated undifferentiated carcinoma (1-3). It is morphologically characterized by heavy lymphocytic infiltration around the nest or nodules of malignant epithelial cells and it usually develops in the nasopharynx, stomach, and lung (4). The major histological types of primary lung cancer are adenocarcinoma, squamous cell carcinoma, and small cell carcinoma, and primary LELC of the lung is very rare among primary lung cancers (5-7).
Dermatomyositis (DM) is a systemic autoimmune disease characterized by inflammation in multiple organs, most commonly in the skin and muscle (8). The cause of DM is still unclear, but several potential triggers, such as cancer (9-12) and viral infection (13-16), have been reported. Especially, regarding the relationship between the pathophysiology of DM and cancer, the treatment of cancer which is associated with DM sometimes leads to an improvement in the DM-related symptoms. Therefore, in patients with DM, it is important to perform cancer screening and timely therapeutic intervention (17). In particular, anti-transcriptional intermediary factor 1γ (anti-TIF1γ) antibody-positive DM is associated with a higher prevalence of cancer (18, 19), although the reason for this association is still unclear.
We herein present an instructive case of primary pulmonary LELC diagnosed by video-assisted thoracoscopic surgery (VATS) rather than computed tomography (CT)-guided biopsy in a patient with anti-TIF1γ antibody-positive DM. Interestingly, immunohistochemistry showed TIF1γ expression in the LELC tissues. This supports the existence of a potential relationship between tumor-derived TIF1γ and anti-TIF1γ antibody-positive DM.
Case Report
An 80-year-old Japanese woman, who was not on medication and had no family history of autoimmune disorders and cancers, presented with gradually progressive dyspnea, dysphagia, and muscle weakness 2 weeks before visiting another hospital. Because she needed artificial respiration management with tracheal intubation due to aspiration pneumonia caused by dysphagia, she was transported to our hospital. Upon physical examination, the patient was found to have bilateral heliotrope edema including the upper eyelids with erythematosquamous plaques. Additionally, she had a pronounced diffuse rash on the upper chest (shawl sign) and discrete red papules over the finger joints of both hands (Gottron's papules; Fig. 1). Blood tests were positive for anti-TIF1γ antibody. Creatine kinase was slightly elevated (268 IU/L), whereas alanine aminotransferase, aspartate aminotransferase, and C-reactive protein were normal (Table). An electromyogram and muscle biopsy could not be performed. According to these findings, she was diagnosed with probable DM because three of five requirements for the diagnostic criteria of DM proposed by Bohan and Peter (20) were met. Takotsubo cardiomyopathy was a coexisting complication, potentially because of excessive stress due to her respiratory failure. Therefore, she first received antibiotic treatment and catecholamine support under artificial respiration. For DM, we also started intravenous pulse steroid therapy (methylprednisolone, 1,000 mg for 3 consecutive days) followed by 1 mg/kg/day of corticosteroids. In addition, chest CT showed a mass in the left lower lobe (Fig. 2A). Increased levels of serum cytokeratin 19 fragment (CYFRA) were observed, however, the serum levels of pro-gastrin releasing peptide (Pro GRP), carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) were within the normal range(Table). After 1 week of therapy, she was removed from the artificial ventilator because her respiratory failure had improved. The symptoms of DM, including dysphasia, had also partially improved. However, CT images showed that the mass in the left lower lobe had not changed in size for 1 month, and therefore, the mass was strongly suspected to be a malignancy. A CT-guided biopsy for the nodule revealed only lymphocyte infiltration and slight fibrosis, but no malignant cells (Fig. 2B, C). Because the patient had anti-TIF1γ antibody-positive DM, we strongly suspected that the nodule was lung cancer and decided to perform a VATS biopsy. Finally, a histopathological diagnosis of LELC was obtained; Hematoxylin and Eosin staining showed that the nuclei were round with irregular borders, and both the tumor and stromal tissues contained large reactive lymphoplasmacytic cells (Fig. 3A, B). Immunohistochemistry showed that the expression of Epstein-Barr virus-encoding small RNA (EBER) (Fig. 3C, D) and TIF1γ (Fig. 3E, F) in LELC were elevated compared to that in the normal lung (Fig. 3G). Immunohistochemical staining of TIF1γ was positive based on anti-TIF1γ antibody (sc-101179, 1:500, Santa Cruz Biotechnology, Santa Cruz, USA) as previously described (21). Epidermal growth factor receptor (EGFR) mutations and echinoderm microtubule associated protein-like 4-anaplastic lymphoma kinase (EML 4-ALK) fusion were negative, whereas anti-programmed death Ligand-1 (PD-L1) was detected in 70% of the cells. The LELC was diagnosed as Stage III based on positron emission tomography (PET)-CT showing an accumulation of fluorodeoxyglucose in the primary tumor [standard uptake value (SUV) max of 15.5] (Fig. 4A), para-aortic lymph node metastasis (SUVmax 13.3) (Fig. 4B), and left interlobar lymph node metastasis (SUVmax 9.9) (Fig. 4C, D). Radical radiotherapy was performed, and both her muscle weakness and dysphagia further improved after radiotherapy.
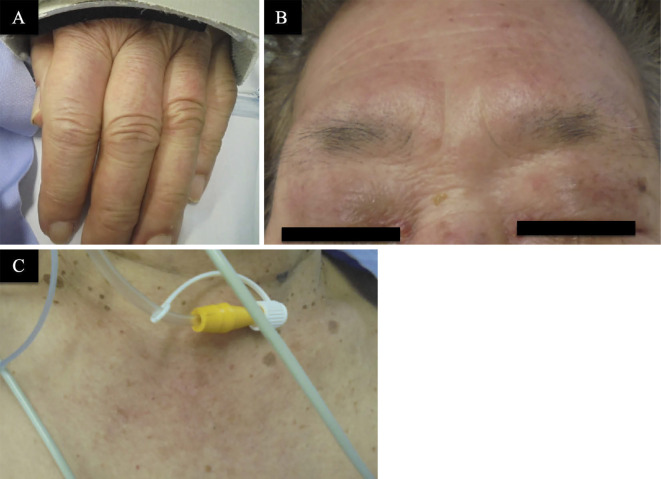
Figure 1.
Clinical skin images. A: Gottron’s sign on the dorsum of the hands. B: Facial erythema (heliotrope rash). C: V-sign (a typical distribution of macular exanthema on the front site of the patient’s chest).
Table.
Laboratory Findings
| Hematology | ||
| WBC | 13,030 | /μL |
| Neutrophils (%) | 91.4 | % |
| Lymphocytes (%) | 4.0 | % |
| Monocytes (%) | 4.4 | % |
| Hb | 12.6 | g/dL |
| PLT | 238,000 | /μL |
| Biochemistry | ||
| Total protein | 5.6 | g/dL |
| Albumin | 3.0 | g/dL |
| Total bilirubin | 0.9 | mg/dL |
| AST | 25 | U/L |
| ALT | 38 | U/L |
| LDH | 301 | U/L |
| BUN | 35.4 | mg/L |
| Cre | 0.44 | mg/dL |
| Na | 136 | mEg/L |
| K | 3.8 | mEg/L |
| Cl | 94 | mEg/L |
| CK | 268 | IU/L |
| CRP | 0.02 | mg/dL |
| Tumor marker | ||
| CEA | 3.0 | ng/mL |
| CYFRA | 8.9 | ng/mL |
| ProGRP | 80.7 | pg/mL |
| NSE | 13.1 | ng/mL |
| Serology | ||
| ANA | ×160 | |
| Speckled | ||
| Anti-ARS antibody | Undetectable | |
| Anti-MDA-5 antibody | Undetectable | |
| Anti-Mi-2 antibody | Undetectable | |
| Anti-TIF1γ-antibody | 125 | index |
| Anti-RNP antibody | Undetectable | |
| Infection | ||
| β-D-glucan | Undetectable |
WBC: white blood cells, Hb: hemoglobin, PLT: platelets, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, Na: sodium, K: potassium, Cl: chlorine, Cre: creatinine, BUN: blood urea nitrogen, CRP:C-reactive protein, CEA: carcinoembryonic antigen, CYFRA: cytokeratin 19 fragment, ProGRP: Pro-gastrin-releasing peptide, NSE: neuron-specific enolase, ANA: antinuclear antibodies, ARS: aminoacyl-tRNA synthetase, MDA-5:melanoma differentiation-associated gene-5, Mi-2: complex nucleosome remodeling histone deacetylase, TIF1γ: transcription intermediary factor 1 gamma, RNP: ribonucleoprotein
Figure 2.

Chest CT findings. Chest CT showed a lung mass in the left lower lobe (A). Chest CT during needle biopsy shows the guide needle of the coaxial system within the mass (B). The histological findings indicated no malignancy (C).
Figure 3.
Histopathology and immunohistochemistry of primary lung lymphoepithelial carcinoma. Hematoxylin and Eosin staining is presented at ×200 and ×400 magnification, respectively (A, B). Epstein-Barr virus-encoded small RNA in situ hybridization is presented at ×200 and ×400 magnification, respectively (C, D). Immunostaining for transcriptional intermediary factor 1γ (TIF1γ) is presented at ×200 and ×400 magnification, respectively (E, F). Immunostaining for TIF1γ in normal lung tissues from the same patient is presented at ×200 magnification (G). Scale bar=100 μm.
Figure 4.
A PET-CT scan of the patient, which revealed para-aortic and left interlobar metastasis of primary lung cancer. In the primary focus (A), para-aortic metastasis (B), and left interlobar metastasis (C, D), hypermetabolic activities were seen.
However, LELC recurred 6 months after the end of cancer treatment, and both her muscle weakness and dysphagia worsened in association with the recurrence. Therefore, we prescribed an increased dose of corticosteroids to control the symptoms of DM. We considered cancer treatment with an anti-programmed death 1 (PD-1) antibody based on the high PD-L1 expression. However, the patient was elderly, had autoimmune disease-associated dysphagia, and was being treated with high-dose corticosteroids. These factors are associated with a lower efficacy (22) and more severe adverse effects of anti-PD-1 antibody therapy (23-25). Therefore, cytotoxic chemotherapy was recommended, but the patient instead chose best supportive care.
Discussion
This is the first report of a patient diagnosed with the coexistence of primary pulmonary LELC and anti-TIF1γ antibody-positive DM. Remarkably, the LELC in this case was diagnosed by a VATS biopsy rather than by a CT-guided biopsy. It was previously reported that the sensitivity of CT-guided biopsy is 90% or greater for the diagnosis of lung cancer (26), suggesting that VATS biopsy is unnecessary when CT-guided biopsy does not show malignant findings. However, we performed VATS biopsy for two reasons. First, the patient was diagnosed with anti-TIF1γ antibody-positive DM, which is associated with a higher prevalence in patients with cancer. Patients with DM who are positive for the anti-TIF1γ antibody have an 8-fold higher risk of cancer than patients who are negative (27, 28). In particular, the standardized incidence ratio of lung cancer is 20.58 in patients with anti-TIF1γ antibody-positive DM (12). Additionally, in most patients with cancers related to DM, cancers are diagnosed within 1 year of the diagnosis of DM (17, 19, 29). Second, the tissue obtained through CT-guided biopsy showed lymphocyte infiltration, and we expected the pulmonary nodule to have decreased in size due to the administration of corticosteroids. However, the pulmonary mass did not regress at 1 month after the start of treatment. The lack of a diagnosis by CT-guided biopsy might be explained by the characteristic tissue morphology of LELC, which was accompanied by lymphocyte infiltration around malignant cells; the tissue sampled via CT-guided biopsy was smaller than that sampled through a VATS biopsy and included only lymphocyte infiltration but not any malignant tumor cells. This study reaffirmed the importance of active screening for malignancy for patients with DM, especially anti-TIF1γ antibody-positive patients.
In this case, TIF1γ-positive LELC coexisted with anti-TIF1γ antibody-positive DM, which might suggest a relationship between cancer and DM via the immune response to TIF1γ. It is unclear why patients with DM frequently develop malignancies, which is especially true for patients positive for the anti-TIF1γ antibody. TIF1γ is a unique molecule that has been reported to not only be a tumor suppressor but also an enhancer of tumorigenesis (27, 28, 30). Addtionally, genetic alterations in TIF1γ might lead to neoantigen formation, thereby activating the adaptive and innate anti-tumor immune response (31). Furthermore, previous reports have described patients with TIF1γ-positive endometrial cancer and colorectal cancer with anti-TIF1γ antibody-positive DM (32, 33). These data indicate that TIF1γ expression in the tumor can be recognized as an antigen by the immune system, resulting in anti-TIF1γ antibody production, followed by DM development. Alternatively, LELC is an EBV-associated cancer, and EBV was also reported to induce DM and myositis (14, 34, 35). Additionally, high standardized incidence rates of EBV-associated cancer such as nasopharynx cancer and lymphoma/leukemia have been reported in patients with DM (12). However, there have been no reports of EBER- and TIF1γ-positive cancer except for this case, and therefore, further investigations are needed to elucidate the relationship between EBV and TIF1γ expression in the tumor by investigating the frequencies of TIF1γ expression in EBV-associated cancer.
Conclusion
This is the first report of a patient with TIF1γ-positive primary pulmonary LELC with anti-TIF1γ antibody-positive DM in which our findings suggested that TIF1γ in tumors is associated with the development of anti-TIF1γ antibody-positive DM. Additionally, our results emphasize the potential for the misdiagnosis of primary pulmonary LELC when using small samples, such as those obtained by a CT-guided biopsy.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Xie MM, Wu X, Wang F, et al. Clinical significance of plasma Epstein-barr virus DNA in pulmonary lymphoepithelioma-like carcinoma (LELC) patients. J Thorac Oncol 13: 218-227, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Ma BBY , Chan ATC, Chan FKL, Murray P, Tao Q. Epstein-barr virus-induced epigenetic pathogenesis of viral-associated lymphoepithelioma-like carcinomas and natural killer/T-cell lymphomas. Pathogens 7: 63, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie C, Xu X, Wu B, Yang KY, Huang J. Primary pulmonary lymphoepithelioma-like carcinoma in non-endemic region: a case report and literature review. Medicine (Baltimore) 97: e9976, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kobayashi M, Ito M, Sano K, Honda T, Nakayama J. Pulmonary lymphoepithelioma-like carcinoma: predominant infiltration of tumor-associated cytotoxic T lymphocytes might represent the enhanced tumor immunity. Intern Med 43: 323-326, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Castro CY, Ostrowski ML, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol 32: 863-872, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 11: 539-545, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Chang YL, Wu CT, Shih JY, Lee YC. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol 26: 715-723, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med 325: 1487-1498, 1991. [DOI] [PubMed] [Google Scholar]
- 9. Chow WH, Gridley G, Mellemkjaer L, McLaughlin JK, Olsen JH, Fraumeni JF Jr. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control 6: 9-13, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med 134: 1087-1095, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Barnes BE, Mawr B. Dermatomyositis and malignancy. A review of the literature. Ann Intern Med 84: 68-76, 1976. [DOI] [PubMed] [Google Scholar]
- 12. Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL, Chang YT. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther 12: R70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang PY, Zhong ZL, Luo DH, et al. Paired study of 172 cases of nasopharyngeal carcinoma with or without dermatomyositis. Acta Otolaryngol 134: 824-830, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Chen DY, Chen YM, Lan JL, et al. Polymyositis/dermatomyositis and nasopharyngeal carcinoma: the Epstein-Barr virus connection? J Clin Virol 49: 290-295, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Carroll MB, Holmes R. Dermatomyositis and HIV infection: case report and review of the literature. Rheumatol Int 31: 673-679, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Chou JW, Lin YL, Cheng KS, Wu PY, Reanne Ju T. Dermatomyositis induced by hepatitis B virus-related hepatocellular carcinoma: a case report and review of the literature. Intern Med 56: 1831-1837, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357: 96-100, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Fiorentino D, Casciola-Rosen L. Autoantibodies to transcription intermediary factor 1 in dermatomyositis shed insight into the cancer-myositis connection. Arthritis Rheum 64: 346-349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hida A, Yamashita T, Hosono Y, et al. Anti-TIF1-γ antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology 87: 299-308, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med 292: 344-347, 1975. [DOI] [PubMed] [Google Scholar]
- 21. Horimasu Y, Ishikawa N, Taniwaki M, et al. Gene expression profiling of idiopathic interstitial pneumonias (IIPs): identification of potential diagnostic markers and therapeutic targets. BMC Med Genet 18: 88, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol 13: 1771-1775, 2018. [DOI] [PubMed] [Google Scholar]
- 23. Kudo F, Watanabe Y, Iwai Y, et al. Advanced lung adenocarcinoma with nivolumab-associated dermatomyositis. Intern Med 57: 2217-2221, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune disease by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11: 141-151, 1999. [DOI] [PubMed] [Google Scholar]
- 25. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol 36: 1905-1912, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143: e142S-e165S, 2013. [DOI] [PubMed] [Google Scholar]
- 27. Trallero-Araguás E, Rodrigo-Pendás JÁ, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 64: 523-532, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Fujimoto M, Hamaguchi Y, Kaji K, et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum 64: 513-522, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Masiak A, Kulczycka J, Czuszyńska Z, Zdrojewski Z. Clinical characteristics of patients with anti-TIF1-γ antibodies. Reumatologia 54: 14-18, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu C, Ding Z, Liang H, Zhang B, Chen X. The roles of TIF1γ in cancer. Front Oncol 9: 979, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tate JG, Bamford S, Jubb HC, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res 47: D941-D947, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kasuya A, Hoshino T, Aoshima M, Tatsuno K, Fujiyama T, Tokura Y. TGFβ/SMAD4 signalling is inhibited in tumour cells and infiltrating lymphocytes of a patient with colon cancer-associated dermatomyositis. J Eur Acad Dermatol Venereol 29: 2265-2267, 2015. [DOI] [PubMed] [Google Scholar]
- 33. Kasuya A, Hamaguchi Y, Fujimoto M, Tokura Y. TIF1γ-overexpressing, highly progressive endometrial carcinoma in a patient with dermato-myositis positive for malignancy-associated anti-p155/140 autoantibody. Acta Derm Venereol 93: 715-716, 2013. [DOI] [PubMed] [Google Scholar]
- 34. Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya JM, Shoenfeld Y. Epstein-Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci 1108: 567-577, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Yamashita K, Hosokawa M, Hirohashi S, et al. Epstein-Barr virus-associated gastric cancer in a patient with dermatomyositis. Intern Med 40: 96-99, 2001. [DOI] [PubMed] [Google Scholar]