Abstract
The Amaryllidaceae alkaloids have been a target of synthesis for decades due to their complex architectures and biological activity. A central feature of these natural product cores is a quaternary substituted hydroindole heterocycle. Building off the foundation of our previous multicomponent approach to highly functionalized pyrrolidinones, herein we report a highly convergent, diastereoselective, multicomponent approach to access the hydroindole cores present within crinine, haemanthamine, pretazettine, and various other bioactive alkaloids. These scaffolds are additionally useful as building blocks for druglike molecules and natural product like library generation.
Graphical Abstract
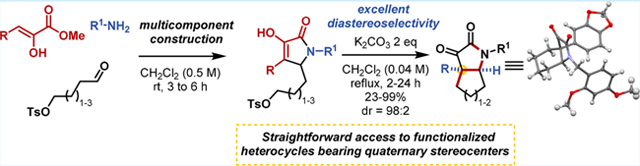
Natural products serve as a rich source of inspiration for a significant portion of approved pharmaceuticals.1,2 Their intricate, three-dimensional structures provide structural diversity and often exhibit a wide range of functionality that translates to bioactivity. As natural product structural complexity increases, synthetic difficulty often increases as well, creating barriers to the practical use of natural products in traditional drug discovery programs. On top of that, natural products often serve as unoptimized hit/lead compounds requiring further synthetic modification to transition to the clinic. These alterations require the generation of functionally diverse chemical libraries to probe valuable chemical space around the natural product scaffold.3 In some cases, the complete natural product structure is required for activity, while in others a fragment can be utilized as a simplified scaffold.4 If the molecule is accessible in suitable quantities from natural sources, semisynthetic methods can provide an efficient approach to confined library generation based on the core scaffold of the natural product. For natural products with less sustainable natural sources or the need for severe molecular modification, total synthesis provides another option for accessing the necessary molecules for development; however, in many cases total syntheses provides low quantities of final product or does not allow for analogue preparation required for effective SAR studies.5
Amaryllidaceae alkaloids (AA) are well-known for their bioactivity with regard to diseases such as malaria, Alzheimer’s, viral infections, and inflammation.6–8 Some members of the family are recognized for their activity against cancer, including multidrug resistant cancer cell lines. A key example is haemanthamine (HAE), an AA belonging to the crinane alkaloid family, which has recently been cocrystallized with the eukaryotic 80S ribosome at the p53 binding clef providing significant insight for further rational analogue design.9 When comparing the structures of the various crinane alkaloids, they all share a common core scaffold known as N,6-seco-crinine (Figure 1). Thus, efficient access to this scaffold would be of high utility for future crinane-based medicinal chemistry efforts and natural product synthesis as well as natural product like library generation.
Figure 1.

(a) Amaryllidaceae alkaloid natural products that possess the N,6-seco-crinine core; (b) potential synthetic intermediates.
As part of a broader program targeting the Amaryllidaceae alkaloids for synthetic and biological studies, we initiated synthetic efforts toward the cores of these molecules via a convergent multicomponent approach. Previously, we have reported a method to access highly functionalized pyrrolidinone cores through a multicomponent reaction.10 Among the various multicomponent approaches to access highly functionalized heterocycles, including pyrrolidinones, imines serve as a common intermediate.11–13 In addition to our efforts, there has been a long history of elegant methods toward these versatile building blocks. Gein and co-workers have contributed significant efforts to the synthesis of these molecules via a similar multicomponent reaction that requires an acyl electron-withdrawing group and prolonged reaction time.14–24 A number of other groups have used analogous strategies, all employing activated precursors and aggressive reaction conditions.25–30 Joo and co-workers and Yavari and Souri independently synthesized related pyrrolidinones utilizing symmetrical alkynes.31,32
In addition to the synthesis of these highly functionalized pyrrolidinones, decades of synthetic work has focused on their functionalization and utilization as synthetic building blocks (Figure 2a). We recently established a method to access highly functionalized pyrrolidinones that provided the foundation for the synthesis of diverse quaternary substituted pyrrolidine-2,3-diones and bicyclic N-heterocycles.10,33,34 These studies were inspired by many previous efforts that demonstrated the value in these building blocks. Early reports include the unique bicycles generated in the presence of formalin accomplished by Southwick and Vida.35 Other bicycles were synthesized by Rapoport,36,37 Kurosawa,38 and Crimmins39 independently preparing β-lactams, peroxides, and cyclobutanes, respectively. There have also been multiple examples of functional group manipulation on these scaffolds, including enamine generation, alkylation, reduction, and use of the enol moiety as a nucleophile.40–43 Our efforts have shown that these pyrrolidones can also be engaged in sigmatropic rearrangements to prepare all-carbon quaternary stereocenters bearing pyrrolidinones capable of further conversion to β-amino acids and β-lactams.10 Herein, we report a concise route to hydroindole cores by incorporating a leaving group in our multicomponent substrate to allow for facile displacement providing a two-step multicomponent protocol to form four key bonds in the crinane alkaloid core (Figure 2b).
Figure 2.

(a) Previous synthetic applications of oxidized pyrrolidinones; (b) this work.
In our initial reaction development, we planned to perform this transformation as a two-step protocol to access these hydroindole cores, with the intermediate pyrrolidone substrate accessed by our previously reported method while circumventing the risk of unwanted amine alkylation by our −OTs aldehyde fragment.10 To focus on the 6-exo-tet cyclization strategy, we selected 4a as a model substrate (Table 1). From our initial screening, we obtained encouraging results with cesium carbonate as the base and dimethylformamide as solvent. We next screened solvents that would promote SN2 reactions; unfortunately, the yield decreased when the solvent was changed from DMF to MeCN at reflux and further decreased in THF at reflux. To our delight, upon conducting the reaction in dichloromethane at reflux, we observed a significant increase in yield. From there, we next screened alternative carbonate bases. Potassium carbonate and sodium carbonate both gave a decrease in reaction rate but a significant increase in yield in the case of potassium carbonate and no reaction with the use of sodium carbonate, highlighting the importance of the counterion in these cyclizations.
Table 1.
Optimization of 6-exo-tet Cyclization
 | ||||
|---|---|---|---|---|
| entry | base | solvent | temp (°C) | yield of 5aa (%) |
| 1 | Cs2CO3 | DMF | 70 °C, 1 h | 32 |
| 2 | Cs2CO3 | MeCN | reflux, 1 h | 23 |
| 3 | Cs2CO3 | THF | reflux, 1 h | 15 |
| 4 | Cs2CO3 | CH2C12 | reflux, 1 h | 55 |
| 5 | K2CO3 | CH2C12 | reflux, 2 h | 93 (92)b |
| 6 | Na2CO3 | CH2C12 | reflux, 2 h | 0 |
All optimization reactions were performed by adding the corresponding base (0.052 mmol, 2.0 equiv) to a solution of compound 4a (15 mg, 0.026 mmol, 0.04 M) in solvent. All reactions were refluxed until consumption of starting material 4a by TLC. All reaction yields were determined by qNMR utilizing ethylene carbonate as standard.
Isolated yield.
With optimized conditions in hand, it was necessary to synthesize the required pyrrolidone substrates. Our previous multicomponent approach provided access to compounds 4a–4n, tolerating a variety of functional groups (Figure 3). In addition to the Dmb-amine utilized in the model system (4a), the reaction also tolerated simple benzylamine (4c), electron-deficient ortho-substituted 2-nitrobenzylamine (4b), and bromide-substituted benzylamine (4d). Additionally, more electron-rich or heteroaromatic benzylamines, such as a protected catechol and thiophene, were also tolerated in this reaction, providing acceptable yields (4e and 4f). With nonaryl groups on the amine fragment, the reaction proceeded in suitable yield, both with allylic (4g), propargylic (4h), and simple methyl amine (4i) with slightly modified conditions (see the SI for further details). We also probed the scope of the multicomponent reaction with nonstyryl pyruvic acid methyl ester derivatives. The reaction tolerated simple phenyl (4j) as well as an electron-deficient ortho nitro functional group (4k). Additionally, the reaction tolerated a protected catechol in the pyruvic ester fragment (4l), representing the aryl group present in many AA natural products. Lastly, we also synthesized substrates to attempt a 5- and 7-exo-tet cyclization. Surprisingly, these three-component reactions did not perform as well as the preceding examples with 5-oxopentyl 4-methylbenzenesulfonate 2a, and we were only able to isolate 4m (7-exo precursor). With regard to the 5-membered ring precursor, we prepared this intermediate and directly used this substrate for the subsequent cyclization.
Figure 3.

Preparation of substrates from the optimized three-component reaction. (a) All reactions were performed by adding 3 (1.1 equiv) to a solution of 1 (1.0 equiv) and 2 (1.1 equiv) in anhydrous dichloromethane (0.5 M). Reactions were monitored by TLC for the consumption of 1, upon which reaction was concentrated to volume and purified by flash column chromatography. (b) For 4i 1.1 equiv of Et3N and 200 μL of methanol was added. (c) Reaction was performed on a 5.16 mmol scale.
With substrates 4a–4m in hand, we next employed our optimized conditions from Table 1 to perform the intramolecular cyclization (Figure 4). Aside from the excellent yield obtained with 5a, we observed slightly diminished yields with an o-nitro substituent on the amine fragment (5b), while simple benzyl and p-bromobenzyl substitution both resulted in excellent yields of products 5c and 5d, respectively. Slightly reduced yields were observed with the highly electron-rich ring fragment 5e, although an excellent yield was obtained when the thiophene was present (5f). All cases proceeded in ≥98:2 dr, highlighting the utility of this strategy to access these fused heterocycles.
Figure 4.

All reactions were performed by adding K2CO3 (2.0 equiv) to a solution of 4 (1.0 equiv, 0.026 mmol) in anhydrous dichloromethane (0.04 M). All products were obtained in ≥98:2 dr. (a) Reaction was performed at a 1.0 mmol scale; (b) reaction utilized 6.0 equiv of K2CO3; (c) reaction time was 12 h; (d) reaction time was 24 h; (e) reaction was performed at a 1.27 mmol scale; (f) reaction time was 5 h; (g) reaction was performed at a 0.32 mmol scale.
While all of the nonaryl amine fragments containing substrates provided excellent yields (5g–5i), when we switched from a styryl substrate to a phenyl substrate we noticed that the reaction rate decreased significantly, generally extending to 24 h (5j). We propose that the nucleophilicity and electron density at the α position of the pyrrolidone ring dictates the rate and success of this reaction. Thus, when an o-nitro was present, the reaction did not proceed at all (5k). Conversely, when we utilized a more electron-rich aryl ring, the rate of reaction increased to previously observed reaction times as seen with the styryl group (5l). Finally, we screened varying ring-size cyclizations. We did not observe a 7-exo-tet cyclization (5m), although we did accomplish a 5-exo-tet cyclization (5n) but in diminished yield over two steps. The difficulties in these cyclizations are somewhat unexpected and appear to be the result of slow product decomposition. The stereoselectivity of this method was confirmed by X-ray analysis (5l) as well as NOE interactions (5a) (Figure 5). To our delight, the product exhibiting a cis ring fusion between the 5–6 ring system found in the AA natural products is the major product in high dr in all cases.
Figure 5.

Confirmation of the stereochemistry of the all-carbon quaternary center stereochemistry at the [5.6] ring junction. Thermal ellipsoid plot of 5l. Ellipsoid drawn at 50% probability.
Having the data derived from the substrate scope of our multicomponent reaction in this and previous work,10,33,34 we propose that the facile nature of this reaction is achieved through a rapid acid-base reaction between electron rich imines (6, Scheme 1) and the acidic enol tautomer of the pyruvic esters (1, Scheme 1). This rapid Mannich-type addition provides an intermediate capable of intramolecular lactamization to form the α-ketolactam that subsequently tautomerizes to the observed multicomponent product 4. The mechanism is supported through the observations that preformed electron-deficient imines do not undergo this mild reaction in agreement with literature reports that require harsh reaction conditions for such reactions.14–30 It is also possible that upon imine protonation a more concerted (albeit asynchronous) process ensues as we do not observe the amino ester in any of the reaction mixtures. Further exploration in the reaction mechanism and efforts to render it asymmetric are ongoing.
Scheme 1.

Proposed Mechanism for the Multicomponent Process
In conclusion, en route to the Amaryllidaceae alkaloids and related natural product-like scaffolds, we have reported a method to prepare functionalized hydroindoles. This synthetic method is a concise and modular approach to the cores of these molecules with excellent stereoselectivity without the need for transition-metal catalysis, elevated temperatures, or the need for exclusion of water or air from reaction mixtures. Further studies building on these scaffolds and further harnessing the potential of the pyrrolidinone cores will be reported in due course.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the NIH (R01GM117570) for partial support of this work and to NC State University for support of our program. Mass spectrometry data, NMR data, and X-ray data were obtained at the NC State Molecular, Education, Technology and Research Innovation Center (METRIC).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c01650.
Experimental details, spectroscopic and analytical data of all new compounds (PDF)
Accession Codes
CCDC 1991570 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.0c01650
Contributor Information
Nicholas P. Massaro, Department of Chemistry, College of Sciences and Comparative Medicine Institute, North Carolina State University, Raleigh, North Carolina 27695, United States
Joshua G. Pierce, Department of Chemistry, College of Sciences and Comparative Medicine Institute, North Carolina State University, Raleigh, North Carolina 27695, United States.
REFERENCES
- (1).Newman DJ; Cragg GM Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod 2016, 79, 629–661. [DOI] [PubMed] [Google Scholar]
- (2).Newman DJ; Cragg GM Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod 2020, 83, 770–803. [DOI] [PubMed] [Google Scholar]
- (3).Lipinski C; Hopkins A Navigating Chemical Space for Biology and Medicine. Nature 2004, 432 (7019), 855–861. [DOI] [PubMed] [Google Scholar]
- (4).Crane EA; Gademann K Capturing Biological Activity in Natural Product Fragments by Chemical Synthesis. Angew. Chem., Int. Ed 2016, 55, 3882–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sun AW; Lackner S; Stoltz BM Modularity: Adding New Dimensions to Total Synthesis. Trends Chem. 2019, 1, 630–643. [Google Scholar]
- (6).Fürst R Narciclasine - an Amaryllidaceae Alkaloid with Potent Antitumor and Anti-Inflammatory Properties. Planta Med. 2016, 82, 1389–1394. [DOI] [PubMed] [Google Scholar]
- (7).Şener B; Orhan I; Satayavivad J Antimalarial Activity Screening of Some Alkaloids and the Plant Extracts from Amaryllidaceae. Phytother. Res 2003, 17, 1220–1223. [DOI] [PubMed] [Google Scholar]
- (8).Jin Z; Yao G Amaryllidaceae and Sceletium Alkaloids. Nat. Prod. Rep 2019, 36, 1462–1488. [DOI] [PubMed] [Google Scholar]
- (9).Pellegrino S; Meyer M; Zorbas C; Bouchta SA; Saraf K; Pelly SC; Yusupova G; Evidente A; Mathieu V; Kornienko A; Lafontaine DLJ; Yusupov M The Amaryllidaceae Alkaloid Haemanthamine Binds the Eukaryotic Ribosome to Repress Cancer Cell Growth. Structure 2018, 26, 416–425. [DOI] [PubMed] [Google Scholar]
- (10).Shymanska NV; Pierce JG Stereoselective Synthesis of Quaternary Pyrrolidine-2,3-Diones and β-Amino Acids. Org. Lett 2017, 19, 2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Martin SF Recent Applications of Imines as Key Intermediates in the Synthesis of Alkaloids and Novel Nitrogen Heterocycles. Pure Appl. Chem 2009, 81, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Layer RW The Chemistry of Imines. Chem. Rev 1963, 63, 489–510. [Google Scholar]
- (13).Li C-J; Trost BM Green Chemistry for Chemical Synthesis. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 13197–13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gein VL; Kasimova NN; Potemkin KD Simple Three-Component Synthesis of 4-Acyl-1-(2-Aminoethyl)-5-Aryl-3-Hydroxy-2,5-Dihydropyrrol-2(1H)-Ones. Russ. J. Gen. Chem 2002, 72, 1150–1151. [Google Scholar]
- (15).Gein VL; Odegova TF; Korol’ AN; Varkentin LI; Bobyleva AA; Gein LF; Vakhrin MI Synthesis and Antibacterial Activity of 5-Aryl-4-Acyl-3-Hydroxy-1-(2-Hydroxyethyl)-3-Pyrrolin-2-Ones. Pharm. Chem. J 2014, 47, 536–538. [Google Scholar]
- (16).Gein VL; Vychegzhanina VN; Levandovskaya EB; Syropyatov BY; Vakhrin MI; Voronina EV; Danilova NV Synthesis and Antibacterial and Analgesic Activity of 5-Aryl-4-Acyl-3-Hydroxy-1(2,2-Dimethoxyethyl)-3-Pyrrolin-2-Ones. Pharm. Chem. J 2010, 44, 370–373. [Google Scholar]
- (17).Gein VL; Yushkov VV; Kasimova NN; Shuklina NS; Vasil’eva MY; Gubanova MV Synthesis and Antiinflammatory and Analgesic Activity of 1-(2-Aminoethyl)-5-Aryl-4-Acyl-3-Hydroxy-3-Pyrrolin-2-Ones. Pharm. Chem. J 2005, 39, 484–487. [Google Scholar]
- (18).Gein VL; Kasimova NN; Voronina ÉV; Gein LF Synthesis and Antimicrobial Activity of 5-Aryl-4-Acyl-1-(N, N-Dimethylaminoethyl)-3-Hydroxy-3-Pyrrolin-2-Ones. Pharm. Chem. J 2001, 35, 151–154. [Google Scholar]
- (19).Gein VL; Odegova TF; Rogachev SN; Bobyleva AA; Gein LF Synthesis and Antimicrobial Activity of 5-Aryl-4-Acyl-3-Hydroxy-1-[2-(2-Hydroxyethoxy)-Ethyl]-3-Pyrrolin-2-Ones. Pharm. Chem. J 2015, 49, 175–177. [Google Scholar]
- (20).Gein VL; Fedorova NL; Levandovskaya EB; Syropyatov BY; Voronina EV; Danilova NV; Kovaleva MY Synthesis and Pharmacological Activity of 1-Alkoxyaryl-5-Aryl-4-Acyl-3-Hydroxy-3-Pyrrolin-2-Ones. Pharm. Chem. J 2011, 45, 355–358. [Google Scholar]
- (21).Gein VL; Buldakova EA; Korol AN; Veikhman GA; Dmitriev MV Synthesis of 5-Aryl-4-Aroyl-3-Hydroxy-1-Cyano-methyl-3-Pyrrolin-2-Ones. Russ. J. Gen. Chem 2018, 88, 908–911. [Google Scholar]
- (22).Gein VL; Kasimova NN Three-Component Condensation of Methyl Acylpyruvates with Aromatic Aldehydes and Ethylenediamine. Chemical Properties of the Products. Russ. J. Gen. Chem 2005, 75, 254–260. [Google Scholar]
- (23).Gein VL; Kasimova NN; Aliev ZG; Vakhrin MI Three-Component Reaction of Methyl 2,4-Dioxo-4-Phenylbutanoate and Methyl 2,4-Dioxopentanoate with Aromatic Aldehydes and Propane-1,2-Diamine and Chemical Properties of the Products. Russ. J. Org. Chem 2010, 46, 875–883. [Google Scholar]
- (24).Gein VL; Shumilovskikh EV; Andreichikov YS; Saraeva RF; Korobchenko LV; Vladyko GV; Boreko EI Synthesis of 4-Substituted 1-Methyl-5-Aryl- and 1,5-Diaryltetrahydropyrrole-2,3-Diones and Their Antiviral Action. Pharm. Chem. J 1991, 25, 884–887. [Google Scholar]
- (25).Reddy TRK; Li C; Guo X; Myrvang HK; Fischer PM; Dekker LV Design, Synthesis, and Structure-Activity Relationship Exploration of 1-Substituted 4-Aroyl-3-Hydroxy-5-Phenyl-1 H -Pyrrol-2(5 H)-One Analogues as Inhibitors of the Annexin A2-S100A10 Protein Interaction. J. Med. Chem 2011, 54, 2080–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ma K; Wang P; Fu W; Wan X; Zhou L; Chu Y; Ye D Rational Design of 2-Pyrrolinones as Inhibitors of HIV-1 Integrase. Bioorg. Med. Chem. Lett 2011, 21, 6724–6727. [DOI] [PubMed] [Google Scholar]
- (27).Morozova AD; Muravyova EA; Shishkina SV; Sysoiev D; Glasnov T; Musatov VI; Desenko SM; Chebanov VA Features of 3-Amino-5-Methylisoxazole in Heterocyclizations Involving Pyruvic Acids. Chem. Heterocycl. Compd 2019, 55, 78–89. [Google Scholar]
- (28).Sakhno YI; Desenko SM; Shishkina SV; Shishkin OV; Sysoyev DO; Groth U; Kappe CO; Chebanov VA Multicomponent Cyclocondensation Reactions of Aminoazoles, Arylpyruvic Acids and Aldehydes with Controlled Chemoselectivity. Tetrahedron 2008, 64, 11041–11049. [Google Scholar]
- (29).Murlykina MV; Sakhno YI; Desenko SM; Shishkina SV; Shishkin OV; Sysoiev DO; Kornet MN; Schols D; Goeman JL; Van der Eycken J; Van der Eycken EV; Chebanov VA Study of the Chemoselectivity of Multicomponent Heterocyclizations Involving 3-Amino-1,2,4-Triazole and Pyruvic Acids as Key Reagents, and Biological Activity of the Reaction Products. Eur. J. Org. Chem 2015, 2015, 4481–4492. [Google Scholar]
- (30).Tye H; Whittaker M Use of a Design of Experiments Approach for the Optimisation of a Microwave Assisted Ugi Reaction. Org. Biomol. Chem 2004, 2, 813–815. [DOI] [PubMed] [Google Scholar]
- (31).Ahankar H; Ramazani A;Ślepokura K; Lis T; Joo SW Synthesis of Pyrrolidinone Derivatives from Aniline, an Aldehyde and Diethyl Acetylenedicarboxylate in an Ethanolic Citric Acid Solution under Ultrasound Irradiation. Green Chem. 2016, 18, 3582–3593. [Google Scholar]
- (32).Yavari I; Souri S Synthesis of Functionalized 5-Oxo-2,5-Dihydro-1 H -Pyrroles from Primary Alkylamines, Oxalyl Chloride, and Dimethyl Acetylenedicarboxylate. Synlett 2008, 2008, 1208–1210. [Google Scholar]
- (33).Cusumano AQ; Pierce JG 3-Hydroxy-1,5-Dihydro-2H-Pyrrol-2-Ones as Novel Antibacterial Scaffolds against Methicillin-Resistant Staphylococcus Aureus. Bioorg. Med. Chem. Lett 2018, 28, 2732–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Cusumano AQ; Boudreau MW; Pierce JG Direct Access to Highly Functionalized Heterocycles through the Condensation of Cyclic Imines and α-Oxoesters. J. Org. Chem 2017, 82, 13714–13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Southwick PL; Vida JA Enols of 4-Bromo- and 4-Methyl-2,3-Dioxopyrrolidines. Ketone α-Monomethylation under Acidic or Neutral Conditions 1. J. Org. Chem 1962, 27, 3075–3079. [Google Scholar]
- (36).Bender DR; Brennan J; Rapoport H Periodate Oxidation of Alpha.-Keto. Gamma.-Lactams. Enol Oxidation and. Beta.-Lactam Formation. Mechanism of Periodate Hydroxylation Reactions. J. Org. Chem 1978, 43, 3354–3362. [Google Scholar]
- (37).Bender DR; Bjeldanes LF; Knapp DR; Rapoport H Rearrangement of Pyruvates to Malonates. Synthesis of Beta.-Lactams. J. Org. Chem 1975, 40, 1264–1269. [Google Scholar]
- (38).Nguyen V-H; Nishino H; Kurosawa K Mn(III)-Induced Molecular Oxygen Trapping Reaction of Alkenes with 2,3-Pyrrolidinedione Derivatives. A Novel Entry to 1-Hydroxy-8-Aza-2,3-Dioxabicyclo[4.3.0]Nonan-9-Ones. Tetrahedron Lett. 1997, 38, 1773–1776. [Google Scholar]
- (39).Crimmins MT; Reinhold TL Enone olefin [2 + 2] photochemical cycloadditions. Org. React. (N.Y.) 1993, 44, 297–588. [Google Scholar]
- (40).Bailey K; Rees AH Some Pyrrolidone Derivatives. Can. J. Chem 1970, 48, 2512–2516. [Google Scholar]
- (41).Sano T; Horiguchi Y; Tsuda Y Dioxopyrrolines. XXIX. Solvolytic Behavior of 3-Ethoxycarbonyl-2-Phenyl-.DELTA.2-Pyrroline-4,5-Diones in Protic Solvents. Chem. Pharm. Bull 1985, 33, 110–120. [Google Scholar]
- (42).Mohammat MF; Mansor NS; Shaameri Z; Hamzah AS Diastereoselective Reduction of 2,3-Dioxo-4-Carboxy-5-Substituted Pyrrolidines Using NaBH4/AcOH and Heterogenous Hydrogenation Reactions. J. Korean Chem. Soc 2015, 59, 31–35. [Google Scholar]
- (43).Badiola E; Olaizola I; Vázquez A; Vera S; Mielgo A; Palomo C β 2,2 -Amino Acid N -Carboxyanhydrides Relying on Sequential Enantioselective C(4)-Functionalization of Pyrrolidin-2,3-Diones and Regioselective Baeyer-Villiger Oxidation. Chem. - Eur. J 2017, 23, 8185–8195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


