Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by deficits in social communication and stereotypical behaviours. ASD’s aetiology remains mostly unclear, because of a complex interaction between genetic and environmental factors. Recently, a strong consensus has developed around ASD’s immune‐mediated pathophysiology, which is the subject of this review. For many years, neuroimmunological studies tried to understand ASD as a prototypical antibody‐ or cell‐mediated disease. Other findings indicated the importance of autoimmune mechanisms such as familial and individual autoimmunity, adaptive immune abnormalities and the influence of infections during gestation. However, recent studies have challenged the idea that autism may be a classical autoimmune disease. Modern neurodevelopmental immunology shows the double‐edged nature of many immune effectors, which can be either beneficial or detrimental depending on tissue homeostasis, stressors, neurodevelopmental stage, inherited and de novo gene mutations and other variables. Nowadays, mother–child interactions in the prenatal environment appear to be crucial for the occurrence of ASD. Studies of animal maternal–foetal immune interaction are being fruitfully carried out using different combinations of type and timing of infection, of maternal immune response and foetal vulnerability and of resilience factors to hostile events. The derailed neuroimmune crosstalk through the placenta initiates and maintains a chronic foetal neuroglial activation, eventually causing the alteration of neurogenesis, migration, synapse formation and pruning. The importance of pregnancy can also allow early immune interventions, which can significantly reduce the increasing risk of ASD and its heavy social burden.
Keywords: autism spectrum disorder, maternal immune activation, inflammation, autoimmunity, gestation, neurodevelopment
In this review, we argue over the significance of the several immune signatures associated with both susceptibility to autistic disorder and susceptibility to its core social and behavioural traits. Despite conclusive evidence, an impaired mother–child immune interplay during pregnancy is thought to be aetiopathogenetically crucial for the atypical neurodevelopment as observed in autism, and an appropriate management of immune responses during pregnancy may positively impact on autism.

Introduction
In the last few decades, a strong consensus has developed around the immune aetiology of autism spectrum disorder (ASD), particularly after the description of a chronic microglial activation in brains of individuals with ASD. 1 From then on, an increasing amount of studies have described the association and causative links between ASD and several aberrant immune functions. In this review, we summarise some of the results accumulated in the last 20 years and discuss whether the (auto)immune theorisation on ASD is a solid argument, a self‐perpetuating tautology or a still undetermined issue.
Autism spectrum disorder
Autism is a multifaceted neuropsychiatric disorder defined by a constellation of early‐appearing social communication deficits and repetitive sensory‐motor behaviours, with a strong male preponderance. 2 ASD frequency has progressively increased in the last decades up to the current rate of 1 of 59 children in the USA. 3 This escalation is generating a substantial burden on public health care 2 , 3 and is only partly attributable to a higher public awareness and better diagnostic criteria. Yet, the majority of autistic disorders still remains of undetermined origin. 4 ASD causation and clinical pleiotropy involve a complex combination of interacting genetic, epigenetic and immunological factors, which in isolation are non‐causative. 2 , 4 , 5 , 6
The core autistic features, its onset and clinical trajectories may vary substantially, allowing clinicians to define distinct ASD subtypes. 2 Many individuals present with cognitive or psychiatric comorbidities and minor neurological or morphological atypia (e.g. microcephaly). This subset, termed 'complex autism', is phenotypically distinguishable from the non‐dysmorphic and less‐comorbid 'essential autism'. 7 Individuals with 'complex' ASD are more likely to carry pathogenic de novo mutations and copy‐number variants. 8 Also heterogeneous is the timing of clinical appearance, according to which ASD is categorised into 'early' or 'regressive' patterns of onset. In the early‐onset type, children display early atypical behaviour and social communication delay; in the regressive pattern, loss of communication is experienced later, conventionally after the child has learned 5 words and used them for at least 3 months. 9 What substantially distinguishes 'complex' versus 'essential' or 'early' versus 'regressive' ASD forms is yet to be uncovered. It is believed that most children with early‐onset ASD have more frequently a 'complex' pattern, with lower intelligence quotient and a male/female ratio close to 1, while the regressive onset is prevalent in the 'essential' subtype and in male children. 7 , 8 , 9
Interestingly, infants 'at risk' of ASD (i.e. with an autistic sib) and later diagnosed with autism have a transient excess volume of extra‐axial cerebrospinal fluid (CSF) confirmed using brain magnetic resonance imaging (MRI), in which ASD precedes the onset of behavioural symptoms (between 6 and 24 months of age) and does not correlate with reduced parenchymal size, which, in fact, is increased in most cases. 10
MRI studies also show that abnormal brain enlargement is more common in male children with regressive ASD onset, while brain size in boys without regression does not differ from controls. 11 Retrospectively, head circumference in boys with regressive autism was normal at birth and diverged at about 4–6 months. 11 The rapid, although transient, head growth in the regressive subtype is associated not only with known genes such as phosphatase and tensin homolog (PTEN) and chromodomain helicase DNA‐binding protein 8, 4 but also with de novo mutations or other candidate genes or immunological dysfunctions. 12 These findings suggest a strategy of phenotype–genotype–immunotype combination to further elucidate the complex relationships leading to ASD clinical heterogeneity.
On the immune‐mediated origin of ASD
Familial autoimmunity and poly‐autoimmunity in ASD patients
Families with at least one autistic child tend to display a high autoimmune burden such as type 1 diabetes, thyroiditis and maternal rheumatoid arthritis (RA). The risk of ASD in offspring is particularly increased when maternal autoimmunity is on an active phase during pregnancy, 13 suggesting that an active inflammatory state during gestation may negatively influence the foetal neurodevelopmental trajectory.
Individuals with ASD very often manifest allergic and autoimmune comorbidities early in life or during adolescence. Type 1 diabetes, asthma, allergic rhinitis, atopic dermatitis and gastrointestinal problems, including Crohn’s and coeliac diseases, are over‐represented and, in some individuals with ASD, may even influence their behaviour and the severity of core clinical features. 14 Notably, gene expression of blood–brain barrier and intestinal tight junction proteins are found to be similarly affected on post‐mortem tissues of subjects with ASD, which raised the hypothesis, termed the 'gut–brain axis', 15 that microbiota and intestinal metabolites could directly influence the brain when the blood–brain barrier is not sufficiently intact.
Gestational infections and foetal neurodevelopment
The placenta is a selective barrier that enables nutrient absorption and waste elimination, provides protection from pathogens and allows defensive maternal immunoglobulins to positively cross into the amniotic fluid compartment. Overall, a healthy pregnancy requires a fine balance of the maternal immune activation to maintain a protective, homeostatic, non‐inflammatory environment, to ensure a tolerance state and to avoid rejection of the semi‐allogeneic foetal–placental unit. 16
However, as the developing foetal brain goes into an expedited neuroplasticity process (i.e. neurogenesis, neuronal differentiation, migration, synapse formation and apoptosis), it is particularly vulnerable to intrauterine (e.g. maternal undernutrition or diabetes) and, perhaps, early postnatal adversities. 17 It is increasingly recognised that even minor perturbations of the gestational immune environment (e.g. common infections or a mere cytokine imbalance) may have deleterious consequences on early neurodevelopment. 17 , 18
The vast majority of women experience at least one infection during gestation, and thus, most foetuses undergo a modification in their immune environment. Unfortunately, in the least resilient foetuses the infection may alter the growing trajectory, an alteration that, combined with other genetic and environmental susceptibility factors, may eventually result in an ASD diagnosis during early years. 18
Many studies of prenatal exposure to rubella and cytomegalovirus have linked maternal infections with neurological, psychiatric, cognitive and mood disorders in descendants, the strongest association being with schizophrenia and ASD. 19 A large Danish study reported a threefold increase in risk of ASD in the offspring of pregnant mothers with viral infection in the first trimester or bacterial infection in the second trimester, suggesting the importance of both the infectious agent itself and the foetal maturation stage. 20 Regardless of the type of infection (viral, bacterial, severe or moderate) and regardless of pregnancy stage, febrile episodes longer than one week have been associated with a high risk of ASD in offsprings. 21
From a mechanistic perspective derived from studies on human amniotic fluid 22 and particularly from animal models, 23 infection‐driven maternal serum cytokines, such as interleukin (IL)‐6 and IL‐17, can cross the placenta and stimulate the downstream production of other detrimental immune mediators of brain damage in the human foetal compartment, thus inducing behavioural and cognitive characteristics, strictly resembling human ASD features. 24 , 25 , 26 Most studies agree on the concept that, depending on the embryonic/foetal developmental stage, the more severe the infection, the stronger the inflammatory response (and the fever), and the higher the risk of ASD in the developing child (Figure 1). 27
Figure 1.
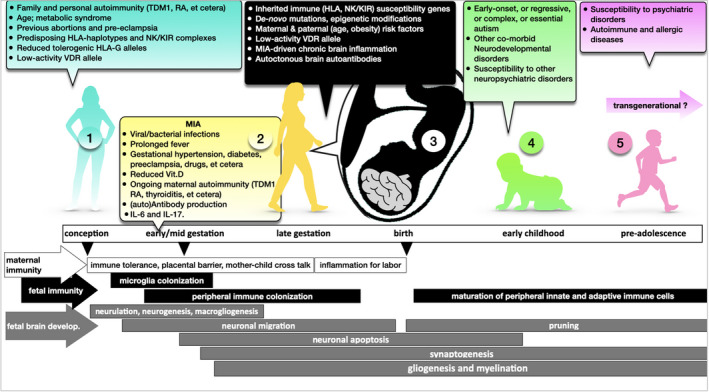
Multistep immunological road to autism is as follows: the first step (1) starts from the maternal immunogenetic predisposition to MIA (KIR/HLA), familial and personal autoimmunity and epigenetic forces such as age and gestational metabolic syndrome; the second step (2) takes place at the intrauterine level where pathogens and other events can trigger a detrimental immune activation at the maternal–foetal interface; and the third crucial step (3) takes place at the brain foetal level, at a crossroad between foetal immune and non‐immune genetic susceptibility, chronic microglial activation and persistent maternal immune activation. As a result of the complex interaction of different risk factors and depending on several variables, (3) and (4), offspring may present with various clinical forms of ASD (early onset, complex, regressive and essential). The same immune and structural brain alterations may predispose a toddler, or even an adolescent (5), to a late onset of neuropsychiatric illnesses. The transgenerational effect of the previous multistep process is, in humans, only hypothesised. TDM1 = type 1 diabetes mellitus; RA = rheumatoid arthritis; NK/KIR = NK/killer cell immunoglobulin‐like receptor; VDR = vitamin D receptor; MIA = maternal immune activation; Vit.D = vitamin D; IL = interleukin.
Maternal immune activation (MIA) and mother–child immune crosstalk
The maternal immune activation (MIA) during pregnancy in mammals, including humans, is being used as an experimental procedure to explore the phenomenology underlying risk and/or resilience of the foetus to neurodevelopmental disorders under gestational infectious/inflammatory circumstances. 23 , 24 , 25 , 26 , 27 , 28 MIA in experimental animals is classically modelled by injecting viral‐like or bacterial‐like molecules in pregnant rodents. Progeny displays both structural brain modification and behavioural anomalies explicitly evocative of the human autistic disorder, which can persist into adulthood. 29 , 30
In MIA‐induced ASD‐like behaviours, a combination of maternal chemokines and cytokines (e.g. IL‐6, IL‐17, IL‐4) 29 , 31 crosses the placenta, acts on the developing foetal brain either directly or by altering its epigenetic regulation, and leads to detrimental actions on its plasticity, precursor migration and synaptic pruning. 32 About 10% of mothers of children with autism has been found to produce anti‐foetal brain antibodies. 33 These autoantibodies can induce ASD‐like pathology and ASD‐like behaviour in animal models 33 and target a combination of foetal brain antigens such as lactate dehydrogenase‐A/B, stress‐induced phosphoprotein‐1, collapsin response mediator protein 1/2 and Y‐box binding protein, acting by reducing the dendritic spine formation in the mouse cortex. 34
Studies of experimental models of MIA now take into account different infection types (e.g. viral versus bacterial, acute versus chronic), different gestational periods in which exposure occurs, additional maternal stressors such as hypernutrition and starvation 35 or known human maternal risk factors for neurodevelopmental disorders such as ageing or gestational metabolic syndromes. 5 , 6 In mice, offspring response to hostile postnatal events, such as maternal care by a surrogate mother, and gender‐specific factors are being evaluated. 36 When impacted by prenatal immune disruption, female offspring exhibit a less severe neurodevelopmental disorder than male offspring, which may be explained by a sex‐driven modulation of several immune genes in response to metabolic and inflammatory stress. 36 Thus, the MIA model is evolving into a translational model for human research on transgenerational transmission of atypical neurodevelopment 37 and on mental health at large. 31 , 35 As the vast majority of pregnant women exposed to infections give birth to neurotypical offspring, understanding which pregnancies are fragile and which are resilient could substantially reduce the risk of ASD, attention‐deficit/hyperactivity disorder (ADHD), intellectual disability, and other neurodevelopmental disorders and common human psychiatric disorders such as schizophrenia and bipolar disorders (Figure 1).
Microglia and innate immunity
Microglia are innate immune cells that colonise the brain in the early pregnancy. They have a crucial role in the correct neurodevelopment of the child: they modulate astrocytic differentiation from neuronal precursor cells and, later, contribute to synaptic pruning and clearance of apoptotic neuronal precursors. 38 , 39 , 40 Thus, a cyclic activation of microglia is positively required for a typical neurodevelopment to occur; on the contrary, persistent microglial activation causes brain cell death and reduced or abnormal interneuronal connectivity. 41 , 42
The 2005 paper by Vargas and coll. 1 might represent the foundational moment in the theorisation of the inflammatory causes of ASD. 43 Here, for the first time, a chronic activation process of microglial and astroglial cells was documented in several cortical regions of autoptic brains of subjects with ASD. Interestingly, the loss of Purkinje neurons complemented the presence of anti‐inflammatory cytokines and astroglia, suggesting a compensatory immune‐modulating process aimed at reducing the chronic inflammation of the damaged tissue. 1 , 44
Many studies confirmed these seminal findings and added the information that ASD pathology is neither age‐related nor colocalised with markers of acute inflammation, confirming a long‐standing rather than acute immune brain alteration. 45 Furthermore, pro‐inflammatory cytokines were found elevated within the CSF and many brain regions, and the presence of CD68+ perivascular cells suggested a peripheral macrophage/monocyte brain infiltration through the blood–brain barrier. 46
Subsequent transcriptomic analyses of autoptic brains from autistic subjects confirmed a strong upregulation of the brain’s innate immune pathways. 47 , 48 , 49 Other analyses showed a dysregulated complement‐mediated synaptic pruning in brains from patients with ASD, presumably through microglia, and emphasised that the upregulation of interferon response and gene products of microglia and astrocytes is peaking during early development and coincident with clinical onset (Figure 2). 50 In contrast, in schizophrenia and bipolar disorders, the microglial transcriptomic module appeared to be downregulated. 50 , 51
Figure 2.
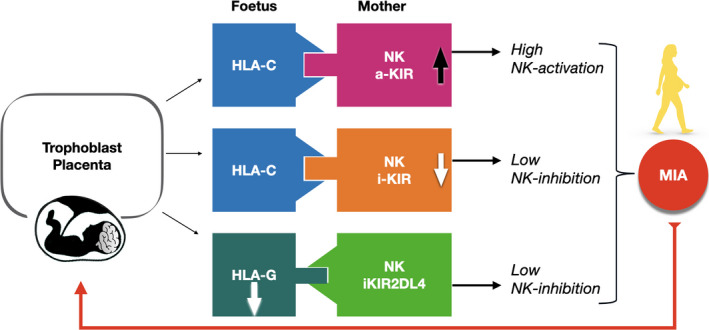
KIR‐HLA‐C and KIR/HLA‐G complexes are skewed in ASD children and their mothers because of the significant increase in NK cell pro‐activatory complexes and reduction in the tolerogenic ones. The net result causes NK‐activating molecules to prevail. 63 We also found that cognitive and behavioural impairments are correlated with particular pro‐inflammatory KIRs‐HLA‐C complexes/HLA‐G*14bp + polymorphisms. 65 In principle, the resulting NK activation negatively influences the development and the plasticity of the foetal central nervous system. NK = innate natural killer cells; a‐KIR = NK‐activatory killer cell immunoglobulin‐like receptor; iKIR = NK‐inhibitory killer cell immunoglobulin‐like receptor.
The presence of a chronic microglial activation in brains of individuals with ASD has been also substantiated in vivo by brain positron emission tomography using specific microglial radiotracers, which showed a significant activated microglial binding in cerebellum, in corpus callosum and in other cortical regions. 52 Hypomethylation of some immune genes causing over‐transcription of TNF‐α, complement and microglial‐derived factors has also been found in several cortical areas of brains of individuals with ASD. 53
Genes, immunity and autism
Inherited factors have an undeniable role in both the susceptibility to and the clinical complexity of ASD, 4 , 8 as shown by the higher concordance rate among monozygotic compared with dizygotic twins. However, inherited genes may account for only 20% of ASD cases, as they are also present in individuals with typical neurodevelopment. 4 , 54 Since the concordance in dizygotic twins is higher than that of non‐twin siblings and is progressively increasing, other drivers are necessarily operating during gestation. 6 Among these, de novo mutations are emerging as a new key factor, and are enriching the genome of many individuals with neurodevelopmental disorders, in particular, ASD. 54
Discussions of ASD‐associated or putatively causative immune genes are extraordinarily abundant in literature. An early dispute was about the involvement of the human leucocyte antigen (HLA) in ASD, since different associations were reported in different studies. Class‐I HLA‐B44 and HLA‐B15, HLA‐DRB1*04 (the major susceptibility allele for rheumatoid arthritis) and other class II alleles sharing the third hypervariable region were associated with ASD in various studies among Caucasians, 55 , 56 with DRB1*13 having some protective functions. 55 HLA‐DRB1*03‐DQB1*02 and DRB*07‐DQB1*08 haplotypes of individuals with ASD were associated with high IgA response to tissue transglutaminase II, an enzyme involved in the immune‐mediated pathophysiology of the coeliac disease, 57 confirming a link between HLA and autoimmune ASD comorbidities. Our studies in families with at least one affected child suggested significant associations with the α and β blocks, conserved HLA regions mapping classical HLA‐A, HLA‐B and non‐classical HLA‐G alleles. 58 , 59
Immune genes and cells at the maternal–foetal interface
The mother–child interface allows the foetus to safely develop in the uterus, in spite of being identified as half‐allogenic by the maternal immune system. A large variety of decidual leucocytes are crucial in this control, including innate natural killer (NK) and lymphoid cells, dendritic cells, NKT cells, and regulatory T and B cells (Tregs and Bregs). Of particular interest is an aberrant leucocyte balance in the placenta, which is significantly associated with pregnancy loss and infertility. 60
Innate CD56+CD3‐ NK cells are possibly the largest cell subpopulation at the maternal–foetal interface during early pregnancy, with several properties, including immunosurveillance and promotion of foetal growth. 61 By targeting activated immune cells, virally infected cells and also placental cells through an interaction between their killer cell immunoglobulin‐like receptors (KIRs) and the cognate HLA ligands, NK cells play a crucial role in the protection of the foetus. 62
However, when conditioned by a particular KIR‐HLA ligand, activated NK cells can produce pro‐inflammatory cytokines, perforin and granzyme B, and induce detrimental innate immune responses.
Torres and coll. first described an increased frequency of KIR‐activating genes and their HLA ligands (HLA‐C and HLA‐G, mapping in the α and β blocks) in autistic children. 55 We confirmed that pro‐inflammatory KIR/HLA are higher, whereas tolerogenic KIR/HLA gene complexes are lower in children with ASD and, more significantly, in their mothers. 63 This suggests that the true HLA‐ASD association should be viewed as a dysregulated interaction between maternal KIRs and filial HLA (Figure 2). We also evaluated HLA‐G allelic frequency in families of autistic children and found that the tolerogenic HLA‐G*01:01 allele is less common, whereas the NK‐activating HLA‐G*01:05N allele is more frequent in affected children and their mothers than controls (Figure 2), 64 and correlated with the extent of behavioural disorder in autistic children. 65 Also, mothers of children with ASD show a HLA‐G allele distribution similar to that of women with recurrent miscarriages, reinforcing the idea that an intrauterine pro‐inflammatory milieu may threaten a typical neurodevelopment or, at worst, cause miscarriage. 64
Peripheral innate and adaptive immunity
Compared with control children, markers of a dysregulated innate immunity appear in autism also at the peripheral level with increased number of NK cells, increased production of perforin and granzyme B, 66 and upregulated expression of KIR, IL‐1RA and HLA‐DR, which correlates with regressive ASD features and bigger amygdala size. 12 , 43 , 67 IL‐1β, IL‐6, IL‐8, interferon‐γ and monocyte chemoattractant protein‐1 are also abnormally elevated, with a significant reduction in the concentration of the anti‐inflammatory transforming growth factor‐β1. 68 A transcriptomic analysis of blood samples showed that, overall, differences in gene expression between sibling pairs discordant for ASD mainly reflect changes in NK cell subpopulation. 69
In contrast to the innate counterpart, an alteration of the adaptive immunity in the peripheral blood is perhaps marginal in ASD phenomenology. Serum anti‐cardiolipin, anti‐2‐glycoprotein, anti‐M1 ganglioside, anti‐endothelial cells, anti‐double‐stranded DNA and anti‐mitochondrial DNA autoantibodies have been observed in individuals with ASD along with antibodies targeting brain self‐antigens. 43 , 70 However, as they are also detected in typically developing controls, their role may be merely epiphenomenal rather than causative. 71 Nevertheless, compared with no reactivity in controls, 20% of autistic children have serum immunoreactivity to GABAergic neurons of the primate cerebellum, which correlates with a worse score on their Child Behavior Checklist, 72 cognitive impairment and motor stereotypies (Figure 3). 73
Figure 3.
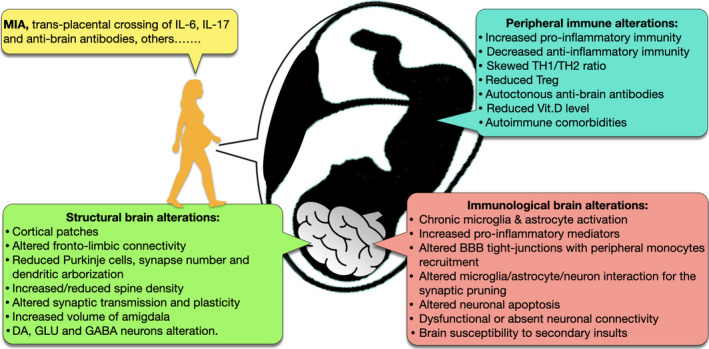
Main structural and immunological brain alterations in autism are linked to an abnormal maternal immune activation (MIA) and, in humans, are also possibly linked to the transdecidual crossing of pro‐inflammatory cytokines and autoantibodies against foetal brain antigens. Peripheral immune alterations of the foetus are mainly of the innate type. IL = interleukin; DA = dopaminergic; GLU = glutamatergic; GABA = γ‐aminobutyric acid; NK/KIR = NK/killer cell immunoglobulin‐like receptor; TH = T helper cells; Treg = T regulatory cells; Vit.D = vitamin D; BBB = blood–brain barrier.
As for the T‐cell compartment, newborns who were later diagnosed with ASD show early immune dysregulation such as increased monocyte chemoattractant protein‐1, IL‐4 and IL‐1β. 74 Later in life, autistic children present with other dysregulated T‐cell activities, including the excess production of IL‐5, IL‐12, IL‐13, IL‐17, IL‐21, IL‐22 and IL‐23, and a skewed CD4/CD8 ratio associated with decreased executive function. 12 , 14 , 75 Clustering subjects into TH1 and TH2 endophenotypes revealed that the TH1 profile correlates with a more severe ASD behavioural phenotype. 76 A reduced apoptotic signal (Fas) receptor CD95 together with increased HLA‐DR and CD26 suggests a persistent peripheral T‐cell activation in ASD. 12 Other studies found circulating CD4+ CD25 high Tregs to be functionally impaired particularly in children with clinically severe form of ASD (Figure 3). 77
However, recent large biomarker profilings in discordant sibling pairs do not confirm this adaptive immune dysregulation nor its association with ASD clinical severity. Only one correlation was found between the level of the innate cytokines IL‐1α/β and quantitative traits at the Vineland Adaptive Behavior Scale. 78 , 79
Towards an immunological treatment of ASD
Prenatal translational studies
On an experimental level, serum IL‐6 and IL‐17 elevations in MIA dams are necessary and sufficient to induce brain and behavioural alterations in offspring, since their treatment with specific antibodies prevents behavioural abnormalities in the progeny. 23 , 80 The translational value of these studies has been supported by in vitro 22 and structural and functional MRI studies in humans. 22 , 24 , 25 , 26 , 30
Similar experimental results, with a high translational value, are achieved with non‐classical immunological treatments, such as dietary interventions. The (omega‐3) docosahexaenoic acid (DHA) is important for foetal development, and neuronal and immune functions as it reduces detrimental IL‐6 production. In humans, low maternal DHA during gestation has been associated with cognitive and behavioural abnormalities. 81 In animals, comparing dams assigned to a DHA‐deficient diet with those with a DHA‐enriched diet showed that DHA‐enriched animals have offspring with less behavioural problems compared with the DHA‐deficient counterpart. 82 Likewise, memory impairment of MIA model offspring was restored by zinc supplementation during pregnancy, 83 although the translational relevance of this experiment for therapeutic interventions in humans is low. Pre‐treatment of vitamin D (Vit.D; 25‐hydroxycholecalciferol) during experimental MIA induction prevents stereotyped digging and social withdrawal in the progeny. 84 Unfortunately, as Vit.D cannot be used in human pregnancy because of its detrimental hypercalcaemic effects on the foetus, 85 postnatal studies have been performed with the safer form cholecalciferol (vide infra). Finally, oral probiotics given to experimental MIA dams prevent offspring from developing stereotypical behaviours, social withdrawal and reduction in cortical parvalbumin–GABA neurons. 86
Postnatal intervention in humans
The use of corticosteroids or immunosuppressive agents achieves the amelioration of expressive language disorder, social withdrawal and behavioural disruption in single cases or brief case series. 87 Supplementation with the cyclooxygenase‐2‐non‐steroidal celecoxib and, more recently, with prednisolone as add‐on to D2‐blocker risperidone on randomised, controlled trials has remarkably improved core clinical features in children with a regressive form of autism. 88 Mesenchymal stem cell transplantation, known to have a strong immune regulatory potential, has also significantly improved behavioural disorders in some children with ASD. 88 On the contrary, intravenous immunoglobulin has been inconclusively tested as immunotherapy for autism. This result speaks against a peripherally maintained B‐cell immune process and suggests that we need more careful pathophysiological studies. 89
Dietary intervention with Vit.D is based on a supposed, but not always confirmed, 79 , 90 Vit.D deficiency in the serum of children with ASD, and on the fact that the level of Vit.D is inversely correlated with language and behavioural scores on Childhood Autism Rating Scale. 91 Vit.D has immunomodulatory and neuroprotective functions, particularly during pregnancy; its serum shortfall may therefore contribute to the persistence of both immune and behavioural abnormalities in post‐gestational age. Vit.D’s biological activity depends on the Vit.D receptor (VDR) gene polymorphisms. We evaluated VDR polymorphic allele distribution in a large cohort of families with autistic children and found that the Vit.D/VDR complex with low biological activity is prevalent in children with ASD and their mothers. 92 Our findings encourage Vit.D supplementation in preventative and therapeutic protocols for ASD. Conformingly, dietary Vit.D supplementation in children with ASD seems to improve significantly their behavioural outcomes and, concomitantly, to reduce the CD5 T‐cell blood level. 93
Discrepancies in the (auto)immune hypothesis of ASD
A footprint of the immune system is evident in every biological process, including typical neurodevelopment. Historically, several immune proteins, including cytokines, have been conventionally divided into pro‐ or anti‐inflammatory functional classes and considered able of skewing the immune system towards a good or a bad response. Against this traditional view, modern neuroimmunology indicates that immune effectors, also produced by glial cells and neural precursors, 94 can have two possible antithetical roles, as they are either beneficial or detrimental depending on several factors: homeostasis of the neural tissue, duration of the stressor, neurodevelopmental stage (e.g. embryonic, foetal, early postnatal) and other influencing variables (inherited or de novo‐mutated genes, epigenome, environment and gender).
On the basis of the above‐described ASD risk factors such as familial autoimmunity, common non‐neurological autoimmune comorbidities and gestational immune activation because of an active maternal autoimmune disease, several studies put forward the aetiological hypothesis of a classical autoimmune environmental–genetic interaction for ASD. 95 Thus, the adaptive immune branch has been extensively studied, although, after the strong initial claim, the evidence that autism may be an intrinsically autoimmune disorder seems not to have stood the test of time.
In contrast to most autoimmune diseases, the gender ratio of affected individuals with ASD is exceedingly in favor of male individuals. 2 Despite the fact that autoantibodies to brain antigens have been repeatedly described both in children with ASD and their mothers, 70 , 96 the level of evidence is low, and we lack validation by independent research groups and the specificities of maternal and offspring antibodies. A recent systematic review suggests that there is currently insufficient evidence to develop a guideline for routine autoantibody testing in ASD patients. 71 As for other supposed biomarkers of adaptive immunity, recent profilings in sporadic cases and in ASD discordant sibling pairs do not confirm the initial claim of specific, Th1‐skewed profiling in ASD. 78 , 79 Also, dysregulation of the adaptive T‐cell immunity seems not to associate with clinical severity, head circumference, gastrointestinal or allergic issues of children with ASD and the only correlation is found between the level of innate cytokines IL‐1α/β and quantitative traits at the Vineland Scale. 78
The limits of studies on peripheral blood markers can also be attributed to between‐study heterogeneity, to small sample sizes, to techniques with different sensitivity and specificity and to epiphenomenal factors such as the psychological distress of reluctant children with autism before a venipuncture. Another critical issue is that immune parameters measured in vitro in the periphery can be misleading with regard to the relevant regulation in vivo and in the central nervous system. Nonetheless, fragmentary immune findings are said to be associated with a peculiar onset type of autism or with symptom severity. These findings, however, only account for a part of the disorder and not for the whole clinical picture.
Data on the immune dysregulation in ASD are beginning to be consolidated, but given its clinical heterogeneity, it remains difficult to explain different behavioural outcomes based solely on immune phenotype. Therefore, to avoid spurious conclusions, it would be important to aim at well‐defined and homogeneous experimental populations with homogeneous ASD core symptoms.
Many of the reviewed results have been obtained in the pre‐modern neuroimmunology era, a time when neuroscientific approaches aimed at including ASD among the prototypical antibody‐ or cell‐mediated diseases, and when the 'immunological brain privilege' was believed to prevent the crosstalk between the brain and the immune system. This long‐standing dogma, recently disproven by the discovery of glymphatic and meningeal lymphatic vessels, 97 is a misconception that slowed down scientific progress in neurodevelopmental sciences and contributed to spurious conclusions. Thanks to the fruitful MIA models, research in autism has recently evolved and now involves a less 'disease‐oriented' analysis, focusing instead on how the maternal–foetal interface really works, how the immune system impacts a developing brain during pregnancy, how foetal neuroglia cells play an immune regulatory role, and how this can affect the susceptibility to neurological, behavioural and mood disorders in neonates, toddlers and adolescents.
Perspectives
Our immunological understanding of ASD aetiology is still at the beginning, and this review probably asks more questions than it answers. The traditional view of 'one disease–one individual' is changing in favor of a new 'multifaceted' paradigm, since the prenatal environment is shared by two half‐different individuals, the mother and the child. The maternal–foetal interface appears to be the most vulnerable site for the appearance of neurodevelopmental disorders, with gestational events contributing to atypical microglial activation, neurogenesis, migration, differentiation, synapse formation and pruning. Neuroglial function is still under evaluation in ASD, but it is likely crucial in maintaining, and perhaps initiating, some of the brain abnormalities observed in autism: there is evidence that complement C1q is expressed by synapses of perinatal (and not adult) neurons. 40
New studies also bring new ideas on how emerging microbes such as Zika 98 or gut microbiome 15 can influence brain shaping during development and predispose to lifelong immune‐mediated neurological and psychiatric diseases. The application of genetic panels and new genotype–phenotype mapping 99 will diversify patient populations and enhance our understanding of the cryptic genetic composition of ASD. The interface generated by merging next‐generation sequencing, modern neuroimmunology and neuroimaging advancements will help developing a more personalised treatment for autistic children.
Nonetheless, pregnancy is also a time in which interventions may be most effective in preventing ASD. Preventative strategies include treatment of prolonged fevers, maternal diet and correction of possible epigenetic forces such as maternal obesity, diabetes and hypertension. 95 Millions of pregnant women are exposed to infections each year. Thus, reducing exposure to infections, controlling risk factors and implementing an appropriate management of the maternal immune response could have a significant impact on public health. 100
Conflict of interest
The authors declare no conflict of interest.
Authors’ Contributions
All authors participated in the writing of the manuscript and were involved in drafting the manuscript or revising it critically for important intellectual content. Each author has participated sufficiently to take public responsibility for appropriate portions of the content. All gave final approval of the submitted version.
Ethical approval
The article was written in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The manuscript is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals and aims for the inclusion of representative human populations (sex, age and ethnicity) as per those recommendations.
Acknowledgments
This work was supported by Ricerca Corrente 2018 and Ricerca Finalizzata 2013: RF‐2013‐02358607, Italian Ministry of Health. Thanks to Elisa Sotgiu and Thomas Leonard‐Roy, Departments of Comparative Literature and English, Harvard University, Cambridge, MA (USA), for help with proofreading.
References
- 1. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005; 57: 67–81. [DOI] [PubMed] [Google Scholar]
- 2. Lord C, Elsabbagh M, Baird G, Veenstra‐Vanderweele J. Autism spectrum disorder. Lancet 2018; 392: 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baio J, Wiggins L, Christensen DL et al Prevalence of autism spectrum disorder among children aged 8 years ‐ Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 2018; 67: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persico AM, Napolioni V. Autism genetics. Behav Brain Res 2013; 251: 95–112. [DOI] [PubMed] [Google Scholar]
- 5. Kim JY, Son MJ, Son CY et al Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry 2019; 6: 590–600. [DOI] [PubMed] [Google Scholar]
- 6. Loke YJ, Hannan AJ, Craig JM. The role of epigenetic change in autism spectrum disorders. Front Neurol 2015; 6: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miles JH, Takahashi TN, Bagby S et al Essential versus complex autism: definition of fundamental prognostic subtypes. Am J Med Genet A 2005; 135: 171–180. [DOI] [PubMed] [Google Scholar]
- 8. Tammimies K, Marshall CR, Walker S et al Molecular diagnostic yield of chromosomal microarray analysis and whole‐exome sequencing in children with autism spectrum disorder. JAMA 2015; 314: 895–903. [DOI] [PubMed] [Google Scholar]
- 9. Zwaigenbaum L. Perspectives on regressive onset in autism: Looking forward on looking back. Neurosci Biobehav Rev 2019; 103: 399–400. [DOI] [PubMed] [Google Scholar]
- 10. Shen MD, Nordahl CW, Li DD et al Extra‐axial cerebrospinal fluid in high‐risk and normal‐risk. children with autism aged 2–4 years: a case‐control study. Lancet Psychiatry 2018; 5: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordahl CW, Lange N, Li DD et al Brain enlargement is associated with regression in preschool‐age boys with autism spectrum disorders. Proc Natl Acad Sci USA 2011; 108: 20195–20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashwood P, Krakowiak P, Hertz‐Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 2011; 25: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen SW, Zhong XS, Jiang LN et al Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta‐analysis. Behav Brain Res 2016; 296: 61–69. [DOI] [PubMed] [Google Scholar]
- 14. Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, Croen LA. Immune mediated conditions in autism spectrum disorders. Brain Behav Immun 2015; 46: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13: 701–712. [DOI] [PubMed] [Google Scholar]
- 16. Yang F, Zheng Q, Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal‐fetal interface. Front Immunol 2019; 10: 2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life‐long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 2014; 148: R111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016; 353: 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder‐specific pathogenesis via perinatal inflammation? Pediatr Res 2011; 69: 26R–33R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atladottir HO, Thorsen P, Ostergaard L et al Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 21. Al‐Haddad BJS, Jacobsson B, Chabra S et al Long‐term risk of neuropsychiatric disease after exposure to infection In utero. JAMA Psychiatry 2019; 76: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdallah MW, Larsen N, Grove J et al Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry 2013; 14: 528–538. [DOI] [PubMed] [Google Scholar]
- 23. Choi GB, Yim YS, Wong H et al The maternal interleukin‐17 pathway in mice promotes autism‐like phenotypes in offspring. Science 2016; 51: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham AM, Rasmussen JM, Rudolph MD et al Maternal systemic interleukin‐6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2‐ years‐of‐age. Biol Psych 2018; 83: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudolph MD, Graham AM, Feczko E et al Maternal IL‐6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci 2018; 21: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasmussen JM, Graham AM, Entringer S et al Maternal Interleukin‐6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. NeuroImage 2019; 185: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hornig M, Bresnahan MA, Che X et al Prenatal fever and autism risk. Mol Psychiatry 2018; 23: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knuesel I, Chicha L, Britschgi M et al Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 2014; 10: 643–660. [DOI] [PubMed] [Google Scholar]
- 29. Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurogibol 2019; 175: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spann MA, Monk C, Scheinost D, Peterson BS. Maternal immune activation during the third trimester is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. J Neurosci 2018; 38: 2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boulanger‐bertolus J, Pancaro C, Mashour GA. Increasing role of maternal immune activation in neurodevelopmental disorders. Front Behav Neurosci 2018; 12: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rose DR, Careaga M, Van de Water J, McAllister K, Bauman MD, Ashwood P. Long‐term altered immune responses following fetal priming in a non‐human primate model of maternal immune activation. Brain Behav Immun 2017; 63: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones KL, Pride MC, Edmiston E et al Autism‐specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen‐driven mouse model of autism. Mol Psychiatry 2020;25:2994–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ariza J, Hurtado J, Rogers H et al Maternal autoimmune antibodies alter the dendritic arbor and spine numbers in the infragranular layers of the cortex. PLoS One 2017; 12: e0183443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psychiatry 2018; 175: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carlezon WA Jr, Kim W, Missig G et al Maternal and early postnatal immune activation produce sex ‐specific effects on autism‐like behaviors and neuroimmune function in mice. Sci Rep 2019; 9: 16928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pollak DD, Weber‐Stadlbauer U. Transgenerational consequences of maternal immune activation. Semin Cell Dev Biol 2020; 97: 181–188. [DOI] [PubMed] [Google Scholar]
- 38. Paolicelli RC, Bolasco G, Pagani F et al Synaptic pruning by microglia is necessary for normal brain development. Science 2011; 333: 1456–1458. [DOI] [PubMed] [Google Scholar]
- 39. Hong S, Stevens B. Microglia: phagocytosing to clear, sculpt, and eliminate. Dev Cell 2016; 38: 126–128. [DOI] [PubMed] [Google Scholar]
- 40. Stevens B, Allen NJ, Vazquez LE et al The classical complement cascade mediates CNS synapse elimination. Cell 2007; 131: 1164–1178. [DOI] [PubMed] [Google Scholar]
- 41. Smith JA, Das A, Ray SK, Banik NL. Role of pro‐inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 2012; 87: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 2012; 71: 444–457. [DOI] [PubMed] [Google Scholar]
- 43. Hughes H, Mills E, Rose D, Ashwood P. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front Cell Neurosci 2018; 12: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matta SM, Hill‐Yardin EL, Crack PJ. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav Immun 2019; 79: 75–90. [DOI] [PubMed] [Google Scholar]
- 45. Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res 2012; 1456: 72–81. [DOI] [PubMed] [Google Scholar]
- 46. Vogel DYS, Vereyken EJF, Glim JE et al Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 2013; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Voineagu I, Wang X, Johnston P et al Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gupta S, Ellis SE, Ashar FN et al Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity‐dependent genes in autism. Nat Commun 2014; 5: 5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parikshak NN, Swarup V, Belgard TG et al Genome‐wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 2016; 540: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gandal MJ, Zhang P, Hadjimichael E et al Transcriptome‐wide isoform‐level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018; 362: eaat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gandal MJ, Haney JR, Parikshak NN et al Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 2018; 359: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suzuki K, Sugihara G, Ouchi Y et al Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 2013; 70: 49–58. [DOI] [PubMed] [Google Scholar]
- 53. Nardone S, Sharan Sams D, Reuveni E et al DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl Psychiatry 2014; 4: e433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 2008; 9: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Torres AR, Sweeten TL, Johnson RC et al Common genetic variants found in HLA and KIR immune genes in autism spectrum disorder. Front Neurosci 2016; 10: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wojcik S, Bernatsky S, Platt RW et al Risk of autism spectrum disorders in children born to mothers with rheumatoid arthritis: a systematic literature review. Arthritis Care Res (Hoboken) 2017; 69: 1926–1931. [DOI] [PubMed] [Google Scholar]
- 57. Rosenspire A, Yoo W, Menard S, Torres AR. Autism spectrum disorders are associated with an elevated autoantibody response to tissue transglutaminase‐2. Autism Res 2011; 4: 242–249. [DOI] [PubMed] [Google Scholar]
- 58. Guerini FR, Bolognesi E, Manca S et al Family‐based transmission analysis of HLA genetic markers in Sardinian children with autistic spectrum disorders. Human Immunol 2009; 70: 184–190. [DOI] [PubMed] [Google Scholar]
- 59. Guerini FR, Bolognesi E, Chiappedi M et al HLA polymorphisms in Italian children with autism spectrum disorders: results of a family based linkage study. J Neuroimmunol 2011; 230: 135–142. [DOI] [PubMed] [Google Scholar]
- 60. Jafarpour R, Pashangzadeh S, Mehdizadeh S, Bayatipoor H, Shojaei Z, Motallebnezhad M. Functional significance of lymphocytes in pregnancy and lymphocyte immunotherapy in infertility: A comprehensive review and update. Int Immunopharmacol 2020; 87: 106776. [DOI] [PubMed] [Google Scholar]
- 61. Fu B, Zhou Y, Ni X et al Natural killer cells promote fetal development through the secretion of growth‐promoting factors. Immunity 2017; 47: 1100–1113.e6. [DOI] [PubMed] [Google Scholar]
- 62. Cook KD, Waggoner SN, Whitmire JK. NK cells and their ability to modulate T cells during virus infections. Crit Rev Immunol 2014; 34: 359–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guerini FR, Bolognesi E, Chiappedi M et al Activating KIR molecules and their cognate ligands prevail in children with a diagnosis of ASD and in their mothers. Brain Behav Immun 2014; 36: 54–60. [DOI] [PubMed] [Google Scholar]
- 64. Guerini FR, Bolognesi E, Chiappedi M et al HLA‐G coding region polymorphism is skewed in autistic spectrum disorders. Brain Behav Immun 2018; 67: 308–313. [DOI] [PubMed] [Google Scholar]
- 65. Guerini FR, Bolognesi E, Chiappedi M et al HLA‐G∗14bp insertion and the KIR2DS1‐HLAC2 complex impact on behavioral impairment in children with autism spectrum disorders. Neuroscience 2018; 370: 163–169. [DOI] [PubMed] [Google Scholar]
- 66. Enstrom AM, Lit L, Onore CE et al Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun 2009; 23: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Breece E, Paciotti B, Nordahl CW et al Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain Behav Immun 2013; 31: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta‐analysis. Mol Psychiatry 2015; 20: 440–446. [DOI] [PubMed] [Google Scholar]
- 69. Filosi M, Kam‐Thong T, Essioux L et al Transcriptome signatures from discordant sibling pairs reveal changes in peripheral blood immune cell composition in Autism Spectrum Disorder. Transl Psychiatry 2020; 1: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol 2012; 69: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zou T, Liub J, Zhang X, Tang H, Song Y, Kong X. Autoantibody and autism spectrum disorder: A systematic review. Res Autism Spectr Disord 2020; 75: 101568. [Google Scholar]
- 72. Rossi CC, Fuentes J, Van de Water J, Amaral DG. Brief report: antibodies reacting to brain tissue in Basque Spanish children with autism spectrum disorder and their mothers. J Autism Dev Disord 2013; 44: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goines P, Haapanen L, Boyce R et al Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun 2011; 25: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zerbo O, Yoshida C, Grether JK et al Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case‐control study. J Neuroinflammation 2014; 11: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahmad SF, Nadeem A, Ansari MA et al Imbalance between the anti‐ and pro‐inflammatory milieu in blood leukocytes of autistic children. Mol Immunol 2017; 82: 57–65. [DOI] [PubMed] [Google Scholar]
- 76. Careaga M, Rogers S, Hansen RL, Amaral DG, Van de Water J, Ashwood P. Immune endophenotypes in children with autism spectrum disorder. Biol Psychiatry 2015; 81: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mostafa GA, Al Shehab A, Fouad NR. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child Neurol 2010; 25: 328–335. [DOI] [PubMed] [Google Scholar]
- 78. Napolioni V, Ober‐Reynolds B, Szelinger S et al Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J Neuroinflammation 2013; 14: 10–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Egorova O, Myte R, Schneede J et al Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: a study of 62 serum biomarkers. Mol Autism 2020; 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through Interleukin‐6. J Neurosci 2007; 27: 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Colombo J, Shaddy DJ, Gustafson K et al The Kansas University DHA Outcomes Study (KUDOS) clinical trial: long‐term behavioral follow‐up of the effects of prenatal DHA supplementation. Am J Clin Nutrition 2019; 109: 1380–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weiser MJ, Mucha B, Denheyer H et al Dietary docosahexaenoic acid alleviates autistic‐like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot Essent Fatty Acids 2016; 106: 27–37. [DOI] [PubMed] [Google Scholar]
- 83. Alizadeh F, Davoodian N, Kazemi H, Ghasemi‐Kasman M, Shaerzadeh F. Prenatal zinc supplementation attenuates lipopolysaccharide‐induced behavioral impairments in maternal immune activation model. Behav Brain Res 2020; 377: 112247. [DOI] [PubMed] [Google Scholar]
- 84. Luan W, Hammond LA, Vuillermot S, Meyer U, Eyles DW. Maternal vitamin D prevents abnormal dopaminergic development and function in a mouse model of prenatal immune activation. Sci Rep 2018; 8: 9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Forbes GB. Vitamin D in pregnancy and the infantile hypercalcemic syndrome. Pediatr Res 1979; 13: 1382. [DOI] [PubMed] [Google Scholar]
- 86. Wang X, Yang J, Zhang H, Yu J, Yao Z. Oral probiotic administration during pregnancy prevents autism‐related behaviors in offspring induced by maternal immune activation via anti‐inflammation in mice. Autism Res 2019; 12: 576–588. [DOI] [PubMed] [Google Scholar]
- 87. Duffy FH, Shankardass A, Mcanulty GB et al Corticosteroid therapy in regressive autism: a retrospective study of effects on the Frequency Modulated Auditory Evoked Response (FMAER), language, and behavior. BMC Neurol 2014; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malek M, Ashraf‐Ganjouei A, Moradi K, Bagheri S, Mohammadi MR, Akhondzadeh S. Prednisolone as adjunctive treatment to risperidone in children with regressive type of autism spectrum disorder: a randomized, placebo‐controlled trial. Clin Neuropharmacol 2020; 43: 39–45. [DOI] [PubMed] [Google Scholar]
- 89. Lv YT, Zhang Y, Liu M et al Transplantation of human cord blood mononuclear cells and umbilical cord‐derived mesenchymal stem cells in autism. J Transl Med 2013; 11: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Desoky T, Hassan MH, Fayed HM, Sakhr HM. Biochemical assessments of thyroid profile, serum 25‐hydroxycholecalciferol and cluster of differentiation 5 expression levels among children with autism. Neuropsychiatr Dis Treat 2017; 13: 2397–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Krause I, He XS, Gershwin ME, Shoenfeld Y. Brief report: immune factors in autism: a critical review. J Autism Dev Disord 2002; 32: 337–345. [DOI] [PubMed] [Google Scholar]
- 92. Guerini FR, Bolognesi E, Chiappedi M et al Vitamin D receptor polymorphisms associated with autism spectrum disorder. Autism Res 2020; 13: 680–690. [DOI] [PubMed] [Google Scholar]
- 93. Feng J, Shan L, Du L et al Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder. Nutr Neurosci 2017; 20: 284–290. [DOI] [PubMed] [Google Scholar]
- 94. Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross‐talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci 2012; 15: 1078–1087. [DOI] [PubMed] [Google Scholar]
- 95. Scott O, Shi D, Andriashek D, Clark B, Goez HR. Clinical clues for autoimmunity and neuroinflammation in patients with autistic regression. Dev Med Child Neurol 2017; 59: 947–951. [DOI] [PubMed] [Google Scholar]
- 96. Bauman MD, Van de Water J. Translational opportunities in the prenatal immune environment: Promises and limitations of the maternal immune activation model. Neurobiol Dis 2020; 141: 104864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015; 36: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vianna P, Gomes JDA, Boquett JA et al Zika virus as a possible risk factor for autism spectrum disorder: neuroimmunological aspects. NeuroImmunoModulation 2018; 25: 320–327. [DOI] [PubMed] [Google Scholar]
- 99. Satterstrom FK, Kosmicki JA, Wang J et al Large‐scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 2020; 180: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Boivin MJ, Kakooza AM, Warf BC, Davidson LL, Grigorenko EL. Reducing neurodevelopmental disorders and disability through research and interventions. Nature 2015; 527: S155–S160. [DOI] [PubMed] [Google Scholar]


