Abstract
Background
Immune-modulatory treatments have so far shown limited clinical activity in primary brain tumours. We aimed to investigate soluble programmed death receptor ligand 1 (sPD-L1) as systemic inflammation parameter in patients with brain tumour.
Methods
EDTA plasma was collected from 81 glioma (55 glioblastoma (GBM), 26 lower-grade glioma (LGG)), 17 meningioma and 44 brain metastasis (BM) patients and 24 controls. sPD-L1 concentrations were determined by ELISA. Correlations with the local tumour microenvironment were assessed by immunohistochemical analysis for PD-L1, CD3 and CD8.
Results
sPD-L1 was detected in 62 out of 166 (37.7%) patients (glioma: 41/81, 50.6%; meningioma: 5/17, 29.4%; BM: 7/44, 15.9%; controls: 9/24, 37.5%; p=0.002). sPD-L1 concentrations were lower in BM than in LGG (p=0.003) or GBM (p<0.001). Membranous PD-L1 expression on tumour cells was not associated with sPD-L1 concentrations (p=0.953). sPD-L1 concentration was inversely correlated with the density of CD8+ (r=−0.713, p=0.001) and CD3+ (r=−0.484, p=0.042) tumour-infiltrating lymphocytes in LGG. sPD-L1 is correlated with neutrophil counts (r=−0.318, p=0.045) and C reactive protein levels (r=−0.363, p=0.008) in GBM. sPD-L1+ patients had longer overall survival in GBM (p=0.006) and worse OS in LGG (p=0.028).
Conclusions
sPD-L1 is detectable in a fraction of patients with brain tumour. Although it is not correlated with tissue PD-L1 expression, correlations with other local and systemic inflammation parameters could be detected in LGG and GBM.
Keywords: glioma, tumour microenvironment, systemic inflammation, PD-L1, soluble PD-L1
Key questions.
What is already known about this subject?
Immune checkpoint blockade showed durable responses in asymptomatic patients with brain metastases, while the clinical benefit of immune-modulating therapies is very limited in patients with primary brain tumours. In extracranial tumour entities, the levels of circulating soluble programmed death receptor ligand 1 (sPD-L1) have been shown to correlate with prognosis and response towards drugs targeting the PD-1/PD-L1 axis.
What does this study add?
Here, we show that soluble PD-L1 is measurable in the blood of patients with brain tumour and varies over distinct brain tumour entities. Furthermore, we observed that sPD-L1 correlated with other local and systemic inflammatory markers. While sPD-L1 was independently linked with longer overall survival in glioblastoma, sPD-L1 detectability was associated with worse prognosis in lower-grade glioma.
How might this impact on clinical practice?
Our results support the inclusion of both local and systemic inflammatory markers in trials of immune-modulating agents in primary brain tumours which may translate to future clinical practice in neuro-oncology.
Background
Immune-modulating therapies have so far shown only limited efficacy in primary brain tumours, while durable responses were observed in patients with asymptomatic to oligosymptomatic brain metastases (BM).1–3 Indeed, the inflammatory microenvironment differs substantially between the different types of central nervous system (CNS) malignancies. While dense infiltration with tumour-infiltrating lymphocytes (TILs) can be observed in many of patients with BM, glioblastoma (GBM) presents with significantly less pronounced infiltration.4–6 Lower-grade glioma (LGG), especially in the presence of isocitrate dehydrogenase (IDH) mutation, presents with even less TILs, potentially due to the immunosuppressive effect of 2-hydroxyglutarate.6–9 In addition, systemic factors including C reactive protein (CRP) levels10 or leucocyte subsets and their respective ratios11 12 have repetitively been shown to be associated with clinical response to immune checkpoint inhibitors.
Circulating soluble programmed cell death ligand 1 (sPD-L1) is primarily generated by proteolytic cleavage from membrane-bound PD-L1 and has immune suppressive functions.13 Higher sPD-L1 concentrations were associated with impaired prognosis in hepatocellular carcinoma,14 15 non-small cell lung cancer (NSCLC),16 gastric cancer,17 lymphoma,18 renal cell carcinoma (RCC),19 pancreatic adenocarcinoma20 and melanoma.21 High pretreatment levels of sPD-L1 were associated with increased likelihood of progression in patients with metastatic melanoma under immune checkpoint-based treatment.21 Reduction of sPD-L1 under treatment with immune checkpoint inhibitors is associated with tumour regression in patients with NSCLC.22 Therefore, we aimed to investigate the correlations of sPD-L1 to established inflammatory parameters and its prognostic value in patients with brain tumour.
Methods
Patient cohort
Non-pregnant patients aged ≥18 years with a histologically proven diagnosis of GBM, WHO grade II–III astrocytoma or oligodendroglioma (lower-grade glioma, LGG), meningioma (WHO grade I), atypical and anaplastic meningioma (WHO grade II–III) or BM which were not treated with immune checkpoint inhibitors were included in this study. Subjects with the differential diagnosis of neurological disease but no diagnosis or suspicion of cancer suffering were included as control group. EDTA plasma samples were left over after regular blood examination which was performed before a new treatment was established, requiring no extra blood take for the present study. Routinely assessed markers of systemic inflammation in the concurrently performed routine blood examination were measured at the Department of Laboratory Medicine of the Medical University of Vienna according to institutional practice and neutrophil-to-lymphocyte ratios (NLR) were accordingly calculated. Patient data were stored in a password-secured, encrypted database on a firewall-protected server of the Medical University of Vienna (FileMaker Server/Pro Advanced 17, FileMaker, Santa Clara, California, USA) and were handled anonymously.
sPD-L1 ELISA
sPD-L1 levels were determined by ELISA in plasma. EIA/RIA plates were coated overnight with polyclonal goat anti-rabbit antibody (Invitrogen Goat anti-Rabbit igG (H+L) Secondary Antibody #31210, ThermoFisher Scientific, Cheshire, UK) at 4°C. Subsequently and after a brief wash with phosphate-buffered saline (PBS), the plate was incubated for 1.5 hours to capture rabbit anti-PD-L1 antibody (polyclonal anti-PD-L1 antibody/CD274 ABF133, Merck Millipore, Billerica, Massachusetts, USA). Following anti-PD-L1 antibody binding and two subsequent washes with PBS, samples and standard PD-L1 human recombinant protein (Sino Biologicals 10084-H02H, Wayne, Pennsylvania, USA) were incubated for 12 hours at 4°C. The plate was rinsed with wash buffer (T-PBS) and incubated for 2 hours with anti-PD-L1 monoclonal antibody (clone 5H1, kindly provided by Dr Lieping Chen, Yale University, New Haven, Connecticut, USA) diluted in 1x Assay Diluent (Item E2, RayBiotech, Peachtree Corner, Georgia, USA) under constant shaking at 22°C. After three washes with T-PBS, the plate was incubated with horseradish peroxidase-conjugated polyclonal goat anti-mouse Ab (clone P0447, DakoCytomation, Glostrup, Denmark) diluted in 1x Assay Diluent containing goat serum for 1.5 hours at 22°C under constant shaking. The ELISA was developed with TMB 2-component microwell peroxidase substrate kit (Seracare KPL, Milford, Massachusetts, USA) following six washes with T-PBS. The reaction was stopped after incubation for 20 min at 22°C using 100 µL of 1 M HCl. The extinction was measured at 450 nm and values were calculated according to the standard curve run together with each ELISA plate using Gen5 Data Analysis software (BioTek Instruments, Winooski, Vermont, USA). The lower limit of sPD-L1 detection was 0.05 ng/mL as determined by serial dilutions of recombinant human PD-L1.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on a Ventana Benchmark Ultra immunostainer platform (Roche Ventana Medical Systems, Tucson, Arizona, USA) as described previously.4 In patients in whom IDH mutational status was not assessed in clinical routine and formalin-fixed, paraffin-embedded (FFPE) tissue was available, IHC for the IDH1 R132H mutation was performed. Used antibodies are listed in online supplemental table 1.
esmoopen-2020-000863supp001.pdf (25.8KB, pdf)
IHC slides were digitalised using a NanoZoomer slide scanner (Hamamatsu Photonics, Hamamatsu, Japan). Computer-based TIL quantification was performed using Definiens Tissue Studio V.4.4.3 (Definiens AG, Munich, Germany). TIL densities are given as CD3/CD8-positive cells/mm2 tumour tissue (#/mm2).
Statistical analysis
χ2 or Fisher’s exact test, Mann-Whitney U or Kruskal-Wallis tests were applied as appropriate. Correlations between continuous variables were assessed using Spearman’s rho, where a correlation coefficient r>0.7 is interpreted as strong, 0.7≥r>0.5 as medium and 0.5≥r>0.3 as weak correlation. Overall survival (OS) was calculated as the time span between first radiological diagnosis of intracranial disease and all-cause death or last follow-up. Survival estimates were calculated using the Kaplan-Meier method, while survival differences between groups were analysed using the log-rank test. Multivariate analysis was performed applying a Cox proportional hazard model. Results were considered significant at p≤0.05. As this exploratory study was aimed at the generation of hypotheses, no adjustment for multiple testing was performed.23 Statistical analysis was performed using IBM SPSS Statistics V.25 (IBM, Armonk, New York, USA), GraphPad Prism V.6.0h for Mac (La Jolla, California, USA) and R V.3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria) with RStudio V.1.2.1335 (RStudio, Boston, Massachusetts, USA) and the packages ‘haven’ (V.2.1.1), survival (V.2.44–1.1) and survminer (V.0.4.6).
Results
Patients’ characteristics
One hundred and sixty-six patients were included in the present study: 81 out of 166 (48.8 %) patients with glioma, 17 out of 166 (10.2%) with meningioma and 44 out of 166 (26.5%) with BM as well as 24 out of 166 (14.5%) controls. Further, baseline characteristics are given in table 1.
Table 1.
Baseline characteristics
| Glioma (n=81) |
Meningioma (n=17) |
Brain metastases (n=44) |
Controls (n=24) |
|
| Gender | ||||
| Male | 60 (74.1%) | 8 (47.1%) | 15 (34.1%) | 7 (29.2%) |
| Female | 21 (25.9%) | 9 (52.9%) | 29 (65.9%) | 17 (70.8%) |
| Age (years) | ||||
| Median (range) | 54 (20–83) | 54 (38–79) | 62 (39–79) | 39* (21 – 58) |
| Karnofsky Performance Scale at diagnosis | ||||
| Median (range) | 90% (40%–100%) | 90% (80%–100%) | 80% (40%–100%) | – |
| WHO grade | ||||
| Grade I | 0 (0.0%) | 12 (70.6%) | – | – |
| Grade II–III | 26 (32.1%) | 5 (29.4%) | – | – |
| Grade IV | 55 (67.9%) | – | – | – |
| IDH status | ||||
| IDH1 R132H mutation | 12 (14.8%) | – | – | – |
| No IDH-1 R132H mutation | 56 (69.1%) | – | – | – |
| Not available | 13 (16.1%) | – | – | – |
| MGMT promoter methylation status | ||||
| Methylated | 7 (8.6%) | – | – | – |
| Unmethylated | 8 (9.9%) | – | – | – |
| Unknown | 66 (81.5%) | – | – | – |
| Largest tumour diameter (mm)‡ | ||||
| Median (range) | 45 (15–83) | 40 (15–100) | 23 (5–65) | – |
| Extent of resection | ||||
| Gross total resection (GTR) | 21 (25.9%) | 14 (82.3%) | – | – |
| Subtotal resection (STR) | 27 (33.3%) | 1 (5.9%) | – | – |
| Biopsy | 31 (38.3%) | 0 (0.0%) | – | – |
| Unknown | 2 (2.5%) | 0 (0.0%) | – | – |
| Stereotactic radiosurgery | – | 2 (11.8%) | – | – |
| Any systemic antitumoral treatment prior to serum sampling | ||||
| Yes | 41 (50.6%) | 0 (0.0%) | 29 (65.9%) | – |
| No | 40 (49.4%) | 100 (100.0%) | 15 (34.1%) | – |
| Dexamethasone use at time of serum sampling | ||||
| Yes | 40 (49.4%) | 6 (35.3%) | 34 (77.3%) | – |
| No | 18 (22.2%) | 10 (58.8%) | 10 (22.7%) | – |
| Unknown | 23 (28.4%) | 1 (5.9%) | 0 (0.0%) | – |
| No of brain metastases at diagnosis of brain metastases | ||||
| Median (range) | – | – | 1.5 (1–8) | – |
| Extracranial metastases at diagnosis of brain metastases | ||||
| Present | – | – | 31 (70.5%) | – |
| Absent | – | – | 13 (29.5%) | – |
| Median OS from diagnosis in months | 21.3 (95% CI 16.7 to 26.0) | not reached | 7.5 (95% CI 6.0 to 9.0) | – |
*Age at study inclusion in the control group.
†Largest tumour diameter (or diameter of largest metastasis in patients with brain metastasis).
IDH, isocitrate dehydrogenase; OS, overall survival.
The control group comprised 20 out of 24 patients with multiple sclerosis (MS), 3 patients with non-specific neurological symptoms and unremarkable diagnostic workup, as well as one patient with non-tumorous structural epilepsy. No other autoimmune disorders were documented except for limited systemic sclerosis, uveitis and psoriasis in one patient, each. Samples in control patients were mostly drawn at regularly scheduled follow-up visits. Disease exacerbation at time of serum sampling was noted in 5 out of 20 (25.0%) patients with MS, one patient was admitted for systemic sclerosis-associated digital ischaemia, while no active infections were documented.
sPD-L1 detectability and concentrations
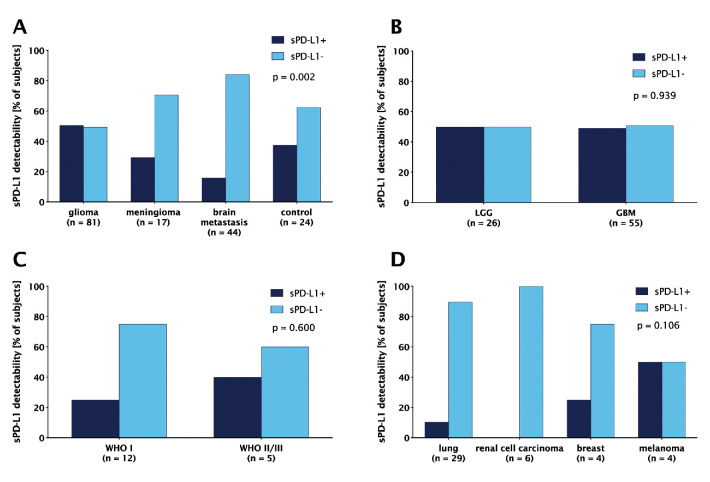
sPD-L1 was detectable in 62 out of 166 (37.3%) patients (figure 1). Patients with glioma presented most frequently with sPD-L1, followed by controls and patients with meningioma and BM (p=0.002, χ2 test). No difference was observed between GBM and LGG (WHO II–III glioma) (p=0.939, χ2 test, figure 1B), as well as between WHO I and WHO II/III meningioma (p=0.6, Fisher’s exact test, figure 1C). In patients with BM, the highest fraction of sPD-L1+ samples was seen in melanoma (2/4, 50.0%), followed by breast cancer (1/4, 25.0%) and NSCLC (3/29, 10.3%), while all patients with RCC were sPD-L1- and one patient with follicular thyroid carcinoma was sPD-L1+ (figure 1D). Furthermore, the fraction of sPD-L1+ samples did not significantly differ between IDH-mutated (IDH-mt) (8/12, 66.7%) and IDH-wild-type (IDH-wt) (26/56, 46.4%) glioma (p=0.203, χ2test, online supplemental figure 1A).
Figure 1.
Soluble programmed death receptor ligand 1 (sPD-L1) detectability in (A) the overall cohort according to histology; patients with (B) glioma and (C) meningioma according to WHO grade; (D) patients with brain metastasis according to the primary tumour. The lower limit of sPD-L1 detectability was 0.05 ng/mL.
esmoopen-2020-000863supp002.pdf (258KB, pdf)
No difference in sPD-L1 detectability was observed between patients on dexamethasone treatment versus patients without corticosteroids at the time when blood samples were drawn in GBM (p=1, Fisher’s exact test), LGG (p=0.684, χ2 test), meningioma (p=0.299, Fisher’s exact test) or BM (p=0.649, Fisher’s exact test). In GBM, 21 out of 31 (67.7%) patients who received previous systemic treatment were sPD-L1+, while this was the case in 7 out of 24 (29.2%) of non-pretreated GBM patients (p=0.007, χ2 test, online supplemental figure 2A). Similarly, 8 out of 10 (80.0%) pretreated LGG patients were sPD-L1+, while only 5 out of 16 (31.3%) non-pretreated LGG patients had detectable sPD-L1 (p=0.041, χ2 test). In contrast, there was no difference according to previous systemic treatment in BM (p=1, Fisher’s exact test).
esmoopen-2020-000863supp003.pdf (282.5KB, pdf)
Median sPD-L1 concentration was 0.412 ng/mL (range 0.050–42.150 ng/mL) in samples with sPD-L1 over the detection threshold. In glioma, median sPD-L1 was 0.055 ng/mL (range 0.00–42.105 ng/mL), while the median concentrations were 0.00 ng/mL in meningioma, BM and controls (figure 2A). sPD-L1 concentrations significantly differed across diagnoses (p=0.007, Kruskal-Wallis). In a pairwise analysis, sPD-L1 concentrations were significantly lower in BM as compared with GBM (p<0.001) and LGG patients (p=0.003). sPD-L1 levels did not differ between GBM and LGG (p=0.803). Similarly, sPD-L1 concentrations did not differ between IDH-wt and IDH-mt glioma (p=0.197, online supplemental figure 1B). There was no statistically significant difference between meningioma and atypical/anaplastic meningioma (figure 2B, p=0.879). Interestingly, we observed alterations in sPD-L1 concentrations according to the primary tumour (p=0.035, Kruskal-Wallis, figure 2C). Pairwise analysis was not performed due to small sample sizes.
Figure 2.
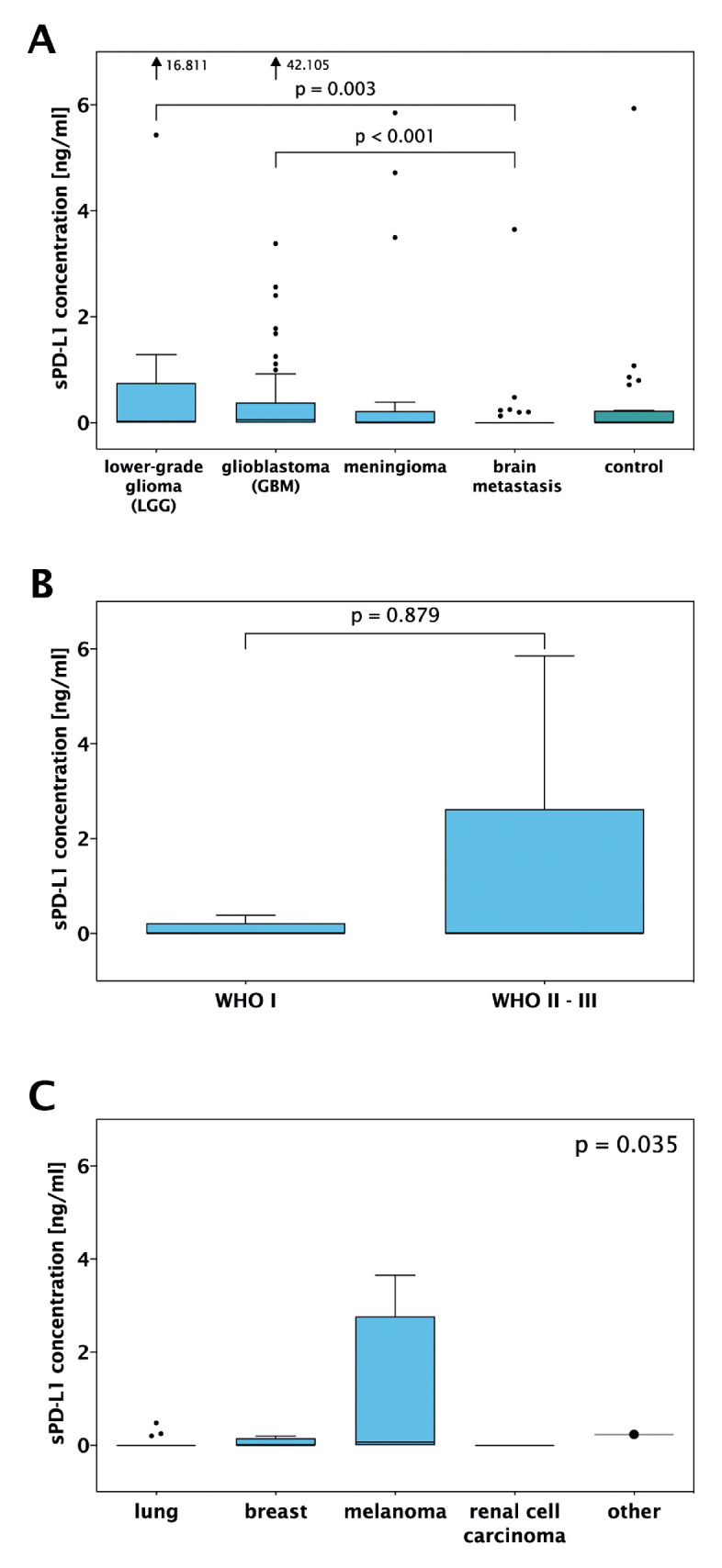
Soluble programmed death receptor ligand 1 (sPD-L1) concentrations in (A) the overall cohort according to histology, (B) patients with meningioma according to WHO grade, (C) patients with metastasis according to the primary tumour.
No differences in sPD-L1 levels were seen between dexamethasone-treated patients versus patients who did not receive steroids in GBM (p=0.782, Mann-Whitney U test), LGG (p=0.880), meningioma (p=0.428) and BM (p=0.815). sPD-L1 concentrations were higher in patients with GBM who received previous systemic treatment than in non-pretreated patients (p=0.026, Mann-Whitney U test, online supplemental figure 2B). However, no difference was seen in patients with LGG (p=0.109) or BM (p=0.712).
Correlation between sPD-L1 concentrations and systemic inflammation markers
A weak negative correlation of sPD-L1 with CRP (Spearman’s rho=−0.363, p=0.008) and neutrophil counts (r=−0.318, p=0.045) was observed in with GBM. In controls, sPD-L1 correlated negatively with leucocyte (r=−0.498, p=0.035) and neutrophil counts (r=−0.477, p=0.045). No further significant correlations could be determined (online supplemental figure 3).
esmoopen-2020-000863supp004.pdf (669.3KB, pdf)
Correlations between sPD-L1 and immune markers in the tumour microenvironment
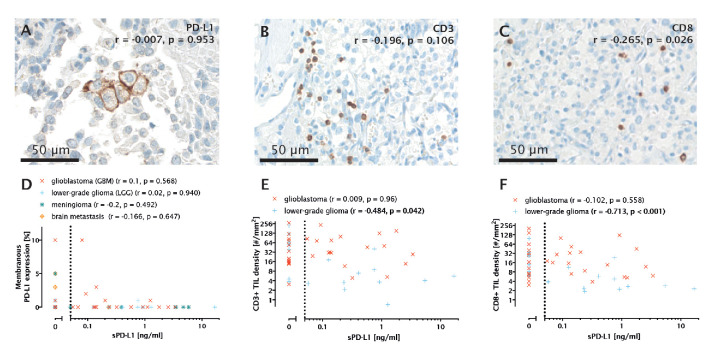
Tumour tissue for correlative analysis was available in 76 out of 142 patients. Overall, 12 out of 76 (15.8%) patients showed membranous PD-L1 expression. Membranous PD-L1 staining was observed in 2 out of 17 (11.7%) LGG, 7 out of 35 (20.0%) GBM (figure 3A), 1 out of 14 (7.1%) meningioma and 2 out of 10 (20.0%) BM. There was no correlation between sPD-L1 levels and membranous PD-L1 expression on tumour cells overall (r=−0.007, p=0.953, n=76) as well as in subgroups according to tumour histology (figure 3D).
Figure 3.
(A) Membranous programmed death receptor ligand 1 (PD-L1) staining in a glioblastoma (GBM) tumour sample. Spearman’s r and p value are given with respect to the overall cohort. (B) CD3+ tumour-infiltrating lymphocytes (TILs) in a GBM sample. (C) CD8+ TILs in a GBM sample. (D) Correlation of membranous PD-L1 expression (%) with soluble PD-L1 (sPD-L1) concentrations in GBM, lower-grade glioma (LGG), meningioma and brain metastasis (BM). (E) Correlation of CD3+ TIL density (cells/mm2) with sPD-L1 concentrations in LGG and GBM. (F) Correlation of CD8+ TIL density (cells/m2) with sPD-L1 concentrations in LGG and GBM.
In addition, there was no association between PD-L1 expression in TIL and sPD-L1 concentrations (p=0.385, Mann-Whitney U) (online supplemental figure 4).
esmoopen-2020-000863supp005.pdf (248.9KB, pdf)
CD3+ TIL density was not linked to sPD-L1 concentrations in the overall cohort (r=−0.196, p=0.106, n=69, figure 3B). However, a correlation between CD3+ TIL density and sPD-L1 concentrations was found in LGG (r=−0.484, p=0.042, n=18, figure 3E). No other correlations between CD3+ TIL density and sPD-L1 concentrations were found.
The density of CD8+ TIL was not correlated with sPD-L1 concentrations in the entire cohort (Spearman’s rho=−0.265, p=0.026, n=71, figure 3C). However, a correlation between CD8+ TIL and sPD-L1 was observed in the glioma cohort (figure 3C, r=−0.369, p=0.007, n=53), which was even more distinct in the LGG subgroup (r=−0.713, p<0.001, n=18, figure 3F). In contrast, there was no significant correlation between CD8+ cells and sPD-L1 in the GBM cohort (Spearman’s rho=−0.102, p=0.558, n=35).
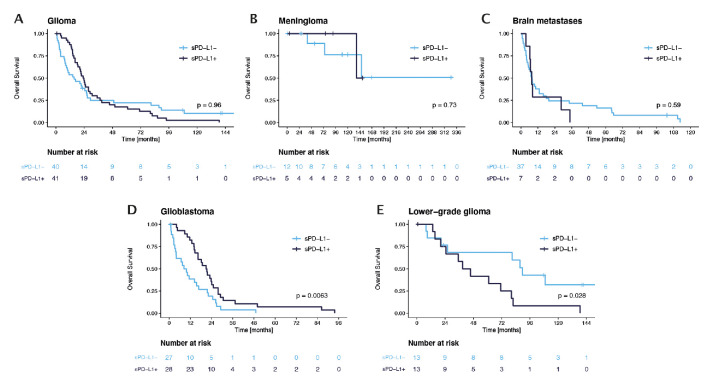
Prognostic impact of sPD-L1 in patients with brain tumours
In the entire cohort except for controls, patients with sPD-L1 presented with a median OS of 23.8 months compared with 14.4 months in patients without sPD-L1 (p=0.838; log-rank test). No association with OS and sPD-L1 was observed in the glioma group, in patients with meningioma or BM (figure 4A–C). Interestingly, patients with GBM presented with improved survival prognosis in the presence of sPD-L1 (median OS 20.9 months; 95% CI 16.5 to 25.3 months) as compared with those without sPD-L1 (median OS 8.4 months; 95% CI 4.0 to 12.7 months, p=0.006, figure 4D). In contrast, LGG patients with detectable sPD-L1 had significantly shorter OS (median OS 38.9 months; 95% CI 21.3 to 56.5 months) compared with patients without sPD-L1 (median OS 89.6 months; 95% CI 75.4 to 103.7 months; p=0.028, figure 4E). sPD-L1 remained a significant prognostic factor in previously untreated patients with GBM (p=0.024), while there was no difference in OS in pretreated GBM (p=0.37). No prognostic impact of sPD-L1 detectability was however seen in untreated LGG (p=0.22) and previously treated LGG (p=0.33, online supplemental figure 5A–D).
Figure 4.
Overall survival according to soluble programmed death receptor ligand 1 (sPD-L1) detectability in (A) glioma, (B) meningioma, (C) brain metastasis, (D) glioblastoma and (E) lower-grade glioma.
esmoopen-2020-000863supp006.pdf (406.3KB, pdf)
For IDH-wt GBM, multivariate survival analysis was performed using a Cox proportional hazard model. Based on previously reported clinical risk factors in GBM,24 we included the prognostic factors age, Karnofsky Performance Scale (KPS) and extent of resection along with sPD-L1 detectability as covariates. Notably, sPD-L1 detectability remained an independent prognostic factor for OS (HR 0.311, p=0.012; table 2). Multivariate analysis was omitted in the LGG and IDH-mt subgroups due to small sample sizes.
Table 2.
Multivariate survival analysis in patients with IDH-wt glioblastoma
| Covariate | HR (95% CI) | P value |
| Age | 1.002 (0.969 to 1.037) | 0.900 |
| Extent of resection (GTR) | (Reference) | |
| STR | 1.321 (0.503 to 3.471) | 0.572 |
| Biopsy | 0.670 (0.253 to 1.774) | 0.670 |
| Karnofsky Performance Scale (≤70% vs >70%) |
0.469 (0.203 to 1.085) | 0.077 |
|
sPD-L1 detectability (sPD-L1+ vs sPD-L1-) |
0.311 (0.125 to 0.774) | 0.012 |
IDH-wt, isocitrate dehydrogenase wild type; sPD-L1, soluble programmed death receptor ligand 1; STR, subtotal resection.
Discussion
Immune-modulatory therapies have shown remarkable response in patients with BM, while efficacy is limited in primary brain tumours.1–3 Insight in cancer–immune system interactions could reveal targetable differences and is needed to govern the further development of immune-modulatory therapy in patients with brain tumour. sPD-L1 was correlated with prognosis as well as response to immune checkpoint inhibitors in extracranial tumours and might be a promising marker to measure systemic immune suppression.14–22 Using a sandwich ELISA, we detected sPD-L1 in the plasma of patients with brain tumour and the highest concentration was evident in patients with glioma. No correlation of sPD-L1 and tissue PD-L1 expression was observed. However, high sPD-L1 was associated with impaired OS prognosis in GBM and improved OS prognosis in LGG, supporting further research of systemic inflammatory processes in glioma. sPD-L1 detectability was heterogeneous across different brain tumours with the numerically highest sPD-L1 levels in LGG, followed by GBM, whereas sPD-L1 could be detected in only few BM, meningioma and control patients. In line, Liu et al25 and Cabezas-Camarero et al26 recently observed significantly higher serum sPD-L1 levels in glioma as compared with meningioma and healthy controls. However, in contrast to our observation, Liu et al detected higher sPD-L1 levels in biologically more aggressive tumours as represented by elevated mitotic activity (Ki67 proliferation index) and higher WHO grade.25 Of note, the cohort in Liu et al—in contrast to our cohort—also included pilocytic astrocytoma (WHO grade I) which is known to exhibit a distinctly lower immune cell infiltration27 as well as more favourable clinical course than GBM28 and therefore could cause the correlation with WHO grade and sPD-L1 concentration. Of note, the majority of the patients in our control group suffered from MS, although only few patients were admitted for disease activity. Reduced levels of sPD-L1 were observed in patients with MS in comparison to patients with non-inflammatory diseases, although increased levels were linked to elevated disease activity.29 Physiologically, sPD-L1 correlates with immunosuppression as particularly high levels can be observed during pregnancy due to immune suppression securing the maternal acceptance of the placenta.30 31 Moreover, altered sPD-L1 was detected in immune-modulating diseases such as type I diabetes mellitus,32 allergic rhinitis33 and severe sepsis.34 35 According to the high levels of sPD-L1 observed in patients with glioma in the present cohort, systemic immune suppression might be more pronounced in patients with glioma as compared with other brain tumours and controls. The studies of Liu et al25 and Cabezas-Camarero et al26 did not include previously treated patients, while the present study included pretreated patients. Here, GBM with previous treatment presented with higher sPD-L1 concentration, indicating that chemotherapeutic treatment potentially impacts systemic inflammation.
In contrast to the previous studies, we were able to also investigate the matched tumour samples to put sPD-L1 in the context with local inflammation. PD-L1 expression in the tumour tissue did not correlate with sPD-L1 in the present cohort, suggesting that sPD-L1 cannot be used as a surrogate marker for membranous PD-L1 expression on tumour cells. Furthermore, this finding underscores that local and systemic inflammation might have autonomous regulatory mechanisms. Indeed, systemic as well as local parameters were previously shown to correlate with response to immune-modulatory therapies, as both—potentially independently—impact the clinical efficacy of an immune response.36 Local characteristics including PD-L1 expression and TIL density were previously identified to potentially predict response to immune checkpoint inhibitors.37 However, despite the frequent expression of PD-L1 in GBM,5 38 first reports of immune checkpoint inhibitors in glioma did not reveal a clinically meaningful efficacy.39 sPD-L1 was shown to correlate with response to immune checkpoint blockade in NSCLC40 41 and melanoma21 patients, underscoring the impact of systemic inflammation on response to immune-modulatory therapies. Therefore, the inclusion of systemic inflammatory biomarkers could potentially give a more accurate prediction of patients most likely to respond to immune modulatory therapies.36
Notably, we observed an association of sPD-L1 with overall survival in GBM and LGG but not in patients with BM and meningioma. Interestingly, the presence of sPD-L1 was linked to longer OS in GBM, whereas an impaired association was found in LGG, indicating that the impact of systemic inflammation might differ between glioma subtypes. In contrast to several other malignancies, LGG are in comparison much slower growing tumours and cancer cells persist over years without relevant growth. The interaction with the immune system might therefore differ from other faster growing tumours, as detectability of sPD-L1 representing systemic immune suppression was associated with improved prognosis. Previous studies from extracranial malignancies suggest that sPD-L1 is negatively associated with survival in various advanced malignant diseases.42 43 sPD-L1 might therefore serve as a valuable marker to measure clinically relevant immune suppression given the association with survival prognosis in various entities. Therefore, sPD-L1 could be further explored as a biomarker for systemic immune suppression in clinical trials on immune-modulatory therapies in patients with brain tumour.
Our study has several limitations that have to be considered in the interpretation of the provided data. The retrospective, exploratory design of the study is inherently linked to relatively small sample sizes in certain subgroups and, as a consequence, limited statistical power to detect significant associations. Second, the prognostic impact of sPD-L1 has to be interpreted cautiously due to the heterogeneity of the cohort, especially in the LGG subgroup where no multivariate analysis could be performed due to small sample sizes. In addition, tissue-based investigations could not be performed in all cases as sufficient tumour material was not available for all patients. We further acknowledge that analyses with respect to molecular glioma subgroups could not be performed as only 12 patients had verified IDH mutations and IDH sequencing was not performed. Included IDH-wt WHO grade II III tumours may therefore display a biological behaviour similar to that of GBM. Moreover, the herein used sandwich ELISA to assess sPD-L1 levels was not systematically validated in other cohorts or along with other methods, although the measured concentrations are well comparable to those previously. Moreover, most patients in the control group suffered from MS as samples from healthy donors with no history of an inflammatory condition were not available. Furthermore, as only one timepoint per patient was assessed, sPD-L1 levels could not be observed over time to further investigate the impact of antitumoral treatments on sPD-L1 in brain tumours. The generated results however suggest that further investigation of sPD-L1 could be of clinical interest in brain tumours and should be included in further prospective trials.
In conclusion, we show that sPD-L1 is detectable in a fraction of patients with brain tumour and is associated with the local tumour microenvironment but not with tissue PD-L1. Our study adds to the evidence that tumour–immune system interactions vary according to the histological lineage of CNS tumours, although no significant differences could be observed between low-grade and high-grade tumours in glioma and meningioma or IDH-mt and IDH-wt glioma. Despite the tight immune-regulatory mechanisms in the brain, the present study further substantiates that systemic immune responses are observable and not necessarily correlate with characteristics of the local tumour microenvironment. Taken together, our results highlight the need for a combination of local and systemic immune-related biomarkers for future trial design on immune-modulating therapies in neuro-oncology.
Acknowledgments
We thank Irene Erber, Stefan Traint, Teresa Hatziioannou and Barbara Neudert for excellent technical assistance. This study was performed within the PhD thesis project of Maximilian Mair with the title “Clinical and immunological characteristics associated with lower-grade glioma prognosis” in the Clinical Neurosciences (CLINS) programme at the Medical University of Vienna.
Footnotes
Twitter: @MJ_Mair
Presented at: Parts of this manuscript were presented at a Poster Discussion session at the European Association of Medical Oncology (ESMO) Congress 2019 and as an oral presentation at the European Association of Neuro-Oncology (EANO) Meeting 2019.
Contributors: Contribution to study design and its implementation: MJM, SP, AI-M, AS, BK, GW, KD, KF, JH, CM, LM, LW, MP, ASB. Data analysis and interpretation: MJM, SP, AI-M, CM, MP, ASB. Manuscript writing and editing: MJM, SP, AI-M, AS, BK, GW, KD, KF, JH, CM, LM, LW, MP, ASB. All authors read and approved the final manuscript.
Funding: This study was partly funded by the unrestricted grant “Immunological characteristics in the tumour microenvironment associated with clinical course and treatment response” from Roche and the research budget of the Medical University of Vienna.
Competing interests: MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Boehringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, AbbVie. ASB has research support from Daiichi Sankyo and honoraria for lectures, consultation or advisory board participation from Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo as well as travel support from Roche, Amgen and AbbVie. AI-M has received honoraria for advisory board participation from Merck Sharp & Dohme and Servier, lecture fees from Servier and Eli Lilly, and travel support from Roche and Bristol-Meyers Squibb.
Patient consent for publication: Not required.
Ethics approval: The study was performed in accordance to the Declaration of Helsinki and its amendments as well as to local and institutional guidelines and was approved by the ethics committee of the Medical University of Vienna (approval no. 351/2005).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. No additional data available.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976–83. 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459–65. 10.1016/S1470-2045(12)70090-6 [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018;19:672–81. 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 4.Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 2016;5:e1057388. 10.1080/2162402X.2015.1057388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 2015;17:1064–75. 10.1093/neuonc/nou307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klemm F, Maas RR, Bowman RL, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 2020;181:1643–60. 10.1016/j.cell.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 2018;24:1192–203. 10.1038/s41591-018-0095-6 [DOI] [PubMed] [Google Scholar]
- 8.Berghoff AS, Kiesel B, Widhalm G, et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol 2017;19:1460–8. 10.1093/neuonc/nox054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest 2017;127:1425–37. 10.1172/JCI90644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrotriya S, Walsh D, Nowacki AS, et al. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PLoS One 2018;13:e0202555. 10.1371/journal.pone.0202555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:1–11. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 12.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204–12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget 2017;8:97671–82. 10.18632/oncotarget.18311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelmeier F, Canli Özge, Tal A, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 2016;59:152–9. 10.1016/j.ejca.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 15.Chang B, Huang T, Wei H, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother 2019;68:353–63. 10.1007/s00262-018-2271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Zhang P, Wang J, et al. Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy. Medicine 2017;96:e6102. 10.1097/MD.0000000000006102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shigemori T, Toiyama Y, Okugawa Y, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol 2019;26:876–83. 10.1245/s10434-018-07112-x [DOI] [PubMed] [Google Scholar]
- 18.Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014;28:2367–75. 10.1038/leu.2014.137 [DOI] [PubMed] [Google Scholar]
- 19.Fukuda T, Kamai T, Masuda A, et al. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med 2016;5:1810–20. 10.1002/cam4.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bian B, Fanale D, Dusetti N, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology 2019;8:e1561120–10. 10.1080/2162402X.2018.1561120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 2017;5:480–92. 10.1158/2326-6066.CIR-16-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando K, Hamada K, Watanabe M, et al. Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res 2019;39:5195–201. 10.21873/anticanres.13716 [DOI] [PubMed] [Google Scholar]
- 23.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol 2001;54:343–9. 10.1016/S0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- 24.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol 2004;6:227–35. 10.1215/S1152851703000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Zhu Y, Zhang C, et al. The clinical significance of soluble programmed cell Death-Ligand 1 (sPD-L1) in patients with gliomas. Front Oncol 2020;10:1–12. 10.3389/fonc.2020.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabezas-Camarero S, García-Barberán V, Pérez-Alfayate R, et al. Plasma PD-L1 levels according to histologic grade and IDH status in patients with gliomas. JCO 2020;38:69 10.1200/JCO.2020.38.5_suppl.69 [DOI] [Google Scholar]
- 27.Yang I, Han SJ, Sughrue ME, et al. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg 2011;115:505–11. 10.3171/2011.4.JNS101172 [DOI] [PubMed] [Google Scholar]
- 28.Mair MJ, Wöhrer A, Furtner J, et al. Clinical characteristics and prognostic factors of adult patients with pilocytic astrocytoma. J Neurooncol 2020;148:187–98. 10.1007/s11060-020-03513-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholas R, Chang K, Romozzi M, et al. P862: programmed cell death ligand 1 (PD-L1) in multiple sclerosis (MS) serum and cerebrospinal fluid (CSF). Mult Scler 2019;25:357–580. [Google Scholar]
- 30.Enninga EAL, Harrington SM, Creedon DJ, et al. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol 2018;79:e12795–6. 10.1111/aji.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuyama M, Mezawa H, Kawai T, et al. Elevated soluble PD-L1 in pregnant women's serum suppresses the immune reaction. Front Immunol 2019;10:86. 10.3389/fimmu.2019.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Guo H, Li S, et al. Soluble programmed death-1 ligand 1(sPD-L1) is significantly reduced in the serum of type 1 diabetes patients. Acta Diabetol 2018;55:515–7. 10.1007/s00592-017-1081-z [DOI] [PubMed] [Google Scholar]
- 33.Nasiri Kalmarzi R, Fattahi N, Kaviani Z, et al. Inverse correlation of soluble programmed cell death-1 ligand-1 (sPD-L1) with eosinophil count and clinical severity in allergic rhinitis patients. Allergol Int 2017;66:326–31. 10.1016/j.alit.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Jia Y, Li C, et al. Predictive value of soluble programmed death-1 for severe sepsis and septic shock during the first week in an intensive care unit. Shock 2019;51:289–97. 10.1097/SHK.0000000000001171 [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Zhang X, Chen H, et al. Serum sPD-L1, upregulated in sepsis, may reflect disease severity and clinical outcomes in septic patients. Scand J Immunol 2017;85:66–72. 10.1111/sji.12509 [DOI] [PubMed] [Google Scholar]
- 36.Blank CU, Haanen JB, Ribas A, et al. Cancer immunology. The "cancer immunogram". Science 2016;352:658–60. 10.1126/science.aaf2834 [DOI] [PubMed] [Google Scholar]
- 37.Music M, Prassas I, Diamandis EP. Optimizing cancer immunotherapy: is it time for personalized predictive biomarkers? Crit Rev Clin Lab Sci 2018;55:466–79. 10.1080/10408363.2018.1499706 [DOI] [PubMed] [Google Scholar]
- 38.Nduom EK, Wei J, Yaghi NK, et al. Pd-L1 expression and prognostic impact in glioblastoma. Neuro Oncol 2016;18:195–205. 10.1093/neuonc/nov172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol 2018;20:674–86. 10.1093/neuonc/nox208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuma Y, Wakui H, Utsumi H, et al. Soluble programmed cell death ligand 1 as a novel biomarker for nivolumab therapy for non-small-cell lung cancer. Clin Lung Cancer 2018;19:410–7. 10.1016/j.cllc.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 41.Costantini A, Julie C, Dumenil C, et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 2018;7:e1452581. 10.1080/2162402X.2018.1452581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei W, Xu B, Wang Y, et al. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: a meta-analysis. Medicine 2018;97:e9617–6. 10.1097/MD.0000000000009617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y, Sun C, Li J, et al. The prognostic significance of soluble programmed death ligand 1 expression in cancers: a systematic review and meta-analysis. Scand J Immunol 2017;86:361–7. 10.1111/sji.12596 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000863supp001.pdf (25.8KB, pdf)
esmoopen-2020-000863supp002.pdf (258KB, pdf)
esmoopen-2020-000863supp003.pdf (282.5KB, pdf)
esmoopen-2020-000863supp004.pdf (669.3KB, pdf)
esmoopen-2020-000863supp005.pdf (248.9KB, pdf)
esmoopen-2020-000863supp006.pdf (406.3KB, pdf)