Abstract
Background
Poor sense of smell in older adults may lead to weight loss, which may further contribute to various adverse health outcomes. However, empirical prospective evidence is lacking. We aimed to longitudinally assess whether poor olfaction is associated with changes in body composition among older adults.
Methods
A total of 2,390 participants from the Health ABC Study had their olfaction assessed using the Brief Smell Identification Test in 1999–2000. Based on the test score, olfaction was defined as poor (0–8), moderate (9–10), or good (11–12). Total body mass, lean mass, and fat mass were measured by dual-energy X-ray absorptiometry annually or biennially from 1999 to 2007.
Results
At baseline, compared to participants with good olfaction, those with poor olfaction weighed on average 1.67 kg less (95% CI: −2.92, −0.42) in total mass, 0.53 kg less (95% CI: −1.08, 0.02) in lean mass, and 1.14 kg less (95% CI: −1.96, −0.31) in fat mass. In longitudinal analyses, compared to participants with good olfaction, those with poor olfaction had a greater annual decline in both total mass (−234 g, 95% CI: −442, −26) and lean mass (−139 g, 95% CI: −236, −43). They also tended to have a greater annual loss of fat mass (−113 g, 95% CI: −285, 59), but the difference was not statistically significant.
Conclusions
Our results indicate poor olfaction is associated with lower body weight and greater weight loss in older adults. It is imperative for future studies to investigate potential underlying mechanisms and associated adverse health consequences.
Keywords: Olfactory impairment, Epidemiology, Body composition
In older adults, olfaction impairment is a common but underrecognized sensory deficit that may precede some age-related adverse health conditions. The prevalence of poor olfaction increases with age, affecting up to 25% of adults aged 50 and older (1), and increases to 60%–75% among those 80 years and older (1). It has been hypothesized that poor olfaction may lead to dietary changes which may further lead to changes in body composition over time (2,3). Olfaction may also be associated with the levels of metabolic hormones, for example, leptin, a chemical that helps regulate satiety (4) and may affect body composition (eg, lean mass and fat mass) (5,6). In older adults, changes in weight and body composition are often important indicators of health status. For example, weight loss, loss of lean mass, and loss of fat mass in older adults have been linked to impaired mobility (7), lower bone mineral density (8), chronic conditions (eg, depression (9), Parkinson’s disease (10), Alzheimer’s disease (11), and dementia (12)), and ultimately higher mortality (13).
Several studies have examined olfaction in relation to body weight or body mass index in older adults. While the overall evidence suggests an inverse association (14–16), study findings have been inconsistent (17,18). Further, these are cross-sectional studies and did not analyze changes in body composition. We therefore examined the relationship between poor olfaction and body composition by conducting two sets of analyses: (i) a cross-sectional approach examining poor olfaction in association with concurrent body weight to confirm and expand the existing literature, and (ii) a prospective analysis of poor olfaction and changes in body composition, using repeated measures of body composition assessed on an annual or biennial basis over a 7-year period.
Method
Study Population
Details of the Health, Aging and Body Composition Study (Health ABC) was published previously (19,20). Briefly, the study enrolled 3,075 community-dwelling, relatively healthy older adults (45% women, 33% black), aged 70–79, during 1997–1998 from Memphis, TN and Pittsburgh, PA. Eligibility criteria included a self-reported ability to climb 10 stairs and walk ¼ mile with no difficulty, no mobility-related problems in performing everyday tasks, cancer-free for the previous 3 years, and no intention of changing residence for at least three years. The health of study participants was subsequently evaluated by means of annual clinical or home visits and semiannual phone interviews. Olfaction was tested at the year 3 clinical visit in 1999–2000, which was considered as the baseline for the current analyses. Institutional Review Boards at the University of Tennessee, the University of Pittsburgh, and the University of California-San Francisco approved all study protocols and written informed consent was obtained for all participants.
Body Composition Measures
Three body composition measures were analyzed in this study: (i) total mass, (ii) total lean mass (ie, fat-free mass), and (iii) total fat mass. All measures were assessed at clinical visits in years 3, 4, 5, 6, 8, and 10, using whole-body dual-energy X-ray absorptiometry (DXA), a noninvasive method that accurately measures both total body composition and regional subsections (21). Two separate x-rays are generated using different energy levels and then, through the comparison of the two results, accurate totals can be assessed for various body composition measures (21). Validation for DXA in assessing lean mass (22) and fat mass measurements (23) has been published previously.
Brief Smell Identification Test
As part of the year 3 clinical visit, study participants completed the Brief Smell Identification Test (BSIT) (24). The BSIT is an abbreviated version of the 40-item Pennsylvania Smell Identification Test and is the most commonly used screening test for olfactory impairment in epidemiological studies (25,26). Briefly, participants are presented 12 common odors one at a time via a scratch-and-sniff card and asked to choose from four options the descriptor that best matches their impression of the odor presented. Each correct answer is given one point and the final score is the total of correct answers, ranging from 0 to 12, with a higher score indicating a better sense of smell (27). This test of olfaction identification has been validated in multiple studies (27,28). For this study, we categorized the BSIT score into tertiles: good (BSIT score 11–12), moderate (BSIT score 9–10), and poor olfaction (BSIT score 0–8).
Covariates Assessment
Covariates in the analyses include demographics (ie, age, sex, race, clinic site, height, education, and family income), smoking status, brisk walking as a surrogate for physical activity, self-reported general health status, and several common chronic diseases as detailed below. Except for age, all demographic variables were from year 1 clinical visit as they were not assessed in year 3. For others, analytic variables were from year 3.
In the analysis, we defined the following health conditions and diseases according to previously published criteria. Briefly, cancer (29) (excluding melanoma), cardiovascular diseases (30) (coronary heart disease, congestive heart failure, cerebrovascular disease, and peripheral vascular disease), and neurodegenerative diseases (Parkinson’s disease (25) and dementia (31)) were adjudicated by the study team after comprehensive reviews of self-reported diagnostic information, hospitalization records, current medication use, and death certificates. Further, cognitive function was assessed in years 1, 3, 5, 8, and 10, using the Modified Mini-Mental State Examination (3MS, score range 0–100) (32). We defined participants as having dementia (31) if (i) their 3MS score in year 1 was <80 or (ii) their race-stratified score decline was ≥1.5 SDs since year 1. We defined depressive symptoms (33) as a score ≥10 on the 10-item Center for Epidemiological Studies-Depression (CES-D) scale. Diabetes (34) was assessed through self-reported diagnosis or antidiabetic medication use or if fasting blood glucose levels were ≥126 mg/dL or 2-hour oral glucose tolerance test levels were ≥200 mg/dL. Finally, hypertension (35) was defined through self-reported diagnosis and medication use, or if systolic blood pressure was ≥140 mmHg or diastolic blood pressure was ≥90 mmHg.
Statistical Analysis
For the purpose of the current analyses, we defined year 3 clinical visit (1999–2000) as the baseline, and the last body composition assessment at the year 10 clinical visit (2006–2007) as the end of follow-up. A total of 2,537 participants took the BSIT test at baseline (year 3 visit). After excluding 136 participants with missing body composition measures and 11 for missing covariates other than family income, the final analytic sample included 2,390 study participants. Since missing family income was relatively common (12%), we created a missing category for the analysis. Means and SDs for continuous variables and frequency for discrete variables are presented as summary statistics.
We examined olfaction in relation to each of the three parameters of body composition (ie, total, lean, and fat mass), using participants with a good sense of smell as the reference group (BSIT score 11–12). The baseline cross-sectional analysis was completed by means of multivariable linear regression, adjusting for demographics, lifestyle choices, and health and disease status as defined above. In the longitudinal analysis, we examined repeated measures for each body composition parameter from clinical visits at years 3, 4, 5, 6, 8, and 10, corresponding to 0, 1, 2, 3, 5, and 7 years of follow-up. We used multivariable linear mixed models estimated by the maximum likelihood method to assess differences in annual changes of body composition across olfaction groups by examining interaction terms between the olfaction and follow-up year variables, assuming an unstructured covariance matrix for residuals. We further utilized a difference-in-differences approach to compare differences in body composition changes at each of the follow-up years when body compositions were measured. Inverse probability weighting was used to address for loss-to-follow-up. We presented this difference-in-differences analysis as Supplementary Materials.
In addition, we conducted subgroup analyses to examine whether associations differ by sex and self-reported race. We did not stratify by age because the age range was only 10 years. Finally, as weight loss is often associated with poor health in older adults and commonly occurs in chronic diseases such as cancer and neurodegenerative diseases, we additionally conducted subgroup analyses stratified by health status, accounting for baseline self-reported health (fair to poor vs excellent to good), and the presence of baseline cancer, dementia, or Parkinson’s disease (10,36,37). We used SAS 9.4 (SAS Institute Inc., Cary, NC) for all statistical analyses.
Results
At baseline, study participants with a poor sense of smell were more likely to be older, slightly taller, male, black, from the Memphis study site, and former smoker, and to report lower education, lower family income, and poor health status (Table 1). For chronic diseases, participants with poor olfaction were more likely to have diabetes, Parkinson’s disease or dementia, and depressive symptoms.
Table 1.
Population Characteristics by Olfaction Status at the Year 3 Clinical Visit
| Poor Olfaction BSIT Score 0–8 n = 782 | Moderate Olfaction BSIT Score 9–10 n = 818 | Good Olfaction BSIT Score 11–12 n = 790 | |
|---|---|---|---|
| Continuous variables, mean ± SD | |||
| Age in years | 76.1 ± 2.91 | 75.5 ± 2.90 | 75.1 ± 2.60 |
| Standing height in meters | 1.68 ± 0.09 | 1.66 ± 0.10 | 1.65 ± 0.09 |
| Categorical variables, number (%) | |||
| Sex | |||
| Female | 317 (40.5) | 425 (52.0) | 490 (62.0) |
| Male | 465 (59.5) | 393 (48.0) | 300 (38.0) |
| Race | |||
| White | 417 (53.3) | 506 (61.9) | 539 (68.2) |
| Black | 365 (46.7) | 312 (38.1) | 251 (31.8) |
| Clinic site | |||
| Pittsburgh | 369 (47.2) | 406 (50.4) | 431 (54.6) |
| Memphis | 413 (52.8) | 412 (49.6) | 359 (45.4) |
| Physical activity | |||
| ≥90 min/wk | 71 (9.1) | 78 (9.5) | 95 (12.0) |
| <90 min/wk | 711 (90.9) | 740 (90.5) | 695 (88.0) |
| Smoking status | |||
| Never smoker | 322 (41.2) | 363 (44.4) | 387 (49.0) |
| Former smoker | 84 (10.7) | 64 (8.0) | 41 (5.2) |
| Current smoker | 376 (48.1) | 390 (47.3) | 362 (45.8) |
| Education | |||
| Less than high school | 255 (32.6) | 177 (21.6) | 125 (15.8) |
| High school graduate | 220 (28.1) | 282 (34.5) | 268 (33.9) |
| Postsecondary | 307 (39.3) | 359 (43.9) | 397 (50.3) |
| Family income | |||
| Less than 10k | 109 (13.9) | 84 (10.3) | 62 (7.8) |
| 10k to 25k | 280 (35.8) | 254 (31.0) | 258 (32.7) |
| 25k to 50k | 209 (26.7) | 255 (31.2) | 233 (29.5) |
| Greater than 50k | 96 (12.3) | 119 (14.5) | 151 (19.1) |
| Missing | 88 (11.3) | 106 (13.0) | 86 (10.9) |
| Health status | |||
| Excellent/very good | 307 (39.3) | 363 (44.4) | 400 (50.6) |
| Good | 296 (37.8) | 329 (40.2) | 270 (34.2) |
| Fair/poor | 179 (22.9) | 126 (15.4) | 120 (15.2) |
| Diabetes | |||
| Yes | 203 (26.0) | 195 (23.8) | 160 (20.3) |
| No | 579 (74.0) | 623 (76.2) | 630 (79.7) |
| Hypertension | |||
| Yes | 588 (75.2) | 623 (76.2) | 571 (72.3) |
| No | 194 (24.8) | 195 (23.8) | 219 (27.7) |
| Cancer | |||
| Yes | 151 (19.3) | 164 (20.1) | 157 (19.9) |
| No | 631 (80.7) | 654 (79.9) | 633 (80.1) |
| Cardiovascular diseases | |||
| Yes | 229 (29.3) | 238 (29.1) | 226 (28.6) |
| No | 553 (70.7) | 580 (70.9) | 564 (71.4) |
| Parkinson or dementia | |||
| Yes | 209 (26.7) | 101 (12.4) | 60 (7.6) |
| No | 573 (73.3) | 717 (87.6) | 730 (92.4) |
| Depressive symptoms | |||
| Yes | 133 (17.0) | 123 (15.0) | 94 (11.9) |
| No | 649 (83.0) | 695 (85.0) | 696 (88.1) |
Note: BSIT = Brief Smell Identification Test.
Cross-sectional Analysis
In the cross-sectional analysis at year 3 clinical visit, poor olfaction was significantly associated with lower total mass and lower fat mass when compared to the reference group (Table 2). Specifically, older adults with poor olfaction had a total body mass that was, on average, 1.67 kg lower (95% CI: −2.92, −0.42) than those with good olfaction, and for total fat mass, the difference was −1.14 kg (95% CI: −1.96, −0.31). Their lean mass was also lower, but the difference (−0.53 kg, 95% CI: −1.08, 0.02) was not statistically significant. There was no difference contrasting moderate with good olfaction for any of the anthropometric outcomes.
Table 2.
Cross-sectional and Longitudinal Analyses of Olfaction in Relation to Body Composition Measures in the Health ABC Study
| Differences in Body Composition Measures at Baseline | |||
|---|---|---|---|
| Body Composition Measures | Difference (SE) | 95% CI | |
| Total mass (kg) | |||
| Poor olfaction | −1.67* (0.64) | (−2.92, −0.42) | |
| Moderate olfaction | 0.09 (0.60) | (−1.10, 1.27) | |
| Good olfaction | Ref | ||
| Total lean mass (kg) | |||
| Poor olfaction | −0.53 (0.28) | (−1.08, 0.02) | |
| Moderate olfaction | 0.02 (0.27) | (−0.50, 0.54) | |
| Good olfaction | Ref | ||
| Total fat mass (kg) | |||
| Poor olfaction | −1.14* (0.42) | (−1.96, −0.31) | |
| Moderate olfaction | 0.06 (0.40) | (−0.72, 0.84) | |
| Good olfaction | Ref | ||
| Differences in Annual Changes in Body Composition Measures Over 7 Years | |||
| Body Composition Measures | Difference in Annual Changes (SE) | 95% CI | |
| Total mass (g) | |||
| Poor olfaction | −234* (105) | (−442, −26) | |
| Moderate olfaction | −189 (98) | (−383, 6) | |
| Good olfaction | Ref | ||
| Total lean mass (g) | |||
| Poor olfaction | −139* (49) | (−236, −43) | |
| Moderate olfaction | −49 (46) | (−141, 43) | |
| Good olfaction | Ref | ||
| Total fat mass (g) | |||
| Poor olfaction | −113 (88) | (−285, 59) | |
| Moderate olfaction | −162 (86) | (−331, 8) | |
| Good olfaction | Ref | ||
Notes: Adjusted for age, sex, race, height, education, clinic site, family income, smoking status, physical activity, self-reported health status, cancer, depression, dementia, cardiovascular diseases, diabetes, and hypertension. Poor olfaction represents BSIT score 0–8, moderate 9–10, and good 11–12. BSIT = Brief Smell Identification Test; CI = confidence interval; SE = standard error.
*p < .05.
Longitudinal Analysis
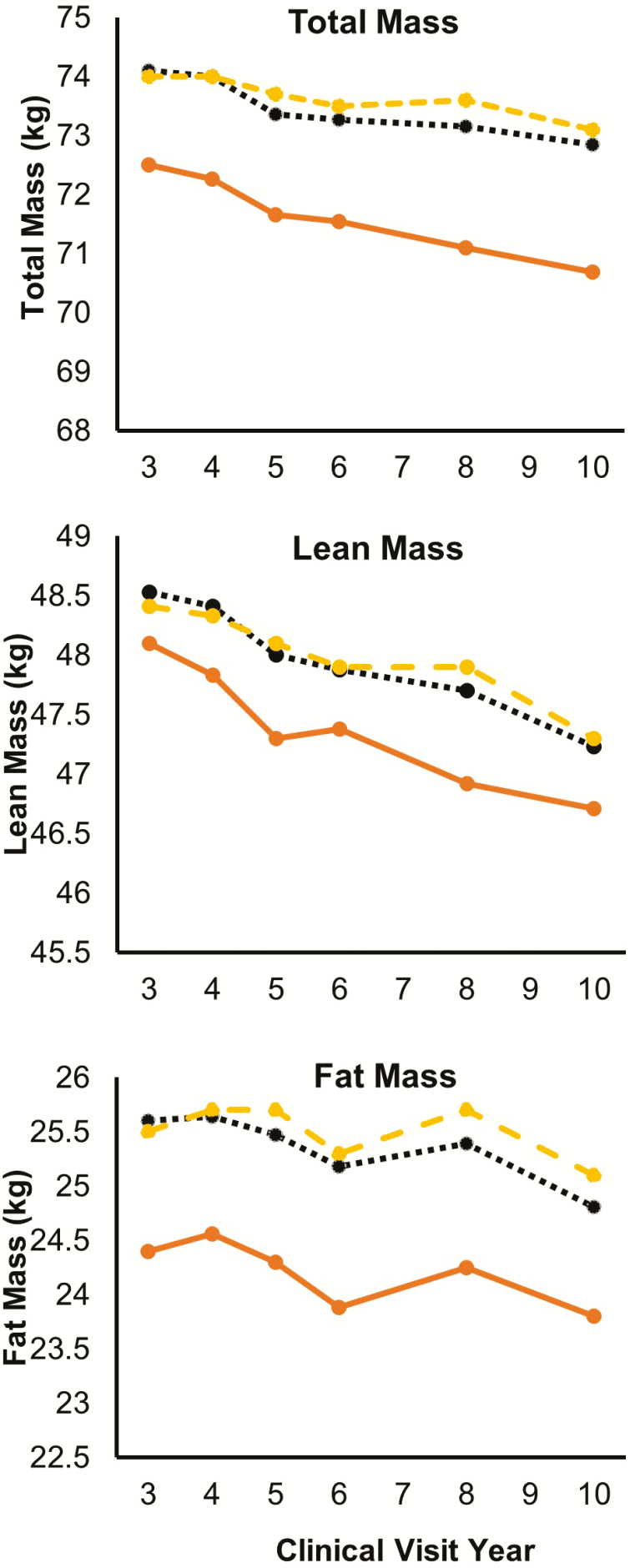
During the follow-up, all three measures of body composition decreased overtime across olfaction groups (Figure 1). In longitudinal analysis (Table 2), compared with the group with good sense of smell, poor olfaction was associated with greater average body composition losses per year, specifically for total mass (−234 g, 95% CI: −442, −26) and lean mass (−139 g, 95% CI: −236, −43). A similar observation was made for fat mass, but the association was not statistically significant. The difference-in-differences analysis that examined year-by-year changes largely confirmed these observations (Supplementary Table 1).
Figure 1.
Changes in body composition measures by olfaction status from clinical visits year 3 to year 10. Solid line: poor olfaction (BSIT score 0–8); dotted line: moderate olfaction (BSIT score 9–10); dashed line: good olfaction (BSIT score 11–12). BSIT = Brief Smell Identification Test.
Results from subgroup analyses by sex and race generally showed similar patterns (Supplementary Table 2), although the estimates are less stable due to smaller sample sizes. Notably, in a few of the analysis, the moderate olfaction group showed statistical differences from the poor olfaction group among women or white participants. The subgroup analyses by health status also showed comparable results (Supplementary Table 3).
Discussion
In this large biracial cohort of community-dwelling older adults, poor olfaction was associated with lower body weight and greater weight loss over time, notably for total and lean masses. To the best of our knowledge, this is the first prospective evidence that documents poor olfaction in relation to changes in body weight and composition. These changes could not be explained by population demographics, behavioral, and health-related factors. Our data clearly demonstrate a pattern of poor olfaction associated weight loss in older adults.
Poor olfaction is prevalent among older adults and may have profound health implications. While the research has focused on poor olfaction in relation to dementia and Parkinson’s disease, our analyses showed these neurodegenerative diseases only explained about 20% of the increased mortality associated with poor olfaction among older adults (38), suggesting poor olfaction is a broader marker for accelerated aging among older adults beyond neurodegenerative diseases. In the same analyses, weight loss accounted for ~6% of the elevated mortality among older adults with a poor sense of smell (38).
Loss of body mass is common among older adults (39), with men generally declining in weight after age 55 and women after age 65 at an average loss of 0.1–0.2 kg per year (40). Examining weight loss solely by total mass, however, leaves an incomplete picture of health for older adults. The loss of lean mass, especially relative to the retention of fat mass, is considered to be a major indicator of poor and declining health risks in older adults. The loss of lean mass can lead to a multitude of health and life quality concerns, including impaired mobility (41) and increased mortality (42). Further, not all weight loss occurs for the same reason or has similar outcomes for body composition. For example, weight loss brought upon by diet and exercise is more likely to preserve lean mass and reduce fat mass (43), while weight loss due to disease or other health-related factors may diminish lean mass as well as total body mass (40).
This study clearly demonstrated an association between poor olfaction and weight loss in older adults, independent of a range of potential confounders and general health status. However, the mechanisms that underlie this association remain unclear. Scientists have speculated that poor olfaction may alter a person’s appetite and dietary intake (2,3) which may in turn lead to changes in body weight and composition. Opinions about the direction of this association differ however, with some speculating that poor sense of smell and associated taste changes results in lower appetite and enjoyment of eating and therefore reduced caloric intake (44), whereas others speculate that poor olfaction may lead to higher intake of caloric-intensive and tastier foods (45), and therefore encourage weight gain. Neither speculation has been rigorously examined. Additionally, with the potential linkage between olfaction and hormones such as leptin (6,46), there are likely complex interactions among satiety, food intakes, sensory inputs of taste and smell, and metabolic rates to affect body composition over time (6,46). A connection between olfaction, metabolism, and body weight was recently suggested by a murine experiment, which found that mice with a poor sense of smell experienced significant weight loss, even though the caloric intake was held constant (47). The authors reported that poor olfaction led to a higher resting metabolic rate in mice, which explained a lower body weight despite consistent calorie intake. There are no comparable empirical data in humans; however, some earlier cross-sectional studies have linked to olfaction to metabolic or eating disorders including obesity (47), anorexia (48), and diabetes (46). Future studies should systematically investigate the potential connections among poor olfaction, dietary changes, metabolic rate, body composition, and health status in the context of aging.
The current study has several notable strengths. Health ABC is a large, well-characterized, community-based, biracial cohort that was designed specifically to prospectively investigate changes in body composition and function in older adults. All these characteristics strengthen the validity and generalizability of study results to similar free-living populations. The cohort had repeated assessments of body composition using DXA and thus enabled precise assessment of change in various components of body composition on an annual or biennial basis for up to 7 years. Finally, the study collected a wide range of covariates that allowed us to account for potential confounding from demographics, lifestyle, and health conditions.
This study also has several limitations. First, Health ABC participants had their olfaction assessed at age 75 years on average over a narrow age range. Therefore, generalizability to relatively younger older adult populations is limited. Further, the sense of smell decreases steadily with age, but we measured olfaction only once at a single time point. Examining the trajectory of olfaction decline in older adults and how it affects body composition and health outcomes would be a potentially important interesting direction to head in further studies. Additionally, the BSIT only assesses smell identification, not threshold or discrimination, and additional research should be done to examine how various modalities of olfaction affect body composition in the context of aging. The BSIT does not differentiate between causes of olfactory impairment, which could be due to unrelated causes such as nasal surgery or head injuries. Finally, while the study identified a significant association between poor olfaction and weight loss in older adults, the clinical significance and implications of this observation remain unknown and need to be further investigated.
In conclusion, we found that poor olfaction in older adults was associated with a decline of body mass, fat mass, and lean mass in this large community-based cohort. This association was robust and independent from lifestyle factors or health status. Future studies should investigate the underlying mechanisms and its health implications in the context of aging.
Supplementary Material
Acknowledgments
Author contributions: H.C. conceived the study, and E.J.S., E.M.S., and T.B.H. provided data. F.P., Z.L., J.C.G., and H.C. designed the analyses, performed data management and statistical analysis. F.P. and H.C. prepared the first draft of the manuscript and all other coauthors provided critical comments. All authors contributed to interpretation of results and critical revision of the manuscript. All authors have contributed to the manuscript and gave final approval for submission.
Funding
The Health ABC Study research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging (NIA), and by NIA contracts N01AG62101, N01AG62103, and N01AG62106. H.C. is supported by a start-up fund from Error! Bookmark not defined.Michigan State University (GE100455), the Parkinson’s Foundation (grant no. PF-IMP-1825), and the Office of the Assistant Secretary of Defense for Health Affairs, through the Parkinson’s Research Program (award no. W81XWH-17-1-0536), and the Error! Bookmark not defined.National Institute of Environmental Health Sciences (R01ES029227). Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense or the National Institutes of Health.
Conflict of Interest
None reported.
References
- 1. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 2. Peng M, Coutts D, Wang T, Cakmak YO. Systematic review of olfactory shifts related to obesity. Obes Rev. 2019;20:325–338. doi: 10.1111/obr.12800 [DOI] [PubMed] [Google Scholar]
- 3. Doty RL. Age-related deficits in taste and smell. Otolaryngol Clin North Am. 2018;51:815–825. doi: 10.1016/j.otc.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 4. Trellakis S, Tagay S, Fischer C, et al. Ghrelin, leptin and adiponectin as possible predictors of the hedonic value of odors. Regul Pept. 2011;167:112–117. doi: 10.1016/j.regpep.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 5. Nicholson T, Church C, Baker DJ, Jones SW. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflamm (Lond). 2018;15:9. doi: 10.1186/s12950-018-0185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandez-Garcia JC, Alcaide J, Santiago-Fernandez C, et al. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS One. 2017;12:e0171204. doi: 10.1371/journal.pone.0171204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy RA, Patel KV, Kritchevsky SB, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014;62:1476–1483. doi: 10.1111/jgs.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lloyd JT, Alley DE, Hochberg MC, et al. ; Health ABC Study Changes in bone mineral density over time by body mass index in the health ABC study. Osteoporos Int. 2016;27:2109–2116. doi: 10.1007/s00198-016-3506-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Callen BL. Nutritional screening in community dwelling older adults. Int J Older People Nurs. 2011;6:272–281. doi: 10.1111/j.1748-3743.2010.00241.x [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Zhang SM, Hernán MA, Willett WC, Ascherio A. Weight loss in Parkinson’s disease. Ann Neurol. 2003;53:676–679. doi: 10.1002/ana.10577 [DOI] [PubMed] [Google Scholar]
- 11. Jimenez A, Pegueroles J, Carmona-Iragui M, et al. ; Alzheimer’s Disease Neuroimaging Initiative Weight loss in the healthy elderly might be a non-cognitive sign of preclinical Alzheimer’s disease. Oncotarget. 2017;8:104706–104716. doi: 10.18632/oncotarget.22218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spauwen PJ, Murphy RA, Jónsson PV, et al. Associations of fat and muscle tissue with cognitive status in older adults: the AGES-Reykjavik Study. Age Ageing. 2017;46:250–257. doi: 10.1093/ageing/afw219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP; Cardiovascular Study Research Group Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x [DOI] [PubMed] [Google Scholar]
- 14. Aiello M, Parma V, De Carlo S, Hummel T, Rumiati RI. Cognitive, olfactory, and affective determinants of body weight in aging individuals. Arch Clin Neuropsychol. 2019;34:637–647. doi: 10.1093/arclin/acy072 [DOI] [PubMed] [Google Scholar]
- 15. Dong J, Pinto JM, Guo X, et al. The prevalence of anosmia and associated factors among U.S. black and white older adults. J Gerontol A Biol Sci Med Sci. 2017;72:1080–1086. doi: 10.1093/gerona/glx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasmussen VF, Vestergaard ET, Hejlesen O, Andersson CUN, Cichosz SL. Prevalence of taste and smell impairment in adults with diabetes: a cross-sectional analysis of data from the National Health and Nutrition Examination Survey (NHANES). Prim Care Diabetes. 2018;12:453–459. doi: 10.1016/j.pcd.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 17. Toussaint N, de Roon M, van Campen JP, Kremer S, Boesveldt S. Loss of olfactory function and nutritional status in vital older adults and geriatric patients. Chem Senses. 2015;40:197–203. doi: 10.1093/chemse/bju113 [DOI] [PubMed] [Google Scholar]
- 18. Patel ZM, DelGaudio JM, Wise SK. Higher body mass index is associated with subjective olfactory dysfunction. Behav Neurol. 2015;2015:675635. doi: 10.1155/2015/675635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 20. National Institute on Aging. Introducing the Health ABC Study: The Dynamics of Health, Aging, and Body Composition https://healthabc.nia.nih.gov/. Accessed December 3, 2020.
- 21. Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12:45–51. doi: 10.1016/0899-9007(95)00017-8 [DOI] [PubMed] [Google Scholar]
- 22. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985). 1999;87:1513–1520. doi: 10.1152/jappl.1999.87.4.1513 [DOI] [PubMed] [Google Scholar]
- 23. Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol (1985). 2000;89:345–352. doi: 10.1152/jappl.2000.89.1.345 [DOI] [PubMed] [Google Scholar]
- 24. Doty RL. The Brief Smell Identification TestTM Administration Manual. Haddon Heights, NJ: Sensonics, Inc.; 2001. [Google Scholar]
- 25. Chen H, Shrestha S, Huang X, et al. ; Health ABC Study Olfaction and incident Parkinson disease in US white and black older adults. Neurology. 2017;89:1441–1447. doi: 10.1212/WNL.0000000000004382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts RO, Christianson TJ, Kremers WK, et al. association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73:93–101. doi: 10.1001/jamaneurol.2015.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021 [DOI] [PubMed] [Google Scholar]
- 28. Doty RL. Olfactory dysfunction and its measurement in the clinic. World J Otorhinolaryngol Head Neck Surg. 2015;1:28–33. doi: 10.1016/j.wjorl.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Izano M, Wei EK, Tai C, et al. ; Health ABC Study Chronic inflammation and risk of colorectal and other obesity-related cancers: the Health, Aging and body composition study. Int J Cancer. 2016;138:1118–1128. doi: 10.1002/ijc.29868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, et al. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175:410–419. doi: 10.1001/jamainternmed.2014.6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2017;88:456–462. doi: 10.1212/WNL.0000000000003558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 33. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. doi: 10.1016/s0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- 34. Yaffe K, Falvey CM, Hamilton N, et al. ; Health ABC Study Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173:1300–1306. doi: 10.1001/jamainternmed.2013.6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shea MK, Booth SL, Weiner DE, et al. ; Health ABC Study Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the health, aging, and body composition study (Health ABC). J Nutr. 2017;147:888–895. doi: 10.3945/jn.117.249375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x [DOI] [PubMed] [Google Scholar]
- 37. Sarhill N, Mahmoud F, Walsh D, et al. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer. 2003;11:652–659. doi: 10.1007/s00520-003-0486-0 [DOI] [PubMed] [Google Scholar]
- 38. Liu B, Luo Z, Pinto JM, et al. Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. 2019;170:673–681. doi: 10.7326/M18-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fillit H, Rockwood K, Young J. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 8th ed. Philadelphia, PA: Elsevier, Inc.; 2017. doi: 10.1016/b978-1-4160-6231-8.x0001-3 [DOI] [Google Scholar]
- 40. Wallace JI, Schwartz RS. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85:15–21. doi: 10.1016/s0167-5273(02)00246-2 [DOI] [PubMed] [Google Scholar]
- 41. Lee JS, Visser M, Tylavsky FA, et al. ; Health ABC Study Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001 [DOI] [PubMed] [Google Scholar]
- 43. Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36:239–262. doi: 10.2165/00007256-200636030-00005 [DOI] [PubMed] [Google Scholar]
- 44. Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278:1357–1362. doi: 10.1001/jama.1997.03550160077042 [DOI] [PubMed] [Google Scholar]
- 45. Aschenbrenner K, Hummel C, Teszmer K, et al. The influence of olfactory loss on dietary behaviors. Laryngoscope. 2008;118:135–144. doi: 10.1097/MLG.0b013e318155a4b9 [DOI] [PubMed] [Google Scholar]
- 46. Palouzier-Paulignan B, Lacroix MC, Aimé P, et al. Olfaction under metabolic influences. Chem Senses. 2012;37:769–797. doi: 10.1093/chemse/bjs059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riera CE, Tsaousidou E, Halloran J, et al. The sense of smell impacts metabolic health and obesity. Cell Metab. 2017;26:198–211.e5. doi: 10.1016/j.cmet.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 48. Islam MA, Fagundo AB, Arcelus J, et al. Olfaction in eating disorders and abnormal eating behavior: a systematic review. Front Psychol. 2015;6:1431. doi: 10.3389/fpsyg.2015.01431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.