Abstract
Balance impairment and falls are among the most prevalent and morbid conditions affecting older adults. A critical contributor to balance and gait function is the vestibular system; however, there remain substantial knowledge gaps regarding age-related vestibular loss and its contribution to balance impairment and falls in older adults. Given these knowledge gaps, the National Institute on Aging and the National Institute on Deafness and Other Communication Disorders convened a multidisciplinary workshop in April 2019 that brought together experts from a wide array of disciplines, such as vestibular physiology, neuroscience, movement science, rehabilitation, and geriatrics. The goal of the workshop was to identify key knowledge gaps on vestibular function and balance control in older adults and develop a research agenda to make substantial advancements in the field. This article provides a report of the proceedings of this workshop. Three key questions emerged from the workshop, specifically: (i) How does aging impact vestibular function?; (ii) How do we know what is the contribution of age-related vestibular impairment to an older adult’s balance problem?; and more broadly, (iii) Can we develop a nosology of balance impairments in older adults that can guide clinical practice? For each of these key questions, the current knowledge is reviewed, and the critical knowledge gaps and research strategies to address them are discussed. This document outlines an ambitious 5- to 10-year research agenda for increasing knowledge related to vestibular impairment and balance control in older adults, with the ultimate goal of linking this knowledge to more effective treatment.
Keywords: Vestibular, Aging, Balance
Balance impairment and falls are among the most prevalent and morbid conditions affecting older adults. Nearly 30% of older adults aged 65 and older fall each year, and recent data report that the mortality rate associated with falls has more than doubled from 51.6 per 100,000 persons in 2000 to 122.2 per 100,000 persons in 2016 (1). In 2008, Americans reportedly experienced 80 million falls, resulting in 10 million fall-related injuries (2). Balance and gait impairments are considered the most important causes of falls in older adults (3). However, a clear understanding of the multiple interacting physiologic and pathophysiologic mechanisms that underlie balance impairments in older adults and that can inform clinical practice is lacking. Notably, a critical contributor to balance and gait function is the vestibular system, and compelling epidemiologic evidence suggests that vestibular impairment is a significant contributor to imbalance and falls in older U.S. adults (4,5). Symptomatic vestibular impairment has been estimated to increase the odds of falling over 12-fold (4). However, these studies have yet to be widely replicated and recognized, and as such in the clinical setting, vestibular impairment is rarely screened for or treated in older adults with balance impairment and falls.
Given these substantial knowledge gaps, the National Institute on Aging and the National Institute on Deafness and Other Communication Disorders recently convened a workshop to evaluate current knowledge and establish research priorities about changes in balance control with aging, with a special emphasis on age-related vestibular impairment. Representatives with expertise in basic, clinical, epidemiologic, and population health research in the fields of gerontology, geriatrics, vestibular physiology, neurology, neuroscience, movement science, movement disorders, and rehabilitation came together for a 2-day workshop in April 2019. This article provides a report of the proceedings of this workshop. We will start with a brief overview of balance control and the anatomy and physiology of the vestibular system. We will then outline the three key questions that emerged from the workshop, specifically: (i) How does aging impact vestibular function?; (ii) How do we know what is the contribution of age-related vestibular impairment to an older adult’s balance problem?; and more broadly, (iii) Can we develop a nosology of balance impairments in older adults that can guide clinical practice? For each of these key questions, we will summarize the current knowledge, then discuss critical knowledge gaps and research strategies to address them over the next 5–10 years.
Overview of Balance Control
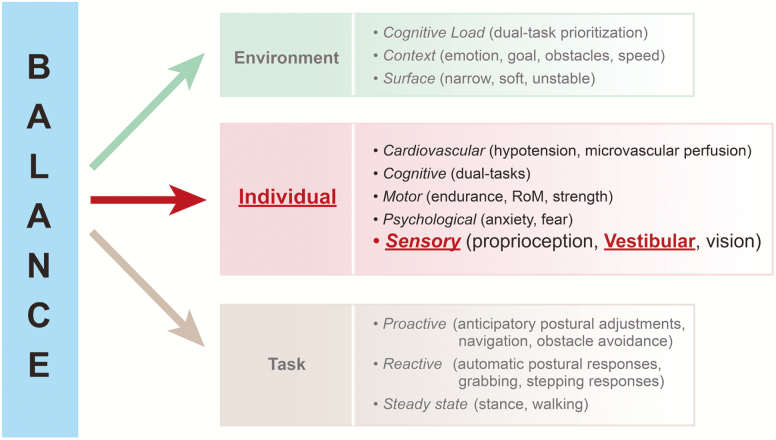
Balance or postural control is a complex process that results from a dynamic interplay between the specific postural tasks to be accomplished, the functional level of an individual’s physiological systems, and the given environment (Figure 1) (6). Different postural tasks are controlled by different central and peripheral nervous system networks and can be categorized into three types: (i) steady state posture (eg, sitting, standing, and walking) (7,8); (ii) reactive postural responses to unanticipated, external postural perturbations (eg, fixed support using ankle, hip, and shoulder torques, or change in support using stepping or reaching strategies) (9–11); and (iii) proactive postural control (ie, anticipatory postural adjustments preceding most voluntary activity, such as postural transitions and navigation) (12). An aging individual’s sensory, motor, cognitive, psychological, and cardiovascular health form the basis for how an individual performs the postural tasks in a particular environment. Moreover, the brain’s integration of various sensorimotor cues is critical for balance control; indeed, several studies have noted that multisensory integration (eg, of visual and somatosensory inputs) are predictive of balance function and falls (13,14). In addition, the cognitive load, environmental, and emotional conditions will affect an individual’s balance control (15–17).
Figure 1.
Model of balance as a dynamic process that is a function of individual characteristics (impairments), the type of balance task, and the environmental conditions. Source: adapted from Shumway Cook and Woollacott (6).
Overview of the Vestibular System
The vestibular system is a sensory organ housed in the inner ear that serves critical functions vital for survival: sensing movement of the head and the orientation of the head with respect to gravity (18,19). The vestibular system was not considered one of Aristotle’s cardinal five senses. However, since the discovery of the vestibular organ in the middle of the 19th century, it is clear that the vestibular system provides sensory information to the brain about head movement and position; as such the vestibular system has been described as the “sixth sense.” (20) Sensory information from the vestibular system is widely distributed in the brain, brainstem, and spinal cord, and contributes to multiple physiological and behavioral responses that utilize information about head movement and orientation (21). The vestibular system is critical for keeping the visual world stable during head movement (eg, while walking) via very fast vestibulo-ocular reflexes (VORs) (22). Vestibular impairment often results in dizziness due to blurring or apparent oscillation of the visual world, because the eyes cannot compensate quickly enough for head movements (23). Vestibular inputs also drive vestibulo-spinal responses (VSRs) that maintain postural control (24). Vestibular impairment results in imbalance due to inadequate postural stabilization during changes in head position and orientation, which can result in falls (25). Additionally, the vestibular system contributes to vestibulo-autonomic responses (VARs), whereby the vestibular signal about head position is used for homeostatic regulation of processes, such as blood pressure, respiratory rate, and gastrointestinal activity during position change (24,26). Vestibular impairment can lead to lightheadedness due to lack of immediate stabilization of intracranial perfusion during position change (27). The vestibular system also contributes to an individual’s perception of their self-motion and self-orientation in space (sometimes referred to as vestibular perception, or spatial orientation) via vestibulo-thalamo-cortical projections (28). Vestibular impairment can lead to spatial disorientation and reduced spatial memory and navigation ability (29). Finally, vestibular inputs also project to the cerebellum, which is thought to play a role in adaptation and calibration of the behavioral responses (motor, autonomic, perceptual, etc.) to vestibular sensory input (20). Loss of vestibular inputs to the cerebellum results in dysmetria and loss of adaptive control of behavioral responses (30).
Key Question 1: How Does Aging Impact the Vestibular System?
Current Knowledge
With respect to age-related structural changes, a few histopathological studies have been conducted in aged animals and in human temporal bone specimens examining age-related changes in peripheral and central vestibular structures. Some studies have noted age-related reduction in both peripheral (eg, hair cells) and central (eg, vestibular afferent nerve, brainstem vestibular nuclei neurons) vestibular cell populations (31–46), although the evidence is not uniform. With respect to age-related changes in clinical vestibular function, the evidence is also equivocal, and varies according to the type of test used for evaluation (47,48). Currently, there is no definitive, universal physiologic test of vestibular function used in vestibular clinical practice or research (eg, unlike the audiogram). Each vestibular physiologic test has certain advantages and disadvantages. The caloric test is perhaps the most widely used vestibular test, and assesses horizontal semicircular canal function. Limits of the caloric test are that it only measures relative function between the right and left side and does not measure absolute vestibular function, and also that the caloric stimulus is an aphysiologic stimulus that is much lower that what is naturally encountered by the vestibular system (49). Rotatory chair testing is another test that has good reproducibility, although also only measures horizontal canal function and also cannot disambiguate between right and left vestibular impairment (50). Video head impulse testing (vHIT) is a newer measure that can assess the function of all semicircular canals (horizontal, anterior, and posterior separately for each side) (51). It is a powerful, portable test; however, testing artifacts can limit the validity of the vHIT, particular in older adults in whom facial skin laxity presents a challenge in achieving the tight fit of the vHIT goggle on the face needed to accurately administer the test (52). Vestibular-evoked myogenic potential tests are the mainstay of otolith function testing; however, vestibular-evoked myogenic potential inter-rater reliability and reproducibility can be low, given that test performance relies on patient effort and examiner factors that can vary across tests (53). Linear movement threshold testing is another method of assessing otolith function; however, the six-degree of freedom platform needed to conduct this testing is available in only a few research laboratories (54). Finally, posturography is another test used to assess the vestibular system, particularly under conditions where other sensory cues to maintain standing balance are eliminated (eg, eyes closed, on foam). However, posturography is a global measure of standing balance and does not directly measure the function of the vestibular end-organ (55). A vestibular test battery that probes both semicircular canal and otolith function, and overcomes the previous technical challenges, and that is universally applied (such as the audiogram) is clearly needed. Numerous studies have observed decrements in VOR function with age (56), although in some studies substantial reductions were noted only over age 75 (57–59). Postural stability declines with aging; however, the extent to which age-related reduction in VSRs contributes to postural stability decline is unclear (48,60–63). Similarly, orthostatic hypotension is significantly more prevalent in older adults (64,65); however, the relative contributions of impaired vestibulo-autonomic reflexes, vascular disease and/or use of specific medications to orthostatic hypotension is unclear (66). Furthermore, spatial orientation and navigation ability is known to decline with aging (67); however, the contribution of declining vestibular inputs to thalamo-cortical and hippocampal spatial cognitive centers to declining spatial cognitive ability is unclear. Finally, very little data exist on potential genetic etiologies for age-related vestibular impairment.
Knowledge Gaps and Key Barriers
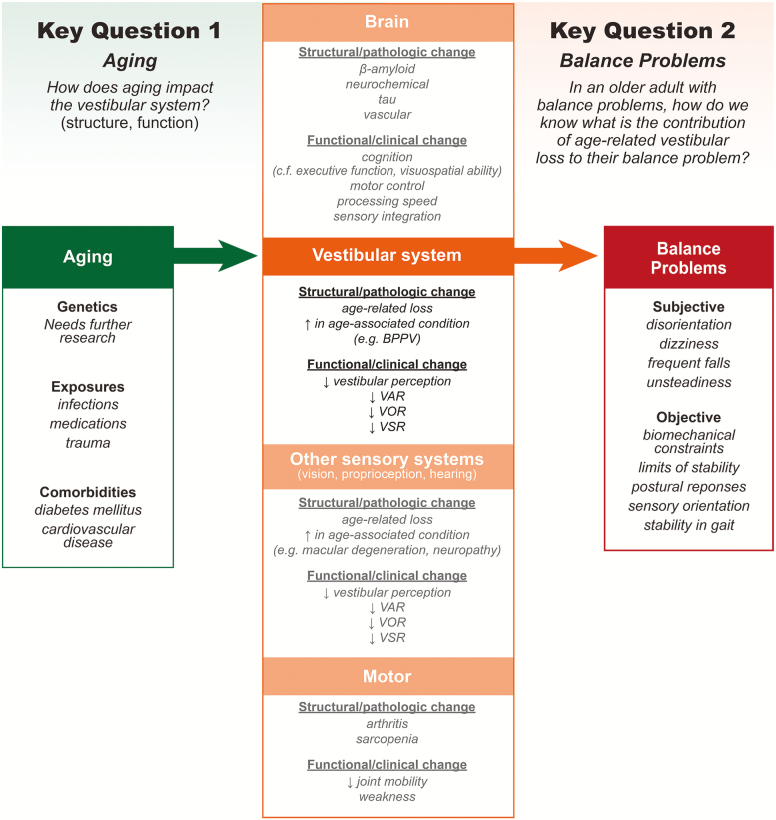
Given the current level of evidence, the group identified three major knowledge gaps related to understanding age-related changes in the vestibular system (Figure 2). First, at this time, we lack a comprehensive understanding of the impact of aging on peripheral and central vestibular structures, with respect to the onset (when do changes start), sequence (which peripheral/central structures are affected and in what temporal order), and timing (what is the time course of the sequence of changes that occur) of age-related changes. Second, we also lack a clear understanding of the time course and severity of age-related changes in vestibular functional pathways (eg, VOR, VSR, VAR) and the impact of these functional changes on clinical symptoms (eg, postural instability, orthostatic hypotension). Third, our understanding of structure–function relationships, that is, how structural changes at each of these levels (peripheral to central vestibular structures) correlate with changes in vestibular functional pathways, is also limited. For example, it has been suggested that functional VOR changes occur much later than would be expected based on the onset of histopathological changes in the vestibular system, possibly due to a broad range of CNS compensation for VOR impairment, and that otolith impairment disproportionately influences vestibulo-autonomic and vestibulo-spatial pathways. However, rigorous evidence of these relationships is lacking.
Figure 2.
Conceptual model of key gaps 1 and 2.
Key barriers to generating this evidence include (i) paucity of animal and human temporal bone data on impacts of aging on peripheral and central vestibular structures; (ii) lack of specific, reliable, reproducible clinical physiologic measures of semicircular canal and otolith function in humans; (iii) lack of any clinical physiologic measures of central vestibular function in humans; (iv) lack of population-based longitudinal studies of vestibular physiologic function extending greater than 5 years; (v) lack of rigorous studies correlating structural-functional changes in animals or in humans pre/post mortem. As a result of these knowledge gaps, emerging new therapies that address vestibular hair cell loss (eg, hair cell regeneration, electrical vestibular stimulation) cannot be effectively deployed because individuals with hair cell structural changes and resulting functional impairment cannot be accurately identified.
Research Strategy
1) Develop and define specific, reliable, reproducible clinical measures of semicircular canal and otolith function in humans that can be linked to pathological change to the vestibular system and to patient symptoms
2) Develop clinical measures of central vestibular function in humans that are also linked to pathology and phenotype
3) Track age-related changes in vestibular physiologic function longitudinally in population-based cohorts for 10+ years
4) Expand animal and human temporal bone data on impacts of aging on peripheral and central vestibular structures to include hundreds to thousands of subjects
5) Expand studies correlating structural-functional changes in animals or in humans pre-/postmortem to include hundreds to thousands of subjects
Key Question 2: In an Older Adult With Balance Problems, How Do We Know What Is the Contribution of Age-Related Vestibular Loss to Their Balance Problem?
In the clinical setting, the balance problems that older adults often present with are dizziness, disequilibrium, unsteady walking, and increased falls (68–70). While vestibular impairment (peripheral and/or central) is a common cause of these symptoms, multiple other age-related deficits may also contribute to these symptoms, including other sensory (eg, vision, somatosensory, hearing), neuromotor and musculoskeletal (eg, weakness, arthritis), cognitive (eg, attentional control (71), sensory integration (71,72), motor coordination/planning), and psychological impairment (eg, fear of falling) (73,74). Additionally, certain systemic conditions that increase in prevalence with age, such as vascular disease and diabetes, have widespread pathological effects and can impact multiple contributors to balance control (eg, sensory loss/peripheral neuropathy, cerebral hypoperfusion/orthostatic hypotension) (75). These risk factors may add to the impact of vestibular impairment on balance control, or may interact with the vestibular impairment to produce a specific balance phenotype. Definitively establishing the extent to which vestibular impairment may contribute to an older individual’s balance problem can be challenging. Determining a vestibular contribution is critical, however, particularly in light of emerging therapies that specifically target the vestibular system, such as vestibular hair cell regeneration and prosthetic electrical vestibular stimulation. In order to effectively deploy these treatments, we need to know that an individual’s balance problem is at least in part caused by their vestibular impairment rather than other coexisting impairments, such that balance function could be improved following vestibular treatment. This section outlines current knowledge about vestibular contributions to the balance phenotype, and identifies gaps in knowledge that need to be filled to successfully manage older adults with balance disorders.
Current Knowledge
Akin to hearing and vision loss in older adults (ie, presbycusis and presbyopia, respectively), vestibular impairment in older adults is thought to occur as a progressive, bilateral, partial loss of vestibular function (56). Age-related vestibular losses can be unilateral or asymmetric though are typically bilateral, given that the exposures related to aging (eg, cumulative toxic, metabolic, ischemic, infectious exposures) are typically systemic. Bilateral vestibular loss is known to affect specific balance tasks in particular environments, such as steady state standing/walking and reactive balance responses when both visual and surface information from the environment are not available or not reliable (76). Indeed, standing balance in the absence of both vision and surface information is impaired in a majority of U.S. adults aged 65 and older (4), and vestibular loss is a significant contributor to the ability to stand without vision or surface information (77,78). With respect to proactive balance tasks that guide transition and navigation, emerging evidence suggests that the vestibular system plays an important role in spatial memory and navigation ability. Older adults with vestibular impairment were more likely to have impaired performance on behavioral tests of spatial navigation, and also more likely to have real-world evidence of spatial deficits including difficulty driving (79–82). Interestingly, these deficits appear to be magnified in patients with Alzheimer’s disease, in whom vestibular loss was linked to an increased rate of spatial disorientation (83–85). However, the extent to which specific vestibular pathways (ie, VOR, VSR, VAR, and/or vestibular perception) mediate the relationship between peripheral vestibular loss and balance impairment is unclear. The most common clinical vestibular tests assess the VOR (eg, caloric testing, rotatory chair testing). Assessing the VSR and vestibular perception requires specialized equipment (electromyography and a motion platform, respectively) that is only available in a few vestibular research centers. Furthermore, there are no clinical tests that specifically assess the VAR (66), although the contribution of the vestibular system to cardiovascular control and orthostatic hypotension has been demonstrated in animal models (27).
The phenotype of vestibular impairment in older adults is characterized not only by the severity and type of vestibular physiologic deficit, but also by the extent of vestibular compensation. Vestibular compensation can be defined as the processes by which the system compensates for loss of vestibular inputs. Specifically, neurons receiving vestibular input can increase their gain to the diminished vestibular input, and/or reweight/upweight signals coming from other more reliable sensory systems (eg, vision) (86–88). There are numerous vestibular-related areas in the brain that are involved in this process, including the brainstem vestibular nuclei, vestibulocerebellar pathways, and higher-level cortical areas involved in sensorimotor processing and cognition (86). Age-related deficits in vestibular compensation can occur due to impairment in neuronal plasticity/reweighting mechanisms, which can occur due to CNS pathology or to the use of CNS-active medications (89). For example, it is known that cholinergic (3%–4% per decade) and dopaminergic (5%–8% per decade) losses also occur as part of normal aging (90–94). These neurochemical losses are most severe in neurodegenerative conditions, such as Parkinson’s Disease (PD) and atypical parkinsonian disorders, which may lie at the end of the spectrum of age-related changes. PD and atypical parkinsonian disorders are associated with reduced cholinergic transmission from brainstem and cerebellar vestibular nuclei to cortical centers involved in motor planning and coordination, and may contribute to both reduced central vestibular signaling and reduced central vestibular compensation. At present, disambiguating whether clinical symptoms are due to physiologic deficits versus compensation deficits is challenging. Clinical tests that comprehensively assess vestibular compensation are lacking. Measures that assess a single aspect of vestibular compensation have been developed, such as the identification of a particular strategy of vestibular adaptation (generating compensatory saccadic eye movements in the setting of an impaired VOR) (95). However, current assessments of sensory reweighting, for example, involve platform perturbation paradigms that are only available in select research centers (96). Identifying vestibular compensation deficits may be particularly relevant for guiding vestibular rehabilitation, which is predicated on enhancing vestibular compensation (97–99).
Knowledge Gaps and Key Barriers
Based on the current evidence, the group identified three major knowledge gaps related to our understanding of the contribution of vestibular declines to imbalance (Figure 2). First, it is unclear how each of the vestibular functional pathways (VOR, VSR, VAR, and vestibular perception) individually and in combination impact the complex process of balance. For example, VOR and VSR deficits may impair reactive postural tasks, while vestibular perceptual deficits may impair anticipatory postural adjustments that guide navigation. However, these relationships have not been comprehensively investigated and remain unclear. Second, there is a lack of understanding of the complex interactions between age-related vestibular loss and other age-related physiological changes. As such, it is unclear how multiple concomitant deficits combine to impact balance, and to what extent an individual’s balance symptoms can be attributed to their vestibular loss. Third, it is unknown how to identify deficits in central compensation for physiologic losses (to the vestibular system as well as other systems), and whether these deficits are contributing to an individual’s balance phenotype. The determinants of effective central compensation are poorly understood, including the impact of neurodegenerative conditions such as PD and Alzheimer’s disease on central vestibular signaling and central compensation.
Key barriers to filling these knowledge gaps include: (i) lack of clinical measures of all vestibular physiologic functions (while candidate measures exist, clear, scalable clinical measures of VAR and vestibular perception are notably absent) (100); (ii) infrequent assessment of balance tasks in different environmental contexts; (iii) infrequent measurement of all contributors to balance control (eg, vestibular function, vision, somatosensory function, cognition) in siloed clinical and research contexts; (iv) lack of specific clinical measures of central compensation deficits; (v) lack of understanding of the contributors to effective central compensation.
Research Strategy
1) Develop and define specific, reliable, reproducible clinical measures of all vestibular functional pathways (including VOR, VSR, VAR and vestibular perception)
2) Develop models that integrate multiple physiologic factors (eg, vestibular function, vision, somatosensory function, medication use, cognitive status, cardiovascular health) to predict balance task performance in specific environments. Model development might need big data/machine learning approaches to leverage data contained in medical records or existing data sets that can be combined and used to generate the predictive models
3) Develop clinical measures of central vestibular processing and central compensation (eg, sensory integration, reweighting ability)
4) Conduct studies in animals or use structural and functional neuroimaging to understand age-related changes in central vestibular processing and sensory reweighting at the level of the cerebellum, vestibular cortex, basal ganglia and thalamus, and central vestibular pathways that integrate with large-scale neural networks
A conceptual model summarizing the current knowledge and knowledge gaps for key questions 1 and 2, as well as research strategies, is presented in Figure 2.
Key Question 3: Can a Nosology of Balance Problems in Older Adults Be Developed to Guide Clinical Management (Diagnosis and Treatment)?
Current Knowledge
As noted previously, recent understanding of balance control define balance as a dynamic process that at any given time is a function of the specific balance task, an individual’s characteristics, and the environmental context (Figure 1). Postural control is no longer considered simply a summation of static reflexes but, rather, a complex skill based on the interaction of dynamic sensorimotor processes. The control of posture involves many different underlying physiological systems that can be affected by pathology or subclinical constraints related to aging. Damage to any of the underlying systems will result in different, context-specific instabilities. Thus, the conditions leading to falls and the most effective rehabilitation of balance to improve mobility and to prevent falls requires a better understanding of the multiple mechanisms underlying postural control (10). However, there is a lack of multimodal models that can classify and characterize a given individual’s balance phenotype in a way that can help identify the contributing factors and guide rational treatment. One example is the Balance Evaluation Systems Test (BESTest), a comprehensive clinical evaluation across six domains of balance control with the goal of focusing rehabilitation on the specific balance domains affected (12). For example, inability to stand in the dark on an unstable surface is suggestive of vestibular impairment, and may be improved with vestibular rehabilitation (101). In contrast, a difficulty recovering equilibrium in response to external perturbations may benefit more from practice responding to surface slips. However, the clinical test could be instrumented for increased objectivity and sensitivity and the relative benefits of rehabilitation targeted to specific balance deficits versus generalized balance training needs to be verified by clinical studies.
Although many wearable sensors are currently available to measure activity, or quantity of mobility in daily life, far fewer systems exist to measure quality of unsupervised gait and turning in daily life (102). Quantity of activity is not a predictor of falls and is not always sensitive to neurological disorders (103). Furthermore, most wearable systems measuring quality of mobility during daily life consist of a single inertial sensor worn on the belt to estimate spatiotemporal parameters of gait (104). These estimates of gait measures from the belt are not as accurate and more limited compared with gait measures from inertial sensors on or in shoes, likely resulting in more variability and therefore poorer effect size as outcome measures (105). A limitation to current body-worn sensors is that they cannot measure increases in stride width, which is a well-known compensation for postural instability while walking. Current body-worn sensor systems also cannot identify the context of mobility, such as whether walking occurred inside or outside the home, limiting potential fall prediction. Since the particular measures of mobility are likely to be different and specific for different pathologies and the reasons for their mobility disability and fall risk, studies are needed that compare objective measures of mobility during daily life across a range of age-related limitations on mobility (106,107).
A nosology of balance control, based on physiological, pathological, and/or functional models will help direct links between comprehensive clinical assessments and specific balance interventions. The clinical and research barriers related to developing useful nosologies of balance control and strategies to overcome these barriers are presented in Table 1.
Table 1.
Barriers and Strategies to Developing Multimodal Models for Comprehensive Clinical Assessments and Interventions for Balance Disorders
| Barriers | Strategies |
|---|---|
| Lack of nosology of balance problems to guide clinical practice |
Short-term: Identify the genetic, physiologic, sensorimotor, behavioral, cognitive, emotional, and environmental contributors to balance assessment. Long-term: Incorporate these individual factors into multimodal classification models to guide the development of a clinical nosology of balance problems and delivery of personalized balance interventions. |
| Lack of clinically accessible, ecologically valid measures to assess different types of balance dysfunction |
Short-term: Develop cost effective, ecologically valid measures that assess the 3 different balance domains. Develop patient-reported quality of balance scales focused on vestibular and other balance disorders that are linked to objective measures of balance control. Long-term: Develop consensus on currently available (eg, Mini-BEST test) (108) and emerging technologies/measures (eg, perturbation-based reactive balance tasks) (109) to include as part of a core set of balance outcomes. |
| Translation of novel balance interventions into clinical and community practice |
Short-term: Develop clinical interventions that incorporate the three different balance domains and are feasible (ie, cost effective, practical, easily implemented, portable/compact, safe) in different subgroups of patients with different ages, sexes, races, and comorbidities Demonstrate efficacy of laboratory-based interventions in different settings, including the community, hospital, nursing homes, etc. Long-term: Integrate effective balance interventions into clinical and community practice. |
| Lack of dosage recommendations for balance interventions |
Short-term: Assess balance dosage prescription based on the FITT principle of interventions (ie, frequency, intensity, time, and type of exercise/activity). Long-term: Establish clinical practice guidelines for the dosage of balance interventions and educate clinicians and researchers in the use of these guidelines. |
Knowledge Gaps and Key Barriers
Given the current state of knowledge, the group identified four key knowledge gaps toward establishing a useful nosology (ie, classification or heuristic system) of balance problems that can guide effective treatment. First, the field currently lacks a widely accepted, multimodal classification model of balance to guide the development and testing of targeted, rational interventions (10,101). Second, there is a lack of clinically accessible performance-based or instrumented outcome measures to assess different types of balance dysfunction in daily life (10,12). Third, effective methods to translate balance interventions into the clinical and community setting are lacking. It is thus currently unclear whether and how balance interventions transfer into meaningful balance and other functional gains in daily life (110). Fourth, little is known about the optimal dosage of balance intervention (111).
Key barriers to developing this knowledge include: (i) lack of large data sets with comprehensive assessment of the multiple dimensions of balance with rigorous characterization of contributors to balance control (eg, vision, vestibular function, cognition, etc.) and of balance phenotype/symptoms; (ii) few trials of balance interventions that are targeted towards specific individual impairments and particular balance tasks and environmental contexts; (iii) the lack of ecologically valid measures of balance (both objective and patient-reported measures) to assess function and response to treatment.
Research Strategy
1) Conduct population-based studies that include older adults as well as comprehensive assessments of balance function that target steady state, proactive, and reactive elements of balance control and also assess genetic, physiologic, sensorimotor, behavioral, cognitive, emotional, and environmental factors that contribute to balance. These data can be used to develop a clinical nosology of balance problems to help guide clinicians in the clinical management of older adults. In line with the first two key questions, a particular focus on age-related vestibular impairment in this clinical nosology will be critical given that age-related vestibular impairment has been less well-recognized or studied in the broader fields of gerontology and geriatrics.
2) Develop cost-effective and validated outcome measures that can be deployed within nonlaboratory, nonclinical settings that assess the three different balance domains (ie, steady state, proactive, and reactive tasks). Such assessments will likely need to stress the balance control system by purposefully manipulating both environmental and task constraints. To this end, the development of “dual-task” or “multi-task” paradigms that measure the capacity to maintain balance performance during execution of ecologically valid cognitive and/or motor tasks, are expected to be of particular importance. Develop and test wearable sensors and instrumented homes capable of accurately measuring and training balance during habitual physical activities in older adults.
3) Develop interventions that target all three balance domains and that can be widely adopted. For example, scalable technologies that incorporate virtual reality, real-time biofeedback, and perturbation training which vary the balance task and environmental context can be considered. Such technologies could enable remote administration (and evaluation) of interventions to large numbers of individuals. For all intervention trials, focused studies will also be needed to establish dosing recommendations based on the FITT principle (ie, frequency, intensity, time, and type of exercise/activity). The effectiveness of balance interventions will need to be evaluated based on ecologically valid objective and patient-reported outcome measures.
In this article, we draft an ambitious agenda for the critical insights that need to be gained in the next 5–10 years to better understand and treat balance and vestibular problems in older adults. Specifically, we identify three key questions that need to be answered to improve balance function in older adults: (i) How does aging impact vestibular function?; (ii) How do we know what is the contribution of age-related vestibular impairment to an older adult’s balance problem?; and (iii) Can we develop a nosology of balance impairments in older adults that can guide clinical practice? We propose a series of research steps that will provide answers to the three key questions, which will involve both basic science and clinical research approaches. Moreover, we emphasize that because balance control involves numerous organ systems in the body, multidisciplinary teams will be needed to advance knowledge and improve outcomes related to the complex but primal process of balance control. Balance impairment and falls are highly common and morbid in older adults; as such the potential public health benefit of better understanding and addressing vestibular and balance problems in older adults is profound.
Acknowledgments
The authors would like to acknowledge Dr. Lyndon Joseph, Dr. Coryse St-Hillaire Clark, and Dr. Matt Sutterer from the National Institute on Aging and Dr. Amy Poremba from the National Institute on Deafness and Other Communication Disorders for convening the workshop and for their valuable contributions to this document.
Funding
This work was supported by the National Institutes of Health (AG057667 to Y.A.).
Conflicts of Interest
None reported.
References
- 1. Hartholt KA, Lee R, Burns ER, Van Beeck EF. Mortality from falls among US adults aged 75 years or older, 2000–2016. JAMA. 2019;321(21):2131–2133. doi: 10.1001/jama.2019.4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verma SK, Willetts JL, Corns HL, Marucci-Wellman HR, Lombardi DA, Courtney TK. Falls and fall-related injuries among community-dwelling adults in the United States. PLoS One. 2016;11:e0150939. doi: 10.1371/journal.pone.0150939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panel on Prevention of Falls in Older Persons AGS, Society BG. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–157. doi: 10.1111/j.1532-5415.2010.03234.x [DOI] [PubMed] [Google Scholar]
- 4. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–944. doi: 10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- 5. Beylergil SB, Karmali F, Wang W, Bermúdez Rey MC, Merfeld DM. Vestibular roll tilt thresholds partially mediate age-related effects on balance. Prog Brain Res. 2019;248:249–267. doi: 10.1016/bs.pbr.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 6. Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 7. Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacKinnon CD. Sensorimotor anatomy of gait, balance, and falls. Handb Clin Neurol. 2018;159:3–26. doi: 10.1016/B978-0-444-63916-5.00001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatt T, Yang F, Pai YC. Learning to resist gait-slip falls: long-term retention in community-dwelling older adults. Arch Phys Med Rehabil. 2012;93:557–564. doi: 10.1016/j.apmr.2011.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077 [DOI] [PubMed] [Google Scholar]
- 11. Westlake KP, Johnson BP, Creath RA, Neff RM, Rogers MW. Influence of non-spatial working memory demands on reach-grasp responses to loss of balance: effects of age and fall risk. Gait Posture. 2016;45:51–55. doi: 10.1016/j.gaitpost.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89:484–498. doi: 10.2522/ptj.20080071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahoney JR, Cotton K, Verghese J. Multisensory integration predicts balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2019;74:1429–1435. doi: 10.1093/gerona/gly245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahoney JR, Verghese J. Does cognitive impairment influence visual-somatosensory integration and mobility in older adults? J Gerontol A Biol Sci Med Sci. 2020;75:581–588. doi: 10.1093/gerona/glz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel P, Lamar M, Bhatt T. Effect of type of cognitive task and walking speed on cognitive-motor interference during dual-task walking. Neuroscience. 2014;260:140–148. doi: 10.1016/j.neuroscience.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 16. Woytowicz EJ, Sours C, Gullapalli RP, Rosenberg J, Westlake KP. Modulation of working memory load distinguishes individuals with and without balance impairments following mild traumatic brain injury. Brain Inj. 2018;32:191–199. doi: 10.1080/02699052.2017.1403045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furman JM, Redfern MS, Jacob RG. Vestibulo-ocular function in anxiety disorders. J Vestib Res. 2006;16:209–215. [PubMed] [Google Scholar]
- 18. Holstein GR. The vestibular system. In: The Human Nervous System. London, UK: Elsevier; 2012:1239–1269. [Google Scholar]
- 19. Wolfe JM, Kluender KR, Levi DM, et al. Sensation & Perception. Sunderland, MA: Sinauer; 2006. [Google Scholar]
- 20. Goldberg JM, Wilson VJ, Angelaki DE, Cullen KE, Fukushima K. The Vestibular System: A Sixth Sense. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 21. Kirsch V, Keeser D, Hergenroeder T, et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221:1291–1308. doi: 10.1007/s00429-014-0971-x [DOI] [PubMed] [Google Scholar]
- 22. Tian J-R, Shubayev I, Baloh RW, Demer JL. Impairments in the initial horizontal vestibulo-ocular reflex of older humans. Exp Brain Res. 2001;137(3–4):309–322. doi: 10.1007/s002210000671 [DOI] [PubMed] [Google Scholar]
- 23. Minor LB. Gentamicin-induced bilateral vestibular hypofunction. JAMA. 1998;279:541–544. doi: 10.1001/jama.279.7.541 [DOI] [PubMed] [Google Scholar]
- 24. McCall AA, Miller DM, Yates BJ. Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front Neurol. 2017;8:112. doi: 10.3389/fneur.2017.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray AJ, Croce K, Belton T, Akay T, Jessell TM. Balance control mediated by vestibular circuits directing limb extension or antagonist muscle co-activation. Cell Rep. 2018;22:1325–1338. doi: 10.1016/j.celrep.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 26. Gagliuso AH, Chapman EK, Martinelli GP, Holstein GR. Vestibular neurons with direct projections to the solitary nucleus in the rat. J Neurophysiol. 2019;122:512–524. doi: 10.1152/jn.00082.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol (1985). 1999;86:1552–1560. doi: 10.1152/jappl.1999.86.5.1552 [DOI] [PubMed] [Google Scholar]
- 28. Smith PF, Zheng Y. From ear to uncertainty: vestibular contributions to cognitive function. Front Integr Neurosci. 2013;7:84. doi: 10.3389/fnint.2013.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agrawal Y, Smith PF, Rosenberg PB. Vestibular impairment, cognitive decline and Alzheimer’s disease: balancing the evidence. Aging Ment Health. 2019:1–4. doi: 10.1080/13607863.2019.1566813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldberg JM, Wilson VJ, Angelaki DE, Cullen KE, Buttner-Ennever J, Fukushima K. The Vestibular System: A Sixth Sense. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 31. Alvarez JC, Díaz C, Suárez C, et al. Aging and the human vestibular nuclei: morphometric analysis. Mech Ageing Dev. 2000;114:149–172. doi: 10.1016/s0047-6374(00)00098-1 [DOI] [PubMed] [Google Scholar]
- 32. Alvarez JC, Díaz C, Suárez C, et al. Neuronal loss in human medial vestibular nucleus. Anat Rec. 1998;251:431–438. doi: [DOI] [PubMed] [Google Scholar]
- 33. Anniko M. The aging vestibular hair cell. Am J Otolaryngol. 1983;4(3):151–160. doi: 10.1016/S0196-0709(83)80037-4 [DOI] [PubMed] [Google Scholar]
- 34. Gleeson M, Felix H. A comparative study of the effect of age on the human cochlear and vestibular neuroepithelia. Acta Otolaryngol. 1987;436:103–109. doi: 10.3109/00016488709124982 [DOI] [PubMed] [Google Scholar]
- 35. Kevetter GA, Leonard RB. Decreased expression of calretinin and calbindin in the labyrinth of old gerbils. Brain Res. 2002;957:362–365. doi: 10.1016/s0006-8993(02)03668-5 [DOI] [PubMed] [Google Scholar]
- 36. Leonard RB, Kevetter GA. Structural and functional changes in the cristae ampullares of aged gerbils. Neuroscience. 2007;147:794–802. doi: 10.1016/j.neuroscience.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 37. Lopez I, Honrubia V, Baloh RW. Aging and the human vestibular nucleus. J Vestib Res. 1997;7:77–85. doi: 10.3233/VES-1997-7107 [DOI] [PubMed] [Google Scholar]
- 38. Lopez I, Ishiyama G, Tang Y, Tokita J, Baloh RW, Ishiyama A. Regional estimates of hair cells and supporting cells in the human crista ampullaris. J Neurosci Res. 2005;82:421–431. doi: 10.1002/jnr.20652 [DOI] [PubMed] [Google Scholar]
- 39. Lyon MJ, King JM. Aging rat vestibular ganglion: II. Quantitative electron microscopic evaluation. Ann Otol Rhinol Laryngol. 1997;106:753–758. doi: 10.1177/000348949710600908 [DOI] [PubMed] [Google Scholar]
- 40. Merchant SN, Tsuji K, Wall C III, Velázquez-Villaseñor L, Glynn RJ, Rauch SD. Temporal bone studies of the human peripheral vestibular system: 1. Normative vestibular hair cell data. Ann Otol Rhinol Laryngol. 2000;109(5 suppl):3–13. doi: 10.1177/00034894001090S502 [DOI] [PubMed] [Google Scholar]
- 41. Park JJ, Tang Y, Lopez I, Ishiyama A. Age‐related change in the number of neurons in the human vestibular ganglion. J Comp Neurol. 2001;431(4): 437–443. doi: [DOI] [PubMed] [Google Scholar]
- 42. Rauch SD, Velazquez-Villaseñor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Ann N Y Acad Sci. 2001;942:220–227. doi: 10.1111/j.1749-6632.2001.tb03748.x [DOI] [PubMed] [Google Scholar]
- 43. Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol. 1973;76:208–220. doi: 10.3109/00016487309121501 [DOI] [PubMed] [Google Scholar]
- 44. Sturrock RR. Age related changes in neuron number in the mouse lateral vestibular nucleus. J Anat. 1989;166:227–232. [PMC free article] [PubMed] [Google Scholar]
- 45. Tang Y, Lopez I, Baloh RW. Age-related change of the neuronal number in the human medial vestibular nucleus: a stereological investigation. J Vestib Res. 2001;11:357–363. [PubMed] [Google Scholar]
- 46. Taylor RR, Jagger DJ, Saeed SR, et al. Characterizing human vestibular sensory epithelia for experimental studies: new hair bundles on old tissue and implications for therapeutic interventions in ageing. Neurobiol Aging. 2015;36:2068–2084. doi: 10.1016/j.neurobiolaging.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maes L, Dhooge I, D’haenens W, et al. The effect of age on the sinusoidal harmonic acceleration test, pseudorandom rotation test, velocity step test, caloric test, and vestibular-evoked myogenic potential test. Ear Hear. 2010;31:84–94. doi: 10.1097/AUD.0b013e3181b9640e [DOI] [PubMed] [Google Scholar]
- 48. Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–839. doi: 10.1097/MAO.0b013e3182545061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mezzalira R, Bittar RSM, do Carmo Bilécki-Stipsky MM, Brugnera C, Grasel SS. Sensitivity of caloric test and video head impulse as screening test for chronic vestibular complaints. Clinics (Sao Paulo). 2017;72:469–473. doi: 10.6061/clinics/2017(08)03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rambold H. Clinical value of rotational-chair testing in vestibular disease. Clin Otorhinolaryngol. 2017;1(1):013. [Google Scholar]
- 51. MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–1141. doi: 10.1212/WNL.0b013e3181bacf85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mantokoudis G, Saber Tehrani AS, Kattah JC, et al. Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol. 2015;20:39–50. doi: 10.1159/000362780 [DOI] [PubMed] [Google Scholar]
- 53. Nguyen KD, Welgampola MS, Carey JP. Test–retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol. 2012;13:381–401. doi: 10.1007/s10162-012-0318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Furman JM. Posturography: uses and limitations. Baillieres Clin Neurol. 1994;3:501–513. [PubMed] [Google Scholar]
- 56. Paige GD. Senescence of human visual–vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res. 1992;2:133–151. [PubMed] [Google Scholar]
- 57. Zalewski CK. Aging of the human vestibular system. Paper presented at: Seminars in Hearing; August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schubert MC, Migliaccio AA. New advances regarding adaptation of the vestibulo-ocular reflex. J Neurophysiol. 2019;122:644–658. doi: 10.1152/jn.00729.2018 [DOI] [PubMed] [Google Scholar]
- 59. Brosel S, Strupp M. The vestibular system and ageing. In: Biochemistry and Cell Biology of Ageing: Part II Clinical Science. Singapore: Springer; 2019:195–225. doi: 10.1007/978-981-13-3681-2_8 [DOI] [PubMed] [Google Scholar]
- 60. Serrador JM, Lipsitz LA, Gopalakrishnan GS, Black FO, Wood SJ. Loss of otolith function with age is associated with increased postural sway measures. Neurosci Lett. 2009;465:10–15. doi: 10.1016/j.neulet.2009.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Teasdale N, Stelmach GE, Breunig A. Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol. 1991;46:B238–B244. doi: 10.1093/geronj/46.6.b238 [DOI] [PubMed] [Google Scholar]
- 62. Peters RM, Blouin JS, Dalton BH, Inglis JT. Older adults demonstrate superior vestibular perception for virtual rotations. Exp Gerontol. 2016;82:50–57. doi: 10.1016/j.exger.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 63. Dalton BH, Blouin JS, Allen MD, Rice CL, Inglis JT. The altered vestibular-evoked myogenic and whole-body postural responses in old men during standing. Exp Gerontol. 2014;60:120–128. doi: 10.1016/j.exger.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 64. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992;19(6 Pt 1):508–519. doi: 10.1161/01.hyp.19.6.508 [DOI] [PubMed] [Google Scholar]
- 65. Forman DE, Lipsitz LA. Syncope in the elderly. Cardiol Clin. 1997;15:295–311. doi: 10.1016/s0733-8651(05)70337-4 [DOI] [PubMed] [Google Scholar]
- 66. Aoki M, Sakaida Y, Tanaka K, Mizuta K, Ito Y. Evidence for vestibular dysfunction in orthostatic hypotension. Exp Brain Res. 2012;217:251–259. doi: 10.1007/s00221-011-2989-0 [DOI] [PubMed] [Google Scholar]
- 67. Lester AW, Moffat SD, Wiener JM, Barnes CA, Wolbers T. The Aging Navigational System. Neuron. 2017;95:1019–1035. doi: 10.1016/j.neuron.2017.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gassmann KG, Rupprecht R; IZG Study Group Dizziness in an older community dwelling population: a multifactorial syndrome. J Nutr Health Aging. 2009;13:278–282. doi: 10.1007/s12603-009-0073-2 [DOI] [PubMed] [Google Scholar]
- 69. Barin K, Dodson EE. Dizziness in the elderly. Otolaryngol Clin North Am. 2011;44:437–454, x. doi: 10.1016/j.otc.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 70. Boult C, Murphy J, Sloane P, Mor V, Drone C. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858–861. doi: 10.1111/j.1532-5415.1991.tb04451.x [DOI] [PubMed] [Google Scholar]
- 71. Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait Posture. 2001;14:211–216. doi: 10.1016/s0966-6362(01)00144-8 [DOI] [PubMed] [Google Scholar]
- 72. Westlake KP, Culham EG. Sensory-specific balance training in older adults: effect on proprioceptive reintegration and cognitive demands. Phys Ther. 2007;87:1274–1283. doi: 10.2522/ptj.20060263 [DOI] [PubMed] [Google Scholar]
- 73. Adkin AL, Frank JS, Carpenter MG, Peysar GW. Fear of falling modifies anticipatory postural control. Exp Brain Res. 2002;143:160–170. doi: 10.1007/s00221-001-0974-8 [DOI] [PubMed] [Google Scholar]
- 74. Hadjistavropoulos T, Carleton RN, Delbaere K, et al. The relationship of fear of falling and balance confidence with balance and dual tasking performance. Psychol Aging. 2012;27:1–13. doi: 10.1037/a0024054 [DOI] [PubMed] [Google Scholar]
- 75. Wetmore SJ, Eibling DE, Goebel JA, et al. Challenges and opportunities in managing the dizzy older adult. Otolaryngol Head Neck Surg. 2011;144:651–656. doi: 10.1177/0194599810397493 [DOI] [PubMed] [Google Scholar]
- 76. Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res. 2001;140:95–111. doi: 10.1007/s002210100802 [DOI] [PubMed] [Google Scholar]
- 77. Anson E, Bigelow RT, Swenor B, et al. Loss of peripheral sensory function explains much of the increase in postural sway in healthy older adults. Front Aging Neurosci. 2017;9:202. doi: 10.3389/fnagi.2017.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM. Vestibular perceptual thresholds increase above the age of 40. Front Neurol. 2016;7:162. doi: 10.3389/fneur.2016.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bigelow RT, Semenov YR, Trevino C, et al. Association between visuospatial ability and vestibular function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2015;63:1837–1844. doi: 10.1111/jgs.13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wei EX, Agrawal Y. Vestibular dysfunction and difficulty with driving: data from the 2001–2004 National Health and Nutrition Examination Surveys. Front Neurol. 2017;8:557. doi: 10.3389/fneur.2017.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wei EX, Agrawal Y. Association between vestibular vertigo and motor vehicle accidents: data from the 2016 National Health Interview Survey. Ear Hear. 2018;39(6):1232–1235. doi: 10.1097/AUD.0000000000000602 [DOI] [PubMed] [Google Scholar]
- 82. Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, Agrawal Y. Vestibular loss in older adults is associated with impaired spatial navigation: data from the triangle completion task. Front Neurol. 2017;8:173. doi: 10.3389/fneur.2017.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wei EX, Oh ES, Harun A, Ehrenburg M, Agrawal Y. Saccular impairment in Alzheimer’s disease is associated with driving difficulty. Dement Geriatr Cogn Disord. 2017;44:294–302. doi: 10.1159/000485123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wei EX, Oh ES, Harun A, Ehrenburg M, Agrawal Y. Vestibular loss predicts poorer spatial cognition in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;61:995–1003. doi: 10.3233/JAD-170751 [DOI] [PubMed] [Google Scholar]
- 85. Wei EX, Oh ES, Harun A, et al. Increased prevalence of vestibular loss in mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2019;16:1143–1150. doi: 10.2174/1567205016666190816114838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist’s best friend. J Neurol. 2016;263(suppl 1):S54–S64. doi: 10.1007/s00415-015-7903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McCabe BF, Ryu JH, Sekitani T. Further experiments on vestibular compensation. Laryngoscope. 1972;82(3):381–396. doi: 10.1288/00005537-197203000-00005 [DOI] [PubMed] [Google Scholar]
- 88. Peterka RJ, Statler KD, Wrisley DM, Horak FB. Postural compensation for unilateral vestibular loss. Front Neurol. 2011;2:57. doi: 10.3389/fneur.2011.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Teasdale N, Stelmach GE, Breunig A, Meeuwsen HJ. Age differences in visual sensory integration. Exp Brain Res. 1991;85:691–696. doi: 10.1007/bf00231755 [DOI] [PubMed] [Google Scholar]
- 90. Volkow ND, Ding Y-S, Fowler JS, et al. Dopamine transporters decrease with age in healthy subjects. J Nucl Med. 1996;37:554–558. [PubMed] [Google Scholar]
- 91. Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276 [DOI] [PubMed] [Google Scholar]
- 92. Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev. 2009;46:1045–1052. doi: 10.1682/jrrd.2009.03.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309 [DOI] [PubMed] [Google Scholar]
- 94. Albin RL, Bohnen NI, Muller MLTM, et al. Regional vesicular acetylcholine transporter distribution in human brain: a [18 F]fluoroethoxybenzovesamicol positron emission tomography study. J Comp Neurol. 2018;526:2884–2897. doi: 10.1002/cne.24541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Macdougall HG, Curthoys IS. Plasticity during vestibular compensation: the role of saccades. Front Neurol. 2012;3:21. doi: 10.3389/fneur.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Assländer L, Peterka RJ. Sensory reweighting dynamics in human postural control. J Neurophysiol. 2014;111:1852–1864. doi: 10.1152/jn.00669.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cohen HS, Kimball KT, Jenkins HA. Factors affecting recovery after acoustic neuroma resection. Acta Otolaryngol. 2002;122: 841–850. doi: 10.1080/003655402_000028039 [DOI] [PubMed] [Google Scholar]
- 98. Whitney SL, Wrisley DM, Marchetti GF, Furman JM. The effect of age on vestibular rehabilitation outcomes. Laryngoscope. 2002;112:1785–1790. doi: 10.1097/00005537-200210000-00015 [DOI] [PubMed] [Google Scholar]
- 99. Gauchard GC, Lion A, Perrin PP, Parietti-Winkler C. Influence of age on postural compensation after unilateral deafferentation due to vestibular schwannoma surgery. Laryngoscope. 2012;122:2285–2290. doi: 10.1002/lary.23497 [DOI] [PubMed] [Google Scholar]
- 100. Dupuits B, Pleshkov M, Lucieer F, et al. A new and faster test to assess vestibular perception. Front Neurol. 2019;10:707. doi: 10.3389/fneur.2019.00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American Physical Therapy Association Neurology Section. J Neurol Phys Ther. 2016;40:124–155. doi: 10.1097/NPT.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson’s disease using body‐worn sensors. Mov Disord. 2013;28(11):1544–1551. doi: 10.1002/mds.25684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shema-Shiratzky S, Hillel I, Mirelman A, et al. A wearable sensor identifies alterations in community ambulation in multiple sclerosis: contributors to real-world gait quality and physical activity. J Neurol. 2020;267:1–10. doi: 10.1007/s00415-020-09759-7 [DOI] [PubMed] [Google Scholar]
- 104. Warmerdam E, Hausdorff JM, Atrsaei A, et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020;19(5):462–470. doi: 10.1016/S1474-4422(19)30397-7 [DOI] [PubMed] [Google Scholar]
- 105. Mariani B, Jiménez MC, Vingerhoets FJ, Aminian K. On-shoe wearable sensors for gait and turning assessment of patients with Parkinson’s disease. IEEE Trans Biomed Eng. 2012;60(1):155–158. doi: 10.1109/TBME.2012.2227317 [DOI] [PubMed] [Google Scholar]
- 106. Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M. How to select balance measures sensitive to Parkinson’s disease from body-worn inertial sensors—separating the trees from the forest. Sensors. 2019;19(15):3320. doi: 10.3390/s19153320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shah VV, McNames J, Mancini M, et al. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J Neurol. 2020;267:1–9. doi: 10.1007/s00415-020-09696-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang Y, Wang S, Bolton R, Kaur T, Bhatt T. Effects of task-specific obstacle-induced trip-perturbation training: proactive and reactive adaptation to reduce fall-risk in community-dwelling older adults. Aging Clin Exp Res. 2019;32:1–13. doi: 10.1007/s40520-019-01268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lee PG, Jackson EA, Richardson CR. Exercise prescriptions in older adults. Am Fam Physician. 2017;95:425–432. [PubMed] [Google Scholar]
- 111. Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51:1750–1758. doi: 10.1136/bjsports-2016-096547 [DOI] [PubMed] [Google Scholar]