Abstract
Objectives
Cefepime/taniborbactam is a cephalosporin/cyclic boronate β-lactamase inhibitor combination under development for the treatment of infections due to MDR Enterobacterales and Pseudomonas aeruginosa. Using a neutropenic murine thigh infection model, we aimed to determine the pharmacokinetic/pharmacodynamic index, relative to taniborbactam exposure, that correlated most closely with the efficacy of the cefepime/taniborbactam combination and the magnitude of index required for efficacy against serine-β-lactamase-producing strains.
Methods
Twenty-six clinical Enterobacterales (expressing ESBLs, plasmid-mediated AmpC and/or carbapenemases of classes A or D; cefepime/taniborbactam combination MICs 0.06–16 mg/L) and 11 clinical P. aeruginosa (AmpC overproducing or KPC expressing; cefepime/taniborbactam combination MICs 1–16 mg/L) were evaluated. A cefepime human-simulated regimen (HSR) equivalent to a clinical dose of 2 g q8h as a 2 h infusion was given in combination with taniborbactam for 24 h. For a subset of P. aeruginosa isolates, a sub-therapeutic cefepime exposure was utilized.
Results
Dose-fractionation studies revealed that dosing frequency had no impact on taniborbactam potentiation of cefepime activity. Relative to the initial bacterial burden, the median taniborbactam fAUC0–24/MIC associated with 1 log kill in combination with the cefepime HSR for Enterobacterales and P. aeruginosa isolates was 2.62 and 0.46, respectively. In combination with sub-therapeutic cefepime, the median taniborbactam fAUC0–24/MIC associated with 1 and 2 log kill against AmpC-overproducing P. aeruginosa was 2.00 and 3.30, respectively, relative to the bacterial burden in the cefepime-treated groups. The taniborbactam HSR (equivalent to 0.5 g q8h as a 2 h infusion) was adequate to attain ≥1 log reduction against all test isolates.
Conclusions
Our data show that the cefepime/taniborbactam combination (2 g/0.5 g q8h as a 2 h infusion) exerts potent in vivo activity against cefepime-resistant isolates, including serine-carbapenemase producers.
Introduction
Owing to their spectrum of activity and favourable safety profile, β-lactams are the most frequently prescribed antibiotic class. These agents share a β-lactam ring as part of their core structure, which is essential for blocking the transpeptidase activity of PBPs and consequently inhibiting bacterial cell wall synthesis. β-Lactam ring hydrolysis by β-lactamases is the most prevalent form of resistance to β-lactams among Gram-negative bacteria.1 Carbapenem resistance attributed to the rapid spread of plasmid-borne genetic determinants encoding carbapenemases, carbapenem-hydrolysing β-lactamases, is of particular importance and represents an urgent health threat.2 The development of β-lactamase inhibitors to partner with the β-lactam agents, shielding them from hydrolysis, is an important strategy to tackle infections due to β-lactamase producers.
Taniborbactam (previously known as VNRX-5133) is a novel cyclic boronate-based β-lactamase inhibitor that lacks antibacterial activity but shows potent in vitro inhibitory activity against serine-β-lactamases and MBLs [classes A, B (VIM and NDM), C and D].3 Taniborbactam in combination with cefepime, a broad-spectrum fourth-generation cephalosporin, is currently being studied in a Phase III clinical trial and has the potential to be a potent treatment option for infections caused by carbapenemase producers as well as a carbapenem-sparing option for infections due to cephalosporinase and ESBL producers.
We herein examined the in vivo activity of cefepime/taniborbactam in the neutropenic murine thigh infection model against serine-β-lactamase-producing Enterobacterales and Pseudomonas aeruginosa, including carbapenemase producers.
Methods
Antimicrobial agents
For in vivo testing, taniborbactam (HCl)2 (Batches DMG00039.169.1 and A/2428/69/1; Venatorx Pharmaceuticals, Inc., Malvern, PA, USA) was reconstituted to a 10 mg/mL concentration with sterile water for injection and subsequently diluted in sterile 0.9% normal saline solution (NS). Cefepime 2 g vials (Lot 106880C; Sagent Pharmaceuticals, Inc., Schaumburg, IL, USA) were reconstituted and diluted with NS. For in vitro testing, taniborbactam master stock was prepared in DMSO. Cefepime HCl (Batch 60003CK88D-A; Tecoland, Irvine, CA, USA) was reconstituted with phosphate buffer, pH 6, 0.1 mol/L.
Bacteria and susceptibility testing
The MICs of cefepime and cefepime/taniborbactam (with taniborbactam at a fixed concentration of 4 mg/L) were determined in triplicate for clinical Enterobacterales (n = 26) and P. aeruginosa (n = 11) isolates expressing class A, C and D β-lactamases using broth microdilution methodology as outlined by the CLSI.4,5
Infection model
Specific pathogen-free, female ICR mice weighing 20–22 g (Envigo RMS, Inc., Indianapolis, IN, USA) were housed in HEPA-filtered cages in groups of six at controlled room temperature, provided food and water ad libitum and allowed to acclimatize for a minimum of 48 h before commencement of experimentation. A 12 h light/12 h dark cycle was maintained. The protocol was approved by the Institutional Animal Care and Use Committee at Hartford Hospital (Assurance #A3185-01). Mice were rendered transiently neutropenic by injecting cyclophosphamide intraperitoneally at a dose of 150 mg/kg of body weight 4 days before inoculation and 100 mg/kg of body weight 1 day before inoculation. Uranyl nitrate at 5 mg/kg was administered intraperitoneally 3 days prior to inoculation to produce a controlled degree of renal impairment to assist with humanizing the target exposures of cefepime and taniborbactam. Thigh infection was produced by intramuscular injection of 0.1 mL of the bacterial inoculum (107 cfu/mL) into each thigh (n = 2) of the mouse 2 h prior to the initiation of antimicrobial therapy via subcutaneous injection as previously described.6
Pharmacokinetic studies
Cefepime pharmacokinetic studies were conducted to identify a regimen in mice that provided an exposure similar to that achieved in humans following the administration of 2 g q8h as a 2 h infusion based on the percentage of the dosing interval during which the unbound drug concentrations remained above a threshold plasma concentration (%fT > CT). Pharmacokinetic data for cefepime in healthy adult volunteers identified from Phase I studies upon the co-administration of taniborbactam [T. Henkel (Venatorx Pharmaceuticals) and J. Dowell (Pharmacology Development Services), personal communication] and previously established murine cefepime pharmacokinetic parameters were used for simulation purposes.7 Cefepime regimens were simulated for mice and humans using WinNonlin (Phoenix version 6.3, Pharsight Corp., Mountain View, CA, USA) after accounting for cefepime protein binding (20% and 0% in humans and mice, respectively).8 Infected animals were administered this cefepime human-simulated regimen (HSR) alone or in combination with taniborbactam at cefepime:taniborbactam ratios of 6:1, 4:1, 3:1 and 2:1. Groups of six mice were euthanized at six predefined timepoints. Terminal blood samples from CO2-asphyxiated mice were collected via cardiac puncture and placed in K2EDTA BD Microtainer® tubes (BD, Franklin Lakes, NJ, USA). Plasma was separated by centrifugation for 10 min at 4°C at 10 000 rpm and stored at −80°C until analysed. Cefepime and taniborbactam plasma concentrations were determined using validated HPLC9 and LC-MS/MS methods, respectively. Taniborbactam exposures achieved with various ratios were estimated and used to identify a taniborbactam regimen that provided an exposure similar to that achieved in humans following the administration of 0.5 g q8h as a 2 h infusion10 (taniborbactam dose in Phase I–III clinical trials11) based on %fT>CT and fAUC0–24 using taniborbactam protein-binding data provided by the sponsor [0% and 19.4% in humans and mice, respectively;12 T. Henkel (Venatorx Pharmaceuticals) and B. Geibel (Venatorx Pharmaceuticals), personal communication].
In vivo efficacy studies
For in vivo efficacy studies, treatment and control groups contained three mice each (six thighs per group). Control mice were sacrificed just prior to antibiotic initiation (0 h controls) and 24 h later (24 h controls receiving NS). Cefepime monotherapy served as a negative control. Treatments were continued for 24 h, after which all animals were euthanized by CO2 asphyxiation followed by cervical dislocation. The thighs were removed and individually homogenized in NS. Serial dilutions were plated on Tryptic Soy Agar with 5% sheep blood for cfu enumeration. Changes in log10 cfu per thigh at 24 h, relative to 0 h controls, were evaluated.
Taniborbactam dose fractionation
The purpose of these studies was to determine the pharmacokinetic/pharmacodynamic index (fCmax/MIC, fAUC0–24/MIC or %fT>CT), relative to taniborbactam exposure, which correlated most closely with efficacy of the cefepime/taniborbactam combination using two KPC-producing clinical isolates. All treatment groups were administered the predefined cefepime HSR. Two total daily doses of taniborbactam were evaluated per isolate: 5 mg/kg/day and 1 mg/kg/day, each fractionated into three regimens, i.e. 4 times, 2 times or once over 24 h. Comparisons were made between the three regimens of the same total daily dose relative to bacterial densities after 24 h using a one-way analysis of variance (ANOVA) test followed by Tukey’s test where the P value was <0.05.
Taniborbactam dose ranging
The purpose of these studies was to determine the magnitude of taniborbactam exposure, relative to the pharmacokinetic/pharmacodynamic driver of activity, required for cefepime/taniborbactam to achieve various efficacy endpoints against serine-β-lactamase-producing Gram-negative bacteria.
Taniborbactam dose ranging utilizing the cefepime HSR
The in vivo bactericidal activity of the cefepime HSR alone and in combination with escalating taniborbactam exposures was assessed against 26 Enterobacterales and 4 P. aeruginosa. Taniborbactam was administered at various cefepime:taniborbactam ratios, relative to the doses of the cefepime HSR, including 24:1, 12:1, 6:1, 4:1, 3:1 and/or 2:1 (i.e. the doses of the cefepime HSR were fixed in all experiments, while the doses of taniborbactam were escalated providing the aforementioned ratios). For taniborbactam pharmacodynamic analyses, fAUC0–24/MIC was estimated for the different taniborbactam exposures and each isolate using bioactive, free taniborbactam exposures and the MICs of the cefepime/taniborbactam combination. A sigmoidal inhibitory Emax model was fitted to the data using WinNonlin. The effective taniborbactam index (fAUC0–24/MIC) required to achieve net stasis and 1 log bacterial killing from the starting bacterial burden (0 h control groups) for each isolate was estimated and the goodness of fit for each relationship was characterized.
Taniborbactam dose ranging utilizing a sub-therapeutic cefepime regimen
Ten P. aeruginosa isolates were examined, of which eight had confirmed AmpC overproduction. Treatment mice received 25% of the doses of the cefepime HSR alone or in combination with escalating taniborbactam exposures (cefepime:taniborbactam ratios ranging between 24:1 and 3:1, relative to the cefepime HSR doses). The effective taniborbactam fAUC0–24/MIC targets required to achieve 1 and 2 log bacterial killing, relative to the bacterial burden in the thighs of the mice receiving the cefepime HSR alone, at 24 h were estimated.
In vivo activity of the cefepime/taniborbactam HSR
The in vivo bactericidal activities of the cefepime HSR alone and in combination with the taniborbactam HSR (cefepime:taniborbactam ratio, 2:1) were assessed against the 30 isolates described in the ‘Taniborbactam dose ranging utilizing the cefepime HSR’ section. As a subset analysis, the change in log10 cfu/thigh at 24 h from 0 h controls was compared between the groups that received the taniborbactam HSR and those that received 8.33% of the doses of the taniborbactam HSR in combination with the cefepime HSR using descriptive statistics.
Results
In vitro susceptibility
Cefepime and cefepime/taniborbactam MICs and the β-lactamases encoded are shown in Table 1. All isolates were cefepime resistant based on MIC breakpoints defined by the CLSI4 and had cefepime/taniborbactam MICs ranging between ≤0.06 and 16 mg/L. The extent of potentiation of cefepime activity by taniborbactam varied from 4- to >256-fold.
Table 1.
Summary of MICs and known β-lactamase gene content for isolates selected for the in vivo efficacy studies
| Isolate | MIC (mg/L) |
β-Lactamase(s) encoded | |
|---|---|---|---|
| cefepime | cefepime + taniborbactam (4 mg/L) | ||
| EC 481 | >512 | 0.125 | CTX-M-15 |
| ECL 96 | >512 | 8 | TEM-OSBL; CTX-M-15; ACT-7 |
| ECL 123 | >512 | 0.5 | TEM-OSBL; CTX-M-15; ACT-New Variant; OXA-48 |
| ECL 124 | >512 | 8 | TEM-OSBL; CTX-M-15; ACT-7 |
| KP 329B | 512 | 0.25 | SHV-11; SHV-5; OXA-9; KPC-2; TEM-1 |
| KP 510 | 256 | 0.06 | CTX-M-11; SHV-11; DHA-1; TEM-1A |
| KP 569 | >512 | 4 | OXA-48; CTX-M-15 |
| KP 575 | >512 | 2 | KPC |
| KP 579 | >512 | 16 | SHV-11; TEM-1; CTX-M-15; OXA-48 |
| KP 580 | >512 | 8 | SHV-OSBL; TEM-OSBL; CTX-M-15; OXA-48 |
| KP 583 | >512 | 4 | SHV-32; TEM-1; CTX-M-15; OXA-48 |
| KP 585 | 512 | 8 | KPC |
| KP 679 | >512 | 4 | OXA-232; OXA-9; TEM-1A; CTX-M-15; OXA-1 |
| KP 686 | >512 | 1 | KPC-3; OXA-9; TEM-1A; SHV-11 |
| KP 731 | >512 | 1 | SHV-11; TEM-1; KPC-3 |
| KP 732 | >512 | 1 | SHV-OSBL; TEM-OSBL; KPC-2 |
| KP 734 | >512 | 2 | KPC-3 |
| KP 735 | >512 | 2 | KPC-2 |
| KP 736 | >512 | 1 | SHV-11; CTX-M-55; OXA-48 |
| KP 737 | >512 | 2 | SHV-OSBL; CTX-M-15; OXA-48 |
| KP 738 | >512 | 4 | KPC-3 |
| KP 739 | >512 | 8 | SHV-11; TEM-1; CTX-M-15; OXA-48 |
| KP 740 | >512 | 8 | SHV-OSBL; TEM-OSBL; CTX-M-15; OXA-48 |
| KP 742 | >512 | 8 | SHV-11; TEM-1; CTX-M-15; OXA-48 |
| KP 743 | >512 | 8 | SHV-11; TEM-1; CTX-M-15; OXA-48 |
| KP 744 | >512 | 4 | SHV-32; TEM-1; CTX-M-15; OXA-48 |
| PSA 1593 | >512 | 4 | KPC |
| PSA 1672 | >512 | 2 | AmpC overexpression |
| PSA 1675 | 32 | 2 | AmpC overexpression |
| PSA 1676 | 32 | 1 | AmpC overexpression |
| PSA 1677 | 32 | 2 | AmpC overexpression |
| PSA 1679 | 256 | 16 | AmpC overexpression |
| PSA 1680 | 32 | 4 | AmpC overexpression |
| PSA 1681 | >512 | 8 | AmpC overexpression |
| PSA 1684 | 64 | 16 | AmpC overexpression |
| PSA JJ8-16 | 32 | 4 | ND |
| PSA JJ11-54 | 64 | 8 | ND |
ND, not determined; EC, Escherichia coli; KP, Klebsiella pneumoniae; PSA, P. aeruginosa; ECL, Enterobacter cloacae.
Pharmacokinetic studies
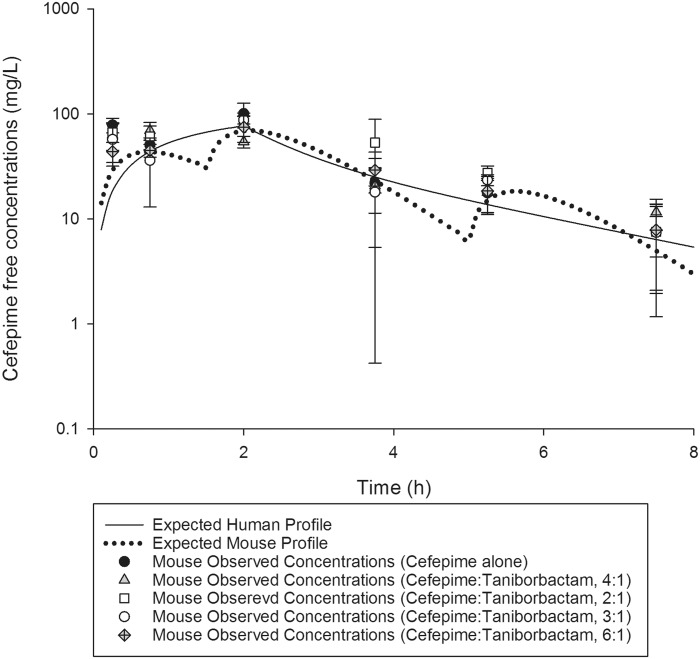
Cefepime exposure in mice similar to that expected in humans following the administration of 2 g q8h as a 2 h infusion was attained when mice were administered 17, 20 and 6 mg/kg at 0, 1.5 and 5 h, respectively, q8h (i.e. the cefepime HSR). Comparison of %fT>CT values achieved with cefepime at CTs of 4 to 512 mg/L in humans and mice receiving the selected HSR are presented in Table 2. Figure 1 illustrates the free plasma concentration–time profile of cefepime, when administered to mice alone and in combination with taniborbactam at various ratios. Co-administration of taniborbactam did not alter the cefepime profile.
Table 2.
Comparison of the exposures achieved with cefepime and taniborbactam in humans and in mice
| %fT>CT | fAUC0–24 (mg·h/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cefepime | |||||||||
| CT (mg/L) | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | |
| humana | 100 | 84 | 58 | 35 | 11 | 0 | 0 | 0 | |
| mouse HSRb | 96 | 83 | 59 | 38 | 8 | 0 | 0 | 0 | |
| Taniborbactam | |||||||||
| CT (mg/L) | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |
| humanc | 100 | 100 | 100 | 100 | 80 | 51 | 17 | 0 | 229 |
| moused 24:1 | 85 | 56 | 23 | 3 | 0 | 0 | 0 | 0 | 17.0 |
| moused 12:1 | 99 | 85 | 56 | 23 | 3 | 0 | 0 | 0 | 33.9 |
| moused 6:1 | 100 | 99 | 85 | 56 | 23 | 3 | 0 | 0 | 67.8 |
| moused 4:1 | 100 | 100 | 88 | 64 | 33 | 8 | 0 | 0 | 101 |
| moused 3:1 | 100 | 100 | 99 | 85 | 56 | 23 | 3 | 0 | 136 |
| moused,e 2:1 | 100 | 100 | 100 | 100 | 84 | 53 | 16 | 0 | 204 |
Human exposures for a dose of 2 g q8h as a 2 h infusion estimated based on best-fit pharmacokinetic parameters of cefepime in healthy subjects from Phase I studies upon co-administration of taniborbactam.
Mouse exposure estimated based on best-fit pharmacokinetic parameters of cefepime in infected mice.
Human exposures for a dose of 0.5 g q8h as a 2 h infusion estimated based on best-fit pharmacokinetic parameters of taniborbactam in healthy subjects from Phase I studies.10
Mouse exposure estimated based on best-fit pharmacokinetic parameters of taniborbactam in infected mice. Ratios represent the proportions of cefepime:taniborbactam, relative to the doses of the cefepime HSR.
Mouse exposure was comparable to that achieved in humans and is denoted in bold.
Figure 1.
Cefepime human-simulated free plasma concentration–time profile in a neutropenic thigh infection model compared with humans receiving a dose of 2 g q8h as a 2 h infusion. Data are presented as mean ± SD.
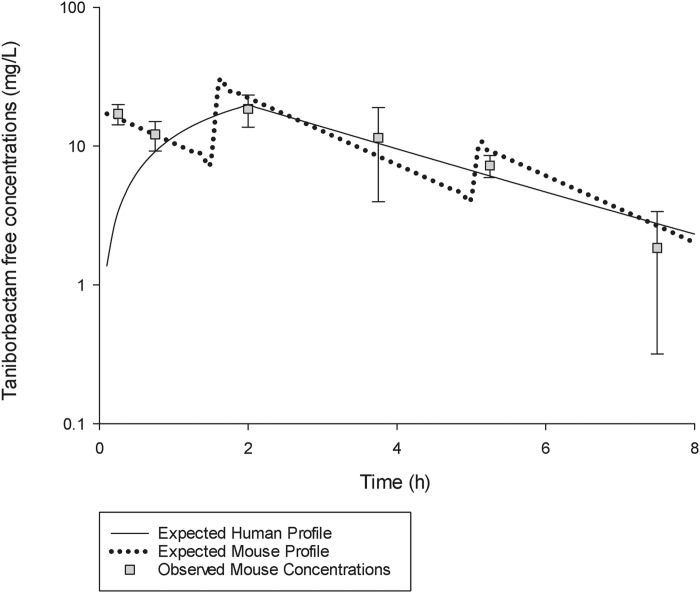
Taniborbactam pharmacokinetics in mice were satisfactorily described by a one-compartment model with first-order absorption and elimination. The best-fit pharmacokinetic parameters of taniborbactam in the murine infection model were: V, 0.4 L/kg; first-order absorption rate constant, 45.5 h−1; and overall elimination rate constant, 0.6 h−1. The taniborbactam unbound plasma concentration–time profile when co-administered with the cefepime predefined HSR at a cefepime:taniborbactam ratio of 2:1 (i.e. 8.5, 10 and 3 mg/kg taniborbactam at 0, 1.5 and 5 h, respectively, q8h; the taniborbactam HSR) was comparable to that observed in humans following administration of 0.5 g q8h as a 2 h infusion (Figure 2). The %fT>CT values for the taniborbactam exposure achieved with this regimen at CTs of 0.25 to 32 mg/L and the fAUC0–24 in humans and mice were comparable (Table 2). Table 2 also shows the comparative %fT>CT and fAUC0–24 values for taniborbactam achieved with various cefepime:taniborbactam ratios (24:1, 12:1, 6:1, 4:1 and 3:1) in mice, demonstrating the wide range of taniborbactam exposures examined in the model.
Figure 2.
Taniborbactam free plasma concentration–time profile when administered with the cefepime HSR at a ratio of 2:1 (taniborbactam HSR) in a neutropenic thigh infection model compared with humans receiving a dose of 0.5 g q8h as a 2 h infusion. Data are presented as mean ± SD.
In vivo efficacy studies
Taniborbactam dose fractionation
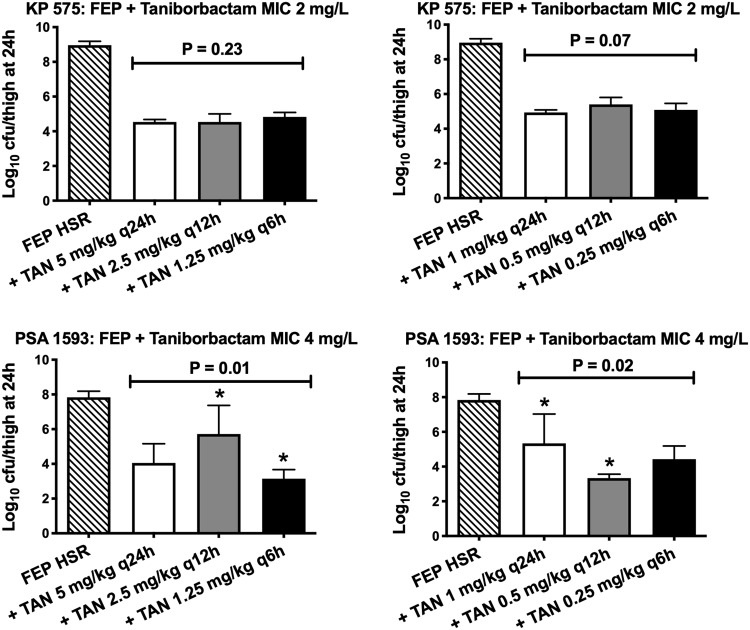
At 0 h, the average bacterial burden was 5.75 ± 0.45 log10 cfu/thigh. For both tested isolates, the cefepime HSR demonstrated lack of efficacy; the bacterial burdens increased over 24 h by 3.33 ± 0.20 and 3.10 ± 0.32 log10 cfu/thigh in the 24 h control and cefepime HSR groups, respectively. Compared with the cefepime HSR alone, the co-administration of taniborbactam at all tested dosing regimens resulted in bacterial reductions. For isolate KP 575, the bacterial burdens observed in the thighs of the groups of mice receiving the same taniborbactam total daily dose were comparable for doses of 5 and 1 mg/kg/day (P > 0.05). For isolate PSA 1593, statistically significant differences in bacterial eradication were observed among some of the treatment groups as shown in Figure 3. Nevertheless, the most fractionated taniborbactam regimen (q6h, providing the highest %fT>CT) was not associated with a statistically significant difference in bacterial eradication compared with the once-daily regimen (q24h, providing the highest fCmax/MIC) of the same total daily dose for both examined doses (P > 0.05).
Figure 3.
Bacterial burdens observed with the cefepime HSR alone and in combination with two total daily taniborbactam doses (1 or 5 mg/kg/day), each given at three dosing frequencies. Asterisks indicate P < 0.05 with the post hoc test. FEP, cefepime; TAN, taniborbactam.
Taniborbactam dose ranging
Taniborbactam dose ranging utilizing the cefepime HSR
At 0 h, the average bacterial burden was 5.77 ± 0.47 log10 cfu/thigh and increased over 24 h by 3.26 ± 0.55 log10 cfu/thigh in the 24 h controls. For 28/30 of the tested isolates, an increase in the bacterial burden was observed in the thighs of the mice receiving the cefepime HSR alone by an average of 2.67 ± 1.01 log10 cfu/thigh.
Compared with cefepime HSR monotherapy, the co-administration of taniborbactam enhanced bacterial killing against all 30 isolates. The average maximal reduction of burden (Imax) at 24 h achieved due to taniborbactam co-administration was 4.93 ± 1.41 log10 cfu/thigh, estimated relative to the bacterial densities in the thighs of the cefepime HSR monotherapy groups.
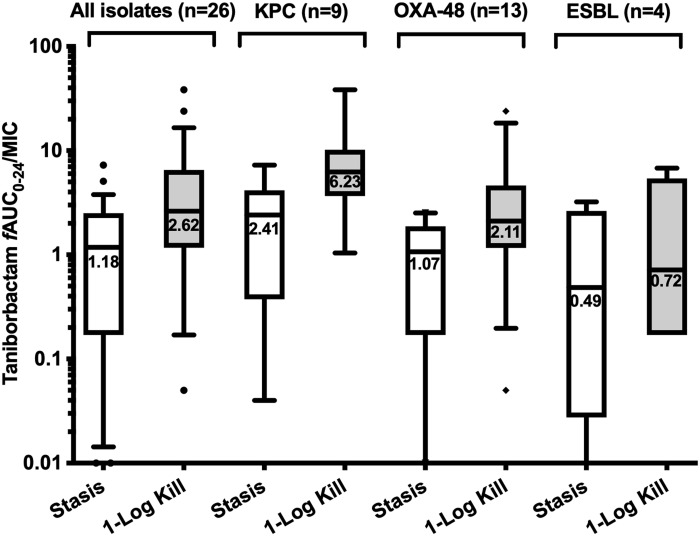
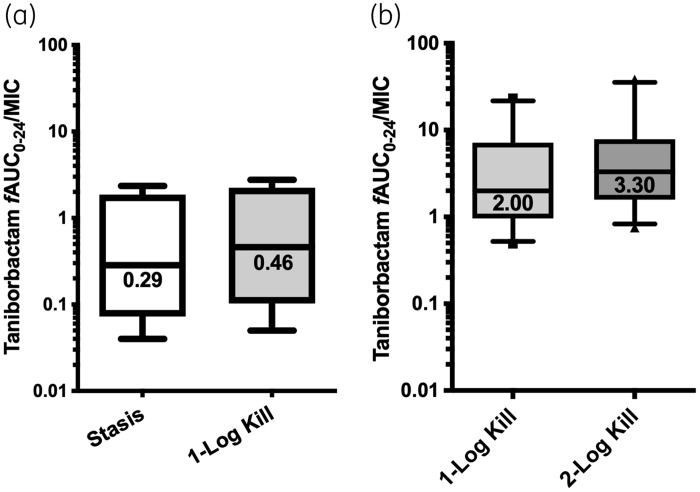
The exposure–response relationships for the majority of the isolates were strong based upon the coefficient of determination (median R2 = 0.96, range = 0.63–0.99). Against Enterobacterales, the median taniborbactam fAUC0–24/MIC values associated with static and 1 log kill endpoints in combination with the cefepime HSR were 1.18 (IQR = 0.17–2.51) and 2.62 (IQR = 1.17–6.52), respectively. The median targets for KPC and OXA-48/OXA-48-like producers were higher than those required for ESBL producers (Figure 4). Against P. aeruginosa, the median fAUC0–24/MIC values associated with static and 1 log kill endpoints were 0.29 (IQR = 0.07–1.86) and 0.46 (IQR = 0.10–2.24), respectively (Figure 5a).
Figure 4.
Taniborbactam fAUC0–24/MIC required to achieve efficacy endpoints against Enterobacterales when co-administered with the cefepime HSR. Median values are displayed on the plots. Hinges represent 25th and 75th percentiles. Whiskers represent 10th and 90th percentiles.
Figure 5.
Taniborbactam fAUC0–24/MIC required to achieve efficacy endpoints against P. aeruginosa when co-administered with (a) the cefepime HSR (n = 4) and (b) the sub-therapeutic cefepime regimen (n = 10). Median values are displayed on the plots. Hinges represent 25th and 75th percentiles. Whiskers represent 10th and 90th percentiles.
Taniborbactam dose ranging utilizing a sub-therapeutic cefepime regimen
At 0 h, the average bacterial burden in the thighs was 4.99 ± 0.50 log10 cfu/thigh and it increased by 3.65 ± 0.58 log10 cfu/thigh at 24 h in the 24 h controls. For the 10 tested isolates, an increase in the bacterial burden of 2.51 ± 1.08 log10 cfu/thigh was observed for the groups receiving sub-therapeutic cefepime (25% HSR) alone. Compared with cefepime alone, the co-administration of taniborbactam at all tested ratios enhanced bacterial killing.
For three isolates examined using both the cefepime HSR and the sub-therapeutic cefepime regimen (PSA 1672, PSA 1679 and PSA 1681), the difference in cefepime regimen did not impact the extent of the bacterial growth among the groups that received cefepime alone as comparable growth was achieved with the two exposures (3.25 ± 0.29 versus 3.37 ± 0.50 log10 cfu/thigh, relative to 0 h bacterial burden). For the lower taniborbactam exposures (cefepime:taniborbactam ratios of 24:1, 12:1 and 6:1, relative to the doses of the cefepime HSR), reduced in vivo activity was observed when co-administered with sub-therapeutic cefepime compared with the cefepime HSR. Nevertheless, for all three isolates, the higher taniborbactam exposure (cefepime:taniborbactam ratio of 3:1) produced substantial in vivo activity achieving ≥1 log reduction in bacterial burdens, relative to the 0 h groups, irrespective of the co-administered cefepime exposure.
The exposure–response relationships between the taniborbactam fAUC0–24/MIC and the change in log10 cfu/thigh at 24 h, relative to the growth among the groups receiving the sub-therapeutic cefepime regimen, were strong (median R2 = 0.95, range = 0.89–0.98). The median fAUC0–24/MIC associated with 1 and 2 log reduction, relative to the growth observed with the sub-therapeutic cefepime exposure, was 2.00 (IQR = 0.96–7.18) and 3.30 (IQR = 1.59–7.86), respectively (Figure 5b).
In vivo activity of the cefepime/taniborbactam HSR
At 0 h, the average bacterial burden was 5.82 ± 0.34 log10 cfu/thigh. For all tested isolates, adequate growth in the neutropenic thigh infection model was achieved; the bacterial burdens increased over 24 h by an average magnitude of 3.59 ± 0.49 log10 cfu/thigh in the 24 h controls. Consistent with the cefepime resistance of the test strains, treatment with cefepime HSR monotherapy was associated with ≥1 log bacterial growth, relative to 0 h controls, against 28 of 30 isolates and stasis (KP 510) to 0.6 log bacterial growth (ECL 123) against the remaining 2 isolates.
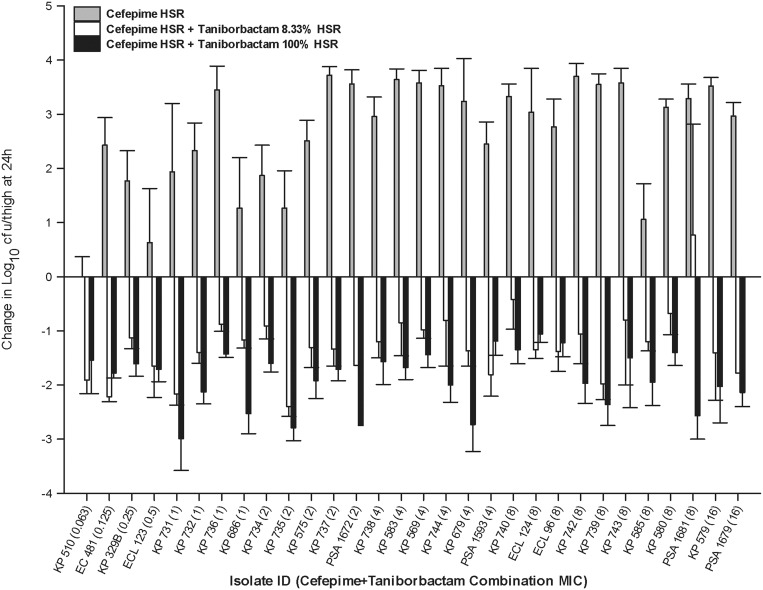
Among the examined 30 isolates, a taniborbactam dosing regimen that is 1/12th the human-equivalent regimen (cefepime:taniborbactam ratio of 24:1 or 8.33% of the taniborbactam HSR) was adequate to achieve ≥1 log reduction in the initial bacterial burden against 21 isolates and at least stasis against 8 isolates, while co-administration of the taniborbactam HSR (cefepime:taniborbactam ratio of 2:1 or 100% of the taniborbactam HSR) achieved ≥2 log reduction against 11 isolates and ≥1 log reduction against the remaining 19 isolates [Figure 6 and Table S1 (available as Supplementary data at JAC Online)].
Figure 6.
Comparative efficacy of the cefepime HSR alone and in combination with either 8.33% or 100% of the taniborbactam HSR. Data are presented as mean ± SD.
Discussion
Cefepime/taniborbactam exerts potent in vitro activity against Enterobacterales and P. aeruginosa isolates; in a global collection of isolates from community and hospital infections that included 3998 Enterobacterales, the MIC50/90 values were 0.06/0.25 mg/L.13 For the subset of carbapenem-non-susceptible Enterobacterales (n = 101), MIC50/90 values were 1/8 mg/L. For 1136 isolates of P. aeruginosa, MIC50/90 values were 2/8 mg/L. Activity was maintained among carbapenem-non-susceptible P. aeruginosa (n = 307); the MIC50/90 values were 8/16 mg/L.13 What distinguishes cefepime/taniborbactam from the currently approved β-lactam/β-lactamase inhibitors is that taniborbactam can inhibit all classes of carbapenemases, including MBLs and novel KPC variants uninhibited by avibactam,14 as well as ESBLs and cephalosporinases, rendering it one of the broadest-spectrum inhibitors under development.
In the current investigation, a population of Enterobacterales and P. aeruginosa expressing various serine-β-lactamases was selected based on their phenotypic profiles to include isolates that were highly resistant to cefepime. The isolates exhibited a wide distribution of cefepime/taniborbactam MICs with substantial reductions in MIC relative to cefepime alone as a function of taniborbactam-mediated β-lactamase inhibition and improved cefepime stability. Moreover, 24/26 Enterobacterales and 4/11 P. aeruginosa selected for the efficacy studies had cefepime/taniborbactam combination MIC values that were equal to or greater than the corresponding MIC90 values from 2018 surveillance,14 thereby representing the most challenging clinical phenotypes. All isolates were pre-screened for their ability to grow in the infection model to ensure adequate fitness and robust expression of the encoded β-lactamases in vivo as evidenced by the lack of activity upon the administration of cefepime monotherapy. Of note, the cefepime regimen utilized, equivalent to the highest clinical dosage of 2 g q8h administered as a prolonged 2 h infusion, provides enhanced probability of pharmacodynamic target attainment at higher MICs (50% fT>MIC) compared with the standard 0.5 h infusion regimen.15
Taniborbactam dose-fractionation studies and dose-ranging studies were conducted to identify the pharmacokinetic/pharmacodynamic index, relative to taniborbactam exposure, that correlated most closely with the taniborbactam-mediated β-lactamase-inhibitory activity and the magnitude of taniborbactam exposure required for efficacy when combined with cefepime against serine-β-lactamase producers in the murine thigh infection model. In order to eliminate the potential confounding effect of altered cefepime activity, a fixed, clinically relevant cefepime exposure was used, while varying the taniborbactam exposure only. This design allowed us to identify the pharmacokinetic/pharmacodynamic index as well as the magnitude of index required to produce activity relative to taniborbactam exposure when administered concomitantly with the cefepime dose currently being studied in combination with taniborbactam in clinical trials.
The results from the dose-fractionation studies against two KPC-producing strains provided no consistent evidence of concentration- or time-dependent bacterial killing. Four additional isolates expressing serine-β-lactamases and MBLs were examined using the same study design and showed similar results (data not shown). We thus concluded that taniborbactam dosing frequency had no impact on the cefepime potentiation capability, which indicated that the index that best correlated with taniborbactam inhibitory activity was fAUC0–24/MIC. Similar findings were reported from in vitro investigation; dose-fractionation studies identified that both fAUC0–24/MIC and %fT>CT (3.75 or 0.03 mg/L) were associated with taniborbactam efficacy.16,17 However, the authors concluded that fAUC0–24/MIC best described efficacy across a larger panel of isolates.17
Subsequent dose-ranging experiments encompassed various taniborbactam regimens that were well differentiated in terms of fAUC0–24. The pharmacodynamic targets of stasis and 1 log bacterial reduction, generally considered surrogate endpoints in preclinical infection models for prediction of clinical efficacy,18 were estimated relative to the ratio of taniborbactam fAUC0–24 to cefepime/taniborbactam combination MIC. The estimated targets showed moderate variability between strains, which is likely attributed to the different levels of expression of the encoded β-lactamase genes in vivo and thus different requirements of inhibitor exposure for enzyme suppression. The target required for 1 log kill against KPC-producing isolates (median taniborbactam fAUC0–24/MIC = 6.23) was the highest among the study population, indicating that these enzymes were relatively less amenable to inhibition in vivo. Nevertheless, given that the fAUC0–24 observed in humans with a taniborbactam dose of 0.5 g q8h was ∼230 mg·h/L,10 our data predicted that this dose should provide sufficient systemic exposure to achieve at least 1 log kill against isolates with a cefepime/taniborbactam MIC at the upper end of the MIC distribution among carbapenem-resistant Enterobacterales and P. aeruginosa from the 2018 global surveillance.13 Consistent with our predictions, the administration of the cefepime/taniborbactam HSR resulted in >1 log kill among all the isolates examined with cefepime/taniborbactam MICs up to 16 mg/L. These data provided further justification for the cefepime/taniborbactam combination dosage of 2 g/0.5 g q8h as a 2 h infusion, as is currently being evaluated in a Phase III study of adult patients with complicated urinary tract infections.11 Moreover, the administration of taniborbactam at an exposure as low as 8.33% of the HSR was associated with >1 log kill among the majority of the examined isolates. For these isolates, the fAUC0–24/MIC achieved with the low taniborbactam dose was sufficient to attain the pharmacodynamic target. This finding is important as it demonstrates the high potency of the combination and provides assurance that robust and therapeutic exposures could still be achieved among patient populations with variable pharmacokinetic profiles.
For AmpC-overproducing P. aeruginosa, a slightly modified design was required. Consistent with the knowledge that cefepime is quite stable in the presence of AmpC β-lactamase,19,20 the growth of the majority of the AmpC-overproducing P. aeruginosa isolates was inhibited in mice that received the full cefepime HSR, with the exception of PSA 1672, PSA 1679 and PSA 1681. This observation hindered our efforts to assess taniborbactam AmpC inhibitory activity against a larger number of isolates in our animal model upon the administration of the combination HSR. Thus, a modified design utilizing a reduced cefepime exposure was adopted. Nevertheless, the activity of the taniborbactam HSR was unencumbered by co-administration of a reduced cefepime dose as the combination still resulted in a high degree of bacterial killing. Given that a sub-therapeutic cefepime exposure was co-administered, conventional estimation of the different taniborbactam target exposures required to achieve stasis or reduction in bacterial burden, relative to 0 h groups, was not attempted as those targets would likely be overestimated. Instead, the taniborbactam target exposures required to achieve efficacy endpoints, relative to the growth among the groups receiving cefepime monotherapy, were estimated. This assessment was conducted to demonstrate the additional bacterial killing that could be achieved upon taniborbactam co-administration and the exposures associated with incremental reduction in bacterial burden compared with cefepime monotherapy. The identified target exposures indicated that the taniborbactam exposure achieved with the 0.5 g dose q8h should be sufficient to attain multi-log reduction in bacterial density compared with cefepime alone against AmpC-producing P. aeruginosa, including isolates with cefepime/taniborbactam MICs at the upper end of the MIC distribution.
It is important to mention that, while these preclinical data provide some insights into the clinical breakpoints of cefepime/taniborbactam, the projections should be interpreted as provisional, as they were estimated based on the exposures achieved among healthy subjects following the selected clinical dose. As previously mentioned, critically ill patients exhibit variable pharmacokinetics,21 which may impact the probability of pharmacodynamic target attainment. Establishing the clinical breakpoints will ultimately require the integration of the identified preclinical targets for efficacy with the population pharmacokinetic information as well as the clinical outcomes of cefepime/taniborbactam therapy among patients.22
In summary, our results showed that the co-administration of taniborbactam with cefepime markedly enhanced the in vivo efficacy of the latter against Enterobacterales and P. aeruginosa isolates expressing a broad range of serine-β-lactamases and lend support for further development of this novel combination. The preclinical pharmacokinetic/pharmacodynamic targets for efficacy identified in our model support a taniborbactam dose of 0.5 g in combination with cefepime 2 g q8h for Phase III studies.
Supplementary Material
Acknowledgements
This study was presented in part at IDWeek 2018, San Francisco, CA, USA (Poster 1405) and the Twenty-Ninth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 2019 (ePoster O1064).
We thank our colleagues at the Center for Anti-Infective Research and Development (Hartford, CT, USA) for the assistance with the conduct of the study.
Funding
This study was funded by Venatorx Pharmaceuticals, Inc., Malvern, PA, USA. This Project was funded in part with Federal funds from The National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services (Contract No. HHSN272201300019C) and the Wellcome Trust (Grant No. WT 101999/Z/13/Z).
Transparency declarations
K.A. and D.P.N. have received research grants from the study sponsor. S.A.A.: none to declare.
References
- 1. Bush K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother 2018; 62: e01076–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Antibiotic Resistance Threats in the United States. 2019.
- 3. Hamrick JC, Docquier JD, Uehara T et al. VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2020; 64: e01963–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Thirtieth Edition: M100 2020.
- 5.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07 2018.
- 6. Abdelraouf K, Stainton SM, Nicolau DP. In vivo pharmacodynamic profile of ceftibuten-clavulanate combination against extended-spectrum-β-lactamase-producing Enterobacteriaceae in the murine thigh infection model. Antimicrob Agents Chemother 2019; 63: e00145–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monogue ML, Tsuji M, Yamano Y et al. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61: e01022–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattie H, Sekh BA, van Ogtrop ML et al. Comparison of the antibacterial effects of cefepime and ceftazidime against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother 1992; 36: 2439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burgess DS, Hastings RW, Hardin TC. Pharmacokinetics and pharmacodynamics of cefepime administered by intermittent and continuous infusion. Clin Ther 2000; 22: 66–75. [DOI] [PubMed] [Google Scholar]
- 10. Geibel B, Dowell J, Dickerson D et al. A randomized, double-blind, placebo-controlled study of the safety and pharmacokinetics of single and repeat doses of VNRX-5133 in healthy subjects. Open Forum Infect Dis 2018; 5 Suppl 1: S431. [Google Scholar]
- 11.Safety and Efficacy Study of Cefepime/VNRX-5133 in Patients with Complicated Urinary Tract Infections. ClinicalTrials.gov Identifier: NCT03840148.
- 12.Safety and Pharmacokinetics of VNRX-5133 in the Epithelial Lining Fluid of Healthy Adult Subjects. ClinicalTrials.gov Identifier: NCT03870490.
- 13. Hackel M, Sahm D, Antimicrobial activity of cefepime in combination with VNRX-5133 against a global 2018 surveillance collection of clinical isolates Twenty-Ninth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 2019. Abstract P1175. [Google Scholar]
- 14. Myers CL, Daigle DM, Kurepina N et al. Cefepime/VNRX-5133 retains potent activity against KPC variants responsible for recent clinical treatment failures of ceftazidime/avibactam in Klebsiella pneumoniae ASM Microbe 2018, Atlanta, GA, USA. Abstract 610. [Google Scholar]
- 15. Nicasio AM, Ariano RE, Zelenitsky SA et al. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 2009; 53: 1476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daigle D, Vernacchio S, Xerri L et al. Defining the magnitude of AUC:MIC driver for efficacy of the β-lactamase inhibitor VNRX-5133 when combined with cefepime against KPC- and VIM/NDM-producing Enterobacteriaceae and P. aeruginosa. Open Forum Infect Dis 2018; 5 Suppl 1: S429. [Google Scholar]
- 17. VanScoy BD, McCauley J, Lakota EA et al. Pharmacokinetics-pharmacodynamics (PK-PD) of VNRX-5133, a broad-spectrum novel β-lactamase inhibitor (BS-BLI), in combination with cefepime in a one-compartment in vitro infection model Twenty-Eighth European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 2018. Abstract P1537. [Google Scholar]
- 18. Bulitta JB, Hope WW, Eakin AE et al. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 2019; 63: e02307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacoby GA. AmpC β-lactamases. Clin Microbiol Rev 2009; 22: 161–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siedner MJ, Galar A, Guzman-Suarez BB et al. Cefepime vs other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin Infect Dis 2014; 58: 1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonçalves-Pereira J, Póvoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care 2011; 15: R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 2007; 20: 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.