Abstract
Despite escalating anthropogenic alteration of food webs, how the carbon cycle in ecosystems is regulated by food web processes remains poorly understood. We quantitatively synthesize the effects of consumers (herbivores, omnivores and carnivores) on the carbon cycle of coastal wetland ecosystems, ‘blue carbon’ ecosystems that store the greatest amount of carbon per unit area among all ecosystems. Our results reveal that consumers strongly affect many processes of the carbon cycle. Herbivores, for example, generally reduce carbon absorption and carbon stocks (e.g. aboveground plant carbon by 53% and aboveground net primary production by 23%) but may promote some carbon emission processes (e.g. litter decomposition by 32%). The average strengths of these effects are comparable with, or even times higher than, changes driven by temperature, precipitation, nitrogen input, CO2 concentration, and plant invasions. Furthermore, consumer effects appear to be stronger on aboveground than belowground carbon processes and vary markedly with trophic level, body size, thermal regulation strategy and feeding type. Despite important knowledge gaps, our results highlight the powerful impacts of consumers on the carbon cycle and call for the incorporation of consumer control into Earth system models that predict anthropogenic climate change and into management strategies of Earth's carbon stocks.
This article is part of the theme issue ‘Integrative research perspectives on marine conservation’.
Keywords: blue carbon, ecosystem functioning, food webs, salt marsh, top-down control
1. Introduction
An inclusive understanding of the ecological factors regulating the ecosystem carbon cycle is critical in the Anthropocene. This understanding is needed to develop Earth system models that can accurately predict climate change [1,2] and to design effective strategies to protect or maximize carbon storage in ecosystems [3]. How abiotic factors (e.g. precipitation changes, warming and nitrogen enrichment) and human-driven land use changes (e.g. forest logging and reforestation) affect various processes of the ecosystem carbon cycle has been investigated in many observational and experimental studies and synthetic analyses [4–7]. These studies have shown that many abiotic factors and human-driven land use changes can strongly affect ecosystem carbon cycling and exacerbate or help mitigate climate warming. Despite the many advances these studies have made in our understanding of the ecosystem carbon cycle and its response to environmental change, important knowledge gaps remain [2].
A major knowledge frontier in current understanding of the ecosystem carbon cycle is whether or not food web processes affect the ecosystem carbon cycle and should be incorporated into Earth system models and protection strategies of Earth's carbon stocks [8]. We know of no current Earth system models or carbon-climate models that have incorporated food web processes. Nevertheless, the response of the ecosystem carbon cycle to human-driven environmental changes cannot be understood or predicted without recognizing the potentially powerful impacts of food web processes. Indeed, accelerating human activities in the Anthropocene not only modify the abiotic mechanisms of the ecosystem carbon cycle but also directly alter the structure of food webs in ecosystems (via overfishing, hunting, pollution or introducing novel consumers such as livestock or invasive predators; [9,10]). It is well known that such alterations often have cascading effects on lower trophic levels such as plants and, in extreme cases, can lead to complete vegetation loss [10]. It is very likely that alterations of food webs also affect the functioning and biogeochemistry of ecosystems, including the carbon cycle. The effects of food web processes (such as grazing) on carbon cycling have been relatively well recognized in phytoplankton-dominated ecosystems (e.g. [11,12]). In vascular plant-dominated ecosystems, there are an increasing number of studies on livestock grazing in terrestrial grasslands [13] and goose grazing in wetlands [14]. How food web processes affect the ecosystem carbon cycle, however, remains poorly understood.
It is particularly important to understand how food web processes affect the carbon cycle in coastal wetland ecosystems including salt marshes and mangroves. These coastal vegetated wetlands are ‘blue carbon’ ecosystems that store the greatest amount of carbon per unit area among all Earth's ecosystems. Coastal wetlands, however, are threatened not only by reclamation, draining, and pollution, but also by human-driven changes in food webs (e.g. overfishing; [15]). Such human-driven changes in food webs have been shown to exert top-down control on vegetation in coastal wetlands globally [16]. There are also an increasing number of studies investigating the effects of consumers on biogeochemistry in coastal wetlands (e.g. [17]). With evidence from only a few case studies, maintaining healthy predator populations has also been argued to be critical to help protect carbon stocks in blue carbon ecosystems [18,19]. The generality and predictability of the effects of consumers on the carbon cycle in coastal wetlands are not well understood. Furthermore, the carbon cycle involves a series of pools and fluxes from photosynthesis and primary production to decomposition and respiration [2]. Most previous studies, however, investigated one or a few of those carbon cycle processes (e.g. [20–22]) or were focused on a specific type of consumers (e.g. livestock; [23]). How consumers in general affect the entire carbon cycle and which carbon processes are most sensitive to changes in consumer pressure are still unknown. Understanding these questions requires a holistic synthesis.
In this paper, we provide a quantitative synthesis on the impacts of consumers on the carbon cycle in coastal wetlands. Using a global dataset compiled from published and unpublished studies, we examined if consumers affect a series of carbon cycle processes, including both carbon pools and fluxes. We also examined whether the effects of consumers on the carbon cycle vary with consumer traits, including trophic level (herbivores, omnivores and carnivores), consumer taxon (e.g. insects, snails, crabs, small mammals, geese and livestock), thermal regulation strategy (endotherms and ectotherms), and feeding type (aboveground herbivores, belowground herbivores, above- plus belowground herbivores, and trampling herbivores which mainly included livestock). Specifically, we tested the following hypotheses: (1) herbivores decrease carbon pools and carbon absorption processes (e.g. photosynthesis and primary production) and promote carbon emission processes (litter decomposition (LDP) and respiration); (2) the effects of herbivores on the carbon cycle are generally evident, but their relative strength varies with herbivore traits, including taxon, thermal regulation strategy and feeding type; and (3) in contrast to herbivores, consumers at higher trophic levels, carnivores in particular, enhance carbon stocks. We also identified knowledge gaps and areas that merit further investigation and discuss the implications of our results for the development of next-generation Earth system models and for the conservation of blue carbon ecosystems and Earth's carbon stocks.
2. Materials and methods
(a). Dataset
To build a dataset on the impacts of consumers on the carbon cycle in coastal wetlands, we compiled data from two sources. First, we considered data and papers analysed in a previous comprehensive meta-analysis we conducted on consumer control of vegetation in coastal wetlands [16]. That meta-analysis focused on consumer control of vegetation performance, although studies on consumer control of the carbon cycle were also included while we were screening the literature and collecting related data. Also, we updated this dataset by adding data in recently published papers between 2015 and 2019. To do so, we first searched Web of Science in June 2019 using the query: TS = (top-down* OR herbivor* OR grazing* OR predat* OR consumer* OR trophic cascade*) AND TS = (salt marsh* OR mangrove* OR coastal wetland* OR coastal marsh*) AND TS = (carbon* OR organic matter* OR biomass* OR productivity* OR decomposition). We screened the resulting papers and retained those that: (1) quantified the effects of consumers (either herbivores, omnivores or carnivores; human ‘grazing’ through salt marsh haying was not considered) in observational or experimental studies; (2) contained a response variable relevant to a carbon process (either pool or flux); and (3) reported mean values of the carbon cycle measure with sample sizes and some measure of variance (standard deviations, standard errors or confidence intervals (CIs)) in treatments with and without consumers. A PRISMA flow diagram showing the literature screening process is given in electronic supplementary material, figure S1. Finally, 125 papers (including a few unpublished studies extracted from [16]) were retained. A list of those papers is given in electronic supplementary material, data S1.
From each retained publication, we extracted carbon cycle data in treatments with and without consumers from text, tables, or by digitizing figures in the publication. We considered the following measures: carbon pools—aboveground plant carbon (APC), belowground plant carbon (BPC), litter carbon (LC), soil carbon stock (soil CS), soil organic carbon density (soil OCden), soil organic carbon concentration (soil OCcon), soil total carbon density (soil TCden), soil total carbon concentration (soil TCcon), soil microbial biomass carbon (soil MBC), and soil dissolved organic carbon (soil DOC); and carbon fluxes—gross ecosystem photosynthesis (GEP), aboveground net primary production (ANPP), belowground net primary production (BNPP), litter decomposition (LDP), canopy respiration (canopyR), soil respiration (soilR), ecosystem respiration (ER), and net ecosystem exchange (NEE). For APC, BPC and LC, we also used biomass data as a proxy, assuming that consumers did not change carbon concentration in plants. This was supported by our meta-analysis of studies that reported plant carbon concentration in treatments with and without herbivores (electronic supplementary material, table S1). Some studies reported the carbon responses of different plant species or a soil carbon measure in multiple soil layers (less than 30 cm deep in most studies) in the same plot/treatment, separately, which were included in our dataset. To account for potential autocorrelation, these data points were pooled using a meta-analysis, and the single pooled mean estimate (with variance) included in all the meta-analyses described below [24]. For soil carbon measures, most studies analysed soil cores of less than 30 cm deep except for a few that were 50–100 cm deep. For studies that reported soil C concentration but not soil carbon density, we estimated soil carbon density by multiplying soil C concentration by soil bulk density (if unavailable, soil bulk density was estimated using the pedotransfer function; [25]).
Following [16], we also recorded the following variables for each experiment/observation: (1) author(s) and year; (2) study venue (field versus laboratory), latitude and longitude; (3) ecosystem (marsh versus mangrove); (4) annual mean temperature and annual precipitation; (5) plant growth form (forb, grass versus shrub); (6) name, taxon, bone type (vertebrate versus invertebrate), trophic level (herbivore, omnivore or carnivore (as defined in the original study)), thermal regulation strategy, and feeding type of the study consumer species; (7) source (natural, transplanted or seeded) of the study plant species; (8) method of consumer manipulation (observation, exclusion or addition); and (9) other factors manipulated (e.g. competition or nutrient) and their treatment level (ambient versus treated).
(b). Meta-analysis
We used log response ratio (and associated variance; [26]) to quantify the effect sizes of consumers on a carbon cycle measure. Log response ratio is among the most widely used effect size metrics in meta-analysis, but does not accept non-positive data, which were present in a relatively small number of cases in our dataset. To resolve this issue, zero values in our dataset were (x + 1)-transformed (three negative data points were excluded from the analysis). Data in the corresponding treatments with or without consumers were transformed similarly. Inclusion or exclusion of these transformed data did not affect the general findings in our study.
Random-effects models were used to estimate the mean effect sizes (and 95% CIs) of consumers on a carbon cycle measure. In each analysis, heterogeneity in the effect size data was quantified using the Q statistic, a measure of weighted squared deviations [26]. Mean effect sizes are considered to be significant if their 95% CIs do not cross zero [26]. All analyses were carried out using R 3.6.1 [27] and its metafor package [28].
Mixed-effects models with a herbivore trait as the moderator were used to determine if the effects of herbivores on a carbon cycle measure varied with consumer traits. Three traits were considered in this analysis: taxon, thermal regulation strategy and feeding type. These traits (including trophic level, analysed below) represent some of the most widely considered traits that can be relatively easily defined for most of the herbivores included in our analysis. Although we did not include a quantitative measure of body size, another important consumer trait, our classification of herbivore taxon described above represents a broad gradient of body size for herbivores, from insects with the smallest body size to livestock with the largest body size. Thermal regulation strategy is a key functional trait underlying the energy maintenance of animals. Endotherms, which consume resources to maintain their body temperature, are expected to have stronger impacts on the carbon cycle relative to ectotherms, which change their body temperature depending on external environments [29]. Finally, feeding type was included as a herbivore trait, given that herbivores that feed primarily on aboveground or belowground material may have differential effects on the carbon cycle. In these mixed-effects models, the between-group heterogeneity statistic QM was used to assess if mean effect sizes differed among herbivores of different taxa, thermal regulation strategies or feeding types.
The above herbivore traits are likely to be non-independent, and other explanatory variables, either plant, climatic or methodological, have also been found in univariate meta-analyses to be significant in mediating variation in the effects of herbivores [16]. To examine potential non-independence and interactions among herbivore traits and other explanatory variables, we further evaluated the performance of a candidate set of multivariate mixed-effects models, where effect sizes were related to the main effects of the three herbivore traits and five other explanatory variables: plant growth form, method of consumer manipulation, source of response plants (whether they were planted, seeded or naturally present) and climate variables (annual mean temperature and annual precipitation). Two-way interactive effects of the four categorical variables on herbivore traits and plant growth form were also considered in modelling. Two of the most widely reported measures of the carbon cycle—APC and BPC—were used as the dependent variables. Only field studies in ambient conditions (i.e. no other factors were disturbed except for the presence of herbivores) were considered in these analyses. The most parsimonious models in predicting variations in effect size were selected using Akaike's information criterion, corrected for small sample sizes (AICc) [30].
Furthermore, to determine if the effects of consumers on a carbon cycle measure vary with consumer trophic level (herbivores, omnivores and carnivores), mixed-effects models with trophic level as the moderator were used. Three carbon cycle measures with data for at least two types of trophic level available were used: APC, BPC carbon and LC. Studies on the impacts of carnivores and omnivores on other measures of the carbon cycle were unavailable. In these mixed-effects models, the between-group heterogeneity statistic QM was used to assess if mean effect sizes differ among herbivores, omnivores and carnivores. Additionally, to examine if carnivores and omnivores affect herbivores, we computed log response ratio (lnRR) effect sizes on herbivore abundance using the dataset in [16]. This included data of herbivore abundances in treatments with and without carnivores (or omnivores) reported in previous studies. We used random-effects models to estimate the mean effect sizes (and 95% CIs) of carnivores and omnivores on herbivore abundance.
Additionally, to check for the influence of potential publication bias on our results, we examined funnel plot asymmetry quantitatively using the trimfill method and estimated Rosenthal's fail-safe number for each of our effect size metrics. These supplementary analyses and results are detailed in electronic supplementary material, text S1 and table S1. These analyses showed that our results were generally robust to publication bias.
3. Results
(a). Effects of herbivores on carbon pools and fluxes
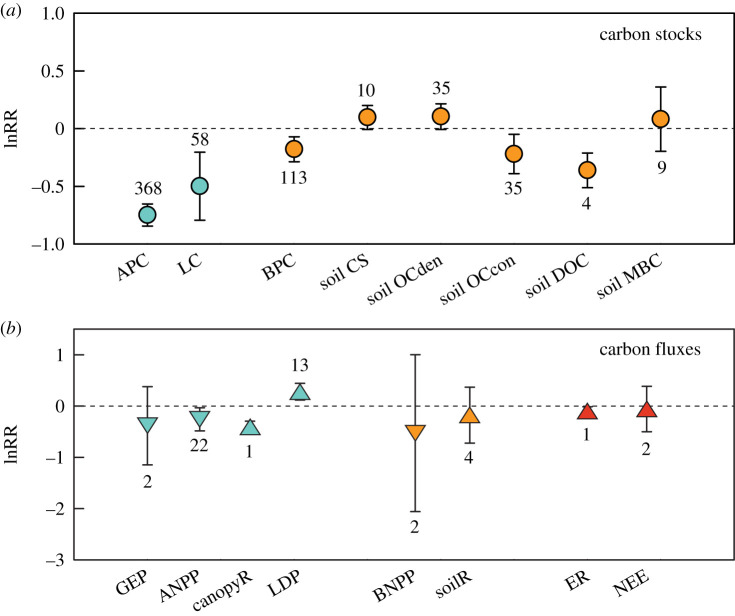
Herbivores significantly affected various components of carbon pools. Herbivores reduced APC by 53% and LC by 39% (figures 1a and 2). Herbivores also significantly reduced BPC by 16%, soil OCcon by 20%, soil TCcon by 18%, and soil DOC by 30%, and nearly significantly increased soil CS and soil OCden, but did not affect soil MBC and soil TCden (figures 1a and 2 and electronic supplementary material, table S1). These results held when a few studies on soil OCden and OCcon in soil cores of greater than 30 cm deep were included (electronic supplementary material, table S1).
Figure 1.
Herbivore effects on the carbon cycle in coastal wetland ecosystems. (a) Measures of carbon stocks and (b) measures of carbon fluxes. Shown are mean log response ratios (lnRR) estimated with random-effects models. Error bars are 95% confidence intervals. Sample sizes for each carbon cycle measure are also shown. Effect sizes are significant when their 95% confidence intervals do not cross zero. Aboveground, belowground and whole-ecosystem carbon measures are indicated with cyan, orange and red symbols, respectively. In (b), for downward triangles, negative and positive effect sizes indicate that herbivores reduce and promote carbon absorption, respectively; for upward triangles, negative and positive effect sizes indicate that herbivores reduce and promote carbon emission, respectively. APC, aboveground plant carbon; BPC, belowground plant carbon; LC, litter carbon; soil CS, soil carbon stock; soil OCden, soil organic carbon density; soil OCcon, soil organic carbon concentration; soil MBC, soil microbial biomass carbon; soil DOC, soil dissolved organic carbon; GEP, gross ecosystem photosynthesis; ANPP, aboveground net primary production; BNPP, belowground net primary production; LDP, litter decomposition; canopyR, canopy respiration; soilR, soil respiration; ER, ecosystem respiration; and NEE, net ecosystem exchange. (Online version in colour.)
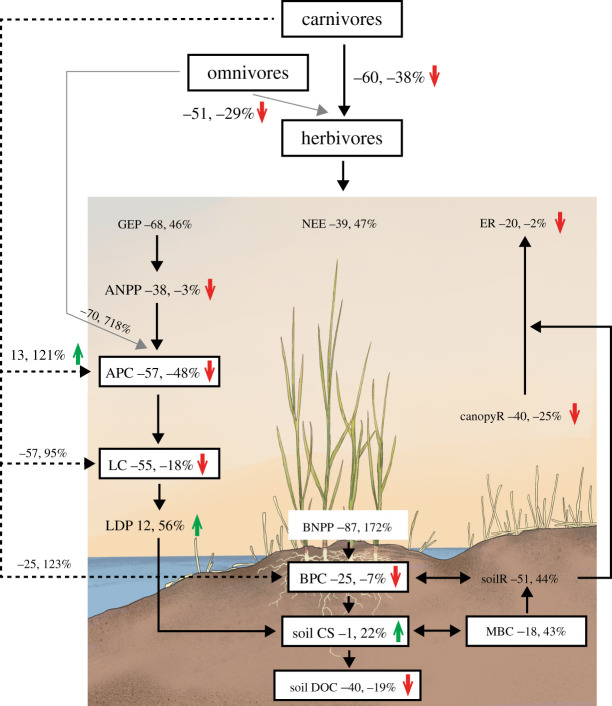
Figure 2.
Consumer control of the carbon cycle in coastal wetland ecosystems. Shown are 95% confidence intervals of percentage changes (back-transformed from lnRR estimates). Significant effects are indicated with a green, upward or red, downward arrow (p < 0.06), and effects without an arrow are insignificant. Upward and downward arrows indicate positive and negative effects of consumers, respectively. Carbon cycle measures with a small sample size (less than 10) are indicated with smaller fonts and represent areas that merit future tests. Abbreviations follow figure 1. (Online version in colour.)
Fewer studies have investigated how consumers affect carbon fluxes. Of the carbon absorption measures, herbivores significantly reduced ANPP by 23%, but no significant effects on GEP and BNPP were found (figures 1b and 2). Although herbivores also significantly reduced carbon emission measures such as canopyR (by 33%) and ER (by 11%), they significantly accelerated LDP by 32% (figures 1b and 2). Herbivores did not significantly affect soilR or NEE (figures 1b and 2).
(b). Variation with herbivore traits
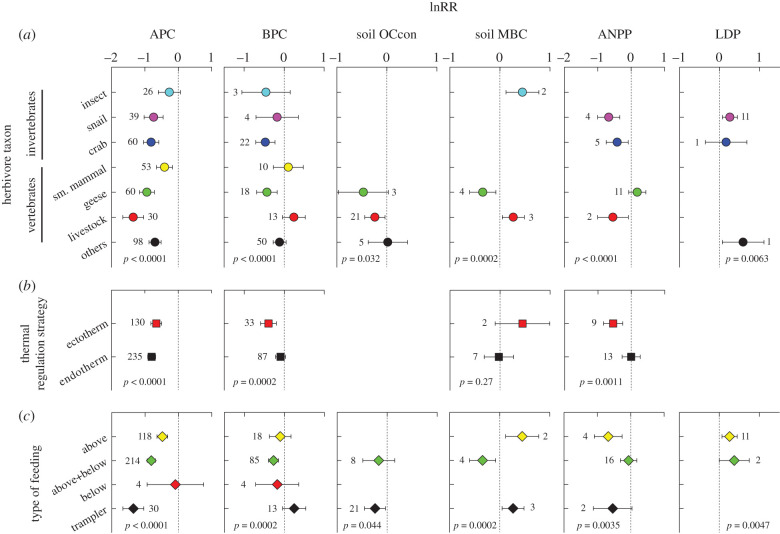
Herbivore taxon, thermal regulation strategy, and feeding type significantly mediated variation in the effect of herbivores on the carbon cycle (figure 3). Herbivore taxon was significant in modulating the strength and the nature of herbivore effects on almost all carbon cycle measures with sufficient data (except for soil OCden, where all types of herbivores with data available showed no significant effects; mixed-effects model, p = 0.32). All types of herbivores except insects with data available significantly reduced APC, and generally effects increased with body size within invertebrate and vertebrate herbivores (figure 3a). BPC was significantly reduced by crabs and geese, LC by livestock and ‘other’ herbivores, soil OCcon by livestock, and ANPP by snails, crabs and livestock (figure 3a; electronic supplementary material, table S1). Insects and livestock significantly increased, while geese significantly reduced soil MBC (figure 3a). Snails and ‘other’ herbivores significantly increased LDP (figure 3a). In all other cases with data available, no significant effects were detected (figure 3a).
Figure 3.
Herbivore traits and top-down control of the carbon cycle in coastal wetlands. (a) Herbivore taxon (except other herbivores, arranged by body size from the smallest (insects) to the largest (livestock)); (b) thermal regulation strategy; and (c) type of feeding (aboveground herbivores, above- plus belowground herbivores, belowground herbivores, and herbivores with strong trampling effects). Shown are mean lnRR effect sizes (and 95% confidence intervals, estimated with mixed-effects models) of six carbon cycle measures: APC, BPC, soil OCcon, soil MBC, ANPP and LDP. In (a), ‘others’ included herbivores of taxa with few data available and those of multiple mixed taxa. Effect sizes are significant when their 95% confidence intervals do not cross zero. p-values indicate the significance of a trait in mediating variation in lnRR. Abbreviations follow figure 1. (Online version in colour.)
Thermal regulation strategy was a significant mediator of variation in the effects of herbivores on APC, BPC, LC and ANPP (p < 0.005 in all cases), but not on soil MBC (p = 0.27). Relative to ectotherms, endotherms more strongly affect APC (figure 3b) and LC (p = 0.002). By contrast, endotherms had much weaker effects on BPC and ANPP than ectotherms (figure 3b).
Feeding type was a significant or nearly significant mediator of variation in the effects of herbivores on all carbon cycle measures with sufficient data (including APC, BPC, LC, soil OCcon, soil MBC, ANPP and LDP) except for soil Cden. All feeding types except belowground herbivores (with a small sample size) significantly reduced APC, and this effect weakened from tramplers to above- plus belowground and to aboveground herbivores (figure 3c). Above- plus belowground herbivores significantly reduced, while herbivores of all other feeding types did not significantly affect BPC (figure 3c). Aboveground herbivores did not significantly affect, above- plus belowground herbivores nearly significantly reduced, while tramplers strongly reduced LC (figure 3c). Above- plus belowground herbivores did not affect while tramplers increased soil CS (electronic supplementary material, table S1). Above- plus belowground herbivores did not significantly affect, while tramplers tended to reduce soil OCcon (figure 3c). Aboveground herbivores and tramplers significantly increased, while above- plus belowground herbivores reduced soil MBC (figure 3c). Aboveground herbivores reduced, tramplers nearly significantly reduced, while above- plus belowground herbivores did not significantly affect ANPP (figure 3c). Similarly, aboveground herbivores reduced, and above- plus belowground herbivores nearly significantly reduced LDP (figure 3c).
Multivariate modelling revealed that (i) herbivore effects on APC were best predicted by a single variable (herbivore taxon), (ii) source of response plants, thermal regulation strategy and temperature were also common predictors, occurring in 4, 3 and 3 of the 10 most parsimonious models, respectively, and (iii) annual precipitation and feeding type might also play a role, as they occurred in 2 and 1 of the 10 most parsimonious models, respectively (ΔAICc < 4; electronic supplementary material, tables S2 and S3). Herbivore effects on BPC were best predicted by a combination of herbivore taxon, plant growth form, method of consumer manipulation and temperature, although precipitation, feeding type and source of response plants could also be common predictors, occurring in 3, 2 and 2 of the 8 most parsimonious models, respectively (electronic supplementary material, tables S4 and S5).
(c). Effects of herbivores, omnivores and carnivores
The effects of consumers on the carbon cycle also varied significantly with trophic level (i.e. herbivores, omnivores and carnivores). Among the carbon cycle measures with data available, herbivores significantly reduced, omnivores did not affect, while carnivores significantly increased APC (by 58%) (figures 2 and 4). Herbivores also significantly reduced, while carnivores did not significantly affect BPC and LC (figures 2 and 4). Carnivores and omnivores significantly reduced herbivore abundance by 51% and 41%, respectively (figure 2).
Figure 4.
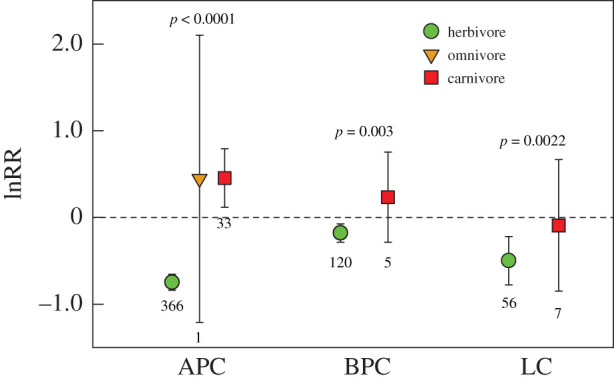
Trophic level and top-down control of the carbon cycle in coastal wetlands. Shown are mean lnRR estimated with mixed-effects models. Error bars are 95% confidence intervals. Sample sizes for each carbon cycle measure are also shown. Effect sizes are significant when their 95% confidence intervals do not cross zero. p-values indicate the significance of trophic level in mediating variation in lnRR. Abbreviations follow figure 1. (Online version in colour.)
4. Discussion
Our results support the hypothesis that herbivores generally decrease carbon absorption processes (e.g. ANPP) and increase carbon emission processes (decomposition). This decreases carbon pools (e.g. APC, BPC, LC and soil Ccon, except for soil CS and soil Cden) in coastal wetland ecosystems. These effects of herbivores on the ecosystem carbon cycle varied with herbivore taxon and traits (body size, thermal regulation strategy and feeding type). Our study also supports the hypothesis that carnivores influence the carbon cycle in coastal wetlands by suppressing herbivores and promoting plant biomass carbon accumulation. Moreover, our study clearly identifies important knowledge gaps and areas that require further investigation.
The effects of herbivores were stronger on aboveground carbon processes (e.g. APC, LC and decomposition) than on belowground processes (e.g. BPC, soil MBC, soil CS and soil OCcon). This finding is consistent with a previous study showing that livestock grazing strongly reduced APC but did not significantly affect BPC [23]. Also, relative to belowground carbon processes, aboveground carbon processes are often more sensitive to warming [31], precipitation changes [31], nutrient addition [32,33] and plant invasions [5]. In our study, herbivores reduced APC regardless of taxon and functional traits, while their effects on BPC were much more variable. Indeed, positive, negative and no effects of herbivores on belowground carbon processes in coastal wetlands have all been reported in individual site-scale studies [22,34,35]. Therefore, it is important to consider differential responses of aboveground and belowground carbon processes to food web alterations. Our study highlights the challenge in generalizing and predicting responses of belowground carbon processes.
Our analysis suggests that a trait-based approach can be highly valuable to helping predict variation in the top-down effects of consumers on the ecosystem carbon cycle. Our analysis of top-down effects of herbivores on APC among herbivore taxa of different body sizes supports the long-held view that body size is an important determinant of consumption and top-down effects of consumers [36–38]. However, previous studies on the relationship between body size and top-down control have often focused on either invertebrates or vertebrates, rather than both, and have focused on the effects of consumers on aboveground processes, rather than belowground and soil processes. Our finding that the relationship between body size and top-down effects of herbivores on APC appeared to hold only within but not across invertebrate and vertebrate herbivores suggests that other factors such as herbivore abundance may modulate their top-down effects on APC. Indeed, invertebrate herbivores are often orders of magnitude more abundant than vertebrate counterparts in coastal wetlands [39]. Furthermore, instead of body size, variation in the effects of herbivores on other processes of the ecosystem carbon cycle (such as BPC and ANPP) can be better explained by feeding type. For example, herbivores that feed above- and belowground (e.g. crabs and geese) consistently reduced BPC, while those that feed primarily aboveground (e.g. snails and small mammals) did not affect BPC (figure 3). Additionally, our results confirm that, relative to ectothermic herbivores, endothermic herbivores with energetic maintenance costs [29] may have stronger impacts on APC. But this was not the case for BPC, likely because endothermic herbivores in coastal wetlands are mostly aboveground feeders (except geese). This finding highlights the need for employing multiple functional traits to predict variation in the effects of consumers on aboveground versus belowground processes. It should be stressed that, as shown in our study (electronic supplementary material, tables S2–S5), predicting variation in the effects of consumers on the carbon cycle across different scales may also require incorporation of other variables such as plant and climate variables.
Our synthesis of the existing literature also identifies important gaps in current understanding of consumer control of the carbon cycle in coastal wetlands. First, although large proportions of carbon in coastal wetlands are stored in soils [40], the effect of consumers on belowground carbon processes has received much less attention than aboveground processes. In particular, existing studies on the impacts of consumers on soil carbon processes have focused on the carbon concentration or density of top soil layers, without accounting for the effects of consumers on soil vertical/lateral erosion or accretion (e.g. [41]), or have focused on large-bodied herbivores such as livestock and geese. Whether small invertebrate herbivores that are often abundant in coastal wetlands have differential effects on soil carbon sequestration is still unknown. Second, managing ecosystem carbon stocks requires understanding when, where and how carbon fluxes (e.g. photosynthesis, respiration and NEE; [42]) are affected by consumers. However, the impacts of consumers on carbon fluxes, NEE in particular (a measure of carbon sequestration), have been investigated in only a few studies, and generality and variability remain to be understood. Furthermore, current studies on consumer control of the carbon cycle in coastal wetland ecosystems have been largely focused on herbivores. Predators and omnivores, especially large-bodied top predators, have been widely advocated to be essential for the functioning of coastal marine ecosystems [19,43], but studies that specially investigate the effects of carnivores, omnivores and consumer diversity on the carbon cycle remain few. Although our synthesis focused on coastal wetlands, these knowledge gaps are likely to be general for all types of ecosystems.
Coastal wetlands are highly valued for their carbon storage function, which helps mitigate climate change [40]. Owing to declines in large predator populations, grazing pressure has been elevated in many coastal wetland ecosystems [9]. For example, overfishing has been shown to elevate grazing by herbivorous crabs in New England [44] and by periwinkle snails in the southeastern USA [45]. Our results suggest that elevated grazing pressure may lead to reductions in the amount of carbon stored in coastal wetlands, compromising the capacity of these ecosystems to help mitigate climate change. In fact, in extreme cases, loss of predators can interact with abiotic stressors (e.g. drought and nitrogen enrichment) to lead to runaway grazing by herbivores, complete denuding of coastal wetlands, and massive loss of carbon [14,34,46]. Therefore, to halt further loss of carbon stocks, coastal wetland managers not only need to restore hydrology and vegetation and control pollutant and nutrient input, but also need to be aware of the potential importance of maintaining healthy food webs [3]. The latter could be achieved either directly or indirectly by controlling environmental factors that mediate herbivore and predator populations, wherever appropriate. We encourage future studies to design more specific measures and test how best to manage herbivores and predators across different scales so that the recovery of carbon stocks in degraded or destroyed coastal wetlands can be maximized.
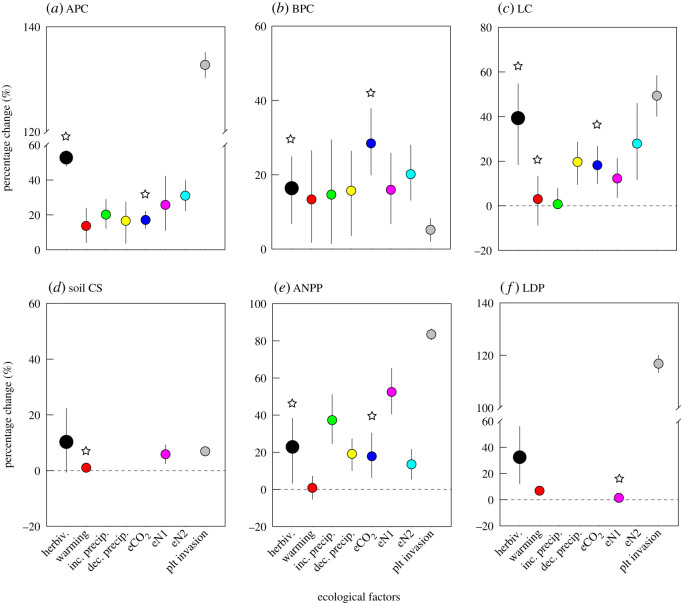
Our finding that consumers strongly affect a series of carbon cycle processes underscores the importance of incorporating consumer control into Earth system models predicting future climate [8]. Current Earth system models are parameterized mainly with physical variables and vegetation properties, and modelling of the carbon cycle mainly considers responses to changes in temperature, precipitation, CO2 concentration and nutrient input [2]. In magnitude (figure 5), many effects of herbivores on the carbon cycle shown in our study, however, are comparable with, even times higher than those of warming, precipitation changes, nitrogen enrichment and even plant invasions (although the effects of plant invasions on APC, ANPP and LDP were much higher than all other ecological factors). Ignoring consumer control in Earth system models can therefore result in considerable deviations of model predictions from empirical observations. Incorporating consumer control into Earth system models, nevertheless, might not be as straightforward as physical variables such as temperature and precipitation. As a kind of biotic interactive process, the effects of consumers on the carbon cycle are often variable with consumer species and functional traits and are also likely to be more responsive to changes in environmental factors (e.g. temperature; [16]). Despite potential challenges, accurate predictions of climate change and ecosystem dynamics cannot be achieved without fully recognizing the profound impacts of consumers and human-driven alterations in food webs on the cycling of carbon in ecosystems. It is hoped that this synthesis will motivate further efforts toward consumer-inclusive predictions of changes in Earth's climate and ecosystems, and mitigation strategies.
Figure 5.
Herbivore control of the carbon cycle in coastal wetlands compared with other ecological factors. Shown are mean percentage changes (and 95% confidence intervals). The star symbols indicate negative mean percentage changes that are shown as positive values to facilitate comparisons of effect strength. The bigger black dots indicate herbivore (herbiv.) effects shown in the present study. Data on warming were extracted from [7] (for APC, BPC, LC and ANPP) and [47] (for soil CS and LDP). Data on increased precipitation (inc. precip.), decreased precipitation (dec. precip.), elevated CO2 (eCO2), and enriched nitrogen (eN2) were also extracted from [7], and those on enriched nitrogen (eN1) and plant invasions (plt invasion) from [33] and [5], respectively. The other abbreviations follow figure 1. (Online version in colour.)
Supplementary Material
Supplementary Material
Acknowledgements
We thank Professor Helmut Hillebrand for the opportunity to contribute this manuscript to Philosophical Transactions B. Our work is supported by the National Natural Science Foundation of China (grant nos 31870414 and 41630528).
Data accessibility
All data supporting this article are included in the electronic supplementary material (data S1).
Authors' contributions
Q.H. and B.R.S. contributed to conception and design; Q.H., H.L., C.X. and Q.S. contributed to acquisition of data, or analysis and interpretation of data; Q.H. drafted the manuscript, and all the authors revised it; and all the authors approved the latest version of the manuscript for publication.
Competing interests
We declare we have no competing interests
References
- 1.Riebesell U, Körtzinger A, Oschlies A. 2009. Sensitivities of marine carbon fluxes to ocean change. Proc. Natl Acad. Sci. USA 106, 20 602–20 609. ( 10.1073/pnas.0813291106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo Y, Keenan TF, Smith M. 2015. Predictability of the terrestrial carbon cycle. Glob. Change Biol. 21, 1737–1751. ( 10.1111/gcb.12766) [DOI] [PubMed] [Google Scholar]
- 3.Macreadie PI, et al. 2017. Can we manage coastal ecosystems to sequester more blue carbon? Front. Ecol. Environ. 15, 206–213. ( 10.1002/fee.1484) [DOI] [Google Scholar]
- 4.Luo Y, Hui D, Zhang D. 2006. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87, 53–63. ( 10.1890/04-1724) [DOI] [PubMed] [Google Scholar]
- 5.Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B. 2008. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol. 177, 706–714. ( 10.1111/j.1469-8137.2007.02290.x) [DOI] [PubMed] [Google Scholar]
- 6.Don A, Schumacher J, Freibauer A. 2011. Impact of tropical land-use change on soil organic carbon stocks – a meta-analysis. Glob. Change Biol. 17, 1658–1670. ( 10.1111/j.1365-2486.2010.02336.x) [DOI] [Google Scholar]
- 7.Song J, et al. 2019. A meta-analysis of 1,119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat. Ecol. Evol. 3, 1309–1320. ( 10.1038/s41559-019-0958-3) [DOI] [PubMed] [Google Scholar]
- 8.Schmitz OJ, et al. 2014. Animating the carbon cycle. Ecosystems 17, 344–359. ( 10.1007/s10021-013-9715-7) [DOI] [Google Scholar]
- 9.Bertness MD, Silliman BR. 2008. Consumer control of salt marshes driven by human disturbance. Conserv. Biol. 22, 618–623. ( 10.1111/j.1523-1739.2008.00962.x) [DOI] [PubMed] [Google Scholar]
- 10.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 11.Calbet A, Landry MR. 2004. Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol. Oceanogr. 49, 51–57. ( 10.4319/lo.2004.49.1.0051) [DOI] [Google Scholar]
- 12.Beaugrand G, Edwards M, Legendre L. 2010. Marine biodiversity, ecosystem functioning, and carbon cycles. Proc. Natl Acad. Sci. USA 107, 10 120–10 124. ( 10.2307/25681740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G, Zhou X, He Y, Shao J, Hu Z, Liu R, Zhou H, Hosseinibai S. 2017. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: a meta-analysis. Glob. Change Biol. 23, 1167–1179. ( 10.1111/gcb.13431) [DOI] [PubMed] [Google Scholar]
- 14.Sjögersten S, van der Wal R, Loonen MJJE, Woodin SJ. 2011. Recovery of ecosystem carbon fluxes and storage from herbivory. Biogeochemistry 106, 357–370. ( 10.2307/41490528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gedan KB, Silliman BR, Bertness MD. 2009. Centuries of human-driven change in salt marsh ecosystems. Annu. Rev. Mar. Sci. 1, 117–141. ( 10.1146/annurev.marine.010908.163930) [DOI] [PubMed] [Google Scholar]
- 16.He Q, Silliman BR. 2016. Consumer control as a common driver of coastal vegetation worldwide. Ecol. Monogr. 86, 278–294. ( 10.1002/ecm.1221) [DOI] [Google Scholar]
- 17.Kelsey KC, Leffler AJ, Beard KH, Schmutz JA, Choi RT, Welker JM. 2016. Interactions among vegetation, climate, and herbivory control greenhouse gas fluxes in a subarctic coastal wetland. J. Geophys. Res. Biogeosci. 121, 2960–2975. ( 10.1002/2016JG003546) [DOI] [Google Scholar]
- 18.Wilmers CC, Estes JA, Edwards M, Laidre KL, Konar B. 2012. Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Front. Ecol. Environ. 10, 409–415. ( 10.1890/110176) [DOI] [Google Scholar]
- 19.Atwood TB, Connolly RM, Ritchie EG, Lovelock CE, Heithaus MR, Hays GC, Fourqurean JW, Macreadie PI. 2015. Predators help protect carbon stocks in blue carbon ecosystems. Nat. Clim. Change 5, 1038–1045. ( 10.1038/nclimate2763) [DOI] [Google Scholar]
- 20.Morris JT, Jensen A. 1998. The carbon balance of grazed and non-grazed Spartina anglica saltmarshes at Skallingen, Denmark. J. Ecol. 86, 229–242. ( 10.1046/j.1365-2745.1998.00251.x) [DOI] [Google Scholar]
- 21.Olsen YS, Dausse A, Garbutt A, Ford H, Thomas DN, Jones DL. 2011. Cattle grazing drives nitrogen and carbon cycling in a temperate salt marsh. Soil Biol. Biochem. 43, 531–541. ( 10.1016/j.soilbio.2010.11.018) [DOI] [Google Scholar]
- 22.Mueller P, Granse D, Nolte S, Do HT, Weingartner M, Hoth S, Jensen K. 2017. Top-down control of carbon sequestration: grazing affects microbial structure and function in salt marsh soils. Ecol. Appl. 27, 1435–1450. ( 10.1002/eap.1534) [DOI] [PubMed] [Google Scholar]
- 23.Davidson KE, Fowler MS, Skov MW, Doerr SH, Beaumont N, Griffin JN. 2017. Livestock grazing alters multiple ecosystem properties and services in salt marshes: a meta-analysis. J. Appl. Ecol. 54, 1395–1405. ( 10.1111/1365-2664.12892) [DOI] [Google Scholar]
- 24.He Q, Silliman BR. 2015. Biogeographic consequences of nutrient enrichment for plant–herbivore interactions in coastal wetlands. Ecol. Lett. 18, 462–471. ( 10.1111/ele.12429) [DOI] [PubMed] [Google Scholar]
- 25.Atwood TB, et al. 2017. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Change 7, 523–528. ( 10.1038/nclimate3326) [DOI] [Google Scholar]
- 26.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. 2011. Introduction to meta-analysis. New York: NY: John Wiley & Sons. [Google Scholar]
- 27.R Development Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 28.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 29.Buckley LB, Hurlbert AH, Jetz W. 2012. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 21, 873–885. ( 10.1111/j.1466-8238.2011.00737.x) [DOI] [Google Scholar]
- 30.Calcagno V, de Mazancourt C. 2010. glmulti: An R package for easy automated model selection with (generalized) linear models. J. Stat. Softw. 34, 1–29. ( 10.18637/jss.v034.i12) [DOI] [Google Scholar]
- 31.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob. Change Biol. 17, 927–942. ( 10.1111/j.1365-2486.2010.02302.x) [DOI] [Google Scholar]
- 32.Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B. 2011. Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agr. Ecosyst. Environ. 140, 234–244. ( 10.1016/j.agee.2010.12.010) [DOI] [Google Scholar]
- 33.Yue K, Peng Y, Peng C, Yang W, Peng X, Wu F. 2016. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: a meta-analysis. Scient. Rep. 6, 19895 ( 10.1038/srep19895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coverdale TC, Brisson CP, Young EW, Yin SF, Donnelly JP, Bertness MD. 2014. Indirect human impacts reverse centuries of carbon sequestration and salt marsh accretion. PLoS ONE 9, e93296 ( 10.1371/journal.pone.0093296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harvey RJ, Garbutt A, Hawkins SJ, Skov MW. 2019. No detectable broad-scale effect of livestock grazing on soil blue-carbon stock in salt marshes. Front. Ecol. Evol. 7, 151 ( 10.3389/fevo.2019.00151) [DOI] [Google Scholar]
- 36.Shackell NL, Frank KT, Fisher JAD, Petrie B, Leggett WC. 2010. Decline in top predator body size and changing climate alter trophic structure in an oceanic ecosystem. Proc. R. Soc. B 277, 1353–1360. ( 10.1098/rspb.2009.1020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legagneux P, et al. 2014. Arctic ecosystem structure and functioning shaped by climate and herbivore body size. Nat. Clim. Change 4, 379–383. ( 10.1038/nclimate2168) [DOI] [Google Scholar]
- 38.Gianuca AT, Pantel JH, De Meester L. 2016. Disentangling the effect of body size and phylogenetic distances on zooplankton top-down control of algae. Proc. R. Soc. B 283, 20160487 ( 10.1098/rspb.2016.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenberg R, Maldonado JE, Droege SAM, McDonald MV. 2006. Tidal marshes: a global perspective on the evolution and conservation of their terrestrial vertebrates. BioScience 56, 675–685. ( 10.1641/0006-3568(2006)56675:TMAGPO.2.0.CO;2) [DOI] [Google Scholar]
- 40.Spivak AC, Sanderman J, Bowen JL, Canuel EA, Hopkinson CS. 2019. Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nat. Geosci. 12, 685–692. ( 10.1038/s41561-019-0435-2) [DOI] [Google Scholar]
- 41.Kirwan ML, Murray AB, Boyd WS. 2008. Temporary vegetation disturbance as an explanation for permanent loss of tidal wetlands. Geophys. Res. Lett. 35, L05403 ( 10.1029/2007GL032681) [DOI] [Google Scholar]
- 42.Schlesinger WH, Andrews JA. 2000. Soil respiration and the global carbon cycle. Biogeochemistry 48, 7–20. ( 10.1023/a:1006247623877) [DOI] [Google Scholar]
- 43.Hammerschlag N, et al. 2019. Ecosystem function and services of aquatic predators in the Anthropocene. Trends Ecol. Evol. 34, 369–383. ( 10.1016/j.tree.2019.01.005) [DOI] [PubMed] [Google Scholar]
- 44.Altieri AH, Bertness MD, Coverdale TC, Herrmann NC, Angelini C. 2012. A trophic cascade triggers collapse of a salt-marsh ecosystem with intensive recreational fishing. Ecology 93, 1402–1410. ( 10.1890/11-1314.1) [DOI] [PubMed] [Google Scholar]
- 45.Silliman BR, Bertness MD. 2002. A trophic cascade regulates salt marsh primary production. Proc. Natl Acad. Sci. USA 99, 10 500–10 505. ( 10.1073/pnas.162366599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silliman BR, Van De Koppel J, Bertness MD, Stanton LE, Mendelssohn IA. 2005. Drought, snails, and large-scale die-off of southern US salt marshes. Science 310, 1803–1806. ( 10.1126/science.1118229) [DOI] [PubMed] [Google Scholar]
- 47.Lu M, Zhou X, Yang Q, Li H, Luo Y, Fang C, Chen J, Yang X, Li B. 2013. Responses of ecosystem carbon cycle to experimental warming: a meta-analysis. Ecology 94, 726–738. ( 10.1890/12-0279.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting this article are included in the electronic supplementary material (data S1).