Abstract
Acoustic approaches have been recently proposed to investigate critical ecological issues, such as biodiversity loss and different typologies of impacts, including climate change. However, the extensive use of acoustic monitoring is hampered by the lack of algorithms enabling the discrimination among different sound sources (e.g. geophysical, anthropogenic and biological). Eco- and bioacoustic indexes have been applied to provide non-invasive information on the temporal and spatial patterns of marine biodiversity and on the anthropogenic impact on marine life. Here, we review the potential of acoustic tools in expanding the monitoring of marine ecosystems from a current three-dimensional perception to a four-dimensional dimension. We also explore the use of acoustic indexes, mostly developed in terrestrial ecology, for the investigation of different marine ecosystems. Their appraisal, strengths and limits, and potential for future investigations in the biological exploration of the oceans are also discussed.
This article is part of the theme issue ‘Integrative research perspectives on marine conservation’.
Keywords: acoustic indexes, ecoacoustics, soundscape ecology, marine biodiversity, ecosystem health
1. Background
The ocean hosts a huge number of diverse and unique ecosystems, which are under an unprecedented threat owing to increasing direct and indirect anthropogenic pressures [1] (i.e. overfishing, climate change, ocean acidification, alien species, habitat destruction and degradation) [2]. A dramatic decline of abundance, biomass, size and biodiversity have already been documented in several exploited systems worldwide [3], but an extensive knowledge on the implications in terms of ecosystem services is still lacking and requires careful quantification [3,4]. The increasing concern on these issues has led to calls for collectively gathering standardized baseline data and acquiring additional and complementary information on biodiversity trends and ecological status of marine ecosystems [5]. However, most of the traditional approaches are invasive and limited to a short sampling time frame (snapshot sampling), which limit the typology and complexity of the information collected [4]. Innovative marine biodiversity indicators and monitoring approaches have been recently developed, spanning from molecular approaches, remote sensing and acoustic methodologies to in situ monitoring instruments [4]. These new tools provide additional insights on the biodiversity and on some specific aspects of ecosystem functioning. In this context, passive acoustic monitoring (PAM) is a non-invasive method for studying marine organisms in their natural environment, without the introduction of any interference or stressing factor. Owing to its high propagation in seawater, the sound is often detectable at much longer distances than other signals of biological activity (i.e. light, chemical cues) by travelling at about 1500 m per second and potentially propagating for hundreds of kilometres, as in the case of low-frequency communication in baleen whales [6]. Besides marine mammals, several other marine species produce sounds (e.g. fishes, crustaceans and other invertebrates) in relation to their behavioural and bio-ecological functions (e.g. communication, mating, defending territories) [7]. Animals depend on ambient sounds as a source of environmental cues for achieving several needs, such as foraging, navigation, predation (or detection of predators or competitors) and habitat selection [8,9]. Sounds are therefore a fundamental component of the ecosystem functioning and can be used as a proxy for assessing species presence/absence, abundance and, given the impact of anthropogenic activities on several life forms, also to assess marine environmental health [10].
Here, we summarize the current status of scientific knowledge on acoustic methodologies used to date for the automatic extraction of relevant biological information from long-term and/or large-scale acoustic datasets.
(a). Sound as a measure of biodiversity
PAM has recently emerged as a promising solution for biodiversity assessment, as this is a cost-effective tool enabling the achievement of accurate assessment of soniferous species, even at large spatial scales. By deploying acoustic sensors and processing the recorded sounds, researchers can recognize species-specific calls, either manually (e.g. [11]) or automatically (e.g. [12]). These analyses can also allow the discovery of cryptic species, which produce species-specific sounds and are overlooked by visual census surveys [13]. PAM has many advantages, including the minimal disturbance caused by operators during the sampling phase, and can be of particular interest for biodiversity exploration in remote environments, which are difficult to access and monitor using classical monitoring approaches [14–16]. Moreover, sound records can be stored and serve as archives of the habitat sounds enabling comparisons over time [17]. Although accurate studies on marine mammals have revealed their complete acoustic repertoire [18], many biological sounds of fishes and other invertebrates cannot be easily associated with a given species. Consequently, biological sounds are assigned to species only when there is certainty of the origin (e.g. experimental approaches) and unicity of the acoustic features. Otherwise sounds are classified in sound type categories on the basis of their acoustic characteristics, which allows the evaluation of the acoustic diversity [19].
(b). Sounds measuring ecosystem functions
An emerging research field known as ecoacoustics [20] considers environmental sounds for the exploration of the functional biodiversity and as an indicator of the ecological processes that determine the distribution of species. This association emerges when we expand the focus of acoustic analyses from single-species sound production, to all sound sources (i.e. biological, anthropogenic or oceanographic/geophysical sounds) present in the environment (i.e. the soundscape [10,21,22]). Ecoacoustics is based on the rationale that some key ecological processes are strictly linked to the patterns of sounds [20,23]. Environmental sounds are explored at a broader scale to gather data on the ecology of populations, communities and ecosystems [20]. Recent research has proven that sounds can be effectively used as indicators of ecosystem functions for their ability to assess environmental quality [24–27]. Some studies have reported significant differences among biological sounds of different habitats, which reveal a spatial heterogeneity and could serve as indicators of habitat type [25,28,29]. Other authors have postulated that climate change is shifting the sounds of our environments [30], and sounds have been proposed as a proxy to measure the effects of changes on biological populations and assemblages. A reduction in frequency and intensity of sounds produced by the world's noisiest marine invertebrate (the snapping shrimp, Alpheus sp.) was associated with elevated CO2 concentrations under an ocean acidification scenario forecasted for the end of the century [26].
(c). Large-scale and long-term surveys
Over the past two decades, investigations based on ecoacoustics have been prompted by the development of new digital and autonomous audio recorders. Fixed, programmable acoustic sensors can be left unattended in the field to record uninterruptedly 24/7 for prolonged periods (i.e. months or years). In addition, the significant reduction in the costs of this equipment enables the acquisition of large numbers of these instruments, which can thus be simultaneously placed in a variety of habitats. As an example, studies conducted over an annual cycle on an Antarctic iceberg during its drift and disintegration provided insights into the seasonal variations in ocean noise at different locations of the Southern Hemisphere [27]. Antarctic blue whales' occurrence and behaviour were modelled via the use of 586 sonobuoys deployed in the austral summers of 1997 through 2009 [31]. Cabled and standalone observatories in the deep sea are the object of the international program LIDO (‘Listen to the Deep Ocean Environment’) aiming at the long-term monitoring of marine sounds [14].
This long-term and large-scale application of the acoustic approach inevitably produces a massive amount of information, with associated difficulties in data management and analysis [32]. Direct inspection of recordings, both visual and/or aural, to annotate signals of the target species represents a labour-intensive and time-consuming procedure that can only be carried out for small datasets. The development of automated tools (i.e. acoustic indexes) is thus a priority for future acoustic monitoring, and the pursuit of appropriate metrics to extract informative data from vast acoustic surveys is nowadays considered a highly relevant field for future marine research.
2. Acoustic indexes
Low-cost and quick tools are nowadays available for the automated analyses of acoustic datasets from marine environments. They include (i) detection and classification metrics to disentangle signals of a target species from other sounds (or background noise)(e.g. [33–35]), (ii) measures of the intensity of sounds produced by marine communities (e.g. [36,37]) and (iii) algorithms designed for direct quantification of the acoustic diversity within a single community or comparison among diverse communities (e.g. [38,39]). Here, we set out a systematic search by compiling all the pertinent literature from the Scopus database from 1950 to October 2019. The following criteria had to be met for retaining articles in the review: (i) studies must have dealt with automated procedures aimed at inferring information on marine species or communities and (ii) studies must have been carried out on recordings performed in marine environments (hence excluding freshwater ecosystems, tank experiments or assessments performed on acoustic files created artificially). All papers that manually counted biological sounds within recordings rather than using indexes were discarded. We reviewed the resulting publications and annotated for each study if a bioacoustic or ecoacoustic index was used, the target (entire community, marine mammals, fishes, crustaceans or others) and the geographical location. For the ecoacoustic indexes, we also detailed the type of index, the environmental setting and the depth of deployment of the hydrophone.
(a). Bioacoustic and ecoacoustic indexes
We retrieved a total of 210 studies that were divided into bioacoustic indexes (hereafter named Bio-AIs) and ecoacoustic indexes (hereafter named Eco-AIs).
What differentiates bioacoustics from ecoacoustics is that the former is largely related to species-centred investigations, which commonly focus on a single or a few species [38]. Bioacoustics mostly investigates sound for the transfer of information at a specific level and is usually assimilated to the science of animal behaviour and not to ecology [20,38]. Accordingly, Bio-AIs have mostly been applied for the detection of single signals (i.e. calls, vocalization, echolocation trains and clicks) in a specific type of noise [33]. A plethora of tools from pattern to voice recognition has been developed to build signal detectors relative to a single species or group of affiliate species [35,40–43]. Automatic detection of biological signals at higher organization levels (communities) has been rarely pursued [12,44] owing to difficulties in dealing with other sources of sounds (anthropogenic or geophysical noise).
On the other hand, ecoacoustics focuses on a higher level structure by investigating sounds produced by entire communities. It approaches all the sound sources (the soundscape) as a means conveying important information about the ecological status of ecosystems [20]. Rather than attempting to recognize species-specific calls, Eco-AIs tackle the problem of diversity assessment at the community (rather than species) level or use spatio-temporal changes of sounds to estimate alterations of the ecosystem functionality [10,20,23,38]. This is achieved by sampling at large observational scales (wide-scale or long-term surveys) and by using automated algorithms conceived to extract the intensity, diversity or complexity of biological sounds [38]. Eco-AIs are commonly meant to operate on all the sounds of an environment but can work on smaller portions of it (i.e. frequency bands) where target species are known to vocalize. This step often becomes necessary when a reduction in the interference of other biological or non-biological sounds is required to obtain informative ecological data. Ecoacoustics also comprehends investigations on a single population when this is done for prolonged periods, or in dense spatial samplings [20].
(b). Past studies: trends, target and biogeography
Scientists began to listen to underwater sounds in the late 1800s [45] and found the first practical application in the 1900s, particularly for war uses [46]. First studies describing biological underwater sounds were published in the mid-1900s [47,48] but investigations on geographical or temporal patterns came only after. Few studies in the 1970s measured the contribution to soundscapes of biological sounds [49,50] by observing circadian and seasonal trends of biological choruses of marine species. Passive acoustics became an important technique for environmental monitoring just after the early 1990s, when technological implementations and cost reduction rendered autonomous acoustic devices more affordable [46]. Subsequently, the technical challenge has been to develop acoustic methods for the automated measurement/detection of underwater sounds, and studies proposing metrics for the analysis of sounds gradually emerged (figure 1a). Ecoacoustic indexes received particular interest after the meeting in Paris in 2014 ‘Ecoacoustics: ecology and acoustics, emergent properties from community to landscape’ where, for the first time, environmental sounds were acknowledged as a means for ecological investigations [20]. After the conference, the application of Eco-AIs in marine environments risen considerably, passing from three studies published in 2014 to 21 studies in 2016.
Figure 1.
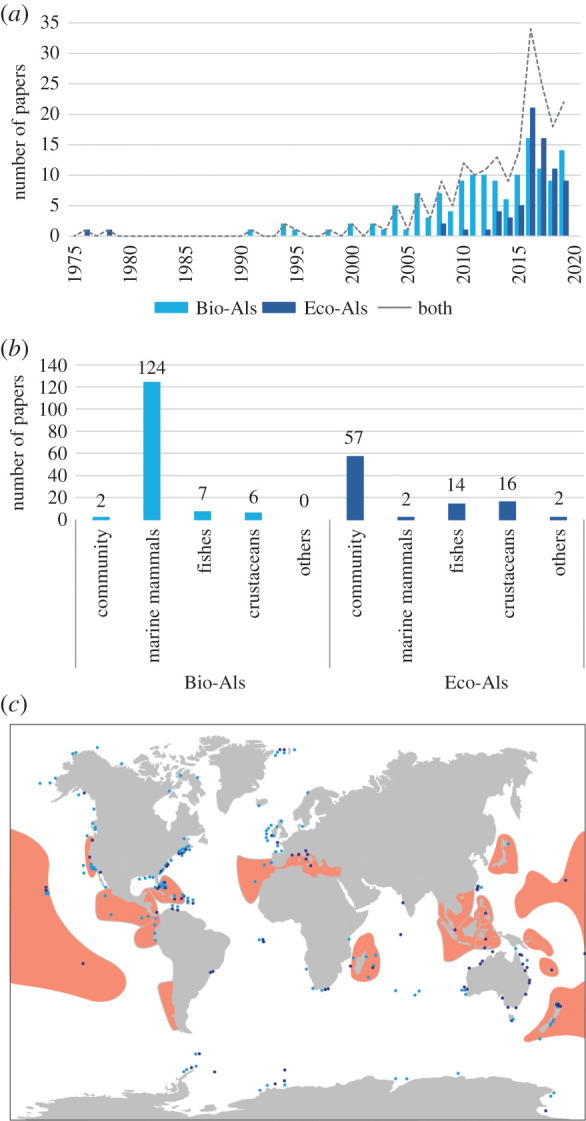
Number of scientific papers published per year related to Bioacoustic (light blue) and Ecoacoustic indexes (dark blue) as sourced from a systematic literature search on Scopus: yearly trend (a), biological target (b) and schematic geographical localization (c). The world map shows the `biodiversity hotspots' as indicated by Myers et al. [51] and revisited by Conservation International [52].
The target of studies that used Bio-AIs and Eco-AIs clearly reflects the main questions they were born to answer. Bio-AIs were mostly conceived for investigating marine mammal communication and behaviour (figure 1b). By contrast, studies using Eco-AIs commonly targeted the community (figure 1b), sometimes pointing at specific frequency bands known to be covered by selected groups of species (i.e. 0–2000 Hz for fishes; 2000–8000 Hz for snapping shrimps; 800–2000 Hz for sea urchins). Eco-AIs rarely focused on marine mammals' sounds, probably owing to the number of Bio-AIs that already covered that topic.
We plotted the geographic locations of these studies and compared their distribution with the ‘biodiversity hotspots’ map provided by [51] and revisited by Conservation International [52] (figure 1c). Studies using Bio-AIs were mainly located along breeding grounds or migration routes of cetaceans and were particularly dense in North America, both on the Western and Eastern side. Ecoacoustics studies particularly occurred in the Eastern North American side, Australia and New Zealand; they were more equally spaced and better-covered hotspot areas all around the globe. Despite the impellent need to prioritize monitoring in the hotspots because of the exceptional concentrations of endemic species and high biodiversity levels, acoustic indexes have never been applied in some of them, namely the Mesoamerica, the Melanesia Islands or Indo-Burma Hotspots.
(c). Most used indexes and fields of applications
The most widely used Eco-AIs were the so-called intensity indexes (for reference, see [38,53]) (figure 2a). These indexes provide simple quantitative or statistical summaries of the acoustic energy of the environmental sounds according the frequency or the time domain. A summary measure known as sound pressure level (SPL, dB re 1 µPa) is commonly applied as a method for representing the broadband or in-band time-varying acoustic energy levels at a receiver. Intensity indexes were often used in terms of the statistical distribution of sound pressure levels in each 1/3 octave band (TOLs) or power spectral density (PSD), which describe the power present in the soundscape at each frequency unit. These indexes have been extensively used in ecoacoustics for creating plots of hourly, daily and seasonal rhythms. Comparisons over time and across multiple habitats allowed researchers to quantify for changes in rhythm and frequency of sounds that were associated with the ecological status of the ecosystems [37,50,54–56]. Unfortunately, intensity indexes can provide reliable information on biological sounds only in non-noisy locations with calm sea state. Noise from human activities or geophonic sounds (i.e. wind, waves, earthquakes) would strongly affect their results, biasing eventual considerations on marine fauna acoustic signals. This aspect led researchers to restrict their analysis to optimal weather conditions [39,54] or to manually delete files in which noise from shipping was detected [44,57]. Alternatively, intensity indexes can be used in restricted frequencies or temporal windows in order to minimize the influence of out-of-target sources of sounds [58].
Figure 2.
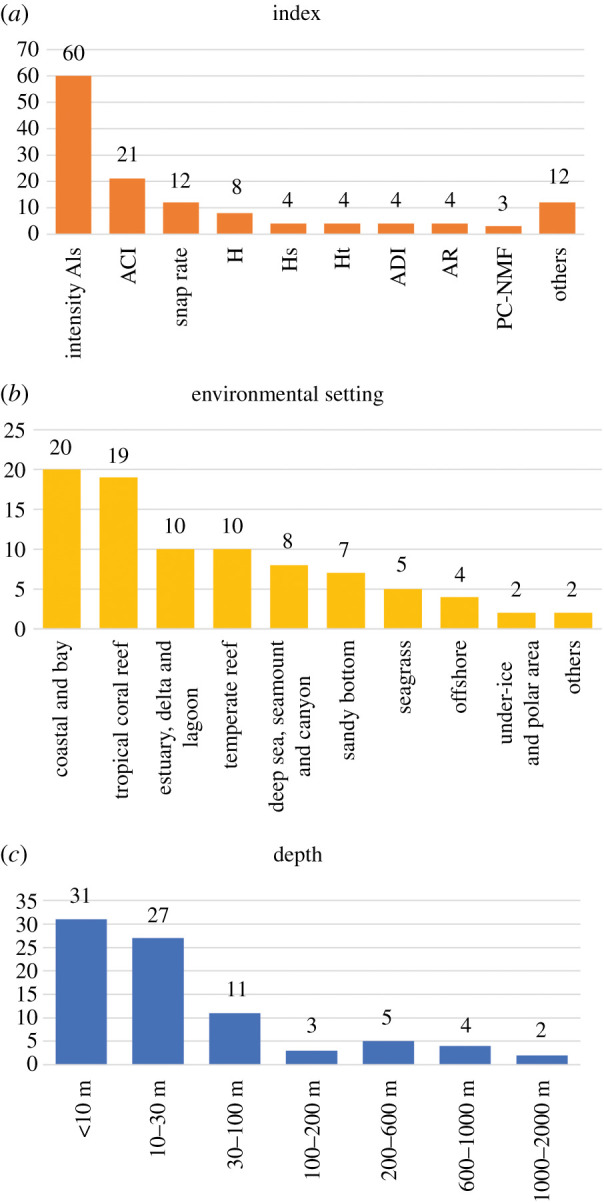
Number of scientific papers published per year involving Eco-AIs divided by (a) index, (b) environmental setting and (c) depth of deployment of the hydrophone. The number above each bar is the number of publications for that class. ACI, acoustic complexity index; H, acoustic entropy; Hs, spectral entropy; Ht, temporal entropy; ADI, acoustic diversity index; AR, acoustic richness; PC-NMF, periodicity coded non-negative matrix factorization.
The second mostly used Eco-AI is the acoustic complexity index (ACI) [59] (figure 2a), an automated metric to describe the biotic contribution to the soundscape. The ACI is an acoustic energy differential algorithm that has recently revealed its utility to track biological pulsed signals, such as snapping and fish calls, being scarcely affected by continuous noise (i.e. vessel passage and wave noise) [39,60]. Investigations found ACI to be a reliable metric for describing biological diversity and habit quality in reef fish communities [61] or to be correlated with key ecosystem functions [62]. It was suggested as the metric that better performed to discriminate species richness in the Southern Ocean [63] and was directly correlated with fish and snapping shrimp sound production in a temperate rocky reef or inshore waters [60,64]. Conversely, other studies could not find clear relationships with fish assemblages [65,66], tonal signals of fishes [60] and proper identification of the peak period of dense fish chorusing [39,58]. The latter was recently solved by new promising Eco-AIs, i.e. the periodicity coded non-negative matrix factorization (PC-NMF) [67] and the complexity-entropy approach (C-H) [58]. Both approaches effectively enhanced biological choruses in recordings containing environmental or anthropogenic noise without requiring training data.
Snapping shrimps (family Alpheidae) produce snaps that are the most common source of marine biological sounds in coastal systems [68,69]. Researchers attempted to produce automated snap detection procedures creating kernels for sound detection (in this review considered as Bio-AIs) [69], or by simply setting a threshold level on the raw data and counting any transient spike that overpassed it (here considered as Eco-AIs) [70]. Measures of frequency and loudness of snaps have been recently applied to determine potential relationships with fish community [66], regime shifts generated by nutrient pollution and ocean acidification [26,71], correlation with diel, lunar and seasonal periodicities [60,70] and to estimate habitat quality or differences between sites [29,64,72]. Numbers of snaps were also found to be significantly reduced in degraded coral reefs [73]. Based on these first pieces of evidence, the snap rate was suggested as an effective ecological indicator for coastal or hard bottom settings.
Other diversity and complexity indexes were explored in marine ecoacoustics studies: the acoustic entropy (H)—with relative subcomponents: spectral entropy (Hs) and temporal entropy (Ht) [74], the acoustic diversity index (ADI) [75] and acoustic richness (AR) [76]. The application of these indexes has resulted in mixed outcomes, sometimes owing to the influence of noise. In particular, the entropy index (H) measures the spectral and temporal entropy of sounds by applying Shannon's Index [74]. H was found to be an appropriate proxy for biodiversity assessment on temperate reefs [61] and to accurately reflect changes in the acoustic activity of biological sounds when loud sources of sound were removed (i.e. seismic airgun surveys) [77]. Adversely, H decreased with sustained fish chorusing [78].
To date, Eco-AIs have been applied in a variety of environmental settings, but few of them have been explored with more detail (i.e. coastal areas, tropical reefs, estuaries) (figure 2b). In fact, most of the studies were carried out in shallow waters within 30 m, while deep-sea habitats were commonly neglected. No Eco-AIs were tested on sounds recorded below 1400 m. Although available technologies allow the recording in the deep sea, logistical constraints (ship time availability) and the high costs limit the number of these studies. Moreover, since deep-sea investigations are generally focused on marine mammals, Bio-AIs targeted on cetaceans were preferred. Intensity indices have been also applied, but mainly as indicators for ambient noise levels. However, recent studies have suggested that several deep-sea fish species might produce sounds, as well as many invertebrates, at depths greater than 700 m [79]. Since knowledge on these sounds is scarce, manual detection and supervised analysis are needed first. Eco-AIs could be applied in the future to help in assessing acoustic patterns of deep-sea biological sounds.
3. Anthropogenic noise impact: the potential of acoustic analyses
Beside biological sounds, PAM also offers the possibility to track marine noise at relevant spatial and temporal scales and its impact on ecosystems [17]. Underwater noise is associated with several human activities (i.e. shipping, sonar systems, dredging and drilling operations, seismic surveys, and cabling). The alteration of the acoustic environment by anthropogenic noise is one of the environmental changes to consider in the monitoring of marine ecosystem health. Several studies have shown that noise has detrimental effects on a number of marine organisms and ecosystems [80,81]. Monitoring programs such as those enforced in Europe through the Marine Strategy Framework Directive (MSFD) and in USA through the Ocean Noise Reference Station Network contemplate the acoustic monitoring for the assessment of marine environmental quality. They aim at defining baseline data and setting long-term PAM stations, which are an essential step to measure the trend and impact of noise over time [81]. Noise monitoring is specifically required to ensure that impact of the acoustic energy introduced by human activities remains below detrimental thresholds.
Noise creates a sort of ‘acoustic fog’ that masks natural sounds, impeding the ecological functions connected to them. From an analytical point of view, it affects the proper functioning of several Eco-AIs. Noise-resilient Eco-Ais are necessary in order to effectively track biological sound patterns. On the other hand, intensity indexes used in ecoacoustics studies (i.e. SPL) were initially created for the purpose of monitoring noise levels across different sites and years [53]. By coupling data on noise levels with biological sounds, Eco-AIs could provide important insights into the health of a habitat, as well as potential stressors in the case of anthropogenic noise [82].
4. Monitoring biodiversity and acoustic trends
Since monitoring trends in biodiversity is nowadays considered as an essential, but still unsolved matter, Eco-AIs have been developed as a relatively cheap and quick method for their indirect evaluation. Instead of focusing on single-species classification or species richness, the monitoring of marine sounds over time or across similar habitats could effectively constitute an indicator of the ecological status of marine communities. Scientists now have access to unprecedented recording equipment that can store long-term data allowing the opportunity of understanding where the abundance and diversity of biological sounds are, and how they are changing over space and time. Understanding normal levels of variation in biological sounds' abundance and diversity could be fundamental for guiding conservation efforts, enabling managers to decide whether changes in acoustic dynamics warrant further investigation [32]. The assessment of daily and seasonal temporal acoustic fluctuations of marine communities could highlight possible anomalies and identify the drivers responsible for such fluctuations/changes.
Bio-AIs applied to long-term or wide-scale studies (42 studies in this review) could complement Eco-AIs measurements. When acoustic repertoires of animals are known, as for many baleen whale species, Bio-AIs can add important insights on distribution and abundance of keystone species [83].
5. Challenges, knowledge gaps and future directions
In marine environments, the main challenge for assessing acoustic shifts from normal soundscape fluctuations is the scarcity or lack of historic baseline data, which hampers the possibility of statistical comparisons with past records. Similarly to noise monitoring, determining solid and multiyear baseline datasets is mandatory in order to build reference acoustic patterns from which changes and trends can be deduced in the future. Every ecosystem has its own ‘spectrum of life forms’ that creates its peculiar acoustic signature across circadian and seasonal rhythms. Consequently, baseline studies should cover a wide array of environments and describe the variability of their fluctuations in relation to the local threats owing to anthropogenic activities. Several marine ecosystems still lack any acoustic information and need to be investigated in the future, particularly deep-sea habitats (figure 2b,c), but also biodiversity hotspots in shallow tropical basins. It would thus be of extreme relevance to provide acoustic baseline data for highly human-impacted areas (see map reported by [1]). These areas are undergoing the effect of multiple anthropogenic drivers of ecological change and Eco-AIs could provide an effective tool to measure the worsening of ecological conditions or the progress toward the achievement of the conservation goals.
Climate change, habitat degradation, colonization of alien species or overexploitation are all threats to biodiversity that can be investigated through an acoustic approach. Shifts in the phenology of plant and animal populations can result in cascading effects on the timing of sound production linked to diverse ecological functions (i.e. reproduction, spawning, predation or migration). Habitat degradation, eutrophication events or overexploitation can produce shifts in the abundance of sounds of fishes and other species. Alien species could likely be identified by the sudden presence of sound types or choruses that were previously not recorded.
There is growing evidence that Eco-AIs show great promise for the automated analysis of vast acoustic surveys. A wide-scale data analysis inevitably leads to marine populations being better understood and more effectively managed. However, most Eco-AIs were originally created for terrestrial ecological studies and successively directly applied on marine ecosystems. Even if this has led to successful outcomes, marine biological sounds differ from the terrestrial ones in acoustic features and propagation. They considerably overlap in frequencies with anthropogenic noise and can form continuous choruses, which makes it difficult to disentangle them from the rest of the sound sources for terrestrial AIs. The development, testing and application of new indexes specifically targeted to the detection of marine biological sounds should be prioritized to overcome the limitations of the terrestrial-derived ones.
Large datasets and baseline evaluations on current and new developed Eco-AIs' potentialities and limitations are essential. The development of any Eco-AI should go through a solid comparison with biological sounds to provide strong evidence that it is a reliable indicator of the sounds of the community or targeted acoustic phenomenon. Since geographical and seasonal variations may occur, the validation of the index should be considered accomplished only if carried out on a long-term dataset and on a critical number of studies. Different settings of the indexes might produce different outcomes, so guidelines and experience-based protocols would therefore need to be developed for Eco-AIs surveys to ensure efficient measurement of biological sounds. All indexes have their strengths and weaknesses and this needs to be recognized in data analysis, especially when dealing with marine biodiversity. To analyse trends in the abundance, distribution and diversity of natural sounds, the simultaneous use of diverse Eco-AIs (with additional Bio-AIs) would certainly provide complementary information. Additional studies from a variety of environments are still needed to identify the most effective combination of indexes to measure the marine biodiversity changes/trends. Information provided by Eco-AIs can also support other conventional underwater survey methods, by generating a more in-depth understanding of the ecological health of underwater habitats.
Acknowledgements
This work was financially supported by the DG ENV project IDEM (Implementation of the MSFD to the Deep Mediterranean Sea; contract EU No 11.0661/2017/750680/SUB/EN V.C2).
Data accessibility
This article has no additional data.
Authors' contributions
N.P. conceived and carried out the study and drafted the manuscript. R.D. critically revised and helped to draft the manuscript. Both authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
References
- 1.Halpern BS, et al. 2008. A global map of human impact on marine ecosystems. Science 319, 948–952. ( 10.1126/science.1149345) [DOI] [PubMed] [Google Scholar]
- 2.Küpper FC, Kamenos NA. 2018. The future of marine biodiversity and marine ecosystem functioning in UK coastal and territorial waters (including UK Overseas Territories) – with an emphasis on marine macrophyte communities. Bot. Mar. 61, 521–535. ( 10.1515/bot-2018-0076) [DOI] [Google Scholar]
- 3.Luypaert T, Hagan JG, McCarthy ML, Poti M. 2020. Status of marine biodiversity in the Anthropocene. In YOUMARES 9 - The oceans: Our research, Our future: proceedings of the 2018 conference for YOUng MArine RESearcher in oldenburg, Germany (eds Jungblut S, Liebich V, Bode-Dalby M), pp. 57–82. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 4.Danovaro R, et al. 2016. Implementing and innovating marine monitoring approaches for assessing marine environmental status. Front. Mar. Sci. 3, 213 ( 10.3389/fmars.2016.00213) [DOI] [Google Scholar]
- 5.Costello MJ, et al. 2017. Methods for the study of marine biodiversity. In The GEO handbook on biodiversity observation networks (eds Walters M, Scholes RJ), pp. 129–163. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 6.Ainslie MA. 2010. Propagation of underwater sound. In Principles of sonar performance modelling (ed. Ainslie M.), pp. 439–512. Berlin, Germany: Springer. [Google Scholar]
- 7.Lindseth AV, Lobel PS. 2018. Underwater soundscape monitoring and fish bioacoustics: a review. Fishes 3, 36 ( 10.3390/fishes3030036) [DOI] [Google Scholar]
- 8.Barth P, Berenshtein I, Besson M, Roux N, Parmentier E, Banaigs B, Lecchini D. 2015. From the ocean to a reef habitat: how do the larvae of coral reef fishes find their way home. VIE MILIEU-LIFE Environ. 95, 91–100. [Google Scholar]
- 9.Simpson SD. 2005. Homeward sound. Science 308, 221 ( 10.1126/science.1107406) [DOI] [PubMed] [Google Scholar]
- 10.Pijanowski BC, Villanueva-Rivera LJ, Dumyahn SL, Farina A, Krause BL, Napoletano BM, Gage SH, Pieretti N. 2011. Soundscape ecology: the science of sound in the landscape. BioScience 61, 203–216. ( 10.1525/bio.2011.61.3.6) [DOI] [Google Scholar]
- 11.Ruppé L, Clément G, Herrel A, Ballesta L, Décamps T, Kéver L, Parmentier E. 2015. Environmental constraints drive the partitioning of the soundscape in fishes. Proc. Natl Acad. Sci. 112, 6092–6097. ( 10.1073/pnas.1424667112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bittencourt L, Barbosa M, Secchi E, Lailson-Brito J, Azevedo A. 2016. Acoustic habitat of an oceanic archipelago in the Southwestern Atlantic. Deep Sea Res. Part Oceanogr. Res. Pap. 115, 103–111. ( 10.1016/j.dsr.2016.06.001) [DOI] [Google Scholar]
- 13.Picciulin M, Kéver L, Parmentier E, Bolgan M. 2019. Listening to the unseen: passive acoustic monitoring reveals the presence of a cryptic fish species. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 202–210. ( 10.1002/aqc.2973) [DOI] [Google Scholar]
- 14.André M, van der Schaar M, Zaugg S, Houégnigan L, Sánchez AM, Castell JV. 2011. Listening to the deep: live monitoring of ocean noise and cetacean acoustic signals. Mar. Pollut. Bull. 63, 18–26. ( 10.1016/j.marpolbul.2011.04.038) [DOI] [PubMed] [Google Scholar]
- 15.Baumann-Pickering S, Trickey JS, Wiggins SM, Oleson EM. 2016. Odontocete occurrence in relation to changes in oceanography at a remote equatorial Pacific seamount. Mar. Mammal Sci. 32, 805–825. ( 10.1111/mms.12299) [DOI] [Google Scholar]
- 16.Erbe C, Verma A, McCauley R, Gavrilov A, Parnum I. 2015. The marine soundscape of the Perth Canyon. Prog. Oceanogr. 137, 38–51. ( 10.1016/j.pocean.2015.05.015) [DOI] [Google Scholar]
- 17.Van Parijs S, Clark C, Sousa-Lima R, Parks S, Rankin S, Risch D, Van Opzeeland I. 2009. Management and research applications of real-time and archival passive acoustic sensors over varying temporal and spatial scales. Mar. Ecol. Prog. Ser. 395, 21–36. ( 10.3354/meps08123) [DOI] [Google Scholar]
- 18.Erbe C, Dunlop R, Jenner KCS, Jenner M-N, McCauley RD, Parnum I, Parsons M, Rogers T, Salgado-Kent C. 2017. Review of underwater and in-air sounds emitted by Australian and Antarctic marine mammals. Acoust. Aust. 45, 179–241. ( 10.1007/s40857-017-0101-z) [DOI] [Google Scholar]
- 19.Desiderà E, Guidetti P, Panzalis P, Navone A, Valentini-Poirrier C-A, Boissery P, Gervaise C, Di Iorio L. 2019. Acoustic fish communities: sound diversity of rocky habitats reflects fish species diversity. Mar. Ecol. Prog. Ser. 608, 183–197. ( 10.3354/meps12812) [DOI] [Google Scholar]
- 20.Sueur J, Farina A. 2015. Ecoacoustics: the ecological investigation and interpretation of environmental sound. Biosemiotics 8, 493–502. ( 10.1007/s12304-015-9248-x) [DOI] [Google Scholar]
- 21.Farina A, Pieretti N. 2012. The soundscape ecology: a new frontier of landscape research and its application to islands and coastal systems. J. Mar. Isl. Cult. 1, 21–26. ( 10.1016/j.imic.2012.04.002) [DOI] [Google Scholar]
- 22.Krause B. 1987. Bioacoustics, habitat ambience in ecological balance. Whole Earth Rev. 57, 14–18. [Google Scholar]
- 23.Risch D, Parks S. 2017. Overview: Ecoacoustics for monitoring freshwater and marine biodiversity. J. Acoust. Soc. Am. 141, 3937–3937. ( 10.1121/1.4988911) [DOI] [Google Scholar]
- 24.Lillis A, Eggleston DB, Bohnenstiehl DR. 2013. Oyster larvae settle in response to habitat-associated underwater sounds. PLoS ONE 8, e79337 ( 10.1371/journal.pone.0079337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertucci F, Parmentier E, Berten L, Brooker RM, Lecchini D. 2015. Temporal and spatial comparisons of underwater sound signatures of different reef habitats in Moorea Island, French Polynesia. PLoS ONE 10, e0135733 ( 10.1371/journal.pone.0135733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi T, Connell SD, Nagelkerken I. 2016. Silent oceans: ocean acidification impoverishes natural soundscapes by altering sound production of the world's noisiest marine invertebrate. Proc. R. Soc. B 283, 20153046 ( 10.1098/rspb.2015.3046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto H, Bohnenstiehl DR, Tournadre J, Dziak RP, Haxel JH, Lau T-KA, Fowler M, Salo SA. 2014. Antarctic icebergs: a significant natural ocean sound source in the Southern Hemisphere. Geochem. Geophys. Geosystems 15, 3448–3458. ( 10.1002/2014GC005454) [DOI] [Google Scholar]
- 28.Lillis A, Eggleston D, Bohnenstiehl D. 2014. Estuarine soundscapes: distinct acoustic characteristics of oyster reefs compared to soft-bottom habitats. Mar. Ecol. Prog. Ser. 505, 1–17. ( 10.3354/meps10805) [DOI] [Google Scholar]
- 29.Piercy J, Codling E, Hill A, Smith D, Simpson S. 2014. Habitat quality affects sound production and likely distance of detection on coral reefs. Mar. Ecol. Prog. Ser. 516, 35–47. ( 10.3354/meps10986) [DOI] [Google Scholar]
- 30.Sueur J, Krause B, Farina A. 2019. Climate change is breaking Earth's beat. Trends Ecol. Evol. 34, 971–973. ( 10.1016/j.tree.2019.07.014) [DOI] [PubMed] [Google Scholar]
- 31.Shabangu FW, Yemane D, Stafford KM, Ensor P, Findlay KP. 2017. Modelling the effects of environmental conditions on the acoustic occurrence and behaviour of Antarctic blue whales. PLoS ONE 12, e0172705 ( 10.1371/journal.pone.0172705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieretti N, Duarte MHL, Sousa-Lima RS, Rodrigues M, Young RJ, Farina A. 2015. Determining temporal sampling schemes for passive acoustic studies in different tropical ecosystems. Trop. Conserv. Sci. 8, 215–234. ( 10.1177/194008291500800117) [DOI] [Google Scholar]
- 33.Erbe C, King AR. 2008. Automatic detection of marine mammals using information entropy. J. Acoust. Soc. Am. 124, 2833–2840. ( 10.1121/1.2982368) [DOI] [PubMed] [Google Scholar]
- 34.Abbot TA, Premus VE, Abbot PA. 2010. A real-time method for autonomous passive acoustic detection-classification of humpback whales. J. Acoust. Soc. Am. 127, 2894–2903. ( 10.1121/1.3365255) [DOI] [PubMed] [Google Scholar]
- 35.Gillespie D, Caillat M, Gordon J, White P. 2013. Automatic detection and classification of odontocete whistles. J. Acoust. Soc. Am. 134, 2427–2437. ( 10.1121/1.4816555) [DOI] [PubMed] [Google Scholar]
- 36.Ahonen H, Stafford KM, de Steur L, Lydersen C, Wiig Ø, Kovacs KM. 2017. The underwater soundscape in western Fram Strait: breeding ground of Spitsbergen's endangered bowhead whales. Mar. Pollut. Bull. 123, 97–112. ( 10.1016/j.marpolbul.2017.09.019) [DOI] [PubMed] [Google Scholar]
- 37.Staaterman E, Rice AN, Mann DA, Paris CB. 2013. Soundscapes from a Tropical Eastern Pacific reef and a Caribbean Sea reef. Coral Reefs 32, 553–557. ( 10.1007/s00338-012-1007-8) [DOI] [Google Scholar]
- 38.Sueur J, Farina A, Gasc A, Pieretti N, Pavoine S. 2014. Acoustic indices for biodiversity assessment and landscape investigation. Acta Acust. United Acust. 100, 772–781. ( 10.3813/AAA.918757) [DOI] [Google Scholar]
- 39.Pieretti N, Martire ML, Farina A, Danovaro R. 2017. Marine soundscape as an additional biodiversity monitoring tool: a case study from the Adriatic Sea (Mediterranean Sea). Ecol. Indic. 83, 13–20. ( 10.1016/j.ecolind.2017.07.011) [DOI] [Google Scholar]
- 40.Huang HC, Joseph J, Ming Jer Huang, Margolina T. 2016. Automated detection and identification of blue and fin whale foraging calls by combining pattern recognition and machine learning techniques. In OCEANS 2016 MTS/IEEE Monterey, CA, 19–23 September, pp. 1–7: Monterey, CA: IEEE; ( 10.1109/OCEANS.2016.7761269) [DOI] [Google Scholar]
- 41.Staaterman E, Brandl SJ, Hauer M, Casey JM, Gallagher AJ, Rice AN. 2018. Individual voices in a cluttered soundscape: acoustic ecology of the Bocon toadfish, Amphichthys cryptocentrus. Environ. Biol. Fishes 101, 979–995. ( 10.1007/s10641-018-0752-0) [DOI] [Google Scholar]
- 42.Brown JC, Smaragdis P, Nousek-McGregor A. 2010. Automatic identification of individual killer whales. J. Acoust. Soc. Am. 128, EL93–EL98. ( 10.1121/1.3462232) [DOI] [PubMed] [Google Scholar]
- 43.Harland EJ, Armstrong MS. 2004. The real-time detection of the calls of cetacean species. Can. Acoust. 32, 76–82. [Google Scholar]
- 44.Coquereau L, Lossent J, Grall J, Chauvaud L. 2017. Marine soundscape shaped by fishing activity. R. Soc. Open Sci. 4, 160606 ( 10.1098/rsos.160606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayleigh JWS. 1877. The theory of sound. London, UK: Macmillan and Co; See http://archive.org/details/theorysound06raylgoog. [Google Scholar]
- 46.Sousa-Lima RS, Norris TF, Oswald JN, Fernandes DP. 2013. A review and inventory of fixed autonomous recorders for passive acoustic monitoring of marine mammals. Aquat. Mamm. 39, 23–53. ( 10.1578/AM.39.1.2013.23) [DOI] [Google Scholar]
- 47.Johnson MW, Everest FA, Young RW. 1947. The role of snapping shrimp (Crangon and Synalpheus) in the production of underwater noise in the sea. Biol. Bull. 93, 122–138. ( 10.2307/1538284) [DOI] [PubMed] [Google Scholar]
- 48.Everest FA, Young RW, Johnson MW. 1948. Acoustical characteristics of noise produced by snapping shrimp. J. Acoust. Soc. Am. 20, 137–142. ( 10.1121/1.1906355) [DOI] [Google Scholar]
- 49.Kibblewhite AC, Jones DA. 1976. Ambient noise under Antarctic sea ice. J. Acoust. Soc. Am. 59, 790–798. ( 10.1121/1.380930) [DOI] [Google Scholar]
- 50.Cato DH. 1978. Marine biological choruses observed in tropical waters near Australia. J. Acoust. Soc. Am. 64, 736 ( 10.1121/1.382038) [DOI] [Google Scholar]
- 51.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. ( 10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 52.Mittermeier RA, Turner WR, Larsen FW, Brooks TM, Gascon C. 2011. Global biodiversity conservation: the critical role of hotspots. In Biodiversity hotspots: distribution and protection of conservation priority areas (eds Zachos FE, Habel JC), pp. 3–22. Heidelberg, Germany: Springer; ( 10.1007/978-3-642-20992-5_1) [DOI] [Google Scholar]
- 53.Merchant ND, Fristrup KM, Johnson MP, Tyack PL, Witt MJ, Blondel P, Parks SE. 2015. Measuring acoustic habitats. Methods Ecol. Evol. 6, 257–265. ( 10.1111/2041-210X.12330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertucci F, Parmentier E, Berthe C, Besson M, Hawkins AD, Aubin T, Lecchini D. 2017. Snapshot recordings provide a first description of the acoustic signatures of deeper habitats adjacent to coral reefs of Moorea. PeerJ 5, e4019 ( 10.7717/peerj.4019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pine M, Radford C, Jeffs A. 2015. Eavesdropping on the Kaipara Harbour: characterising underwater soundscapes within a seagrass bed and a subtidal mudflat. N. Z. J. Mar. Freshw. Res. 49, 247–258. ( 10.1080/00288330.2015.1009916) [DOI] [Google Scholar]
- 56.Sánchez-Gendriz I, Padovese LR. 2016. Underwater soundscape of marine protected areas in the south Brazilian coast. Mar. Pollut. Bull. 105, 65–72. ( 10.1016/j.marpolbul.2016.02.055) [DOI] [PubMed] [Google Scholar]
- 57.Freeman L, Freeman S. 2016. Rapidly obtained ecosystem indicators from coral reef soundscapes. Mar. Ecol. Prog. Ser. 561, 69–82. ( 10.3354/meps11938) [DOI] [Google Scholar]
- 58.Siddagangaiah S, Chen C-F, Hu W-C, Pieretti N. 2019. A complexity-entropy based approach for the detection of fish choruses. Entropy 21, 977 ( 10.3390/e21100977) [DOI] [Google Scholar]
- 59.Pieretti N, Farina A, Morri D. 2011. A new methodology to infer the singing activity of an avian community: The Acoustic Complexity Index (ACI). Ecol. Indic. 11, 868–873. ( 10.1016/j.ecolind.2010.11.005) [DOI] [Google Scholar]
- 60.Buscaino G, et al. 2016. Temporal patterns in the soundscape of the shallow waters of a Mediterranean marine protected area. Sci. Rep. 6, 34230 ( 10.1038/srep34230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris SA, Shears NT, Radford CA. 2015. Ecoacoustic indices as proxies for biodiversity on temperate reefs. Methods Ecol. Evol. 7, 713–724, ( 10.1111/2041-210X.12527) [DOI] [Google Scholar]
- 62.Elise S, Urbina-Barreto I, Pinel R, Mahamadaly V, Bureau S, Penin L, Adjeroud M, Kulbicki M, Bruggemann JH. 2019. Assessing key ecosystem functions through soundscapes: a new perspective from coral reefs. Ecol. Indic. 107, 105623 ( 10.1016/j.ecolind.2019.105623) [DOI] [Google Scholar]
- 63.Roca IT, Van Opzeeland I. 2019. Using acoustic metrics to characterize underwater acoustic biodiversity in the Southern Ocean. Remote Sens. Ecol. Conserv. 6, 262–273. ( 10.1002/rse2.129) [DOI] [Google Scholar]
- 64.McWilliam J. 2016. Spatial patterns of inshore marine soundscapes. In The effects of noise on aquatic life II (eds Popper AN, Hawkins A), pp. 697–703. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan M, Mooney T, Partan J, Solow A. 2015. Coral reef species assemblages are associated with ambient soundscapes. Mar. Ecol. Prog. Ser. 533, 93–107. ( 10.3354/meps11382) [DOI] [Google Scholar]
- 66.Lyon R, Eggleston D, Bohnenstiehl D, Layman C, Ricci S, Allgeier J. 2019. Fish community structure, habitat complexity, and soundscape characteristics of patch reefs in a tropical, back-reef system. Mar. Ecol. Prog. Ser. 609, 33–48. ( 10.3354/meps12829) [DOI] [Google Scholar]
- 67.Lin T-H, Fang S-H, Tsao Y. 2017. Improving biodiversity assessment via unsupervised separation of biological sounds from long-duration recordings. Sci. Rep. 7, 4547 ( 10.1038/s41598-017-04790-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Versluis M, Schmitz B, von der Heydt A, Lohse D. 2000. How snapping shrimp snap: through cavitating bubbles. Science 289, 2114–2117. ( 10.1126/science.289.5487.2114) [DOI] [PubMed] [Google Scholar]
- 69.Bohnenstiehl DR, Lillis A, Eggleston DB. 2016. The curious acoustic behavior of estuarine snapping shrimp: temporal patterns of snapping shrimp sound in sub-tidal oyster reef habitat. PLoS ONE 11, e0143691 ( 10.1371/journal.pone.0143691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radford CA, Jeffs AG, Tindle CT, Montgomery JC. 2008. Temporal patterns in ambient noise of biological origin from a shallow water temperate reef. Oecologia 156, 921–929. ( 10.1007/s00442-008-1041-y) [DOI] [PubMed] [Google Scholar]
- 71.Rossi T, Connell SD, Nagelkerken I. 2017. The sounds of silence: regime shifts impoverish marine soundscapes. Landsc. Ecol. 32, 239–248. ( 10.1007/s10980-016-0439-x) [DOI] [Google Scholar]
- 72.Piercy JJB, Smith DJ, Codling EA, Hill AJ, Simpson SD. 2016. The good, the bad, and the distant: soundscape cues for larval fish. In The effects of noise on aquatic life II (eds Popper AN, Hawkins A), pp. 829–837. New York, NY: Springer New York. [DOI] [PubMed] [Google Scholar]
- 73.Gordon TAC, Harding HR, Wong KE, Merchant ND, Meekan MG, McCormick MI, Radford AN, Simpson SD. 2018. Habitat degradation negatively affects auditory settlement behavior of coral reef fishes. Proc. Natl Acad. Sci. USA 115, 5193–5198. ( 10.1073/pnas.1719291115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sueur J, Pavoine S, Hamerlynck O, Duvail S. 2008. Rapid acoustic survey for biodiversity appraisal. PLoS ONE 3, e4065 ( 10.1371/journal.pone.0004065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villanueva-Rivera LJ, Pijanowski BC, Doucette J, Pekin B. 2011. A primer of acoustic analysis for landscape ecologists. Landsc. Ecol. 26, 1233–1246. ( 10.1007/s10980-011-9636-9) [DOI] [Google Scholar]
- 76.Depraetere M, Pavoine S, Jiguet F, Gasc A, Duvail S, Sueur J. 2012. Monitoring animal diversity using acoustic indices: implementation in a temperate woodland. Ecol. Indic. 13, 46–54. ( 10.1016/j.ecolind.2011.05.006) [DOI] [Google Scholar]
- 77.Parks SE, Miksis-Olds JL, Denes SL. 2014. Assessing marine ecosystem acoustic diversity across ocean basins. Ecol. Inform. 21, 81–88. ( 10.1016/j.ecoinf.2013.11.003) [DOI] [Google Scholar]
- 78.Rice A, Soldevilla M, Quinlan J. 2017. Nocturnal patterns in fish chorusing off the coasts of Georgia and eastern Florida. Bull. Mar. Sci. 93, 455–474. ( 10.5343/bms.2016.1043) [DOI] [Google Scholar]
- 79.Wall CC, Rountree RA, Pomerleau C, Juanes F. 2014. An exploration for deep-sea fish sounds off Vancouver Island from the NEPTUNE Canada ocean observing system. Deep Sea Res. Part Oceanogr. Res. Pap. 83, 57–64. ( 10.1016/j.dsr.2013.09.004) [DOI] [Google Scholar]
- 80.Erbe C, Marley SA, Schoeman RP, Smith JN, Trigg LE, Embling CB. 2019. The effects of ship noise on marine mammals—a review. Front. Mar. Sci. 6, 606 ( 10.3389/fmars.2019.00606) [DOI] [Google Scholar]
- 81.Hawkins AD, Popper AN. 2016. A sound approach to assessing the impact of underwater noise on marine fishes and invertebrates. ICES J. Mar. Sci. J. Cons. 14, 635–651. ( 10.1093/icesjms/fsw205) [DOI] [Google Scholar]
- 82.Heenehan H, et al. 2019. Caribbean Sea soundscapes: monitoring humpback whales, biological sounds, geological events, and anthropogenic impacts of vessel noise. Front. Mar. Sci. 6, 347 ( 10.3389/fmars.2019.00347) [DOI] [Google Scholar]
- 83.Erbe C. 2013. Underwater passive acoustic monitoring & noise impacts on marine fauna-a workshop report. Acoust. Aust. 41, 113–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


