Abstract
Objectives
Symptom screening is important to achieving symptom control. Symptom Screening in Paediatrics Tool (SSPedi) is validated for English-speaking children. Objectives were to translate SSPedi into Spanish, and to evaluate the understandability and cultural relevance of the translated version among Spanish-speaking children with cancer and paediatric haematopoietic stem cell transplant recipients.
Methods
We conducted a multiphase, descriptive study to translate SSPedi into Spanish. The first step was to determine whether one Spanish version would be appropriate for both North America and Argentina. Once this decision was made, forward and backward translations were performed. The translated version was evaluated by Spanish-speaking children 8–18 years of age receiving cancer treatments.
Primary and secondary outcome measures
Primary outcome was child self-reported difficulty with understanding of the entire instrument and each symptom using a 5-point Likert scale. Secondary outcomes were incorrect understanding of SSPedi items identified by cognitive interviews with the children using a 4-point Likert scale and cultural relevance, which was assessed qualitatively.
Results
This report focuses on North American Spanish as a separate version will be required for Argentinian Spanish SSPedi based on different common vocabulary and grammatical structure. There were 20 children from Toronto and San Antonio included in cognitive interviews. The most common types of Spanish spoken were Mexican (13, 65%), Central American (2, 10%) and South American (2, 10%). No child reported that it was hard or very hard to complete Spanish SSPedi. Changes to the instrument itself were not required based on understanding or cultural relevance.
Conclusions
We translated and finalised Spanish SSPedi appropriate for use in North America. Future research will translate and evaluate SSPedi for use in Argentina and other Spanish-speaking countries.
Keywords: paediatric oncology, bone marrow transplantation, paediatric oncology
Strengths and limitations of this study.
Multicentre conduct is a strength as it improves generalisability of the study.
Multiple approaches to assessing understandability is a strength as it improves robustness and validity of the findings.
Use of external adjudicators is a strength as it improves reliability of the results.
The study is limited by conduct in only two countries; this version of Symptom Screening in Paediatrics Tool may not be well understood in other Spanish-speaking countries.
Background
Paediatric patients with cancer experience prevalent and severely bothersome symptoms during the treatment.1–3 Common symptoms experienced include pain, nausea and fatigue.1 More recent studies have also highlighted the prevalence of changes in hunger and taste as bothersome symptoms in this population.4–7 Symptoms are important because there is strong correlation between increasing symptom burden and worse quality of life.8 Active symptom screening and reporting are likely to be central in optimising symptom control. Active symptom screening may identify symptoms early, improve communication of the extent of bother to the healthcare team and increase earlier and more consistent management strategies.
In prior research, we identified the lack of appropriate symptom screening measures for children with cancer based on length, content validity or appropriateness9 and consequently, developed a new instrument named the Symptom Screening in Paediatrics Tool (SSPedi).10 SSPedi asks about the degree to which 15 symptoms bothered the child yesterday or today on a 5-point Likert scale. These symptoms are disappointed or sad, scared or worried, cranky or angry, problems thinking, body or face changes, tiredness, mouth sores, headache, other pain, tingling or numbness, throwing up, hunger changes, taste changes, constipation and diarrhoea.
To evaluate the psychometric properties of SSPedi, we conducted a multicentre study with 502 English-speaking children with sites in both Canada and the USA. All children enrolled in the study were between the ages of 8 and 18 and were receiving cancer therapies. SSPedi was found to be reliable (internal consistency and test retest and inter-rater reliability), valid (construct validity) and responsive to change.10 More precisely, the intraclass correlation coefficients were 0.88 (95% CI 0.82 to 0.92) for test retest reliability, and 0.76 (95% CI 0.71 to 0.80) for inter-rater reliability between children and their parents. The mean difference in SSPedi scores between groups that were hypothesised to be more and less symptomatic was 7.8 (95% CI 6.4 to 9.2; p<0.001).10 Construct validity was demonstrated as all hypothesised relationships among measures were observed. SSPedi was responsive to change; those who reported they were much better or worse on a global symptom change scale had significantly changed from their baseline score (mean absolute difference 5.6, 95% CI 3.8 to 7.5; p<0.001).
Translation into other languages will be an important component of SSPedi adoption within and outside of North America. We initially chose to focus translation on Spanish as it is a common first language of children in the USA.11 The process of translation to Spanish must consider both cultural and linguistic perspectives.12 Consequently, objectives were to translate SSPedi into Spanish and to evaluate the understandability and cultural relevance of the translated version of SSPedi among children with cancer and paediatric haematopoietic stem cell transplant (HSCT) recipients.
Methods
Written informed consent and verbal assent were obtained from all study participants or guardians (in the case of children providing assent). Both Spanish and English consent/assent forms were available. The following reflect the specific steps taken for translation of SSPedi into Spanish. The target countries were the USA, Canada and Argentina. We first determined whether one Spanish version would be appropriate for North America and Argentina by identification of a single translation that would be acceptable and understood in both regions. Next, we conducted translation followed by cognitive interviews as further described below.
With Spanish-speaking investigators and translators from the USA, Canada and Argentina, we identified that at least two versions of Spanish would be required, namely one appropriate for North America and one appropriate for Argentina. More specifically, the local investigators and translators determined that for some symptoms, language that would be commonly used and well understood in one region would not be commonly used or well understood in the other region. In addition, they identified regional differences in terms of grammatical structure and the use of voseo conjugation. Voseo is the use of vos as a second-person singular pronoun, instead of, or alongside tu. In some countries such as Argentina, vos is the written and spoken standard. It can also be found in more casual speech in many other parts of Central and South America. Only the North American version is presented in this manuscript; the Argentinian version will be reported separately. Thus, enrolment sites for this report were The Hospital for Sick Children, Toronto, Canada and University of Texas Health Sciences Centre San Antonio, San Antonio, USA.
Translation
Translation of SSPedi included four distinct steps, namely forward translation, reconciliation, back translation and back translation review. We followed the guiding principles for the translation and cultural adaptation process for patient-reported outcomes from the ISPOR Task Force.13 The generic methods that will be used for SSPedi translations are provided as online supplemental appendix 1.
bmjopen-2020-037406supp001.pdf (32.4KB, pdf)
Forward translation involved the independent translation of SSPedi from English (source language) by two professional medical translators, at least one of whom resided in the country targeted for translation. Reconciliation between the translated versions of SSPedi occurred via a translation panel, which consisted of investigators from the enrolment sites, both translators and the Toronto-based team. The Toronto-based research team included one paediatric oncologist, one paediatric pharmacist, one clinical research manager and one clinical research project assistant.
Next, the product of reconciliation was back translated to English by a third translator who did not have knowledge of English SSPedi and who was a native English speaker. The translation panel then reviewed the back translation against the source instrument to identify any discrepancies in meaning.
In addition to translating SSPedi itself, the professional medical translators also translated the synonym list. The synonym list was created for the English version of SSPedi to facilitate child self-report. It provides alternative words for each SSPedi symptom and was derived primarily through cognitive interviews with children themselves. Examples of synonyms for ‘‘te sientes decepcionado’ (you feel disappointed) included ‘te sientes desilusionado’ (you feel disillusioned) and ‘desencantado’ (disenchanted).
Cognitive interviewing
Overview
The interviews were audio recorded and sent to Toronto for evaluation and adjudication. The goals were to determine whether children found the Spanish translated version of SSPedi difficult to understand, whether they incorrectly understood it, and whether there were cultural issues with the instrument. Interviews were conducted by trained research associates or nurses with experience in cognitive interviewing who are fluent in Spanish and English.
Eligibility criteria
Children were eligible to participate if they were 8–18 years of age; they had a diagnosis of cancer or were HSCT recipients; and Spanish was their first language (permissible for both English and Spanish to be their first language). We excluded participants who had visual or cognitive impairments that precluded completion of SSPedi according to their healthcare provider.
Primary and secondary outcome measures
Primary outcome was child self-reported difficulty with understanding of the entire instrument and each symptom using a 5-point Likert scale. Secondary outcomes were incorrect understanding of SSPedi items identified by cognitive interviews with the children using a 4-point Likert scale and cultural relevance, which was assessed qualitatively.
Procedures
Sampling was purposive to ensure that children of varying age, underlying diagnosis and gender were included. Potential participants were identified on the inpatient ward or outpatient clinic by the healthcare team. On confirmation of eligibility, the patient or family was approached to request participation in this study.
First, the respondent completed the translated version of SSPedi on paper in the presence of the interviewer. SSPedi could be read aloud if the child was having difficulty with reading. We evaluated four aspects, namely ease or difficulty with understanding as reported by the child, correct or incorrect understanding as evaluated by two raters, cultural relevance and missing items. Child respondents rated how easy or hard the translated version of SSPedi was to understand using a 5-point Likert scale ranging from 1=‘very hard’ to 5=‘very easy’. The instrument overall, each of the 15 items and the response scale were evaluated. We reported the number of children who found SSPedi hard or very hard to understand (score of 1 or 2). We also evaluated the child’s understanding of each item and the response scale using cognitive probing. Both the interviewer and an independent rater in Toronto who listened to the audio-recording adjudicated understanding using a 4-point Likert scale ranging from 1=‘completely incorrect’ to 4=‘completely correct’. Discrepancies were resolved by consensus. We described the number of items that were rated as partially or completely incorrect (score of 1 or 2). Next, we asked children whether any questions within SSPedi did not make sense to them in thinking about their day-to-day life in order to assess cultural relevance. These data were evaluated by the Toronto rater and dichotomised into issues with cultural relevance identified versus not identified. Finally, we asked whether any important symptoms were missing from Spanish SSPedi. Children could have responded to questions in English or Spanish according to their preference.
Evaluation of responses and sample size justification
After each group of five children were interviewed, the study team met to review the responses to identify whether the translated version of SSPedi should be modified. Modification could be made to the script, the instrument itself or a synonym list of terms available for each SSPedi item. Formal evaluation of difficulty with understanding and incorrect understanding was performed after each group of 10 children were interviewed (considered one iteration).
Criteria to consider Spanish SSPedi satisfactory were as follows: no more than one of the last 10 participants found the entire instrument and each item hard to understand, no more than one of the last 10 participants were incorrect in their understanding of each item as adjudicated by the raters, and other comments including those pertaining to cultural relevance did not suggest that modification was required. Sample size was based on the suggestion that 7–10 interviews are sufficient to determine understandability of an item.14 We, therefore, intended to enrol up to 10–30 children to allow for up to three iterations consisting of 10 children each. All analyses were descriptive.
Finalisation
The final version of Spanish SSPedi was reviewed by all members of the translation panel to ensure cohesiveness and freedom from minor error. The final version was then formatted.
Patient and public involvement
No patients were involved in study design or conduct apart from being participants in the research.
Results
Between January 2018 and April 2019, we identified 38 children and enrolled 20 participants (figure 1). Table 1 shows the demographics of the included participants. The number of children who were 8–10, 11–14 and 15–18 years of age were 4 (20%), 7 (35%) and 9 (45%), respectively. The most common types of Spanish spoken were Mexican (13, 65%) followed by Central American (2, 10%), South American (2, 10%) and other (3, 15%). After enrolment of 20 children, the North American Spanish SSPedi was considered satisfactory
Figure 1.
North American Spanish SSPedi participant flow diagram. SSPedi, Symptom Screening in Paediatrics Tool.
Table 1.
Demographic characteristics of participants evaluating North American Spanish SSPedi
| Cohort 1 (n=10), (%) | Cohort 2 (n=10), (%) | |
| Sex | ||
| Male | 6 (60) | 6 (60) |
| Female | 4 (40) | 4 (40) |
| Age in years | ||
| 8–10 | 1 (10) | 3 (30) |
| 11–14 | 4 (40) | 3 (30) |
| 15–18 | 5 (50) | 4 (40) |
| Diagnosis | ||
| Leukaemia/lymphoma | 9 (90) | 4 (40) |
| Solid tumour | 1 (10) | 3 (30) |
| Brain tumour | 0 | 2 (20) |
| Other* | 0 | 1 (10) |
| Metastatic disease | 0 | 0 |
| Relapse | 1 (10) | 1 (10) |
| Stem cell transplantation | 1 (10) | 1 (10) |
| Active treatment | 7 (70) | 4 (40) |
| Born in country of interview | 6 (60) | 9 (90) |
| Type of Spanish spoken | ||
| Mexican | 5 (50) | 8 (80) |
| Central American | 2 (20) | 0 |
| South American | 1 (10) | 1 (10) |
| Other | 2 (20) | 1 (10) |
| Inpatient at interview | 0 | 1 (10) |
| Attending school | 5 (50) | 9 (90) |
*Other—primary immunodeficiency (n=1).
SSPedi, Symptom Screening in Paediatrics Tool.
None of the child respondents reported that it was hard or very hard to complete Spanish SSPedi overall. Table 2 shows self-reported difficulty with understanding and adjudicated incorrect understanding of SSPedi items. It shows that after enrolling the first 10 participants, two participants found two items (mouth sores and tingly or numb hands or feet) hard to understand, and therefore, criteria were not met to consider that version satisfactory. Changes made were additions to the synonym list only, based on alternative words given by children during the interview process. No changes to the instrument itself were required. In the last 10 enrolled participants, at most one participant found each item hard to understand and none were incorrect in their understanding of each item. None of the respondents were incorrect in their understanding of the response scale. In terms of cultural relevance, no issues were identified by any of the 20 respondents. None of the children interviewed indicated that there were additional symptoms they felt were missing from the tool.
Table 2.
Self-Reported difficulty with understanding and rater-adjudicated incorrectness with North American Spanish SSPedi*
| Cohort 1 (n=10) | Cohort 2 (n=10) | |||
| Hard to understand | Incorrect | Hard to understand | Incorrect | |
| Disappointed or sad | 0 | 0 | 0 | 0 |
| Scared or worried | 0 | 0 | 0 | 0 |
| Cranky or angry | 1 | 0 | 1 | 0 |
| Difficulty thinking/ remembering | 0 | 0 | 1 | 0 |
| Changes in your face/ body | 0 | 0 | 1 | 0 |
| Tired | 0 | 0 | 0 | 0 |
| Mouth sores | 2 | 1 | 1 | 0 |
| Headache | 0 | 0 | 0 | 0 |
| Hurt or pain | 0 | 1 | 0 | 0 |
| Tingly or numb hands or feet | 2 | 0 | 1 | 0 |
| Throwing up | 0 | 0 | 0 | 0 |
| More or less hungry | 0 | 0 | 0 | 0 |
| Changes in taste | 0 | 0 | 1 | 0 |
| Constipation | 0 | 0 | 1 | 0 |
| Diarrhoea | 0 | 0 | 0 | 0 |
*Hard=rated as hard or very hard to understand by participant. Incorrect=rated as partially or completely incorrect by rater.
SSPedi, Symptom Screening in Paediatrics Tool.
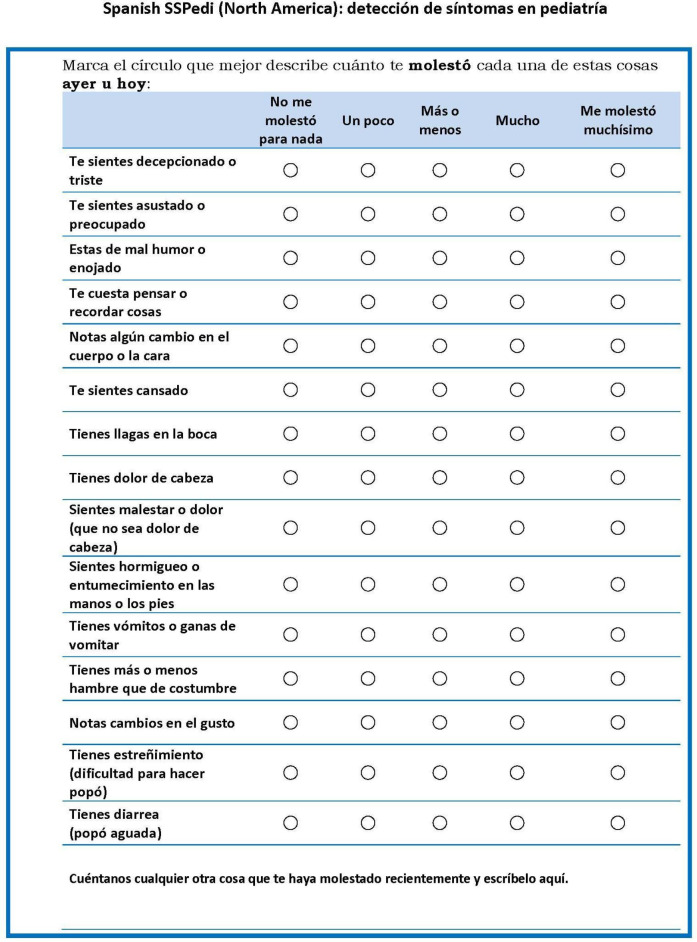
Thus, after 20 participants, the North American Spanish version of SSPedi was considered satisfactory and appropriate for utilisation. Figure 2 shows the final version.
Figure 2.
North American Spanish SSPedi. SSPedi, Symptom Screening in Paediatrics Tool.
Discussion
We translated a self-report symptom screening tool for paediatric patients with cancer and HSCT recipients named SSPedi into Spanish appropriate for use in North America. The final version was acceptable based on self-reported difficulty with understanding, adjudicated incorrect understanding of different aspects of SSPedi and cultural relevance. Many patient-reported outcomes incorporated into oncology clinical trials are only validated in English,15 leading to potential disparities in clinical trial participation. Consequently, translation into non-English languages should be a priority.
We found that at least two versions of Spanish SSPedi will be needed since Argentinian Spanish was considered sufficiently different from North American Spanish to require a distinct version. Interestingly, different quality of life instruments have taken alternate approaches to Spanish translation. For example, the developers of the PedsQL modules have chosen to translate Spanish for several different countries including the United States, Argentina, Columbia and Spain.16 In contrast, the patient-reported outcome measurement information system has a single Spanish translation version.17 It is possible that the Argentinian version would be appropriate for other countries where voseo conjugation is prominent, such as several countries in Central America. However, we cannot be sure without explicit evaluation of the Argentinian version in those countries.
We termed this version of Spanish SSPedi ‘North American’ even though we did not include a site in Mexico. However, we noted that the majority of children self-identified their Spanish type as Mexican, thus providing reassurance that this version should be appropriate in that country. Ideally, further testing in Mexico would be conducted to confirm understandability and cultural relevance in that setting. Some could argue that North American Spanish is not a distinct form of Spanish as it reflects the Spanish spoken in several different originating countries. To emphasise this point, four children identified their Spanish type as Central or South American. However, regardless of Spanish type of origin, there is likely to be changes in how Spanish is understood and used on moving to North America. In addition, a study conducted in the USA or Canada is unlikely to use multiple versions of Spanish. Thus, creating a North America Spanish version addresses a practical clinical and research need in these geographic locations.
During the creation of English SSPedi, we found four items more difficult to understand by children 8–18 years of age, namely ‘changes in how your body and face look’, ‘tingly or numb hands or feet’, ‘feeling more or less hungry than you usually do’, and ‘constipation (hard to poop).18 Interestingly, three of these four items were similarly hard to understand by at least one participant in this current study focused on Spanish translation. This may suggest that difficulty with understanding was not related to Spanish translation but rather, that these are more difficult concepts for children in general to understand, particularly if respondents had no previous experience with the symptom. This hypothesis is supported by the absence or limited number of self-reported instruments for at least peripheral neuropathy among paediatric patients with cancer.19
The main implication of this work is that there is now a symptom assessment tool that can be used among North American Spanish speaking children receiving cancer treatments. Given known disparities based on race, ethnicity and language,20 21 development of such a tool may be an important step toward reducing disparities in terms of both clinical trial enrolment and routine clinical care. Future efforts could evaluate barriers to utilisation of the translated tool as well as translating SSPedi to other Spanish-speaking populations.
The strengths of this study were conduct of the translation according to internationally recognised standards13 and evaluation in two countries. Other strengths include its multicentre conduct to improve generalisability, multiple approaches to assessing understandability to improve validity and use of external adjudicators to improve reliability. However, weaknesses included enrolment of a limited number of children and in only two centres. Evaluation in other locations and with additional children may influence the synonym list further although based on the initial results, it is less likely that changes to the instrument itself will be required. In addition, throughout the SSPedi programme, ease or difficulty in understanding has focused on the number of children describing an item as hard or very hard to understand. Focusing on those who find an item neither easy nor hard to understand could lead to different results.
In summary, we translated and finalised Spanish SSPedi appropriate for use in North America based on self-reported difficulty with understanding, adjudicated incorrect understanding of different aspects of SSPedi and cultural relevance. This work is important as translation of patient-reported outcomes to non-English languages may reduce disparities in clinical trial enrolment and cancer care delivery. Future research will translate and evaluate SSPedi for use in Argentina and other Spanish-speaking countries.
Supplementary Material
Acknowledgments
We thank all the translators who worked with us on this project and whose expertise and insights greatly assisted the translation and evaluation process.
Footnotes
Contributors: LD and LS developed the study concept and design. EP, DN, CS, SG, GG and GD were involved in data collection. LS drafted the manuscript. All authors EP, AG, AS, A-ML, DN, CS, SG, GG, GD, LD and LS participated in data interpretation, reviewed, revised and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: To translate SSPedi into Spanish, we conducted a multi-phase, descriptive study that was approved by The Hospital for Sick Children’s Research Ethics Board (#1000057560) and the Research Ethics Boards of all participating sites.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Baggott C, Dodd M, Kennedy C, et al. . Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs 2010;27:307–15. 10.1177/1043454210377619 [DOI] [PubMed] [Google Scholar]
- 2.Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum 2011;38:E382–93. 10.1188/11.ONF.E382-E393 [DOI] [PubMed] [Google Scholar]
- 3.Pöder U, Ljungman G, von Essen L. Parents’ perceptions of their children’s cancer-related symptoms during treatment: a prospective, longitudinal study. J Pain Symptom Manage 2010;40:661–70. 10.1016/j.jpainsymman.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 4.Loves R, Plenert E, Tomlinson V, et al. . Changes in hunger among pediatric patients with cancer and hematopoietic stem cell transplantation recipients. Support Care Cancer 2020 10.1007/s00520-020-05425-w [DOI] [PubMed] [Google Scholar]
- 5.Loves R, Plenert E, Tomlinson V, et al. . Changes in taste among pediatric patients with cancer and hematopoietic stem cell transplantation recipients. Qual Life Res 2019;28:2941–9. 10.1007/s11136-019-02242-5 [DOI] [PubMed] [Google Scholar]
- 6.Loves R, Tomlinson D, Baggott C, et al. . Taste changes in children with cancer and hematopoietic stem cell transplant recipients. Support Care Cancer 2019;27:2247–54. 10.1007/s00520-018-4509-2 [DOI] [PubMed] [Google Scholar]
- 7.Johnston DL, Hyslop S, Tomlinson D, et al. . Describing symptoms using the symptom screening in pediatrics tool in hospitalized children with cancer and hematopoietic stem cell transplant recipients. Cancer Med 2018;7:1750–5. 10.1002/cam4.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis LL, Johnston DL, Baggott C, et al. . Validation of the symptom screening in pediatrics tool in children receiving cancer treatments. J Natl Cancer Inst 2018;110:661–8. 10.1093/jnci/djx250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuis LL, Ethier M-C, Tomlinson D, et al. . A systematic review of symptom assessment scales in children with cancer. BMC Cancer 2012;12:430. 10.1186/1471-2407-12-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomlinson D, Dupuis LL, Gibson P, et al. . Initial development of the symptom screening in pediatrics tool (SSPedi). Support Care Cancer 2014;22:71–5. 10.1007/s00520-013-1945-x [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Barrera A, Lopez M. Spanish is the most spoken non-English language in U.S. homes, even among non-Hispanics Washington, DC: Pew research center, 2013. Available: https://www.pewresearch.org/fact-tank/2013/08/13/spanish-is-the-most-spoken-non-english-language-in-u-s-homes-even-among-non-hispanics/
- 12.Yang L. Treatment of cultural differences in translation. Stud Literat Lang 2014;8:39–42. [Google Scholar]
- 13.Wild D, Grove A, Martin M, et al. . Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (pro) measures: report of the ISPOR Task force for translation and cultural adaptation. Value Health 2005;8:94–104. 10.1111/j.1524-4733.2005.04054.x [DOI] [PubMed] [Google Scholar]
- 14.Willis G, Cognitive interviewing: a tool for improving questionnaire design. Sage Publications, 2009. [Google Scholar]
- 15.Grant SR, Noticewala SS, Mainwaring W, et al. . Non-English language validation of patient-reported outcome measures in cancer clinical trials. Support Care Cancer 2020;28:2503–5. 10.1007/s00520-020-05399-9 [DOI] [PubMed] [Google Scholar]
- 16.Varni JW. PedsQL translations, 2019. Available: https://www.pedsql.org/translations.html
- 17.HealthMeasures Available translations, 2019. Available: http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis/available-translations/117-available-translations
- 18.O'Sullivan C, Lee Dupuis L, Gibson P, et al. . Evaluation of the electronic self-report symptom screening in pediatrics tool (SSPedi). BMJ Support Palliat Care 2018;8:110–6. 10.1136/bmjspcare-2015-001084 [DOI] [PubMed] [Google Scholar]
- 19.Johnston DL, Sung L, Stark D, et al. . A systematic review of patient-reported outcome measures of neuropathy in children, adolescents and young adults. Support Care Cancer 2016;24:3723–8. 10.1007/s00520-016-3199-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer 2011;56:994–1002. 10.1002/pbc.23078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiol Rev 2009;31:99–112. 10.1093/epirev/mxp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037406supp001.pdf (32.4KB, pdf)