Abstract
Introduction
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is a well-established treatment for peritoneal cancer (PC). However, this kind of combination therapy is associated with a high incidence of complications. Moreover, relative studies have indicated that traditional laboratory testing is insufficient to demonstrate the overall haemostatic physiology of CRS/HIPEC. Thromboelastography (TEG), administered by monitoring dynamic changes in haemostasis, has been shown to contribute to reducing transfusion requirements and improving survival. However, there is no evidence to verify whether TEG can be applied to guide transfusion strategies during CRS/HIPEC. Therefore, we aim to investigate whether TEG-guided blood product transfusion (TEG-BT) therapy is superior to traditional blood product transfusion (T-BT) therapy for guiding perioperative blood transfusion treatment and improving the prognosis of patients undergoing CRS/HIPEC.
Methods and analysis
The TEG-BT versus T-BT study is a single-centre, randomised, blinded outcome assessment clinical trial of 162 patients with PC, aged 18–64 years and undergoing CRS/HIPEC. Participants will be randomly allocated to receive TEG-BT or T-BT. The primary outcome will be the evaluation of perioperative blood transfusion, which refers to the total amount of blood transfusion given from the time patients enter the operating room up to 72 hours postoperatively. The secondary outcomes will include the transfusion volume during surgery, total amount of intraoperative infusion, amount of blood lost during the operation, total blood transfusion between 0 and 72 hours after surgery, lowest haemoglobin level within 72 hours after surgery, intensive care unit duration, overall length of stay, total cost of hospitalisation and adverse events. Data will be analysed according to the intention-to-treat principle.
Ethics and dissemination
The study protocol has been approved by the Scientific Research Ethics Committee of Beijing Shijitan Hospital Affiliated with Capital Medical University (Approval Number: sjtkyll-lx-2020-3). The results will be published in peer-reviewed journals.
Trial registration number
Chinese Clinical Trial Registry (ChiCTR2000028835).
Keywords: bleeding disorders & coagulopathies, anaesthesia in oncology, gastrointestinal tumours
Strengths and limitations of this study.
This is a randomised, controlled, blinded outcome assessment trial to test the efficacy of thromboelastography (TEG)-guided blood transfusion in cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy.
The results of this study will improve a more goal-oriented transfusion strategy to reduce intraoperative blood transfusion, stabilise the perioperative coagulation function and lighten the economic burden.
Future studies should address the importance of the relationship between transfusion thresholds and TEG parameters for the optimal management of coagulopathy.
This is a single centre trial, which may limit the generalisation of conclusions; consequently, multicentre clinical studies with a larger sample size will be required.
Although there is no way for anaesthesiologists to be blinded during the trial, the main outcome evaluators will be blinded.
Background
Peritoneal cancer (PC) was previously considered to be a fatal stage of many gastrointestinal malignancies. Patients receiving palliative care had a median survival of 3–9 months, depending on the initial stage.1 Significant progress has been made in the treatment of peritoneal malignancies over the past decade, and increasing evidence supports the use of cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in an attempt to eradicate the disease macroscopically or microscopically and reduce peritoneal recurrence.2 Currently, CRS/HIPEC is an established treatment for pseudomyxoma peritonei, colorectal cancer, ovarian cancer and gastric cancer with intraperitoneal metastasis.3 The number of patients receiving CRS/HIPEC is expected to rise due to the high mortality of PC and the encouraging long-term benefits of the treatment.4
Although positive results have been observed in the treatment of tumour disease, CRS/HIPEC is characterised by a high incidence of complications that challenge both intraoperative and postoperative management.5 Patients undergo CRS/HIPEC with significant fluid and blood loss, as well as chemotherapy, and severe fluctuations in the core temperature.6 Schmidt et al reported that CRS/HIPEC is a long and intricate surgical procedure accompanied by massive blood and fluid loss, with 51% of patients requiring a blood transfusion.7 Massive intraoperative bleeding and the above factors will certainly lead to severe coagulation disorders. Intraoperative coagulation is a known complication of extensive surgery and HIPEC, and may be caused by a combination of the high fluid requirements for resuscitation, direct effects of intraperitoneal chemotherapy, hepatotoxicity due to antitumour drugs and direct liver injury.8 Coagulation problems can be part of a series of events that can lead to massive blood loss during surgery, which can compromise the quality of the procedure, increase the need for blood transfusions and compromise the patient’s postoperative process.9 Therefore, perioperative management of patients undergoing CRS/HIPEC is a challenge for surgeons, anaesthesiologists and critical care physicians.10
Regarding the perioperative monitoring of coagulation function, the management of coagulation disorders at 90% of HIPEC centres is guided by standard laboratory tests (SLTs), such as the activated partial thromboplastin time (APTT), prothrombin time (PT) or international normalised ratio (INR).11 Laboratory analysis shows that with an increase in the INR, coagulation disorders are observed, antithrombin III and fibrinogen values are reduced, APTT is prolonged and the number of coagulation cells is reduced.12 Originally invented by Hartert in 1948, thromboelastography (TEG) is a viscoelastic, haemostatic assay analyser that imitates sluggish venous flow.13 It is a different test from standard coagulation tests in that it measures the viscoelastic properties of whole blood as it clots, providing comprehensive information about the dynamics of clot development, stabilisation and dissolution, and assesses both thrombosis and fibrinolysis.14 15 TEG has been used increasingly in intensive care units (ICUs) and in cases of acute critical surgery to evaluate coagulation disorders and guide the infusion of blood products for critically ill patients.16 The technique has been tested in clinical scenarios such as heart and liver surgery, and transplantation to reduce the number of transfusions and serve as a screening tool for patients managing hypercoagulant and bleeding disorders.17 One study has shown that the PT, APTT and platelet (PLT) count are insufficient to demonstrate the effect of surgical stress, hyperthermia, chemotherapy and mass fluid transfer on the overall haemostatic physiology of CRS/HIPEC, while more sophisticated TEG monitoring is more accurate for perioperative coagulation function monitoring.18 Another study reported that although traditional clinical monitoring of coagulation disorders was not meaningful in CRS/HIPEC, TEG monitoring confirmed that epidural analgesia after CRS/HIPEC was safe.19 However, whether the effect of CRS/HIPEC on coagulation function can be treated according to TEG guidance and the infusion of various blood products can be guided according to changes in TEG and thereby achieve better outcomes for patients has not been fully verified.
Based on the existing literature, we aim to investigate the advantages of TEG-guided blood product transfusion (TEG-BT) in perioperative blood protection during CRS/HIPEC. Our working hypothesis is that compared with traditional blood product transfusion (T-BT) patients, TEG-BT patients receive fewer transfusions of intraoperative blood products and exhibit a more stable perioperative coagulation function, no reduction in postoperative haemoglobin (HGB) levels and coagulation function, shorter hospital stays and no increase in the incidence of adverse reactions.
Methods and analysis
Trial design
The trial will be conducted at Beijing Shijitan Hospital, Capital Medical University in Beijing, China. The study started recruiting patients in May 2020 and will continue for 1 year. The TEG-BT versus T-BT study is a single-centre, randomised, blinded outcome assessment clinical trial that conforms to the Consolidated Standards of Reporting Trials.20 CRS/HIPEC is planned for two groups of patients, and TEG-BT or T-BT is adopted for perioperative blood transfusion management. The ratio of the two groups is 1:1 (figure 1).
Figure 1.
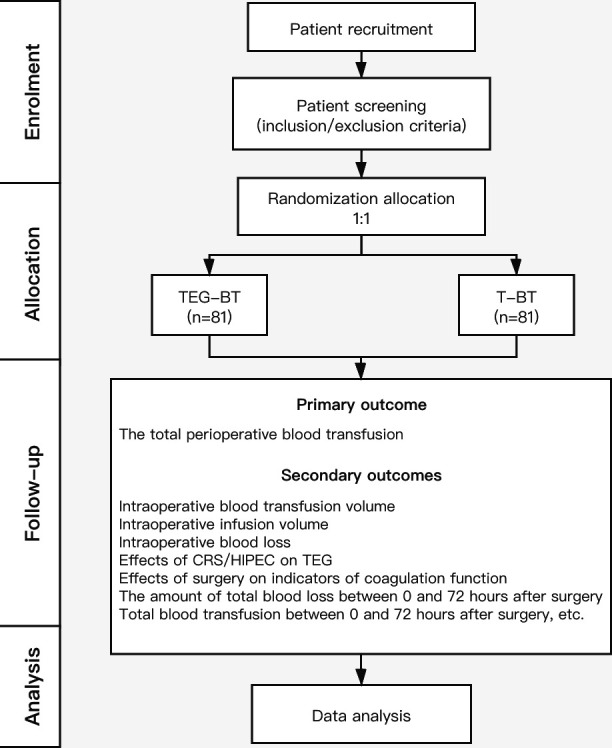
Study flow diagram of the TEG-BT versus T-BT trial. CRS/HIPEC, cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy; T-BT, traditional blood product transfusion; TEG-BT, thromboelastography-guided blood product transfusion.
Objectives
The purpose of this study is to verify whether TEG-BT is better than T-BT for perioperative blood transfusion treatment and the prognosis of patients undergoing CRS/HIPEC.
Participants
Inclusion criteria
Patients who meet all of the following criteria are eligible for inclusion:
Age of 18–64 years.
American Society of Anaesthesiologists grade I or II.
Well-established histological diagnosis of peritoneal disease.
Performance of CRS/HIPEC under general anaesthesia.
Written consent to participate in the study.
Exclusion criteria
Anaemia: HGB <90 g/L.
Abnormal coagulation function before surgery.
Uncontrolled systemic infections.
Antiplatelet or anticoagulant therapy was administered at enrolment or discontinued for less than 7 days prior to study evaluation.
Thrombotic events: any blood clot in the vein or artery has been recorded before or at present.
Severe cardiopulmonary disease.
Hepatic or renal failure.
Pregnancy or lactation.
Patient refusal to sign the informed consent form.
Patient participation in another clinical treatment study.
Randomisation and blinding
All participants will be randomly divided into two groups: TEG-BT for the experimental group and T-BT for the control group. Before the study begins, an independent investigator who is not exposed to any of the participants will use a simple randomised method to divide the two groups in a 1:1 ratio. The random numbers will be saved in sealed opaque envelopes. Before surgery, the anaesthesiologist will evaluate the patient, and after the informed consent form is signed, the envelope will be opened to obtain the grouping information of the patient. Blood transfusion will be performed intraoperatively according to the grouping of the patients. After the operation, independent follow-up staff will collect the data of the patients during and after the operation according to the electronic medical record system. During the whole experiment, the anaesthesiologists will be aware of the patient grouping information, but they will not participate in the postoperative follow-up and data collection. Other individuals and personnel involved, including patients, surgeons and data collectors, will not know the grouping information.
Anaesthesia management
The anaesthesia regimen will be consistent between the two groups. Venous access will be open in all patients in the preparation room, and midazolam will be administered (0.05mg/kg intravenously) to the patients before they enter the operating room. On arrival in the operating room, standard monitoring (pulse oximetry, ECG and non-invasive arterial blood pressure monitoring) will be established. Sufentanil 0.5 µg/kg, propofol 2.5 mg/kg and rocuronium 0.6 mg/kg will be adopted for general anaesthesia induction. After endotracheal intubation, the lungs will be aerated with 50% oxygen and 50% air mixture, and the ventilation level will be adjusted to maintain normocapnia. Radial artery and internal jugular vein puncture will be performed to monitor the invasive arterial pressure and central venous pressure. We will perform an ultrasound-guided bilateral rectus sheath blockade, and 0.375% ropivacaine will be given for analgesia on both sides. Anaesthesia will be maintained with inhalation of sevoflurane and intravenous (IV) remifentanil, and muscle relaxation will be maintained with IV rocuronium. Postoperative IV injection of atropine 0.01 mg/kg and neostigmine 0.05 mg/kg will be used to antagonise residual neuromuscular block. Extubation will be performed after confirming that the patient’s eyes are open and that he or she exhibits adequate spontaneous breathing and purposeful movement.
Intraoperative intervention
All enrolled patients will be assigned to one of the following two study groups. For patients in the T-BT group, the anaesthesiologist will inject blood products according to his or her clinical judgement. Patients entering the TEG-BT group will undergo TEG monitoring four times before surgery, during CRS, before HIPEC and after HIPEC, and blood products such as erythrocytes, plasma, PLTs, prothrombin complex and fibrinogen will be administered according to the monitoring results. The two groups of patients will be given red blood cells (RBCs) to maintain HGB levels of at least 90 g/L.
Blood will be collected from the central vein through a three-way catheter. The first 10 mL of venous blood will be administered to the patient through the peripheral venous pathway, and then 3.5 mL of venous blood will be collected after syringe replacement. According to relevant guidelines, the samples of whole blood should be tested within 5 min after collection.
All blood samples will be tested by the same professional TEG operator. The operator of the TEG machine model (TEG 5000 (haemoscope)) will not know the patient grouping information.
Reaction time (R time) is the incubation period from blood entry into the reactive vessels to initial clot formation. The lack of the R time extension prompt factor can be corrected by fresh frozen plasma (FFP).21 When the R time value is greater than 10 min, the patient will be infused with 2 U of FFP; when the R time value is greater than 15 min, the patient will be infused with 4 U of plasma; when the R time value is greater than 20 min, the patient will be infused with 6 U.22
Angle α is the measurement of fibrin cross linkage mechanics or clot strengthening speed. A low angle may indicate a lack of fibrinogen, which is less affected by the PLT count, and this loss of function may be corrected by the use of FFP or fibrinogen.
Maximum amplitude (MA) is a direct effect of fibrin and PLT binding properties of glycoprotein IIb/IIIA, representing the strength of fibrin clots. Low MA may be corrected by the administration of PLTs.21 At MA<45, PLT transfusion will begin. LY30 values show the rate of thrombus rupture at 30 min after MA. When LY30 value is >8%, it indicates hyperfibrinolysis, which can be corrected by tranexamic acid.
Outcomes
Table 1 provides an overview of the outcomes and intervention or assessment time points.
Table 1.
Study visits of the TEG-BT versus T-BT trial
| Time point | Enrolment | Allocation | Post-allocation | ||||||||||
| Preoperative | 0 d | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | Discharged | |
| Enrolment | |||||||||||||
| Eligibility screen | X | ||||||||||||
| Informed consent | X | ||||||||||||
| Allocation | X | ||||||||||||
| Interventions | |||||||||||||
| TEG-BT | X | X | X | X | |||||||||
| T-BT | |||||||||||||
| Assessments | |||||||||||||
| Demographic data | X | X | |||||||||||
| Baseline variables | X | ||||||||||||
| HGB | X | X | X | X | X | X | |||||||
| Hct | X | X | X | X | X | X | |||||||
| PLTs | X | X | X | X | X | X | |||||||
| PT | X | X | X | X | X | X | |||||||
| APTT | X | X | X | X | X | X | |||||||
| INR | X | X | X | X | X | X | |||||||
| Fibrinogen | X | X | X | X | X | X | |||||||
| Crystalloid fluid | X | ||||||||||||
| Artificial colloid fluid | X | ||||||||||||
| RBCs | X | ||||||||||||
| FFP | X | ||||||||||||
| PLTs | X | ||||||||||||
| Fibrinogen | X | ||||||||||||
| Prothrombin complex | X | ||||||||||||
| Albumin | X | ||||||||||||
| Blood loss | X | ||||||||||||
| Urine output | X | ||||||||||||
| The amount of blood lost between 0 and 72 hours after surgery | X | ||||||||||||
| Total blood transfusion between 0 and 72 hours after surgery | X | ||||||||||||
| The lowest HGB level | X | ||||||||||||
| ICU duration | X | ||||||||||||
| Overall length of stay | X | ||||||||||||
| Total cost of the hospitalisation | X | ||||||||||||
T0, entering the operating room; T1, the performance of CRS; T2, before HIPEC; T3, after HIPEC; T4, at the end of surgery; T5, 2 hours after surgery; T6, postoperative day 1; T7, postoperative day 2; T8, postoperative day 3; T9, postoperative day 5.
APTT, activated partial thromboplastin time; FFP, fresh frozen plasma; Hct, haematocrit; HGB, haemoglobin; ICU, intensive care unit; INR, international normalised ratio; PLTs, platelets; PT, prothrombin time; RBCs, red blood cells; T-BT, traditional blood product transfusion; TEG-BT, thromboelastography-guided blood product transfusion.
Primary outcome
The primary outcome will be the evaluation of perioperative blood transfusion. The amount of blood transfusion during the perioperative period refers to the total amount of blood transfusion given from the time patients enter the operating room (intra-operative) to 72 hours postoperatively (postoperative), including RBCs, FFP and PLTs.
Secondary outcomes
The secondary results mainly include the following aspects:
Transfusion volume during the operation: the total amount of RBCs, FFP and PLTs in the two groups.
The total amount of intraoperative infusion: during the operation, the amount of fluid input from the two groups will include the total amount of crystalloid fluid, artificial colloid fluid, albumin, fibrinogen and prothrombin complex.
The amount of blood lost during the operation: the total output of the two groups will include blood loss and urine output.
Effects of CRS/HIPEC on TEG: before and after CRS/HIPEC, TEG monitoring will assess changes in blood coagulation function, including R time, thrombosis time (K time), fibrinogen function (α angle), thrombus strength (MA) and fibrinolysis (LY30).
Effects of surgery on indicators of coagulation function: the influence of surgery on coagulation function. Based on the routine blood cell count and coagulation test, the values of HGB, PLT count, PT, APTT and INR will be recorded 1, 2, 3 and 5 days after the operation.
The amount of total blood lost between 0 and 72 hours after surgery: total blood lost is calculated using the formula provided by gross,23 total blood loss=peripheral blood volume (PBV)×(haematocrit (Hct)pre−Hctpost)/Hctave. The PBV is then calculated using the formula proposed by Nadler et al:24 PBV=k1×height(m)3+ k2×weight(kg)+k3, where k1=0.3669, k2=0.03219 and k3=0.6041 for men, and k1=0.3561, k2=0.03308 and k3=0.1833 for women.
Total blood transfusion in 0–72 hours after operation: if the patient needs a blood transfusion within 0–72 hours after the operation, we will record the total amount of RBCs, FFP and PLTs. If the patient does not receive a blood transfusion, the amount will be recorded as 0.
The lowest HGB within 72 hours after surgery: within 3 days after the operation, blood samples will be collected every morning to measure the HGB level. Patients who need a second operation due to postoperative bleeding within 72 hours will be excluded from this study.
ICU duration.
Overall length of stay.
Total cost of the hospitalisation.
Adverse events
Adverse events in this study will mainly include intraoperative blood transfusion-related adverse reactions such as non-haemolytic febrile reactions, allergic reactions, haemolytic reactions, circulatory overload and acid–base imbalance. Once the anaesthesiologist identifies an adverse event, all patients should be accurately documented and immediately treated. If the condition progresses to severe intraoperative adverse events, such as shock, heart failure and massive blood loss, we will continue to follow-up the patients to observe the final results.
Data collection
Throughout the trial, the investigator—the anaesthesiologist involved in the operation—will be completely independent of the data collection staff. Data collection personnel are responsible for collecting preoperative and postoperative patient information and all data required in the trial protocol. Anonymous data will be collected in the case report form (CRF), either numerically or alphabetically. After the completion of the anonymous CRF table, the researcher shall confirm the authenticity and validity of all data, give a reasonable explanation for any missing data, or choose to exclude the test scheme.
Sample size calculation
The Pass V.11.0 software package was used for sample size evaluation. According to a small sample (86 cases) observation study in the early stage of the research group, it was found that the allogeneic blood volume used in the operation of PC patients undergoing thermal perfusion chemotherapy was 1664.7±789.3 mL (median: 1600 (1200, 2000)). It is estimated that the blood volume of allogeneic patients used in thromboelastogram monitoring after intervention could be reduced by 20%, that is, 1331.8±631.4 mL, with a set α value of 0.05 and a β value of 0.2, and the sample volume of the two groups was 73 cases. However, the index of allogeneic blood volume is non-normally distributed data, and a non-parametric test is planned to be used for analysis. Compared with the t-test, the efficiency of the Wilcoxon rank-sum test is estimated to be approximately 95%, which means that the sample size required for the Wilcoxon test is 1.053 times the sample size required for the t-test.25 Therefore, the number of patients to be included in the study was increased by 1.053 times, and the drop-out rate was maintained to within 5%. The final target sample size was 81 patients in each group (162 patients in total).
Statistical analysis
Baseline characteristics
Data analyses will be performed by the statistical software SPSS V.25.0. During the trial, the statistical analyst will be unaware of the participants’ personal information and their group assignment. The Kolmogorov-Smirnov test will be used to test the normal distribution of continuous variables. If data values are normally distributed, they will be presented as the mean±SD and will be compared using the independent t-test. If data values are not normally distributed, they will be presented as median and IQR and compared using the non-parametric test. Categorical data will be shown as frequency and percentage, and compared using the χ2 test or Fisher’s exact test.
Primary outcome and secondary outcomes
The primary outcome, the total amount of blood transfusion, will be presented as the mean±SD or median and IQR, and compared using the independent t-test or Wilcoxon’s rank-sum test. For the secondary outcomes (ie, transfusion volume during the operation, total amount of intraoperative infusion, amount of blood lost during the operation, total blood transfusion between 0 and 72 hours after surgery, and lowest HGB within 72 hours after surgery), the t-test will be used to compare the measurement data, and the rank-sum test will be used for ranked data. The χ2 test or Fisher’s exact test will be used to analyse categorical data (adverse events). The effect size, mean differences and their CIs will be reported to make the results comparable. For repeated variables, a repeated-measures analysis of covariance will be performed with visit time as the repeated factor and group as the non-repeated factor.
All analyses will be performed on the intention-to-treat population of participants who are given the randomised treatment. Missing data will be handled using the multiple imputation method. A complete case analysis without imputation of missing data will also be performed to determine whether the results are consistent. The significance level that will be used for statistical analysis with two-tailed testing will be 5%. No interim analyses will be performed.
Discussion
The TEG-BT versus T-BT study is a single centre and randomised clinical trial with a blinded outcome assessment that aims to verify whether TEG-BT is superior to T-BT in the perioperative blood transfusion treatment and prognosis of patients in CRS/HIPEC. If it can be proven that compared with T-BT, TEG-BT can lead to less intraoperative blood transfusion, more stable perioperative coagulation function, no reduction in postoperative HGB levels and coagulation function, and no increase in the incidence of adverse events, then the treatment and transfusion of various blood products can be guided according to changes in the TEG index to achieve a better prognosis.
CRS/HIPEC is a therapeutic method for patients with colorectal, appendiceal, ovarian and gastric cancer with peritoneal metastasis and peritoneal mesothelioma.26–28 A relevant study demonstrated that intraoperative transfusion of RBCs and a possibly increased Peritoneal Carcinomatosis Index are associated with abnormal postoperative coagulation, including changes in the PLT count, INR and PTT.4 Based on the above points, optimal blood product transfusion is of great importance for patients receiving CRS/HIPEC.
SLTs, including the fibrinogen concentration, INR, PT and APTT, were initially used to diagnose intraoperatively acquired coagulopathy and guide the administration of treatment for massive haemorrhage.29 30 However, relevant data suggest that the PT, APTT and PLT count insufficiently demonstrate the impact of surgical stress, hyperthermia, chemotherapy and considerable fluid shifts on the overall haemostatic physiology of CRS/HIPEC.18 Routine laboratory testing is performed in PLT-deficient plasma whose results are not available to the clinician for 45–60 min;30–32 in contrast, TEG can make up for the above deficiencies as a bedside analysis tool, estimating the clotting process in whole blood and providing real-time data.33 Increasing evidence has demonstrated that the application of a TEG-guided transfusion strategy can reduce the demand for blood products and improve the morbidity of bleeding patients, mainly according to trials involving heart surgery with cardiopulmonary bypass and liver transplantation surgery.34 35 After many clinical experiences and the application of TEG, targeted coagulation therapy has become feasible.36 A previous prospective study indicated that conventional coagulation measures had no significance for CRS/HIPEC, but TEG monitoring confirmed the suitability of epidural analgesia after CRS/HIPEC by evaluating perioperative clot kinetics.19 However, there is no relevant study to verify whether TEG can be applied to guide transfusion strategies for treating coagulation disorders due to CRS/HIPEC. Therefore, it is believed that the use of TEG in guiding perioperative blood transfusion treatment and improving prognosis of patients undergoing CRS/HIPEC is definitely worth exploring.
The current study still has several limitations. First, for various reasons, we did not observe certain long-term outcomes, such as overall mortality, the incidence of reoperation, transfusion-related complications and thrombotic/thromboembolic events. Nevertheless, the influence of TEG-BT on these outcomes is worthy of further exploration. Moreover, due to the design of this trial, it is not available to determine the impact of pathophysiological changes in patients with potential diseases; therefore, we will remove severely ill patients from this study for safety reasons. Additionally, further studies may be required to determine whether TEG-BT combined with T-BT is superior to either alone for guiding the perioperative blood transfusion treatment of patients receiving CRS/HIPEC. Last but not least, this study is a single centre trial, which may limit its generalisability; consequently, it is of great importance to perform multicentre clinical studies with a larger sample size to provide higher levels of evidence.
The primary outcome of our study is perioperative blood transfusion. As mentioned earlier, patients treated with CRS/HIPEC undergo an extensive abdominal incision, large fluid shifts, hyperthermic insults and exposure to chemotherapeutic agents, which increases the likelihood of altered coagulation and excessive bleeding.4 37 38 Therefore, rational transfusion strategies are warranted. Extensive literature notes that allogeneic blood transfusion itself is an independent risk factor for increased morbidity (thrombotic/thromboembolic events, anaemia, nosocomial infections and multiorgan dysfunction syndrome), mortality, hospital stay, hospital costs and so on in trauma, cardiovascular surgery and ICU patients.39–42 Nevertheless, TEG can be performed to monitor dynamic changes in haemostasis, which is thought to enable clinicians to distinguish between a surgical cause of bleeding or coagulopathy, to guide and evaluate the choice of haemostatic treatment, and to reduce transfusion requirements and improve survival.34 In contrast, postoperative bleeding and coagulation disorders also increase the transfusion of allogeneic blood products, thereby affecting morbidity and mortality.36 Hence, to further explore their interaction in patients undergoing CRS/HIPEC, the indicators of coagulation function, lowest value of HGB within 72 hours after surgery, ICU duration, overall length of stay and costs incurred during the hospital stay will be the secondary outcomes of this study.
To conclude, the TEG-BT versus T-BT trial will be the first single centre, randomised clinical trial with a blinded outcome assessment undertaken to substantiate the hypothesis that TEG-BT is superior to T-BT for administrating perioperative blood transfusion treatment and improving the prognosis of patients undergoing CRS/HIPEC. If the benefits mentioned in the hypothesis are confirmed, our study will improve a more goal-oriented transfusion strategy to reduce intraoperative blood transfusion, stabilise perioperative coagulation function and lighten the economic burden. Combined with our research results, the potential significance of this trial is that it may influence future guidelines on anaesthesia management of CRS/HIPEC and bring wider application for TEG.
Ethics and dissemination
Ethical and legislative approvals
The research plan was approved by the scientific research ethics committee of Beijing Shijitan Hospital Affiliated with Capital Medical University (Approval Number: sjtkyll-lx-2020-3). We will inform investigators, all participants and the trial registry when there are significant changes to the study protocol. Before each participant enters the study, he/she and the researchers will sign an informed consent form. Patients have the right to refuse or withdraw from the study at any time, which will not affect any of their medical or other interests. The personal information of the participants will be kept confidential, and anonymous personal patient data will be shared according to requirements.
Publication plan
With the consent of the main researchers and methodologists, the research coordinator will be responsible for preparing scientific statements and reports corresponding to the study. Based on the proportion of contribution to the study, the participating researchers and clinicians as well as biostatisticians and related researchers will be the co-authors of the ensuring report and publication. The rules of publication will be in accordance with international recommendations, and the publications will be submitted to peer-reviewed journals.43
Supplementary Material
Footnotes
Contributors: SW and QZ contributed equally to this work and should be considered co-first authors. SW and QZ contributed to the conception and drafting of the first manuscript for this trial. PfL is the principal investigator of the entire study and edited the final manuscript. LC and GL contributed to the conception of the research protocol and will participate in the follow-up for this trial. All authors critically revised and modified the protocol and the article. They all approved the final version to be published.
Funding: This research was supported by The Youth Science Foundation of Beijing Shijitan Hospital Affiliated with Capital Medical University (No. 2019-q19).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Glehen O, Osinsky D, Beaujard AC, et al. . Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg Oncol Clin N Am 2003;12:729–39. 10.1016/S1055-3207(03)00044-9 [DOI] [PubMed] [Google Scholar]
- 2.Sargant N, Roy A, Simpson S, et al. . A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus Med 2016;26:118–22. 10.1111/tme.12301 [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Kumar V, Garg R, et al. . Anesthetic implications in hyperthermic intraperitoneal chemotherapy. J Anaesthesiol Clin Pharmacol 2019;35:3–11. 10.4103/joacp.JOACP_93_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurdle H, Bishop G, Walker A, et al. . Coagulation after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a retrospective cohort analysis. Can J Anaesth 2017;64:1144–52. 10.1007/s12630-017-0952-7 [DOI] [PubMed] [Google Scholar]
- 5.Chua TC, Robertson G, Liauw W, et al. . Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol 2009;135:1637–45. 10.1007/s00432-009-0667-4 [DOI] [PubMed] [Google Scholar]
- 6.Korakianitis O, Daskalou T, Alevizos L, et al. . Lack of significant intraoperative coagulopathy in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) indicates that epidural anaesthesia is a safe option. Int J Hyperthermia 2015;31:857–62. 10.3109/02656736.2015.1075606 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C, Creutzenberg M, Piso P, et al. . Peri-Operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008;63:389–95. 10.1111/j.1365-2044.2007.05380.x [DOI] [PubMed] [Google Scholar]
- 8.Desgranges F-P, Steghens A, Mithieux F, et al. . Potential risks of thoracic epidural analgesia in hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2010;101:442. 10.1002/jso.21485 [DOI] [PubMed] [Google Scholar]
- 9.Wijeysundera DN, Beattie WS, Austin PC, et al. . Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet 2008;372:562–9. 10.1016/S0140-6736(08)61121-6 [DOI] [PubMed] [Google Scholar]
- 10.Webb CA-J, Weyker PD, Moitra VK, et al. . An overview of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for the anesthesiologist. Anesth Analg 2013;116:924–31. 10.1213/ANE.0b013e3182860fff [DOI] [PubMed] [Google Scholar]
- 11.Bell JC, Rylah BG, Chambers RW, et al. . Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol 2012;19:4244–51. 10.1245/s10434-012-2496-y [DOI] [PubMed] [Google Scholar]
- 12.Schmidt C, Creutzenberg M, Piso P, et al. . Peri-Operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008;63:389–95. 10.1111/j.1365-2044.2007.05380.x [DOI] [PubMed] [Google Scholar]
- 13.Hartert H. Blood coagulation studies using thromboelastography, a new evaluation technique (in German). Klin Wochenschr 1948;26:577–83.18101974 [Google Scholar]
- 14.da Luz LT, Nascimento B, Rizoli S, Thrombelastography RS. Thrombelastography (TEG®): practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med 2013;21:29. 10.1186/1757-7241-21-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol 2014;89:228–32. 10.1002/ajh.23599 [DOI] [PubMed] [Google Scholar]
- 16.Meybohm P, Zacharowski K, Weber CF. Point-Of-Care coagulation management in intensive care medicine. Crit Care 2013;17:218. 10.1186/cc12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitlur M, Sorensen B, Rivard GE, et al. . Standardization of thromboelastography: a report from the TEG-ROTEM Working group. Haemophilia 2011;17:532–7. 10.1111/j.1365-2516.2010.02451.x [DOI] [PubMed] [Google Scholar]
- 18.Van Poucke S, Huskens D, Van der Speeten K, et al. . Thrombin generation and platelet activation in cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy - A prospective cohort study. PLoS One 2018;13:e0193657. 10.1371/journal.pone.0193657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teoh DA, Hutton MJH, Else S, et al. . Epidural analgesia? A prospective analysis of perioperative coagulation in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Am J Surg 2019;217:887–92. 10.1016/j.amjsurg.2019.01.034 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Hopewell S, Schulz KF, et al. . Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869–c69.. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson JC. Thromboelastography-Guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized controlled trial. Clin Liver Dis 2019;13:102–5. 10.1002/cld.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh KJ, Nedelcu E, Bai Y, et al. . How do we manage cardiopulmonary bypass coagulopathy? Transfusion 2014;54:2158–66. 10.1111/trf.12751 [DOI] [PubMed] [Google Scholar]
- 23.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology 1983;58:277–80. 10.1097/00000542-198303000-00016 [DOI] [PubMed] [Google Scholar]
- 24.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224–32. [PubMed] [Google Scholar]
- 25.Page VJ, Ely EW, Gates S, et al. . Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1:515–23. 10.1016/S2213-2600(13)70166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am 2001;10:915–33. 10.1016/S1055-3207(18)30039-5 [DOI] [PubMed] [Google Scholar]
- 27.Morano WF, Khalili M, Chi DS, et al. . Clinical studies in CRS and HIPEC: trials, tribulations, and future directions-A systematic review. J Surg Oncol 2018;117:245–59. 10.1002/jso.24813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42. 10.1097/00000658-199501000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Association of Anaesthetists of Great Britain and Ireland, Thomas D, Wee M, et al. . Blood transfusion and the anaesthetist: management of massive haemorrhage. Anaesthesia 2010;65:1153–61. 10.1111/j.1365-2044.2010.06538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samama CM, Leroux G, Fléron M-H, et al. . Point-Of-Care versus central laboratory coagulation testing during haemorrhagic surgery. Thromb Haemost 2017;101:394–401. [PubMed] [Google Scholar]
- 31.Davenport R, Manson J, De'Ath H, et al. . Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 2011;39:2652–8. 10.1097/CCM.0b013e3182281af5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Görlinger K, Shore-Lesserson L, Dirkmann D, et al. . Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth 2013;27:S20–34. 10.1053/j.jvca.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 33.Konstantinidi A, Sokou R, Parastatidou S, et al. . Clinical application of Thromboelastography/Thromboelastometry (TEG/TEM) in the neonatal population: a narrative review. Semin Thromb Hemost 2019;45:449–57. 10.1055/s-0039-1692210 [DOI] [PubMed] [Google Scholar]
- 34.Wikkelsø A, Wetterslev J, Møller AM, et al. . Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev 2016:CD007871. 10.1002/14651858.CD007871.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abeysundara L, Mallett SV, Clevenger B. Point-of-Care testing in liver disease and liver surgery. Semin Thromb Hemost 2017;43:407–15. 10.1055/s-0037-1599154 [DOI] [PubMed] [Google Scholar]
- 36.Bolliger D, Tanaka KA. Point-Of-Care coagulation testing in cardiac surgery. Semin Thromb Hemost 2017;43:386–96. 10.1055/s-0037-1599153 [DOI] [PubMed] [Google Scholar]
- 37.Esquivel J, Angulo F, Bland RK, et al. . Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open "coliseum technique". Ann Surg Oncol 2000;7:296–300. 10.1007/s10434-000-0296-2 [DOI] [PubMed] [Google Scholar]
- 38.Kanakoudis F, Petrou A, Michaloudis D, et al. . Anaesthesia for intra-peritoneal perfusion of hyperthermic chemotherapy. haemodynamic changes, oxygen consumption and delivery. Anaesthesia 1996;51:1033–6. 10.1111/j.1365-2044.1996.tb14998.x [DOI] [PubMed] [Google Scholar]
- 39.Murphy GJ, Reeves BC, Rogers CA, et al. . Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544–52. 10.1161/CIRCULATIONAHA.107.698977 [DOI] [PubMed] [Google Scholar]
- 40.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008;36:2667–74. 10.1097/CCM.0b013e3181844677 [DOI] [PubMed] [Google Scholar]
- 41.Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Anaesthesiol 2008;21:669–73. 10.1097/ACO.0b013e32830dd087 [DOI] [PubMed] [Google Scholar]
- 42.Bjursten H, Dardashti A, Ederoth P, et al. . Increased long-term mortality with plasma transfusion after coronary artery bypass surgery. Intensive Care Med 2013;39:437–44. 10.1007/s00134-012-2723-9 [DOI] [PubMed] [Google Scholar]
- 43.International Committee of Medical Journal Editors (ICMJE) International Committee of medical Journal editors (ICMJE): uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publication. Haematologica 2004;89:264. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


