This nonrandomized controlled trial reports the oncological outcomes of the deintensification (from 50 Gy to 36 Gy) of a preoperative radiotherapy regimen for adults with myxoid liposarcoma.
Key Points
Question
Given the radiosensitivity of myxoid liposarcoma, is it possible to reduce preoperative radiotherapy dose for this histological subtype without jeopardizing oncological outcome?
Findings
In this nonrandomized controlled trial of 79 adults with myxoid liposarcoma of the extremity or trunk, 77 of whom underwent surgical resection, extensive pathological treatment response was found in the surgical specimens of 91% of patients along with a local control rate of 100% and a median follow-up of 25 months.
Meaning
Findings of this trial suggest that a dose reduction is effective and oncologically safe, and thus 36 Gy is proposed as an acceptable alternative preoperative radiotherapy dose for myxoid liposarcoma.
Abstract
Importance
Currently, preoperative radiotherapy for all soft-tissue sarcomas is identical at a 50-Gy dose level, which can be associated with morbidity, particularly wound complications. The observed clinical radiosensitivity of the myxoid liposarcoma subtype might offer the possibility to reduce morbidity.
Objective
To assess whether a dose reduction of preoperative radiotherapy for myxoid liposarcoma would result in comparable oncological outcome with less morbidity.
Design, Setting, and Participants
The Dose Reduction of Preoperative Radiotherapy in Myxoid Liposarcomas (DOREMY) trial is a prospective, single-group, phase 2 nonrandomized controlled trial being conducted in 9 tertiary sarcoma centers in Europe and the US. Participants include adults with nonmetastatic, biopsy-proven and translocation-confirmed myxoid liposarcoma of the extremity or trunk who were enrolled between November 24, 2010, and August 1, 2019. Data analyses, using both per-protocol and intention-to-treat approaches, were conducted from November 24, 2010, to January 31, 2020.
Interventions
The experimental preoperative radiotherapy regimen consisted of 36 Gy in once-daily 2-Gy fractions, with subsequent definitive surgical resection after an interval of 4 or more weeks.
Main Outcomes and Measures
As a short-term evaluable surrogate for local control, the primary end point was centrally reviewed pathologic treatment response. The experimental regimen was regarded as a success when 70% or more of the resection specimens showed extensive treatment response, defined as 50% or greater of the tumor volume containing treatment effects. Morbidity outcomes consisted of wound complications and late toxic effects.
Results
Among the 79 eligible patients, 44 (56%) were men and the median (interquartile range) age was 45 (39-56) years. Two patients did not undergo surgical resection because of intercurrent metastatic disease. Extensive pathological treatment response was observed in 70 of 77 patients (91%; posterior mean, 90.4%; 95% highest probability density interval, 83.8%-96.4%). The local control rate was 100%. The rate of wound complication requiring intervention was 17%, and the rate of grade 2 or higher toxic effects was 14%.
Conclusions and Relevance
The findings of the DOREMY nonrandomized clinical trial suggest that deintensification of preoperative radiotherapy dose is effective and oncologically safe and is associated with less morbidity than historical controls, although differences in radiotherapy techniques and follow-up should be considered. A 36-Gy dose delivered in once-daily 2-Gy fractions is proposed as a dose-fractionation approach for myxoid liposarcoma, given that phase 3 trials are logistically impossible to execute in rare cancers.
Trial Registration
ClinicalTrials.gov Identifier: NCT02106312
Introduction
Myxoid liposarcoma is a rare cancer with an incidence of 2 per million person-years,1 representing approximately 5% of soft-tissue sarcoma (STS) in adults worldwide.2 The predominant tumor location is the extremity, although the cancer can also present primarily in the trunk or head and neck region. Myxoid liposarcoma occurs in a relatively young patient population, with a peak incidence in the fifth decade of life,2 which implies that survivors could experience late-treatment toxic effects for a long time.
The standard of care for most intermediate- and high-grade STS, including myxoid liposarcoma, is surgical resection with radiotherapy,3,4 resulting in more favorable local control than surgical resection alone.5,6,7,8 With respect to timing, preoperative radiotherapy is associated with fewer late adverse effects, such as fibrosis, joint stiffness, and edema, compared with postoperative radiotherapy.9 However, with an incidence of 35%, wound complications are observed more frequently after preoperative radiotherapy.8,10 A shift toward preoperative radiotherapy has been observed within the past decade,11 given the temporary nature of wound complications weighted against the permanent nature of fibrosis, stiffness, and edema.10 However, wound complications, albeit temporary, have an adverse effect on patient quality of life, functional outcome, and health care costs.12,13,14,15,16
Data suggest that myxoid liposarcoma is more sensitive to radiotherapy than other STS histological subtypes.7,17,18,19,20,21,22,23,24,25 During and continuing after radiotherapy until the date of surgical resection, myxoid liposarcoma frequently exhibits a marked tumor volume reduction, in sharp contrast to other subtypes of STS in which the volumes, as a rule, remain fairly constant.17,18,19 In addition, unique to myxoid liposarcoma, in 78% to 100% of the resected specimens, 50% or greater pathological response is observed after a standard dose of 50 Gy preoperative radiotherapy delivered using 25 fractions, each of 2 Gy.20,21,22,23 In addition to these remarkable histological responses, local control after standard preoperative radiotherapy and surgical intervention is exceptionally high, with reported 5-year local control rates of 96% to 98% in a large retrospective series.7,24,25
Given the enhanced radiosensitivity demonstrated by tumor volume reduction, pathological complete response, and excellent local control, we hypothesized that deintensification of preoperative radiotherapy for myxoid liposarcoma would not compromise the outcome. We initiated the Dose Reduction of Preoperative Radiotherapy in Myxoid Liposarcomas (DOREMY) trial to evaluate the oncological safety of decreasing the preoperative radiotherapy dose from 50 Gy to 36 Gy for myxoid liposarcoma. In addition to maintaining an excellent local control rate, this dose reduction may decrease wound complication rates and other toxic effects.
Methods
Design and Participants
DOREMY is a prospective, multicenter, single-group, phase 2 nonrandomized controlled trial being conducted at 9 tertiary sarcoma centers in Europe and the US. The trial opened to accrual on November 24, 2010. All eligible patients provided written informed consent before enrollment, and the conduct of the trial has complied with the Declaration of Helsinki26 and all other applicable laws. The trial protocol (Supplement 1) and all amendments to it were approved by the institutional review board or research ethics committee of each of the 9 participating sarcoma centers. We followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Eligible patients were adults (≥18 years) with nonmetastatic and biopsy-proven myxoid liposarcoma of the extremity or trunk. Molecular confirmation of the diagnosis was mandatory. Exclusion criteria were pregnancy, Eastern Cooperative Oncology Group Performance Status score higher than 2, and previous radiotherapy to the target area.
Procedures
The staging procedures consisted of magnetic resonance imaging (MRI) scans of the primary site followed by image-guided biopsies and computed tomography scans of the chest, abdomen, and pelvis.3,4 The radiotherapy protocol, which conforms to the International Commission on Radiation Units and Measurements 50 and 62 guidelines,27,28 required standardized target volume delineation. The gross tumor volume was defined by using gadolinium-enhanced, T1-weighted MRI. The clinical target volume (CTV) was constructed by expanding the gross tumor volume by 3 cm in the longitudinal axis and 1.5 cm in all other directions. Subsequently, if applicable, the CTV was manually edited to encompass any T2-weighted MRI–identified peritumoral edema in the CTV. The planning target volume was produced by expanding the CTV by 1 cm isotropically in all directions.29 The treatment planning technique was intensity-modulated radiation therapy (IMRT). The total prescribed dose was 36 Gy, given in once-daily fractions of 2 Gy, 5 fractions per week. Definitive surgical resection according to local guidelines was performed a minimum of 4 weeks after the final fraction of radiotherapy, preferably after repeated preoperative MRI scans. The resection specimens were processed routinely. Diagnostic biopsies and the resection specimens were scanned using microscope slides (ScanScope; Aperio) and then uploaded on Slide Score.30
Central pathology review was performed by 3 of us, who are sarcoma pathologists (J.V.M.G.B., H.V.B., K.T.). Diagnostic biopsies were examined, and the percentage of the round cell component was estimated. For every resection specimen, all available sections were reviewed and the percentages of vital tumor cells,31 hyalinization,32 fatty maturation, and necrosis in the specimen were scored systematically, together adding up to 100%. Seventeen cases were independently reviewed by 2 of us (J.V.M.G.B., H.V.B.), and the concordance was 100%. The remaining 53 cases were divided among the expert pathologists for central pathology review. For 7 cases, sections from the resection specimens were not evaluable for central pathology review, and the judgment of the local pathologist was used as a surrogate to determine histological response.
End Points
The primary end point of the trial was extensive treatment response in the definitive resection specimen (thus containing <50% of vital tumor cells). The definition of an extensive treatment response was a resection specimen containing 50% or greater of any histological treatment effect, including hyalinization, fatty maturation, and necrosis. The dose reduction was regarded as a success if 70% or more of the patients achieved an extensive treatment response. At the time the trial was conceptualized, the study group hypothesized that this rate of treatment responses would not jeopardize local control because definitive surgical resection was still to follow after radiotherapy.
Secondary end points included local control, wound complication rate, late toxic effect, progression-free survival, disease-specific survival, and overall survival. Wound complications were classified as minor, requiring no intervention or noninvasive intervention without readmission; moderate, requiring secondary wound management without secondary operation; or major, requiring secondary operation (eTable 1 in Supplement 2).8 Late toxic effect was scored at the time of follow-up visits according to the Radiation Therapy Oncology Group toxicity criteria33 and, in the case of edema, according to the Stern Rating Scale for Edema. Follow-up visits were scheduled every 3 months in years 1 and 2; every 6 months in years 3, 4, and 5; and annually thereafter. At these follow-up visits, the decision to perform appropriate imaging to detect metastases and to repeat the MRI scans of the primary site was left to the discretion of the local investigator.
Statistical Analysis
A bayesian statistical design was used to provide a stopping rule for inefficacy of the decreased dose. The observed pathological response was a binary variable indicating either a 50% or greater response in the treated tumor (success, with probability P) or a less than 50% response (failure). Jeffreys prior was used: a β distribution with parameters of α = .50 and β = 0.50. The posterior mean of the success probability P was calculated, and the 95% highest probability density interval (HPDI) was reported. The final conclusion of the trial will be based on the 95% HPDI and whether it excludes 70% of patients. The trial would have stopped for inefficacy if the posterior probability of P ≥ .70 were P < .05 at any time. In the case of potentially reaching the stopping rule, the trial would have been put on temporary hold until the observation period of the previous case was completed. At the time of the cutoff date (August 1, 2019), the minimum required number of responders to preclude the activation of the stopping rule was reached for the maximum sample size of 100 participants.
To assess the differences in baseline characteristics between responders and nonresponders, Mann-Whitney test was performed for continuous variables and Fisher exact test was performed for categorical variables. Differences in baseline presentation and type of surgical intervention were not tested, as these variables were not considered meaningful given the small numbers of patients in 1 or more of the subgroups.
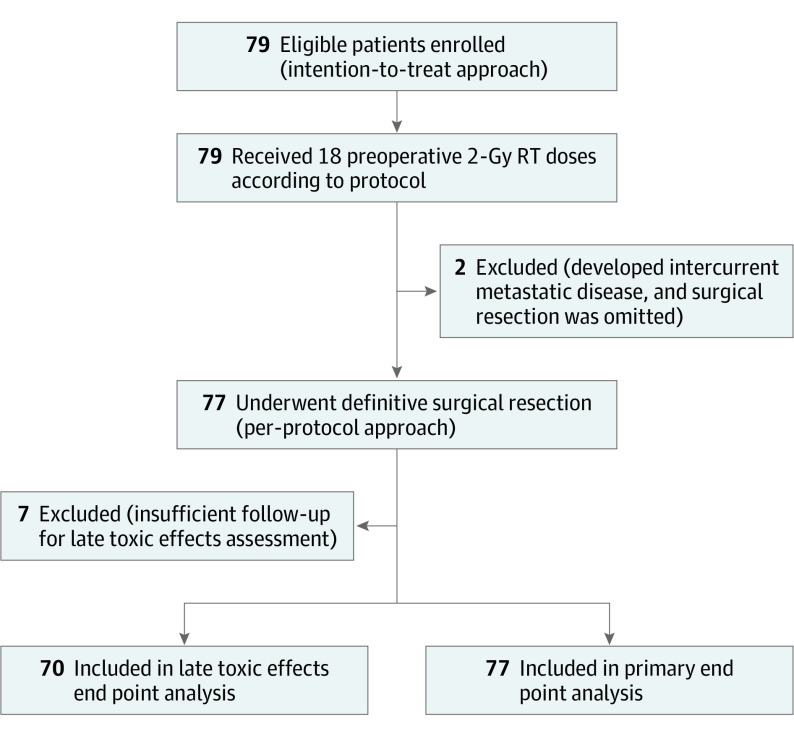
The primary end point was analyzed by both per-protocol and intention-to-treat approaches (Figure 1). Local control and morbidity data were analyzed per protocol. Survival end points were measured from the date of the baseline visit and were analyzed by intention to treat using the Kaplan-Meier method. The level of statistical significance was a 2-sided P ≤ .05 in all tests. Statistical analyses were conducted from November 24, 2010, to January 31, 2020, with SPSS Statistics, version 25 (IBM).
Figure 1. CONSORT Study Flowchart.
RT indicates radiotherapy.
Results
A total of 79 patients were enrolled in the DOREMY trial by August 1, 2019; of these patients, 44 (56%) were men and 35 (44%) were women, with a median (interquartile range [IQR]) age of 45 (39-56) years (Table 1). All patients were treated according to the radiotherapy protocol and received a preoperative dose of 36 Gy in once-daily 2-Gy fractions, with a median (IQR) overall treatment time of 23 (23-23) days and median (IQR) follow-up time of 25 (13-38) months. The median (IQR) interval between completion of radiotherapy and surgical resection was 44 (39-53) days, with 90% of patients undergoing resection within 8 weeks. Surgical margins were negative in 72 of 77 patients (94%) and microscopically positive in 5 of 77 patients (6%) (Table 1).
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Total (n = 79) | Pathological treatment effecta | |||
| ≥50% (n = 70) | <50% (n = 7) | |||
| Follow-up, median (IQR), mo | 25 (13-38) | 25 (13-38) | 35 (8-51) | NS |
| Age, median (IQR), y | 45 (39-56) | 46 (41-55) | 43 (40-67) | NS |
| Sex | NS | |||
| Female | 35 (44) | 30 (43) | 4 (43) | |
| Male | 44 (56) | 40 (57) | 3 (57) | |
| Presentation | NAb | |||
| Primary | 77 (98) | 70 (100) | 6 (86) | |
| Recurrence | 2 (2) | 0 | 1 (14) | |
| Tumor location | NSc | |||
| Lower extremity | ||||
| Proximal | 62 (78) | 57 (81) | 3 (42) | |
| Distal | 10 (13) | 8 (11) | 2 (29) | |
| Upper extremity | 2 (3) | 1 (1) | 1 (14) | |
| Trunk | 5 (6) | 4 (6) | 1 (14) | |
| Tumor diameter, median (IQR), cm | 9.9 (7.3-15.9) | 10.4 (8.0-16.2) | 6.4 (4.3-7.8) | .003 |
| Round cell component | NS | |||
| 0%-5% | 61 (77) | 54 (77) | 5 (71) | |
| >5% | 15 (19) | 13 (19) | 2 (29) | |
| Unknown | 3 (4) | 3 (4) | 0 | |
| Type of surgical intervention | NAb | |||
| Limb sparing or other | 76 (96) | 69 (99) | 7 (100) | |
| Amputation | 1 (1) | 1 (1) | 0 | |
| None | 2 (3) | 0 | 0 | |
| Surgical margins | .004 | |||
| Negative | 72 (94) | 68 (97) | 4 (57) | |
| Positive | 5 (6) | 2 (3) | 3 (43) | |
Abbreviations: IQR, interquartile range; NA, not assessed; NS, not significant.
The cumulative values in the ≥50% and <50% pathological treatment effect columns do not match the total value because 2 patients did not undergo surgical intervention and therefore did not have resection specimens available for analysis.
No statistic testing was performed because of the small number of participants in (at least 1 of) the subgroups.
Fisher exact test of proximal lower extremity and distal lower extremity was performed.
One patient received an additional postoperative 30-Gy dose because of the presence of only 20% pathological treatment effect and multiple positive surgical margins. One patient refused a free flap reconstruction and underwent a limb amputation because of the absence of a volumetric response in a large mass in the distal lower extremity. However, despite the lack of a volumetric response, this tumor showed 80% pathological treatment effect. In 2 patients, surgical resection was omitted because of the development of intercurrent metastatic disease. The first of these 2 patients died from progressive disease 5 months after diagnosis. The second patient continued follow-up at 8 months after diagnosis, without clinical tumor progression of the irradiated primary lesion.
Pathological Response and Local Control
Overall, resection specimens from 70 of 77 patients (91%) showed extensive pathological response. The posterior mean was 90.4% (95% HPDI, 83.8%-96.4%), which demonstrates that the percentage with extensive pathological response was 70% or greater. In addition, when responses from the 2 patients with intercurrent metastatic disease were considered as failures, the posterior mean was 88.1% (95% HPDI, 81.0%-94.7%). An overview of patient, tumor, and treatment characteristics stratified by 50% or greater or less than 50% pathological response status is presented in Table 1. Patients who met the primary end point of extensive pathological response (≥50%) vs those in the other group (<50%) had larger tumors (median [IQR] tumor diameter: 10.4 [8.0-16.2] cm vs 6.4 [4.3-7.8] cm; P = .003) and more frequent negative surgical margins (68 [97%] vs 4 [57%]; P = .004). After a median (IQR) follow-up time of 25 (13-38) months among the patients who underwent a limb-sparing operation, the local control rate was 100% for all patients regardless of the extent of pathological response.
Survival and Morbidity
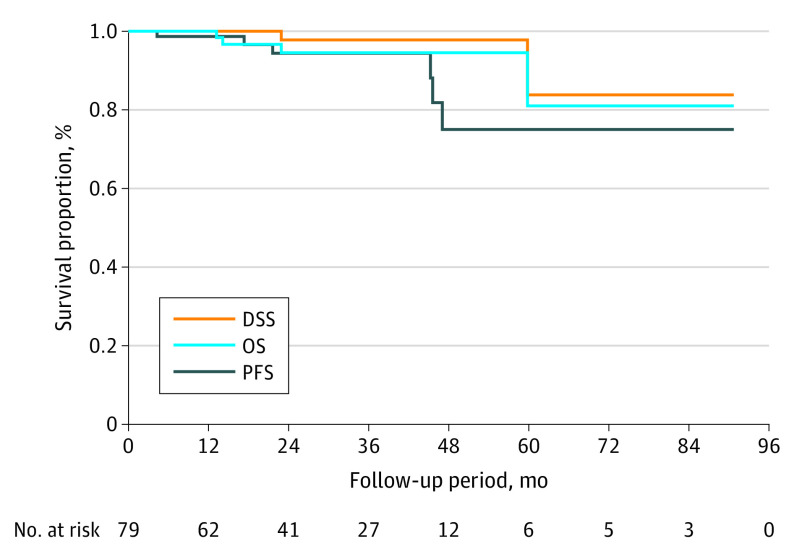
Progression-free survival at year 1 was 97% and at year 3 was 93%. Disease-specific survival at year 1 was 99% and at year 3 was 96%. Overall survival rates were 99% at year 1 and 95% at year 3 (Figure 2).
Figure 2. Kaplan-Meier Survival Analysis of the Intention-to-Treat Population.
DSS indicates disease-specific survival; OS, overall survival; PFS, progression-free survival.
Wound complications of any severity were observed in 17 of 77 patients (22%), with 4 patients (5%) having minor complications, 8 patients (10%) having moderate complications, and 5 (6%) having major complications (Table 2). Of these moderate or major complications, 13 (17%) needed any intervention. Incidences of any grade 1 late toxic effects were 40% (28 of 70 patients) and of any grade 2 late toxic effects were 11% (8 of 70 patients) (eTable 2 in Supplement 2). Grade 3 late toxic effects (edema) were observed in only 2 of 70 patients (3%) . No grade 4 or 5 toxic effects were found. When patients with less than 2 years of follow-up were excluded, the incidence was 7% for grade 2 or higher fibrosis, 0% for joints stiffness, and 5% for edema (eTable 2 in Supplement 2).
Table 2. Wound Complication Overviewa.
| Wound complication type | No. (%) | ||||
|---|---|---|---|---|---|
| Total | Lower extremity | Upper extremity | Other tumor location | ||
| Proximal | Distal | ||||
| Wound complication | |||||
| None | 60 (78) | 48 (80) | 8 (80) | 1 (50) | 3 (60) |
| Any | 17 (22) | 12 (20) | 2 (20) | 1 (50) | 2 (40) |
| Minor | 4 (5) | 1 (2) | 1 (10) | 1 (50) | 1 (20) |
| Moderate | 8 (10) | 7 (12) | 1 (10) | 0 | 0 |
| Major | 5 (6) | 4 (7) | 0 | 0 | 1 (20) |
| Wound complication necessitating any intervention (moderate/major) | 13 (17) | 11 (18) | 1 (10) | 0 | 1 (20) |
| Total surgical cases | 77 (100) | 60 (78) | 10 (13) | 2 (3) | 5 (7) |
Wound complication data were available for all 77 patients, stratified by tumor location.
Discussion
In the DOREMY trial, we found that decreasing the dose of preoperative radiotherapy for myxoid liposarcoma from 50 Gy to 36 Gy was oncologically safe. An extensive pathological treatment response was observed in 91% of patients, which met the primary end point. After a median follow-up time of 25 months, no local relapses occurred. The wound complication rate that required intervention was only 17%, and any grade 2 or higher late toxic effects were found in 14% of patients.
Comparing the experimental preoperative radiotherapy regimen of 36 Gy with historical data of a conventional 50-Gy regimen, we observed similar outcomes and a more favorable toxic effect profile. The dose reduction did not compromise the quality of surgical technique and local control, as reported in previous studies on myxoid liposarcoma with conventional radiotherapy doses.7,24,25 The observed wound complication rate of 17% was substantially lower than the 35% in the preoperative radiotherapy group in the randomized clinical trial by O’Sullivan et al,8 using identical wound complication definitions (Table 3 and eTable 2 in Supplement 2). Furthermore, when patients with less than 2 years of follow-up were not taken into account, the late toxic effect rates seemed to be at least 3-fold lower.9 Some of these differences could be explained by the use of 3-dimensional radiotherapy by O’Sullivan et al,8 whereas the current trial used IMRT. This difference is underscored by the more favorable morbidity rates reported in subsequent single-group phase 2 trials of the value of modern radiotherapy planning techniques.34,35 Although 1 of these trials (RTOG-063035) reported a similar grade 2 or higher late toxic effects rate (11%), from the use of image-guided IMRT and decreased target volumes, the grade 2 or higher late toxic effects rate in the other study (24%)34 and the wound complication rates in both studies (31% and 36%)34,35 were clinically relevant and higher compared with the rates in the current trial. Although we recognize the potential biases that complicate these comparisons, in the setting of rare diseases, we also believe these data from other trials are the most robust available and help put the DOREMY trial findings in perspective.
Table 3. Overview of the Most Relevant Studies on Morbidity and Local Control After Preoperative Radiotherapy in Soft-Tissue Sarcomas.
| Source | No. of patients | Design | Subgroup | Preoperative RT regimen | Rate, % | Follow-up, y | |||
|---|---|---|---|---|---|---|---|---|---|
| Grade ≥2 toxic effect | R0 | WC | LC | ||||||
| DOREMY trial | 77a | Prospective | All | 18 × 2 Gy | 14 | 94 | 17 | 100 | 2.1 |
| O’Sullivan et al,8 2002; Davis et al,9 2005 | 88a | Prospective | Preoperative group, various histological subtypes | 25 × 2 Gy | 37 | 84 | 35 | 92b | 3.3 |
| Lansu et al,10 2019 | 32c | Retrospective | Myxoid liposarcoma subgroup | 25 × 2 Gy | NA | 91 | 38 | 97 | 5 |
| Chung et al,7 2009 | 88d | Retrospective | Myxoid liposarcoma subgroup | 25 × 2 Gy | NA | 81 | NA | 98 | 5 |
| O’Sullivan et al,34 2013 | 59e | Prospective | All, various histological subtypes | 25 × 2 Gy | 24 | 93 | 31 | 93b | 4 |
| Wang et al,35 2015 (RTOG-0630 trial) | 57f | Prospective | All, various histological subtypes | 25 × 2 Gy | 11 | 76 | 36 | 89 | 2 |
Abbreviations: DOREMY, Dose Reduction of Preoperative Radiotherapy in Myxoid Liposarcomas; LC, local control; NA, not applicable; RT, radiotherapy; R0, radical resection; WC, wound complication.
The number of patients in the per-protocol analysis, which is relevant for wound complications.
Most patients in the SR2 trial had other histological subtypes than myxoid liposarcoma, so a comparison with LC rates is not applicable.
All patients with schedules other than 25 × 2 Gy preoperative RT dose were excluded. In Lansu et al,10 the definition of a WC was slightly stricter in comparison to the definition in the SR2 trial (definition: a single seroma aspiration did not meet the criteria for WC).
Only 47% of patients underwent preoperative RT; the remaining patients received postoperative RT dose of 33 × 2 Gy (43%) or preoperative RT dose of 25 × 2 Gy and postoperative RT dose of 8 × 2 Gy (10%).
This cohort included only lower extremity localizations, which is associated with a higher WC risk.
The use of 3-cm craniocaudal and 1-cm radial gross tumor volume–clinical target volume expansions resulted in decreased target volumes.
The pragmatically chosen bayesian statistical design facilitates the best level of evidence possible in rare diseases.36 With a primary end point being a short-term evaluable predictor of outcome,37,38,39,40 continuous reconsideration of trial continuation provides optimal safety for the trial patients (eTable 3 in Supplement 2). This design maximizes the sample size of the experimental treatment. Although the use of external controls is prone to bias, it does not hamper the introduction of new treatment strategies in rare cancers.41,42
We propose the use of 36 Gy in once-daily 2-Gy fractions as an alternative dose-fractionation approach of preoperative radiotherapy for myxoid liposarcoma. This approach may be feasible for resource-limited or high-acuity centers during the ongoing coronavirus disease 2019 pandemic. Demonstrating the noninferiority of this regimen in a conventional phase 3 trial is practically impossible. Therefore, we invite the international sarcoma community to participate in a registry to confirm the findings of the DOREMY trial in daily practice.
Limitations
This trial has several limitations. First, the primary end point may be subject to discussion, with regard to the optimal pathological response evaluation in STS in general and in myxoid liposarcoma in particular.32 Second, in the DOREMY cohort, the median follow-up of 25 months is relatively short. However, in the largest previous series, the median time to local recurrence was reported to be 21 months,43 suggesting that at least half of the potential local recurrences should have occurred by now in this cohort, but we have observed none. Third, we did not assess the potential advantages in quality of life, patient-reported outcomes, and health care costs resulting from the lower overall treatment time, fewer readmissions and reoperations for wound complications, and favorable toxic effect profile.
Conclusions
The DOREMY trial found that a dose reduction of preoperative radiotherapy in myxoid liposarcoma was effective and oncologically safe. Furthermore, the morbidity of the decreased dose regimen appeared to be lower than the rate of the conventional dose regimen. Therefore, we propose the use of 36 Gy in once-daily 2-Gy fractions as an alternative dose-fractionation approach of preoperative radiotherapy for myxoid liposarcoma. This approach could be reasonable for resource-limited or high-acuity centers particularly during the coronavirus disease 2019 pandemic.
Trial protocol
eTable 1. Overview of the Wound Complication Definitions
eTable 2. Late Toxicity Overview
eTable 3. Overview of Bayesian Stopping Rule and Pathological Response Data per Patient
Data Sharing Statement
References
- 1.Bock S, Hoffmann DG, Jiang Y, Chen H, Il’yasova D. Increasing incidence of liposarcoma: a population-based study of National Surveillance Databases, 2001–2016. Int J Environ Res Public Health. 2020;17(8):2710. doi: 10.3390/ijerph17082710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonescu CR, Ladanyi M. Myxoid liposarcoma In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. International Agency for Research on Cancer; 2013:39-41. [Google Scholar]
- 3.Casali PG, Abecassis N, Aro HT, et al. ; ESMO Guidelines Committee and EURACAN . Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4)(suppl 4):iv51-iv67. doi: 10.1093/annonc/mdy096 [DOI] [PubMed] [Google Scholar]
- 4.von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(5):536-563. doi: 10.6004/jnccn.2018.0025 [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197-203. doi: 10.1200/JCO.1998.16.1.197 [DOI] [PubMed] [Google Scholar]
- 6.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14(3):859-868. doi: 10.1200/JCO.1996.14.3.859 [DOI] [PubMed] [Google Scholar]
- 7.Chung PWM, Deheshi BM, Ferguson PC, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009;115(14):3254-3261. doi: 10.1002/cncr.24375 [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241. doi: 10.1016/S0140-6736(02)09292-9 [DOI] [PubMed] [Google Scholar]
- 9.Davis AM, O’Sullivan B, Turcotte R, et al. ; Canadian Sarcoma Group; NCI Canada Clinical Trial Group Randomized Trial . Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48-53. doi: 10.1016/j.radonc.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 10.Lansu J, Groenewegen J, van Coevorden F, et al. Time dependent dynamics of wound complications after preoperative radiotherapy in extremity soft tissue sarcomas. Eur J Surg Oncol. 2019;45(4):684-690. doi: 10.1016/j.ejso.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Panwar U, Sankaye P. Preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma: a changing trend towards preoperative radiotherapy in the UK. Clin Oncol (R Coll Radiol). 2015;27(6):369-370. doi: 10.1016/j.clon.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 12.Payne CE, Hofer SO, Zhong T, Griffin AC, Ferguson PC, Wunder JS. Functional outcome following upper limb soft tissue sarcoma resection with flap reconstruction. J Plast Reconstr Aesthet Surg. 2013;66(5):601-607. doi: 10.1016/j.bjps.2013.01.034 [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107(10):2455-2461. doi: 10.1002/cncr.22298 [DOI] [PubMed] [Google Scholar]
- 14.Davis AM, O’Sullivan B, Bell RS, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20(22):4472-4477. doi: 10.1200/JCO.2002.03.084 [DOI] [PubMed] [Google Scholar]
- 15.Davidge KM, Wunder J, Tomlinson G, Wong R, Lipa J, Davis AM. Function and health status outcomes following soft tissue reconstruction for limb preservation in extremity soft tissue sarcoma. Ann Surg Oncol. 2010;17(4):1052-1062. doi: 10.1245/s10434-010-0915-5 [DOI] [PubMed] [Google Scholar]
- 16.Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1-15. doi: 10.1016/j.jhin.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 17.Pitson G, Robinson P, Wilke D, et al. Radiation response: an additional unique signature of myxoid liposarcoma. Int J Radiat Oncol Biol Phys. 2004;60(2):522-526. doi: 10.1016/j.ijrobp.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Engström K, Bergh P, Cederlund CG, et al. Irradiation of myxoid/round cell liposarcoma induces volume reduction and lipoma-like morphology. Acta Oncol. 2007;46(6):838-845. doi: 10.1080/02841860601080415 [DOI] [PubMed] [Google Scholar]
- 19.Betgen A, Haas RLM, Sonke JJ. Volume changes in soft tissue sarcomas during preoperative radiotherapy of extremities evaluated using cone-beam CT. J Radiat Oncol. 2013;2(1):55-62. doi: 10.1007/s13566-012-0085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vreeze RSA, de Jong D, Haas RL, Stewart F, van Coevorden F. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int J Radiat Oncol Biol Phys. 2008;72(5):1480-1487. doi: 10.1016/j.ijrobp.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 21.Salduz A, Alpan B, Valiyev N, et al. Neoadjuvant radiotherapy for myxoid liposarcomas: oncologic outcomes and histopathologic correlations. Acta Orthop Traumatol Turc. 2017;51(5):355-361. doi: 10.1016/j.aott.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhry V, Goldberg S, DeLaney TF, et al. Myxoid liposarcoma: treatment outcomes from chemotherapy and radiation therapy. Sarcoma. 2018;2018:8029157. doi: 10.1155/2018/8029157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberge D, Skamene T, Nahal A, Turcotte RE, Powell T, Freeman C. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97(3):404-407. doi: 10.1016/j.radonc.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Moreau LC, Turcotte R, Ferguson P, et al. ; Canadian Orthopaedic Oncology Society (CANOOS) . Myxoid/round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann Surg Oncol. 2012;19(4):1081-1088. doi: 10.1245/s10434-011-2127-z [DOI] [PubMed] [Google Scholar]
- 25.Guadagnolo BA, Zagars GK, Ballo MT, et al. Excellent local control rates and distinctive patterns of failure in myxoid liposarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):760-765. doi: 10.1016/j.ijrobp.2007.07.2337 [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 27.Landberg T, Chavaudra J, Dobbs J, et al. Report 50. J ICRU. 1993;os26(1). [Google Scholar]
- 28.Landberg T, Chavaudra J, Dobbs J, et al. Report 62. J ICRU. 1999; os32(1). [Google Scholar]
- 29.Haas RLM, Delaney TF, O’Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84(3):572-580. doi: 10.1016/j.ijrobp.2012.01.062 [DOI] [PubMed] [Google Scholar]
- 30.Slide Score Accessed October 2, 2020. http://www.slidescore.com
- 31.Wardelmann E, Haas RL, Bovée JVMG, et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur J Cancer. 2016;53:84-95. doi: 10.1016/j.ejca.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 32.Schaefer IM, Hornick JL, Barysauskas CM, et al. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group response score. Int J Radiat Oncol Biol Phys. 2017;98(2):375-383. doi: 10.1016/j.ijrobp.2017.02.087 [DOI] [PubMed] [Google Scholar]
- 33.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119(10):1878-1884. doi: 10.1002/cncr.27951 [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Zhang Q, Eisenberg BL, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of Radiation Therapy Oncology Group RTOG-0630 trial. J Clin Oncol. 2015;33(20):2231-2238. doi: 10.1200/JCO.2014.58.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zohar S, Teramukai S, Zhou Y. Bayesian design and conduct of phase II single-arm clinical trials with binary outcomes: a tutorial. Contemp Clin Trials. 2008;29(4):608-616. doi: 10.1016/j.cct.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 37.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203-3209. doi: 10.1200/JCO.2001.19.13.3203 [DOI] [PubMed] [Google Scholar]
- 38.Vaynrub M, Taheri N, Ahlmann ER, et al. Prognostic value of necrosis after neoadjuvant therapy for soft tissue sarcoma. J Surg Oncol. 2015;111(2):152-157. doi: 10.1002/jso.23775 [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Harris J, Kraybill WG, et al. Pathologic complete response and survival outcomes in patients with localized soft tissue sarcoma treated with neoadjuvant chemoradiotherapy or radiotherapy: long-term update of NRG Oncology RTOG 9514 and 0630. J Clin Oncol. 2017;35(15):11012-11012. doi: 10.1200/JCO.2017.35.15_suppl.11012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salah S, Lewin J, Amir E, Abdul Razak A. Tumor necrosis and clinical outcomes following neoadjuvant therapy in soft tissue sarcoma: A systematic review and meta-analysis. Cancer Treat Rev. 2018;69:1-10. doi: 10.1016/j.ctrv.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 41.Gaddipati H, Liu K, Pariser A, Pazdur R. Rare cancer trial design: lessons from FDA approvals. Clin Cancer Res. 2012;18(19):5172-5178. doi: 10.1158/1078-0432.CCR-12-1135 [DOI] [PubMed] [Google Scholar]
- 42.Billingham L, Malottki K, Steven N. Research methods to change clinical practice for patients with rare cancers. Lancet Oncol. 2016;17(2):e70-e80. doi: 10.1016/S1470-2045(15)00396-4 [DOI] [PubMed] [Google Scholar]
- 43.Fiore M, Grosso F, Lo Vullo S, et al. Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2007;109(12):2522-2531. doi: 10.1002/cncr.22720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Overview of the Wound Complication Definitions
eTable 2. Late Toxicity Overview
eTable 3. Overview of Bayesian Stopping Rule and Pathological Response Data per Patient
Data Sharing Statement