Key Points
Question
Does fluvoxamine, a selective serotonin reuptake inhibitor and σ-1 receptor agonist, prevent clinical deterioration in outpatients with acute coronavirus disease 2019 (COVID-19)?
Findings
In this randomized trial that included 152 adult outpatients with confirmed COVID-19 and symptom onset within 7 days, clinical deterioration occurred in 0 patients treated with fluvoxamine vs 6 (8.3%) patients treated with placebo over 15 days, a difference that was statistically significant.
Meaning
In this preliminary study, adult outpatients with symptomatic COVID-19 treated with fluvoxamine, compared with placebo, had a lower likelihood of clinical deterioration over 15 days; however, determination of clinical efficacy would require larger randomized trials with more definitive outcome measures.
Abstract
Importance
Coronavirus disease 2019 (COVID-19) may lead to serious illness as a result of an excessive immune response. Fluvoxamine may prevent clinical deterioration by stimulating the σ-1 receptor, which regulates cytokine production.
Objective
To determine whether fluvoxamine, given during mild COVID-19 illness, prevents clinical deterioration and decreases the severity of disease.
Design, Setting, and Participants
Double-blind, randomized, fully remote (contactless) clinical trial of fluvoxamine vs placebo. Participants were community-living, nonhospitalized adults with confirmed severe acute respiratory syndrome coronavirus 2 infection, with COVID-19 symptom onset within 7 days and oxygen saturation of 92% or greater. One hundred fifty-two participants were enrolled from the St Louis metropolitan area (Missouri and Illinois) from April 10, 2020, to August 5, 2020. The final date of follow-up was September 19, 2020.
Interventions
Participants were randomly assigned to receive 100 mg of fluvoxamine (n = 80) or placebo (n = 72) 3 times daily for 15 days.
Main Outcomes and Measures
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting both criteria of (1) shortness of breath or hospitalization for shortness of breath or pneumonia and (2) oxygen saturation less than 92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Results
Of 152 patients who were randomized (mean [SD] age, 46 [13] years; 109 [72%] women), 115 (76%) completed the trial. Clinical deterioration occurred in 0 of 80 patients in the fluvoxamine group and in 6 of 72 patients in the placebo group (absolute difference, 8.7% [95% CI, 1.8%-16.4%] from survival analysis; log-rank P = .009). The fluvoxamine group had 1 serious adverse event and 11 other adverse events, whereas the placebo group had 6 serious adverse events and 12 other adverse events.
Conclusions and Relevance
In this preliminary study of adult outpatients with symptomatic COVID-19, patients treated with fluvoxamine, compared with placebo, had a lower likelihood of clinical deterioration over 15 days. However, the study is limited by a small sample size and short follow-up duration, and determination of clinical efficacy would require larger randomized trials with more definitive outcome measures.
Trial Registration
ClinicalTrials.gov Identifier: NCT04342663
This randomized trial compares the effects of fluvoxamine, a selective serotonin reuptake inhibitor with immunomodulatory effects vs placebo on a composite of dyspnea or pneumonia and oxygen desaturation among adult outpatients with polymerase chain reaction–confirmed mild coronavirus disease 2019 (COVID-19) illness.
Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can result in serious illness leading to hospitalization, intensive care unit admission, and death.1 Clinical deterioration typically occurs during the second week of illness. Early studies of COVID-19 found that hospitalization most often occurs within 8 to 10 days of initially mild to moderate symptoms.2,3,4 Further evidence suggested that lung damage from COVID-19 was related to an excessive inflammatory response, prompting numerous trials of immunomodulatory drugs.5,6
A potential mechanism for immune modulation is σ-1 receptor (S1R) agonism.7 The S1R is an endoplasmic reticulum chaperone protein with various cellular functions, including regulation of cytokine production through its interaction with the endoplasmic reticulum stress sensor inositol-requiring enzyme 1α (IRE1). Previous studies have shown that fluvoxamine, a selective serotonin reuptake inhibitor (SSRI) with high affinity for the S1R,8 reduced damaging aspects of the inflammatory response during sepsis through the S1R-IRE1 pathway, and decreased shock in murine sepsis models.9
Fluvoxamine is a strong S1R agonist,10,11 is highly lipophilic, and has rapid intracellular uptake.12 This study tested whether fluvoxamine, given as early treatment in individuals with mild COVID-19 illness, may prevent clinical deterioration.
Methods
This was a double-blind, placebo-controlled, randomized clinical trial that compared fluvoxamine with placebo in adult outpatients with confirmed SARS-CoV-2 infection. The trial protocol and statistical analysis plan appear in Supplement 1. The study was approved by the institutional review board at Washington University in St Louis and was conducted in compliance with the Declaration of Helsinki,13 the Good Clinical Practice guidelines, and local regulatory requirements. All participants provided informed consent via e-consent or written consent.
Study Design
This trial was conducted in the greater St Louis metropolitan area (eastern Missouri and southern Illinois). Patients were recruited from April 10, 2020, to August 5, 2020. The 30-day postrandomization follow-up assessment was completed on September 19, 2020. This was a fully remote (contactless) clinical trial.14 Participants were recruited via electronic health records, physician and other health professional referrals, study advertisements near COVID-19 testing centers and in emergency departments, referrals by colleagues, a study website, and communication in local television and newspapers. Participants were enrolled without regard to sex, race, ethnicity, or religion. Potential participants underwent screenings by email and phone, and provided informed consent, typically electronically.
Study supplies were delivered to self-quarantined study patients as a package left at their door and the study materials consisted of the study medication, an oxygen saturation monitor, an automated blood pressure monitor, and a thermometer. Participants then self-assessed using the equipment provided and confirmed vital signs within range (systolic blood pressure between 80 mm Hg and 200 mm Hg, diastolic blood pressure between 40 mm Hg and 120 mm Hg, and pulse rate between 50 beats/min and 120 beats/min), pregnancy status when indicated, and oxygen saturation of 92% or greater. Study staff called participants, informed them of eligibility, and instructed them to take the study medication. The study medication was targeted to start on the same day that participants were first contacted and screened by the research team.
All data collection was done by twice-daily REDCap surveys sent to patients via email, with phone-based data collection as backup to ensure that individuals without internet access were able to participate. The surveys recorded oxygen saturation, vital signs, medication adherence, and COVID-19 symptoms. Fixed race and ethnicity categories were used by interviewers as part of the demographic information collected to characterize the sample.
Dyspnea (shortness of breath) was measured using a continuous scale (0 = symptom is not present and 10 = symptom is very severe) with the Ecological Momentary Assessment15 (ie, “how bad is your symptom right now?”). Phone contact was attempted daily during the first 3 days of the trial to address participants’ questions, address any medication-related issues, and encourage assessment completion. Additional phone calls were conducted on a case-by-case basis when participants’ survey data indicated values outside the ranges. For participants who had worsening COVID-19 illness, study staff recommended they seek medical attention.
Participants
The study included adults living in the community with SARS-CoV-2 infection confirmed by polymerase chain reaction assay and who were symptomatic within 7 days of the first dose of study medication (Figure 1). Exclusion criteria included having COVID-19 that required hospitalization or evidence of the primary end point with oxygen saturation less than 92% on room air at the time of randomization. Other exclusion criteria were severe underlying lung disease (eg, chronic obstructive pulmonary disease or required home oxygen, interstitial lung disease, pulmonary hypertension), decompensated cirrhosis, congestive heart failure (New York Heart Association class III or IV), or immunocompromised (eg, solid organ transplant recipient or donor, bone marrow transplant recipient, AIDS, or taking immunosuppressant biologic drugs or high-dose corticosteroids [>20 mg/d of prednisone]; additional details appear in Supplement 1).
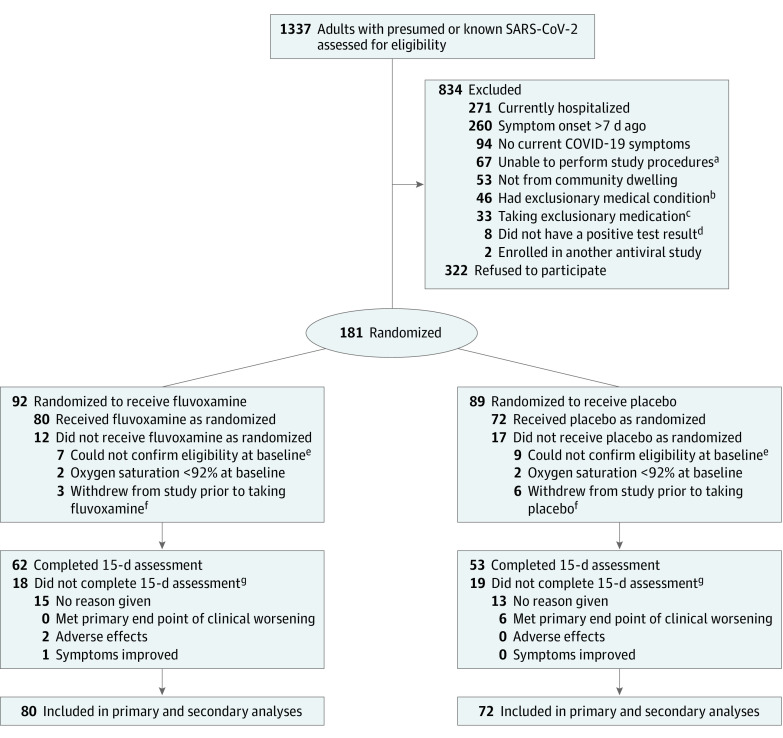
Figure 1. Enrollment and Patient Flow.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aDid not speak English, lived outside delivery area of the study, or unable to provide data via phone or internet.
bInterstitial lung disease, immunocompromised, actively suicidal or psychotic, cognitive impairment (dementia or Alzheimer disease), metastatic cancer, or end-stage congestive heart failure.
cPrednisone dose greater than 20 mg/d (most common exclusionary medication), azithromycin (not allowed at start of the study, but later allowed), hydroxychloroquine (not allowed at start of study, but later allowed), or some immunosuppressant biologic medications (such as belimumab).
dCOVID-19 suspected and patient either had a negative test result or unable to obtain test.
eStaff unable to contact potential participants.
fReceived medication and study supplies, but then research staff were unable to contact participants further.
gIncluded in analysis but censored early.
Randomization
Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated that stratified by age (18-44, 45-54, 55-64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment.
Intervention
Participants received a dose of 50 mg of fluvoxamine (or matching placebo) in the evening immediately after the baseline assessment and confirmation of eligibility, then for 2 days at a dose of 100 mg twice daily as tolerated, and then increasing to a dose of 100 mg 3 times daily as tolerated through day 15 then stopped (additional details appear in Supplement 1). This dose range was determined based on the binding affinity of fluvoxamine for the S1R.17 After the completion of 15 days of fluvoxamine or placebo, participants were given the option to receive a 6-day open-label course of fluvoxamine. This optional open-label phase was a change from the original study protocol.
Primary and Secondary End Points
The primary end point was clinical deterioration defined by both the (1) presence of dyspnea (ie, shortness of breath) or hospitalization for shortness of breath or pneumonia and (2) decrease in oxygen saturation (<92%) on room air or supplemental oxygen requirement to maintain oxygen saturation of 92% or greater. The primary end point was corroborated by phone discussion with participants and review of the medical records.
For the secondary end points, episodes of clinical deterioration were rated on a novel 7-point scale with 0 indicating none; 1, shortness of breath and oxygen saturation less than 92% but no supplemental oxygen needed; 2, shortness of breath and oxygen saturation less than 92% plus supplemental oxygen needed; 3, oxygen saturation less than 92% plus supplemental oxygen needed and hospitalization related to dyspnea or hypoxia; 4, oxygen saturation less than 92% plus supplemental oxygen needed and hospitalization related to dyspnea or hypoxia plus ventilator support needed for less than 3 days; 5, oxygen saturation less than 92% plus supplemental oxygen needed and hospitalization related to dyspnea or hypoxia plus ventilator support needed for at least 3 days; and 6, death. The number of days requiring supplemental oxygen, hospitalization, and ventilator support also were assessed.
A prespecified secondary end point in the study protocol was symptomatic severity during the 15 days of the trial using a continuous scale of each patient’s most severe baseline symptom on an 11-point scale (0 = symptom is not present and 10 = symptom is very severe). This analytic strategy was flawed (eFigure 1 in Supplement 2) and we did not pursue further analyses. As a post hoc analysis, self-reported anxiety levels were examined and were measured on the same 11-point scale because anxiety may relate to shortness of breath (eFigure 2 in Supplement 2). Clinical deterioration was ranked using the World Health Organization ordinal scale for COVID-19 trials (eTable in Supplement 2).18
The primary and secondary end points were measured using participants’ self-reported responses on twice-daily surveys during the 15 days after randomization that were corroborated by research staff with phone contact. For participants who had stopped responding to the surveys prior to day 15 or who had met the primary end point, medical records and subsequent calls to these participants were used to determine whether they met the primary end point. For participants who met the primary end point, hospital records were used to confirm specific health care use (eg, supplemental oxygen use, hospital length of stay, ventilator support). Adverse events and serious adverse events were recorded each day via participant self-report for 15 days after randomization.
At 30 days after the conclusion of the 15-day trial, a follow-up survey was performed asking, “Have you visited a hospital or emergency department since your last study survey 30 days ago?” This nonprespecified end point was confirmed by phone, email, or electronic medical record review.
Statistical Analysis
Patients were analyzed according to randomization group. Based on 80% power, an α level of .05, a rate of 20% for clinical deterioration in the placebo group, and a reduction of 75% in the risk of clinical deterioration in the fluvoxamine group, a total sample size of 152 participants was required. This magnitude of risk reduction was chosen because discovery of a large effect would be of major clinical importance and warrant further study.
As prespecified in the study protocol, the full analysis set included only participants who were confirmed eligible and started taking the study medication, which is consistent with the principles of infectious disease clinical trials.19 A study statistician (L.Y.) conducted the blinded analysis under the supervision of a senior biostatistician (J.P.M.) prior to unblinding. No interim analysis was conducted.
The primary analysis was the survival analysis for the primary outcome (clinical deterioration) using a log-rank test. This analysis treated participants a priori as censored on the day that they met the primary outcome, or on the last day that they filled out an outcome assessment. The rate of missingness for survey completion was measured. To determine if missingness was nonrandom, the available scores immediately before and after each missing score and their mean were compared with the total mean score for both shortness of breath and oxygen saturation.
Because of the potential for type I error due to multiple comparisons, the analysis of the secondary end points was exploratory. SAS version 9.4 (SAS Institute Inc) was used for all the analyses. Significance was set as a 2-tailed α level of .05.
Results
Patient Characteristics
Of 1337 patients screened, 834 (62%) were excluded, 322 (24%) were contacted and declined participation, and 181 (14%) were randomized and provided with study materials (Figure 1). Of the 181 patients randomized, 20 were excluded (9 in the fluvoxamine group and 11 in the placebo group), 9 never began taking the study medication (3 in the fluvoxamine group and 6 in the placebo group), and 152 started the study and constituted the primary analysis set. Among the 152 patients, 140 (92%) took the first dose of study medication on the same day they were first contacted by study staff (the rest started it the day after contact). A total of 35 participants opted to take open-label fluvoxamine after the double-blind phase, but no data collection was conducted for this phase.
Participants were well matched in demographic and clinical characteristics (Table 1). Of the 152 participants, 38 (25%) were Black adults and the mean age was 46 years (SD, 13 years). The most severe presenting COVID-19 symptom varied, with fatigue (23%) and loss of sense of smell (29%) being the most common. The baseline oxygen saturation level did not differ between the groups (median of 97% [interquartile range, 96%-98%] for fluvoxamine vs 97% [interquartile range, 96%-98%] for placebo (distributions shown in eFigure 3 in Supplement 2).
Table 1. Baseline Characteristics.
| Fluvoxamine (n = 80) | Placebo (n = 72) | |
|---|---|---|
| Age, median (IQR) [range], y | 46 (35-58) [20-75] | 45 (36-54) [21-69] |
| Sex at birth, No. (%) | ||
| Female | 56 (70) | 53 (74) |
| Male | 24 (30) | 19 (26) |
| Race, No. (%)a | ||
| White | 56 (70) | 50 (69) |
| Black | 18 (23) | 20 (28) |
| Asian | 3 (4) | 1 (1) |
| Other | 2 (3) | 1 (1) |
| Unknown | 1 (1) | 0 |
| American Indian/Alaska Native | 0 | 1 (1) |
| Ethnicity, No. (%)a | ||
| Non-Hispanic/Non-Latino | 75 (94) | 66 (92) |
| Hispanic/Latino | 3 (4) | 2 (3) |
| Unknown/not reported | 2 (3) | 4 (5) |
| Coexisting conditions, No. (%)a | ||
| Asthma | 17 (21) | 9 (13) |
| Hypertension | 15 (19) | 15 (21) |
| Diabetes | 9 (11) | 8 (11) |
| High cholesterol | 7 (9) | 7 (10) |
| Hyperthyroidism | 6 (8) | 6 (8) |
| Anxiety | 5 (6) | 1 (1) |
| Arthritisb | 4 (5) | 3 (4) |
| Depression | 1 (1) | 4 (6) |
| Body mass index category, No. (%)c | ||
| Underweight (<18.5) | 1 (1) | 1 (1) |
| Normal (18.5-24.9) | 14 (18) | 7 (10) |
| Overweight (25-29.9) | 22 (28) | 22 (31) |
| Obese (≥30) | 43 (54) | 42 (58) |
| Duration of COVID-19 symptoms, median (IQR) [range], da | 4 (3-5) [1-7] | 4 (3-5) [1-7] |
| Oxygen saturation, median (IQR) [range], % | 97 (96-98) [93-99] | 97 (96-98) [92-99] |
| Most severe COVID-19 symptom at baseline, No. (%)a | ||
| Loss of sense of smell | 26 (33) | 18 (25) |
| Fatigue | 17 (21) | 18 (25) |
| Body aches | 9 (11) | 13 (18) |
| Cough | 9 (11) | 1 (1) |
| Subjective fever | 8 (10) | 4 (6) |
| Loss of appetite | 3 (4) | 8 (11) |
| Chills | 3 (4) | 6 (8) |
| Shortness of breath | 2 (3) | 1 (1) |
| Loss of taste | 2 (3) | 2 (3) |
| Nausea | 1 (1) | 1 (1) |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range.
Per participant self-report.
Osteoarthritis or rheumatoid arthritis.
Calculated as weight in kilograms divided by height in meters squared.
Efficacy of Fluvoxamine vs Placebo
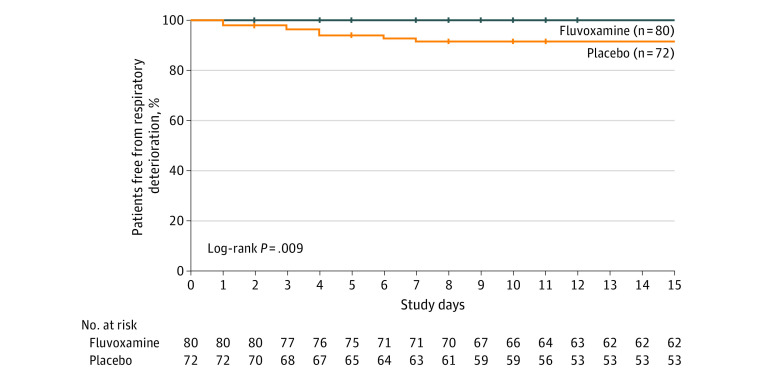
Clinical deterioration occurred in 0 of 80 patients in the fluvoxamine group and in 6 of 72 (8.3%) patients in the placebo group (absolute difference, 8.7% [95% CI, 1.8%-16.4%] by survival analysis, log-rank χ2 = 6.8 and P = .009; Table 2 and Figure 2). In the placebo group, cases of clinical deterioration ranged from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of 6 patients were hospitalized for COVID-19 illness, with the length of stay ranging from 4 to 21 days. One patient required mechanical ventilation for 10 days (Table 2) and no patients died. Detailed vignettes of clinical deterioration appear in eResults 1 in Supplement 2.
Table 2. Primary, Secondary, and Nonprespecified Outcomes.
| Fluvoxamine (n = 80) | Placebo (n = 72) | Absolute difference (95% CI)a |
P valueb | |
|---|---|---|---|---|
| Primary end point | ||||
| Clinical deterioration (met both criteria), No. (%)c | 0 | 6 (8.3) | 8.7 (1.8 to 16.4) | .009 |
| Secondary end points | ||||
| Clinical status on 7-point scale, No. (%)d | ||||
| 0 (none) | 80 (100) | 66 (91.7) | 8.3 (0.6 to 18.4) | .009 |
| Any nonzero value | 0 | 6 (8.3) | −8.3 (−18.4 to −0.6) | .009 |
| 1 (shortness of breath and oxygen saturation <92% but no supplemental oxygen needed) | 0 | 2 (2.8) | −2.8 (−10.8 to 3.5) | .15 |
| 3 (oxygen saturation <92% plus supplemental oxygen needed and hospitalization related to dyspnea or hypoxia) | 0 | 3 (4.2) | −4.2 (−13.2 to 2.0) | .07 |
| 5 (oxygen saturation <92% plus supplemental oxygen needed and hospitalization related to dyspnea or hypoxia plus ventilator support needed for ≥3 days) | 0 | 1 (1.4) | −1.4 (−8.4 to 4.4) | .36 |
| Clinical status on 7-point scale, mean (SD) | 0 | 0.22 (0.84) | −0.22 (−0.41 to −0.04) | .02 |
| Clinical deterioration, No. of dayse | NA | NA | NA | NA |
| Most severe baseline symptom change score (difference between baseline and final rating)f | −5.6 | −5.8 | 0.3 (−0.8 to 1.4) | .63 |
| Nonprespecified end points | ||||
| 30-d post trial observation events (emergency department visit, hospitalization, or both)g | 1 (1.3) | 1 (1.4) | −0.1 (−6.7 to 5.1) | >.99 |
Abbreviation: NA, not applicable (see footnote “e” for explanation).
For outcomes reported as No. (%), the absolute difference is a difference in proportions. For other variables, the difference between group means is reported. Most analyses were conducted using BinomCI from the R package ExactCIdiff.
Most were calculated using the exact.test from the R package Exact. The log-rank χ2 was used (χ2 = 6.8) for the primary end point. The t test was used for clinical status on 7-point scale (t = −2.4) and the most severe baseline symptom change (t = 0.5).
Shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation dropped below 92% or supplemental oxygen was required to keep oxygen saturation at or above 92%. The prespecified primary outcome analysis was determined instead by survival analysis (time to clinical worsening). The absolute difference and 95% CI are for the Kaplan-Meier estimate of the placebo group at day 15. The test of difference is the log-rank statistic (χ2 = 6.8).
No study participants were rated 2 (shortness of breath and oxygen saturation <92% plus supplemental oxygen needed), 4 (oxygen saturation <92% plus supplemental oxygen needed and hospitalization related to dyspnea or hypoxia plus ventilator support needed for <3 days), or 6 (death).
The protocol included a plan to examine number of days (1) requiring oxygen, (2) requiring hospitalization, and (3) requiring ventilator support. This type of outcome measure turned out to be invalid for this study because few patients required these interventions; therefore, a statistical analysis comparing the number of days was not appropriate.
Change from day 0 to day 15. The mean of the highest daily symptom score for each participant that was reported most severe at baseline (62 for fluvoxamine group and 54 for placebo group). This analysis was not pursued further because the curves showed no substantial differences and because the baseline most severe symptom was heterogeneous across participants (Table 1) and likely did not adequately capture overall symptom burden. eFigure 1 in Supplement 2 is a box and whisker plot of the symptom data over the 15 days.
During the 30-day observation period after the 15-day randomized clinical trial, 1 participant from the fluvoxamine group was hospitalized for post-COVID headache and 1 participant from the placebo group had an emergency department visit for chest pain (costochondritis COVID-19 sequela). Details appear in eResults 2 in Supplement 2.
Figure 2. Time to Clinical Deterioration in the Fluvoxamine and Placebo Groups.
The median observation time was 15 days (interquartile range, 15-15 days) for the fluvoxamine group and 15 days (interquartile range, 15-15 days) for the placebo group. Study day 0 indicates the day of randomization.
Among fluvoxamine-treated participants, 18 of 80 stopped responding to the surveys prior to day 15 compared with 19 of 72 who were randomized to placebo. For the nonprespecified outcome of hospital or emergency department care received during the 30 days after day 15 of the trial, among fluvoxamine-treated participants, 1 of 80 received care (hospitalized for headache) compared with 1 of 72 placebo-treated participants (emergency department visit for costochondritis) (eResults 1 in Supplement 2).
Adverse Events
The fluvoxamine group had 1 serious adverse event and 11 other adverse events, whereas the placebo group had 6 serious adverse events and 12 other adverse events (Table 3 and eResults 1 in Supplement 2). Pneumonia and gastrointestinal symptoms (such as nausea and vomiting) occurred more often in the placebo group compared with those who received fluvoxamine.
Table 3. Adverse Events.
| No. of adverse events (%)a | ||
|---|---|---|
| Fluvoxamine (n = 80) | Placebo (n = 72) | |
| Pneumonia | 3 (3.8) | 6 (8.3) |
| Shortness of breath | 2 (2.5) | 4 (5.6) |
| Headache or head pain | 2 (2.5) | 1 (1.4) |
| Gastroenteritis, nausea, or vomiting | 1 (1.3) | 5 (6.9) |
| Muscle aches | 1 (1.3) | 0 |
| Bacterial infection | 1 (1.3) | 0 |
| Vasovagal syncope | 1 (1.3) | 0 |
| Teeth chattering | 1 (1.3) | 0 |
| Dehydration | 1 (1.3) | 0 |
| Low oxygen saturation or hypoxia | 0 | 6 (8.3) |
| Chest pain or tightness | 0 | 2 (2.8) |
| Fever | 0 | 2 (2.8) |
| Acute respiratory failure | 0 | 1 (1.4) |
| Hypercapnia | 0 | 1 (1.4) |
| Flank pain | 0 | 1 (1.4) |
| By No. of patients | ||
| Serious adverse eventsb | 1 (1.3) | 5 (6.9) |
| Other adverse eventsc | 11 (13.8) | 6 (8.3) |
In some cases, there was more than 1 symptom or problem that occurred as part of 1 adverse event. Additional details of adverse events appear in eResults 1 in Supplement 2.
One patient in the placebo group had more than 1 serious adverse event. The total No. of serious adverse events was 1 in the fluvoxamine group and 6 in the placebo group.
There were patients in the placebo group who had more than 1 other adverse event. The total No. of other adverse events was 11 in the fluvoxamine group and 12 in the placebo group.
Missing Data
In terms of missing data, 517 of 3943 follow-up surveys (13%) were not filled out by participants. The mean score for those with missing data (0.80) was not different from the overall mean score for shortness of breath (0.83) and the median was 0 for both missing data and overall. The mean score for oxygen saturation was 97.3% for both those with missing data and overall and the median was 98% for both. Therefore, the data appeared to be missing at random and no data imputation was conducted. For the participants who stopped responding to the surveys prior to day 15 because they met the primary end point or for other reasons (Figure 1), the data were censored. In 31 individuals who stopped responding to the surveys prior to day 15 for other reasons, we confirmed that none received medical care at a hospital or emergency department for worsening COVID-19. However, for 6 of these individuals, we could not exclude the possibility that they received care at an urgent care center that was outside the major regional hospital systems.
Discussion
In this preliminary randomized clinical trial, fluvoxamine (an S1R agonist) was associated with a reduction in clinical deterioration in adult outpatients with COVID-19. No fluvoxamine-treated patients met criteria for clinical deterioration as defined in the study, whereas 8.3% of patients taking placebo met this end point. However, because of study limitations, these findings need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.
This double-blind, placebo-controlled, randomized clinical trial demonstrated the feasibility of a fully remote (contactless) study during the COVID-19 pandemic. Adult outpatients with COVID-19 are in self-quarantine, but few studies have focused on the care of this vulnerable population. This design included a short time from symptom onset to first dose of medication (median, 4 days), efficient study treatment initiation (92% took the first dose on the same day as they were contacted), and representative sample of race and sex.20 The study required approximately 4500 hours of staff time and 30 hours of time per participant.
If fluvoxamine is determined to be effective in treating COVID-19, the underlying mechanism needs further clarification. The study was prompted by a hypothesis involving the influence of fluvoxamine on the S1R-IRE1 pathway. Anti-inflammatory (cytokine reduction) actions resulting from S1R activation would fit with recent findings of benefits of other anti-inflammatory drugs, such as colchicine and corticosteroids, for COVID-19.21,22 However, a recent study found lower levels of cytokines in patients with severe COVID-19 vs patients with bacterial sepsis.23 Alternative mechanisms of a potential fluvoxamine benefit include direct antiviral effects via its lysosomotropic properties,24 modulation of the effect of IRE1 effects on autophagy,25 and SSRI inhibition of platelet activation.26
The potential advantages of fluvoxamine for outpatient treatment of COVID-19 include its safety,27 widespread availability, low cost, and oral administration. Fluvoxamine does not promote QT prolongation unlike other SSRIs.28 However, fluvoxamine has adverse effects and can cause drug-drug interactions, particularly via inhibition of cytochromes P450 1A2 and 2C19.29
Limitations
This study has several limitations. First, it was a small study and it was conducted within a single geographic area, so these findings should be regarded as preliminary. The study needs to be replicated in larger trials with a more heterogeneous study population.
Second, there was a small number of end point events, which makes the findings extremely fragile. Third, it is possible that the differences in clinical deterioration may have been a reflection of the comparative baseline distributions of oxygen saturation rather than an effect of treatment.
Fourth, the method of measuring the most severe baseline symptom over time did not appear to provide valid data, so potential effects of fluvoxamine on symptomatic improvement are unknown. Fifth, 20% of study participants stopped responding to surveys during the 15-day trial. Although it was confirmed that none of these participants required medical care, such as hospitalization or an emergency department visit, it is possible that some received care at an urgent care center outside the major regional hospital systems.
Sixth, the follow-up duration was short and did not measure the effect of fluvoxamine on persistent symptoms or late deterioration. For example, individuals with COVID-19 may develop cardiac injury,30 which may be common and persistent, even in otherwise mild or recovered cases.31 Because S1R agonists have cardioprotective effects in rodents32 and protective effects in other tissues,33 future COVID-19 treatment trials should examine long-term outcomes and measures of cardiopulmonary function. Seventh, the 7-point ordinal scale created for this study to classify clinical deterioration has not been validated.
Conclusions
In this preliminary study of adult outpatients with symptomatic COVID-19, patients treated with fluvoxamine, compared with placebo, had a lower likelihood of clinical deterioration over 15 days. However, the study is limited by a small sample size and short follow-up duration, and determination of clinical efficacy would require larger randomized trials with more definitive outcome measures.
Trial protocol and statistical analysis plan
eFigure 1. Improvement in most severe baseline COVID-19 symptom
eFigure 2. Anxiety ratings in the fluvoxamine vs. placebo groups during the 15-day RCT
eFigure 3. Distributions of baseline oxygen saturation in the fluvoxamine and placebo groups and clinical deterioration as a function of baseline oxygen saturation
eTable. Clinical deterioration findings ranked on study-specific 7-point (0-6) ordinal scale of clinical deterioration (prespecified secondary endpoint) and the World Health Organization (WHO) nine-point (0-8) ordinal scale for clinical improvement (post-hoc analysis)
eResults 1. Summaries of the six participants who showed Clinical Deterioration (all Placebo, all who were hospitalized were also considered SAEs)
eResults 2. Additional hospitalizations/ER visits during 30-day post-RCT observation period
Data sharing statement
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). JAMA. 2020;324(8):782-793. [DOI] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe. Lancet Infect Dis. 2020;20(6):697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad A, Prasad M. Single virus targeting multiple organs. Front Med (Lausanne). 2020;7:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol GE, Karp JF, Reiersen AM, et al. “What were you before the war?” J Clin Psychiatry. Published online April 7, 2020. doi: 10.4088/JCP.20com13373 [DOI] [PubMed] [Google Scholar]
- 8.Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167-173. [DOI] [PubMed] [Google Scholar]
- 9.Rosen DA, Seki SM, Fernández-Castañeda A, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11(478):eaau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J Pharmacol Sci. 2015;127(1):6-9. [DOI] [PubMed] [Google Scholar]
- 11.Cobos EJ, Entrena JM, Nieto FR, et al. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr Neuropharmacol. 2008;6(4):344-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallifax D, Houston JB. Saturable uptake of lipophilic amine drugs into isolated hepatocytes. Drug Metab Dispos. 2007;35(8):1325-1332. [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 14.Nicol GE, Piccirillo JF, Mulsant BH, Lenze EJ. Action at a distance. J Am Geriatr Soc. 2020;68(5):922-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mofsen AM, Rodebaugh TL, Nicol GE, et al. When all else fails, listen to the patient. JMIR Ment Health. 2019;6(5):e11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19). Morbid Mortal Weekly Rep. 2020;69(12):343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa M, Ishiwata K, Ishii K, et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine. Biol Psychiatry. 2007;62(8):878-883. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Novel coronavirus. Accessed November 2, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
- 19.Gillings D, Koch G. The application of the principle of intention-to-treat to the analysis of clinical trials. Drug Information J. 1991;25:411-424. doi: 10.1177/009286159102500311 [DOI] [Google Scholar]
- 20.Chastain DB, Osae SP, Henao-Martínez AF, et al. Racial disproportionality in Covid clinical trials. N Engl J Med. 2020;383(9):e59. [DOI] [PubMed] [Google Scholar]
- 21.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019. JAMA Netw Open. 2020;3(6):e2013136-e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020;324(13):1330-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kox M, Waalders NJB, Kooistra EJ, et al. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020;324(15):1565-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homolak J, Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int J Antimicrob Agents. 2020;56(2):106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung TS, Liu DX. The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus. Virology. 2019;533:34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlienger RG, Meier CR. Effect of selective serotonin reuptake inhibitors on platelet activation. Am J Cardiovasc Drugs. 2003;3(3):149-162. [DOI] [PubMed] [Google Scholar]
- 27.Omori IM, Watanabe N, Nakagawa A, et al. Efficacy, tolerability and side-effect profile of fluvoxamine for major depression. J Psychopharmacol. 2009;23(5):539-550. [DOI] [PubMed] [Google Scholar]
- 28.Assimon MM, Brookhart MA, Flythe JE. Comparative cardiac safety of selective serotonin reuptake inhibitors among individuals receiving maintenance hemodialysis. J Am Soc Nephrol. 2019;30(4):611-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen M, Tybring G, Mihara K, et al. Low daily 10-mg and 20-mg doses of fluvoxamine inhibit the metabolism of both caffeine (cytochrome P4501A2) and omeprazole (cytochrome P4502C19). Clin Pharmacol Ther. 2002;71(3):141-152. [DOI] [PubMed] [Google Scholar]
- 30.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19). Prog Cardiovasc Dis. 2020;63(3):390-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. Published online July 27, 2020. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor. Prog Neurobiol. 2013;100:15-29. [DOI] [PubMed] [Google Scholar]
- 33.Hosszu A, Antal Z, Lenart L, et al. σ1-receptor agonism protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2017;28(1):152-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eFigure 1. Improvement in most severe baseline COVID-19 symptom
eFigure 2. Anxiety ratings in the fluvoxamine vs. placebo groups during the 15-day RCT
eFigure 3. Distributions of baseline oxygen saturation in the fluvoxamine and placebo groups and clinical deterioration as a function of baseline oxygen saturation
eTable. Clinical deterioration findings ranked on study-specific 7-point (0-6) ordinal scale of clinical deterioration (prespecified secondary endpoint) and the World Health Organization (WHO) nine-point (0-8) ordinal scale for clinical improvement (post-hoc analysis)
eResults 1. Summaries of the six participants who showed Clinical Deterioration (all Placebo, all who were hospitalized were also considered SAEs)
eResults 2. Additional hospitalizations/ER visits during 30-day post-RCT observation period
Data sharing statement