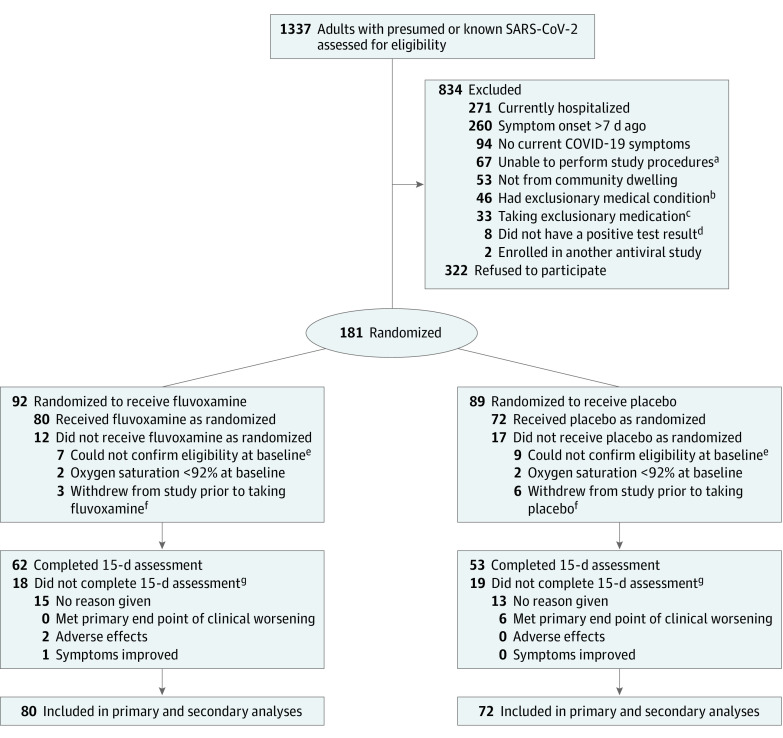
Figure 1. Enrollment and Patient Flow.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aDid not speak English, lived outside delivery area of the study, or unable to provide data via phone or internet.
bInterstitial lung disease, immunocompromised, actively suicidal or psychotic, cognitive impairment (dementia or Alzheimer disease), metastatic cancer, or end-stage congestive heart failure.
cPrednisone dose greater than 20 mg/d (most common exclusionary medication), azithromycin (not allowed at start of the study, but later allowed), hydroxychloroquine (not allowed at start of study, but later allowed), or some immunosuppressant biologic medications (such as belimumab).
dCOVID-19 suspected and patient either had a negative test result or unable to obtain test.
eStaff unable to contact potential participants.
fReceived medication and study supplies, but then research staff were unable to contact participants further.
gIncluded in analysis but censored early.