Abstract
This survey study examines the attitudes of a large group of cancer survivors toward trial participation during the coronavirus disease 2019 outbreak.
The coronavirus disease 2019 (COVID-19) outbreak substantially reduced cancer clinical trial accrual.1 Many sites temporarily paused enrollment owing to state, local, sponsor, or institutional restrictions intended to prevent the spread of COVID-19.2 Once site and enrollment restrictions lift, it is unclear whether patients will be as willing to participate in clinical research as before the outbreak, especially if community COVID-19 transmission is still occurring. To examine this, we surveyed a large group of cancer survivors about their attitudes toward trial participation during the COVID-19 pandemic.
Methods
Participants were included from the American Cancer Society Cancer Action Network’s Survivor Views panel (established September 2019). Panelists were 18 years or older, had been diagnosed with and/or treated for cancer within the last 5 years, and were US residents. We designed a series of COVID-19–related questions regarding disposition toward trials, willingness to participate, and reasons for nonparticipation. These questions were incorporated into the existing survey program3 and sent to 3054 participants on May 27, 2020, through June 17, 2020. A total of 933 responses (30.6%) were received.
The survey study was deemed exempt by the Morehouse School of Medicine Institutional Review Board. Patient informed consent was required of participants in the study. The sample size enabled estimation of any particular response to within 3.3%. We used χ2 tests for comparisons, and α = .05 indicated statistical significance.
Results
Among the 933 respondents with known data, 675 of 924 (73.1%) were female, 33 of 920 (3.6%) self-reported as Black, and 284 of 924 (36.6%) had an annual household income of $60 000 or less. Overall, 316 of the 933 (33.9%) respondents reported a prior conversation with their physician about clinical trials, and 192 (20.6%) were offered trial participation. Among the 192 respondents offered a trial, 150 (78.1%) said yes and 116 (60.4%) eventually enrolled, resulting in an overall participation rate of 12.4%. Among 662 respondents not offered trial participation, 519 (78.4%) reported being somewhat or very likely to enroll if offered a trial.
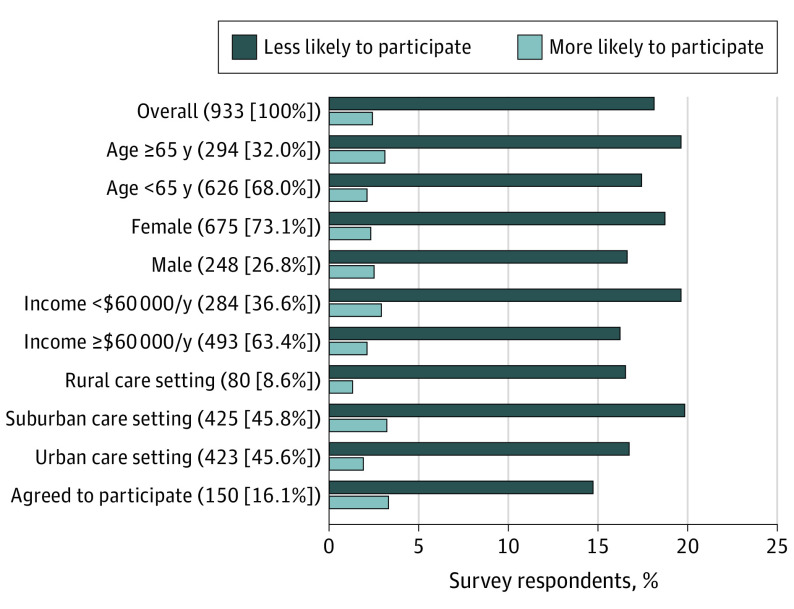
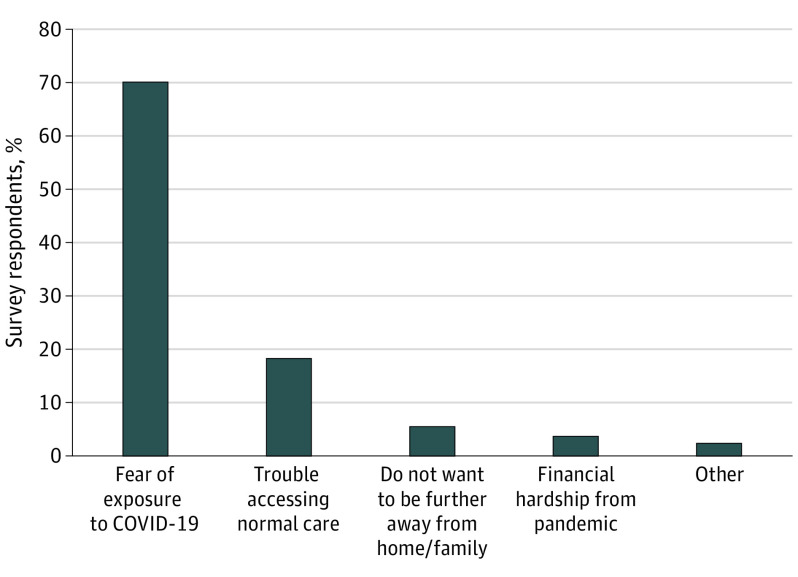
All respondents were asked if the pandemic made them more or less likely to participate in a clinical trial, or if it made no difference. Among 907 respondents, the majority (721 [79.5%]) indicated no difference; remaining respondents were more than 7 times more likely to indicate that the pandemic made them less likely to enroll in a clinical trial (164 [18.1%] vs 22 [2.4%]). Response patterns were similar across demographic, socioeconomic, and care settings, and in the subset of 150 participants who previously agreed to trial participation (Figure 1). Among the 164 respondents less likely to enroll, the most common reasons were fear of increased COVID-19 exposure (115 [70.1%]) or difficulty accessing care during the pandemic (30 [18.3%]) (Figure 2).
Figure 1. Change in Likelihood to Participate in Cancer Clinical Trials in Light of COVID-19 Pandemic.
Responses indicating no difference are not shown. There were no differences in the rates of those replying that they were more likely to participate vs less likely to participate by levels of age, gender, household income, or care setting. Percentages are based on respondents with known data.
Subgroup examinations by race were not included owing to the small sample of non-White patients, including Black (n = 33), Asian/Pacific Islander (n = 19), and Native American (n = 14). Unknown patient data included age (n = 13), race (n = 13), household income (n = 9), gender (n = 9), and care setting (n = 5). One participant indicated that they were transgender. For 147 participants, household income was recorded as prefer not to answer. The majority of participants (69.1%) had 1 of the 4 most common cancers (breast [45.9%], colorectal [7.4%], lung [7.7%], or prostate [8.1%]).
Figure 2. Reasons for Reduced Likelihood of Participating in Clinical Trials.
Discussion
Despite a strong predisposition to participate in clinical research, nearly one-fifth of patients with cancer reported that they would be less likely to participate in a trial because of fears surrounding COVID-19. This observation was limited by reliance on a survey of volunteers with cancer, whose demographics and attitudes toward trial participation may not be representative of all patients with cancer, as well as by a 30.6% overall response rate. Nonetheless, the difference between those less vs more willing to participate in trials in light of the pandemic was stark, was consistently observed across evaluable demographic/socioeconomic patient groups, and extended even to those who previously agreed to participate and who had the most imminent opportunity to enroll.
The present findings suggest that as long as high rates of COVID-19 cases exist, patients with cancer will be less likely to consider trial participation even when sites return to prepandemic status. The National Cancer Institute and the US Food and Drug Administration have provided guidance on increasing flexibility for trial investigators during the COVID-19 pandemic.4,5 These guidelines focus on reducing COVID-19 exposure or offering alternative care settings.6 Trial sponsors will need to take full advantage of the approaches indicated in these guidelines to better address patient fears about clinical trial participation while the COVID-19 pandemic endures.
References
- 1.Unger JM, Blanke CD, LeBlanc M, Hershman DL. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open. 2020;3(6):e2010651. doi: 10.1001/jamanetworkopen.2020.10651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1:1-3. doi: 10.1038/s43018-020-0074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society Cancer Action Network . COVID survey questions. Accessed October 8, 2020. https://www.fightcancer.org/sites/default/files/docs/resources/CT_COVID_Survey%20Quetions.pdf
- 4.National Cancer Institute . Coronavirus guidance. Updated April 1, 2020. Accessed April 9, 2020. https://ctep.cancer.gov/investigatorResources/corona_virus_guidance.htm
- 5.US Food and Drug Administration . FDA guidance on conduct of clinical trials of medical products during COVID-19 public health emergency. Updated September 21, 2020. Accessed October 8, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-pandemic
- 6.Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290. doi: 10.1126/science.abd3377 [DOI] [PubMed] [Google Scholar]