Abstract
A 23-year-old Caucasian woman, presented with recurrent fevers, elevated liver function tests and pancytopenia. Her labs at presentation were white blood cells 1.5 ×109/L, haemoglobin 8 g/L, platelets 59 k/mcl, lactate dehydrogenase (LDH) over 2000 U/L, aspartate aminotransferase 593 U/L, alanine aminotransferase 1321 U/L, alkaline phosphatase 223 U/L and ferritin 7665 µg/L. Epstein-Barr virus (EBV) IgM and IgG antibodies were positive in serum. A soluble interleukin 2 receptor was elevated at 2458. A bone marrow biopsy revealed scattered macrophages containing erythrocytes and other cellular elements. Immunohistochemistry for CD68 highlighted macrophages with erythrophagocytosis and in situ hybridisation was positive for EBV. She met the diagnostic criteria for haemophagocytic lymphohistiocytosis (HLH). She was initially treated with broad spectrum antibiotics which were eventually discontinued once the diagnosis was established. Over a period of 2–3 weeks her fever, transaminitis, ferritin and LDH improved spontaneously. She continued to improve clinically and was subsequently discharged. HLH is an aggressive, life-threatening hyper-inflammatory syndrome which, if not promptly recognised and treated, can be fatal. Treatment involves etoposide-based chemotherapy and possible stem-cell transplantation. This patient showed signs of improvement spontaneously and a decision was made to not treat her. This was a rare case of EBV-associated HLH which resolved spontaneously without any intervention. This young patient was not subjected to unnecessary chemotherapy. So far only few cases of spontaneous resolution of EBV-associated HLH have been reported.
Keywords: infections, haematology (incl blood transfusion), pathology, contraindications and precautions
Background
Haemophagocytic lymphohistiocytosis (HLH) is an aggressive, life-threatening hyper-inflammatory syndrome, which if not promptly recognised and treated, can be fatal. Primary HLH is hereditary and seen most commonly in children as an autosomal recessive disease affecting immune regulation.1 Secondary HLH, also known as macrophage-activating syndrome if caused by a rheumatological disorder, develops at any age and is a response to a strong immunological trigger such as infection. Epstein-Barr virus (EBV) is one such virus that integrates in B lymphocytes acting as a trigger for this exaggerated immune response. Cardinal features of HLH include fever, hepatosplenomegaly, pancytopenia and haemophagocytosis.2
In the following case, we present a patient with EBV infection who developed secondary HLH. Classically, the treatment of EBV-associated HLH involves immunosuppressive agents, chemotherapy, and in severe cases, stem-cell transplantation.3 Interestingly, our patient improved spontaneously without any treatment which is something not reported previously to our knowledge.
Case presentation
A 23-year-old Caucasian woman with no significant medical history presented with shortness of breath and cough. She was given inhaler therapy and 40 mg oral prednisone taper for 10 days believing she had an asthma attack. Over the next 2 days, she developed fatigue, recurrent fevers, nausea and vomiting. She returned to the hospital where physical examination was only remarkable for exudate in her throat. Her labs at presentation were significant for white blood cells 1.5 k/mcl, haemoglobin 8 g/dL, platelets 59 k/mcl, lactate dehydrogenase (LDH) over 2000 U/L, aspartate aminotransferase 593 U/L, alanine aminotransferase 1321 U/L, alkaline phosphatase 223 U/L and ferritin 7665 µg/L. EBV IgM and IgG antibodies were positive in serum. A soluble interleukin 2 (IL-2) receptor (IL2R) was elevated at 2458 U/L. Ultrasound of the spleen showed a normal-sized spleen. She was admitted to the hospital for management. Over the next few days, she continued to have fatigue and had persistent fever despite being on broad spectrum antibiotics. Her transaminitis and complete blood count also continued to be abnormal.
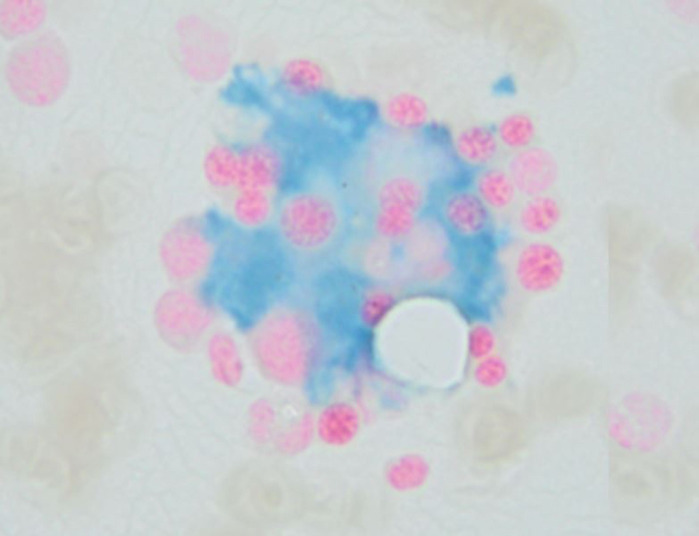
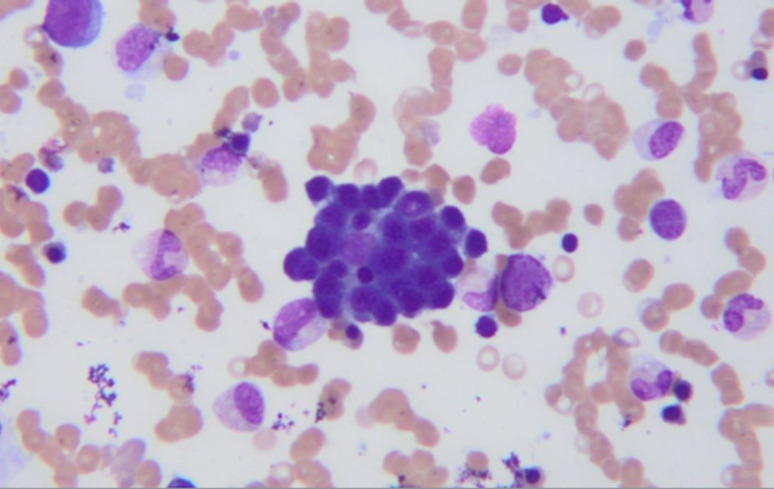
A bone marrow biopsy revealed scattered macrophages containing erythrocytes and other cellular elements (figure 1). Immunohistochemistry for CD68 highlighted macrophages with erythrophagocytosis and in situ hybridisation was positive for EBV (figure 2). She met 6/8 of HLH-2004 criteria for HLH (5/8 criteria required and her H-score was 215 (93%–96% probability of HLH).
Figure 1.
A bone marrow biopsy showing scattered macrophages containing erythrocytes and other cellular elements.
Figure 2.
Immunohistochemistry for CD68 highlighted macrophages with erythrophagocytosis seen.
At the time of diagnosis, the patient was already slowly clinically improving. She no longer had fevers so steroids were never initiated. Broad spectrum antibiotics were eventually discontinued once the diagnosis was established. Over the next few days, she spontaneously began to improve and her transaminitis and LDH levels were beginning to drop. Decision was made not to treat and monitor closely since it was felt that she may not require primary treatment of HLH. Over a period of 2–3 weeks, her fever, transaminitis, ferritin and LDH improved significantly with no intervention. Soon after, she became clinically stable for discharge with outpatient haematology follow-up and remains completely asymptomatic and disease free.
Outcome and follow-up
The patient was followed during her entire hospitalisation by the haematology team. The patient had complete resolution of her symptoms.
She continues to have 6-month follow-up with the haematology team with repeat blood work to assure her complete blood count and comprehensive metabolic panel are within normal limits.
Discussion
HLH is characterised by a multisystem inflammation that is a result of activation of antigen-presenting cells, such as macrophages and histiocytes, and CD8+ T cells. It has also been associated with abnormalities in natural killer (NK) cells which are responsible for maintaining a homeostasis between immune responsiveness and control of autoimmune conditions. This severe autoimmune response is responsible for the clinical and laboratory findings associated with HLH. The clinical syndrome seen in HLH is a result of this excessive activation of lymphocytes and macrophages.4 5 The mechanism with which EBV infection causes HLH is not very clear. EBV-infected B cells trigger cytotoxic T lymphocytes (known as CD8+ T cells) and NK cells to proliferate as a response to the infection. It is believed that these activated cytotoxic T lymphocytes lead to fulminant proliferation of T cells that leads to HLH.6 7
Diagnosis for both primary and secondary HLH was previously based on the diagnostic criteria proposed by Henter et al in 2004 for paediatric population.8 While some investigators proposed minor changes to adjust for the adult population such as a higher cut-off for ferritin (>3000 µg/L), nonetheless there was concern that HLH was underdiagnosed due to the lack of well-defined diagnostic criteria.9 10 For example, haemophagocytosis on biopsy was previously considered a gold standard; however it is often seen in patients with sepsis in a critical care setting. These patients have significant histiocytic hyperplasia with no clinical findings of HLH.11 This prompted the need to develop a diagnostic score, such as the H-score in 2014, which is more specific when compared with the HLH-2004 and has become a new standard of diagnosis for HLH in adults.12 13 H-score consists of nine variables including clinical, cytological and biological measurements and the score ranges from 0 to 302. In a study by Fardet et al, an H-score of 169 or above had sensitivity of 93% and specificity of 86% for the diagnosis of HLH.12 Our patient met 6/8 of HLH-2004 criteria and had H-score of 215 which corresponds to a specificity of 93%–96% for the diagnosis of HLH.12 The other differentials considered were primary EBV infection that can result in a lot of symptoms including fever, hepatitis, elevated ferritin and cytopenia, but haemophagocytosis on bone marrow biopsy and elevated IL2R pointed away from the diagnosis. We did not find any other infection or evidence of malignancy in the patient. An elevated H-score also is consistent with diagnosis of HLH.
Treatment of HLH involves controlling the cytokine release known as ‘the cytokine storm’ that causes coagulopathy and multiple organ failure as well as eradicating the EBV-infected T cells. The HLH-94 and HLH-2004 guidelines both rely on a combination of dexamethasone, etoposide, cyclosporine, and, in select patients, intrathecal methotrexate (HLH-94). In some cases, eradication of the proliferating EBV-containing T cells or NK cells can only be done with a haemopoietic stem cell/bone marrow transplant especially in primary, severe or relapsing HLH.14 15 EBV-associated HLH is often fatal and was previously treated with intravenous immunoglobulin and steroids alone. Due to its limited benefit in many cases, the introduction of etoposide-containing regimens was necessary. It significantly improved survival and was effective in controlling the progression of the disease, although, the risk of neutropenia-associated opportunistic infections was significantly higher.16 In our patient, due to spontaneous improvement in her clinical condition, improvement in transaminitis and LDH levels, we planned to observe her closely in our inpatient unit. The plan was to start steroid treatment in case her clinical condition deteriorates; however, she continued to improve and did not require any treatment.
Learning points.
This is a rare case of Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis (HLH) that resolved spontaneously without any intervention.
This young patient was not subjected to unnecessary immunosuppressive treatment or chemotherapy. Treatment of HLH can be life-saving but also interferes with the normal functioning of the immune system.
In severe cases, the early introduction of high-dose steroids, immunosuppressant and/or etoposide-based chemotherapy is necessary and can be life-saving. However, we make the argument that such aggressive therapy is not universally needed after diagnosis is made.
It is important to follow the patient closely in order to determine whether they are improving and chemotherapy can be avoided.
Footnotes
Contributors: ZC conducted the case review of this case and prepared the manuscript draft with important intellectual input from YS and TJ. All authors approved the final manuscript. SF edited the final manuscript. We would like to thank SF for his editorial support during preparation of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jordan MB, Hildeman D, Kappler J, et al. An animal model of hemophagocytic lymphohistiocytosis (hLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004;104:735–43. 10.1182/blood-2003-10-3413 [DOI] [PubMed] [Google Scholar]
- 2.Emmenegger U, Schaer DJ, Larroche C, et al. Haemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med Wkly 2005;135:299–314. doi:2005/21/smw-10976 [DOI] [PubMed] [Google Scholar]
- 3.Imashuku S, Hibi S, Ohara T, et al. Effective control of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. histiocyte Society. Blood 1999;93:1869–74. [PubMed] [Google Scholar]
- 4.Kumakura S. Hemophagocytic syndrome. Intern Med 2005;44:278–80. 10.2169/internalmedicine.44.278 [DOI] [PubMed] [Google Scholar]
- 5.Filipovich AH. Hemophagocytic lymphohistiocytosis (hLH) and related disorders. Hematology Am Soc Hematol Educ Program 2009;2009:127–31. 10.1182/asheducation-2009.1.127 [DOI] [PubMed] [Google Scholar]
- 6.Kasahara Y, Yachie A, Takei K, et al. Differential cellular targets of Epstein-Barr virus (EBV) infection between acute EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Blood 2001;98:1882–8. 10.1182/blood.V98.6.1882 [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi H, Miyashita T, Herbst H, et al. Epstein-Barr virus-infected T lymphocytes in Epstein-Barr virus-associated hemophagocytic syndrome. J Clin Invest 1993;92:1444–50. 10.1172/JCI116721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 9.Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program 2013;2013:605–11. 10.1182/asheducation-2013.1.605 [DOI] [PubMed] [Google Scholar]
- 10.Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv 2017;1:2529–34. 10.1182/bloodadvances.2017012310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss R, Neureiter D, Westenburger B, et al. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients--a postmortem clinicopathologic analysis. Crit Care Med 2004;32:1316–21. 10.1097/01.CCM.0000127779.24232.15 [DOI] [PubMed] [Google Scholar]
- 12.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014;66:2613–20. 10.1002/art.38690 [DOI] [PubMed] [Google Scholar]
- 13.Debaugnies F, Mahadeb B, Ferster A, et al. Performances of the H-Score for diagnosis of hemophagocytic lymphohistiocytosis in adult and pediatric patients. Am J Clin Pathol 2016;145:862–70. 10.1093/ajcp/aqw076 [DOI] [PubMed] [Google Scholar]
- 14.Henter J-I, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002;100:2367–73. 10.1182/blood-2002-01-0172 [DOI] [PubMed] [Google Scholar]
- 15.Larroche C. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine 2012;79:356–61. 10.1016/j.jbspin.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 16.Imashuku S, Kuriyama K, Teramura T, et al. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol 2001;19:2665–73. 10.1200/JCO.2001.19.10.2665 [DOI] [PubMed] [Google Scholar]