Abstract
Caries is the most common and extensive oral chronic disease. Due to the lack of anti-caries properties, traditional caries filling materials can easily cause secondary caries and lead to treatment failure. Nanomaterials can interfere with the bacteria metabolism, inhibit the formation of biofilm, reduce demineralization, and promote remineralization, which is expected to be an effective strategy for caries management. The nanotechnology in anti-caries materials, especially nano-adhesive and nano-composite resin, has developed fast in recent years. In this review, the antibacterial nanomaterials, remineralization nanomaterials, and nano-drug delivery systems are reviewed. We are aimed to provide a theoretical basis for the future development of anti-caries nanomaterials.
Keywords: anti-caries, nanotechnology, nanomaterials, antibacterial, remineralization
1. Introduction
Caries is one of the most important diseases, which has seriously threatened human oral health, and even the whole body, for a long time [1]. According to statistics associated with 328 major diseases from “The Lancet-Global Burden of Disease Study 2016, GBD”, the prevalence of permanent teeth caries was the highest [2]. Based on modern theory in caries etiology, the imbalance of oral flora could result in acid accumulation and lead to tooth demineralization, inducing the caries formation. At present, caries treatment is still based on filling restoration. However, clinical studies showed that dental filling and bonding materials’ lack of anti-caries properties could result in a high incidence of secondary caries. The five-year failure rate in filling restoration is up to 50%. The replacement of restorations caused by filling failure leads to many public health resources waste. Therefore, the development of new anti-caries materials is a hot spot in the field of caries prevention and treatment.
Given the current problems and challenges in caries prevention and treatment, anti-caries nanomaterials have become a breakthrough in caries research. Nanotechnology was first proposed by Richard Feynman, which is a technology that studied the properties and applications of materials in the range of 1–100 nm. Nanomaterials are superior to traditional materials due to their physical/chemical advantages, such as volume effect, surface effect, quantum size, quantum tunnel, and dielectric confinement [3]. At present, the nanoparticles used include nanorings, nanopores, nanotubes, carbon, nanocapsules, nanospheres, and dendrimers [4]. Nanoparticles can be combined with polymers or coated on different biomaterials. The smaller the diameter of nanoparticles, the larger the specific surface area, and the stronger mechanical properties and antibacterial effect [5]. They can be used as the carrier of antibacterial drugs since nanoparticles have the characteristics of targeting antibacterial with the least side effects on the host. At present, a variety of antibacterial mechanisms of nanoparticles have been proposed, including metal ion release [6], oxidative stress [7], and non-oxidative mechanisms [8]. It is widely believed that the positively charged nanoparticles are attracted to the negatively charged cell membrane of bacteria by static electricity, which changes the permeability of the cell wall, leading to cell membrane rupture and organelle leakage.
In recent years, nanotechnology has shown great application prospects in the development of anti-caries materials, especially in nano-adhesive and nano-composite resin. In this paper, the antibacterial nanomaterials, remineralization nanomaterials, nano-drug delivery systems will be reviewed. We are aimed to provide the theoretical basis for the future development of anti-caries nanomaterials.
2. Metal Nanoparticles Used in Caries Infections
2.1. Silver Nanoparticle (NAg)
Silver has a broad spectrum of antibacterial properties, which can inactivate enzymes and prevent DNA replication in bacteria. NAg further increases surface area ratio, making silver particles smaller and antibacterial effects better. When NAg was added to the adhesives, its antibacterial property increased significantly, but whether it had any effect on the bonding strength is still controversial. Some scholars have found that the antibacterial effect of NAg increased in a dose-dependent manner from 0.05%~0.1% with no effect on bonding strength or color [9]. However, ElkassasDW et al. [10] indicated that self-etching adhesive containing 0.05%~0.1% NAg can affect the bonding strength at pH 2.7. Cheng et.al [11] demonstrated that 0%~0.175% of NAg composite resin can significantly reduce biofilm growth and metabolic activity, in which antibacterial activity increased in a dose-dependent manner. Moreover, when the mass fraction of NAg is 0%~0.088%, the flexure strength and elastic modulus of modified composite resin are not significantly different from the control group. However, the flexural strength of modified composite resin decreased significantly when the nano-silver mass fraction was higher than 0.175% [12]. Mohadese Azarsina examined the antibacterial activity of NAg added in Z250 composite. The results showed that NAg could significantly inhibit the growth of Streptococcus mutans and Lactobacillus on the composite surface [13]. NAg can be released slowly from the materials and has good biocompatibility in a certain concentration range [14].
In recent years, NAg has been added to the resin in cooperation with other antibacterial agents, which has a good antibacterial effect against caries-related bacteria (Table 1). In other research, Shenggui Chen et al. studied the antibacterial effect of polymethyl methacrylate (PMMA)-cellulose nanocrystals (CNCs)-NAg modified resin against Staphylococcus aureus and Escherichia coli. The authors concluded that PMMA-CNCs-NAg modified resin exhibited excellent mechanical properties, desirable biocompatibility, and excellent antibacterial activities [14]. Fang Li et al. found that the novel antibacterial adhesives containing quaternary ammonium dimethacrylate (QADM) and NAg are promising anti-caries nanomaterials. QADM and NAg can be used as antibacterial agents on the resin surface with good bacteriostatic effect [15]. In vitro artificial enamel caries model, reduced graphene oxide-silver nanoparticles (rGO/NAg) composite showed shallower lesion depth and less mineral loss compared with the control groups [16]. In the artificial dentine caries model, Irene et al. found that polyethylene glycol-coated silver nanoparticles (PEG-NAg) can remineralize dentine caries and inhibit collagen degradation without causing significant tooth staining [17]. In the rat caries model, NAg coated orthodontic brackets inhibited Streptococcus mutans for 30 days and reduced caries on the smooth surfaces [18].
Table 1.
The anti-caries component of nanoparticles.
| Type | Component | Modified Materials | Concentration | Model In Vitro Experiment | Mechanism of Anti-Caries | Ref. |
|---|---|---|---|---|---|---|
| Metal Nanoparticles | Silver Nanoparticle (NAg) | Resin Adhesive |
Resin: 0%~0.088% Adhesive: 1–5% |
Streptococcus mutans and Lactobacillus | ①Ag ions penetrate the cell membrane and enter the microbial body. ②Mechanism of reactive oxygen species (ROS). |
[103] |
| NAg-NZnO | Resin | NAg, 1% NZnO, 1% |
Streptococcus mutans and Lactobacillus | [104] | ||
| NAg-Laden Hydroxyapatite | Resin | 6–8 wt.% | Streptococcus mutans | ①Antibacterial mechanism of NAg. ②Hydroxyapatite can precipitate on the surface of demineralized enamel to form a new layer and promote remineralization. |
[105][45] | |
| NAg- Polyamidoamine (PAMAM)-Cellulose Nanocrystals (CNCs) | Resin | 0.1 wt.% | Staphylococcus aureus and Escherichia coli | Antibacterial mechanism of NAg and remineralized mechanism of PAMAM. | [14] | |
| NAg-Quaternary Ammonium Dimethacrylate (QADM) | Adhesive | NAg, 0.05% QADM, 10% |
Streptococcus mutans | ①Antibacterial mechanism of NAg. ②Mechanism of quaternary ammonium salt QAS. CHK1-mediated two-component regulatory system results in the accumulation of ROS which induces cell apoptosis. |
[106] | |
| NAg- 12-methacryloxydodecylpyridium bromide (MDPB) | Adhesive | NAg, 0.1% MDPB, 2.5% |
Human saliva biofilms | [107] | ||
| NAg- Dimethylaminododecyl methacrylate (DMADDM) | Adhesive | NAg, 0.1% DMADDM, 5% |
Dental plaque microcosm biofilm model | [108] | ||
| Nano-Zinc (NZn) | Adhesive | 2.15 ± 0.05 µg Zn/mg NPs | Extracted unerupted human third molars | Inhibit MMP activity, reduction of the decomposition of dentin collagen bundle, protection of mineral crystal at the interface of resin-tooth formation. | [109] | |
| Nano-Zincoxide (NZnO) | Resin | 1% | Streptococcus mutans | ①NZnO has higher surface potential energy and can release more zinc ions to kill bacteria. ②NZnO can also activate the photocatalytic antibacterial mechanism and produce a large number of free radicals to interact with bacteria. |
[21] | |
| TiO2 nanoparticles (NTiO2) | Glass-ionomer | 3% and 5% | Streptococcus mutans | ①Contact inhibited mechanism. ②Mechanism of reactive oxygen species (ROS). |
[28] | |
| Nano-SiO2 (NSiO2) | Acrylic resin | 1% | Lactobacillus acidophilus and Streptococcus mutans | [31] | ||
| Copper Nanoparticles (NCuO) | Adhesive | 0.01, 0.5, and 1 wt.% | Streptococcus mutans | [32] | ||
| MgO Nanoparticles (NMgO) | Glass-ionomer | 1% and 2.5% | Streptococcus mutans and Streptococcus sobrinus | [35] | ||
| QAS | Quaternary Ammonium Salt Polyethylenimine (QAS-PEI) Nanoparticles | Resin | 1–2 wt.% | Streptococcus mutans and Lactobacillus | The electrostatic interaction between positively charged QAS-PEI and negatively charged bacterial cell walls. | [43] |
| Remineralized Nanopaticles | Nano Particulate Hydroxyapatite (NHAP) | Resin | 2–5–10% | Sound premolars fixed in acrylic blocks and coated with nail polish | NHAP can stably release Ca2+ and PO43− to promote remineralization. | [48] |
| Nanosized Calcium Fluoride (NCaF2) | Resin | 17% | Biofilm by Streptococcus mutans on the tooth surface | NCaF2 materials can keep fluorine release at a better level for a long time to promote tooth remineralization. | [51] | |
| Nanosized Amorphous Calciumphosphate Particle (NACP) | Resin | Ca and P with concentrations of 8 mmol/L and 5.333 mmol/L | Dental plaque microcosm biofilm model | NACP can release higher levels of Ca2+ and PO43− at low pH, with the acid invasion neutralization, increasing the pH value from 4 to 6.5 to resist dental caries. | [61,62] | |
| Bioactive Glass Nanoparticle (NBG) | Resin | 20 wt% | Bioglass | ①It will release Ca2+ and PO43− to form a mineralized layer with a porous network. ②NBG can interfere with the degradation of collagenase, formation of high alkaline pH, resulting in antibacterial ions (such as Ag+) release to achieve an antibacterial effect. |
[73] | |
| Drug Delivery System | Mesoporous Silica Nanoparticle (MSN) | Adhesive | 34 wt% | Multi-species biofilms | As a carrier, the system can slowly release antibacterial/remineralization particles. | [81] |
| Liposome | - | 0.05%, 0.2% | Dental enamel, Saliva | [83] | ||
| Halloysite Nano-Tube (HNT) | Adhesive | 20% | Streptococcus mutans | [86] | ||
| PAMAM | Adhesive/resin | 0.3% (w/v) | Dentin disks, Artificial saliva | [87] | ||
| Caries Vaccine | - | 50 mmol/L | Plasmid | [100] | ||
| Nanocatalyst | Catalytic iIon Oxide Nanoparticles (CAT-NP) | gargle | 4% | a rodent caries model | As a catalyst, it can catalyze the effect of H2O2 against cariogenic bacteria. | [101] |
| Dextran-Coated Iron Oxide Nanoparticles termed Nanozymes (Dex-NZM) | gargle | - | a rodent caries model | As a catalyst, it can catalyze the effect of Fe4O3 against cariogenic bacteria. | [102] |
2.2. Nano-Zinc (NZn) and Nano-Zincoxide (NZnO)
NZn has a wide antibacterial spectrum, which antibacterial ability mainly comes from the quantum size effect of dissolving and releasing zinc nanoparticles. Matrix metalloproteinases (MMPs) can be activated in both total-etch and self-etch adhesives, which induce degradation of resin and dentin matrix, shortening the service life of adhesives. NZn can reduce the expression of MMPs and prolong the lifespan of adhesive. It has been reported that the addition of Zn2+ to total-etch adhesive can inhibit MMPs activity, reduce the decomposition of dentin collagen bundle, and protect mineral crystal formation at the eosin–tooth interface, improving the nano-mechanical properties [19,20]. Compared with micron ZnO, NZnO has higher surface potential energy and can release more zinc ions to kill bacteria. Besides, some scholars believed that NZnO could also activate the photocatalytic antibacterial mechanism and produce a large number of free radicals to interact with bacteria [21]. Tavasoli et al. [22] studied 0–5% NZnO composite resin and found that 1% NZnO had no obvious effect on the mechanical properties of the composite resin. With the increase of mass fraction (0–5%), the number of Streptococcus mutans was decreased significantly in 24 h [23]. NZnO has been added to resin or binder alone or in cooperation with other antibacterial agents (Table 1). As recent researches asserted the antimicrobial effects of NZnO and chitosan (CS) nanoparticles, which results demonstrated that the two components of nanoparticles in the composite resin can be synergistically beneficial in reducing the number of microorganisms [24]. Yazi Wang et al. explored the mechanical and antibacterial properties of cellulose nanocrystal/zinc oxide (CNC/ZnO) nanohybrids of the dental resin composites [25]. They concluded that CNC/ZnO nanohybrids positively affected the mechanical and antibacterial properties on dental composite resin which are promising to resist secondary caries. In the dentine caries model, Mario et al., found that NZnO and copper nanoparticles (NCuO) addition in universal adhesive systems provided antimicrobial, anti-MMP activities and improved interface stability on caries-affected dentin [26].
2.3. Other Metal Nanoparticles
TiO2 nanoparticles (NTiO2) were incorporated into glass-ionomer at 3% and 5% (w/w), exhibiting an antibacterial activity in direct contact test against Streptococcus mutans [27,28]. Moreover, NTiO2-containing dental adhesives (80% v/v) had strong antibacterial efficacy against Streptococcus mutans biofilms [29] with good biocompatibility [30]. In vitro experiment, an acrylic resin containing NTiO2 (0.5%) and nano-SiO2 (NSiO2, 1%) in acrylic liquid reduced the cariogenic bacterial count (Lactobacillus acidophilus and Streptococcus mutans) by 3.2–99% in a time-dependent manner [31]. Toodehzaeim MH et al. indicated that incorporating NCuO into adhesive (0.01, 0.5, and 1 wt.%) added antimicrobial effects to the adhesive with no adverse effects on shear bond strength [32]. Besides, the incorporation of Cu nanoparticles into adhesive renders the adhesive antibacterial to Streptococcus mutans for at least 1 year [33,34]. The MgO nanoparticles (NMgO) modified glass-ionomer cement showed effective antibacterial and antibiofilm activity against two cariogenic microorganisms (Streptococcus mutans and Streptococcus sobrinus) [35].
2.4. The Anti-Caries Mechanism of Mental Nanoparticles
The antibacterial mechanism of mental nanoparticles is summarized in Figure 1.
Figure 1.
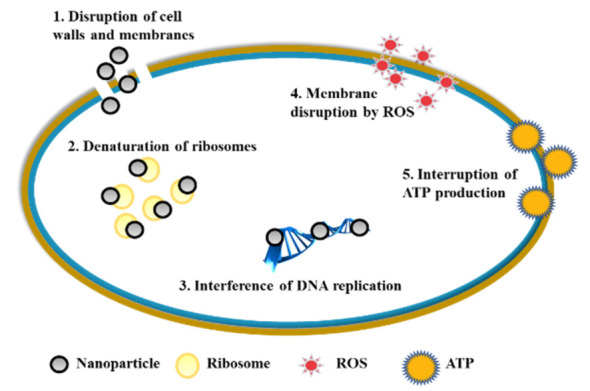
The antibacterial mechanism of mental nanoparticles.
The first is “Contact Inhibited Mechanism”. For metal nanoparticles, the positively charged metal ions contact with negatively charged cell membranes on microorganisms, which is called the micro-dynamic effect. Metal ions penetrate the cell membrane and enter the microbial body. They react with the thiol group (-SH) on the microbial protein, inhibiting the synthesis of protein and nucleic acid. Metal ions destroy the electron transport system, respiratory system, and material transfer system of microorganisms, resulting in microbial death. Metal ions are generally loaded on sustained-release carriers and gradually released in the reaction process. When the bacteria were killed by metal ions (such as Ag+), the metal ions are free from the bacterial corpse and can contact with other bacteria again. In this way, metal ions can maintain a durable antibacterial effect. The bactericidal and inhibitory activities of metal ions decreased in the following order: Ag+ > Hg2+ > Cu2+ > Cd2+ > Cr3+ > Ni2+ > Pb2+ > Co3+ > Zn2+ > Fe3+.
The second is “Reactive Oxygen Species (ROS) Mechanism”. According to the hypothesis of the ROS mechanism, the trace metal elements distributed on the surface of the materials are the active centers that play a catalytic role. The active center can absorb energy in the environment, activating the oxygen on the material surface, and generating hydroxyl radicals and reactive oxygen atoms. Their strong redox effect would destroy the cell proliferation ability to inhibit or kill bacteria [36]. Yoshihiro [37] et al. showed that only under aerobic conditions can silver loaded zeolite to exhibit antibacterial activity, which provided strong evidence for the mechanism of ROS.
3. Quaternary Ammonium Salt Polyethylenimine (QAS-PEI) Nanoparticles
QAS is a highly active cationic agent with a wide antibacterial spectrum [38,39]. QAS-PEI nanoparticles were prepared based on polyethyleneimine cross-linked structure which makes the modified composite with high chemical stability and antibacterial properties under different oxidants and storage conditions, without effect on the oral micro ecological balance. The antibacterial mechanism of QAS-PEI is related to the electrostatic interaction between positively charged QAS-PEI and negatively charged bacterial cell walls [40]. QAS-PEI nanoparticles incorporated in resin-based composite at 2% wt/wt have demonstrated prolonged inhibition of bacterial growth [41]. Yudovin Farber et al. [42] added 1% QAS-PEI nanoparticles into the composite resin. Through 3 months of long-term experiments in vitro, it was confirmed that the composite resin had strong sustained antibacterial activity against Streptococcus mutans, and the alkyl chain length of polyethyleneimine had a significant influence on the antibacterial effect. In addition, some scholars have added QAS-PEI nanoparticles into glass ionomer polymer and found it has an obvious antibacterial effect on Streptococcus mutans and Lactobacillus [43]. Moreover, Nurit Beyth et al. investigated the biocompatibility of QAS-PEI nanoparticles incorporated in a resin composite [30,44], revealing that TNFα secretion and macrophage viability were not altered by QAS-PEI nanoparticles.
4. Remineralized Nano Anti-Caries Materials
Remineralized nano anti-caries materials are adhesives or resins that added with bioactive nanoparticles which can remineralize the exposed collagen in the mixed layer and prevent collagen degradation, improving the bonding durability. Remineralized nanoparticles modified resins can release Ca2+ and PO43− in low pH values and promote the remineralization of tooth tissue to prevent caries.
4.1. Nano Particulate Hydroxyapatite (NHAP)
The physical and chemical properties of the synthesized NHAP are similar to those of natural tooth hydroxyapatite. NHAP can stably release Ca2+ and PO43− to promote remineralization. Vyavhare et al. [45] found that NHAP can precipitate on the surface of demineralized enamel to form a new layer. NHAP enhanced the shear bond strength to remineralized enamel in the etched enamel model [46]. Moreover, in the recurrent enamel caries model, resin infiltration doped with NHAP caused higher enamel resistance against demineralization compared to the control group [47]. Roza Haghgoo et al. studied the efficacy of different NHAP concentrations in mouthwash for the remineralization of incipient caries [48]. The results showed that NHAP can significantly enhance tooth remineralization and increase microhardness in a dose-dependence manner. NHAP added in total etching adhesive can increase the micro shear bond strength of teeth [49]. However, it will weaken the mechanical interlock between the adhesive and the tooth when added in the self-etching adhesive. In vivo and in vitro, toothpaste containing NHAP has proven to be a valuable prevention measure against dental caries in primary dentition [50].
4.2. Nanosized Calcium Fluoride (NCaF2)
Compared with the traditional fluoride, NCaF2 materials can keep fluorine release at a better level for a long time. NCaF2 can be added to dental composites as dental filling compo glass with a size of 58 ± 21 nm [51]. The results showed that the fluorine release concentration in NCaF2-composite resin is similar to or even higher than that of commercial modified glass ionomer. Moreover, after thermal cycling and 2-year aging treatment, the flexural strength of NCaF2-composite resin is 5 times higher than that of commercial modified glass ionomer [52]. However, the aesthetic properties still need to be improved due to the mismatching of the refractive index between filler and matrix. In a white spot lesion model, NCaF2-containing orthodontic cement increased the enamel hardness by 56% and decreased the lesion depth by 43%, compared to control groups [53]. In caries prevention, a novel pit and fissure sealant containing NCaF2 and antibacterial component achieved high F release and strong antibacterial performance [54]. In a rat caries model, NCaF2 was observed to substantially decrease caries by scanning electron micrographs [55]. Thus, NCaF2 materials is promising to inhibit enamel demineralization, white spot lesions, and caries.
4.3. Nanosized Amorphous Calciumphosphate Particle (NACP)
Compared with amorphous calcium phosphate, NACP can release higher levels of Ca2+ and PO43− with lower filler. Lee et al. [56] found that NACP can release more ions at low pH, with the acid invasion neutralization, increasing the pH value from 4 to 6.5 to resist dental caries. Besides, studies have shown that the addition of NACP in adhesive can improve the pulp biological reaction and promote tertiary dentin formation [57]. When NACP combined with antibacterial components are added to the adhesive, the new binder has dual functions of both antibacterial and remineralization [58]. Novel adhesive containing MBDP and NACP exhibited an application prospect in resisting enamel white spot lesions and even caries [59]. Although NACP composite resin has strong remineralization ability, Weir et al. [60] revealed that the ion release of NACP can only last for a few months. To solve this problem, Zhang et al. [61,62] developed a composite resin which can re-release Ca2+ and PO43−. The new resin has the function of long-term anti-caries and auxiliary remineralization, in which mechanical properties have no significant difference with commercial composite materials. Moreover, Yousif A. Al Dulaijan et al. investigated a novel rechargeable calcium phosphate synthesized with NACP and dimethylaminohexadecyl methacrylate (DMAHDM), which can recharge and re-release Ca and P ion [61]. Moreover, this release remains at the same level as the charge cycle increases, indicating the long-term ability of ion release and remineralization. In the artificial enamel caries model, an adhesive containing NACP could achieve higher enamel remineralization of artificial caries than the control by surface and cross-sectional hardness test in vitro [59,63,64]. In the artificial dentin caries model, the NACP adhesive remineralized dentin lesions with exceptional efficacy [65,66] and showed excellent water-aging durability for 12 months [67]. In a biofilm-based recurrent root caries model, NACP-containing nanocomposite substantially reduced demineralization and protected root dentin hardness around the restorations [68]. In a rat tooth cavity model, the antibacterial and remineralizing properties of adhesive containing NACP and dimethylaminododecyl methacrylate (DMADDM) were tested in vivo. The novel adhesive exhibited much greater tertiary dentin formation and milder pulpal inflammation than the control [57]. Besides, NACP combined into an antibacterial sealant protected the enamel against demineralization in vivo [69]. Thus, pH-responsive Ca2+ and PO43− releasing sealants may be a reliable complementary approach for caries management [69,70].
4.4. Bioactive Glass Nanoparticle (NBG)
When the bioactive glass contacts with water or saliva, it will release Ca2+ and PO43− to form a mineralized layer with a porous network, which is similar to hydroxyapatite. NBG has better physical and chemical activities due to the decrease of grain size, the increase of surface area, the surface free energy, and the binding energy. The research showed that NBG can interfere with the degradation of collagenase and maintain a high alkaline pH, resulting in antibacterial ions (such as Ag+) release to achieve antibacterial effect [71]. Camila et al. prepared nanocomposite cements based on the incorporation of NBGs into BiodentineTM and to assess their bioactive properties. They concluded that the incorporation of NBGs into BiodentineTM improved in vitro bioactivity of modified resin, accelerating the formation of a crystalline apatite layer on the resin surface [72]. Tauböck et al. [73] demonstrated that the composite resin containing 20% NBG formed a mineralized layer after 21 days, while the composite resin without NBG had slight corrosion and no sediment formation. However, it needs further study that whether the increase of BGN content would affect the hardness of the composite resin since its hydrophilicity and water absorption rate were increased.
4.5. Remineralization Mechanism of Nanoparticles
Caries demineralization involves loss of minerals occurring in the early stage of the lesion, in the deep below the enamel surface, with the migration of acid ions from the plaque to the deep lesion and mineral ions from the deep lesion toward the plaque [74]. Caries remineralization is a natural repair process to restore the minerals in ionic forms to achieve the hydroxyapatite crystal lattice [75,76]. It occurs at near-neutral physiological pH, and calcium and phosphate mineral ions in nanoparticles are redeposited from saliva and dental plaque fluid to form new hydroxyapatite crystals, which are larger and more acid-soluble.
5. Nanodrug Delivery System
Nano drug delivery system is a new drug controlled-release system with a particle size of 1–100 nm, which is made of carrier materials and anti-caries drugs by nanotechnology. The anti-caries effect can be enhanced by adding a nanodrug loading system into composite resin or adhesive. In recent years, the system showed a promising application in the clinic with the advantages of targeting and sustained drug release.
5.1. Mesoporous Silica Nanoparticle (MSN)
The surface of MSN can be modified by functional groups, which made MSN can be compatible with various solutions and stored as different types of molecules. Besides, due to the high affinity of MSN, it is easy to adhere to the dentin surface. Calcium and phosphate can be slowly released from MSN, which can improve the bonding durability and remineralization effect [77,78]. The study of chlorhexidine on MSN showed that MSN had a high inhibitory ability on a variety of oral bacterioplankton and biofilm [79]. Zhang et al. [80] found that compared with the composite resin containing chlorhexidine only, MSN loaded chlorhexidine composite resin has the characteristics of slow-release, longer antibacterial effect, and relatively stable flexural strength. Moreover, Cameron A. et al. have developed new broad-spectrum antimicrobial agent MSNs co-assembled with the template drug, octytening salt. They found that the steady-state release of template agent in adhesive killed caries-related bacteria, inhibiting biofilm formation on the adhesive surface and non-toxic [81].
5.2. Liposomes
A liposome is a microvesicle formed by encapsulating drugs in the lipid bilayer. Gregoriadis et al. [82] used liposomes as drug carriers for the first time. Liposomes can encapsulate lipophilic or water-soluble drugs which have been widely studied as anti-cancer, antibacterial, anti-inflammatory drug carriers as well as for gene delivery due to its advantages of targeting, slow-released and tissue affinity after encapsulating drugs. In the caries prevention and treatment, liposome drug delivery can induce calcium, phosphorus, and other minerals to precipitate on the surface of tooth hard tissue which can promote mineralization. Fat-soluble or water-soluble antimicrobial agents encapsulated in liposomes have a good effect on plaque biofilms. The latest study [83] found that in addition to the role of drugs carried by liposomes, a new type of pectin coated liposome carrier can permanently adsorb on the enamel surface to protect the enamel.
5.3. Halloysite Nano-Tube (HNT)
Halloysite is a kind of natural silicate mineral, which mainly exists in nature in the form of nanotubes. HNT can be used as a carrier to control the delivery of therapeutic agents with excellent biocompatibility, hydrophilicity, and high mechanical strength [10]. NZnO and NAg were loaded into HNT by some scholars. It was found that HNT promoted the dispersion of NZnO, made ZnO contact closely with bacteria and increased the concentration of zinc, which achieved a synergistic antibacterial effect with NAg [84]. Bottino et al. [85] found that HNT can be used as a carrier of MMP inhibitor in a modified total-etching adhesive to increase the adhesion durability, and the addition amount can be up to 20%, without affecting the physical and chemical properties. S.A. Feitosa et al. investigated the biological activity and bonding properties of doxycycline (DOX)-encapsulated HNT-modified adhesive. The results showed the growth of Streptococcus mutans was successfully inhibited by direct contact. In addition, compared with the control group, the DOX-encapsulated HNT group had stronger inhibitory activity on MMP-1 [86].
5.4. Polyamidoamine (PAMAM)
A dendrimer is a novel type of high branched nano polymer with a cavity in the molecule and easy to modify surface groups. It has good biocompatibility, low toxicity, and non-immunogenicity. PAMAM terminal groups can be modified into various functional groups as drug delivery carriers to control drug release and play dual functions of both antibacterial and remineralization in caries. Zhou et al. [87] found that PAMAM-COOH can be used as a carrier of triclosan to improve the antibacterial effect of triclosan and induce remineralization. Moreover, a bovine enamel carious lesions model was created to investigate the remineralization ability of PAMAM with different terminal groups, among which PAMAM-NH2 showed the most prominent competence, followed by PAMAM-COOH and PAMAM-OH, in that order. In the dentin caries model, the PAMAM combined with NACP adhesive completely remineralized demineralized dentin for long-term fluid challenges to protect tooth structures [88,89]. In a biofilm-based recurrent root caries model, the combined bioactive multifunctional composite (BMC) with PAMAM substantially induced root dentin remineralization and increased the hardness of pre-demineralized root dentin [90]. Liang et al. [91] studied the effects of PAMAM, amorphous nano calcium phosphate, and their mixed adhesives on remineralization. The result showed that compared with single-component adhesives, mixed adhesives can promote dentin remineralization more effectively in an acidic environment. Researchers loaded amphotericin B into the MSN-incorporated PMMA and observed a long-term antimicrobial effect for two weeks, which suggested that MSN-incorporated PMMA resin had clinical application prospect [92].
5.5. Dental Caries Vaccine
It is an effective strategy in caries prevention to induce oral mucosal immune systems through the nasal tract. Vaccination has the following advantages: High patient compliance, induction of systemic immunity, and convenient administration [93,94]. Recently, promising results have been achieved in the study of anti-caries DNA vaccine inoculation [95]. However, due to the lack of effective targeting, the effectiveness of the mucosal administration of “naked” DNA is limited. In order to improve the function of the “naked” DNA vaccine, a plasmid was loaded into chitosan nanoparticles [96]. Chitosan is a rich natural biopolymer extracted from the exoskeleton of crustaceans [97]. In animal experiments, chitosan has been proved to be an excellent vaccine carrier system [96] and a vehicle suitable for oral drug delivery systems [98]. Chitosan is a valuable gene carrier in oral [99]. The chitosan-DNA nanoparticles are suitable for mucosal anti-caries DNA vaccination, which could induce the oral specific immune responses with biocompatible and non-cytotoxic [100]. In further research, human clinical trials are needed to implement regular anti-caries strategies.
6. Biomimetic Nanocatalyst
The application of nanocatalyst is a new approach to combat cariogenic plaque-biofilm in nanotechnology. In a previous study, catalytic iron oxide nanoparticles (CAT-NP) have been shown exceptional topical anti-biofilm effects in vitro [101]. Moreover, CAT-NP/H2O2 was observed to suppress the onset and severity of dental caries in vivo in a rodent model [101]. With an in-depth study, Pratap C. Naha et al. reported that dextran-coated iron oxide nanoparticles termed nanozymes (Dex-NZM) displayed strong catalytic activity at acidic pH values and targeted biofilms with high specificity, preventing the development of dental caries in a pH-dependent manner in vivo [102].
7. Conclusions
The anti-caries components of nanoparticles were summarized in Table 1. The roles of anti-caries nanomaterials were exhibited in Figure 2. With the development of caries diagnosis and prevention, nanotechnology will significantly improve medical treatment. The use of nanoparticles against caries infection is very important because of its antibacterial, remineralization, and drug loading capacity. All the studies in this review have shown that nanoparticles have an enhanced role in the prevention and treatment of caries infection. However, it is necessary to understand their disadvantages and their potential cytotoxicity and environmental effects. In the future, we should develop better technology to prepare highly effective anti-caries nanoparticles with the highest safety for patients.
Figure 2.
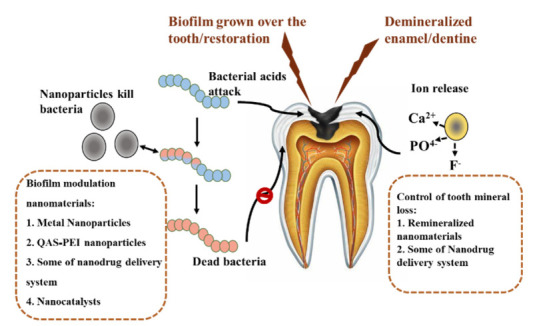
Schematic is indicating the roles of anti-caries nanomaterials.
Acknowledgments
We greatly appreciate the help from Hockin H. K. Xu for the critical advice on this paper.
Author Contributions
Conceptualization, H.C. and X.Z.; writing—review and editing, H.C. and B.L.; supervision, L.G., L.C., and B.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number: 81870778, 81600858, 81870759, and by Applied Basic Research Programs of Sichuan Province, grant number 2020YJ0227.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li X., Kolltveit K.M., Tronstad L., Olsen I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000;13:547–558. doi: 10.1128/CMR.13.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T., Allen C., Arora M., Barber R.M., Bhutta Z.A., Brown A., Carter A., Casey D.C., Charlson F.J., Chen A.Z. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhury H., Gorain B., Karmakar S., Biswas E., Dey G., Barik R., Mandal M., Pal T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014;460:131–143. doi: 10.1016/j.ijpharm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Guardiola A., Ortiz-Cano R., Sandoval-Salinas M.E., Fernández-Rossier J., Casanova D., Pérez-Jiménez A.J., Sancho-García J.C. From cyclic nanorings to single-walled carbon nanotubes: Disclosing the evolution of their electronic structure with the help of theoretical methods. J. Phys. Chem. Chem. Phys. 2019;21:2547–2557. doi: 10.1039/C8CP06615A. [DOI] [PubMed] [Google Scholar]
- 5.Song W., Ge S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules. 2019;24:1033. doi: 10.3390/molecules24061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakharova O.V., Godymchuk A.Y., Gusev A.A., Gulchenko S.I., Vasyukova I.A., Kuznetsov D.V. Considerable Variation of Antibacterial Activity of Cu Nanoparticles Suspensions Depending on the Storage Time, Dispersive Medium, and Particle Sizes. Biomed. Res. Int. 2015;8:11. doi: 10.1155/2015/412530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurunathan S., Han J.W., Dayem A.A., Eppakayala V., Kim J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012;7:5901–5914. doi: 10.2147/IJN.S37397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung Y.H., Ng A.M., Xu X., Shen Z., Gethings L.A., Wong M.T., Chan C.M., Guo M.Y., Ng Y.H., Djurisic A.B., et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small. 2014;10:1171–1183. doi: 10.1002/smll.201302434. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira C.A., Campos R.M., Macedo J.P., Silva A.R., Maximo L.N., Silva T.M., Franca F.M., Turssi C.P., Basting R.T., Goncalves S.E.P., et al. Incorporation of ZnCl2 into an etch-and-rinse adhesive system on flexural strength, degree of conversion and bond durability to caries-affected dentin. Am. J. Dent. 2019;32:299–305. [PubMed] [Google Scholar]
- 10.Elkassas D., Arafa A. The innovative applications of therapeutic nanostructures in dentistry. Nanomedicine. 2017;13:1543–1562. doi: 10.1016/j.nano.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y.J., Zeiger D.N., Howarter J.A., Zhang X., Lin N.J., Antonucci J.M., Lin-Gibson S. In situ formation of silver nanoparticles in photocrosslinking polymers. J. Biomed. Mater. Res. B Appl. Biomater. 2011;97:124–131. doi: 10.1002/jbm.b.31793. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L., Weir M.D., Xu H.H., Antonucci J.M., Lin N.J., Lin-Gibson S., Xu S.M., Zhou X. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100:1378–1386. doi: 10.1002/jbm.b.32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azarsina M., Kasraei S., Yousef-Mashouf R., Dehghani N., Shirinzad M. The antibacterial properties of composite resin containing nanosilver against Streptococcus mutans and Lactobacillus. J. Contemp. Dent. Pr. 2013;14:1014–1018. doi: 10.5005/jp-journals-10024-1442. [DOI] [PubMed] [Google Scholar]
- 14.Chen S., Yang J., Jia Y.G., Lu B., Ren L. A Study of 3D-Printable Reinforced Composite Resin: PMMA Modified with Silver Nanoparticles Loaded Cellulose Nanocrystal. Materials. 2018;11:2444. doi: 10.3390/ma11122444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng L., Zhang K., Zhou C.C., Weir M.D., Zhou X.D., Xu H.H. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016;29:172–181. doi: 10.1038/ijos.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu R., Zhao Q., Lu S., Fu Y., Yu D., Zhao W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J. Appl. Oral Sci. 2018;27:e20180042. doi: 10.1590/1678-7757-2018-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao I.S., Yin I.X., Mei M.L., Lo E.C.M., Tang J., Li Q., So L.Y., Chu C.H. Remineralising Dentine Caries Using Sodium Fluoride with Silver Nanoparticles: An In Vitro Study. Int. J. Nanomed. 2020;15:2829–2839. doi: 10.2147/IJN.S247550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metin-Gursoy G., Taner L., Akca G. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur. J. Orthod. 2017;39:9–16. doi: 10.1093/ejo/cjv097. [DOI] [PubMed] [Google Scholar]
- 19.Toledano M., Sauro S., Cabello I., Watson T., Osorio R. A Zn-doped etch-and-rinse adhesive may improve the mechanical properties and the integrity at the bonded-dentin interface. Dent. Mater. 2013;29:e142–e152. doi: 10.1016/j.dental.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Toledano M., Aguilera F.S., Osorio E., Cabello I., Toledano-Osorio M., Osorio R. Self-etching zinc-doped adhesives improve the potential of caries-affected dentin to be functionally remineralized. Biointerphases. 2015;10:031002. doi: 10.1116/1.4926442. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L., Weir M.D., Xu H.H., Antonucci J.M., Kraigsley A.M., Lin N.J., Lin-Gibson S., Zhou X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavassoli Hojati S., Alaghemand H., Hamze F., Ahmadian Babaki F., Rajab-Nia R., Rezvani M.B., Kaviani M., Atai M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent. Mater. 2013;29:495–505. doi: 10.1016/j.dental.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mosawi R.M., Al-Badr R.M. The Study Effects of Dental Composite Resin as Antibacterial Agent Which Contain Nanoparticles of Zinc Oxide on the Bacteria Associated with Oral Infection. J. Dent. Med. Sci. 2017;16:49–55. doi: 10.9790/0853-1601014955. [DOI] [Google Scholar]
- 24.Yusof N.A.A., Zain N.M., Pauzi N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019;124:1132–1136. doi: 10.1016/j.ijbiomac.2018.11.228. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Hua H., Li W., Wang R., Jiang X., Zhu M. Strong antibacterial dental resin composites containing cellulose nanocrystal/zinc oxide nanohybrids. J. Dent. 2019;80:23–29. doi: 10.1016/j.jdent.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez M.F., Bermudez J., Davila-Sanchez A., Alegria-Acevedo L.F., Mendez-Bauer L., Hernandez M., Astorga J., Reis A., Loguercio A.D., Farago P.V., et al. Zinc oxide and copper nanoparticles addition in universal adhesive systems improve interface stability on caries-affected dentin. J. Mech. Behav. Biomed. Mater. 2019;100:103366. doi: 10.1016/j.jmbbm.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Contreras R., Scougall-Vilchis R.J., Contreras-Bulnes R., Sakagami H., Morales-Luckie R.A., Nakajima H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. Rev. FOB. 2015;23:321–328. doi: 10.1590/1678-775720140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsaka S.E., Hamouda I.M., Swain M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011;39:589–598. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Esteban Florez F.L., Hiers R.D., Larson P., Johnson M., O’Rear E., Rondinone A.J., Khajotia S.S. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;93:931–943. doi: 10.1016/j.msec.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esteban Florez F.L., Kraemer H., Hiers R.D., Sacramento C.M., Rondinone A.J., Silverio K.G., Khajotia S.S. Sorption, solubility and cytotoxicity of novel antibacterial nanofilled dental adhesive resins. Sci. Rep. 2020;10:13503. doi: 10.1038/s41598-020-70487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodagar A., Khalil S., Kassaee M.Z., Shahroudi A.S., Pourakbari B., Bahador A. Antimicrobial properties of poly (methyl methacrylate) acrylic resins incorporated with silicon dioxide and titanium dioxide nanoparticles on cariogenic bacteria. J. Orthod. Sci. 2016;5:7–13. doi: 10.4103/2278-0203.176652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toodehzaeim M.H., Zandi H., Meshkani H., Hosseinzadeh Firouzabadi A. The Effect of CuO Nanoparticles on Antimicrobial Effects and Shear Bond Strength of Orthodontic Adhesives. J. Dent. 2018;19:1–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Sabatini C., Mennito A.S., Wolf B.J., Pashley D.H., Renne W.G. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J. Dent. 2015;43:546–555. doi: 10.1016/j.jdent.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez M.F., Malaquias P., Matos T.P., Szesz A., Souza S., Bermudez J., Reis A., Loguercio A.D., Farago P.V. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent. Mater. 2017;33:309–320. doi: 10.1016/j.dental.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Noori A.J., Kareem F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon. 2019;5:e02568. doi: 10.1016/j.heliyon.2019.e02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao S., Zhang Y., Pan X., Zhu F., Jiang C. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019;14:1469–1487. doi: 10.2147/IJN.S191340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosono H., Abe Y. Silver ion selective porous lithium titanium phosphate glass-ceramics cation exchanger and its application to bacteriostatic materials. Mater. Res. Bull. 1994;29:1157–1162. doi: 10.1016/0025-5408(94)90185-6. [DOI] [Google Scholar]
- 38.Chen H., Zhou Y., Zhou X., Liao B., Xu H.H.K., Chu C.-H., Cheng L., Ren B. Dimethylaminododecyl methacrylate inhibits Candida albicans and oropharyngeal candidiasis in a pH-dependent manner. Appl. Microbiol. Biotechnol. 2020;104:3585–3595. doi: 10.1007/s00253-020-10496-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen H., Han Q., Zhou X., Zhang K., Wang S., Xu H.H.K., Weir M.D., Feng M., Li M., Peng X., et al. Heat-Polymerized Resin Containing Dimethylaminododecyl Methacrylate Inhibits Candida albicans Biofilm. Materials. 2017;10:431. doi: 10.3390/ma10040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou Y., Li D., Shen M., Shi X. Polyethylenimine-Based Nanogels for Biomedical Applications. Macromol. Biosci. 2019;19:e1900272. doi: 10.1002/mabi.201900272. [DOI] [PubMed] [Google Scholar]
- 41.Zaltsman N., Kesler-Shvero D., Weiss E.I., Beyth N. Synthesis Variants of Quaternary Ammonium Polyethyleneimine Nanoparticles and Their Antibacterial Efficacy in Dental Materials. J. Appl. Biomater. Funct. Mater. 2018;14:e205–e211. doi: 10.5301/jabfm.5000269. [DOI] [PubMed] [Google Scholar]
- 42.Yudovin-Farber I., Beyth N., Weiss E.I., Domb A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanoparticle Res. 2009;12:591–603. doi: 10.1007/s11051-009-9628-8. [DOI] [Google Scholar]
- 43.Melo M.A., Guedes S.F., Xu H.H., Rodrigues L.K. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013;31:459–467. doi: 10.1016/j.tibtech.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyth N., Houri-Haddad Y., Baraness-Hadar L., Yudovin-Farber I., Domb A.J., Weiss E.I.J.B. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008;29:4157–4163. doi: 10.1016/j.biomaterials.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Vyavhare S., Sharma D.S., Kulkarni V.K. Effect of three different pastes on remineralization of initial enamel lesion: An in vitro study. J. Clin. Pediatr. Dent. 2015;39:149–160. doi: 10.17796/jcpd.39.2.yn2r54nw24l03741. [DOI] [PubMed] [Google Scholar]
- 46.Memarpour M., Shafiei F., Rafiee A., Soltani M., Dashti M.H. Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral Health. 2019;19:92. doi: 10.1186/s12903-019-0785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrade Neto D.M., Carvalho E.V., Rodrigues E.A., Feitosa V.P., Sauro S., Mele G., Carbone L., Mazzetto S.E., Rodrigues L.K., Fechine P.B. Novel hydroxyapatite nanorods improve anti-caries efficacy of enamel infiltrants. Dent. Mater. 2016;32:784–793. doi: 10.1016/j.dental.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Haghgoo R., Rezvani M.B., Salehi Zeinabadi M. Comparison of nano-hydroxyapatite and sodium fluoride mouthrinse for remineralization of incipient carious lesions. J. Dent. 2014;11:406–410. [PMC free article] [PubMed] [Google Scholar]
- 49.Leitune V.C., Collares F.M., Trommer R.M., Andrioli D.G., Bergmann C.P., Samuel S.M. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013;41:321–327. doi: 10.1016/j.jdent.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Bossu M., Saccucci M., Salucci A., Di Giorgio G., Bruni E., Uccelletti D., Sarto M.S., Familiari G., Relucenti M., Polimeni A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019;17:17. doi: 10.1186/s12951-019-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghafar H., Khan M.I., Sarwar H.S., Yaqoob S., Hussain S.Z., Tariq I., Madni A.U., Shahnaz G., Sohail M.F. Development and Characterization of Bioadhesive Film Embedded with Lignocaine and Calcium Fluoride Nanoparticles. AAPS Pharmscitech. 2020;21:60. doi: 10.1208/s12249-019-1615-5. [DOI] [PubMed] [Google Scholar]
- 52.Weir M.D., Moreau J.L., Levine E.D., Strassler H.E., Chow L.C., Xu H.H. Nanocomposite containing CaF(2) nanoparticles: Thermal cycling, wear and long-term water-aging. Dent. Mater. 2012;28:642–652. doi: 10.1016/j.dental.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi J., Dai Q., Weir M.D., Melo M.A.S., Lynch C.D., Oates T.W., Zhang K., Zhao Z., Xu H.H.K. A nano-CaF2-containing orthodontic cement with antibacterial and remineralization capabilities to combat enamel white spot lesions. J. Dent. 2019;89:103172. doi: 10.1016/j.jdent.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Fei X., Li Y., Weir M.D., Baras B.H., Wang H., Wang S., Sun J., Melo M.A.S., Ruan J., Xu H.H.K. Novel pit and fissure sealant containing nano-CaF2 and dimethylaminohexadecyl methacrylate with double benefits of fluoride release and antibacterial function. Dent. Mater. 2020;36:1241–1253. doi: 10.1016/j.dental.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Kulshrestha S., Khan S., Hasan S., Khan M.E., Misba L., Khan A.U. Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: An in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 2016;100:1901–1914. doi: 10.1007/s00253-015-7154-4. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.H., Seo S.J., Kim H.W. Bioactive glass-based nanocomposites for personalized dental tissue regeneration. Dent. Mater. J. 2016;35:710–720. doi: 10.4012/dmj.2015-428. [DOI] [PubMed] [Google Scholar]
- 57.Li F., Wang P., Weir M.D., Fouad A.F., Xu H.H. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014;10:2804–2813. doi: 10.1016/j.actbio.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu J., Weir M.D., Melo M.A., Xu H.H. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J. Dent. 2015;43:317–326. doi: 10.1016/j.jdent.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Zhang L., Niu L.N., Yu T., Xu H.H.K., Weir M.D., Oates T.W., Tay F.R., Chen J.H. Antibacterial and remineralizing orthodontic adhesive containing quaternary ammonium resin monomer and amorphous calcium phosphate nanoparticles. J. Dent. 2018;72:53–63. doi: 10.1016/j.jdent.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Weir M.D., Chow L.C., Xu H.H. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res. 2012;91:979–984. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Dulaijan Y.A., Cheng L., Weir M.D., Melo M.A.S., Liu H., Oates T.W., Wang L., Xu H.H.K. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018;72:44–52. doi: 10.1016/j.jdent.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L., Weir M.D., Chow L.C., Antonucci J.M., Chen J., Xu H.H. Novel rechargeable calcium phosphate dental nanocomposite. Dent. Mater. 2016;32:285–293. doi: 10.1016/j.dental.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Y., Liang K., Weir M.D., Gao J., Imazato S., Tay F.R., Lynch C.D., Oates T.W., Li J., Xu H.H.K. Enamel remineralization via poly(amido amine) and adhesive resin containing calcium phosphate nanoparticles. J. Dent. 2020;92:103262. doi: 10.1016/j.jdent.2019.103262. [DOI] [PubMed] [Google Scholar]
- 64.Zhou W., Peng X., Zhou X., Weir M.D., Melo M.A.S., Tay F.R., Imazato S., Oates T.W., Cheng L., Xu H.H.K. In vitro evaluation of composite containing DMAHDM and calcium phosphate nanoparticles on recurrent caries inhibition at bovine enamel-restoration margins. Dent. Mater. 2020;36:1343–1355. doi: 10.1016/j.dental.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Tao S., He L., Xu H.H.K., Weir M.D., Fan M., Yu Z., Zhang M., Zhou X., Liang K., Li J. Dentin remineralization via adhesive containing amorphous calcium phosphate nanoparticles in a biofilm-challenged environment. J. Dent. 2019;89:103193. doi: 10.1016/j.jdent.2019.103193. [DOI] [PubMed] [Google Scholar]
- 66.Yue S., Wu J., Zhang Q., Zhang K., Weir M.D., Imazato S., Bai Y., Xu H.H.K. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. J. Dent. 2018;75:48–57. doi: 10.1016/j.jdent.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Wu J., Zhou C., Ruan J., Weir M.D., Tay F., Sun J., Melo M.A.S., Oates T.W., Chang X., Xu H.H.K. Self-healing adhesive with antibacterial activity in water-aging for 12 months. Dent. Mater. 2019;35:1104–1116. doi: 10.1016/j.dental.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Zhou W., Zhou X., Huang X., Zhu C., Weir M.D., Melo M.A.S., Bonavente A., Lynch C.D., Imazato S., Oates T.W., et al. Antibacterial and remineralizing nanocomposite inhibit root caries biofilms and protect root dentin hardness at the margins. J. Dent. 2020;97:103344. doi: 10.1016/j.jdent.2020.103344. [DOI] [PubMed] [Google Scholar]
- 69.Ibrahim M.S., Balhaddad A.A., Garcia I.M., Collares F.M., Weir M.D., Xu H.H.K., Melo M.A.S. pH-responsive calcium and phosphate-ion releasing antibacterial sealants on carious enamel lesions in vitro. J. Dent. 2020;97:103323. doi: 10.1016/j.jdent.2020.103323. [DOI] [PubMed] [Google Scholar]
- 70.Xie X.J., Xing D., Wang L., Zhou H., Weir M.D., Bai Y.X., Xu H.H. Novel rechargeable calcium phosphate nanoparticle-containing orthodontic cement. Int. J. Oral Sci. 2017;9:24–32. doi: 10.1038/ijos.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vichery C., Nedelec J.M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials. 2016;9:288. doi: 10.3390/ma9040288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corral Nunez C., Covarrubias C., Fernandez E., Oliveira O.B.J. Enhanced bioactive properties of BiodentineTM modified with bioactive glass nanoparticles. J. Appl. Oral Sci. 2017;25:177–185. doi: 10.1590/1678-77572016-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taubock T.T., Zehnder M., Schweizer T., Stark W.J., Attin T., Mohn D. Functionalizing a dentin bonding resin to become bioactive. Dent. Mater. 2014;30:868–875. doi: 10.1016/j.dental.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 74.Robinson C., Shore R.C., Brookes S.J., Strafford S., Wood S.R., Kirkham J. The chemistry of enamel caries. Crit. Rev. Oral Biol. Med. 2000;11:481–495. doi: 10.1177/10454411000110040601. [DOI] [PubMed] [Google Scholar]
- 75.Arifa M.K., Ephraim R., Rajamani T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019;12:139–144. doi: 10.5005/jp-journals-10005-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philip N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019;53:284–295. doi: 10.1159/000493031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung J.H., Park S.B., Yoo K.H., Yoon S.Y., Bae M.K., Lee D.J., Ko C.C., Kwon Y.H., Kim Y.I. Effect of different sizes of bioactive glass-coated mesoporous silica nanoparticles on dentinal tubule occlusion and mineralization. Clin. Oral Investig. 2019;23:2129–2141. doi: 10.1007/s00784-018-2658-9. [DOI] [PubMed] [Google Scholar]
- 78.Tian L., Peng C., Shi Y., Guo X., Zhong B., Qi J., Wang G., Cai Q., Cui F. Effect of mesoporous silica nanoparticles on dentinal tubule occlusion: An in vitro study using SEM and image analysis. Dent. Mater. J. 2014;33:125–132. doi: 10.4012/dmj.2013-215. [DOI] [PubMed] [Google Scholar]
- 79.Seneviratne C.J., Leung K.C., Wong C.H., Lee S.F., Li X., Leung P.C., Lau C.B., Wat E., Jin L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS ONE. 2014;9:e103234. doi: 10.1371/journal.pone.0103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J.F., Wu R., Fan Y., Liao S., Wang Y., Wen Z.T., Xu X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014;93:1283–1289. doi: 10.1177/0022034514555143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stewart C.A., Hong J.H., Hatton B.D., Finer Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018;76:283–294. doi: 10.1016/j.actbio.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 82.Gregoriadis G., Ryman B.E. Liposomes as carriers of enzymes or drugs: A new approach to the treatment of storage diseases. Biochem. J. 1971;124:58P. doi: 10.1042/bj1240058P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen S., Hiorth M., Rykke M., Smistad G. Polymer coated liposomes for dental drug delivery--interactions with parotid saliva and dental enamel. Eur. J. Pharm. Sci. 2013;50:78–85. doi: 10.1016/j.ejps.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Shu Z., Zhang Y., Yang Q., Yang H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett. 2017;12:135. doi: 10.1186/s11671-017-1859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bottino M.C., Batarseh G., Palasuk J., Alkatheeri M.S., Windsor L.J., Platt J.A. Nanotube-modified dentin adhesive--physicochemical and dentin bonding characterizations. Dent. Mater. 2013;29:1158–1165. doi: 10.1016/j.dental.2013.08.211. [DOI] [PubMed] [Google Scholar]
- 86.Feitosa S.A., Palasuk J., Kamocki K., Geraldeli S., Gregory R.L., Platt J.A., Windsor L.J., Bottino M.C. Doxycycline-Encapsulated Nanotube-Modified Dentin Adhesives. J. Dent. Res. 2014;93:1270–1276. doi: 10.1177/0022034514549997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y., Yang J., Lin Z., Li J., Liang K., Yuan H., Li S., Li J. Triclosan-loaded poly(amido amine) dendrimer for simultaneous treatment and remineralization of human dentine. Colloids Surf. B Biointerfaces. 2014;115:237–243. doi: 10.1016/j.colsurfb.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 88.Liang K., Gao Y., Xiao S., Tay F.R., Weir M.D., Zhou X., Oates T.W., Zhou C., Li J., Xu H.H.K. Poly(amido amine) and rechargeable adhesive containing calcium phosphate nanoparticles for long-term dentin remineralization. J. Dent. 2019;85:47–56. doi: 10.1016/j.jdent.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Liang K., Weir M.D., Xie X., Wang L., Reynolds M.A., Li J., Xu H.H. Dentin remineralization in acid challenge environment via PAMAM and calcium phosphate composite. Dent. Mater. 2016;32:1429–1440. doi: 10.1016/j.dental.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Xiao S., Liang K., Weir M.D., Cheng L., Liu H., Zhou X., Ding Y., Xu H.H.K. Combining Bioactive Multifunctional Dental Composite with PAMAM for Root Dentin Remineralization. Materials. 2017;10:89. doi: 10.3390/ma10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang K., Weir M.D., Reynolds M.A., Zhou X., Li J., Xu H.H.K. Poly (amido amine) and nano-calcium phosphate bonding agent to remineralize tooth dentin in cyclic artificial saliva/lactic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;72:7–17. doi: 10.1016/j.msec.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 92.Lee J.H., El-Fiqi A., Jo J.K., Kim D.A., Kim S.C., Jun S.K., Kim H.W., Lee H.H. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent. Mater. 2016;32:1564–1574. doi: 10.1016/j.dental.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Moyle P.M., Mcgeary R.P., Blanchfield J.T., Toth I. Mucosal immunization: Adjuvants and delivery systems. Curr. Drug Deliv. 2004;1:385–396. doi: 10.2174/1567201043334588. [DOI] [PubMed] [Google Scholar]
- 94.Jin Z., Gao S., Cui X., Sun D., Zhao K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019;572:118731. doi: 10.1016/j.ijpharm.2019.118731. [DOI] [PubMed] [Google Scholar]
- 95.Guo J.H., Jia R., Fan M.W., Bian Z., Chen Z., Peng B. Construction and Immunogenic Characterization of a Fusion Anti-caries DNA Vaccine against PAc and Glucosyltransferase I of Streptococcus mutans. J. Dent. Res. 2004;83:266–270. doi: 10.1177/154405910408300316. [DOI] [PubMed] [Google Scholar]
- 96.Rong J., Ji H.G., Ming W.F., Bian Z., Zhi C., Peng B., Bing F.J.V. Mucosal immunization against dental caries with plasmid DNA encoding pac gene of Streptococcus mutans in rats. Vaccine. 2004;22:2511–2516. doi: 10.1016/j.vaccine.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 97.Shrestha A., Fong S.W., Khoo B.C., Kishen A. Delivery of Antibacterial Nanoparticles into Dentinal Tubules Using High-intensity Focused Ultrasound. J. Endod. 2009;35:1028–1033. doi: 10.1016/j.joen.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 98.Calvo P., Remuñán-López C., Vila-Jato J.L., Alonso M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997;63:125–132. doi: 10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4. [DOI] [Google Scholar]
- 99.Mcneela. E.A., O’Connor D., Jabbal-Gill. I., Mills. K.H.G. A mucosal vaccine against diphtheria: Formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19:1188–1198. doi: 10.1016/S0264-410X(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 100.Li Y.H., Fan M.W., Bian Z., Chen Z., Zhang Q., Yang H.R. Chitosan-DNA microparticles as mucosal delivery system: Synthesis, characterization and release in vitro. Chin. J. Med. 2005;118:936–941. [PubMed] [Google Scholar]
- 101.Gao L., Liu Y., Kim D., Li Y., Hwang G., Naha P.C., Cormode D.P., Koo H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272–284. doi: 10.1016/j.biomaterials.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naha P.C., Liu Y., Hwang G., Huang Y., Gubara S., Jonnakuti V., Simon-Soro A., Kim D., Gao L., Koo H., et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano. 2019;13:4960–4971. doi: 10.1021/acsnano.8b08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noronha V.T., Paula A.J., Durán G., Galembeck A., Cogo-Müller K., Franz-Montan M., Durán N. Silver nanoparticles in dentistry. Dent Mater. 2017;33:1110–1126. doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Kasraei S., Sami L., Hendi S., Alikhani M.Y., Rezaei-Soufi L., Khamverdi Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014;39:109–114. doi: 10.5395/rde.2014.39.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ai M., Du Z., Zhu S., Geng H., Zhang X., Cai Q., Yang X. Composite resin reinforced with silver nanoparticles-laden hydroxyapatite nanowires for dental application. Dent. Mater. 2017;33:12–22. doi: 10.1016/j.dental.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 106.Li F., Weir M.D., Chen J., Xu H.H. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 2013;29:450–461. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang K., Li F., Imazato S., Cheng L., Liu H., Arola D.D., Bai Y., Xu H.H. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J. Biomed. Mater. Res. B Appl. Biomater. 2013;101:929–938. doi: 10.1002/jbm.b.32898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang K., Cheng L., Wu E.J., Weir M.D., Bai Y., Xu H.H. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J. Dent. 2013;41:504–513. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toledano M., Osorio R., Osorio E., Medina-Castillo A.L., Toledano-Osorio M., Aguilera F.S. Ions-modified nanoparticles affect functional remineralization and energy dissipation through the resin-dentin interface. J. Mech. Behav. Biomed. Mater. 2017;68:62–79. doi: 10.1016/j.jmbbm.2017.01.026. [DOI] [PubMed] [Google Scholar]


