Abstract
Introduction:
Pathological cardiac fibrosis, through excessive extracellular matrix protein deposition from fibroblasts and pro-fibrotic immune responses and vascular stiffening is associated with most forms of cardiovascular disease. Pathological cardiac fibrosis and stiffening can lead to heart failure and arrythmias and vascular stiffening may lead to hypertension. ROCK, a serine/threonine kinase downstream of the Rho-family of GTPases, may regulate many pro-fibrotic and pro-stiffening signaling pathways in numerous cell types.
Areas covered:
This article outlines the molecular mechanisms by which ROCK in fibroblasts, T helper cells, endothelial cells, vascular smooth muscle cells, and macrophages mediate fibrosis and stiffening. We speculate on how ROCK could be targeted to inhibit cardiovascular fibrosis and stiffening.
Expert opinion:
Critical gaps in knowledge must be addressed if ROCK inhibitors are to be used in the clinic. Numerous studies indicate that each ROCK isoform may play differential roles in regulating fibrosis and may have opposing roles in specific tissues. Future work needs to highlight the isoform- and tissue-specific contributions of ROCK in fibrosis, and how isoform-specific ROCK inhibitors in murine models and in clinical trials affect the pathophysiology of cardiac fibrosis and stiffening. This could progress knowledge regarding new treatments for heart failure, arrythmias and hypertension and the repair processes after myocardial infarction.
Keywords: Rho, ROCK, fibrosis, heart failure, vascular stiffness, arrhythmias, myocardial infarction
1. INTRODUCTION
Myocardial fibrosis and vascular stiffening underlie a broad spectrum of cardiovascular diseases.[1] In the myocardium, pathological remodeling caused by the deposition of extracellular matrix (ECM) proteins by cardiac fibroblasts may lead to changes in the cardiac interstitium. These changes often either cause or accompany myocardium-related cardiovascular disease, including heart failure, diastolic dysfunction, and cardiac arrhythmias. In the vasculature, endothelial and smooth muscle cells modulate arterial stiffness and myocardial fibrosis through the production of cytokines, growth factors, and other enzymes, as well as further deposition of ECM proteins. Parallel to these processes, the innate and adaptive immune responses participate to induce cardiac fibrosis and arterial stiffening in the myocardium and vasculature, respectively.
The Rho-associated coiled-coil forming protein kinases (ROCKs) are serine/threonine kinases directly activated by RhoA, a protein belonging to the Rho family of small GTPases. The RhoA/ROCK pathway has received a great deal of attention by researchers due to its central role in a multitude of cellular and pathogenic processes. ROCK was first identified as an important target of RhoA[2] and a regulator of the actin cytoskeleton in 1995 and 1996 through inhibitory phosphorylation at Thr696 and Thr853 of the myosin binding subunit (MBS) of myosin light chain (MLC) phosphatase (now more commonly referred to as the myosin phosphatase binding subunit 1 [MYPT1]),[3] thereby leading to MLC phosphorylation and actomyosin stress fiber formation. Following papers suggested that these actions affected vascular tone, cell migration, gene expression., inflammation, and oxidative stress.[4] As cell migration, inflammation, and oxidative stress play important roles in the pathogenesis of fibrosis, it is likely that ROCK could also affect fibrosis through these cellular mechanisms.
Due to the pathophysiological significance of fibrosis in the cardiovascular system, identifying the fibrotic contributions of important molecular pathways, including Rho/ROCK, may yield new therapies that can decrease cardiovascular mortality and morbidity. Such findings could be beneficial as cardiovascular diseases related to fibrosis are major public health burdens. For instance, heart failure alone afflicts 26 million people worldwide with an estimated economic burden of over $100 billion a year.[5] Moreover, a variety of fibrosis-related cardiovascular diseases, including heart failure with preserved ejection fraction (HFpEF), remain without effective therapeutic interventions. In this review on the role of ROCK in cardiovascular fibrosis and stiffening, we will begin by summarizing the structure and general functions of ROCK and then discussing the role of ROCK in the molecular mechanisms of fibrosis and stiffening. In addition, we will provide an overview of how ROCK contributes to fibrosis and stiffening in a diverse array of cell types involved in cardiovascular disease. We conclude by considering current and future developments of ROCK inhibitors as therapeutic agents for cardiovascular fibrosis and stiffening.
2. STRUCTURE AND FUNCTIONS OF ROCK
The structure of ROCK, and its two isoforms ROCK1 and ROCK2, are well characterized (Figure 1). ROCK1 and ROCK2 are highly homologous, sharing 92% of the amino acids in the kinase domain, 55% in the coiled-coil region, and 65% in the overall amino acid sequence.[4] The C-terminus of ROCK contains an autoinhibitory region, composed of a Pleckstrin-homology domain and a Rho-binding domain. Binding of lipids, particularly phosphatidylinositol (3,4,5)-trisphosphate (PIP3), to the Pleckstrin-homology domain and interaction of the active form of Rho with the Rho-binding domain induces the open and catalytically-active conformation of ROCK (Figure 1). The open conformation may also be induced by cleavage of the C-terminus by caspase-3 or granzyme B. Moreover, the N-terminal kinase domain and the coiled-coil domain may mediate dimerization of the open form of ROCK, linking each kinase domain together. It is likely that dimerization of ROCK is necessary for some of its downstream functions. While the primary mechanism of activation for both isoforms of ROCK is through RhoA, other proteins are also implicated in the activation of ROCK. For instance, ROCK appears to be activated by other members of the Rho family, including RhoB and RhoC,[2] as well as polo-like kinase 1.[6] A range of other proteins, however, may inhibit ROCK, including the GTPases RhoE (Rnd3),[7] Gem and Rad[8], and the chaperone protein Morgana/chp-1.[9] It is likely that there are additional, yet undiscovered, pathways involved in the regulation of ROCK activity.
Figure 1. Structure and activation of ROCK.
ROCK is composed of a N-terminal kinase domain followed by a coiled-coil region. The coiled-coil region of ROCK contains the Rho-binding domain (RBD) and is followed by a Pleckstrin-homology (Ph) domain and a C-terminal cysteine rich domain (CRD). The RBD and Ph domain associate independently with the N-terminal kinase domain for ROCK autoinhibition. The binding of RhoA to the RBD disrupts the autoinhibition of ROCK, causing its unfolding and activation. Cleavage of ROCK by caspase-3 and granzyme B can also activate ROCK independent of RhoA.
Both ROCK isoforms mediate changes in cytoskeletal dynamics through the phosphorylation of numerous downstream actin cytoskeleton-mediating proteins. In particular, from an intense period of research conducted predominantly in the late 1990s and early 2000s, it was found that ROCK phosphorylates LIM kinase-1 and -2, adducin, ezrin/radixin/moesin (ERM) proteins, formin homology domain protein 1 (FHOD1), MLC, and MYPT1.[10] Furthermore, ROCK can mediate the polymerization of G-actin to F-actin though the action of LIM kinase-1 on cofilin.[11] These interactions lead to force generation and morphological changes, contributing to a broad range of important actin-myosin mediated processes, including cell adhesion, polarity, motility, cytokinesis, and phagocytosis. Both ROCK isoforms also appear to play important roles in gene expression, proliferation, and apoptosis, largely mediated by the kinase’s effects on the proteins involved in stress fiber formation. As examples, ROCK regulates the cell cycle regulatory proteins cyclin A via LIM kinase-2 and also regulates cyclin D via Ras and the mitogen-activated protein kinase.[12] In addition, through its effects on MLC, ROCK mediates apoptotic processes such as membrane blebbing, cellular fragmentation, and nuclear disintegration.[13] Consequently, ROCK plays a central role across a broad spectrum of fundamental cellular functions, often attributed to its actions on actomyosin function.
Despite similarities in the kinase domain, ROCK1 and ROCK2 may play different cellular functions. Indeed, ROCK2 and ROCK1 may have different subcellular localizations. ROCK2 appears to have a predominantly cytosolic localization but also accumulates in the cleavage furrow, nucleus, intermediate filaments, and centrosomes.[13] ROCK1 also accumulates in the cytosol, but may be less likely to be distributed at the plasma membrane compared with ROCK2.[13] Likewise, ROCK1 and ROCK2 are differentially expressed: ROCK1 is ubiquitously expressed in all tissues, although less in brain and skeletal muscle, while ROCK2 is more abundantly expressed than ROCK1 in the brain, muscle, heart, lung, and placenta.[14] ROCK1 also may play a more important pro-fibrotic role than ROCK2 across a wide variety of tissues, particularly in the heart,[15–19] as evidenced by global knockout and haploinsufficient ROCK1 mice. A recent study published in 2017, however, indicates that ROCK2 deletion in cardiac fibroblasts leads to decreased cardiac hypertrophy and fibrosis,[20] suggesting that each ROCK isoform may possess varying pro-fibrotic functions depending on cell type. Indeed, a study published a year later suggested that ROCK1 and ROCK2 in cardiomyocytes may in fact play opposing roles in cardiac hypertrophy and fibrosis.[19] Consequently, while an emerging body of research may suggest that both ROCK1 and ROCK2 play important roles in cardiac fibrosis, the role of each ROCK isoform and their knockout/knockdown phenotypes in murine models of diastolic dysfunction, pressure overload, or heart failure remain to be fully characterized.
Intriguingly, upstream negative regulators of ROCK, including the GTPases RhoE and Rad, appear to also mediate cardiac fibrosis and heart failure. One study suggests that RhoE haploinsufficient mice develop hyperactivation of ROCK activity, myocardial apoptosis, and heart failure, a phenotype that was partially attenuated by administration of fasudil, a well-established ROCK inhibitor, or through deletion of ROCK1.[21] Moreover, Rad knockout in mice led to severe cardiac fibrosis through upregulated expression of connective tissue growth factor (CTGF).[22] Therefore, while research into the Rho subfamily and its related GTP-binding proteins has often focused on RhoA and ROCK itself, regulators of ROCK may also be potential therapeutic targets to ameliorate cardiac fibrosis and vascular stiffening. Further research into these proteins may help clarify whether these negative regulators of ROCK affect cardiovascular fibrosis or stiffening through ROCK-dependent or -independent mechanisms.
3. ROCK-MEDIATED PATHWAYS IN FIBROBLASTS
ROCK appears to mediate many of the cellular responses of fibroblasts and plays an important role in the pathological inflammatory environment that persists after injury. Knowledge of these pathways are central towards developing strategies that target ROCK in myocardial fibrosis and vascular stiffening. Hence, in this section of the review, we explore pathways through which ROCK may modulate fibroblast activation and differentiation.
3.1. MRTF/SRF signaling
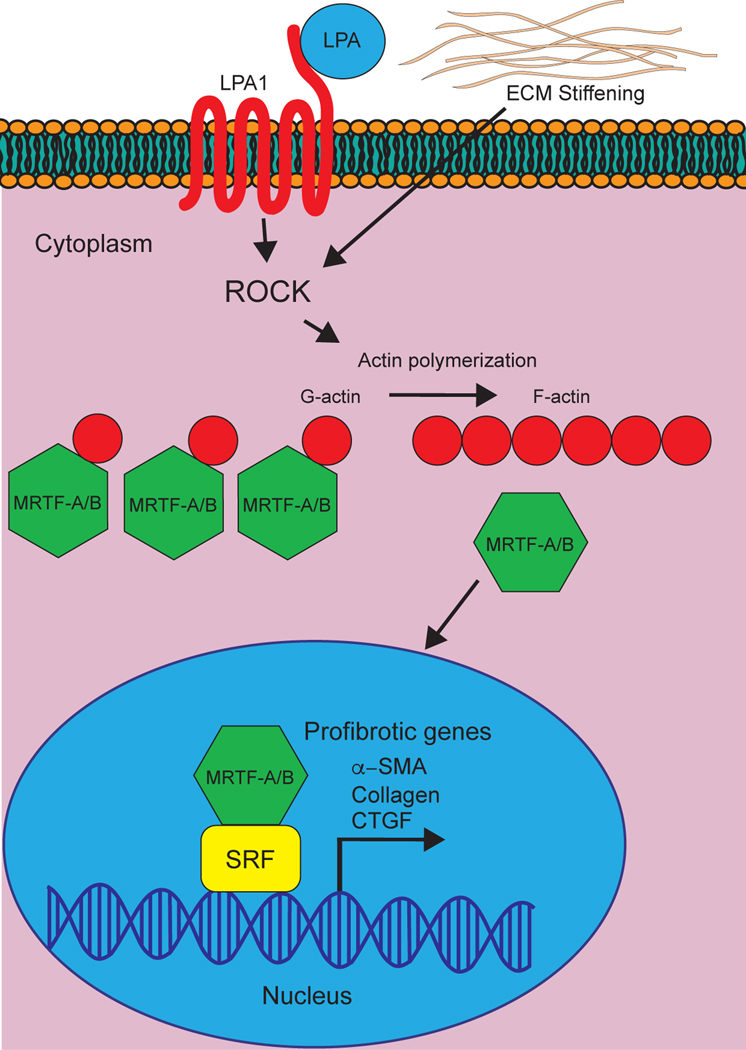
Serum response factor (SRF), belonging to the MADS box-containing family of transcription factors, is critically involved in fibroblast differentiation, proliferation, and activation.[23] SRF interacts with cofactors from the myocardin-related transcription factor (MRTF) and ternary complex factor (TCF) families, thus coupling growth factor signaling to gene transcription.[24] In particular, MRTF-A and MRTF-B recruitment is crucial for SRF signaling: of the >3100 SRF binding sites identified through chromatin immunoprecipitation sequencing (ChIP-seq), >2600 exhibited MRTF-dependent SRF binding.[24] Indeed, numerous MRTF-SRF-binding sites are located in the promoter regions governing fibroblast activation and inhibition of MRTF/SRF prevents scar tissue formation.[25] The MRTF/SRF pathway is regulated by actin dynamics.[20] For example, G-actin binds to MRTF-A and MRTF-B, sequestering the transcription factor in the cytoplasm (Figure 2). Polymerization of G-actin to F-actin liberates the MRTFs, allowing it to enter the nucleus to associate with SRF and to activate target pro-fibrotic target genes.
Figure 2. Involvement of ROCK in MRTF/SRF signaling.
The binding of G-actin to myocardin-related transcription factors (MRTFs) sequesters MRTFs in the cytosol. The polymerization of G-actin to F-actin liberates MRTFs to enter the nucleus and bind serum response factors (SRFs) to induce transcription of pro-fibrotic genes. A variety of upstream activators, including lysophosphatidic acid (LPA) and extracellular matrix (ECM) stiffening, increase Rho-kinase activity to enhance G-actin to F-actin polymerization, thus enhancing the translocation of MRTFs into the nucleus.
Since the polymerization of G-actin into F-actin is regulated by ROCK through the ROCK-LiM kinase-cofilin pathway,[11, 26] and RhoA was first identified as a regulator of transcriptional activity of SRFs in 1995,[27] ROCK may play a central role in controlling the MRTF-SRF pathway. This appears to be the case, as Esnault et al. (2014) have shown that RhoA/ROCK signaling through actin drives SRF binding and MRTF recruitment.[24] Numerous studies have also shown that pro-fibrotic and MRTF/SRF-dependent transcription in the myocardium, including expression of CTGF,[28] differentiation of fibroblasts into myofibroblasts,[29] activation of myofibroblasts,[30] and ECM stiffening and production,[31] are regulated by ROCK. In consequence, some studies have suggested that MRTF-mediated upregulation of pro-fibrotic target genes are broadly regulated by activation of RhoA and ROCK.[32] Indeed, the use of ROCK inhibitors prevented the nuclear accumulation of MRTF-A across a wide variety of pro-fibrotic activators (e.g. lysophosphatidic acid [LPA] or transforming growth factor- β [TGF-β]).[28, 30]
Pro-fibrotic activators, such as LPA, were first found to activate RhoA and ROCK through G-protein coupled receptor (GPCRs) in 1999 by Kranenburg et al.[33] Subsequent studies throughout the early 2000s identified numerous RhoGEFs that mediate this mechanism, including p115-RhoGEF, PSD-95/Disc-large/ZO-1 homology-RhoGEF, leukemia-associated RhoGEF and lymphoid blast crisis-RhoGEF.[34] Intriguingly, ROCK has also been shown to be activated downstream of Gq through a pathway involving phospholipase C, Ca2+, and protein kinase C, although this induction of RhoA activation was through thrombin and not LPA.[35] Nonetheless, as LPA also activates Gq pro-fibrotic pathways, it is possible that LPA can activate ROCK through alternative GPCR signaling pathways to induce MRTF/SRF-mediated fibrosis.
Intriguingly, ROCK itself may be upregulated downstream of matrix stiffening.[36] Consequently, in a feed-forward loop, increased ECM stiffening may increase ROCK activity, which in turn enhances fibroblast differentiation, myofibroblast activation, and further ECM production and stiffening.
3.2. YAP/TAZ/TEAD signaling
Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding domain (TAZ) are key downstream transcriptional coactivators of the Hippo kinase cascade[37] and are important mediators of fibroblast activation and fibrosis.[38] In the canonical Hippo signaling pathway, the serine/threonine kinases MST1 and MST2, orthologs of the Drosophila Hippo kinase, interact with and phosphorylate the proteins SAV1, MOB1A/B, and LATS1/2 to activate LATS1/2 kinases (Figure 3). The activated enzyme complex directly phosphorylates YAP and TAZ on multiple serine residues via interaction with 14-3-3 (Figure 3). After phosphorylation, YAP and TAZ are retained in the cytoplasm and are primed for future proteasomal degradation. When the Hippo kinase cascade is inhibited, however, the unphosphorylated YAP and TAZ translocate into the nucleus to bind with transcription factors, of which the most studied are the TEA domain family members 1–4 (TEAD1–4). Importantly, TEAD, together with YAP and TAZ, mediate the upregulation of important pro-fibrotic target genes, including CTGF,[39] plasminogen activator inhibitor-1 (PAI-1),[40] and TGF-β.[38]
Figure 3. Involvement of ROCK in YAP/TAZ/TEAD signaling.
Yes-associated protein (YAP) and transcriptional coactivator with a PDZ-binding domain (TAZ) are retained in the cytoplasm by the phosphorylation of YAP and TAZ by large tumor suppressor (LATS) kinases, mediated by 14-3-3. The activation of ROCK by upstream G-proteins, including lysophosphatidic acid receptor-1 (LPA1), and by extracellular matrix (ECM) stiffening leads to the polymerization of actin stress fibers that increases YAP/TAZ nuclear translocation and transcription of pro-fibrotic genes. How ROCK regulate LATS kinases and YAP/TAZ translocation is not well characterized, although may involve capping proteins such as cofilin.
An important aspect of the Hippo pathway is its role as a transducer of alterations in cell structure, including changes in cell shape, cell-cell interactions, mechanotransduction, and matrix stiffness. Indeed, numerous studies, primarily from the early 2010s, have shown that this is where ROCK plays a role in regulating YAP/TAZ signaling.[41] For instance, studies have shown that disruptions in F-actin stress fibers (e.g. through ROCK inhibition) prevents nuclear TAZ/YAP translocation (Figure 3).[42] Likewise, F-actin capping or cleavage proteins, such as cofilin, retain TAZ/YAP in the cytoplasm and the inactivation of these factors (as mediated by ROCK) induces YAP/TAZ-mediated transcription.[43] The regulation of YAP/TAZ signaling by ROCK is also evidenced in studies using inhibitors of ROCK, such as Y-27632, which reduce nuclear YAP translocation and inhibit YAP/TAZ-mediated fibroblast differentiation[41, 42] and myofibroblast activation.[44] Regulation of YAP/TAZ signaling through RhoA and ROCK, in a similar manner to MRTF/SRF signaling, may also occur through Gα12/13. Yu et al. (2012) showed that overexpression of active Gα12/13 led to YAP dephosphorylation while knockdown of Gα12/13 blocked LPA-mediated YAP dephosphorylation.[45] Moreover, use of dominant negative mutants of RhoA blocked the LPA-mediated YAP dephosphorylation while constitutively active mutants of RhoA resulted in YAP dephosphorylation.[45] Hence, ROCK may act as an inhibitor of LATS1/2 kinase activity downstream of Gα12/13. While the precise mechanisms mediating ROCK’s regulation of LATS1/2 remains undetermined, a recently published paper by Ibar et al. (2018) suggested that LIMD1, a member of the Ajuba family proteins known to associate with LATS kinases, is required for Rho-mediated regulation of LATS1/2 and YAP.[46]
While ROCK itself may regulate cellular cytodynamics to influence YAP/TAZ signaling, it is possible that changes in cellular actomyosin dynamics themselves may regulate ROCK in a feed forward loop or affect YAP/TAZ signaling through mechanisms independent of ROCK.[36, 42] Indeed, it may be difficult to divorce the effect of changes in myosin-actin dynamics and ROCK function on YAP/TAZ signaling from the effect of each on the other. One study suggests that in human glioblastoma cells, YAP dephosphorylation and activation was abrogated by RhoA inhibition but not affected by the use of Y-27632.[32] Moreover, the same study suggested that YAP dephosphorylation could be achieved through the use of cytochalasin D, an inhibitor of actin polymerization.[32] Consequently, while most studies appear to indicate that the Rho/ROCK signaling pathway is a robust regulator of YAP/TAZ/TEAD signaling and, in turn, its pro-fibrotic target genes, conflicting reports of ROCK on YAP phosphorylation may indicate that further mechanistic studies are necessary to clarify how ROCK governs LATS kinase activity and subsequent YAP phosphorylation.
3.3. TGF-β signaling
The cytokine TGF-β is an important mediator of all aspects of cardiovascular fibrosis.[47] TGF-β is composed of three isoforms (TGF-β1, TGF-β2, and TGF-β3) and all three can bind to TGF-β receptor 2 (TGFR2) as homodimers to recruit and activate TGF-β receptor 1. In the canonical signaling pathway, the binding of TGF-β to its receptors leads to the phosphorylation and activation of Smad2 and Smad3, which complex with Smad4 to translocate to the nucleus. The Smad3 portion of the enzyme complex binds directly with promoters to induce transcription of well-characterized pro-fibrotic agents, including collagen I,[48] tissue inhibitor of matrix metalloproteinases (TIMPs),[49] α-smooth muscle actin (α-SMA),[50] and PAI-1.[51]
ROCK is involved in both the canonical TGF-β signaling pathway and in its non-canonical pathways. ROCK’s role in the canonical TGF-β pathway was first identified by Kamaraju et al. (2005).[52] In the canonical pathway, transfection of dominant negative RhoA or the use of ROCK inhibitors prevented the phosphorylation of Smad2 and Smad3 on its TGF-β-regulated linker region,[52] inhibited Smad nuclear translocation, and decreased Smad-binding element promoter activity.[53, 54] Moreover, use of Y-27632 and statins, well-characterized ROCK inhibitors, have been shown to inhibit Smad2 expression and TGF-β-mediated synthesis of type I collagen mRNA in fibroblasts and epithelial cells.[55, 56] Consequently, ROCK may be an important regulator of TGF-β-mediated SMAD signaling.
Outside of the canonical TGF-β signaling pathway, numerous studies have shown ROCK activity is increased in response to TGF-β stimulation, which may mediate the expression of pro-fibrotic genes.[57] Indeed, the increase in ROCK activity in response to TGF-β may cause polymerization of F-actin to drive MRTF/SRF-dependent myofibroblast activation and differentiation.[58] Intriguingly, the activation of latent TGF-β by the αvβ6 integrin via the effect of thrombin on protease activated receptor 1 is ROCK-dependent.[58] These studies are supported by reports indicating that use of TGF-β/Smad inhibitors can downregulate RhoA and ROCK1 expression, suggestive of crosstalk between the two pathways.[56] Thus, ROCK may regulate the canonical TGF-β pathway and also mediate non-canonical TGF-β pathways both in the activation of and downstream of TGF-β.
4. OTHER CELLULAR TARGETS OF ROCK IN CARDIOVASCULAR FIBROSIS
4.1. T helper cells
Inflammation is an important etiology of fibrosis. Prolonged or acute tissue damage and chronic diseases in the cardiovascular system (e.g. hypertension) result in activation of immune cells that in turn release cytokines that induce the activation and differentiation of fibroblasts. In this section of the review, we cover how ROCK may mediate pro-fibrotic cytokine release from CD4+ T helper (Th) cells, including from Th2, Th1, and Th17 cells.
Th cells are important mediators of the adaptive immune response and produce distinct repertoires of cytokines that are critical for stimulating tissue repair and fibrosis. For instance: Th2 releases three pro-fibrotic cytokines, IL-4, IL-5, and IL-13; Th1 releases anti-fibrotic IFN-γ and IL-12; Th17 releases pro-fibrotic IL-17. ROCK appears to mediate the expression or release of many of these cytokines. For example, in ROCK1 haplo-insufficient mice, IL-13 and IL-5 were significantly decreased[59] while the use of Y-27632, a potent pan ROCK inhibitor, attenuated expression or promoter activity of IL-4, IL-6, and IL-13.[60] Furthermore, use of Y-27632 appeared to increase expression of IFN-γ[61] and IL-12.[62] Finally, administration of ROCK inhibitors Y-27632 and fasudil in mice[63] and the ROCK2-specific inhibitor KD025 in humans[64] decreased production of IL-17. As each of these inflammatory cytokines has been linked with cardiac fibrosis,[65–67] ROCK likely plays an additional role in the regulation of cardiac fibrosis via inhibition of anti-fibrotic or upregulation of pro-fibrotic cytokines. However, despite the importance of ROCK in the production or upregulation of a broad array of cytokines, the precise molecular mechanisms by which ROCK performs these actions remain to be determined and remains a subject of active research.
ROCK may also mediate inflammatory cytokine pathways through regulation of JAK/STAT signaling. Binding of cytokines from the interleukin (IL-1 through IL-31) or interferon (e.g. IFN-γ) families to their cognate receptors leads to receptor aggregation and recruitment of two JAK molecules in approximate proximity. This results in trans-phosphorylation of JAK, augmenting their kinase activities to phosphorylate tyrosine residues on their cognate receptors. These phosphorylated tyrosine residues can subsequently serve as docking sites for numerous signaling and adaptor proteins, among which the most studied are the STAT proteins. The JAK-receptor complex can phosphorylate tyrosine residues on STAT, leading to homo/heterodimerization of two STAT proteins and consequent translocation to the nucleus to active pro-inflammatory or pro-fibrotic genes. Several recent papers have highlighted a potential crosstalk between the Rho/ROCK and JAK/STAT pathways, where ROCK appears to modulate phosphorylation of various JAK and STAT proteins. For example, ROCK1 may be able to directly phosphorylate JAK2 in certain cell types, leading to phosphorylation and activation of STAT3 and FOXO1.[68] Interestingly, in T cells, ROCK2 has been linked to dephosphorylation of STAT3 but phosphorylation of STAT5 to regulate cytokine production.[64] It remains to be seen, however, how exactly ROCK increases phosphorylation of JAK and STAT (e.g. if ROCK modulates STAT phosphorylation directly or through regulation of JAK) and how cross-talk between Rho/ROCK and JAK/STAT may mediate cardiovascular fibrosis.
4.2. Endothelial cells
The vascular endothelium is an important endocrine organ that controls vascular tone through the release of vasoconstrictive or vasodilating factors. Moreover, the endothelium is a major mediator of inflammation, angiogenesis, and fibrosis through the upregulation of cell adhesion molecules and secretion of a variety of growth factors, including vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs). Principal to the modulation of these endothelial functions is the synthesis and release of endothelial-derived nitric oxide (NO). Indeed, endothelial dysfunction is characterized by reduced NO bioavailability. In the myocardium, decreased NO production by coronary endothelial cells has been linked with vascular stiffness,[69] ventricular remodeling,[70] and myocardial fibrosis [71, 72]. Moreover, studies have shown that inhibition of endothelial nitric oxide synthase (eNOS) induces and enhances cardiac fibrosis through stromal-derived-factor-1/C-X-C chemokine receptor type 4-mediated upregulation of ECM production in cardiac fibroblasts and can lead to apoptosis of both cardiac fibroblasts and cardiomyocytes.[71] In the vasculature, arterial contraction is regulated by nitric oxide through the direct effect of NO on vascular smooth muscle cells (VSMCs). Thus, endothelial dysfunction may lead to cardiac fibrosis and vessel stiffening through depressed NO production.
ROCK was first reported as a negative regulator of eNOS and endothelial NO production in 2001 and 2002 by several groups.[73–75] Follow-up studies indicated that the use of ROCK inhibitors, such as Y-27632 and fasudil, dominant negative mutants of ROCK, and ROCK knockout mice increase eNOS mRNA half-life and expression.[76] Aside from transcriptional regulation of eNOS, ROCK may directly inhibit phosphorylation of eNOS through inhibition of the PI3K/Akt signaling pathway, thus altering eNOS activity.[77] The Rho/ROCK pathway and the PI3K/Akt pathway may intersect at PTEN, a phosphatase that dephosphorylates phosphoinositide (PIP) substrates.[77] PTEN acts in opposition to PI3K-mediated Akt signaling by dephosphorylating PIP 3,4,5-triphosphate (PIP3) to generate PIP 4,5-biphosphate (PIP2), consequently inhibiting the ability of Akt to associate with the plasma membrane through its Pleckstrin-homology domain. Moreover, ROCK phosphorylation of PTEN appears to be necessary for the phospholipid phosphatase activity of PTEN.[78] Consequently, ROCK may regulate eNOS at the level of expression and activity, thus governing an important aspect of endothelial function-mediated fibrosis.
ROCK has also been shown to be involved in the endothelial-to-mesenchymal transition (EndMT), which is a form of epithelial-to-mesenchymal transition that primarily occurs during embryonic development of the myocardium and is hypothesized to play a role in the progression of cardiac fibrosis.[79] For instance, the regulation of the cytoskeleton through Rho/ROCK signaling is a necessary component of cytoskeletal rearrangements and migration that allows the cell to delaminate from the endothelial layer to induce EndMT.[80–82] In these experiments, it appears that ROCK inhibitors prevent EndMT while upregulation of ROCK increases EndMT. Moreover, important signaling pathways orchestrating this arrangement in cell structure include the TGF-β signaling pathways, such as the ones involving ROCK.[79] Additional pathways include the transcription of Snail, Slug, and Twist, which are vital genes in EndMT,[79] the downstream effects of which also appear to be mediated by ROCK.[83] It is important to note that while a few papers have suggested EndMT is involved in cardiac fibrosis in adult animals, other studies have suggested that de novo EndMT plays but a minor role in adult cardiac fibrosis.[84] While ROCK plays an important role in EndMT, further studies are necessary to clarify the role of EndMT in cardiac fibrosis and how ROCK may modulate EndMT-induced cardiac fibrosis.
4.3. Vascular smooth muscle cells
ROCK may regulate arterial stiffness through VSMC-ECM interactions, VSMC plasticity, and vascular tone. In particular, mechanical homeostasis between VSMCs and ECM proteins is a crucial element of arterial stiffness.[85] Cellular-ECM interactions involve collagen and elastin proteins in the ECM, adhesion proteins, and crucially, integrins which link at focal adhesion (FA) sites and the accompanying integrin linker proteins that associate with the actin-myosin cytoskeleton and GPCRs.[86] FAs are involved in inside-out or outside-in signaling, defined as the transmission of internal cell contractile forces outside the cell or the transmission of external mechanical forces to cause intracellular changes, respectively. For instance, the binding of linker proteins, such as talin, to the β-cytoplasmic tail of the integrin alters the conformation of the integrin in a form that allows strong binding with ECM proteins.[86] In contrast, binding of talin to actin filaments, such as G-actin, via vinculin promotes nascent FA complexes[86] in a process that appears to be mediated by ROCK’s effects on FA turnover.[87] Depending on the distribution of ECM proteins and the clustering of integrins and recruitment of additional adhesome proteins to the FA, signal transduction through ROCK may alter actin polymerization and MLC phosphorylation to induce changes in arterial stiffness.[86, 88] Thus, ROCK plays an vital role in VSMC-ECM interactions and arterial stiffness through the formation of FAs and stress fiber formation.
VSMC plasticity refers to the ability of smooth muscle cells to switch from a quiescent, contractile phenotype to a migratory, proliferative phenotype that accompanies ECM remodeling and arterial stiffness.[86] Protein markers for the contractile phenotype include α-SMA, myosin heavy chain, calponin, h-caldesmon, desmin, and smoothelin.[86] Along with increased expression of contractile molecular markers, studies have indicated that there is an increase in the number caveolae and their associated proteins, caveolin-1 and cavelolin-2, in the contractile phenotype.[89] Intriguingly, RhoA and ROCK possess a conserved caveolin-binding domain that can putatively interact with the caveolin-scaffolding domain of caveolin-1.[90, 91] Indeed, ROCK appears to phosphorylate caveolin-1 to regulate FA dynamics.[87] Consequently, caveolin may link smooth muscle plasticity, VSMC-EC interactions, and the Rho/ROCK pathway to regulate arterial stiffness. A variety of other mechanisms governing VSMC phenotype switching may also be mediated by ROCK, either through the direct actions of ROCK on MRTF/SRF-mediated transcription of VSMC contractile genes or through other ROCK-mediated pathways (such as Smad and PTEN/PI3K/AKT).[92]
Vascular tone is the intrinsic level of vessel vasoconstriction and compliance and is modulated by the position of the VSMC within the cellular milieu, its interactions with ECM proteins, and through signaling pathways involved with VSMC constriction.[86] ROCK plays a direct role in controlling vascular tone through its actions on cellular contractility, as previously detailed in this review. For instance, ROCK phosphorylates MLC, phosphoinhibits MYPT1 of myosin light chain phosphatase, and controls eNOS expression and activity in order to regulate vasoconstriction.
4.4. Macrophages
Macrophages play central roles in wound healing and fibrosis. As examples, macrophages produce matrix metalloproteinases, cytokines, growth factors, and other inflammatory mediators that contribute to the initial cellular response after injury. Indeed, depletion of macrophages early in the inflammatory cascade can blunt the inflammatory response to injury, leading to reduced scarring and fewer myofibroblasts.[93] In contrast, removal of certain populations of macrophages can also result in an opposing response, such as less efficient repair and more fibrosis.[93] Consequently, intense research over the past decade into macrophages has focused on identifying the various macrophage populations that regulate the stages of cellular response to injury. Two major macrophage phenotypes are broadly identified as M1 (pro-inflammatory) and M2 (pro-regeneration and pro-fibrotic). The study of ROCK regulation of macrophage polarity is recent: Zandi et al. (2015) suggests that ROCK2 inhibition in a murine model or through ROCK pharmacological inhibition may decrease M2-like macrophages and upregulate M1 markers.[94] Liu et al. (2013) suggests the opposite: that ROCK inhibition with fasudil may instead shift macrophages to the M2 phenotype.[95] Lee et al. (2018) supports the later: that nicorandil, a drug able to activate protein kinase G (PKG) to phosphoinhibit RhoA and ROCK, or fasudil can polarize macrophages to M2, leading to an attenuation of myofibroblast-mediated cardiac fibrosis.[96] Despite these reports, the molecular role in which ROCK plays in macrophage polarization remains unclear, but may be due to ROCK-mediated regulation of JAK/STAT signaling[68, 97] or ROCK’s actions on IRF4,[63] a well characterized effector in M2 polarization.[98]
5. ROLE OF ROCK IN FIBROSIS- OR STIFFENING-RELATED CARDIOVASCULAR DISEASES
5.1. Vascular stiffness and hypertension
The above signaling pathways and secretory molecules in endothelial cells and vascular smooth muscle cells suggest that ROCK plays a principal role in vascular stiffness. As a significant body of literature shows that vascular stiffness plays a causal or associative role in hypertension, for instance, in longitudinal analyses of patients (as measured by pulse wave velocity [PWV])[99] or in mouse models[100] that indicate arterial stiffening precedes hypertension, it is possible that ROCK also plays a pivotal role in hypertension. Indeed, numerous studies have connected ROCK with aortic stiffness and hypertension in the clinical setting and in murine models. For instance, Noma et al. (2007), by measuring carotid-femoral PWV in patients, linked ROCK activity with aortic stiffness and aging.[101] Moreover, a plethora of seminal studies on the effects of ROCK inhibition in mice have shown that inhibition of ROCK in mice, either through Y-27632 or fasudil, reduce blood pressure and attenuate endothelial dysfunction.[76, 102] The use of ROCK inhibitors in small clinical trials also reduce pulmonary hypertension.[103] Altogether, these lines of evidence suggest that ROCK is an important mediator of arterial stiffness and in the subsequent development of hypertension and that use of ROCK inhibitors in humans may be a potential therapeutic avenue for alleviating various forms of hypertension.
5.2. Heart Failure: HFpEF, HFrEF, and cardiomyopathies
Research into heart failure has led to widespread acknowledgement that myocardial fibrosis and cardiac hypertrophy play major roles in both HFpEF and heart failure with reduced ejection fraction (HFrEF) and various types of cardiomyopathies.[104] As with all forms of fibrosis, myocardial fibrosis is characterized histologically by excess deposition of fibrous tissue (e.g. collagen I and II) and can be induced by cardiomyocyte death or by mechanical, ischemic, or metabolic injuries to the myocardium.[104] Furthermore, accumulation of fibrosis in the myocardium can cause structural changes that lead to increased left ventricular end-diastolic pressure, impairment of left ventricular compliance and relaxation, reduced ejection fraction, and cardiomyocyte hypertrophy.[104] As diastolic dysfunction is a common etiology of HFpEF and cardiomyocyte hypertrophy and interstitial fibrosis are associated with hypertrophic and dilated cardiomyopathies, myocardial fibrosis plays an important role in mediating the development of HFpEF, HFrEF, and various cardiomyopathies.
Consequently, the importance of ROCK in heart failure has been largely explored through its role in cardiac fibrosis. Early studies using pan ROCK inhibitors such as Y-27632 and fasudil limited the progression of adverse cardiac remodeling and fibrosis after angiotensin II (AngII) and NG-nitro-L-arginine methyl ester (L-NAME, an inhibitor of nitric oxide synthase) treatment and after myocardial infarction.[70] Subsequent studies using ROCK1+/− and ROCK1−/− mice showed that ROCK downregulation decreases cardiac fibrosis, left ventricular dilation, and contractile dysfunction after treatment with AngII or after myocardial infarction.[15] More recent studies have focused on cell-specific and isoform-specific knockouts of ROCK to more precisely evaluate the role of ROCK in heart failure. Sunamura et al. (2018) suggested that deletion of ROCK1 in cardiomyocytes may decrease cardiac fibrosis, MMP activity, and ECM remodeling in response to pressure overload, albeit deletion of ROCK2 had no effect on cardiac fibrosis.[19] In contrast, another study suggested that global ROCK2+/− and cardiomyocyte-specific ROCK2-deficient mice demonstrated less cardiomyocyte hypertrophy and apoptosis and cardiac fibrosis, which may be related to upregulation of four-and-a-half LIM-only protein 2 (FHL2) and FHL2-mediated inhibition of SRF and extracellular signal-regulated mitogen-activated protein kinase (ERK).[105] Shimizu et al. (2017) also suggested that ROCK2 may play a role in heart failure: ROCK2-specific deletion in fibroblasts attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction through inhibition of CTGF and FGF production.[20] Furthermore, Shi et al. (2019) reports that disruption of ROCK1 and ROCK2 in cardiomyocytes can promote autophagy to inhibit cardiac fibrotic remodeling, albeit ablation of only ROCK2 in cardiomyocytes led to more fibrosis.[18] Interestingly, the same study suggested that knocking out ROCK2 can lead to a compensatory increase in ROCK1 activity, which may explain these results. Altogether, these recent studies support earlier in vitro results that suggest ROCK1 and ROCK2 in fibroblasts and cardiomyocytes play central roles in cardiac fibrosis underlying heart failure and hypertrophic and dilated cardiomyopathies.
Additional in vivo evidence that ROCK-mediated fibrosis may be involved or be modulated to improve heart failure may come from pre-clinical and clinical trials of LCZ696, which is a drug composed of an angiotensin receptor blocker (valsaltran) and a neprilysin inhibitor (sacubitril). In the PARADM-HF trial published in 2014, LCZ696 was shown to offer an “overwhelming benefit” over standard therapy of care in patients with HFrEF and New York Heart Association Class II, III, and IV heart failure symptoms by a median follow-up of 27 months.[106] A follow-up pre-clinical trial attempting to elucidate the mechanisms of LCZ696 in 2019 suggested that valsaltran/sacubitril can reduce pathological accumulation and activation of cardiac fibroblasts both in vitro and in vivo through protein kinase G-dependent inhibition of RhoA at Ser188.[107] As the clinical efficacy of LCZ696 cannot be fully explained by a reduction in systemic blood pressure or increased atrial natriuretic peptide levels,[107] ROCK may play an important role in the major clinical benefits seen with LCZ696. Follow-up clinical studies, perhaps assaying for changes in ROCK activity in patients taking LCZ696, may provide more direct evidence for ROCK-mediated benefits in cardiovascular fibrosis in patients with HFrEF.
5.3. Cardiac Arrythmias: Atrial fibrillation and ventricular tachycardia
Cardiomyocytes can propagate electrical signals to adjacent cells through connexin channels that connect the cytoplasm of cardiomyocytes.[108] Abrogation of this cell-to-cell contact, for instance, through cardiac remodeling or increased cardiac fibrosis, is arrhythmogenic and can result in atrial fibrillation (AF) and ventricular tachycardia (VT). This is supported by studies showing that in patients with hypertrophic cardiomyopathy, cardiac fibrosis as measured by late gadolinium enhancement cardiovascular magnetic resonance is an independent predictor for VT.[109] Moreover, increased cardiac fibrosis may limit the efficacy of catheter ablation of AF or VT.[110] Thus, therapies improving the effectiveness of AF or VT ablation or by lessening the presence of AF and VT may involve anti-fibrotic agents.
A few mouse models have suggested that ROCK inhibition may be cardioprotective against ventricular arrhythmias [111] and that ROCK may mediate atrial remodeling and fibrosis prior to AF.[112] However, it is doubtful that these anti-arrhythmogenic effects are mediated solely through the actions of ROCK on fibrosis. Ellawindy et al. (2015) reports that overexpression of a dominant-negative ROCK in the developing heart (SM22α or αMHC-restricted) leads to spontaneous development of ventricular arrhythmias.[111] There was indeed increased fibrosis in both the left and right ventricles as early as postnatal day 3, which progressed to involve the entire right ventricle free wall in some areas of the ventricle by week 19.[111] Nonetheless, given that there were also desmosomal abnormalities, it is likely that arrhythmias through ROCK inhibition are not solely due to ROCK’s role in reducing cardiac fibrosis, but also due to its role in regulating cell junctions.
5.4. Myocardial infarction
The pro-fibrotic response after myocardial infarction, such as the production of ECM proteins by activated fibroblasts after injury, is important in the pathophysiology of heart failure and arrhythmias.[113] The fibrosis induced post-myocardial infarction can be broadly classified into two types: (1) replacement fibrosis (i.e. scar tissue), which can may initially provide structural support to the injured myocardium but eventually may lead to maladaptive alterations to myocardial mechanics that increases the amount and phenotype of collagen fibers in areas remote from the initial infarction[114] and (2) reactive fibrosis, which refers to the increase in ECM proteins synthesized by fibroblasts at the site of injury and in the infarct border zone.[1]
Inhibition of ROCK through pharmacological inhibition or through mouse models has indicated that ROCK plays an important role in governing the extent of the infarct after acute myocardial injury. For instance, pre-treatment of mice with Y-27632 before coronary artery occlusion led to a large reduction in infarct size, attenuation of the deterioration in cardiac function, and a decrease in cardiomyocyte apoptosis.[115, 116] The benefits of ROCK inhibition after myocardial infarction appears to be partially mediated by upregulation of the PI3K/AKT/NO pathway[115] and leads to significantly lower cardiac fibrosis.[116] Consequently, the reduction of infarct size through ROCK inhibition may prevent maladaptive increases in collagen synthesis due to abnormalities in myocardial mechanics as well as decrease fibrosis proximal to the site of the injury itself.
6. CONCLUSION
ROCK is an important regulator of cardiovascular fibrosis through its effects on fibroblasts, Th cells, endothelial cells, vascular smooth muscle cells, and macrophages (Figure 4). From its pro-fibrotic role in these cell types, ROCK may mediate fibrosis and stiffening underlying vascular stiffness, hypertension, heart failure, cardiac arrhythmias, and recovery after myocardial infarction. Further clinical studies, however, are needed to validate ROCK as a therapeutic target for cardiac fibrosis and vascular stiffening.
Figure 4. Targets of ROCK in cardiac fibrosis and stiffening.
The cellular targets of ROCK in fibrosis and stiffening include T helper cells, vascular smooth muscle cells, fibroblasts, endothelial cells, and macrophages. Subsequently, through the role of ROCK on these cellular targets, ROCK may mediate many cardiovascular diseases associated with pathological fibrosis and stiffening, including hypertension, heart failure, and arrythmias.
7. EXPERT OPINION
The diverse role of ROCK in mediating cardiovascular fibrosis identifies the kinase as an important therapeutic target in cardiovascular disease, suggesting the need for future clinical trials that determine the efficacy of ROCK inhibitors in limiting cardiac fibrosis or vascular stiffening in the cardiovascular system. In the absence of direct clinical trials on the effect of ROCK inhibitors on cardiac fibrosis, the benefits may perhaps be ascertained by statin clinical trials through the so-called “pleiotropic effects of statins.” Statins inhibit HMG-CoA reductase, preventing downstream synthesis of isoprenoids such as fernesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP). The isoprenylation, or addition of hydrophobic molecules such as FPP or GGPP, of small Rho GTPases (including RhoA) facilitates their localization, activation, and function. Consequently, statin trials may provide insight into RhoA/ROCK inhibition if some of the beneficial effects of statins are lipid-independent. Indeed, evidence of the benefit of ROCK inhibitors on cardiac fibrosis may come from statin trials on heart failure. For instance, in a large meta-analysis of both published and non-published data from statin trials, Preiss et al. suggested that statins reduce non-fatal heart failure hospitalization and heart failure death.[117] Moreover, this reduction was not associated with reduced risk of non-fatal myocardial infarction or LDL-C levels, suggesting that the beneficial effects of statins in heart failure occurs through a mechanism not related to LDL-C lowering. Furthermore, a few small clinical trials on the use of ROCK inhibitors in humans have supported findings from animal studies, describing a decrease in coronary vasoconstrictor responses to acetylcholine in patients with vasospastic angina[118], an improvement in endothelial dysfunction[119], and an improvement in diastolic dysfunction.[120] Despite these encouraging reports on the benefits of ROCK inhibition, more direct clinical studies on the role of ROCK in cardiovascular fibrosis are required to better characterize the potential benefits of ROCK as an anti-fibrotic therapeutic target in humans.
It is important to note that the majority of preclinical and clinical studies with ROCK inhibitors use isoform-non-specific inhibitors such as fasudil or Y-27632. Due to the importance of ROCK in cytoskeleton dynamics and its expansive roles in other signaling pathways, use of pan-ROCK inhibitors may lead to cellular toxicity and other adverse effects. Indeed, targeting proteins both upstream and downstream of ROCK, such as by LCZ696’s targeting of PKG, or the abundance of phase II clinical trials that target IL-6 or IL-6 receptors to reduce fibrotic inflammation,[121] may provide simpler therapeutic modalities than targeting ROCK itself. In addition, an emerging body of literature suggests that ROCK1 and ROCK2 may play varying fibrotic roles dependent on cell type, and indeed, may play opposing roles in certain tissues such as macrophages and cardiomyocytes.[18, 19, 94] Consequently, future studies may need to highlight the importance of each ROCK isoform in specific tissues, possibly through continued experiments on tissue- and isoform-specific knockout or haploinsufficient murine models. Moreover, in order to facilitate translation to the clinical setting, isoform-specific inhibitors should be developed and better characterized.
The breadth of pre-clinical reports on ROCK inhibition as a potential therapeutic strategy in cardiovascular disease has led to the development of a large number of ROCK inhibitors over the past three decades. The classic ROCK inhibitor for clinical use is fasudil, which has been approved in Japan and China since 1995 for treatment of cerebral vasospasm, a potentially fatal result of subarachnoid hemorrhage.[122] Despite only moderate specificity for ROCK (Ki of ∼0.33 μM towards ROCK2) and off-target inhibition of other kinases, including protein kinase A and other members of the AGC kinase family,[123] subsequent follow-up trials in thousands of patients have suggested that fasudil is relatively safe, notwithstanding adverse events such as changes in hepatic function and hypotension. [122, 124, 125] In the context of cardiac fibrosis and vascular stiffening, small clinical trials in the early to mid-2000s provided evidence that fasudil can improve symptoms and outcomes for patients with systemic hypertension[126] and chronic heart failure.[127] Moreover, a recent clinical trial published in 2015 suggested that fasudil can improve outcomes in patients with severe hypertension and right heart failure[128] while a trial in 2018 indicated that fasudil can improve diastolic function in HFpEF patients.[120] Both trials suggested that fasudil was well-tolerated by patients, although the long-term effects of fasudil administration is still unknown. Nonetheless, perhaps due to fasudil’s lack of specificity for ROCK and the potential for ROCK isoform-specific inhibitors to offer greater therapeutic benefit and less side effects than fasudil, research over the last decade has also focused on the creation of hundreds of new ROCK inhibitors, which have been covered in exhaustive detail by Feng et al. (2015).[123] An important ROCK isoform-specific inhibitor is KD025, a specific ROCK2 inhibitor (IC50 values of 60–110 nM for ROCK2 and >20 μM for ROCK1) [64] undergoing phase II clinical trials for various immune-related diseases and for idiopathic pulmonary fibrosis. A phase I clinical trial using KD025 in a small number of patients suggested that there are few to no side effects from taking the drug.[64] However, the effect of many of these inhibitors, including KD025, in cardiac fibrosis and vascular stiffening remain largely unknown and remain firmly in the preclinical and discovery phase of research.
Another avenue of ROCK clinical research has been through measurements of the ratio of phosphorylated MYPT1 to total MYPT1, a direct downstream effector of ROCK, in peripheral blood leukocytes as an easily obtainable readout for ROCK activity. For example, patients with left ventricular hypertrophy [129] or congestive heart failure [130, 131] diseases associated with myocardial fibrosis, have elevated ROCK activity compared with corresponding controls. These studies may allow clinicians to use ROCK activity as a biomarker for prognostic outcomes or may allow future clinical trials to administer ROCK therapy to those with elevated ROCK activity. There are a few drawbacks to using ROCK activity from peripheral blood leukocytes. First, activation of ROCK in peripheral blood leukocytes may not necessarily correlate with activation of ROCK in cardiac fibroblasts. Studies, however, have indicated that the ROCK activity of circulating leukocytes does indeed closely correlate with the ROCK activity of tissue extracted from the left ventricle and aortic wall.[132] Intriguingly, in the same study, fasudil also decreased the ROCK activity from all three protein types.[132] Second, the ROCK activity of peripheral blood leukocytes may be altered by inflammation or damage in other organs. Future studies may seek to validate the use of ROCK activity in peripheral blood leukocytes as a readout of cardiac (or systemic) ROCK activity, perhaps through biopsies in patients undergoing heart transplantation.
In conclusion, we believe that ROCK is an important regulator of cardiovascular fibrosis. Future studies characterizing the isoform- and tissue-specific roles of ROCK are required to fully validate the kinase as a clinical target to reduce cardiovascular fibrosis. Nonetheless, the last two decades of ROCK research has greatly informed our understanding of the pro-fibrotic cellular mechanisms underlying cardiovascular disease. Future clinical trials on the use of ROCK inhibitors to decrease cardiac fibrosis or improve vascular stiffening are required to fully assess ROCK as a therapeutic target.
ARTICLE HIGHLIGHTS.
ROCK plays a key role in the regulation of fibrosis and stiffening in hypertension, heart failure, cardiomyopathies, arrythmias, and recovery after myocardial infarction
ROCK activates pro-fibrotic genes in fibroblasts and myofibroblasts through MRTF/SRF, YAP/TAZ/TEAD, and TGF-β signaling
ROCK plays an important role in the release of both pro-fibrotic and anti-fibrotic cytokines from Th cells and may also play a role in macrophage polarization
ROCK regulates endothelial function, including NO production and EndMT, to cause cardiac fibrosis and stiffening
ROCK governs VSMC-ECM dynamics, VSMC phenotype switching, and vascular tone
Future clinical trials on the use of ROCK inhibitors to decrease cardiac fibrosis or improve vascular stiffening are required to fully assess ROCK as a therapeutic target.
Acknowledgments
Funding
The work of the authors is funded by American Heart Association and the National Institutes of Health, National Heart, Lung and Blood Institute, USA.
Footnotes
Declaration of interest
JE Blair has received research and equipment grants from Abbott and Phillips Healthcare. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014. February;71(4):549–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung T, Chen XQ, Manser E, et al. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 1996. October;16(10):5313–27.•• First paper identifying ROCK as downstream of Rho.
- 3.Kimura K, Ito M, Amano M, et al. Regulation of Myosin Phosphatase by Rho and Rho-Associated Kinase (Rho-Kinase). Science 1996;273(5272):245.•• First paper showing ROCK mediates myosin light chain through myosin light chain phosphatase.
- 4.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci 2011. March;32(3):167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol 2014. February 15;171(3):368–76. [DOI] [PubMed] [Google Scholar]
- 6.Lowery DM, Clauser KR, Hjerrild M, et al. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. Embo J 2007. May 2;26(9):2262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riento K, Guasch RM, Garg R, et al. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol 2003. June;23(12):4219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward Y, Yap SF, Ravichandran V, et al. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol 2002. April 15;157(2):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferretti R, Palumbo V, Di Savino A, et al. Morgana/chp-1, a ROCK inhibitor involved in centrosome duplication and tumorigenesis. Dev Cell 2010. March 16;18(3):486–95. [DOI] [PubMed] [Google Scholar]
- 10.Knipe RS, Tager AM, Liao JK. The Rho Kinases: Critical Mediators of Multiple Profibrotic Processes and Rational Targets for New Therapies for Pulmonary Fibrosis. Pharmacol Rev 2015;67(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arber S, Barbayannis FA, Hanser H, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998. 1998/06/01;393(6687):805–09. [DOI] [PubMed] [Google Scholar]
- 12.Croft DR, Olson MF. The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol Cell Biol 2006. June;26(12):4612–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007. Mar-Apr;55(2):61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sladojevic N, Yu B, Liao JK. ROCK as a therapeutic target for ischemic stroke. Expert Rev Neurother 2017. December;17(12):1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rikitake Y, Oyama N, Wang CY, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation 2005. November 8;112(19):2959–65.• Early paper suggesting ROCK is involved in cardiac fibrosis.
- 16.Zhang YM, Bo J, Taffet GE, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J 2006. May;20(7):916–25. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Zhang YW, Summers LJ, et al. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol 2008. March;44(3):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Surma M, Yang Y, et al. Disruption of both ROCK1 and ROCK2 genes in cardiomyocytes promotes autophagy and reduces cardiac fibrosis during aging. FASEB J 2019. June;33(6):7348–62.• Recent paper indicating ROCK1 and ROCK2 regulate autophagy in cardiomyocytes, leading to cardiac fibrosis.
- 19.Sunamura S, Satoh K, Kurosawa R, et al. Different roles of myocardial ROCK1 and ROCK2 in cardiac dysfunction and postcapillary pulmonary hypertension in mice. Proc Natl Acad Sci U S A 2018;115(30):E7129.• Recent paper suggesting ROCK1 and ROCK2 in cardiomyocytes have differential effects in cardiac fibrosis and dysfunction.
- 20.Shimizu T, Narang N, Chen P, et al. Fibroblast deletion of ROCK2 attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction. JCI Insight 2017. July 6;2(13).• Recent paper showing ROCK2 in fibroblasts regulates cardiac fibrosis and diastolic dysfunction.
- 21.Yue X, Yang X, Lin X, et al. Rnd3 haploinsufficient mice are predisposed to hemodynamic stress and develop apoptotic cardiomyopathy with heart failure. Cell Death Dis 2014. June 5;5:e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Chang L, Chen C, et al. Rad GTPase inhibits cardiac fibrosis through connective tissue growth factor. Cardiovasc Res 2011. July 1;91(1):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol 2010. May;11(5):353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esnault C, Stewart A, Gualdrini F, et al. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev 2014. May 1;28(9):943–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu-Wai-Man C, Spencer-Dene B, Lee RMH, et al. Local delivery of novel MRTF/SRF inhibitors prevents scar tissue formation in a preclinical model of fibrosis. Sci Rep 2017. 2017/03/31;7(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohashi K, Nagata K, Maekawa M, et al. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem 2000. February 4;275(5):3577–82. [DOI] [PubMed] [Google Scholar]
- 27.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 1995. June 30;81(7):1159–70.• First paper to suggest RhoA is linked to MRTF/SRF signaling.
- 28.Sakai N, Chun J, Duffield JS, et al. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J 2013. May;27(5):1830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis J, Burr AR, Davis GF, et al. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell 2012. October 16;23(4):705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small EM, Thatcher JE, Sutherland LB, et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 2010. July 23;107(2):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhmetshina A, Dees C, Pileckyte M, et al. Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum 2008. August;58(8):2553–64. [DOI] [PubMed] [Google Scholar]
- 32.Yu OM, Miyamoto S, Brown JH. Myocardin-Related Transcription Factor A and Yes-Associated Protein Exert Dual Control in G Protein-Coupled Receptor- and RhoA-Mediated Transcriptional Regulation and Cell Proliferation. Mol Cell Biol 2016. January 1;36(1):39–49.• Interesting paper on how MRTF/STF and YAP/TAZ crosstalk is mediated by Rho/ROCK.
- 33.Kranenburg O, Poland M, van Horck FPG, et al. Activation of RhoA by Lysophosphatidic Acid and Gα12/13 Subunits in Neuronal Cells: Induction of Neurite Retraction. Mol Cell Biol 1999. 1999/06/01;10(6):1851–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siehler S Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol 2009. September;158(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh I, Knezevic N, Ahmmed GU, et al. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem 2007. March 16;282(11):7833–43. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Yang N, Fiore VF, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 2012. September;47(3):340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 2015. September;25(9):499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szeto SG, Narimatsu M, Lu M, et al. YAP/TAZ Are Mechanoregulators of TGF-β-Smad Signaling and Renal Fibrogenesis. J Am Soc Nephrol 2016;27(10):3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008. July 15;22(14):1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomasy SM, Morgan JT, Wood JA, et al. Substratum stiffness and latrunculin B modulate the gene expression of the mechanotransducers YAP and TAZ in human trabecular meshwork cells. Exp Eye Res 2013. August;113:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada K-I, Itoga K, Okano T, et al. Hippo pathway regulation by cell morphology and stress fibers. Development 2011;138(18):3907. [DOI] [PubMed] [Google Scholar]
- 42.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011. June 8;474(7350):179–83. [DOI] [PubMed] [Google Scholar]
- 43.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013. August 29;154(5):1047–59. [DOI] [PubMed] [Google Scholar]
- 44.Milenkovic U, Ilg MM, Zuccato C, et al. Simvastatin and the Rho-kinase inhibitor Y-27632 prevent myofibroblast transformation in Peyronie’s disease-derived fibroblasts via inhibition of YAP/TAZ nuclear translocation. BJU Int 2019. Apr;123(4):703–15. [DOI] [PubMed] [Google Scholar]
- 45.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012. August 17;150(4):780–91.• First paper to show ROCK may mediate the activation of TGF-β.
- 46.Ibar C, Kirichenko E, Keepers B, et al. Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J Cell Sci 2018. March 2;131(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu G, Ma C, Yang H, et al. Transforming growth factor β and its role in heart disease. Exp Ther Med 2017. May;13(5):2123–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A 1986. June;83(12):4167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamanaka M, Shegogue D, Pei H, et al. Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta and mediates TIMP-1 up-regulation. J Biol Chem 2004. December 24;279(52):53994–4001. [DOI] [PubMed] [Google Scholar]
- 50.Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993. July;122(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta -induced physical and functional interactions between smads and Sp1. J Biol Chem 2000. December 22;275(51):40014–9. [DOI] [PubMed] [Google Scholar]
- 52.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem 2005. January 14;280(2):1024–36.• First paper suggesting ROCK regulates TGF-beta signaling.
- 53.Chen S, Crawford M, Day RM, et al. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem 2006. January 20;281(3):1765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu T, Wu M, Feng J, et al. RhoA/Rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int J Mol Med 2012. November;30(5):1119–25. [DOI] [PubMed] [Google Scholar]
- 55.Itoh Y, Kimoto K, Imaizumi M, et al. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res 2007. March;84(3):464–72. [DOI] [PubMed] [Google Scholar]
- 56.Ji H, Tang H, Lin H, et al. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep 2014. November;2(6):787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandbo N, Lau A, Kach J, et al. Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-beta. Am J Physiol Lung Cell Mol Physiol 2011. November;301(5):L656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenkins RG, Su X, Su G, et al. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 2006. June;116(6):1606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu M, Liu PY, Kasahara DI, et al. Role of Rho kinase isoforms in murine allergic airway responses. Eur Respir J 2011;38(4):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tharaux P-L, Bukoski RC, Rocha PN, et al. Rho Kinase Promotes Alloimmune Responses by Regulating the Proliferation and Structure of T Cells. Eur Respir J 2003;171(1):96. [DOI] [PubMed] [Google Scholar]
- 61.Hasan Z, Palani K, Zhang S, et al. Rho kinase regulates induction of T-cell immune dysfunction in abdominal sepsis. Infect Immun 2013. July;81(7):2499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi M, Azuma E, Ido M, et al. A pivotal role of Rho GTPase in the regulation of morphology and function of dendritic cells. J Immunol 2001. October 1;167(7):3585–91. [DOI] [PubMed] [Google Scholar]
- 63.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest 2010. 09/01/;120(9):3280–95.• Paper linking ROCK to IRF4 and cytokine production.
- 64.Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A 2014;111(47):16814.• Paper linking ROCK to STAT and cytokine production.
- 65.Bansal SS, Ismahil MA, Goel M, et al. Activated T Lymphocytes are Essential Drivers of Pathological Remodeling in Ischemic Heart Failure. Circ Heart Fail 2017. March;10(3):e003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, Zhu H, Su Z, et al. IL-17 contributes to cardiac fibrosis following experimental autoimmune myocarditis by a PKCbeta/Erk1/2/NF-kappaB-dependent signaling pathway. Int Immunol 2012. October;24(10):605–12. [DOI] [PubMed] [Google Scholar]
- 67.Peng H, Sarwar Z, Yang XP, et al. Profibrotic Role for Interleukin-4 in Cardiac Remodeling and Dysfunction. Hypertension 2015. September;66(3):582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang H, Kong D, Byun KH, et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci 2012. October;15(10):1391–8.• First paper to suggest ROCK can phosphorylate JAK proteins to activate STAT and FOXO1.
- 69.Bellien J, Favre J, Iacob M, et al. Arterial Stiffness Is Regulated by Nitric Oxide and Endothelium-Derived Hyperpolarizing Factor During Changes in Blood Flow in Humans. Hypertension 2010;55(3):674–80. [DOI] [PubMed] [Google Scholar]
- 70.Kataoka C, Egashira K, Inoue S, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 2002. February;39(2):245–50. [DOI] [PubMed] [Google Scholar]
- 71.Kazakov A, Hall R, Jagoda P, et al. Inhibition of endothelial nitric oxide synthase induces and enhances myocardial fibrosis. Cardiovasc Res 2013. November 1;100(2):211–21. [DOI] [PubMed] [Google Scholar]
- 72.Kim NN, Villegas S, Summerour SR, et al. Regulation of cardiac fibroblast extracellular matrix production by bradykinin and nitric oxide. J Mol Cell Cardiol 1999. February;31(2):457–66. [DOI] [PubMed] [Google Scholar]
- 73.Takemoto M, Sun J, Hiroki J, et al. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 2002. July 2;106(1):57–62. [DOI] [PubMed] [Google Scholar]
- 74.Eto M, Barandier C, Rathgeb L, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res 2001. September 28;89(7):583–90.•• First paper suggesting a link between the Rho/ROCK pathway and eNOS.
- 75.Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 2002. December;22(24):8467–77.• Early paper on how ROCK may regulate PI3K/AKT signaling to influence NO production.
- 76.Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 2005. October;36(10):2251–7.• Paper indicating ROCK inhibition may lead to vasodilation and cardioprotection through eNOS.
- 77.Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 2004. October;24(10):1842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vemula S, Shi J, Hanneman P, et al. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood 2010. March 4;115(9):1785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovacic JC, Mercader N, Torres M, et al. Epithelial-to-Mesenchymal and Endothelial-to-Mesenchymal Transition. Circulation 2012;125(14):1795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krizbai IA, Gasparics Á, Nagyőszi P, et al. Endothelial-Mesenchymal Transition of Brain Endothelial Cells: Possible Role during Metastatic Extravasation. PLOS ONE 2015;10(3):e0119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng H, Li Y, Wang C, et al. ROCK1 Induces Endothelial-to-Mesenchymal Transition in Glomeruli to Aggravate Albuminuria in Diabetic Nephropathy. Sci Rep 2016. 02/04/online;6:20304.• Link between ROCK and EndMT.
- 82.Wu Q, Ouyang C, Xie L, et al. The ROCK inhibitor, thiazovivin, inhibits human corneal endothelialtomesenchymal transition/epithelialtomesenchymal transition and increases ionic transporter expression. Int J Mol Med 2017. October;40(4):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shields MA, Krantz SB, Bentrem DJ, et al. Interplay between beta1-integrin and Rho signaling regulates differential scattering and motility of pancreatic cancer cells by snail and Slug proteins. J Biol Chem 2012. February 24;287(9):6218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kovacic JC, Dimmeler S, Harvey RP, et al. Endothelial to Mesenchymal Transition in Cardiovascular Disease. J Am Coll Cardiol 2019;73(2):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014. December;15(12):802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lacolley P, Regnault V, Segers P, et al. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol Rev 2017;97(4):1555–617. [DOI] [PubMed] [Google Scholar]
- 87.Margadant F, Chew LL, Hu X, et al. Mechanotransduction In Vivo by Repeated Talin Stretch-Relaxation Events Depends upon Vinculin. PLOS Biology 2011;9(12):e1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joshi B, Strugnell SS, Goetz JG, et al. Phosphorylated Caveolin-1 Regulates Rho/ROCK-Dependent Focal Adhesion Dynamics and Tumor Cell Migration and Invasion. Cancer Res 2008;68(20):8210. [DOI] [PubMed] [Google Scholar]
- 89.Davies LM, Purves GI, Barrett-Jolley R, et al. Interaction with caveolin-1 modulates vascular ATP-sensitive potassium (KATP) channel activity. J Physiol 2010. September 1;588(Pt 17):3255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rashid-Doubell F, Tannetta D, Redman CW, et al. Caveolin-1 and lipid rafts in confluent BeWo trophoblasts: evidence for Rock-1 association with caveolin-1. Placenta 2007. Feb-Mar;28(2–3):139–51. [DOI] [PubMed] [Google Scholar]
- 91.Gingras D, Gauthier F, Lamy S, et al. Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem Biophys Res Commun 1998. June 29;247(3):888–93. [DOI] [PubMed] [Google Scholar]
- 92.Halayko AJ, Tran T, Gosens R. Phenotype and Functional Plasticity of Airway Smooth Muscle. Proc Am Thorac Soc 2008. 2008/01/01;5(1):80–88. [DOI] [PubMed] [Google Scholar]
- 93.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016. March 15;44(3):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zandi S, Nakao S, Chun KH, et al. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep 2015. February 24;10(7):1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu C, Li Y, Yu J, et al. Targeting the Shift from M1 to M2 Macrophages in Experimental Autoimmune Encephalomyelitis Mice Treated with Fasudil. PLOS ONE 2013;8(2):e54841.• First paper linking ROCK with macrophage polarization.
- 96.Lee TM, Lin SZ, Chang NC. Nicorandil regulates the macrophage skewing and ameliorates myofibroblasts by inhibition of RhoA/Rho-kinase signalling in infarcted rats. J Cell Mol Med 2018. February;22(2):1056–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanz-Moreno V, Gaggioli C, Yeo M, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 2011. August 16;20(2):229–45. [DOI] [PubMed] [Google Scholar]
- 98.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol 2013. June;33(6):1135–44. [DOI] [PubMed] [Google Scholar]
- 99.Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012. September 5;308(9):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weisbrod RM, Shiang T, Al Sayah L, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 2013. December;62(6):1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noma K, Goto C, Nishioka K, et al. Roles of rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol 2007. February 13;49(6):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997. October 30;389(6654):990–4.•• Seminal paper on the effects of ROCK in vasodilation.
- 103.Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 2005. March;91(3):391–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.González A, Schelbert EB, Díez J, et al. Myocardial Interstitial Fibrosis in Heart Failure. J Am Coll Cardiol 2018;71(15):1696. [DOI] [PubMed] [Google Scholar]
- 105.Okamoto R, Li Y, Noma K, et al. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J 2013. April;27(4):1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014. September 11;371(11):993–1004. [DOI] [PubMed] [Google Scholar]
- 107.Burke RM, Lighthouse JK, Mickelsen DM, et al. Sacubitril/Valsartan Decreases Cardiac Fibrosis in Left Ventricle Pressure Overload by Restoring PKG Signaling in Cardiac Fibroblasts. Circ Heart Fail 2019. April;12(4):e005565.• Recent paper suggesting clinically available drug to improve heart failure (sacubitril/valsartan) may have anti-fibrotic effects through Rho/ROCK.
- 108.de Jong S, van Veen TA, van Rijen HV, et al. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol 2011. June;57(6):630–8. [DOI] [PubMed] [Google Scholar]
- 109.O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010. September 7;56(11):867–74. [DOI] [PubMed] [Google Scholar]
- 110.Dzeshka MS, Lip GYH, Snezhitskiy V, et al. Cardiac Fibrosis in Patients With Atrial Fibrillation. J Am Coll Cardiol 2015;66(8):943. [DOI] [PubMed] [Google Scholar]
- 111.Ellawindy A, Satoh K, Sunamura S, et al. Rho-Kinase Inhibition During Early Cardiac Development Causes Arrhythmogenic Right Ventricular Cardiomyopathy in Mice. Arterioscler Thromb Vasc Biol 2015. October;35(10):2172–84.• Paper linking ROCK with arrhythmias.
- 112.Liu L-J, Yao F- J, Lu G- H, et al. The Role of the Rho/ROCK Pathway in Ang II and TGF-β1-Induced Atrial Remodeling. PLOS ONE 2016;11(9):e0161625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Travers Joshua G, Kamal Fadia A, Robbins J, et al. Cardiac Fibrosis. Circ Res 2016. 2016/03/18;118(6):1021–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van den Borne SWM, Diez J, Blankesteijn WM, et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 2009. 12/01/online;7:30. [DOI] [PubMed] [Google Scholar]
- 115.Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am J Physiol Heart Circ Physiol 2007. 2007/06/01;292(6):H2598–H606. [DOI] [PubMed] [Google Scholar]
- 116.Lee TM, Lin SZ, Chang NC. Membrane ERalpha attenuates myocardial fibrosis via RhoA/ROCK-mediated actin remodeling in ovariectomized female infarcted rats. J Mol Med (Berl) 2014. January;92(1):43–51. [DOI] [PubMed] [Google Scholar]
- 117.Preiss D, Campbell RT, Murray HM, et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J 2015. June 21;36(24):1536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Masumoto A, Mohri M, Shimokawa H, et al. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002. April 2;105(13):1545–7.• One of the first published clinical trials on ROCK.
- 119.Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 2006. December 8;99(12):1426–32.• Paper suggesting ROCK may improve endothelial function in patients.
- 120.Zhang X, Zhang X, Wang S, et al. Effects of Fasudil on Patients with Pulmonary Hypertension Associated with Left Ventricular Heart Failure with Preserved Ejection Fraction: A Prospective Intervention Study. Can Respir J 2018;2018:3148259.• Recent paper suggesting ROCK may improve diastolic function in patients.
- 121.Kang S, Tanaka T, Narazaki M, et al. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019. April 16;50(4):1007–23. [DOI] [PubMed] [Google Scholar]
- 122.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol 2007. July;50(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng Y, LoGrasso PV, Defert O, et al. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J Med Chem 2016. March 24;59(6):2269–300.• Extremely thorough review on the hundreds of currently available/developing ROCK inhibitors.
- 124.Shibuya M, Hirai S, Seto M, et al. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci 2005. November 15;238(1–2):31–9. [DOI] [PubMed] [Google Scholar]
- 125.Suzuki Y, Shibuya M, Satoh S, et al. Safety and efficacy of fasudil monotherapy and fasudil-ozagrel combination therapy in patients with subarachnoid hemorrhage: sub-analysis of the post-marketing surveillance study. Neurol Med Chir (Tokyo) 2008. June;48(6):241–7;discussion 47–8.• Large (n=4828) clinical trial suggesting ROCK inhibitors are well-tolerated by patients.
- 126.Masumoto A, Hirooka Y, Shimokawa H, et al. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension 2001. December 1;38(6):1307–10. [DOI] [PubMed] [Google Scholar]
- 127.Kishi T, Hirooka Y, Masumoto A, et al. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation 2005. May 31;111(21):2741–7. [DOI] [PubMed] [Google Scholar]
- 128.Jiang R, Ai Z-S, Jiang X, et al. Intravenous fasudil improves in-hospital mortality of patients with right heart failure in severe pulmonary hypertension. Hypertension Research 2015. 2015/08/01;38(8):539–44.• Recent paper suggesting ROCK inhibitors can improve heart failure mortality.
- 129.Gabrielli L, Winter JL, Godoy I, et al. Increased rho-kinase activity in hypertensive patients with left ventricular hypertrophy. Am J Hypertens 2014. June;27(6):838–45. [DOI] [PubMed] [Google Scholar]
- 130.Dong M, Liao JK, Fang F, et al. Increased Rho kinase activity in congestive heart failure. Eur J Heart Fail 2012. September;14(9):965–73.• First paper to suggest ROCK activity in patients may serve as a marker for heart failure.
- 131.Do e Z, Fukumoto Y, Sugimura K, et al. Rho-kinase activation in patients with heart failure. Circ J 2013;77(10):2542–50. [DOI] [PubMed] [Google Scholar]
- 132.Ocaranza MP, Fierro C, Jalil JE, et al. Rho kinase activation in circulating leukocytes is related to hypertensive myocardial remodeling. Clin Sci (Lond) 2018. August 31;132(16):1837–53. [DOI] [PubMed] [Google Scholar]