Abstract
Primary sarcomatoid urothelial carcinoma of the ureter with heterologous elements is rare and carries a poor prognosis. Although there is some literature on primary bladder sarcomatoid urothelial carcinoma, ureteric involvement has been reported infrequently, and this case report describes this unusual histological finding with concurrent divergent squamous differentiation. Despite laparoscopic radical nephroureterectomy, our patient died within six months of diagnosis with local recurrence and metastatic spread. A more thorough understanding of this disease process and consideration of standardised guidelines for treatment are needed to improve patient outcomes.
Keywords: Sarcomatoid urothelial carcinoma, Chondrosarcoma, Osteosarcoma, Ureter
Introduction
Sarcomatoid urothelial carcinoma is a rare and aggressive variant of urothelial carcinoma histology.1, 2, 3, 4, 5 Of the limited cases reported, the majority are found in the urinary bladder as opposed to a primary malignancy of the ureter.3,4 This case report details a 72-year-old male with a primary sarcomatoid urothelial carcinoma of the ureter with heterologous chondrosarcoma and osteosarcoma elements, and concurrent divergent squamous differentiation. To our knowledge, there is only one published report of osteosarcomatoid and chondrosarcomatoid differentiation within the same malignancy of the ureter.1,2 Our patient died with local recurrence and metastatic spread within six months of diagnosis despite laparoscopic radical nephroureterectomy. This case highlights the aggressive nature of this malignancy and likely need for multimodal management to achieve better long-term patient outcomes.
Case presentation
A 72-year-old male presented with macroscopic haematuria and right-sided abdominal pain. He had no significant medical history and was a non-smoker. Urine cytology showed atypical cells and serum creatinine was 137μmol/L. Contrast-enhanced computed tomography of the abdomen and pelvis with delayed phases revealed a solid enhancing 2 × 5cm right distal ureteric lesion, with mild upper tract dilatation and no abdominal or pelvic lymphadenopathy (Fig. 1). Cystoureteroscopy revealed a normal bladder and a solid lesion just within the right ureter, unable to be traversed with a ureteroscope, with ureteral washings returning benign spindle shaped squamous cells. Staging chest imaging was normal.
Fig. 1.
Axial and Coronal contrast enhanced computed tomography abdominopelvic cross-sectional imaging showing a 2 × 5cm distal enhancing solid ureteric lesion extending down to the vesicoureteric junction with mild upper tract dilatation.
The patient underwent an uncomplicated laparoscopic radical right nephroureterectomy with an open distal ureter dissection approach. The bladder cuff and ureter were excised en bloc with the kidney. No lymphadenectomy was performed and there was no urinary spillage. On histopathology, macroscopically there was a distal ureteric fusiform swelling with a 75 × 14mm friable haemorrhagic lesion within the lumen (Fig. 2). Microscopically, poorly differentiated high-grade urothelial carcinoma with extensive necrosis was identified extending into the inner muscular layer. The specimen showed sarcomatoid variant histology with heterologous chondrosarcoma and osteosarcoma elements. Extensive divergent squamous differentiation was also noted (Fig. 3). There was concurrent carcinoma-in-situ throughout the ureter, extending to the distal bladder cuff surgical margin, but no sign of invasive carcinoma at this section. There was no vascular or perineural invasion and no malignancy within the kidney. The malignancy was staged pT2NxM0.
Fig. 2.
Gross appearance of the right kidney and ureter showing a distal ureteric fusiform solid swelling with proximal ureter dilation. The distal ureter contains a friable haemorrhagic mass attached to the wall.
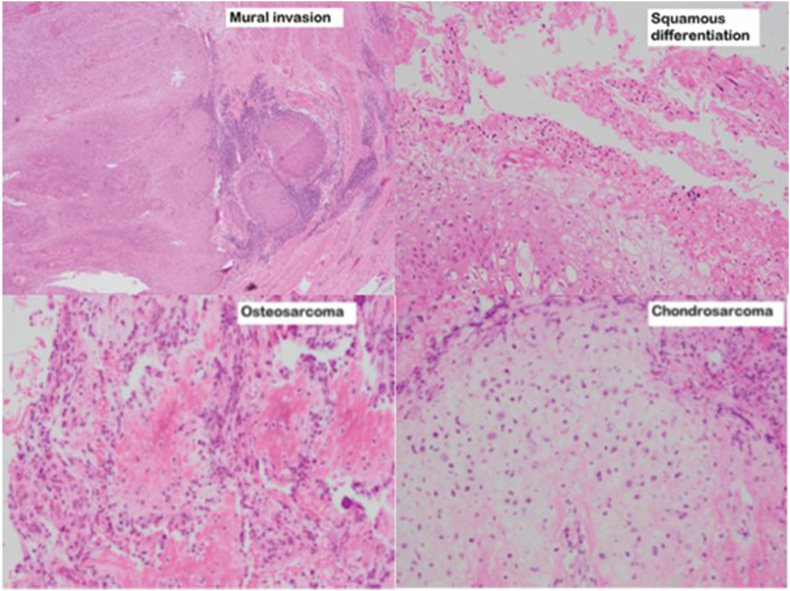
Fig. 3.
Histopathology slides from the nephroureterectomy specimen showing urothelial carcinoma with muscularis invasion, sarcomatoid variant histology with osteosarcoma and chrondrosarcoma heterologous elements, and a component of squamous divergent differentiation.
Two months post radical nephroureterectomy, while the patient was undergoing planning imaging for radiotherapy for localised intermediate risk prostate cancer and adjuvant radiation to the surgical bed, a right-sided bladder lesion was noted. Cystoscopic examination revealed a 5cm solid lesion over the region of the previous right ureteric orifice and a further small satellite trigonal lesion. Histopathology from the resection showed high-grade muscle invasive urothelial carcinoma with sarcomatoid variant histology, again with chondrosarcomatous and osteosarcomatous elements, consistent with disease recurrence.
Restaging with Fluorodeoxyglucose positron emission tomography showed no evidence of metastases, however, there was increased tracer uptake of a peripheral zone lesion in the prostate in keeping with known prostate adenocarcinoma. Multidisciplinary team meeting consensus recommended radical cystectoprostatectomy and ileal conduit surgical management. Intra-operatively, extensive spread to the right pelvic sidewall along with metastatic deposits in the mesentery and the bowel wall were noted, and subsequently a diverting ileal conduit was formed and the resection abandoned. The patient was referred for palliative chemotherapy and unfortunately passed away shortly after commencing this.
Discussion
Sarcomatoid urothelial carcinoma is a rare malignancy with a poor prognosis. This urothelial carcinoma variant accounts for less than one percent of all bladder cancers.3,4 Patients typically present with higher-grade and more advanced disease.4 Sarcomatoid urothelial carcinoma of the ureter is rarer still with only 26 reports in the PubMed database.2,3 Urothelial carcinoma with divergent differentiation with squamous cell differentiation occurs in 20–40% of bladder cancer cases, and also appears to confer less favourable response rates to therapy.4
Sarcomatoid urothelial carcinoma exhibits biphasic properties of both epithelial tumours (carcinomas) and mesenchymal tumours (sarcomas).3,4 There are two hypotheses for the histogenesis of such tumours. The first hypothesis proposes that the carcinomatous and sarcomatous components are derived from the urothelial cancer stem cells with divergent differentiation.4 The second hypothesis suggests that the components arise independently from urothelial and mesenchymal stem cells and ‘collide’ at the same organ location.4 The mesenchymal component may show heterologous elements of osteosarcoma, chondrosarcoma, rhabdomyosarcoma, leiomyosarcoma, liposarcoma, angiosarcoma or a combination.4 Immunohistochemistry was not needed in our case, however, it has been proposed that expression of GTA3 and PAX8 as well as mesenchymal transition markers including vimentin, FoxC2, SNAIL and ZEB1 may help identify cases.3,4
Heterologous elements of osteosarcoma and chondrosarcoma in tandem is a unique finding in this case of sarcomatoid urothelial carcinoma of the ureter, with Johnin et al.1 having described the only similar case in a 58-year-old female, without concurrent squamous divergent differentiation. To the best of our knowledge, no further cases have been reported in a primary malignancy of the ureter. There is a striking similarity of disease progression of the two case reports: both patients underwent nephroureterectomy with tumour recurrence in the urinary bladder thereafter and died of extensive local and metastatic disease spread within six months of diagnosis, illustrating the aggressive nature of this malignancy.
Though outcomes for patients with sarcomatoid urothelial carcinoma are generally unfavourable, there have been a few cases of patients with complete responses to treatment of primary bladder malignancy.5 Most of these patients underwent aggressive multimodal management including surgical intervention and chemotherapy with or without radiotherapy.5 While there are no standardised treatments for patients with sarcomatoid urothelial carcinomas, especially of the ureter, improved patient outcomes may require adoption of aggressive multimodal therapy and not radical surgical intervention alone.5
Conclusion
A primary sarcomatoid urothelial carcinoma of the ureter with heterologous osteosarcomatoid and chondrosarcomatoid differentiation, and concomitant divergent squamous differentiation is a rare occurrence. In general, urothelial carcinoma with sarcomatoid variant histology denotes a poor prognosis, and requires a high suspicion of invasive and metastatic disease at diagnosis, and careful monitoring for recurrence. Further understanding of this uncommon variant will help to standardise treatment pathways and improve patient long-term outcomes.
Consent
Informed consent was gained from the patient for writing this paper.
Sources of funding
No funding was obtained for this paper.
Declaration of competing interest
None to declare.
References
- 1.Johnin K., Kadowaki T., Kushima M., Ushida H., Koizumi S., Okada Y. Primary heterologous carcinosarcoma of the ureter with necrotic malignant polyps. Report of a case and review of the literature. Urol Int. 2003;70:232–235. doi: 10.1159/000068757. [DOI] [PubMed] [Google Scholar]
- 2.Lu W., Wang Y., Li Y., Cao Y., Han H., Zhou F. Sarcomatoid urothelial carcinoma with chondrosarcomatous differentiation of the ureter: a case report and review of the literature. Oncology Letters. 2017;13(3):1331–1337. doi: 10.3892/ol.2017.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Hanchao L., Wang P. Primary sarcomatoid urothelial carcinoma of the ureter: a case report and review of the literature. World J Surg Oncol. 2018;16(77):1–4. doi: 10.1186/s12957-018-1383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Beltran A., Henriques V., Montironi R., Cimadamore A., Raspollini M., Cheng L. Variants and new entities of bladder cancer. Histopathology. 2019;74:77–96. doi: 10.1111/his.13752. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Gillaspie C., Kunadharaju R., Talmon G., Enke C. Sarcomatoid urothelial carcinoma: a single cancer center experience. World J Oncol. 2011;2(4):175–180. doi: 10.4021/wjon370w. [DOI] [PMC free article] [PubMed] [Google Scholar]