Abstract
The demand for artificial organs has greatly increased because of various aging-associated diseases and the wide need for organ transplants. A recent trend in tissue engineering is the precise reconstruction of tissues by the growth of cells adhering to bioscaffolds, which are three-dimensional (3D) structures that guide tissue and organ formation. Bioscaffolds used to fabricate bionic tissues should be able to not only guide cell growth but also regulate cell behaviors. Common regulation methods include biophysical and biochemical stimulations. Biophysical stimulation cues include matrix hardness, external stress and strain, surface topology, and electromagnetic field and concentration, whereas biochemical stimulation cues include growth factors, proteins, kinases, and magnetic nanoparticles. This review discusses bioink preparation, 3D bioprinting (including extrusion-based, inkjet, and ultraviolet-assisted 3D bioprinting), and regulation of cell behaviors. In particular, it provides an overview of state-of-the-art methods and devices for regulating cell growth and tissue formation and the effects of biophysical and biochemical stimulations on cell behaviors. In addition, the fabrication of bioscaffolds embedded with regulatory modules for biomimetic tissue preparation is explained. Finally, challenges in cell growth regulation and future research directions are presented.
Keywords: 3D bioprinting, Bioscaffolds, Cell growth regulation, Organ reconstruction, Synergetic stimulation
Graphical abstract
The functions of different simulations on cells, including biophysical simulations and biochemical simulations are expounded. The regulatory methods for cell growth and tissue formation are organized mainly by three points, bioinks, 3D bioprinting methods, the effects of different simulations on cell behavior regulation. Also, the limitation of biological printing and regulation process and future research matters, are pointed out. We envisage this approach will offer an effective way to tissue reconstruction.
Highlights
-
•
The state of the art on scaffolds in regulating cell behaviors is reviewed.
-
•
The functions of different stimulations on cells are expounded.
-
•
The limitation of regulatory techniques and future directions, are presented.
-
•
The method of synergetic regulation for cell behaviors, are proposed.
1. Introduction
Over the past few decades, the incidence of myocardial infarction, skin inflammation, and arteriosclerosis has increased [[1], [2], [3]]. Removal of diseased tissue and implantation of new tissue are reliable treatment options. To meet the growing demand for human tissues and organs because of disease or accidents, scientific research is increasingly focusing on human tissue engineering and regenerative medicine. The two common methods of preparing tissues and organs are (i) the addition of bioscaffolds, growth factors, and cells into a culture medium for in vitro organization and (ii) the implantation of bioscaffolds and growth factors into the body for organization [4,5]. Cartilage, muscle, fat, skin, liver, and other organs have been successfully fabricated this way [6]. However, there are a few challenges in natural cell growth on bioscaffold surfaces: a lack of vascularized networks for transporting nutrients and waste and a lack of regulation of cell behaviors, such as the growth, proliferation, and differentiation of cells adhering to bioscaffolds [7,8]. Therefore, studies are investigating the regulatory mechanisms underlying cell behaviors [9]. Ideal tissues can be fabricated in vitro or even in vivo, not only because cells are the basis for tissue and organ formation but also based on mechanisms underlying regulation of cell behaviors, bringing new hope for disease treatment, tissue repair and regeneration, drug delivery, and medical diagnosis. Cells are usually cultured on bioscaffolds to form tissues and organs. Cells serve as the basis for tissue organization, and research on cell behaviors under stimulation is of vital importance for tissue reconstruction.
A key issue in tissue regeneration is bioscaffold fabrication. Bioscaffolds are complex structures. Their 3D topography, surface topology, and biophysical and biochemical properties affect cell adhesion, proliferation, migration, and differentiation. With the extensive application of 3D printing technologies, the research on bioscaffold manufacturing has made significant progress. 3D bioprinting is one type of 3D printing and involves the deposition of biomaterials or cells [10,11]. With regard to bioprinting, this review discusses two modalities: 3D printing of acellular bioinks with subsequent cell seeding [12,13], and direct 3D printing of cell-laden bioinks [14,15].
In recent years, microfluidic technologies have been used in in vitro culture and tissue reconstruction. The flow channel accuracy of microfluidic chips matches the mammalian cell diameter, ensuring sufficient oxygen, and nutrient transport to cells [16]. In addition, compared to conventional culture devices, it is easier to achieve experimental variables and accurate simulations of microenvironments for cells in 3D. Cell behaviors can be regulated through biochemical and biophysical stimulations. Biophysical stimulation cues include matrix hardness, surface topology, stress and strain, hydrostatic pressure, electromagnetic field, ultrasound, and light, while biochemical stimulation cues include growth factors, proteins, kinases, and magnetic nanoparticles (MNPs). The effect of biochemical stimulations on cells needs to be considered because of the introduction of chemical factors. Surface morphology affects cell adhesion and growth. Mechanical stimulations affect cell proliferation, migration, and phenotypes.
3D bioprinting technologies adopt the concept of discrete stacking in order to directly integrate microfluidic chips with micron-level accuracy, which provides a tool for studying cell growth and tissue formation. Compared to 3D bioprinting, conventional manufacturing methods, such as molding-pouring-demolding, can neither prepare relatively complex structures nor ensure a sterile microenvironment for cell growth [17]. Conventional manufacturing methods also often have the disadvantages of chemical reagents remaining, complex processes, or long preparation cycles [18]. Although other emerging technologies, such as soft lithography and electrospinning/electrowriting, have become essential tools of tissue engineering, there are limitations in bioscaffold fabrication because of the multiscale morphological size of 3D bioscaffolds and the significantly different sizes of macrostructure and microstructure layers. Not only can the surface topology for cell adhesion and mechanical be arbitrarily changed using 3D bioprinting, we can also achieve synergetic regulations with different stimulations. Using micro–computed tomography (μCT), 3D software, or corrosion casting of reverse engineering [19,20], bioscaffolds can be fabricated through 3D printing, and sufficient regulation of cell growth by biophysical and biochemical stimulations might be achieved.
3D bioprinting technologies and regulation of cell behaviors involving cells, bioinks, cell culture bioscaffolds, and tissue reconstruction are widely used in tissue engineering. However, the mechanism underlying regulation of cell behaviors is still unclear. Although much progress has been made in tissue reconstruction research, studies mainly focus on the pure mechanism underlying regulation of cell behaviors within the scope of bioengineering and bioscaffold modeling in tissue engineering. Several studies have reported strategies based on bioscaffold fabrication and regulation of cell behaviors. However, to our knowledge, there is no systematic, comprehensive research on cell behaviors integrating bioscaffold loaded with cells and regulation modules. There is a need for implanting cell-loaded bioscaffolds into the human body and regulate cells into ideal tissues. This review discusses tissue engineering, microfluidic chips, 3D bioprinting, and multidisciplinary knowledge of mechanics, materials science, and biomedical engineering to study the methods of fabricating bioscaffolds that conform to implantation standards in regenerative medicine.
Methods include bioscaffold fabrication and regulation of cell behaviors (Fig. 1). The mechanism underlying regulation of cell behaviors can be determined by studying the effects of single and multiple stimulations on cell growth. Specifically, cell culture bioscaffolds embedded with regulatory modules may be fabricated on the basis of biophysical and biochemical stimulations and 3D bioprinting technologies. The review also provides an overview of state-of-the-art bioink preparation methods and common 3D bioprinting processes, as well as biophysical and biochemical stimulation cues for regulating cell behaviors. Especially, biophysical and biochemical stimulation cues are reviewed as examples to illustrate synergetic stimulations for cell behaviors. In addition, regulating devices and methods related to different stimulations are described. Finally, future directions and challenges in cell growth regulation and tissue reconstruction are discussed. The findings will provide new technical references for tissue preparation in tissue engineering and regenerative medicine.
Fig. 1.
Schematic diagram of cell culture and tissue reconstruction strategies in tissue engineering. (A) Conventional tissue engineering bioscaffolds. (B) Tissue engineering bioscaffolds embedded with biochemical and biophysical regulation modules, including surface microhardness, external stress and strain, noncontact stimulation cues, and hydrostatic pressure.
2. Bioscaffold fabrication
Broadly speaking, the two indispensable aspects in bioscaffold fabrication are raw materials and processes. A bioscaffold is the application of a scaffold in bioengineering. There are two types of bioscaffolds, cell-loaded, and cell-free. Cell-loaded bioscaffolds are formed by loading biomaterials into cells, while cell-free bioscaffolds are formed by cell adhesion. Different biomaterials and manufacturing methods contribute to the diversity of bioscaffolds. We first discuss bioinks and bioscaffold fabrication. Next, relevant techniques, such as 3D bioprinting, electrospinning/electrowriting, and soft lithography, are reviewed.
2.1. Bioinks for bioscaffold fabrication
2.1.1. Definition and types of bioinks
Bioinks are inks used for 3D bioprinting. They are cell-laden biomaterials that are cell friendly, implantable, and degradable, with a desirable immune response [18]. Accurate selection of bioinks directly affects cell growth and tissue formation, and bioinks should have biophysical and biochemical properties close to the original tissue. In addition, as bioscaffold raw materials, bioinks should ensure cell survival during molding and match the molding process. In some cases, to successfully fabricate high-precision bioscaffolds, bioinks need to have specific properties, for example, shear-thinning properties (liquid characteristics and solid characteristics at different shear rates) and cross-linking properties that contribute to process diversity and structure stability. The usual cross-linking reactions include alginate and calcium ion cross-linking [21], temperature-sensitive cross-linking [22], and light cross-linking [23] (Table 1).
Table 1.
Properties of crosslinking reactions of hydrogels.
| Hydrogel | Principle | Cross-linking method | Reversible or not | Forming efficiency |
|---|---|---|---|---|
| Alginate [37] | Linking between calcium ion and alginate | Chemical cross-linking | Irreversible | High-speed |
| Gelatin [38] | Thermosensitive cross-linking | Physical cross-linking | Reversible | Low-speed |
| GelMA [39] | Thermosensitive cross-linking | Physical cross-linking | Reversible | Low-speed |
| GelMA [40] | Photo-crosslinking | Chemical cross-linking | Irreversible | High-speed |
Many biomaterials are used for cell growth and tissue formation, such as hydrogels. Some commonly used bioinks include natural biomaterials, synthetic materials, composite biomaterials, and decellularized extracellular matrices (dECMs) (Fig. 2). Natural biomaterials include gelatin, alginate hydrogel, collagen, and silk fibroin (SF) [24]. Natural biomaterials have good biocompatibility, numerous sources, and low cost. However, they are unstable and easily degradable. Synthetic materials include polylactic acid (PLA), polyglycolic acid copolymer (PLGA), polycaprolactone (PCL), hydroxyapatite (HA), polyurethane, polyacrylamide gel, graphene hydrogel, poly(glycerol sebacate) (PGS), and modified biomaterials, such as methacrylic acid gelatin (GelMA), metal-based biomaterials, and phosphorylated poly(sebacoyl diglyceride) [[25], [26], [27], [28]]. Compared to natural biomaterials, synthetic materials, especially polymer hydrogels, are relatively stable, but they are still unsuitable for cell-loaded bioinks because of their high melting point. Because of functional requirements, such as cell organization and formability requirement of biomaterials, composite biomaterials and dECMs [29] have become attractive choices. Composite biomaterials include composite natural [30], synthetic [31], bio-, and nanomaterials [32] and also composite biological features [33]. These materials improve the strength and diversity of bioscaffolds. For example, one material in a composite biomaterial is sacrificial, providing a perfect solution for fabricating porous bioscaffolds. In particular, gelatin, sucrose [34], and sodium chloride [[35], [36]] are used as sacrificial materials. Adding a photoinitiator makes a composite biomaterial cross-linkable. Although dECMs contain growth factors, enzymes, and biological macromolecules, which are beneficial for cell growth and tissue formation, it is difficult to fabricate bioscaffolds that meet mechanical requirements and structural strength using only dECMs. Therefore, usually, dECMs are combined with other materials to overcome their limitations. In humans, biomaterials are not limited to these aforementioned biomaterials, and polydimethylsiloxane (PDMS), titanium alloy, etc., are also used.
Fig. 2.
Types of bioinks for bioscaffold fabrication.
2.1.2. Preparation of bioinks
Bioink preparation involves different component designs and different processes. Bioinks are biomaterials, which can be extracted and synthesized by physical or chemical processes. To prepare synthetic materials, processes usually include dissolving raw materials using chemical reagents, mixing raw material solutions, and performing dialysis and freeze-drying to filter out and extract solutes [41]. We prepared GelMA with a specific degree of methacryloyl group substitution as follows:
-
1.
Gelatin was dissolved in phosphate-buffered saline (PBS) through magnetic stirring and temperature control.
-
2.
Methacrylic anhydride was added dropwise to the solution, and preheated PBS was added to stop the chemical reaction.
-
3.
The solution obtained was packed into dialysis bags and dialyzed in deionized water for ~1 week.
-
4.
The solute obtained was wrapped in Petri dishes and stored in a freeze dryer at ~80 °C for ~3–4 days.
To prepare metal-based [27] and synthetic polymer biomaterials [28], quenching and evaporation are usually used. To prepare dECMs, ECMs are decellularized, powdered, and pregelled to form dECM-derived hydrogels [42]. For multiple mixed materials, such as poly(ethylene) glycol-alginate [43] and alginate-gelatin–GelMA-gelatin hybrid hydrogels [44], different ingredients determine the different properties of bioinks, such as viscosity, forming ability, and mechanical properties. After material selection, components are designed. There are two methods of determining the ratio of various materials selected: repeated experiments and artificial intelligence [45]. You et al. [46] investigated the alginate dialdehyde–gelatin hydrogel ratio to achieve good printability and mechanical properties of the fabricated structures, while Lee et al. [45] used collagen, hyaluronic acid, and fibrin as biomaterials. Similar methods have been reported by other studies [47,48]. Many experiments on different ratios of bioinks have been conducted. On the basis of multiple regression analysis, machine learning is used to determine the elastic modulus of structures and the printability of different bioinks.
Mixed materials are prepared by two common methods, thorough mixing of all solutions with stirring and heating and full mixing of solid materials in the molten state. Lei et al. prepared mixed materials comprising PGS and salt particles [36]. They ground salt particles into smaller sizes and then mixed in melted PGS prepolymer at different ratios to obtain bioinks. Using the cross-linking characteristics of hydrogels, combined with a variety of biomaterials and their composite materials, mixtures of cells, and bioinks with different ratios are prepared to meet specific requirements.
2.1.3. Relationship between bioinks and bioscaffolds
Accurate selection of a bioink during bioscaffold fabrication is critical. Successfully applying a solution to the design of a bioscaffold requires a certain understanding of the structures and compositions of bioscaffolds as well as the selection of a suitable bioink. Using bioinks, bioscaffolds are fabricated by the mold-casting process, 3D bioprinting, electrowriting, electrospinning, and soft lithography. Bioinks are constituent materials of bioscaffolds and directly determine the biochemical (e.g., degradability, biocompatibility) and biophysical (e.g., hydrophilicity, mechanical strength, stiffness) properties of bioscaffolds, and the properties of bioinks can be changed. Although 3D structures of bioscaffolds depend on the processes, bioinks directly contribute to the manufacturability of the structures. In addition, connectivity of the internal structures of bioscaffolds is easily achieved by sacrificial materials as part of the bioink mixture. One bottleneck in fabricating ideal bioscaffolds is the limited number of bioinks, which, in turn, limits the types and parameters of fabrication and affects cell differentiation and adhesion on the bioscaffold surface, and tissue formation [49]. For example, the forming ability of bioscaffolds is limited by the rheological properties, freezing point, melting point, and solubility of bioinks.
2.2. Bioscaffold fabrication by 3D bioprinting
In addition to meeting the biocompatibility and biodegradation requirements considered during bioink preparation, 3D morphology and surface properties of bioscaffolds also significantly affect cell behaviors. The bioscaffold-forming precision significantly affects cell adhesion, migration, differentiation, and organization. Therefore, bioscaffold fabrication has stricter requirements. The conventional bioscaffold-forming method is molding-pouring-demolding. Although this method can be used to fabricate 2D or 3D bioscaffolds, it has a few limitations, such as the inability to ensure vascular connectivity requirements, the introduction of chemical reagent residues, complex processes, and long preparation cycles. In addition, it cannot realize the direct forming and manufacturing of complex and heterogeneous bioscaffold networks and does not regulate the 3D cell distribution in bioscaffolds.
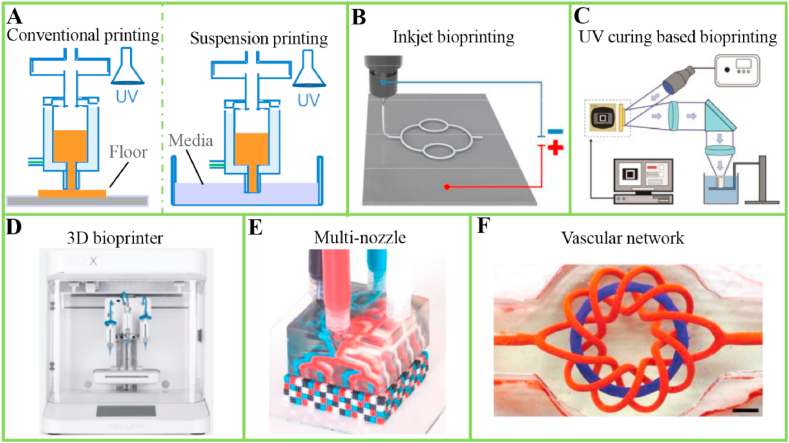
These limitations of the conventional method can be overcome by the application of 3D bioprinting technologies. Skylar-Scott et al. developed new 3D printing devices [50], while other groups investigated bioink materials [16,51,52]. Using existing equipment, He developed new forming technologies to meet the specific requirements of bioscaffolds [53]. At present, there are three common methods of bioprinting cells: extrusion technology, inkjet technology, and ultraviolet (UV)-assisted technology.
2.2.1. Extrusion-based 3D bioprinting
Extrusion-based 3D bioprinting (Fig. 3A) is widely used to print gradient cell-loaded heterostructures. In extrusion-based 3D bioprinting, bioinks form continuous microfilaments under an air pressure or mechanical forces. The microfilaments contain cells and are continuously stacked and formed under a specified printing path. The printing of complex structures is of two types, coaxial [54] and multiple nozzle [55]. Heinrich et al. [56] mixed GelMA and cells into bioinks. On the basis of the physical properties of GelMA, the extruded bioinks were cured by the substrate–bioink temperature difference. Ozbolat et al. [57] used extrusion-based 3D bioprinting with sacrificial bioinks to fabricate a microfluidic device. Gao et al. [47] used coaxial nozzle-assisted 3D bioprinting to create a cell-laden hydrogel 3D structure with built-in microchannels for nutrient delivery. In addition, Andrique et al. used extrusion-based 3D bioprinting with alginate hydrogel as a bioink to investigate cell self-organization [58]. Lei et al. [36] reported a general strategy for 3D printing thermosets. Using sodium chloride particles, they bioprinted various thermoset constructs with extrusion-based 3D bioprinting.
Fig. 3.
Schematic diagram of common 3D bioprinting technologies, bioprinting devices, and fabricated bioscaffolds. (A) Extrusion-based and (B) inkjet 3D bioprinting technologies. Reproduced with permission [60]. Copyright 2020, IOP Publishing. (C) UV-assisted 3D bioprinting. Reproduced with permission [64]. Copyright 2019, Springer Nature. (D) Multihead 3D bioprinting device of our laboratory. (E) Multinozzle 3D printing head. Reproduced with permission [50]. Copyright 2019, Springer Nature. (F) Complex 3D vascular bioscaffolds. Reproduced with permission [52]. Copyright 2019, The American Association for the Advancement of Science. UV, ultraviolet.
However, extrusion-based technology has limited bioprinting resolution. Future directions may focus on 3D bioprinting in suspension baths [59], which will enable bioprinting 3D complex structures with high-precision. In addition, a too small nozzle diameter during molding easily causes blockage and subjects cells to severe pressure, which affects cell survival. Although extrusion materials can be formed on a solid support or directly in a liquid environment, the forming speed in a liquid environment is relatively slow, and the problem of bioink salivation needs to be overcome. However, extrusion-based 3D bioprinting is still the most common bioprinting technology.
2.2.2. Inkjet 3D bioprinting
The principle of inkjet 3D bioprinting (Fig. 3B) is based on droplet formation under an electric field. Droplets overcome surface tension and are sprayed. Compared to extrusion-based 3D bioprinting, inkjet 3D bioprinting can print with small-diameter nozzles and high-concentration cell-loaded bioinks. Xie et al. fabricated GelMA microspheres using inkjet 3D bioprinting [41]. Lv et al. used inkjet 3D bioprinting to study microfluidic chip–based bioscaffolds [60]. Experimental results show that electric field forces can realize the 3D spatial positioning of cell-loaded bioinks and maintain the integrity and better accuracy of corresponding structures. By adding electrostatic jet deflection devices, Liashenko et al. obtained submicrometer features using inkjet 3D bioprinting [61]. These findings indicate that living cells can achieve ideal 3D deposition by a change in printing parameters, which obtains accurate tissue reconstruction through microfluidic chip–based bioscaffolds. However, 3D bioscaffolds fabrication is not just the accumulation and superposition of bioinks; it is also the fabrication of high-gradient, multimaterial, multicomponent heterostructures. Inkjet 3D bioprinting has a few limitations, which need to be further studied.
2.2.3. UV-assisted 3D bioprinting
UV curing is noncontact and nozzleless. It is directly applied to print high-precision, heterogeneous, and complex structures. It avoids problems such as nozzle blockage and viscosity limitation, and is used for in situ printing [62]. Photosensitive hydrogel materials are directly used to fabricate cell-loaded structures or microfluidic devices used for cell culture. UV-assisted 3D bioprinting is of two types, stereolithography (SLA) and digital light processing (DLP) [63]. SLA uses a laser to directly scan patterns through a point, line, and surface. DLP (Fig. 3C) is more complex.
Compared to extrusion-based 3D bioprinting, UV curing can print biological powders with a higher cell concentration. For example, after the photosensitive hydrogel GelMA was mixed with fibroblasts, a 3D bifurcated tubular structure was fabricated using a dynamic light projection–based SLA system by UV irradiation [64]. Hong et al. fabricated cartilage tissues using DLP with a SF hydrogel [65]. However, the high cost, limited photoinitiator materials, and relatively low forming rate limit the wide application of UV-assisted 3D bioprinting.
2.2.4. Comparing pros and cons
In general, of the three types of 3D bioprinting, extrusion-based 3D bioprinting has the highest forming efficiency. The effects of inkjet and UV curing on cells are small, so the cell density is large. UV curing has the highest forming accuracy and the best forming stability. All three types can ensure sterility. The materials used in extrusion-based 3D bioprinting are the most extensive, while UV-assisted 3D bioprinting is limited by printing materials. In addition, 3D bioprinting tissues and organs is not a simple voxel increase. The low cell and biomaterial throughput limits the application of UV-assisted 3D bioprinting in the fabrication of macro cell-loaded 3D structures. Melt electrowriting, a type of inkjet 3D bioprinting, usually uses polymers. This method was expanded with the advent of hydrogel materials [66].
The tissue networks printed by bioprinters are not limited to simple tissues; tissues with complex vascularized networks, such as the heart, are also being bioprinted. Our laboratory has a multihead 3D bioprinting device (Fig. 3D), which bioprints tissues and organs using the synergy of multiple nozzles. To improve bioprinting efficiency, bioprint complex structures, and increase the types of cell bioprinting, both bioink materials and printing devices [50] have been researched and developed (Fig. 3E). Lei et al. [67] built a four-axis bioprinting system with rotary receivers to fabricate tubular bioscaffolds. In addition, a highly complex network of vascularization has been successfully fabricated (Fig. 3F) [52]. For cell-free biomimetic bioscaffold fabrication, other 3D printing technologies, such as fused deposition modeling (FDM) 3D printing [34,35], are usually involved. In addition, multiple bioprinting technologies are combined into a single-step approach, such as inkjet 3D bioprinting and extrusion-based 3D bioprinting [68], in order to expand the freedom of architecture design and the use of multiple materials and cell types.
3. Stimulation cues for cell adhesion
Tissues and organs are formed through cell differentiation [69]. Cell adhesion plays a key role in forming the shape and direction of tissues, laying the foundation for tissue formation [70,71]. In vivo microenvironments are complex, and conventional animal tests cannot accurately simulate cell adhesion in the human body [11]. Therefore, cell adhesion is widely studied in vitro. In tissue engineering, the cell adhesion degree is easily affected by the treatment of corresponding adhesion surfaces: surface modifying or surface coating. Superior cell adhesion is the basic condition to maintain the stability of tissue structures and promote cell proliferation and differentiation. However, abnormal cell adhesion leads to vascular disorders and even tissue-related diseases, such as cardiovascular diseases and cancer [72,73].
Bioprinting technologies can significantly control cell positioning. Using cell-laden bioinks, 3D bioprinting of proper process paths can achieve an ideal spatial cell distribution. Lei et al. fabricated a vascularized tissue using coaxial 3D bioprinting [74]. Briefly, cells were loaded in a sacrificial material, which was then dissolved and a structure was prepared. The cells automatically deposited and adhered to the bioscaffold surface. However, one limitation of this method is the lack of bioinks matching corresponding bioprinting processes. Microneedles are widely used in biomedicine. Cell microneedles [75,76] are used for quantitative injection of cells at designated adhesion surfaces. Although cell-free bioprinting and subsequent cellularization provide a way for fabricating cellular microneedles, they cannot control patterned cell distribution on bioscaffolds. The other two commonly used control methods during cell adhesion are bioscaffold surface modification with topography and bioscaffold surface coating with other materials.
3.1. Stimulation with surface modification for cell adhesion
Surface modification created by surface topography, including the pillar, pit, and grating shape; feature size; spacing; and substrate arrangement, plays a key role in the induction of cell adhesion [63]. Generally, cell adhesion can respond to surface topological structures at the micrometer and even the nanometer scale. For example, the presence of nanotopography on microscale wrinkles of substrates promotes the adhesion of fibroblasts, endothelial cells, osteoblasts, and human mesenchymal stem cells (MSCs) [77]. Cell adhesion is the basis for cell proliferation, cell migration, cell differentiation, and tissue formation, and a comprehensive understanding of the effects of surface modification on cell adhesion is important for regenerative medicine and diagnosis. Therefore, the adhesion of cells on bioscaffold surfaces has attracted increasing attention in tissue engineering. Bioprinting of tissue engineering bioscaffolds paves the way for the creation of surface structures. After determining models and bioprinting materials, a high-precision-topology bioscaffold can be easily fabricated using 3D bioprinting. For example, Xie et al. [78] fabricated heterogeneous bioscaffolds with different fiber diameters and pore sizes using inkjet 3D bioprinting (Fig. 4A). They systematically investigated cell adhesion affected by bioscaffolds with controllable pore sizes, the difference in the adhesion characteristics of bioscaffolds with different fiber diameters, and the different adhesion behaviors of large and small cells on the same bioscaffold. Results showed that cells in bioscaffolds with small pores and large fibers have superior adhesion behavior. Compared to large cells, small cells are more inclined to adhere and bridge adjacent fiber spaces. In addition to fabrication of grid structures, 3D bioprinting can also fabricate bioscaffolds with pillars and pits. Choi et al. [79] fabricated microwrinkle circular pits on the inner surface of a bioscaffold to achieve accurate cell adhesion. 3D bioprinting provides many methods of fabricating this type of bioscaffold surfaces. Common methods include auxiliary printing of support materials or sacrificial materials, bioprinting in suspension baths, and auxiliary molding-pouring-demolding. In addition to surface topologies, posttreatment processes may also affect cell adhesion. Researchers plasma-treated a microchannel of a fabricated bioscaffold to increase its adhesion properties in an oxygen plasma chamber [80]. Compared to surface topology modification, these methods are relatively simple. Yet, precise treatments of bioscaffold surfaces are difficult to achieve because of the requirement of micron-level structures for cell adhesion, leading to inaccurate adhesion positions. Therefore, bioscaffolds with surface topography are preferred. Surface topography modification is a physical treatment that achieves cell adhesion without introducing other materials on the bioscaffold surface. So, bioscaffolds with surface topography modification, processed by 3D bioprinting, and appropriate postprocessing, have good cell compatibility, in addition to direct and effective promotion of cell adhesion.
Fig. 4.
Regulation of cell adhesion. (A) Induction of surface topology for cell adhesion: (i) heterogeneous bioscaffolds with variable pore sizes and their effects influence on cells and (ii) heterogeneous bioscaffolds with variable fiber diameters and their effects on cells. Reproduced with permission [78]. Copyright 2019, Elsevier. (B) Induction of surface coating for cell adhesion: (i) cell adhesion density and morphology of cell seeding on bioscaffold surfaces with different concentrations of magnetic graphene oxide–ferroferric oxide–graphene oxide complex for 48 h and (ii) high-magnification images of cell adhesion density and morphology of cells cultivated with different concentrations for 48 h. Reproduced with permission [83]. Copyright 2020, John Wiley and Sons.
3.2. Stimulation with surface coating for cell adhesion
Unlike the direct effects of surface topography on cells, new chemical factors and material coatings on 3D-bioprinted bioscaffold surfaces also induce corresponding cell adhesion behaviors. In contrast to surface topography modification, surface coating is a conventional and easily controlled method because of its similarity to conventional experimental and clinical injection. In an in vivo setting, it may not be necessary to add additional chemical factors into bioscaffolds. However, coating chemical materials on bioscaffold surfaces is required in order to change the hydrophilicity and hydrophobicity of bioscaffold surfaces in vitro. Surface coating is realized not only manually but also by 3D bioprinting. Although the manual method is simple, accurate surface coating is difficult. Gao et al. [81] used extrusion-based 3D bioprinting to deposit a coating material on a bioscaffold surface. The position of the coating material on the bioscaffold was controllable. Natural materials, such as poly-l-lysine [82], and synthetic materials are widely used for surface coating. Surface coating regulates cell adhesion. There is no need to form complex structures of coating materials. Hence, surface coating by 3D bioprinting, including but not limited to extrusion-based 3D bioprinting, is feasible. In addition, gradient cell adhesion on the same bioscaffold surface can be achieved without time-consuming and extraordinarily complex processes. For example, 3D-bioprinted gradient coating can be easily achieved by controlling the pressure and moving speed of the nozzle in extrusion-based 3D bioprinting, the droplet velocity in inkjet 3D bioprinting, and the exposure time in UV-assisted 3D bioprinting.
Do different concentrations of coating materials also affect cell adhesion? He et al. developed composite bioscaffold surfaces using diverse combinations of magnetic graphene oxide, ferroferric oxide, and graphene oxide in order to determine the relationship between cell adhesion behavior and the corresponding chemical composition of bioscaffold materials (Fig. 4B) [83]. Within a specific concentration range, the cell adhesion density increased with an increase in the concentration of magnetic graphene oxide. However, beyond a specific concentration threshold, the cell adhesion density significantly decreased. There are significant differences in the regulation of cell adhesion with different concentrations of materials used for coating cell adhesion surfaces. Therefore, an appropriate material concentration is important. Microfluidic technologies have the advantage of controlling, operating, and inspecting complex fluids at the microscale, which enables a controllable gradient distribution of concentration by designated microchannels [16]. Combining 3D bioprinting and microfluidic technologies enables fabrication of surface-coated bioscaffolds with controllable distribution of material concentration, promoting the regulation of cell adhesion and tissue regeneration in vitro. In addition, for more accurate cell distribution, other technologies, such as casting, may also be involved, partly because the fabrication of molds for surface coating can be done by 3D printing.
3.3. Biophysical stimulations for cell growth and tissue formation
In conventional tissue engineering, bioscaffolds are successfully fabricated by combining bioink preparation and 3D bioprinting. Cell-loaded or cell-loaded bioscaffolds are distributed in 3D space according to the location given by the 3D bioprinting device. Subsequently, tissues are prepared in a culture medium or bioreactors for regenerative medicine and cell therapies; however, cell proliferation, migration, and differentiation in specified growth directions cannot be guided [84]. The tissues formed are quite different from ideal tissues. Studying the mechanism underlying regulatory mechanism of bioscaffold-based approaches for cell behaviors provides a new way to solve this problem. The common regulation methods include biophysical and biochemical stimulations. The ultimate goal of bioscaffolds is to be implanted into the human body. However, biochemical stimulations might lead to cytotoxicity for cells. Applying biophysical stimulations to cells can effectively avoid this problem. To better define the regulatory behavior of stimulations, it is important to investigate biophysical stimulations. Several factors contribute to biophysical stimulations, such as change in surface microhardness, external force and strain, and non-contact-dependent factors (light, ultrasound, electrical, and magnetic stimulations).
3.3.1. Surface microhardness
Surface microhardness contributes to the migration behavior of cells. A change in microhardness is achieved by controlling 3D bioprinting processes and adding external supports [85], electric and magnetic fields [86], and gamma ray radiation [87] to the substrates formed. Matrix hardness for cells is good for tissue regeneration, but too low hardness is not conducive to structural stability and cell adhesion on hard matrices is difficult. To some extent, the migration behavior of vascular cells to stiffer regions is observed on uniform substrates, promoting the regulatable direction, and distribution of newly formed tissues on bioscaffolds. For example, Song et al. fabricated fibrous networks with different stiffness values and found that meniscal fibrochondrocytes migrate to stiff fibers through the networks after day 6 (Fig. 5A) [88]. In addition, they found better repair effects of damaged meniscuses in stiff fibrous networks.
Fig. 5.
Regulation surface microhardness for cell behaviors. (A) Migration behavior of meniscal fibrochondrocytes on a soft/stiff fibrous network. Reproduced with permission [88]. Copyright 2019, Wiley-VCH. (B) Fabrication of bioscaffolds with regulatable surface microhardness and its effect on cell behaviors: (i) DLP 3D bioprinting of bioscaffolds with regulatable surface microhardness and (ii) cell migration and proliferation on bioscaffold layers with different surface hardness values. Reproduced with permission [89]. Copyright 2019, American Chemical Society. (C) Extrusion-based 3D printing of structures to modulate surface microhardness using electric fields. Reproduced with permission [91]. Copyright 2017, Elsevier. (D) Design strategy of hardness gradient with controllable material distribution and composition for extrusion-based 3D bioprinting. Reproduced with permission [92]. Copyright 2020, Giachini et al. DLP, digital light processing; MR, magnetorheological materials.
To regulate stiffness-responsive cell behaviors, accurate fabrication of bioscaffolds with surface microhardness is necessary. Xue et al. used DLP 3D bioprinting to fabricate a hydrogel bioscaffold with regionally varied stiffness [89]. The surface microhardness of the bioscaffold was tuned by different exposure times on print layers (Fig. 5B–i), and fibroblasts were seeded onto the bioscaffold to investigate cell behaviors. Results showed a higher cell population (Fig. 5B–ii) and fibrous-like tissue layers in the area of a longer exposure in culture. Similarly, in inkjet 3D bioprinting, curing jetted ink droplets at different UV exposure times facilitates the formation of bioscaffold with different hardness values. In addition to photo-cross-linking, ionic cross-linking is also involved. Idaszek et al. [90] used extrusion-based 3D bioprinting to develop a microfluidic print head and formed inks with different calcium chloride–alginate mixing ratios. They achieved different surface microhardness values by regulating the cross-linking reaction between calcium ions and alginate. Studies have also reported indirect regulation of bioscaffold stiffness. Bastola et al. [91] added a magnetorheological material in matrices to fabricate structures with tunable stiffness using extrusion-based 3D printing. They encapsulated magnetorheological materials in the structures layer by layer, and regulated the bioscaffold hardness by applying electric fields (Fig. 5C).
Ramya et al. presented a similar method of changing matrix hardness [87]. They subjected the prepared composite films to gamma ray irradiation at various dosages. By using hybrid materials embedded with materials responsive to electrical stimulation, magnetic stimulation, or light, microhardness can be changed using 3D bioprinting and external fields. Another representative method of regulating surface microhardness is to add external supports [85]. Cell substrates bonded with underlying patterned supports can be fabricated by 3D bioprinting, followed by corresponding assemblies. Therefore, cell migration can be observed and quantified by varying the geometry underlying corresponding cell substrates. Usually, connectors integrated in these two modules may be used to achieve high-precision assembly and microscale regulation.
3.3.1.1. Discussion of pros and cons
Overall, the reported methods have a few advantages and disadvantages. Both controlling 3D bioprinting processes and applying an electric field, magnetic field, and gamma ray radiation have specific requirements for bioinks and materials. In addition, the time and cost of complex processes for adding external supports require extra designing and preparation. Compared to other treatments, realizing different hardness distributions in cell matrices during forming is the most effective and precise method. On the basis of cross-linking reactions (ionic or photosensitive cross-linking), ideal hardness can be achieved by extrusion-based, inkjet, or UV-assisted 3D bioprinting. For example, Giachini et al. [92] used extrusion-based 3D bioprinting to design and print different structures with a controllable hardness gradient (Fig. 5D). They considered the material distribution and composition and cross-linking processes in the structural design. Adding external supports has low requirements for 3D bioprinting to obtain the ideal matrix hardness for cells. The fabrication of these bioscaffolds with surface microhardness involves two Lego-like modules, including a bioscaffold and its corresponding external supports, so there no need to consider the regulatory factor when bioprinting bioscaffolds and it is widely used in the study of in vitro cell culture. Non-contact-dependent methods, including applying an electric field, magnetic field, and gamma ray radiation, regulate cell behaviors in noncontact and remote ways, which are conducive to in vivo tissue formation. In addition, controllable distribution of materials related to external fields into bioscaffolds is easily achieved by 3D bioprinting. However, most reports about regulation of cell migration by surface hardness are still focused on phenomenon. An in-depth study of the regulatory mechanism, such as the redox state [93], needs to be considered.
3.3.2. External force and strain
External force and strain affect the secretion of growth factors, proteins, and gene expression by acting on cells through force conduction. They also affect the direction of cell growth, proliferation, migration, and differentiation. External forces include shear force, hydrostatic pressure, and cyclic stress, generated by a controlled flow rate of the cell culture medium [[94], [95], [96]]. Bioscaffolds with self-designed regulation, including microchannels and resilience materials, provide the mechanical strain stimulation [[97], [98], [99]]. Shear force acting on cells is realized by controlling fluids. Trachtenberg et al. [100] fabricated bioscaffolds with different pore sizes using 3D bioprinting (Fig. 6A–i), forming a shear stress gradient within the bioscaffolds after perfusion of the culture medium. Molladavoodi et al. [101] found that a low shear stress created by the flow velocity of the culture medium promotes wound healing (Fig. 6A–ii). Microfluidic methods that generate cyclic stress are similar to those that generate shear stress; the only difference is the pump that drives the flow velocity of the culture medium. For example, researchers generated a cyclic fluid stress with different reciprocating frequencies by a self-designed mini-oscillator [102]. They found that a specific frequency of reciprocating force promotes fibroblast arrangement and polarization (Fig. 6B–i) but that another frequency promotes fibroblast differentiation (Fig. 6B–ii). Optimization of the reciprocating frequency is important for cell differentiation and tissue regeneration. In addition, there are reports on hydrostatic pressure. Park et al. [103] used extrusion-based 3D bioprinting to fabricate composite bioscaffolds with different mass fractions of beta-tricalcium phosphate (β-TCP) (Fig. 6C–i). After MSC seeding, they generated a more marked ECM by applying hydrostatic pressure (Fig. 6C–ii).
Fig. 6.
Regulation of external force and strain for cell behaviors. (A) Formation of shear force and its effect on cell behaviors: (i) cross-sectional view of bioscaffolds with gradient pores fabricated by 3D bioprinting (L, M, and S represent large, medium, and small, respectively). Reproduced with permission [100]. Copyright 2017, American Chemical Society; (ii) wound healing under low shear stress. Reproduced with permission [101]. Copyright 2017, Molladavoodi et al. (B) Effect of cyclic stress on cell behaviors: (i) fibroblast arrangement and polarization promoted by a specific frequency of reciprocating force and (ii) fibroblast differentiation promoted by another specific frequency of reciprocating force. Reproduced with permission [102]. Copyright 2020, Elsevier. (C) Formation of hydrostatic pressure and its effect on cell behaviors: (i) bioscaffolds with different fractions of β-TCP and (ii) marked ECM generated after applying hydrostatic pressure (SX and SO represent cell culture without or with hydrostatic pressure, respectively). Reproduced with permission [103]. Copyright 2017, Springer Nature. (D) Formation of strain and its effect on cell behaviors: (i) bioscaffolds containing flanking channels fabricated by extrusion-based 3D bioprinting. Reproduced with permission [104]. Copyright 2019, The Royal Society of Chemistry; (ii) elongated cells under the function of cyclic circumferential strain. Reproduced with permission [105]. Copyright 2018, The Royal Society of Chemistry. (E) Formation of graded strain for cells by actions of the resilience and gravity of a deformed bioscaffold. Reproduced with permission [4]. Copyright 2017, Journal of Visualized Experiments. β-TCP, beta-tricalcium phosphate; ECM, extracellular matrix.
In contrast with the stimulation of shear and stress for direct cell regulation, strain indirectly acts on cells by changing the shape of cell matrices. Usually, methods of applying strain include external and self-generating control. The expansion and contraction due to a change in the air pressure in microchannels provides external control, while the resilience and gravity of bioscaffolds provide self-generating control. Kim et al. [104] used UV-assisted 3D bioprinting to fabricate a microfluidic chip that enabled the real-time control of strain in capillary channels surrounded by source and sink channels (Fig. 6D–i). The strain was generated by stretching the flanking channels. Engeland et al. [105] applied a cyclic circumferential strain on vascular and smooth muscle cells and found both types of cells arranged and elongated in the vertical direction of strain (Fig. 6D–ii). Hsieh et al. used self-generating control to design a model to generate gradient strain for cells [4] by the actions of the resilience and gravity of a deformed bioscaffold (Fig. 6E). To achieve this type of regulation, bioscaffolds and regulatory modules can be fabricated separately using 3D bioprinting, followed by corresponding assemblies.
3.3.2.1. Discussion of pros and cons
3D Bioprinting has outstanding applications in fabricating complex bioscaffolds embedded with the regulatory modules of external force and strain. Taking external force regulation as an example, precise bioscaffolds embedded with regulatory modules are easily fabricated by 3D bioprinting. Because of the direct control of fluids acting on cells, there is usually no need to integrate the regulatory modules into bioscaffolds, but the modules are needed for bioscaffold strain-mediated regulation. 3D bioprinting of high-precision structures facilitates not only integration between bioscaffolds and regulatory modules but also the fabrication of microchannel bioscaffolds. UV-assisted 3D bioprinting is a feasible method of fabricating these structures. Especially, for fabricating microchannel bioscaffolds, inkjet and extrusion-based 3D bioprinting of sacrificial materials provide supports during forming. These sacrificial materials can be dissolved postprocessing. Numerous regulation methods can be generated by combining the interdisciplinary knowledge of mechanics and physics. In addition, the regulation of parameters by these methods helps clarify the mechanism underlying cell behaviors. However, long-term studies need to consider the degradation of bioscaffolds and avoid the problems of pollution and operational errors generated during bioscaffold operation, which may be harmful to cells. Mechanical regulation of self-generating control does not need to consider the introduction of harmful reagents, so self-generating control has unique advantages in in vivo regulation, although there are few reports compared to external regulation. Overall, bioscaffold design has been widely studied, and it is expected that researchers will conduct in-depth studies of cell behaviors by stress and strain stimulation.
3.3.3. Non-contact-dependent factors
Non-contact-dependent factors affect cell migration, proliferation, and differentiation, in addition to intracellular microenvironments. Non-contact-dependent factors include light, ultrasound, electrical, and magnetic stimulations [[106], [107], [108], [109]]. These stimulation cues regulate cell behaviors by acting directly on cells or changing cell matrices, such as surface microhardness. Direct actions on cells activate intracellular signaling pathways and enhance intracellular delivery, while indirect stimulations regulate cells by controlling cell microenvironments. Zhu et al. [110] used UV-assisted 3D bioprinting to fabricate a composite GelMA and polyethylene (glycol) diacrylate bioscaffold. They applied red laser light to stimulate neural stem cells seeded on the bioscaffold (Fig. 7A–i). After culturing for a few days, they observed neuronal differentiation of neural stem cells (Fig. 7A–ii). Ramya et al. fabricated methyl methacrylate, iron-zinc, and HA bioscaffolds [87] and subjected them to different doses of gamma rays, which changed the surface roughness, hydrophobicity, and conductivity and enhanced the viability of NIH-3T3 fibroblasts. Osborn et al. [111] used 3D bioprinting to fabricate porous PLA bioscaffolds for ultrasound stimulation (Fig. 7B–i) and seeded human MSCs on the bioscaffolds. Compared to controls, cell morphologies showed a significant increase in proliferation under low-intensity pulsed ultrasound stimulation (Fig. 7B–ii). An et al. reported an indirect stimulation method of using ultrasound stimulation [112]. They cultured cells on bioscaffold surfaces and modified the surfaces for cell adhesion through low-intensity pulsed ultrasound stimulation. After cell culture, microfilaments, pseudopodia, and ECM mineralization nodules of cells increased, and the related protein expression for osteoblast differentiation was upregulated.
Fig. 7.
Regulation of non-contact-dependent factors for cell behaviors. (A) Effects of light stimulation on cell growth: (i) bioscaffold fabrication by UV-assisted 3D bioprinting and schematic illustration of light stimulation and (ii) enhanced neuronal differentiation of neural stem cells under low-level light stimulation. Reproduced with permission [110]. Copyright 2017, IOP Publishing. (B) Effects of ultrasound stimulation on cell proliferation: (i) 3D-bioprinted PLA bioscaffolds visualized with SEM and (ii) enhanced osteogenic differentiation of human MSCs under low-intensity pulsed ultrasound stimulation. Reproduced with permission [111]. Copyright 2018, John Wiley and Sons. (C) Effects of electrical stimulation on cell proliferation: (i) 3D-bioprinted PPF bioscaffolds before and after functionalization with nanocomplex material and (ii) improved cell adhesion and proliferation of pre-osteoblasts under electrical stimulation. Reproduced with permission [113]. Copyright 2020, Elsevier. (D) Effects of magnetic stimulation on cell behaviors: (i) 3D-bioprinted (left) chitosan bioscaffolds and (right) chitosan bioscaffolds embedded with IONPs and (ii) improved proliferation and mineralization of osteoblasts under magnetic stimulation. Reproduced with permission [116]. Copyright 2020, Elsevier. UV, ultraviolet; PLA, polylactic acid; SEM, scanning electron microscopy; MSCs, mesenchymal stem cells; PPF, poly(propylene fumarate); IONPs, iron oxide nanoparticles.
Stimulation based on bioscaffolds and material coating has also been reported. Liu et al. team [113] used extrusion-based 3D bioprinting to fabricate functionalized poly(propylene fumarate) (PPF) bioscaffolds coated with a nanocomplex of single-stranded DNA (ssDNA) and carbon nanotubes (CNTs) (Fig. 7C–i). Pre-osteoblast adhesion and proliferation were enhanced by electrical stimulation after cell seeding (Fig. 7C–ii). Compared to external stimulation cues, Chen et al. [114] formed a built-in stimulation of a nanoscale electric field on bioscaffold surfaces by changing the piezoelectricity of materials using polarization. Continuous electrical stimulation was provided without additional external fields. In addition, cell behaviors can also be regulated through nanovibrational stimulation using piezoelectric materials or piezo actuators [115]. Bioscaffold surfaces for cell adhesion are deformed by an electric field force. A vibration stimulation can be created by applying a time-varying electric field. Lin et al. [116] applied a magnetic field to study cell behaviors with surface-functionalized iron oxide nanoparticles (IONPs). They used extrusion-based 3D bioprinting to fabricate bioscaffolds embedded with IONPs (Fig. 7D–i). A magnetic field can improve the proliferation and mineralization of osteoblasts (Fig. 7D–ii).
3.3.3.1. Discussion of pros and cons
Various stimulations enable remote regulation of cell behaviors without cell contact, decreasing the pollution of cell culture chambers caused by conventional regulation. Some factors are required for imitating physiological microenvironments. For example, electrical stimulation stimulates myocardial cell growth. There is a closed relationship between electric and magnetic fields in physics, leading to many similarities in their stimulations in the design methods, principles, and regulation modes of cell behaviors. Ultrasound stimulations have been applied to study cell behaviors both in vitro and in vivo [117]. In addition, UV light irradiation kills bacteria. Compared to conventional methods, a bioscaffold can serve as a source of stimulations including but not limited to electrical, magnetic, ultrasound, and light stimulations [118].
External fields usually act on cells through corresponding media materials, and these cell stimulations may be mediated by bioscaffolds embedded with these media materials. To target regulation of cell behaviors, piezoelectric and light-responsive materials and MNPs, need to be reasonably distributed in the 3D space of compositive bioscaffolds by 3D bioprinting. In contrast to UV-assisted 3D bioprinting, the distribution of these materials in bioscaffolds is more diversified by inkjet and extrusion-based 3D bioprinting with multinozzle printheads. So far, reports on the remote regulation of cell behaviors have focused on bioscaffolds for cell culture because of the combination of external field–responsive materials and 3D bioprinting providing the possibility for precise cell regulation. However, there are few reports on direct cell stimulation, except for ultrasound stimulation. Future studies need to shift their focus from the regulation of bioscaffolds to the direct regulation of cells.
3.4. Biochemical stimulations for cell growth and tissue formation
Tissue formation is similar to seedling growth. Nutrient solutions contain nutrients that induce the cell activity of trees and provide active substances required for tree growth. Nutrient solutions for trees are similar to biochemical components for cells, and the infusion control devices of trees are like the biochemical control bioscaffolds of cells. Biochemical components induce cell differentiation and reprogramming. Biochemical stimulations mainly involve three aspects: types of biochemical factors, regulatory bioscaffolds, and concentration regulation.
3.4.1. Biochemical factors
Biochemical factors coming from internal bodies mainly include cell growth factors, cellular proteins, kinases, and others, such as heparin, cellulose, and vitamins [119]. Chemical stimulants are from outside the body, such as MNPs [120]. In vivo biochemical stimulation cues, which have good cell compatibility and do not need to consider immune effects, are usually the best choice for regulating cell behaviors. For example, Song et al. investigated the effects of transmembrane 4 L six family member 5 (TM4SF5) on cell metabolism [121]. Compared to untreated cells, after TM4SF5 treatment, cells in 3D collagens showed more aggressive growth, in addition to aggressive pseudopodia, invasive foci, invadopodia, and endothelial-like networks. Poukkula et al. analyzed the migration behavior of border cells using receptor tyrosine kinases and platelet-derived growth factor/vascular endothelial growth factor–related receptors (PVRs) [122]. They found that receptor tyrosine kinases affect the presence and size of border cells, while PVRs induce front extensions and cell migration. However, in vivo stimulation cues sometimes cannot completely meet experimental requirements. To regulate cell behaviors in different ways, synthetic reagents, including composite materials, are used. For example, antioxidants [123], poly(vinyl alcohol)–chitosan [124], and cannabinoid [125] are used to regulate cell proliferation. However, their degradation characteristics and biocompatibility need to be considered using stimulants from outside the body. Biochemical factor delivery and concentration regulation are new aspects of biochemical regulation of cell behaviors. The delivery of biochemical factors and the distribution of chemical factor concentration on demand are realized through the design of bioscaffolds. The function and implementation principle of bioscaffolds are similar to those of drug delivery, which accurately determines the injection position and corresponding controllable gradient concentration of biochemical reagents without creating bulk flows.
3.4.2. Regulatory bioscaffolds
Inductive bioscaffold materials, bioscaffolds loaded with biochemical factors, and biochemical factors generated by degradation of bioscaffolds provide stimulations. Zhao et al. [126] used extrusion-based 3D bioprinting to fabricate bioscaffolds with the regenerative material of bioactive glass (Fig. 8A–i). Cell culture experiments showed that stimulation with bioactive glass bioscaffolds enhances osteoblast migration and extramembranous osteogenesis (Fig. 8A–ii). A more common method of regulating cell behaviors is to use bioscaffolds loaded with biochemical factors. Liu et al. [127] bioprinted biomimetic gelatin/alginate hydrogel bioscaffolds loaded with nanosilicates (Fig. 8B–i). They exploited the bioinert property of alginate to avoid rapid degradation of the bioscaffolds and ensure effective regulation. They found that the addition of nanosilicates significantly promotes the formation of mineralized matrices in the culture medium (Fig. 8B–ii).
Fig. 8.
Regulation of biochemical stimulations for cell behaviors. (A) 3D-bioprinted bioscaffolds of inductive materials for regulation of cell behaviors: (i) fabrication of bioactive glass bioscaffolds using extrusion-based 3D bioprinting and (ii) enhanced osteoblast migration (top) and extramembranous osteogenesis (bottom) after stimulation with bioactive glass bioscaffolds. RAW is a type of cells. Reproduced with permission [126]. Copyright 2018, John Wiley and Sons. (B) 3D-bioprinted bioscaffolds loaded with biochemical factors for regulation of tissue behaviors: (i) fabrication of gelatin/alginate hydrogel bioscaffolds loaded with nanosilicates using extrusion-based 3D bioprinting and (ii) enhanced formation of mineralized matrices in the culture medium after stimulation with bioscaffolds loaded with nanosilicates. Reproduced with permission [127]. Copyright 2020, Elsevier. (C) 3D-bioprinted bioscaffolds loaded with generable biochemical factors for regulation of tissue behaviors: (i) fabrication of strontium-containing HA/PCL bioscaffolds using extrusion-based 3D bioprinting and (ii) enhanced proliferation of MSCs and formation of new bone tissues. Reproduced with permission [128]. Copyright 2019, Elsevier. (D) 3D-bioprinted miniaturized needle arrays to release drugs for regulation of tissue behaviors: (i) 3D-bioprinted microneedle structures for regulation of tissue behaviors by drug delivery and (ii) enhanced effect on tissue repair by microneedles; granulation tissue is denoted with black arrowheads, and hair is denoted with white arrows. Reproduced with permission [132]. Copyright 2020, John Wiley and Sons. (E) 3D-bioprinted modular microfluidic bioscaffolds to create a gradient concentration for regulation of cell behaviors: (i) several formulations with different concentrations of the culture medium created by modular microfluidic bioscaffolds and (ii) increased spherical size of fibroblasts (left) and enhanced proliferation and mutual contact of osteoblasts (right) under perfusion of an appropriate concentration of the culture medium. Reproduced with permission [134]. Copyright 2018, IOP Publishing. BGSE, bioactive glass bioscaffold extract; NM, normal medium; HA, hydroxyapatite; PCL, polycaprolactone; MSCs, mesenchymal stem cells.
Biochemical factors can be not only loaded by bioscaffolds, but also generated by degradation. To generate biochemical factors, Liu et al. [128] 3D-bioprinted strontium-containing HA/PCL bioscaffolds (Fig. 8C–i). Strontium and calcium ions were released from the bioscaffolds in a sustained manner. MSC proliferation and new tissue formation on the composite bioscaffolds increased compared to PCL bioscaffolds (Fig. 8C–ii). In addition, material synthesis processes are involved before bioscaffold fabrication [27]. Bioscaffolds are also used as a delivery vehicle for biochemical reagents. Paolini et al. [129] used extrusion-based 3D bioprinting to fabricate PLA bioscaffolds and coated them with poly-amidoamine hydrogel. They observed improved cell proliferation on the coated bioscaffolds as a function of time compared to PLA bioscaffolds. However, biochemical factors have an uncontrollable release rate. Dang et al. [130] improved this by fabricating macroscale and microscale porous bioscaffolds using 3D bioprinting, and the burst release decreased. In addition, microneedles for delivery of reagents have also been investigated [75,131]. To release drugs for cell regulation, Derakhshandeh et al. prepared resin-based miniaturized needle arrays by 3D printing in support material (Fig. 8D–i) [132]. After perfusing cefazolin salt through microneedles, granulation tissues, new blood vessels, and hair significantly improved in the microneedle-assisted group (Fig. 8D–ii). Unfavorable flows created by bioscaffold delivery can be improved compared to conventional delivery methods [80].
3.4.3. Concentration regulation
The concentration of biochemical factors also significantly affects cell behaviors. A reasonable concentration of biochemical factors is required for cell proliferation, cell migration, cell differentiation, and tissue formation [133]. Gradient concentration regulation is beneficial for cell research. Microchannels can be similarly converged and branched to form a concentration gradient on the basis of the principle of series and parallel networks of circuits in electrotechnique. Nie et al. [134] fabricated Lego-like modular microfluidic devices using 3D bioprinting to create a concentration gradient for the regulation of cell behaviors. The concentration gradient was controlled by the location of microchannels and the flow velocity of the culture medium or other biochemical substances (Fig. 8E–i), and formulations with different concentrations were created by assembled microfluidic bioscaffolds. Cell culture experiments showed that the spherical size of fibroblasts increases and osteoblasts proliferate and form mutual contact under an appropriate concentration of the culture medium (Fig. 8E–ii). To create a diversified concentration gradient, auxiliary devices, such as a rocker platform, may be involved [133].
3.4.3.1. Discussion of pros and cons
Overall, in contrast to biophysical stimulations, biochemical stimulations provide unique stimulation cues for cell growth and tissue formation. The regulation of biochemical factors by bioscaffolds involves the development and delivery and the concentration control of reagents. Reagents come from direct or indirect chemical factors, such as in vivo acellularization and in vitro–synthesized composite materials. Because of good cellular compatibility and immunity, primary biochemical factors, and their derivatives in vivo will probably be the main regulatory factors. Yet, the mechanism underlying biochemical regulation of cell behaviors is relatively clear. Therefore, the focus is on the way these biochemical stimulations work and the concentration control of biochemical factors.
For reagent delivery, there are three application aspects of bioscaffolds. First, bioscaffold with materials functions of biochemical stimulations or embedded with reagents provide direct regulation of cell behaviors. 3D Bioprinting technologies have unsurpassed advantages in controlling material distribution and fabrication of materials loaded with reagents. 3D bioprinting achieves precise targeting and sustained release of stimulations. Delivery by microneedles is convenient for regulation of cell behaviors because of the advantage of minimal invasion during drug delivery. The types and injection locations of biochemical reagents can be easily adjusted by changing the microneedle position. Second, indirect delivery can be achieved by releasing biochemical stimulation cues during the degradation of bioscaffolds. Occasionally, sustainable release of biochemical stimulation cues is required. In some cases, the method of embedding growth factors into bioscaffolds is not feasible because of the limitations of 3D bioprinting and the requirements of targeted regulation. An alternative is to generate interesting and meaningful regulatory cues through the degradation of bioscaffolds. Some biochemical factors, such as calcium, iron, zinc, and magnesium ions, are beneficial for cell growth and tissue formation. If these factors are considered during the design and preparation of bioscaffold materials, cells can be stably regulated for a long time. Third, bioscaffolds can act as transportation tools for delivering biochemical reagents to cells. Cells are commonly disrupted by conventional targeted injections of reagents. These defects can be eliminated by following bioscaffold-based approaches through guiding the smooth flow and precise delivery of biochemical reagents. The only requirements are an appropriate geometry design and 3D bioprinting. In addition, microchannel bioscaffolds are involved in concentration control. Cross sections of microchannels and connections between microchannels affect the final concentration of prepared reagents. Multiple-component control puts high demands on the structures of these microchannels. Modular fabrication facilitates various types of preparation and decreased the complexity of the overall structures and manufacturing difficulties. As discussed previously, 3D-printed connector modules can help achieve the assembly between modules. Therefore, this method is operable and has application prospects.
Realization of these aforementioned regulation methods requires not only the structural accuracy of bioscaffolds but also the function of biochemical factors. Biochemical factors may be modified by 3D bioprinting, such as high-intensity UV exposure and chemical reagents (e.g., calcium chloride) added during cross-linking of extrusion-based, inkjet, and UV-assisted 3D bioprinting. These aspects must be considered to avoid inaccurate regulation of cell behaviors. In addition, the degradation rate of bioscaffold materials also needs to be investigated to match the release of biochemical stimulation cues and corresponding tissue formation. For interesting research on regulatory bioscaffolds and the achievement of concentration gradients, multidisciplines, such as physics and biology, should be integrated to deeply imitate the in vivo cell microenvironments.
3.5. Synergetic stimulations for cell growth and tissue formation
Synergetic stimulation refers to the regulation for cell behaviors by combining at least two stimulants, including biophysical and biochemical stimulation cues [135]. The cellular microenvironments in the body are complex, and they are simultaneously stimulated by various stimulants. Existing regulatory methods have been reported in recent years, and we have a general understanding of the mechanism underlying regulation of cell behaviors under single stimulation. However, the mechanism underlying regulation of cell behaviors under multiple stimulations is still unclear. To provide suitable in vitro or in vivo stimulating microenvironments to promote tissue and organ formation, it is important to research the corresponding mechanism underlying regulation of synergistic stimulations. Common synergistic stimulations include multiple biophysical, multiple biochemical, and chemical and mechanical stimulations. As mentioned before, biophysical stimulation cues include surface topology, surface microhardness, external force and strain, and non-contact-dependent factors (light, ultrasound, electricity, and magnetic stimulations). Biochemical stimulation cues include biochemical factors, such as cells growth factors, cellular proteins, kinases, and synthetic materials (and their concentrations). Synergistic stimulations are regulated by combining regulatory bioscaffolds with research methods mentioned before. Because the regulation of multiple biochemical factors by bioscaffolds is similarly consistent with single-biochemical-factor regulation, synergistic stimulations mainly focus on two aspects, multiple biophysical stimulations, and stimulation by biophysical and biochemical stimulation cues.
3.5.1. Multiple biophysical stimulations
Multiple biophysical stimulations comprehensively regulate cell behaviors by combining at least two stimulation cues from surface topology, surface microhardness, external force and strain, and non-contact-dependent factors. Methods of providing a single stimulation to regulate cell growth and tissue formation have been extensively discussed previously. This section mainly discusses synergetic regulation of cell behaviors by 3D-bioprinted bioscaffolds on the basis of six points: (i) surface topology and external force or strain, (ii) surface topology and external fields, (iii) surface microhardness and surface topology, (iv) surface microhardness and external force or strain, (v) surface microhardness and external fields, and (vi) external fields and external force or strain.
The topological structure of bioscaffolds is usually involved in multiple biophysical stimulations. Matsugaki et al. [136] used extrusion-based 3D bioprinting to fabricate collagen bioscaffolds with different topologies, including oriented scaffolds and scaffolds with random orientation (Fig. 9A–i). Stimulation of shear stress was created by fluid flow. Primary osteocytes showed obvious enhanced directional distribution under the synergetic stimulation by surface topology and shear stress (Fig. 9A–ii). Synergetic stimulation by other external forces and surface topology can also be applied by adjusting the fluid state. In addition, 3D-bioprinted microchannel bioscaffolds pave the way for controllable strain regulation. A corresponding synergetic regulation can be achieved by fabricating microchannel bioscaffolds with surface topology and perfusing fluids. Aliabouzar et al. [137] investigated the synergistic effects of ultrasound stimulation and surface topology. They used UV-assisted 3D bioprinting to fabricate nonporous hydrogel bioscaffolds and porous bioscaffolds with square or hexagonal geometry (Fig. 9B–i). Cell-loaded bioscaffolds were placed in an ultrasound stimulation environment. Synergetic stimulation promoted the spread and growth of MSCs (Fig. 9B–ii). Such regulation can also be achieved by embedding non-contact-dependent-factor-responsive materials into bioprinting materials. Xu et al. [138] used extrusion-based 3D bioprinting to fabricate pattern nanocellulose hydrogel bioscaffolds with different lattice sizes on the basis of the synergetic regulation of surface microhardness and surface topology (Fig. 9C). Bioscaffold stiffness was modified by postprinting chemical cross-linking. Within a certain range, fibroblast cell proliferation significantly improved with an increase in surface microhardness and lattice size.
Fig. 9.
Regulation methods of the synergistic stimulation of multiple biophysical cues for cell behaviors. (A) Synergistic stimulation by surface topology and shear stress and its effect on cell behaviors: (i) collagen bioscaffolds with different topologies fabricated by extrusion-based 3D bioprinting and (ii) enhanced directional cell distribution under synergetic stimulation. Reproduced with permission [136]. Copyright 2020, Matsugaki et al. (B) Synergistic stimulation by surface topology and ultrasound and its effect on cell behaviors: (i) hydrogel bioscaffolds with different topologies fabricated by UV-assisted 3D bioprinting and (ii) enhanced cell distribution and growth under synergetic stimulation. Reproduced with permission [137]. Copyright 2018, John Wiley and Sons. (C) Fabrication of bioscaffolds embedded with regulatory modules of surface microhardness and surface topology for synergistic regulation of cell behaviors. Reproduced with permission [138]. Copyright 2018, The Royal Society of Chemistry. (D) Synergistic stimulation by surface microhardness and electrical stimulation and its effect on cell behaviors: (i) fabrication of bioscaffolds embedded with regulatory modules of surface microhardness and electrical stimulation for synergistic regulation of cell behaviors. Reproduced with permission [140]. Copyright 2019, Elsevier; (ii) increased cell distribution and cell clustering and alignment. Reproduced with permission [141]. Copyright 2018, IOP Publishing. (E) Synergistic stimulation by external strain and electrical stimulation and its effect on cell behaviors: (i) Fabrication of multichannel bioscaffolds embedded with regulatory modules of electrical stimulation. The synergistic regulation is achieved by perfusing fluid into the channels and applying electrical stimulation. Reproduced with permission [142]. Copyright 2019, Elsevier. (ii) Significantly changed connexin43 expression under synergistic stimulation. Reproduced with permission [143]. Copyright 2015, Andrea Pavesi et al. UV, ultraviolet.
For synergetic stimulation by surface microhardness and external force or strain, many of the design and manufacturing processes are the same as those for synergetic stimulation by surface microhardness and surface topology, except for the additional consideration of external force or strain. Dan et al. [139] investigated the coordinate effects of cyclic stretch and substrate stiffness on endothelial cells and found that stimulation improves the integrity of cell monolayers and promotes monolayer recovery. Synergistic stimulation by surface microhardness and external fields has also been researched. Heo et al. [140] use UV-assisted 3D bioprinting to fabricate conductive hydrogel bioscaffolds (Fig. 9D–i) and determined surface microhardness by the degree of cross-linking. After seeding cells and applying electric fields, cells on bioscaffolds with different surface microhardness values were synergistically regulated. Imaninezhad et al. [141] investigated the same method. They found that cell distribution increases and cell clustering and alignment occurs (Fig. 9D–ii). Similarly, bioprinting inks loaded with conductive materials are used for coordinated regulation of electrical stimulation and external strain. Wang et al. [142] fabricated conductive composite multiple-channel bioscaffolds using inkjet 3D bioprinting with graphene-containing bioinks (Fig. 9E–i). Such regulation can be achieved after additional stimulation by external strain, as discussed before. Pavesi et al. [143] investigated this regulation of cell behaviors and found significantly change in the expression of a few genes, such as connexin43 (Fig. 9E–ii).
3.5.1.1. Discussion of pros and cons
Synergetic stimulation by biophysical stimulation cues directly regulates the shape of new tissue and affects its functions. Cell adhesion and cell growth directions are initially determined and guided by the bioscaffold surface topology. The cell distribution on bioscaffolds can be regulated by inducing cell migration through surface microhardness. Exerting stress and strain is a way to regulate cell proliferation, cell differentiation, and tissue formation. Because of parameterized implements and wireless real-time controllable operations, non-contact-dependent factors are widely involved in cell regulation, whether direct or indirect, based on transformation into other stimulation cues. 3D topology structures of bioscaffolds can be fabricated by 3D bioprinting. Surface microhardness can be determined by 3D bioprinting processes, such as cross-linking, or external loads, such as external-field-induced surface modification. Cell regulation by an external force is usually achieved by fluid flow, external strain is exerted by microchannel bioscaffolds with the help of fluids, and noncontact regulation can be achieved by applying an external field. It may be difficult to achieve synergetic stimulation by multiple biophysical stimulation cues using only one 3D bioprinting process or technology. Step-by-step preparation by combining multiple processes (extrusion, inkjet and UV) is a feasible approach to fabricating bioscaffolds embedded with regulatory modules. After integrating multiple biophysical stimulations, nearly the entire processes of cell growth and tissue formation can be regulated. Especially, synergetic stimulation by three or more biophysical stimulation cues for regulation of cell behaviors [144] injects new vitality into tissue engineering, although there are few reports on this. In the foreseeable future, multiple biophysical stimulations are expected to play a crucial role in personalized functional tissue reconstruction.
3.5.2. Stimulations by biophysical and biochemical cues
Cell behaviors can be regulated by combining at least two stimulation cues from biophysical and biophysical stimulations. Biochemical stimulations can be selected intuitively. On the basis of whether an external field is involved, research on coordinated regulations based on methodologies mainly focuses on two aspects. One aspect excluding biochemical stimulation includes surface topology, surface microhardness, and external stress or strain. The other excluding biochemical stimulation includes ultrasound, magnetic, electrical, and light stimulations. In addition, a few uncommon but important synergistic stimulations, such as indirect stimulus by conversing stimulation cues and cell co-culture, are discussed.
Song et al. [145] investigated surface topology and biochemical stimulation cues for cell behaviors. Bioscaffolds with macro-/microstructures were fabricated by 3D bioprinting, and a biochemical stimulation cue was introduced by chemical etching of HA. The authors observed a large area of bone regeneration under synergistic stimulation. Studies have also researched surface microhardness and biochemical stimulation cues. A simple method is to design a bioscaffold the same way as with surface microhardness regulation and then to introduce biochemical factors. Another important approach was developed by Shi et al. [146], who cross-linked alginate hydrogels with different concentrations during 3D bioprinting (Fig. 10A–i). Distler et al. [147] fabricated bioscaffolds with tunable stiffness based on ionical and enzymatical dual cross-linking. Synergistic stimulation increased NIH-3T3 and ATDC-5 cell attachment (Fig. 10A–ii). In addition, cells are subject to fluid shear in dynamic culture. Chen et al. [148] analyzed the stimulation by external strain and nerve growth factors. They fabricated cell-loaded bioscaffolds using extrusion-based 3D bioprinting and applied them to a dynamic tensile culture system (Fig. 10B–i). Synergetic stimulation promoted nerve cell proliferation and the formation of aligned, spindled, and stretched shapes (Fig. 10B–ii). Co-stimulation by external stress and biochemical stimulation can likewise be achieved by using a fluid as a biochemical stimulus or by incorporating biochemical stimulation cues into bioscaffolds.
Fig. 10.
Regulation of synergistic stimulation by biophysical and biochemical stimulation cues for cell behaviors. (A) Synergistic stimulation by surface microhardness and biochemical stimulation cues and its effect on cell behaviors: (i) schematic diagram of surface microhardness changed by cross-linking. Reproduced with permission [146]. Copyright 2017, John Wiley and Sons; (ii) increased NIH-3T3 and ATDC-5 cell attachment under synergetic stimulation. Reproduced with permission [147]. Copyright 2020, American Chemical Society. (B) Synergistic stimulation by external strain and biochemical stimulation cues and its effect on cell behaviors: (i) cell-loaded bioscaffolds applied to a dynamic tensile culture system and (ii) enhanced nerve cell proliferation and formation of aligned, spindled, and stretched shapes under synergetic stimulation. Reproduced with permission [148]. Copyright 2020, Chen et al. (C) Synergistic stimulation by ultrasound and biochemical stimulation cues and its effect on cell behaviors: (i) schematic diagram of the synergistic stimulation exposure setup and (ii) enhanced cell proliferation under synergetic stimulation. Reproduced with permission [149]. Copyright 2016, Aliabouzar et al. (D) Synergistic stimulation by electric field and biochemical stimulation cues and its effect on cell behaviors: (i) conductive CNT-incorporated bioscaffolds fabricated by UV-assisted 3D bioprinting and (ii) enhanced neural stem cell proliferation under synergetic stimulation. Reproduced with permission [151]. Copyright 2018, IOP Publishing. (E) Synergistic stimulation by photothermal effect and biochemical stimulation cues and its effect on tissue behaviors: (i) 3D-bioprinted composite bioscaffolds composed of 30CS and ROS and (ii) inhibited tumor tissue formation in vivo under synergetic stimulation. Reproduced with permission [153]. Copyright 2018, Ma et al. ADA, alginate; GEL 7.5%, 7.5% w/v gelatin; mTG, microbial transglutaminase; CNT, carbon nanotube; UV, ultraviolet; 30CS, Fe–CaSiO3; ROS, reactive oxygen species.
Noncontact factors are also usually involved. Non-invasive ultrasound and lipid-coated microbubbles (MBs) were analyzed by Aliabouzar et al. [149] (Fig. 10C–i). Cells were seeded on the 3D-bioprinted bioscaffolds. Coordinated treatment of ultrasound and MBs significantly promoted cell proliferation (Fig. 10C–ii). Chen et al. [150] investigated the effects of combined quetiapine and magnetic stimulation on cells. Synergetic stimulation increased brain-derived neurotrophic factor and protein kinase levels.
In addition to direct introduction, biochemical stimulation cues can also be integrated into bioscaffolds. Lee et al. [151] investigated electric field and biochemical stimulation cues. CNTs were added to a poly(ethylene glycol) diacrylate (PEGDA) hydrogel, and corresponding bioscaffolds were fabricated using UV-assisted 3D bioprinting (Fig. 10D–i). Synergetic stimulation promoted neural stem cell proliferation (Fig. 10D–ii) and improved neuronal differentiation and maturity. Studies have also investigated co-stimulation of light-responsive bioscaffolds. Imato et al. [152] fabricated bioscaffold materials by incorporating spiropyran into a photoresponsive polymer chain. Biochemical stimulation was adjusted by changing the spiropyran–polymer ratio. Proper UV irradiation and biochemical stimulation significantly inhibited cell adhesion. On the basis of light-responsive materials, this co-stimulation can also be exerted by introducing additional biochemical factors.
Ma et al. [153] investigated indirect stimulation by conversion of stimulation cues. They fabricated iron–calcium silicate composite bioscaffolds using 3D bioprinting (Fig. 10E–i) and analyzed the photothermal effect of iron particles and the catalytic effect of iron ions. Synergistic stimulation inhibited tumor cell growth (Fig. 10E–ii). Indirect stimulation can also be achieved by cell co-culture through cell secretion of biochemical stimulation cues [154]. Choi et al. [79] investigated the effect of fluid stress and cell co-culture. Smooth muscle cells were adhered to the inner surface of bioscaffold microchannels, and endothelial cells were perfused into the microchannels under different flow rates. Co-cultivation promoted endothelial cell–mediated vascularization.
3.5.2.1. Discussion of pros and cons
Compared to multiple biophysical stimulations, synergistic stimulation of biophysical and biochemical stimulation cues achieves more accurate simulation of microenvironments in vivo and diversified regulation of cell behaviors. Biophysical stimulations directly guide cell adhesion, migration, and oriented population and cell morphology. Biochemical stimulations significantly regulate protein secretion and gene expression and enhance cell and tissue functionalization. In addition to direct stimulation cues, there are two ways of regulation, indirect stimulus by conversion of stimulation cues and cell co-culture. The common conversion of stimulation cues involves piezoelectric, photoelectric, and photothermal effects. Conversion provides a means to integrate the positive points of each stimulation cue. In addition, the body's microenvironments are complex, and interactions between co-cultured cells lead to new ideas for regulating corresponding cell behaviors.
Recent studies have reported the effects of microsegment materials of bioscaffolds on cell behaviors [155]. This is synergistic regulation of cell behaviors by biophysical and biochemical stimulations. The chemical properties of microsegment materials provide biochemical stimulation cues, while topological structures provide biophysical stimulation cues. This is an interesting research direction because of the possibility of the intervals between materials promoting cell migration and growth. In addition, composite bioscaffolds are involved in this regulation.
3D Bioprinting technologies provide unique manufacturability for fabricating these bioscaffolds, whether embedded with biophysical or biochemical stimulation modules. Especially, extrusion-based 3D bioprinting is adapted for various materials. Inkjet and UV-assisted 3D bioprinting provide high resolution. Coordinated stimulations created by 3D-bioprinted bioscaffolds pave the way for engineering morphological and functional tissues, which is the ultimate direction of tissue engineering research. These approaches are of great importance and significance in clinical application.
3.5.3. Application prospects of synergetic stimulations
Although tissue engineering research has made significant progress, it is still limited to the simple fabrication of tissues and organs from a shape point of view. Functional tissue fabrication is still in its early stages. Cells in vivo are in complex microenvironments, and many stimulating factors simultaneously regulate the growth process. Synergetic stimulations can simulate cellular niches. In addition, there are many types of stimulation cues, contributing to numerous different synergetic regulation methods. Therefore, regulation of cell behaviors is of significant importance to ensure functional engineered tissues. However, research still focuses on single stimulation schemes. There are few reports on three or more stimulations. There are difficulties in researching the preparation process of biochemical reagents, the forming process of surface structures, the designs of external stress and strain bioscaffolds, and the build methods of non-contact-dependent biophysical stimulations.
3D bioprinting promotes high-precision fabrication of tissue engineering bioscaffolds, which provides a powerful tool for coordinated regulation of multiple stimulations. Application of the 3D-bioprinted bioscaffold technique to synergistically regulate cell growth and tissue formation is promising. First, compared to conventional approaches, bioscaffold-based regulation provides better spatial structure and growth microenvironments for cell propagation. In addition, the properties of engineering tissues, like fabrication, biomechanics, and biochemistries, regulated by 3D bioprinting approaches are better compared to conventional approaches. For example, blood vessels have a hierarchical organization, and blood cells can easily climb and grow on 3D-bioprinted bioscaffolds. In addition, the internal spatial structure and its functionalization, including the initial laminar structure of blood vessels and the tunica intima, media, and externa, can be controlled by 3D bioprinting processes and subsequent regulation methods. Second, some prerequisites are met. Especially, ultrasound and magnetic stimulation and biochemical stimulation cues have been widely applied in medicine. Bioreactors can provide regulation of cell behaviors. These stimulation cues and fluid-driven external stress can be easily achieved by nonprofessionals. Other complex stimulation cues, such as bioscaffold topology, require communication, and cooperation between engineering personnel and medical practitioners. Third, more and more regulatory measures for medical implant bioscaffolds, such as standardized management, are available. It is feasible to regulate cell behaviors in vivo. Although animal experiments are limited by ethical norms and may give inaccurate results in mice, rabbits, and pigs, it is still possible to investigate the conditions of the mechanisms regulating cell growth and tissue formation in vivo. After determining these mechanisms and using cell culture by bioscaffold-based regulation approaches, personalized functional artificial tissues can be fabricated. This is expected to transform bioscaffold-based regulation techniques to clinical productivity.
4. Outlook and conclusion
The advantages of different cell regulation methods promote the individualized and biomimetic regulation of cell behaviors by combining the knowledge of computer science, mechanics, materials science, 3D bioprinting, and biomedical engineering. In addition, the advantages and disadvantages of these methods facilitate the development of regulation methods for cell behaviors in vitro (Table 2).
Table 2.
Comparison of advantages and disadvantages of different regulation methods for cell behaviors.
| Methods | Mechanisms | Advantages | Disadvantages |
|---|---|---|---|
| Surface topography modifying | Optimizing the surface topography of microchannels at nano-scale | ||
| Surface coating | Introducing other favorable materials on the surface of microchannels | Considered biocompatibility and degradation characteristics of materials [164] | |
| External force applying | Changing the morphology of cells and stimulating secretion [165] | Improved proliferation, migration, differentiation and alignment of cells [166,167] |
|
| Strain applying | Influencing the direction of cell growth and stimulating secretion [169] | Easily-controlling, improved alignment and differentiation of cells [[170], [171], [172]] |
|
| Medium Concentration changing | Modulating the speed of cell differentiation and reprogramming [173] | Controllable concentration gradient | Complex preparation and regulation processes |
| Surface microhardness changing | Inducing cell migration [174] | Controllable patterning [175] and no chemical components introduced |
|
| Biochemical cues adding | Inducing cell differentiation and reprogramming [177] | Synergistic regulation of multiple chemical factors |
|
| Light irradiating | Inducing cell proliferation and migration [178] |
|
|
| Ultrasound exerting | Inducing cell proliferation and differentiation [181,182] | No contact on cells and no modify on scaffold surfaces |
|
| Electricity stimulating | Activating intracellular signaling pathways, and influencing microenvironments [184] | Precise cellular regulation by the combination of specific materials/structures and electricity stimulating [185,186] |
|
| Magnetic stimulating | Enhanced intracellular delivery and changed cell stiffness [187] | No contact on cells and controlled matrixes for targeting cells [188] |
|
4.1. Outlook
The complex structure of in vivo ECMs provides a good microenvironment for cell growth. Conventional manufacturing methods cannot accurately simulate cell microenvironments. This problem can be effectively solved by the development of new materials and processes of 3D bioprinting. Currently, the regulation of cell behaviors is not limited to biochemical reagents. Medium concentration and biophysical stimulation cues, such as external force, strain, and electric field, are emerging as regulation methods for cell behaviors. However, there are challenges in bioprinting and regulation processes, especially the fabrication of high-precision cell-loaded bioscaffolds and accurate regulation of cell growth. In addition, image technologies, such as μCT, may be used in bioscaffold design and tissue analysis.
4.1.1. 3D bioprinting technologies
Challenges in 3D bioprinting include bioink materials, bioprinting speed and resolution, and large-scale tissue preparation. Because of cellular requirements of biocompatibility, nutrients, and immunity, bioinks are limited to natural materials or acellular ECMs. Low-speed fabrication cannot meet the requirements of mass production. So far, high resolution bioprinting limited to 2D or simple 3D structures cannot achieve high-precision bioprinting of multilayered or complex large-scale tissues. In addition, the compatibility between the degradation characteristics of bioscaffolds and cell growth needs to be considered. To obtain biomimetic bioscaffolds, composite biomaterials and 3D bioprinting processes, including process parameters and forming accuracy, need to be further studied and optimized. On the basis of the principles of bioprinting, such as extrusion-based, inkjet, and UV-assisted 3D bioprinting, personalized bioprinting bioscaffolds should be fabricated to prepare target tissue structures in the future.
4.1.2. Cell regulation by bioscaffolds
With regard to regulation, regulation methods, and bioscaffold fabrication are the main difficulties. Regulation methods include biophysical and biochemical stimulations of cells. The fabrication of regulatory bioscaffolds involves interdisciplinary technologies, including computer-aided design and manufacturing (CAD/CAM), materials science, control science, chemistry, and biomedical engineering. Many factors regulate cell growth in vivo. Current regulations for cell growth focus on a single regulatory factor. Attention should be paid to synergistic regulation of cell behaviors by various stimulation cues. In addition, the match between the degradation performance of bioscaffolds and the tissue regeneration rate should be studied in the future.
4.1.3. Integration of bioscaffolds loaded with cells and regulation modules
Structures integrated with bioscaffold modules and regulatory modules can be applied to the research on the mechanism underlying regulation of cell growth and tissue formation. There are two ways to integrate modules:
•Bioscaffold modules and control modules are prepared separately and then integrated on the basis of Boolean operation. Compared to the challenges in preparing corresponding modules, the outstanding challenges are integrated methods, assembly accuracy, and introduction of pollution. 3D bioprinting technologies, conventional mold preparation and demolding technologies, Lego-like modular building, and assembly technologies may be combined to fabricate and reassemble bioscaffolds.
•Integrated 3D models with bioscaffold modules and control modules are directly fabricated. The structures are prepared by 3D bioprinting technologies, which have higher requirements for their preparation processes because of the increased complexity of print models.
4.1.4. Regulation of cell behaviors in vitro and in vivo
Due to material properties of bioscaffolds and rheological properties of the culture medium, there are differences between stimulations delivered to cells and externally applied stimulations. Therefore, it is necessary to evaluate the relationship between the actual stimulations acting on cells and corresponding cell behaviors. In addition, cell growth factor secretion, protein expression, related gene phenotypes, and immunity should be evaluated and quantified under single and synergetic stimulations. There are no clear test methods or standards for implanted bioscaffolds. Current studies are based on animal experiments, but animals and humans respond differently to bioscaffolds. On the basis of clarifying the mechanism underlying regulation of cell behaviors and the characteristics of bioscaffold degradation, bioscaffolds integrated with regulatory modules are expected to achieve remote regulation of in vivo cell growth. For large-scale application of bioscaffolds in a clinical setting, the evaluation criteria of implantable bioscaffolds should be investigated and the tissues prepared should be evaluated for clinical application.
4.2. Conclusion
To accurately simulate the growth microenvironments of cells and study the mechanism underlying cell growth and tissue formation, 3D bioprinting and regulation technologies have been applied to the fabrication of bioscaffolds embedded with regulatory modules. Tissue reconstruction has undergone three stages of development: (i) free cell growth on bioscaffolds, (ii) cell growth under the action of a single regulatory factor, and (iii) cell growth under the action of multiple regulatory factors. First, conventional bioscaffold fabrication is based on fabrication of bioscaffolds by 3D bioprinting and cell adhesion to grow into tissues without additional induction. However, the tissues prepared are usually quite different from requirements. To reconstruct satisfactory tissues and organs, single-cell regulatory factors are introduced. Finally, multiple cell regulatory factors are used in tissue preparation to reconstruct bionic tissues, and the mechanism underlying regulation of cell behaviors needs to be clarified and standardized. Bioscaffolds embedded with regulatory modules have shown potential in tissue reconstruction and regenerative medicine.
CRediT authorship contribution statement
Pengju Wang: Writing - original draft. Yazhou Sun: Supervision. Xiaoquan Shi: Giving advices about presentation of the manuscript. Huixing Shen: Searching for the references. Haohao Ning: Searching for the references. Haitao Liu: Giving advices about organization and presentation of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research is supported by the Innovative Public Service Center of High-End Manufacturing Technology for Technical Service of High-Tech Zone, Qiqihar, China.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Inohara T., Liang L., Kosinski A.S., Smith E.E., MPH, Schwamm L.H., Hernandez A.F., Bhatt D.L., Fonarow G.C., Peterson E.D., Xian Y. Recent myocardial infarction is associated with increased risk in older adults with acute ischemic stroke receiving thrombolytic therapy. J. Am. Heart Assoc. 2019;8(15) doi: 10.1161/JAHA.119.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenberger R., Contassot E., French L.E., Navarini A.A. Auto-inflammation and the skin. In: Wallach D., Vignon-Pennamen M.-D., Valerio Marzano A., editors. Neutrophilic Dermatoses. Springer International Publishing; Cham: 2018. pp. 301–318. [Google Scholar]
- 3.Tabib A., Leroux C., Mornex J.F., Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron. Artery. Dis. 2017;11(1):41–46. doi: 10.1097/00019501-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh H.Y., Chu C.W., Chiu M.H., Chu S.Y., Huang T.W., Tseng F.G. Gradient strain chip for stimulating cellular behaviors in cell-laden hydrogel. JoVE. 2017;126:53715. doi: 10.3791/53715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syedain Z.H., Graham M.L., Dunn T.B., O'Brien T., Johnson S.L., Schumacher R.J., Tranquillo R.T. A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons. Sci. Transl. Med. 2017;9(414) doi: 10.1126/scitranslmed.aan4209. [DOI] [PubMed] [Google Scholar]
- 6.Park K.M., Shin Y.M., Kim K., Shin H. Tissue engineering and regenerative medicine 2017: a year in review. Tissue Eng. B Rev. 2018;24(5):327–344. doi: 10.1089/ten.TEB.2018.0027. [DOI] [PubMed] [Google Scholar]
- 7.Xie M., Gao Q., Zhao H., Nie J., Fu Z., Wang H., Chen L., Shao L., Fu J., Chen Z., He Y. Electro-assisted bioprinting of low-concentration GelMA microdroplets. Small. 2019;15(4):1804216. doi: 10.1002/smll.201804216. [DOI] [PubMed] [Google Scholar]
- 8.Pahnke A., Conant G., Huyer L.D., Zhao Y., Radisic M. The role of Wnt regulation in heart development, cardiac repair and disease: a tissue engineering perspective. Biochem. Bioph. Res. Co. 2015;473(3):698–703. doi: 10.1016/j.bbrc.2015.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin H., Qiu X., Du Q., Li Q., Wang O., Akert L., Wang Z., Anderson D., Liu K., Gu L., Zhang C., Lei Y. Engineered microenvironment for manufacturing human pluripotent stem cell-derived vascular smooth muscle cells. Stem Cell Rep. 2019;12(1):84–97. doi: 10.1016/j.stemcr.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui H., Nowicki M., Fisher J.P., Zhang L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017;6(1):1601118. doi: 10.1002/adhm.201601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y., Xie M., Gao Q., Fu J. Why choose 3D bioprinting? Part I: a brief introduction of 3D bioprinting for the beginners. Bio-des. Manuf. 2019;2(4):221–224. [Google Scholar]
- 12.Habib A., Khoda B. Development of clay based novel hybrid bio-ink for 3D bio-printing process. J. Manuf. Process. 2019;38:76–87. doi: 10.1016/j.jmapro.2022.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cidonio G., Cooke M., Glinka M., Dawson J.I., Grover L., Oreffo R.O.C. Printing bone in a gel: using nanocomposite bioink to print functionalised bone scaffolds. Mater. Today Bio. 2019;4:100028. doi: 10.1016/j.mtbio.2019.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 2018;3(2):144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffanti G., Rezabeigi E., Li J., Murshed M., Nazhat S.N. Rapid biofabrication of printable dense collagen bioinks of tunable properties. Adv. Funct. Mater. 2020;30(4):1903874. [Google Scholar]
- 16.Nie M., Takeuchi S. Bottom-up biofabrication using microfluidic techniques. Biofabrication. 2018;10(4) doi: 10.1088/1758-5090/aadef9. [DOI] [PubMed] [Google Scholar]
- 17.Nie J., Gao Q., Xie C., Lv S., Qiu J., Liu Y., Guo M., Guo R., Fu J., He Y. Construction of multi-scale vascular chips and modelling of the interaction between tumours and blood vessels. Mater. Horiz. 2020;7:82–92. [Google Scholar]
- 18.Ahadian S., Khademhosseini A. A perspective on 3D bioprinting in tissue regeneration, bio-des. Man. 2018;1(3):157–160. doi: 10.1007/s42242-018-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P., Wang S. Computer-aided CT image processing and modeling method for tibia microstructure. Bio-des. Manuf. 2020;3(1):71–82. [Google Scholar]
- 20.Huling J., Ko I.K., Atala A., Yoo J.J. Fabrication of biomimetic vascular scaffolds for 3D tissue constructs using vascular corrosion casts. Acta Biomater. 2016;32:190–197. doi: 10.1016/j.actbio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Rodriguez P., Garcia-Triñanes P., López M.M.E., Santoveña A., Landin M. Mineralized alginate hydrogels using marine carbonates for bone tissue engineering applications. Carbohydr. Polym. 2018;195(1):237–241. doi: 10.1016/j.carbpol.2018.04.101. [DOI] [PubMed] [Google Scholar]
- 22.Wu S.W., Liu X., Miller A.L., Cheng Y.S., Lu L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohyd. Polymer. 2018;192:308–316. doi: 10.1016/j.carbpol.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenn S.L., Oldinski R.A. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J. Biomed. Mater. Res. B. 2016;104(6):1229–1236. doi: 10.1002/jbm.b.33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanasa I.A., Minuti A.E., Ivan F.D., Vasiliu S., Verestiuc L. 2017. Novel Natural-Synthetic Hydrogel Scaffolds with Applications in Skin Tissue Repair and Engineering, 2017 E-Health and Bioengineering Conference (EHB) pp. 709–712. Sinaia. [Google Scholar]
- 25.Samal S.K., Dash M., Dubruel P., Van Vlierberghe S. 8 - smart polymer hydrogels: properties, synthesis and applications. In: Aguilar M.R., San Román J., editors. Smart Polymers and Their Applications. Woodhead Publishing; 2014. pp. 237–270. [Google Scholar]
- 26.Xiao B., Yang W., Lei D., Huang J., Yin Y., Zhu Y., You Z., Wang F., Sun S. PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv. Healthc. Mater. 2019;8(5):1801455. doi: 10.1002/adhm.201801455. [DOI] [PubMed] [Google Scholar]
- 27.Sun L., Wang M., Chen S., Sun B., Guo Y., He C., Mo X., Zhu B., You Z. Molecularly engineered metal-based bioactive soft materials - neuroactive magnesium ion/polymer hybrids. Acta Biomater. 2019;85:310–319. doi: 10.1016/j.actbio.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Huang P., Bi X., Gao J., Sun L., Wang S., Chen S., Fan X., You Z., Wang Y. Phosphorylated poly(sebacoyl diglyceride) - a phosphate functionalized biodegradable polymer for bone tissue engineering. J. Mater. Chem. B. 2016;4(12):2090–2101. doi: 10.1039/c5tb02542g. [DOI] [PubMed] [Google Scholar]
- 29.Yu C., Ma X., Zhu W., Wang P., Miller K.L., Stupin J., Koroleva-Maharajh A., Hairabedian A., Chen S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–13. doi: 10.1016/j.biomaterials.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Synchronous 3D bioprinting of large-scale cell-laden constructs with nutrient networks. Adv. Healthc. Mater. 2019:1901142. doi: 10.1002/adhm.201901142. [DOI] [PubMed] [Google Scholar]
- 31.Hoque M.E., Meng T.T.H., Chuan Y.L., Chowdhury M., Prasad R.G.S.V. Fabrication and characterization of hybrid PCL/PEG 3D scaffolds for potential tissue engineering applications. Mater. Lett. 2014;131:255–258. [Google Scholar]
- 32.Diloksumpan P., de Ruijter M., Castilho M., Gbureck U., Vermonden T., van Weeren P.R., Malda J., Levato R. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab69d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faramarzi N., Yazdi I.K., Nabavinia M., Gemma A., Fanelli A., Caizzone A., Ptaszek L.M., Sinha I., Khademhosseini A., Ruskin J.N. Patient-specific bioinks for 3D bioprinting of tissue engineering scaffolds. Adv. Healthc. Mater. 2018;7(11):1701347. doi: 10.1002/adhm.201701347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei D., Yang Y., Liu Z., Yang B., Gong W., Chen S., Wang S., Sun L., Song B., Xuan H., Mo X., Sun B., Li S., Yang Q., Huang S., Chen S., Ma Y., Liu W., He C., Zhu B., Jeffries E.M., Qing F.-L., Ye X., Zhao Q., You Z. 3D printing of biomimetic vasculature for tissue regeneration. Mater. Horiz. 2019;6(6):1197–1206. [Google Scholar]
- 35.Yang Y., Lei D., Huang S., Yang Q., Song B., Guo Y., Shen A., Yuan Z., Li S., Qing F.L., Ye X., You Z., Zhao Q. Elastic 3D‐printed hybrid polymeric scaffold improves cardiac remodeling after myocardial infarction. Adv. Healthc. Mater. 2019;8(10):1900065. doi: 10.1002/adhm.201900065. [DOI] [PubMed] [Google Scholar]
- 36.Lei D., Yang Y., Liu Z., Chen S., Song B., Shen A., Yang B., Li S., Yuan Z., Qi Q., Sun L., Guo Y., Zuo H., Huang S., Yang Q., Mo X., He C., Zhu B., Jeffries E.M., Qing F.-L., Ye X., Zhao Q., You Z. A general strategy of 3D printing thermosets for diverse applications. Mater. Horiz. 2019;6(2):394–404. [Google Scholar]
- 37.Deepthi S., Jayakumar R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2017;3(2):194–200. doi: 10.1016/j.bioactmat.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norikazu K., Shuko S., Yukiteru O., Maiko T., Masaharu S., Takashi T., Noriyoshi S., Masahide Y., Yoshito I., Shigeki T. Effect of covering with cross-linked gelatin glue on tissue regeneration in a rat lung injury model. Interact. Cardiovasc. Thorac. Surg. 2019;29(1):1–7. doi: 10.1093/icvts/ivy297. [DOI] [PubMed] [Google Scholar]
- 39.Yin J., Yan M., Wang Y., Fu J., Suo H. 3D bioprinting of low concentration cell-laden gelatin methacrylate (GelMA) bioinks with two-step crosslinking strategy. Acs Appl. Mater. Inter. 2018;10(8):6849–6857. doi: 10.1021/acsami.7b16059. [DOI] [PubMed] [Google Scholar]
- 40.Xu W., Zhang Molino B., Cheng F., Molino P.J., Yue Z., Su D., Wang X., Willför S.M., Xu C., Wallace G.G. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing towards wound healing application. Acs Appl. Mater. Inter. 2019;11(9):8838–8848. doi: 10.1021/acsami.8b21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie M., Yu K., Sun Y., Shao L., Nie J., Gao Q., Qiu J., Fu J., Chen Z., He Y. Protocols of 3D bioprinting of gelatin methacryloyl hydrogel based bioinks. JoVE. 2019;154:60545. doi: 10.3791/60545. [DOI] [PubMed] [Google Scholar]
- 42.Qiu P., Li M., Chen K., Fang B., Chen P., Tang Z., Lin X., Fan S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials. 2020;227:119552. doi: 10.1016/j.biomaterials.2019.119552. [DOI] [PubMed] [Google Scholar]
- 43.Van Belleghem S., Torres L., Santoro M., Mahadik B., Wolfand A., Kofinas P., Fisher J.P. Hybrid 3D printing of synthetic and cell‐laden bioinks for shape retaining soft tissue grafts. Adv. Funct. Mater. 2019;30(3):1907145. doi: 10.1002/adfm.201907145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Synchronous 3D bioprinting of large-scale cell-laden constructs with nutrient networks. Adv. Healthc. Mater. 2019:1901142. doi: 10.1002/adhm.201901142. [DOI] [PubMed] [Google Scholar]
- 45.Lee J., Oh S.J., An S.H., Kim W.D., Kim S.H. Machine learning-based design strategy for 3D printable bioink: elastic modulus and yield stress determine printability. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab8707. [DOI] [PubMed] [Google Scholar]
- 46.You F., Wu X., Kelly M., Chen X. Bioprinting and in vitro characterization of alginate dialdehyde–gelatin hydrogel bio-ink. Bio-des. Manuf. 2020;3(1):48–59. [Google Scholar]
- 47.Gao Q., He Y., Fu J.Z., Liu A., Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Kankala R.K., Zhu K., Wang S.-B., Zhang Y.S., Chen A.-Z. Coaxial extrusion of tubular tissue constructs using a gelatin/GelMA blend bioink. ACS Biomater. Sci. Eng. 2019;5(10):5514–5524. doi: 10.1021/acsbiomaterials.9b00926. [DOI] [PubMed] [Google Scholar]
- 49.Cascone S., Lamberti G. Hydrogel-based commercial products for biomedical applications: a review. Int. J. Pharm. 2020;573:118803. doi: 10.1016/j.ijpharm.2019.118803. [DOI] [PubMed] [Google Scholar]
- 50.Skylar-Scott M.A., Mueller J., Visser C.W., Lewis J.A. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature. 2019;575(7782):330–335. doi: 10.1038/s41586-019-1736-8. [DOI] [PubMed] [Google Scholar]
- 51.Daly A.C., Riley L., Segura T., Burdick J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020;5:20–43. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grigoryan B., Paulsen S.J., Corbett D.C., Sazer D.W., Fortin C.L., Zaita A.J., Greenfield P.T., Calafat N.J., Gounley J.P., Ta A.H., Johansson F., Randles A., Rosenkrantz J.E., Louis-Rosenberg J.D., Galie P.A., Stevens K.R., Miller J.S. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y., Gu Z., Xie M., Fu J., Lin H. Why choose 3D bioprinting? Part II: methods and bioprinters. Bio-des. Manuf. 2020;3:1–4. [Google Scholar]
- 54.Bella C.D., Duchi S., O'Connell C.D., Blanchard R., Augustine C., Yue Z., Thompson F.W., Richards C., Beirne S.T., Onofrillo C. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. M. 2017;12(3):611–621. doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- 55.Wei S. 2018. Method and Apparatus for Computer-Aided Tissue Engineering for Modeling, Design, and Freeform Fabrication of Tissue Scaffolds, Constructs, and Devices. U.S. Patent. [Google Scholar]
- 56.Heinrich M.A., Bansal R., Lammers T., Zhang Y.S., Schiffelers R.M., Prakash J. 3D-Bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater. 2019;31(14):1806590. doi: 10.1002/adma.201806590. [DOI] [PubMed] [Google Scholar]
- 57.Ozbolat V., Dey M., Ayan B., Ozbolat I.T. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab10ae. [DOI] [PubMed] [Google Scholar]
- 58.Andrique L., Recher G., Alessandri K., Pujol N., Feyeux M., Bon P., Cognet L., Nassoy P., Bikfalvi A. A model of guided cell self-organization for rapid and spontaneous formation of functional vessels. Sci. Adv. 2019;5(6) doi: 10.1126/sciadv.aau6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCormack A., Highley C.B., Leslie N.R., Melchels F.P.W. 3D printing in suspension baths: keeping the promises of bioprinting afloat. Trends Biotechnol. 2020;38(6):584–593. doi: 10.1016/j.tibtech.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 60.Lv S., Nie J., Gao Q., Xie C., Zhou L.-Y., Qiu J., Fu J., Zhao X., He Y. Micro/nanofabrication of brittle hydrogels using 3D printed soft ultrafine fiber molds for damage-free demolding. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab57d8. [DOI] [PubMed] [Google Scholar]
- 61.Liashenko I., Rosell-Llompart J., Cabot A. Ultrafast 3D printing with submicrometer features using electrostatic jet deflection. Nat. Commun. 2020;11(1):753. doi: 10.1038/s41467-020-14557-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morss Clyne A., Swaminathan S., Diaz Lantada A. Biofabrication strategies for creating microvascular complexity. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z., Li P., Chu F., Shen G. Influence of the three-dimensional printing technique and printing layer thickness on model accuracy. J. Orofac. Orthop. 2019;80(4):1–11. doi: 10.1007/s00056-019-00180-y. [DOI] [PubMed] [Google Scholar]
- 64.Wadnap S., Krishnamoorthy S., Zhang Z., Xu C. Biofabrication of 3D cell-encapsulated tubular constructs using dynamic optical projection stereolithography. J. Mater. Sci. Mater. Med. 2019;30(3):36. doi: 10.1007/s10856-019-6239-5. [DOI] [PubMed] [Google Scholar]
- 65.Hong H., Seo Y.B., Kim D.Y., Lee J.S., Lee Y.J., Lee H., Ajiteru O., Sultan M.T., Lee O.J., Kim S.H., Park C.H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- 66.Nahm D., Weigl F., Schaefer N., Sancho A., Frank A., Groll J., Villmann C., Schmidt H.-W., Dalton P.D., Luxenhofer R. A versatile biomaterial ink platform for the melt electrowriting of chemically-crosslinked hydrogels. Mater. Horiz. 2020;7(3):928–933. [Google Scholar]
- 67.Lei D., Luo B., Guo Y., Wang D., Yang H., Wang S., Xuan H., Shen A., Zhang Y., Liu Z., He C., Qing F.-L., Xu Y., Zhou G., You Z. 4-Axis printing microfibrous tubular scaffold and tracheal cartilage application. Sci. China Mater. 2019;62(12):1910–1920. [Google Scholar]
- 68.de Ruijter M., Ribeiro A., Dokter I., Castilho M., Malda J. Simultaneous micropatterning of fibrous meshes and bioinks for the fabrication of living tissue constructs. Adv. Healthc. Mater. 2019;8(7):1800418. doi: 10.1002/adhm.201800418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ovsianikov A., Yoo J., Mironov V. Springer International Publishing; 2018. 3D Printing and Biofabrication. [Google Scholar]
- 70.De Bartolo L. Cell adhesion. In: Drioli E., Giorno L., editors. Encyclopedia of Membranes. Springer Berlin Heidelberg; Berlin, Heidelberg: 2015. pp. 1–2. [Google Scholar]
- 71.Mccarthy J.B., Vachhani B., Iida J. Cell adhesion to collagenous matrices. J. Pept. Sci. 2015;40(4):371–381. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C371::AID-BIP3%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 72.Bruner H.C., Derksen P.W.B. Loss of E-cadherin-dependent cell–cell adhesion and the development and progression of cancer. Cold Spring Harb. Perspect. Biol. 2017;10(3):a029330. doi: 10.1101/cshperspect.a029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruth D., Daniela I., A J.L., O W.J., Katie S., R R.C., Lang D., A J.S., S W.A., Ernest C. O41 Serum vascular cell adhesion molecule 1 levels are associated with vascular dysfunction and increased cardiovascular risk in an animal model and patients with rheumatoid arthritis. Rheumatology. 2016;55(suppl_1):i54. [Google Scholar]
- 74.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Biofabrication; 2020. Directly Coaxial 3D Bioprinting of Large-Scale Vascularized Tissue Constructs. [DOI] [PubMed] [Google Scholar]
- 75.Chi J., Zhang X., Chen C., Shao C., Zhao Y., Wang Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020;5(2):253–259. doi: 10.1016/j.bioactmat.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen S., Matsumoto H., Moro-oka Y., Tanaka M., Miyahara Y., Suganami T., Matsumoto A. Microneedle-Array patch fabricated with enzyme-free polymeric components capable of on-demand insulin delivery. Adv. Funct. Mater. 2019;29(7):1807369. [Google Scholar]
- 77.Seonwoo H., Bae W.G., Park S., Kim H.N., Chung J.H. Hierarchically micro- and nanopatterned topographical cues for modulation of cellular structure and function. IEEE T. Nanobiosci. 2016;15(8):835–842. doi: 10.1109/TNB.2016.2631641. [DOI] [PubMed] [Google Scholar]
- 78.Xie C., Gao Q., Wang P., Shao L., Yuan H., Fu J., Chen W., He Y. Structure-induced cell growth by 3D printing of heterogeneous scaffolds with ultrafine fibers. Mater. Des. 2019;181(5):108092. [Google Scholar]
- 79.Choi J.S., Seo T.S. Orthogonal co-cultivation of smooth muscle cell and endothelial cell layers to construct in vivo-like vasculature. Biomicrofluidics. 2019;13(1) doi: 10.1063/1.5068689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roman H.N., Juncker D., Lauzon A.M. A microfluidic chamber to study the dynamics of muscle-contraction-specific molecular interactions. Anal. Chem. 2015;87(5):2582–2587. doi: 10.1021/ac503963r. [DOI] [PubMed] [Google Scholar]
- 81.Gao Q., Zhao P., Zhou R., Wang P., Fu J., He Y. Rapid assembling organ prototypes with controllable cell-laden multi-scale sheets. Bio-des. Man. 2019;2(1):1–9. [Google Scholar]
- 82.Hong C.A., Son H.Y., Nam Y.S. Layer-by-layer siRNA/poly(L-lysine) multilayers on polydopamine-coated surface for efficient cell adhesion and gene silencing. Sci. Rep.-UK. 2018;8(1):7738. doi: 10.1038/s41598-018-25655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He Y., Li Y., Chen G., Wei C., Zhang X., Zeng B., Yi C., Wang C., Yu D. Concentration-dependent cellular behavior and osteogenic differentiation effect induced in bone marrow mesenchymal stem cells treated with magnetic graphene oxide. J. Biomed. Mater. Res. 2020;108(1):50–60. doi: 10.1002/jbm.a.36791. [DOI] [PubMed] [Google Scholar]
- 84.Truskina J., Vernoux T. The growth of a stable stationary structure: coordinating cell behavior and patterning at the shoot apical meristem. Curr. Opin. Plant Biol. 2018;41:83–88. doi: 10.1016/j.pbi.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Cortese B., Gigli G., Riehle M. Mechanical gradient cues for guided cell motility and control of cell behavior on uniform substrates. Adv. Funct. Mater. 2009;19(18):2961–2968. [Google Scholar]
- 86.Jeon S., Subbiah R., Bonaedy T., Van S., Park K., Yun K. Surface functionalized magnetic nanoparticles shift cell behavior with on/off magnetic fields. J. Cell. Physiol. 2018;233(2):1168–1178. doi: 10.1002/jcp.25980. [DOI] [PubMed] [Google Scholar]
- 87.Ramana Ramya J., Thanigai Arul K., Sathiamurthi P., Asokan K., Rajmuhon Singh N., Narayana Kalkura S. Enhanced magnetic behaviour and cell proliferation of gamma irradiated dual metal ions co-doped hydroxyapatite – poly (methyl methacrylate) composite films. React. Funct. Polym. 2018;123:34–43. [Google Scholar]
- 88.Song K.H., Heo S.J., Peredo A.P., Davidson M.D., Mauck R.L., Burdick J.A. Influence of fiber stiffness on meniscal cell migration into dense fibrous networks. Adv. Healthc. Mater. 2020;9:1901228. doi: 10.1002/adhm.201901228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xue D., Zhang J., Wang Y., Mei D. Digital light processing-based 3D printing of cell-seeding hydrogel scaffolds with regionally varied stiffness. ACS Biomater. Sci. Eng. 2019;5(9):4825–4833. doi: 10.1021/acsbiomaterials.9b00696. [DOI] [PubMed] [Google Scholar]
- 90.Idaszek J., Costantini M., Karlsen T.A., Jaroszewicz J., Colosi C., Testa S., Fornetti E., Bernardini S., Seta M., Kasarello K., Wrzesien R., Cannata S., Barbetta A., Gargioli C., Brinchman J.E., Swieszkowski W. 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication. 2019;11(4) doi: 10.1088/1758-5090/ab2622. [DOI] [PubMed] [Google Scholar]
- 91.Bastola A.K., Hoang V.T., Li L. A novel hybrid magnetorheological elastomer developed by 3D printing. Mater. Des. 2017;114:391–397. [Google Scholar]
- 92.Giachini P.A.G.S., Gupta S.S., Wang W., Wood D., Yunusa M., Baharlou E., Sitti M., Menges A. Additive manufacturing of cellulose-based materials with continuous, multidirectional stiffness gradients. Sci. Adv. 2020;6(8) doi: 10.1126/sciadv.aay0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y., Lang J., Ye Z., Wang M., Yang Y., Guo X., Zhuang J., Zhang J., Xu F., Li F. Effect of substrate stiffness on redox state of single cardiomyocyte: a scanning electrochemical microscopy study. Anal. Chem. 2020;92(7):4771–4779. doi: 10.1021/acs.analchem.9b03178. [DOI] [PubMed] [Google Scholar]
- 94.Ballester-Beltran J., Biggs M.J.P., Dalby M.J., Salmeron-Sanchez M., Leal-Egana A. Sensing the difference: the influence of anisotropic cues on cell behavior. Front. Mater. 2015;2:39. [Google Scholar]
- 95.Naskar S., Kumaran V., Basu B. Reprogramming the stem cell behavior by shear stress and electric field stimulation: lab-on-a-chip based biomicrofluidics in regenerative medicine. Regen. Eng. Transl. Med. 2019;5(2):99–127. [Google Scholar]
- 96.Armistead F.J., Gala De Pablo J., Gadelha H., Peyman S.A., Evans S.D. Cells under stress: an inertial-shear microfluidic determination of cell behavior. Biophys. J. 2019;116(6):1127–1135. doi: 10.1016/j.bpj.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sophie C.L., Hubert J.R., Michel G., Pelling A.E. Cellular orientation is guided by strain gradients. Integr. Biol.-UK. 2017;9(7):607–618. doi: 10.1039/c7ib00019g. [DOI] [PubMed] [Google Scholar]
- 98.Kowsari-Esfahan R., Jahanbakhsh A., Saidi M.S., Bonakdar S. A microfabricated platform for the study of chondrogenesis under different compressive loads. J. Mech. Behav. Biomed. Mater. 2017;78:404–413. doi: 10.1016/j.jmbbm.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y.C., Liou Y.D., Wu K.H., Wang C.K., Chu Y.H. Study of strain-modulated effects on CoFe2O4 epitaxial films. Aps Meeting APS Meeting Abstracts. 2017:62. [Google Scholar]
- 100.Trachtenberg J.E., Santoro M., Williams C., Piard C.M., Smith B.T., Placone J.K., Menegaz B.A., Molina E.R., Lamhamedi-Cherradi S.-E., Ludwig J.A., Sikavitsas V.I., Fisher J.P., Mikos A.G. Effects of shear stress gradients on ewing sarcoma cells using 3D printed scaffolds and flow perfusion. ACS Biomater. Sci. Eng. 2017;4(2):347–356. doi: 10.1021/acsbiomaterials.6b00641. [DOI] [PubMed] [Google Scholar]
- 101.Molladavoodi S., Robichaud M., Wulff D., Gorbet M. Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0178981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lei X., Wu H., Song Y., Liu B., Zhang S.S., Li J.Q., Bi L., Pei G.X. Effects of cyclic fluid stress at different frequencies on behaviors of cells incubated on titanium alloy. Biochem. Biophys. Res. Commun. 2019;522(1):100–106. doi: 10.1016/j.bbrc.2019.11.070. [DOI] [PubMed] [Google Scholar]
- 103.Park S.H., Park S.A., Kang Y.G., Shin J.W., Park Y.S., Gu S.R., Wu Y.R., Wei J., Shin J.W. PCL/beta-TCP composite scaffolds exhibit positive osteogenic differentiation with mechanical stimulation. Tissue Eng. Regen. Med. 2017;14(4):349–358. doi: 10.1007/s13770-017-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim Y.T., Bohjanen S., Bhattacharjee N., Folch A. Partitioning of hydrogels in 3D-printed microchannels. Lab Chip. 2019;19(18):3086–3093. doi: 10.1039/c9lc00535h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Engeland N.C.A., Pollet A., den Toonder J.M.J., Bouten C.V.C., Stassen O., Sahlgren C.M. A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab Chip. 2018;18(11):1607–1620. doi: 10.1039/c8lc00286j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saha N., Monge C., Boudou T., Picart C., Glinel K. In: Photocrosslinked Polyelectrolyte Films of ControlledStiffness to Direct Cell Behavior, Layer‐by‐Layer Films for Biomedical Applications. Picart C., Caruso F., Voegel J.‐C., editors. 2015. pp. 45–64. [Google Scholar]
- 107.Grygotis E., Dalecki D., Hocking D.C. Ultrasound exposure during collagen polymerization produces pro-migratory fiber structures. J. Acoust. Soc. Am. 2017;141(5):4014. [Google Scholar]
- 108.Zhou Z., Yu P., Zhou L., Tu L., Tan G. Polypyrrole nanocones and dynamic piezoelectric stimulation-induced stem cell osteogenic differentiation. ACS Biomater. Sci. Eng. 2019;5(9):4386–4392. doi: 10.1021/acsbiomaterials.9b00812. [DOI] [PubMed] [Google Scholar]
- 109.Poller W., Löwa N., Wiekhorst F., Taupitz M., Wagner S., Möller K., Baumann G., Stangl V., Trahms L., Ludwig A. Magnetic particle spectroscopy reveals dynamic changes in the magnetic behavior of very small superparamagnetic iron oxide nanoparticles during cellular uptake and enables determination of cell-labeling efficacy. J. Biomed. Nanotechnol. 2016;12(2):337–346. doi: 10.1166/jbn.2016.2204. [DOI] [PubMed] [Google Scholar]
- 110.Zhu W., George J.K., Sorger V.J., Grace Zhang L. 3D printing scaffold coupled with low level light therapy for neural tissue regeneration. Biofabrication. 2017;9(2) doi: 10.1088/1758-5090/aa6999. [DOI] [PubMed] [Google Scholar]
- 111.Osborn J., Aliabouzar M., Zhou X., Rao R., Zhang L.G., Sarkar K. Enhanced osteogenic differentiation of human mesenchymal stem cells using microbubbles and low intensity pulsed ultrasound on 3D printed scaffolds. Adv. Biosyst. 2019;3(2):1800257. doi: 10.1002/adbi.201800257. [DOI] [PubMed] [Google Scholar]
- 112.An Y., Song Y., Wang Z., Wang J., Wu G., Zhu G., Chen L. Effect of low-intensity pulsed ultrasound on the biological behaviors of bone marrow mesenchymal stem cells on titanium with different surface topographies. Am. J. Transl. Res. 2018;10(1):67–76. [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X., George M.N., Park S., Miller A.L., Ii, Gaihre B., Li L., Waletzki B.E., Terzic A., Yaszemski M.J., Lu L. 3D-printed scaffolds with carbon nanotubes for bone tissue engineering: fast and homogeneous one-step functionalization. Acta Biomater. 2020;111:129–140. doi: 10.1016/j.actbio.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen J., Li W., Zhou L., Zhou Z., Tan G., Chen D., Wang R., Yu P., Ning C. A built-in electric field with nanoscale distinction for cell behavior regulation. J. Mater. Chem. B. 2018;6(18):2723–2727. doi: 10.1039/c8tb00063h. [DOI] [PubMed] [Google Scholar]
- 115.Robertson S.N., Campsie P., Childs P.G., Madsen F., Donnelly H., Henriquez F.L., Mackay W.G., Salmeron-Sanchez M., Tsimbouri M.P., Williams C., Dalby M.J., Reid S. Control of cell behaviour through nanovibrational stimulation: nanokicking. Philos. Trans. A Math. Phys. Eng. Sci. 2018;376(2120):20170290. doi: 10.1098/rsta.2017.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin H.Y., Huang H.Y., Shiue S.J., Cheng J.K. Osteogenic effects of inductive coupling magnetism from magnetic 3D printed hydrogel scaffold. J. Magn. Magn Mater. 2020;504:166680. [Google Scholar]
- 117.Rubin D., Anderton N., Smalberger C., Polliack J., Nathan M., Postema M. On the behaviour of living cells under the influence of ultrasound. Fluid. 2018;3(4):3040082. [Google Scholar]
- 118.Piech D.K., Johnson B.C., Shen K., Ghanbari M.M., Li K.Y., Neely R.M., Kay J.E., Carmena J.M., Maharbiz M.M., Muller R. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 2020;4(2):207–222. doi: 10.1038/s41551-020-0518-9. [DOI] [PubMed] [Google Scholar]
- 119.Choi B., Kim D., Han I., Lee S.-H. Microenvironmental regulation of stem cell behavior through biochemical and biophysical stimulation. Adv. Exp. Med. Biol. 2018;1064:147–160. doi: 10.1007/978-981-13-0445-3_9. [DOI] [PubMed] [Google Scholar]
- 120.Shahrousvand M., Hoseinian M.S., Ghollasi M., Karbalaeimahdi A., Salimi A., Tabar F.A. Flexible magnetic polyurethane/Fe2O3 nanoparticles as organic-inorganic nanocomposites for biomedical applications: properties and cell behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;74:556–567. doi: 10.1016/j.msec.2016.12.117. [DOI] [PubMed] [Google Scholar]
- 121.Song D.G., Lee G.H., Nam S.H., Cheong J.G., Jeong D., Lee S.J., Pan C.H., Jung J.W., Kim H.J., Ryu J., Kim J.E., Kim S., Cho C.Y., Kang M.K., Lee K.M., Lee J.W. TM4SF5 promotes metastatic behavior of cells in 3D extracellular matrix gels by reducing dependency on environmental cues. Oncotarget. 2017;8(48):83480–83494. doi: 10.18632/oncotarget.17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poukkula M., Cliffe A., Changede R., Rorth P. Cell behaviors regulated by guidance cues in collective migration of border cells. J. Cell Biol. 2011;192(3):513–524. doi: 10.1083/jcb.201010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kornienko J.S., Smirnova I.S., Pugovkina N.A., Ivanova J.S., Shilina M.A., Grinchuk T.M., Shatrova A.N., Aksenov N.D., Zenin V.V., Nikolsky N.N., Lyublinskaya O.G. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci. Rep.-UK. 2019;9(1):1296. doi: 10.1038/s41598-018-37972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Y.F., Zhao J.Y., Hu W.Z., Ma K., Chao Y., Sun P.-J., Fu X.-B., Zhang H. Synthetic poly(vinyl alcohol)–chitosan as a new type of highly efficient hemostatic sponge with blood-triggered swelling and high biocompatibility. J. Mater. Chem. B. 2019;7(11):1855–1866. doi: 10.1039/c8tb03181a. [DOI] [PubMed] [Google Scholar]
- 125.Hohmann T., Feese K., Greither T., Ghadban C., Jäger V., Dehghani F., Grabiec U. Synthetic cannabinoids influence the invasion of glioblastoma cell lines in a cell- and receptor-dependent manner. Cancers. 2019;11(2):161. doi: 10.3390/cancers11020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao F., Xie W., Zhang W., Fu X., Gao W., Lei B., Chen X. 3D printing nanoscale bioactive glass scaffolds enhance osteoblast migration and extramembranous osteogenesis through stimulating immunomodulation. Adv. Healthc. Mater. 2018;7(16):1800361. doi: 10.1002/adhm.201800361. [DOI] [PubMed] [Google Scholar]
- 127.Liu B., Li J., Lei X., Cheng P., Song Y., Gao Y., Hu J., Wang C., Zhang S., Li D., Wu H., Sang H., Bi L., Pei G. 3D-bioprinted functional and biomimetic hydrogel scaffolds incorporated with nanosilicates to promote bone healing in rat calvarial defect model. Mater. Sci. Eng. C. 2020;112:110905. doi: 10.1016/j.msec.2020.110905. [DOI] [PubMed] [Google Scholar]
- 128.Liu D., Nie W., Li D., Wang W., Zheng L., Zhang J., Zhang J., Peng C., Mo X., He C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019;362:269–279. [Google Scholar]
- 129.Paolini A., Leoni L., Giannicchi I., Abbaszadeh Z., D'Oria V., Mura F., Dalla Cort A., Masotti A. MicroRNAs delivery into human cells grown on 3D-printed PLA scaffolds coated with a novel fluorescent PAMAM dendrimer for biomedical applications. Sci. Rep.-UK. 2018;8(1):13888. doi: 10.1038/s41598-018-32258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dang H.P., Shabab T., Shafiee A., Peiffer Q.C., Fox K., Tran N., Dargaville T.R., Hutmacher D.W., Tran P.A. 3D printed dual macro-, microscale porous network as a tissue engineering scaffold with drug delivering function. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab14ff. [DOI] [PubMed] [Google Scholar]
- 131.Yu J., Wang J., Zhang Y., Chen G., Mao W., Ye Y., Kahkoska A.R., Buse J.B., Langer R., Gu Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020;4(5):499–506. doi: 10.1038/s41551-019-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Derakhshandeh H., Aghabaglou F., McCarthy A., Mostafavi A., Wiseman C., Bonick Z., Ghanavati I., Harris S., Kreikemeier‐Bower C., Moosavi Basri S.M., Rosenbohm J., Yang R., Mostafalu P., Orgill D., Tamayol A. A wirelessly controlled smart bandage with 3D‐printed miniaturized needle arrays. Adv. Funct. Mater. 2020;30:1905544. doi: 10.1002/adfm.201905544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Duinen V., Zhu D., Ramakers C., van Zonneveld A.J., Vulto P., Hankemeier T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis. 2019;22(1):157–165. doi: 10.1007/s10456-018-9647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nie J., Gao Q., Qiu J.J., Sun M., Liu A., Shao L., Fu J.Z., Zhao P., He Y. 3D printed Lego((R))-like modular microfluidic devices based on capillary driving. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aaadd3. [DOI] [PubMed] [Google Scholar]
- 135.Panadero J.A., Lanceros-Mendez S., Ribelles J.L.G. Differentiation of mesenchymal stem cells for cartilage tissue engineering: individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016;33:1–12. doi: 10.1016/j.actbio.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 136.Matsugaki A., Matsuzaka T., Murakami A., Wang P., Nakano T. 3D printing of anisotropic bone-mimetic structure with controlled fluid flow stimuli for osteocytes: flow orientation determines the elongation of dendrites. Int. J. Bioprint. 2020;6(4):293. doi: 10.18063/ijb.v6i4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aliabouzar M., Lee S.-j., Zhou X., Zhang G.L., Sarkar K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol. Bioeng. 2018;115(2):495–506. doi: 10.1002/bit.26480. [DOI] [PubMed] [Google Scholar]
- 138.Xu C., Zhang Molino B., Wang X., Cheng F., Xu W., Molino P., Bacher M., Su D., Rosenau T., Willför S., Wallace G. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B. 2018;6(43):7066–7075. doi: 10.1039/c8tb01757c. [DOI] [PubMed] [Google Scholar]
- 139.Dan A., Huang R.B., Leckband D.E. Dynamic imaging reveals coordinate effects of cyclic stretch and substrate stiffness on endothelial integrity. Ann. Biomed. Eng. 2016;44(12):3655–3667. doi: 10.1007/s10439-016-1677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heo D.N., Lee S.J., Timsina R., Qiu X., Castro N.J., Zhang L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C. 2019;99:582–590. doi: 10.1016/j.msec.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 141.Imaninezhad M., Pemberton K., Xu F., Kalinowski K., Bera R., Zustiak S.P. Directed and enhanced neurite outgrowth following exogenous electrical stimulation on carbon nanotube-hydrogel composites. J. Neural. Eng. 2018;15(5) doi: 10.1088/1741-2552/aad65b. [DOI] [PubMed] [Google Scholar]
- 142.Wang B., Chen X., Ahmad Z., Huang J., Chang M.-W. 3D electrohydrodynamic printing of highly aligned dual-core graphene composite matrices. Carbon. 2019;153:285–297. [Google Scholar]
- 143.Pavesi A., Adriani G., Rasponi M., Zervantonakis I.K., Fiore G.B., Kamm R.D. Controlled electromechanical cell stimulation on-a-chip. Sci. Rep.-UK. 2015;5:11800. doi: 10.1038/srep11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Z., Jorgensen M.L., Wang Z., Amagat J., Wang Y., Li Q., Dong M., Chen M. 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration. Biomaterials. 2020;253:120108. doi: 10.1016/j.biomaterials.2020.120108. [DOI] [PubMed] [Google Scholar]
- 145.Song P., Hu C., Pei X., Sun J., Sun H., Wu L., Jiang Q., Fan H., Yang B., Zhou C. Dual modulation on crystallinity and macro/micro structures of 3D printed porous titanium implants to enhance the stability and osseointegration. J. Mater. Chem. B. 2019;7(4):2865–2877. doi: 10.1039/c9tb00093c. [DOI] [PubMed] [Google Scholar]
- 146.Shi P., Laude A., Yeong W.Y. Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness. J. Biomed. Mater. Res. 2017;105(4):1009–1018. doi: 10.1002/jbm.a.35971. [DOI] [PubMed] [Google Scholar]
- 147.Distler T., McDonald K., Heid S., Karakaya E., Detsch R., Boccaccini A.R. Ionically and enzymatically dual cross-linked oxidized alginate gelatin hydrogels with tunable stiffness and degradation behavior for tissue engineering. ACS Biomater. Sci. Eng. 2020;6(7):3899–3914. doi: 10.1021/acsbiomaterials.0c00677. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y.W., Wang K., Ho C.C., Kao C.T., Ng H.Y., Shie M.Y. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020;195:108982. [Google Scholar]
- 149.Aliabouzar M., Zhang L.G., Sarkar K. Lipid coated microbubbles and low intensity pulsed ultrasound enhance chondrogenesis of human mesenchymal stem cells in 3D printed scaffolds. Sci. Rep.-UK. 2016;6(1):37728. doi: 10.1038/srep37728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y.H., Zhang R.G., Xue F., Wang H.N., Chen Y.C., Hu G.T., Peng Y., Peng Z.W., Tan Q.R. Quetiapine and repetitive transcranial magnetic stimulation ameliorate depression-like behaviors and up-regulate the proliferation of hippocampal-derived neural stem cells in a rat model of depression: the involvement of the BDNF/ERK signal pathway. Pharmacol., Biochem. Behav. 2015;136:39–46. doi: 10.1016/j.pbb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 151.Lee S.J., Zhu W., Nowicki M., Lee G., Heo D.N., Kim J., Zuo Y.Y., Zhang L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural. Eng. 2018;15(1) doi: 10.1088/1741-2552/aa95a5. [DOI] [PubMed] [Google Scholar]
- 152.Imato K., Nagata K., Watanabe R., Takeda N. Cell adhesion control by photoinduced LCST shift of PNIPAAm-based brush scaffolds. J. Mater. Chem. B. 2020;8(12):2393–2399. doi: 10.1039/c9tb02958c. [DOI] [PubMed] [Google Scholar]
- 153.Ma H., Li T., Huan Z., Zhang M., Yang Z., Wang J., Chang J., Wu C. 3D printing of high-strength bioscaffolds for the synergistic treatment of bone cancer. NPG Asia Mater. 2018;10:31–44. [Google Scholar]
- 154.Järvinen P., Bonabi A., Jokinen V., Sikanen T. Simultaneous culturing of cell monolayers and spheroids on a single microfluidic device for bridging the gap between 2D and 3D cell assays in drug research. Adv. Funct. Mater. 2020;30(19):2000479. [Google Scholar]
- 155.Liu H., Kitano S., Irie S., Levato R., Matsusaki M. Collagen microfibers induce blood capillary orientation and open vascular lumen. Adv. Biosyst. 2020;4(5):2000038. doi: 10.1002/adbi.202000038. [DOI] [PubMed] [Google Scholar]
- 156.Pennacchio F.A., Caliendo F., Iaccarino G., Langella A., Santoro F. 3D-patterned scaffolds modulate the biointerface at the nanoscale. Nano Lett. 2019;19(8):5118–5123. doi: 10.1021/acs.nanolett.9b01468. [DOI] [PubMed] [Google Scholar]
- 157.Kihara H., Silva J.D., Kim D.M., Nagai M., Nojiri T., Nagai S., Chen C.-Y., Lee C., Hatakeyama W., Kondo H. Epithelial cell adhesion efficacy of a novel peptide identified by panning on a smooth titanium surface. Int. J. Oral Sci. 2018;(3):174–181. doi: 10.1038/s41368-018-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen X.L., Chen Q.H., Zhuang Y., Yan T.T. KGM/Gelatin/Nano HAP scaffolds for tissue engineering of intervertebral disc annulus fibrosus. J. Inorg. Mater. 2018;33(1):60–66. [Google Scholar]
- 159.Gupta S.K., Mishra N.C., Dhasmana A. Decellularization methods for scaffold fabrication. In: Turksen K., editor. Decellularized Scaffolds and Organogenesis: Methods and Protocols. Springer New York; New York, NY: 2018. pp. 1–10. [Google Scholar]
- 160.Xu X., Zhou J., Jiang Y., Zhang Q., Liu D. 3D printing process of oxidized nanocellulose and gelatin scaffold. J. Biomater. Sci. Polym. Ed. 2018;29(12):1–24. doi: 10.1080/09205063.2018.1472450. [DOI] [PubMed] [Google Scholar]
- 161.Gao L., Kupfer M.E., Jung J.P., Yang L., Zhang J. Myocardial tissue engineering with cells derived from human induced-pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ. Res. 2017;120(8):1318–1325. doi: 10.1161/CIRCRESAHA.116.310277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu Q., Miyamoto S., Lam K.S. A novel approach to chemical microarray using ketone-modified macromolecular scaffolds: application in micro cell-adhesion assay. Mol. Divers. 2004;8(3):301–310. doi: 10.1023/b:modi.0000036238.42200.3d. [DOI] [PubMed] [Google Scholar]
- 163.Griffin M. University College London; 2016. Development of a Nanocomposite Polymer for Craniofacial Reconstruction. [Google Scholar]
- 164.Lin X., Wang W., Wen J.Z., Zhang Z.Y., Wei L. Hyaluronic acid coating enhances biocompatibility of nonwoven PGA scaffold and cartilage formation. Tissue Eng. C Methods. 2017;23(2):86–97. doi: 10.1089/ten.TEC.2016.0373. [DOI] [PubMed] [Google Scholar]
- 165.Santoro M. Rice University; Santoro, Marco: 2016. Development of a high-throughput 3D tumor model for bone sarcomas. Diss. [Google Scholar]
- 166.Naskar S., Kumaran V., Basu B. Reprogramming the stem cell behavior by shear stress and electric field stimulation: lab-on-a-chip based biomicrofluidics in regenerative medicine, regenerative medicine. Regen. Eng. Transl. Med. 2019;5(2):99–127. [Google Scholar]
- 167.Bilgen B., Odabas S. John Wiley & Sons, Inc.; 2016. Effects of Mechanotransduction on Stem Cell Behavior, Advanced Surfaces for Stem Cell Research; pp. 45–65. [Google Scholar]
- 168.Vieira D., Mata F., Moita A., Moreira A. Vol. 1. BIODEVICES; Porto, Portugal: 2017. Microfluidic prototype of a lab-on-chip device for lung cancer diagnostics, international conference on biomedical electronics & devices; pp. 63–68. (Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies - Volume). (BIOSTEC 2017) [Google Scholar]
- 169.Kamble H., Vadivelu R., Barton M., Boriachek K., Munaz A., Park S., Shiddiky M., Nguyen N.T. An electromagnetically actuated double-sided cell-stretching device for mechanobiology research. Micromachines. 2017;8(8):256. doi: 10.3390/mi8080256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang M., Li J., Li H., Wu G., Cao C., Wu W., Li L. Effect of biaxial tensile strain on expression of osteogenic specificity markers of rat bone marrow-derived mesenchymal stem cells in vitro. J. Biochem. Eng. 2016;33(3):499–505. [PubMed] [Google Scholar]
- 171.Bonito V., de Kort B.J., Bouten C.V., Smits A.I. Cyclic strain affects macrophage cytokine secretion and extracellular matrix turnover in electrospun scaffolds. Tissue Eng. 2019;25(17–18):1310–1325. doi: 10.1089/ten.TEA.2018.0306. [DOI] [PubMed] [Google Scholar]
- 172.Reznikov N., Boughton O.R., Ghouse S., Weston A.E., Collinson L., Blunn G.W., Jeffers J., Cobb J.P., Stevens M.M. Individual response variations in scaffold-guided bone regeneration are determined by independent strain- and injury-induced mechanisms. Biomaterials. 2019;194:183–194. doi: 10.1016/j.biomaterials.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ezquerro C.U., Friedli M., Trono D., Wildhaber B. Google Patents; 2019. Method for Preparing Induced Hepatic Progenitor Cells. [Google Scholar]
- 174.Grier W.K., Iyoha E.M., Harley B.A.C. The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen-GAG scaffolds. J. Mech. Behav. Biomed. Mater. 2016;65:295–305. doi: 10.1016/j.jmbbm.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gilev M.V., Yu N.L., Naschetnikova I.A., Karabanalov M.S., Stepanov S.I. Osteointegration characterization of additive manufactured porous titanium scaffold based on microhardness and Ca/P ratio. KnE Eng. 2019;4(1):65–73. [Google Scholar]
- 176.Van Helvert S., Storm C., Friedl P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018;20(1):8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee S., Moon S., Oh J.Y., Seo E.H., Kim Y.H., Jun E., Shim I.K., Kim S.C. Enhanced insulin production and reprogramming efficiency of mesenchymal stem cells derived from porcine pancreas using suitable induction medium. Xenotransplantation. 2019;26(1):12451. doi: 10.1111/xen.12451. [DOI] [PubMed] [Google Scholar]
- 178.Li Y., Xu Q., Shi M., Gan P., Huang Q., Wang A., Tan G., Fang Y., Liao H. Low-level laser therapy induces human umbilical vascular endothelial cell proliferation, migration and tube formation through activating the PI3K/Akt signaling pathway. Microvasc. Res. 2020;129:103959. doi: 10.1016/j.mvr.2019.103959. [DOI] [PubMed] [Google Scholar]
- 179.Caires C.S.A., Farias L.A.S., Gomes L.E., Pinto B.P., Gonçalves D.A., Zagonel L.F., Nascimento V.A., Alves D.C.B., Colbeck I., Whitby C., Caires A.R.L., Wender H. Effective killing of bacteria under blue-light irradiation promoted by green synthesized silver nanoparticles loaded on reduced graphene oxide sheets. Mater. Sci. Eng. C. 2020;113:110984. doi: 10.1016/j.msec.2020.110984. [DOI] [PubMed] [Google Scholar]
- 180.Belashov A.V., Zhikhoreva A.A., Belyaeva T.N., Nikolsky N.N., Semenova I.V., Kornilova E.S., Vasyutinskii O.S. Quantitative assessment of changes in cellular morphology at photodynamic treatment in vitro by means of digital holographic microscopy. Biomed. Optic Express. 2019;10(10):4975–4986. doi: 10.1364/BOE.10.004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee I.C., Wu H.-J., Liu H.-L. Dual-frequency ultrasound induces neural stem/progenitor cell differentiation and growth factor utilization by enhancing stable cavitation. ACS Chem. Neurosci. 2019;10(3):1452–1461. doi: 10.1021/acschemneuro.8b00483. [DOI] [PubMed] [Google Scholar]
- 182.Xu T., Gu J., Li C., Guo X., Tu J., Zhang D., Sun W., Kong X. Low-intensity pulsed ultrasound suppresses proliferation and promotes apoptosis via p38 MAPK signaling in rat visceral preadipocytes. Am. J. Transl. Res. 2018;10(3):948–956. [PMC free article] [PubMed] [Google Scholar]
- 183.Hsi P., Christianson R.J., Dubay R.A., Lissandrello C.A., Fiering J., Balestrini J.L., Tandon V. Acoustophoretic rapid media exchange and continuous-flow electrotransfection of primary human T cells for applications in automated cellular therapy manufacturing. Lab Chip. 2019;19(18):2978–2992. doi: 10.1039/c9lc00458k. [DOI] [PubMed] [Google Scholar]
- 184.Naskar S., Kumaran V., Markandeya Y.S., Mehta B., Basu B. Neurogenesis-on-Chip: electric field modulated transdifferentiation of human mesenchymal stem cell and mouse muscle precursor cell coculture. Biomaterials. 2020;226:119522. doi: 10.1016/j.biomaterials.2019.119522. [DOI] [PubMed] [Google Scholar]
- 185.Leppik L., Bhavsar M.B., Oliveira K.M.C., Eischen-Loges M., Mobini S., Barker J.H. Construction and use of an electrical stimulation chamber for enhancing osteogenic differentiation in mesenchymal stem/stromal cells in vitro. JoVE. 2019;143:59127. doi: 10.3791/59127. [DOI] [PubMed] [Google Scholar]
- 186.Hu W., Wei X., Zhu L., Yin D., Wei A., Bi X., Liu T., Zhou G., Qiang Y., Sun X., Wen Z., Pan Y. Enhancing proliferation and migration of fibroblast cells by electric stimulation based on triboelectric nanogenerator. Nanomater. Energy. 2019;57:600–607. [Google Scholar]
- 187.Wang X., Law J., Luo M., Gong Z., Yu J., Tang W., Zhang Z., Mei X., Huang Z., You L., Sun Y. Magnetic measurement and stimulation of cellular and intracellular structures. ACS Nano. 2020;14(4):3805–3821. doi: 10.1021/acsnano.0c00959. [DOI] [PubMed] [Google Scholar]
- 188.Hepel M. Magnetic nanoparticles for nanomedicine. Magnetochemistry. 2020;6(1):3. [Google Scholar]