Abstract
Carbon-based materials have attracted research interest worldwide due to their physical and chemical properties and wide surface area, rendering them excellent carrier molecules. They are widely used in biological applications like antimicrobial activity, cancer diagnosis, bio-imaging, targeting, drug delivery, biosensors, tissue engineering, dental care, and skin care. Carbon-based nanomaterials like carbon nanotubes and graphene have drawn more attention in the field of phototherapy due to their unique properties such as thermal conductivity, large surface area, and electrical properties. Phototherapy is a promising next-generation therapeutic modality for many modern medical conditions that include cancer diagnosis, targeting, and treatment. Phototherapy involves the major administration of photosensitizers (PSs), which absorb light sources and emit reactive oxygen species under cellular environments. Several types of nontoxic PSs are functionalized on carbon-based nanomaterials and have numerous advantages in cancer therapy. In this review, we discuss the potential role and combined effect of phototherapy and carbon nanomaterials, the mechanism and functionalization of PSs on nanomaterials, and their promising advantages in cancer therapy.
Keywords: cancer, carbon nanotubes, graphene, photodynamic therapy, photosensitizers, phototherapy
1. Introduction
Cancer is a deadly disease, where cells grow in an enormous amount and kill a huge population worldwide. Both developed and developing countries are largely affected and the death rate is high due to food habits, lack of exercise, genetic reasons, etc. Every year, the death rate increases in breast, lung, stomach, and liver cancers [1]. The exact cause of cancer is difficult to understand, and continuous research is underway to find cancer growths due to genetic disorders or external chemical compounds altering the gene by either addition or deletion, or overexpression of a gene, leading to the uncontrolled growth of cells [2]. Human papillomavirus, Helicobacter pylori, and hepatitis infections also lead to cancer incidence and progression [3]. Commercially available chemotherapy to treat cancer cells has numerous side effects such as high cost, low bioavailability, and poor targeting.
The ancient medical technique to cure diseases with the help of sunlight was followed in various countries like Egypt, China, and India, and this treatment was slowly forgotten over time due to modern medicine. In the 15th to mid-19th century, people used to cure skin diseases following sunlight therapy named heliotherapy [4]. The Atharvaveda Indians used plant extract and different Ayurvedic oil or seed extracts on the affected area for various diseases and then treated them with sunlight [5]. Nowadays, modern phototherapy is emerging faster in the medical field due to its efficient curing rate. The father of ultraviolet therapy, Niels Ryberg Finsen, designed a chemical ray lamp to treat Lupus vulgaris and succeeded [6]. Then, treatments like ultra vitalux lamps, fluorescent tubes [7], photochemotherapy, and psoralen and ultraviolet A (PUVA) slowly evolved in the 19th century [8]. During the 1980s, the term phototherapy was first named as extra corporeal photochemotherapy (extracorporeal photopheresis, ECP), and this was introduced to treat palliation of erythrodermic cutaneous T-cell lymphoma (CTCL) and was approved by the FDA in 1988 [9]. Thus, phototherapy attracted the research field and many treating methods were invented for various diseases like skin disease, cancer, dental care, and eye treatment.
2. Types of Phototherapy
2.1. Photobiomodulation (PBM)
Photobiomodulation (PBM) is an alternative medicine to treat various diseases by applying a low-level laser or low-power or light-emitting diodes with a limited wavelength level on the affected area of the body [10]. In the late 1960s, this technique was first introduced by Mester for hair growth in mice and potential medical applications [11]. PBM has been used to treat acute and chronic pain, wound healing [12], inflammatory disorders, dentistry [13], neurological disorders, head and neck cancer [14], Parkinson’s disease [15], colorectal cancer, carpel tunnel syndrome [16], and musculoskeletal syndrome [17]. PBM has both inhibitory and simulating effects when the light source is introduced into the cells [18,19]. The great opportunities of using blue and green light on stem cells in regenerative medicine [20], red light in spermatozoa motility [21], and visible light are being developed to visualize neuronal cells therapy [22].
The photobiomodulation effects on cell proliferation on most of the cells like fibroblasts, endothelial cells, keratinocytes, and lymphocytes are based on photo-stimulation of the mitochondrial signalling pathways, and increased production of growth factors by the regulation of transcription process [23,24,25]. Recent studies have focused on head and neck cancer therapy using PBM. The common side-effect of head and neck cancer was oral mucositis, and it was treated using PBM, achieving a positive response, which controlled the radiotherapy-induced oral mucositis [26].
2.2. Photodynamic Therapy (PDT)
Photodynamic therapy is an emerging medical treatment, and research is still ongoing in this century. PDT is a treatment modality that utilizes light of a specific wavelength to activate photosensitizers (PS) to destroy tumours. This works through a PS molecule or drug upon activation by a specific light to produce reactive oxygen species and that specifically kills the targeted tumours. [27,28]. PDT has a higher therapeutic efficacy and improves outcomes for cancer treatment in comparison to commercially available chemo and radiotherapy [29]. The first clinical PDT was tested by Dougherty and co-workers (1978), successfully treating cancer in preclinical models at Rosewell park cancer institute [30]. The PS drug has toxic effects on cancer cells when activated by the light source, but the drawback is that most of the PS molecule is water-insoluble in nature [31]. Therefore, to overcome the drawback, PS molecules have recently been coupled with different nanomaterials for significantly enhanced efficacy and tumour selectivity in cells using PDT [32,33]. The different mechanisms of phototherapy using carbon-based nanomaterials are shown in Figure 1.
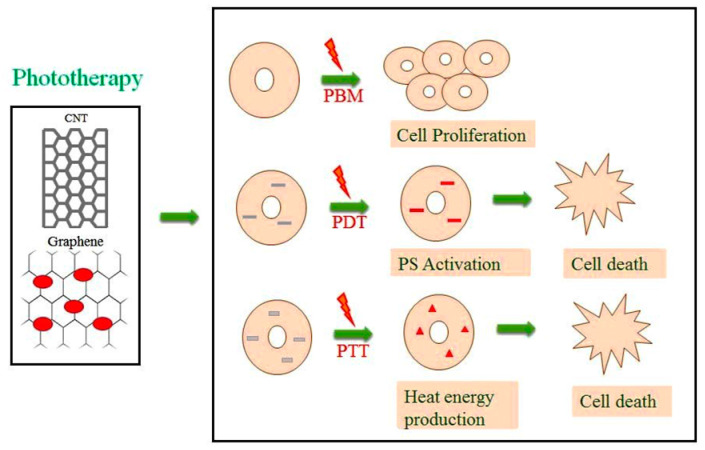
Figure 1.
Mechanism action of phototherapy. CNT—carbon nanotubes, PS—photosensitizers, PBM—photobiomodulation mechanism of light source on cells leads to cell proliferation. PDT—photodynamic therapy mechanism of incorporation of PS into the cells with light source emission leading to PS molecule activation induces singlet oxygen production and cell death. PTT—photothermal therapy mechanism of addition of nanomaterials, which has thermal properties after the introduction of a light source, produces heat energy, leading to cell death.
2.3. Photothermal Therapy (PTT)
At present, photothermal therapy has attracted increasing attention in research due to the targeted ablation of cells using heat energy mainly on cancer. In PTT, the photosensitizer is subjected to a light source, and the PS molecule is activated for heat energy that leads to cell death [34]. When there is a rise in body temperature during the recovery of certain diseases, the elevated heat energy will slow down the multiplication of bacteria, viruses and pathogens, and cancer cells, which also lead to death in the heat environment. Recent research on PTT has been focusing on introducing nanoparticles into the cells, which would have the thermal effect and be activated by laser irradiation, mainly to treat cancer cells using particles like gold, silver, fullerene, carbon nanotubes, and graphene [35]. Photothermal therapy and commercially available chemotherapy combine to improve the therapeutic effects on the treatment of cancer, hence the attracting of researchers in recent years [36]. The types of phototherapy with commonly used wavelengths, lamps, mechanisms of action, and its applications are presented in Table 1.
Table 1.
Types of phototherapy.
| Wavelengths | Lamps | Mechanism of Action | Applications | Reference | |
|---|---|---|---|---|---|
| PBM | 500–1100 nm | Neodymium:yttrium-aluminium-garnet (Nd:YAG) laser, helium-neon laser (He-Ne), Diode laser. | The low-level light source at a particular wavelength applied into the cells will stimulate or enhance the cells. | Rheumatoid arthritis, osteoarthritis, wound healing, low back pain. | [37,38,39,40] |
| PDT | 400–800 nm | NIR lasers, diode lasers, UV lights. | The PS molecule in the ground state; when the molecule activated by the light source reaches the excited the state, it converts to the triplet state by electron spinning. The triplet state interacts with the surrounding oxygen molecule and produces ROS through type I and type II reaction | Antimicrobial, fungal, viral activity, acne vulgaris, malignant tumour (lung, skin, head and neck, prostate cancer), wound healing. | [41,42,43,44,45,46] |
| PTT | 800–980 nm | NIR lasers, UV lights. | The particle (PS or nanoparticles) will be activated by the light source and produce heat energy. | Prostate cancer, melanoma skin cancer, Alzheimer’s disease. | [47,48,49] |
PBM—photobiomodulation, PDT—photodynamic therapy, PTT—photothermal therapy, NIR—near-infrared, PS—photosensitizers, UV—ultraviolet.
3. Photosensitizers (PS)
Photosensitizers are light-sensitive molecules, which are available in natural and synthetic compounds. PSs are molecules widely used in PDT, and when the light source emitted at a particular wavelength transfers into the PS molecule, it reaches an excited state, and electron transfer will occur in the chemical reaction and produce cytotoxicity [50]. The photosensitizers are well-soluble in body tissues, and when coupled with the targeted nanomaterial, they easily reach the cells.
Photosensitizers are sensitive at a specific wavelength, where the minimum wavelength range between 400 and 600 nm prevents excessive sensitivity from sunlight, and the maximum absorption wavelength is between 600 and 800 nm; those above 800 nm will not provide excess oxygen production. It has minimal cytotoxicity in the dark, so when the nanoparticle carries the PS molecule, it will affect the normal cells until activating it [51,52,53]. In cancer therapy commonly, the used PS molecule is from tetrapyrrole compounds, which have a similar structure as protoporphyrin prosthetic group has in haemoglobin for their ease of efficacy to navigate inside living cells [54]. The photosensitizers are classified based on the evolution such as first, second, and third generation. The first-generation PS molecule was introduced by Oscar Raab in 1904, who explained to his professor Von Tappeiner that acridine dyes kill protozoa when irradiated [55]. A commercially available PS molecule was introduced in the 1970s by Dr. Thomas Dougherty and colleagues [56]. In the 1980s, second-generation PS molecule studies began and a few PS molecules were used in clinical trials for anticancer activity. The molecules were hematoporphyrin derivatives and synthetic photosensitizers [57]. These molecules deeply penetrate into the tissues, have a high yield for oxygen molecules in pure form, and get activated at wavelengths between 650 and 800 nm [58]. The third-generation PS molecules have a high affinity to the tumour cells; it will not affect the normal cells and can target the specific area in the body during PDT. More molecules are derived and synthesized to treat cancer cells combined with nanoparticles to improve the targeting of specific cells and to increase the bioavailability of PS [59]. Ormond et al., 2013 discussed the structures and activation energy of different PS molecules and the properties of photosensitizers, and its application in cancer is given in Table 2 [60]. In the use of cancer treatment, most of the PS molecules have porphyrinoid and nonporphyrin dyes, and the modified form of this group is used in cancer cells. Some examples of porphyrinoid and nonporphyrin PS are shown in Figure 2 and Figure 3.
Table 2.
Properties of photosensitizers and its application in cancer.
| PSs | Structure | Λ(nm) and (Εmax) |
Application |
|---|---|---|---|
| HpD |
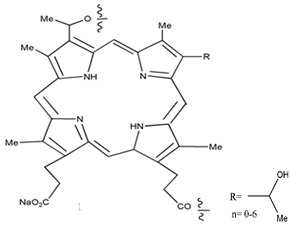
|
630 nm and 3000 (M−1 cm−1) |
Brain, Lung cancer [60,61] |
| Photofrin |
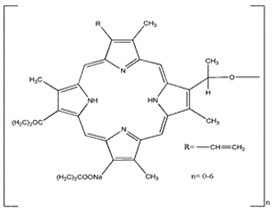
|
630 nm and 3000 (M−1 cm−1) |
Bladder, Lung, Oesophagus cancer [62,63] |
| 5-Aminole-vulinic acid |
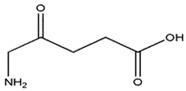
|
632 nm and 5000 (M−1 cm−1) | Cancer diagnosis [64,65,66] |
| Methyl aminolevulinate |
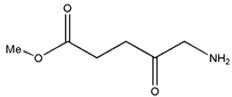
|
632 nm and 5000 (M−1 cm−1) | Nonmelanoma cancer, Basal cell carcinoma [67,68,69] |
| Hexaminolevulinate |
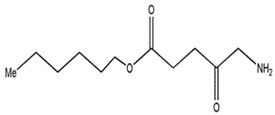
|
632 nm and 5000 (M−1 cm−1) | Bladder cancer diagnosis [70,71,72] |
| Lu-Tex |
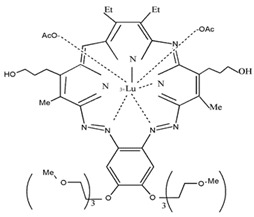
|
732 nm and 42,000 (M−1 cm−1) | Prostate cancer, Breast cancer, Cervical cancer [73,74,75] |
| Meta-tetra(hy-droxyphe-nyl)por-phyrin |
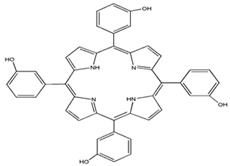
|
648 nm | Basal cell Carcinoma, Head and neck cancer and Skin cancer [76,77,78] |
| 5,10,15,20-tetrakis(4-sulfanato-phenyl)-21H,23H-porphyrin |
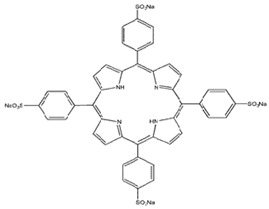
|
645 nm | Basal cell Carcinoma [27,63,79,80] |
| N-aspartyl chlorin e6 |
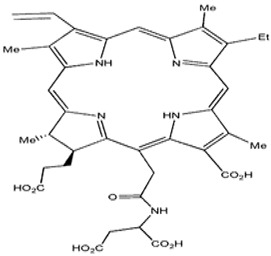
|
664 nm and 40,000 (M−1 cm−1) | Lung cancer [81,82] |
| 2-(1-hexylo-xyethyl)-2-devinyl Pyropheo-phorbide |
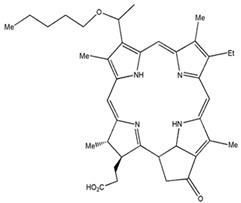
|
665 nm and 47,000 (M−1 cm−1) | Oesophageal cancer, Basal cell carcinoma, Lung cancer [83,84] |
| Phenothia-zines |
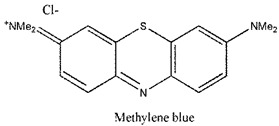
|
666 nm and 82,000 (M−1 cm−1) | Basal cell Carcinoma, Kaposi’s sarcoma, Cervical cancer [85,86] |
| Padoporfin |

|
763 nm and 88,000 (M−1 cm−1) | Prostate cancer [87] |
| Aluminium phthalo-cyanine-tetrasulfo-nate |
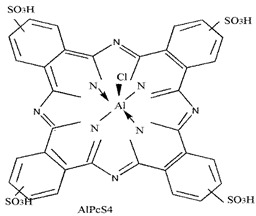
|
676 nm and 200,000 (M−1 cm−1) | Stomach, Skin, Oral, Breast cancer [88] |
| Curcumi-noids |
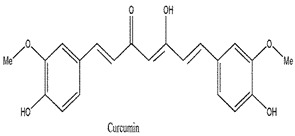
|
420 nm and 55,000 (M−1 cm−1) | Breast, Skin cancer [89,90] |
| Xanthenes |
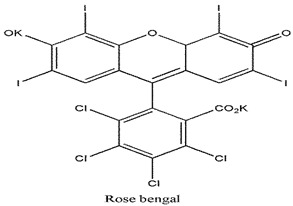
|
549 nm and 100,000 (M−1 cm−1) | Breast carcinoma and Metastatic melanoma [91] |
| 4,5-Dibromorhodamine methyl ester |
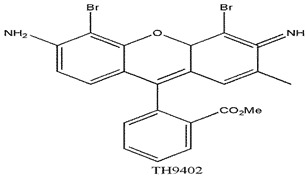
|
514 nm and 100,000 (M−1 cm−1) | Breast cancer [92] |
| Anthraquinones |
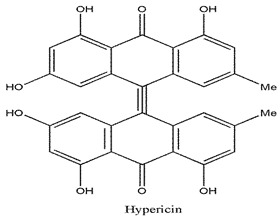
|
590 nm and 44,000 (M−1 cm−1) | Squamous cell carcinoma and Basal cell carcinoma [93,94] |
| Meta-tetra(hydroxyphenyl)chlorin |
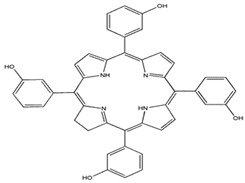
|
652 nm and 35,000 (M−1 cm−1) | Breast, Prostate, Pancreatic cancer, Head and neck cancer [77,80,95] |
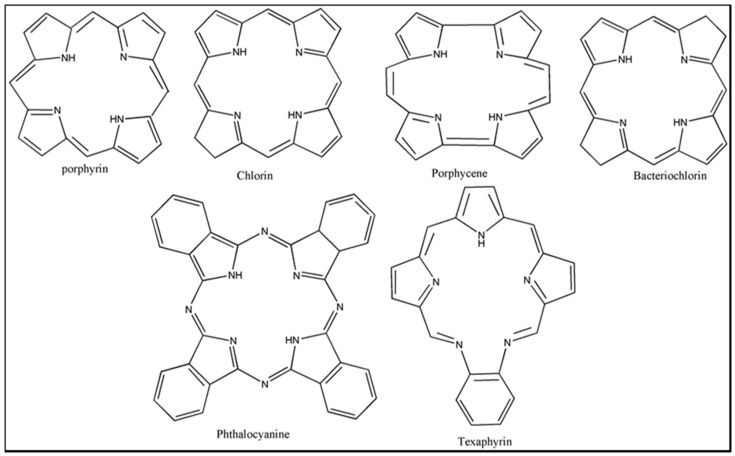
Figure 2.
Examples of porphyrinoids: Porphyrin, Chlorin, Porphycene, Bacteriochlorin, Phthalocyanine, Texaphyrin.
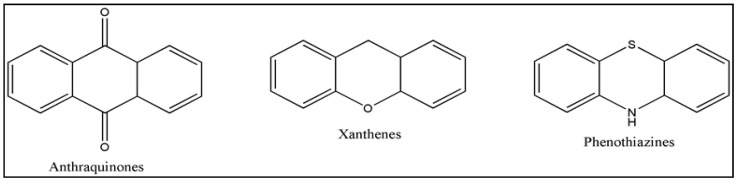
Figure 3.
Examples of nonporphyrin: Anthraquinones, Xanthenes, Phenothiazines.
4. Carbon Nanomaterials
The carbon nanomaterials are classified as zero-dimensional (0D)-structure fullerene, one-dimensional (1D) carbon nanotubes (CNT) and two-dimensional (2D) graphene nano-sized molecules. They are widely used in electronic and electrical fields, biosensors, medical treatments, environmental, etc. [78]. This nanomaterial has unique structures and physiochemical characteristics in the biomedical field to avoid current chemotherapeutic toxicity and to provide new effective therapies. In the drug delivery system, CNTs and graphene play significant roles compared to other nanomaterials due to the large surface area, so their loading efficiency of drugs or biomolecules or PS are high [96].
4.1. One Dimensional Carbon Nanotubes (CNT)
Carbon nanotubes are like rolled-up graphene sheets in a hollow cylindrical shape where both the ends are opened, and carbon atoms are exclusively arranged like benzene rings. The structural representation of CNT is armchair, zigzag, and chiral, with allotropic forms of both sp2 planar and sp3 cubic [97]. When the single sheet is rolled up and forms a tube, it is named a single-walled carbon nanotube (SWCNT) sized between 1 and 3 nm in diameter, with a length of several micrometres. Multiple sheets that roll up and form a tube are named multi-walled carbon nanotubes (MWCNTs) sized between 1 and 3 nm inner diameter, 2 and 100 nm outer diameter, and a length varying from 0.2 to several micrometres [98]. Synthesis of CNTs is mainly in three processes, namely the discharge, chemical vapor deposition (CVD), and laser ablation techniques. Some natural techniques are available but have no proper yield or standardized method [99]. The purity of the CNT is achieved by the acid reflux method, air oxidation, and surfactant-based sonication to remove the extra metals, for when it goes to biological applications as purity is very important [100]. Carbon nanotubes play a unique role as nanocarriers to deliver drugs, polymers, photosensitizers, and specific ligands to target siRNA and DNA. In cancer therapy, conventional treatment like surgery, chemotherapy or radiotherapy has numerous side effects and does not completely get rid of the disease, due to poor targeting, bioavailability, and damaging organs [101]. Now, scientists are focusing on combination therapy like nanomaterials with phototherapy for effective and target cancer treatment. The photosensitizers are coupled with CNTs to increase the solubility, PS bioavailability, and targeting only the cancer cells [102]. The photothermal activity of the CNTs activated at the wavelength of 808 nm and PSs-coupled CNTs are used to treat cancer cells by photodynamic effects [103].
4.2. Two-Dimensional Graphene
Graphene nanomaterials have attracted more attention in several fields due to the presence of more functional groups, wide surface area, and biocompatibility. Geim and Nosovelov were the first to separate a single graphene sheet layer from graphite material in 2004, where they followed the mechanical cleaving technique for isolation from the graphite crystal [41]. It has an sp2-hybridized honeycomb structure with a two-dimensional carbon lattice, which has unique electronic properties. It has a relatively high Young’s modulus, faster electron mobility, and high electric and thermal conductivity. Graphene is widely used in the form of graphene oxide (GO), carboxyl graphene (GCOOH), and reduced graphene oxide (rGO) [104]. Graphene oxide synthesis from graphite gives a high yield of production and cost-effectiveness. Graphene has a wide range of applications in the electronic and electrical, biomedical, environmental, and nanotechnology fields. Graphene is a good nanovehicle to carry more drugs or PS molecules due to the wide surface area. Graphene produces photothermal activity when introduced at a wavelength of 808 nm [105]. The functionalized graphene nanomaterial surface bonds will be broken due to high-temperature vibration and the carrier molecules released inside the cells, which helps to target the cells and act as a good nanocarrier in biological applications [106].
5. Mechanism and Loading of PS on Nanomaterials
5.1. Physical Loading of PS
In the drug delivery system, nanoparticles will be loaded with chemotherapeutic drugs, biomolecules, PS, etc. and the loading of the molecule will be based on the physical and chemical characteristics. Physical loading, also called noncovalent bonding, is a process that has a different mechanism like hydrophobic interaction, van der Waal’s force, and π–π stacking. 1D and 2D particles without any functional groups are hydrophobic in nature, and when interacting with a nonpolar PS molecule, both adsorb on the surface, but it is not strong enough to enter into the body to treat cancer cells [107]. The van der Waal’s force involves dipole interaction between the nanoparticle and PS molecule by the intermolecular force, as if the PS aggregated on the surface of the nanoparticle. Both mechanisms are not well preferred in the drug delivery system, due to poor stability, loading efficiency and release moiety [108]. π–π stacking is another physical adsorption method to load PS on the nanoparticle. Both 1D and 2D have an aromatic ring carbon particle, and when the PS molecule has a similar structure, both stack on the surface, and noncovalent bonding interaction takes place by π bonds. Hence, this mechanism is named π–π stacking. The long-chain polymers or peptides will fold on the nanoparticles by π–π stacking to load drugs [109]. The advantages of noncovalent bonding is that there is no structural damage of the drugs; the properties of the nanoparticle will remain the same. Examples of π–π-stacked molecules are proteins and DNA [110], si-RNA [111], polyacrylic acid, and pyrene [112]. Most of the PS molecules like m-tetrahydroxyl phenylchlorin (mTHPC), zinc phthalocyanine, zinc monoamino phthalocyanine, and Chlorin e6 are coupled noncovalently by π–π stacking to treat various cancer cells using phototherapy, and PDT and PTT are commonly used in cancer cells [113].
In recent research, the graphene oxide composite was prepared for phototheranostic application purposes, and some of the composites were discussed. Photosensitizer chlorin e6 is coupled noncovalently by the ultrasonication technique for photodynamic and photothermal effects, and GO is coupled with PEG-coupled gold nanostars. This composite has a combined effect of PDT, PTT, and photoimaging both in vitro and in vivo to treat breast cancer with successful results [114]. Single-walled carbon nanotubes were coupled with an encapsulated albumin chlorin e6 PS molecule by the ultra-homogenization technique for PTT, having an effect on squamous cell carcinoma (SCC 7) cell lines for in vitro and in vivo studies on the BALB/c (Bagg and Albino laboratory-bred mouse strain) nude female mice model [115]. The multiwalled carbon nanotubes were utilized for both the PDT and PTT effect by coupling foscan® (mTHPC) π–π stacking using a continuous stirring technique for 3 days, and the cell death mechanism occurred when irradiated at 650 and 880 nm on human ovarian cancer SKOV 3 cell lines [102]. The loading mechanism of PS on nanomaterials is depicted in Figure 4.
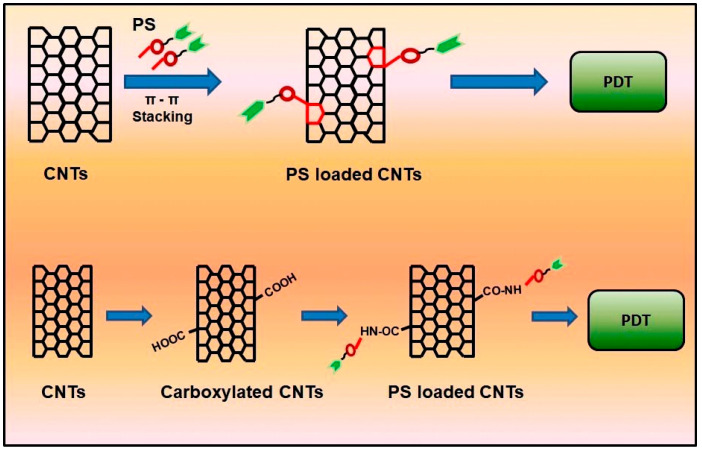
Figure 4.
Loading mechanism of PS on nanomaterials. The attachment of the PS molecule on aromatic rings of CNTs by π–π stacking, and the carboxyl group into amide formation of PS on the walls of CNTs, followed by the molecules being subjected to PDT. (CNTs—carbon nanotubes, PS—photosensitizers, PDT—photodynamic therapy, COOH—carboxyl group, CONH—amide group).
5.2. Chemical Loading of PS
The covalent functionalization is like a defect in the sidewalls of the nanomaterial, where there will be an addition of different functional groups [116]. The main aim of the covalent functionalization is to avoid the change in physical properties like solubility, purity, and sp2 and sp3 hybridization of carbon molecules. Those properties of carbon have a wide range of applications in the field of medical science and nanotechnology [117,118,119,120]. The functional groups are added to the CNTs like the carboxyl group, hydroxyl, amine, fluoride, and disulphide bonds [121]. The carboxyl group of graphene oxide and the amine group of polyethylene glycol (PEG) are coupled using the EDC-NHS method to act as a high loading nanocarrier [122]. Due to the solubilizing nature and biocompatibility of the functionalized carbon nanomaterials, it is opted to add different polymeric chains, proteins, DNA, and drugs. In a recent study, polyethyleneimine-functionalized SWCNTs are tested on melanoma cells in vitro and in vivo using PDT. The single-walled carbon nanotubes covalently functionalized composite showed excellent photocytotoxic action against cancer cells, and the activity of the composite was based on the functionalization method [123].
6. Application of Phototherapy Using CNT and Graphene on Cancer Therapy
Various research works are ongoing using the photo effect to treat new diseases to overcome the side-effects of present chemotherapy mainly on cancer. Using CNT and graphene nanomaterials for PDT, PTT, and photo imaging by coupling with different PS targets cancer cells and fluorescent molecules. The different nanoparticles with photosensitizers and their applications are listed in Table 3.
Table 3.
Various applications of phototherapy using 1D and 2D carbon nanomaterials on cancer.
| Nanoparticle | Photosensitizer | Bonding | Photo Therapy | Applications | Targeted | References |
|---|---|---|---|---|---|---|
| SWCNTs | - | Covalent (Carboxyl-amine cross-linking) |
PTT | Glioblastoma cells | Anti CD133 | [124] |
| SWCNTs | SWCNT-PEI SWCNT-PVPk30 |
Covalent (cationic polymerization) and noncovalent (physical attachment) |
PDT | Mus musculus skin melanoma cells (B16-F10 cells) | - | [102] |
| Metallic-SWCNT Semiconducting-SWCNT |
- | - | PDT PTT |
Lung cancer cells (NCI-H460) | - | [125] |
| MWCNT | m-tetrahydroxylphenylcholrin | Non-covalent (physical attachment) |
PDT PTT |
Ovarian cancer cells (SKOV3) | - | [103] |
| SWCNT (Evans blue and albumin) | Chlorin e6 | Non-covalent (physical attachment) |
PDT/PTT | Mouse squamous cancer cells (SCC-7) and In vivo |
- | [116] |
| SWCNT | Zinc phthalocyanine | Covalent (carboxyl-amine cross-linking) and non-covalent (physical attachment) |
PDT | Breast cancer cells (MCF 7) | Spermine | [126] |
| SWCNT (chitosan) | Chlorin e6 | Non-covalent (π-π interaction) |
PDT | HeLa cancer cells | - | [127] |
| SWCNT, GO, Fullerene |
- | Covalent (Carboxyl-amine cross linking) |
PTT | Breast cancer cells (MCF 7) and In vivo | Hyaluronic acid | [128] |
| SWCNT | Zinc monoamino phthalocyanine | Covalent (Carboxyl-amine cross linking) and non- covalent (π-π interaction) |
PDT/PTT | Melanoma cells (A375) | Folic acid | [129] |
| SWCNT (Docetaxel NGR peptide) | - | Non-covalent (physical attachment) |
PTT | Human prostate cancer (PC3) and in vivo | - | [130] |
| CNT Graphene sheet | Hydroxyapatite | Non-Covalent (physical attachment) |
PTT | - | - | [131] |
| Graphene oxide-PEG-DOX | - | Covalent (Carboxyl-amine cross-linking) |
PTT | Murine mammary cancer cells (EMT6) and in vivo | - | [123] |
| Graphene oxide-gold nanorods-Doxorubicin | - | Noncovalent (physical attachment) |
PTT | Lung cancer cells (A549) | - | [132] |
| Reduced graphene oxide | - | Noncovalent (physical attachment) |
PTT | Human breast cancer (MCF 7) | Hyaluronic acid | [133] |
| Graphene oxide Palladium |
- | Covalent (Carboxyl-amine cross-linking) |
PDT PTT |
Human prostate cancer (PC3) | - | [134] |
| Graphene | - | - | PDT PTT |
Cervical cancer cells (HeLa) | - | [135] |
| Graphene oxide-PEG | - | Covalent (Carboxyl-amine cross-linking) |
PDT PTT |
Melanoma cells (B16F0) | Folate | [106] |
| Graphene oxide (Quantum dots) | TRITC | Covalent (Carboxyl-amine cross-linking) Noncovalent (physical attachments) |
PDT | Mouse mammary tumour cells (4T1) | Upconversion nanoparticle (UCNP) | [136] |
| Graphene | - | Covalent (Carboxyl-amine cross-linking) |
PTT | Skin cancer (in vivo) | Hyaluronic acid | [137] |
| Graphene oxide | Chlorin e6 | Noncovalent (π-π interaction) |
PDT/ PTT |
Mouse breast mammary carcinoma cells (EMT6) | - | [115] |
| Graphene oxide-PEG-Folic acid | - | Covalent (Carboxyl-amine cross-linking) |
PTT | Human breast cancer (MCF 7 and MDA-MB-231) | Folate | [138] |
| SWCNTs | Chlorin e6 | Non-covalent (π-π interaction) |
PDT | Human colon cancer cells (Caco 2) | Hyaluronic acid | [139] |
7. Conclusions and Future Perspectives
Carbon-based nanomaterials graphene and carbon nanotubes play a vital role as nanocarriers at present, and more research articles have been published as they are a good carrier to treat cancer cells both in vivo and in vivo. The unique structure of CNT and graphene will increase the loading efficiency of PS molecules, the biocompatibility, bioavailability, and thermal effect. The phototherapy using carbon nanomaterials to treat cancer is a new approach and is effective with reduced side effects. The loading mechanism of photosensitizers is clearly studied based on the structure and functional group present in the PS molecule. Most of the PS molecules have a benzene ring in their structure that will attach on the nanomaterials by π–π stacking noncovalent functionalization. The chemical functionalization with the PS molecule has very few research works being done to treat cancer cells. The surface functionalization will improve the targeting of the therapeutic efficiency of the photosensitizers for the treatment of cancer in PDT. More research work is needed on this perspective of surface modifications to develop novel targeted carbon nanomaterials for the treatment of cancer. Furthermore, more in vitro, in vivo, and clinical trials are recommended to unlock the medicinal applications of carbon-based nanomaterials for the treatment of cancer. The carbon nanotube and graphene-based nanocomposite with the PS molecule are used to treat various cancer cells, and the mechanism of loading with target molecules is detailed in this review. Emerging phototherapy combined with carbon nanomaterials will be an alternative treatment for cancer with fewer side effects.
Abbreviations
| 0D | Zero dimensions |
| 1D | One dimension |
| 2D | Two dimensions |
| CD | Compact disk |
| CNT | Carbon nanotubes |
| COOH | Carboxyl group |
| CTCL | Cutaneous T-cell lymphoma |
| CVD | Chemical vapor deposition |
| DVD | Digital video disk |
| ECP | Extracorporeal photopheresis |
| EDC | Ethyl(dimethylaminopropyl) carbodiimide |
| FDA | Food and drug administration |
| GCOOH | Carboxylated graphene |
| GO | Graphene oxide |
| He-Ne | Helium and neon |
| HpD | Haematoporphyrin Derivative |
| IEC | International Engineering Consortium |
| LLLT | Low-level laser therapy |
| M−1 cm−1 | Per moles per centimetre |
| mTHPC | m-tetra hydroxyl phenylchlorin |
| MWCNT | Multi-walled carbon nanotubes |
| Nd:YAG | Neodymium-doped yttrium aluminium garnet |
| NHS | N -Hydroxysuccinimide |
| NIR | Near-infrared |
| Nm | Nanometre |
| OH | Hydroxyl group |
| PBM | Photobiomodulation |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PEI | Polyetherimide |
| PS | Photosensitizers |
| PTT | Photothermal therapy |
| PUVA | Psoralens and ultraviolet A |
| PVPk30 | Polyvinylpyrrolidone |
| rGO | Reduced graphene oxide |
| ROS | Reactive oxygen species |
| siRNA | Small interfering ribonucleic acid |
| DNA | Double-stranded nucleic acid |
| SWCNT | Single-walled carbon nanotubes |
| TRITC | Tetramethylrhodamine |
| UCNP | Upconverting nanoparticles |
| UVA | Ultraviolet A |
| UVB | Ultraviolet B |
| εmax | Molar extinction coefficient |
Author Contributions
Conceptualization, P.S. and H.A.; methodology, P.S.; software, P.S.; validation, P.S., H.A.; formal analysis, P.S.; investigation, P.S.; resources, P.S.; data curation, P.S.; writing—original draft preparation, P.S.; writing—review and editing, P.S. and H.A.; visualization, P.S. and H.A.; supervision, H.A.; project administration, H.A.; funding acquisition, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98337), as well as grants received from the University of Johannesburg (URC), the National Research Foundation (NRF), and the CSIR (Council for Scientific and industrial Research)—NLC (National Laser Centre) Rental Pool Program.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anand P., Kunnumakkara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B., Aggarwal B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naira M., Sandhub S.S., Sharma A.K. Cancer molecular markers: A guide to cancer detection and management. Semin. Cancer Biol. 2018;52:39–55. doi: 10.1016/j.semcancer.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Cancer Fact Sheet. World Health Organization; Geneva, Switzerland: 2015. No. 297. [Google Scholar]
- 4.Grzybowski A., Sak J., Pawlikowski J. A brief report on the history of phototherapy. Clin. Dermatol. 2016;34:532–537. doi: 10.1016/j.clindermatol.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Pathak M.A., Fitzpatrick T.B. The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD. J. Photochem. Photobiol. B. 1992;14:3–22. doi: 10.1016/1011-1344(92)85080-E. [DOI] [PubMed] [Google Scholar]
- 6.Hönigsmann H. History of phototherapy in dermatology. Photochem. Photobiol. Sci. 2013;12:16–21. doi: 10.1039/C2PP25120E. [DOI] [PubMed] [Google Scholar]
- 7.Wiskemann A. Die neuere Entwicklung der Lichttherapie [Recent developments in light therapy] Dermatol. Wochenschr. 1963;20:377–383. [PubMed] [Google Scholar]
- 8.Wolff K.W., Fitzpatrick T.B., Parrish J.A., Gschnait F., Gilchrest B., Hönigsmann H., Pathak M.A., Tanenbaum L. Photochemotherapy for psoriasis with orally administered methoxsalen. Arch. Dermatol. 1976;112:943–950. doi: 10.1001/archderm.1976.01630310005001. [DOI] [PubMed] [Google Scholar]
- 9.Trautinger F., Knobler R., Willemze R., Peris K., Stadler R., Laroche L., D’Incan M., Ranki A., Pimpinelli N., Ortiz-Romero P., et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur. J. Cancer. 2006;42:1014–1030. doi: 10.1016/j.ejca.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Bjordal J.M., Lopes-Martins R.A., Joensen J., Couppe C., Ljunggren A.E., Stergioulas A., Johnson M.I. A systematic review with procedural assessments and meta-analysis of Low Level Laser Therapy in lateral elbow tendinopathy (tennis elbow) BMC Musculoskelet. Disord. 2008;9 doi: 10.1186/1471-2474-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mester E., Ludany G., Sellyei M., Szende B., Gyenes G., Tota G.J. Studies on the inhibiting and activating effects of laser beams. Langenbecks Arch. Chir. 1968;322:1022–1027. doi: 10.1007/BF02453990. [DOI] [PubMed] [Google Scholar]
- 12.Da Silva J.P., Da Silva M.A., Almeida A.P.F., Junior I.L., Matos A.P. Laser Therapy in the Tissue Repair Process: A Literature Review. Photomed. Laser Surg. 2010;28:17–21. doi: 10.1089/pho.2008.2372. [DOI] [PubMed] [Google Scholar]
- 13.Cobb C.M. Lasers in Periodontics: A Review of the Literature. J. Periodontol. 2006;77:545–564. doi: 10.1902/jop.2006.050417. [DOI] [PubMed] [Google Scholar]
- 14.Antunes H.S., Herchenhorn D., Small I.A., Araújo C.M.M., Viégas C.M.P., de Assis Ramos G., Dias F.L., Ferreira C.G. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or without low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol. 2017;71:11–15. doi: 10.1016/j.oraloncology.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Willis G.L., Moore C., Armstrong S.M. Parkinson’s disease, lights and melanocytes: Looking beyond the retina. Sci. Rep. 2014;4 doi: 10.1038/srep03921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhi-Jun L., Yao W., Hua-Feng Z., Xin-Long M., Peng T., Yuting H. Effectiveness of low-level laser on carpal tunnel syndrome. Medicine. 2016;95 doi: 10.1097/MD.0000000000004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotler H.B., Chow R.T., Hamblin M.R., Carroll J. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop. Rheumatol. 2015;2 doi: 10.15406/mojor.2015.02.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mester E., Mester A.F., Mester A. The biomedical effects of laser application. Lasers Surg. Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 19.Rezende A.B., Ribeiro M.S., Nunez S.C., Garcia V.G., Maldonando M.P. Effects of a single near-infrared laser treatment on cutaneous wound healing: Biometrical and histological study in rats. J. Photochem. Photobiol. B. 2007;87:145–153. doi: 10.1016/j.jphotobiol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Huang Y.Y., Wang Y., Lyu P., Hamblin M.R. Photobiomodulation (blue and green light) encourages osteoblastic differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016;6 doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preece D., Chow K.W., Gomez-Godinez V., Gustafson K., Esener S., Ravida N., Durrant B., Berns M.W. Red light improves spermatozoa motility and does not induce oxidative DNA damage. Sci. Rep. 2017;7 doi: 10.1038/srep46480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohil C.J., Alicea B., Biocca F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurol. 2011;12:752. doi: 10.1038/nrn3122. [DOI] [PubMed] [Google Scholar]
- 23.Frigo L., Fávero G.M., Lima H.J., Maria D.A., Bjordal J.M. Low-level laser irradiation (InGaAlP-660 nm) increases fibroblast cell proliferation and reduces cell death in a dose-dependent manner. Photomed. Laser Surg. 2010 doi: 10.1089/pho.2008.2475. [DOI] [PubMed] [Google Scholar]
- 24.Basso F.G., Oliveira C.F., Kurachi C., Hebling J., Costa C.A. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med. Sci. 2013 doi: 10.1007/s10103-012-1057-8. [DOI] [PubMed] [Google Scholar]
- 25.Saygun I., Nizam N., Ural A.U., Serdar M.A., Avcu F. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed. Laser Surg. 2012;30:149–154. doi: 10.1089/pho.2011.3079. [DOI] [PubMed] [Google Scholar]
- 26.Guedes C.D.C.F.V., de Freitas Filho S.A.J., de Faria P.R., Loyola A.M., Sabino-Silva R., Cardoso S.V. Variation of Energy in Photobiomodulation for the Control of Radiotherapy-Induced Oral Mucositis: A Clinical Study in Head and Neck Cancer Patients. Int. J. Dent. 2018 doi: 10.1155/2018/4579279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003 doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 28.Hopper C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–219. doi: 10.1016/S1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 29.Detty M.R., Gibson S.L., Wagner S.J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004;47:3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 30.Patrice T., Moan J., Peng Q. An outline of the history of PDT. In: Patrice T., editor. Comprehensive Series in Photochemical and Photobiological Sciences. Royal Society of Chemistry Publishing; Cambridge, UK: 2003. pp. 1–18. [DOI] [Google Scholar]
- 31.Bechet D., Couleaud P., Frochot C., Viriot M.L., Guillemin F., Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–621. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Konan Y.N., Gurny R., Allémann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B. 2002;66:89–106. doi: 10.1016/S1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 33.Lim C.K., Heo J., Shin S., Jeong K., Seo Y.H., Jang W.D., Park C.R., Park S.Y., Kim S., Kwon I.C. Nanophotosensitizers toward advanced photo-dynamic therapy of Cancer. Cancer Lett. 2013;334:176–187. doi: 10.1016/j.canlet.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Tu X., Wang L., Cao Y., Ma Y., Shen H., Zhang M., Zhang Z. Efficient cancer ablation by combined photothermal and enhanced chemo-therapy based on carbon nanoparticles/doxorubicin@SiO2 nanocomposites. Carbon. 2016;97:35–44. doi: 10.1016/j.carbon.2015.05.043. [DOI] [Google Scholar]
- 35.Yu-Wei C., Yu-Lin S., Shang-Hsiu H., San-Yuan C. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016;105:190–204. doi: 10.1016/j.addr.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Chen L., Feng W., Zhou X., Qiu K., Miao Y., Zhang Q., Qin M., Li L., Zhang Y., He C. Facile synthesis of novel albumin-functionalized flower-like MoS2 nanoparticles for in vitro chemo photothermal synergistic therapy. RSC Adv. 2016;6:13040–13049. doi: 10.1039/C5RA27822H. [DOI] [Google Scholar]
- 37.Houreld N., Abrahamse H. Low-Intensity Laser Irradiation Stimulates Wound Healing in Diabetic Wounded Fibroblast Cells (WS1) Diabetes Technol. Ther. 2010;12:971–978. doi: 10.1089/dia.2010.0039. [DOI] [PubMed] [Google Scholar]
- 38.Asgari M., Gazor R., Abdollahifar M., Fathabady F.F., Zare F., Norouzian M., Amini A., Khosravipour A., Kiani P., Atashgah R.B., et al. Combined therapy of adipose-derived stem cells and photobiomodulation on accelerated bone healing of a critical size defect in an osteoporotic rat model. Biochem. Biophys. Res. Commun. 2020;530:173–180. doi: 10.1016/j.bbrc.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Cherkasova E., Babak K., Belotelov A., Labutina J., Yusupov V., Vorobieva N., Nerush A., Maslennikova A. Effects of photobiomodulation in relation to HeLa kyoto tumor cells exposed to ionizing radiation. J. Photochem. Photobiol. B Biol. 2020;209:111936. doi: 10.1016/j.jphotobiol.2020.111936. [DOI] [PubMed] [Google Scholar]
- 40.Dhilip Kumar S.S., Houreld N.N., Abrahamse H. Selective laser efficiency of green-synthesized silver nanoparticles by aloe arborescens and its wound healing activities in normal wounded and diabetic wounded fibroblast cells: In vitro studies. Int. J. Nanomed. 2020;15:6855–6870. doi: 10.2147/IJN.S257204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong E.J., Choi D.G., Shim M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B. 2016;6:297–307. doi: 10.1016/j.apsb.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G., Zhao Y., Xu Y., Zhu C., Liu T., Wang K. Chitosan nanoparticles for oral photothermally enhanced photodynamic therapy of colon cancer. Int. J. Pharm. 2020;589:119763. doi: 10.1016/j.ijpharm.2020.119763. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C., Qin W., Bai X., Zhang X. Nanomaterials to relieve tumor hypoxia for enhanced photodynamic therapy. Nano Today. 2020;35:100960. doi: 10.1016/j.nantod.2020.100960. [DOI] [Google Scholar]
- 44.Xu D.D., Leong M.M.L., Wong F., Lam H.M., Hoeven R. Photodynamic therapy on prostate cancer cells involve mitochondria membrane proteins. Photodiagn. Photodyn. Ther. 2020;31:101933. doi: 10.1016/j.pdpdt.2020.101933. [DOI] [PubMed] [Google Scholar]
- 45.Nassar A., Mostafa M., Khashaba S.A. Photodynamic therapy versus candida antigen immunotherapy in plane wart treatment: A comparative controlled study. Photodiagn. Photodyn. Ther. 2020;32:101973. doi: 10.1016/j.pdpdt.2020.101973. [DOI] [PubMed] [Google Scholar]
- 46.Qamar Z., Bamousa B., AlSheikh R., Ali S., Ali Baeshen H., Noushad M., Niazi F.H. A bibliometric review of top 50 cited articles published in the photodiagnosis and photodynamic therapy from 2004 to 2019. Photodiagn. Photodyn. Ther. 2020;31:101927. doi: 10.1016/j.pdpdt.2020.101927. [DOI] [PubMed] [Google Scholar]
- 47.Junqi C., Chengyun N., Zhengnan Z., Peng Y., Ye Z., Guoxin T., Chuanbin M. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019;99:1–26. doi: 10.1016/j.pmatsci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhi D., Yang T., O’Hagan J., Zhang S., Donnelly R.F. Photothermal therapy. J. Control. Release. 2020;325:52–71. doi: 10.1016/j.jconrel.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 49.Tian L., Tao L., Li H., Zhao S., Zhang Y., Yang S., Xue J., Zhang X. Hollow mesoporous carbon modified with cRGD peptide nanoplatform for targeted drug delivery and chemo-photothermal therapy of prostatic carcinoma. Colloids Surf. A Physicochem. Eng. Asp. 2019;570:386–395. doi: 10.1016/j.colsurfa.2019.03.030. [DOI] [Google Scholar]
- 50.Allison R.R., Downie G.H., Cuenca R., Hu X.-H., Childs C.J., Sibata C.H. Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 51.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kou J., Dou D., Yang L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget. 2017;8:81591–81603. doi: 10.18632/oncotarget.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyman E.S., Hynninen P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B. 2004;73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Abrahamse H., Hamblin M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allison R.R., Sibata C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010;7:61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Dougherty T.J., Kaufman J.E., Goldfarb A., Weishaupt K.R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumours. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- 57.Yoon I., Li J.Z., Shim Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013;46:7–23. doi: 10.5946/ce.2013.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nowak-Stepniowska A., Pergoł P., Padzik-Graczyk A. Photodynamic method of cancer diagnosis and therapy–mechanisms and applications. Postepy Biochem. 2013;59:53–63. [PubMed] [Google Scholar]
- 59.Kataoka H., Nishie H., Hayashi N., Tanaka M., Nomoto A., Yano S., Joh T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017;5 doi: 10.21037/atm.2017.03.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ormond A.B., Freeman H.S. Dye Sensitizers for Photodynamic Therapy. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato H., Romka C., Ono J., Saito T., Matsushima Y., Iimura I., Tahara M., Kawate N., Yoneyama K., Nishimiya K., et al. Cancer localization by detection of fluorescence by means of HpD administration and krypton ion laser photoradiation in canine lung cancer. Lung Cancer. 1981;21:439–445. [Google Scholar]
- 62.Madan V., Loncaster J., Allan D., Lear J., Sheridan L., Leach C., Allan E. Systemic photodynamic therapy with photofrin for naevoid basal cell carcinoma syndrome—A pilot study. Photodiagn. Photodyn. Ther. 2005;2:273–281. doi: 10.1016/S1572-1000(05)00101-8. [DOI] [PubMed] [Google Scholar]
- 63.Pushpan S.K., Venkatraman S., Anand V.G., Sankar J., Parmeswaran D., Ganesan S., Chandrashekar T.K. Porphyrins in photodynamic therapy—A search for ideal photosensitizers. Curr. Med. Chem. Anti-Cancer Agents. 2002;2:187–207. doi: 10.2174/1568011023354137. [DOI] [PubMed] [Google Scholar]
- 64.Batlle A. Porphyrins, porphyrias, cancer and photodynamic therapy—A model for carcinogenesis. J. Photochem. Photobiol. B. 1993;20:5–22. doi: 10.1016/1011-1344(93)80127-U. [DOI] [PubMed] [Google Scholar]
- 65.Morton C.A., Brown S.B., Collins S., Ibbotson S., Jenkinson H., Kurwa H., Langmack K., McKenna K., Moseley H., Pearse A.D., et al. Guidelines for topical photodynamic therapy: Report of a workshop of the British Photodermatology Group. J. Dermatol. 2002;146:552–567. doi: 10.1046/j.1365-2133.2002.04719.x. [DOI] [PubMed] [Google Scholar]
- 66.Peng Q., Berg K., Moan J., Kongshaug M., Nesland J.M. 5-Aminolevulinic acid-based photodynamic therapy: Principles and experimental research. Photochem. Photobiol. 1997;65:235–251. doi: 10.1111/j.1751-1097.1997.tb08549.x. [DOI] [PubMed] [Google Scholar]
- 67.U.S. Food and Drug Administration, Center for Drug Evaluation and Research Metvixia NDA 21-415 Approval Letter, 25 May 2004. [(accessed on 28 February 2013)]; Retrieved 5 January 2013. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021415s000_Metvixia_Aprov.pdf.
- 68.Gilchrest B.A. Photodynamic Therapy and Selected Off-label Uses. In: Tuleya S., editor. Proceedings of the Winter Clinical Dermatology Conference; Kohala Coast, HI, USA. 23–28 January 2010; Malvern, PA, USA: HMP Communications, LLC; 2010. pp. 10–12. [Google Scholar]
- 69.PhotoCure . ClinicalTrials.Gov [Internet] National Library of Medicine (US); Bethesda, MD, USA: [(accessed on 6 January 2013)]. A Double Blinded, Prospective, Randomized, Vehicle Controlled Multi-Center Study of Photodynamic Therapy with Visonac® Cream in Patients with Acne Vulgaris. NLM Identifier: NCT01347879. Available online: http://clinicaltrials.gov/show/NCT01347879. [Google Scholar]
- 70.Furre I.E., Shahzidi S., Luksiene Z., Moller M.T.N., Borgen E., Morgan J., Tkacz-Stachowska K., Nesland J.M., Peng Q. Targeting PBR by hexaminolevulinate-mediated photodynamic therapy induces apoptosis through translocation of apoptosis-inducing factor in human leukemia cells. Cancer Res. 2005;65:11051–11060. doi: 10.1158/0008-5472.CAN-05-0510. [DOI] [PubMed] [Google Scholar]
- 71.PhotoCure . ClinicalTrials.Gov [Internet] National Library of Medicine (US); Bethesda, MD, USA: [(accessed on 5 January 2012)]. Dose-Finding Study of Hexaminolevulinate (HAL) Photodynamic Therapy (PDT) to Treat Cervical Neoplasia. NLM Identifier: NCT01256424. Available online: http://clinicaltrials.gov/show/NCT01256424. [Google Scholar]
- 72.Oslo University Hospital . ClinicalTrials.Gov [Internet] National Library of Materials 2013, 6 837 Medicine (US); Bethesda, MD, USA: [(accessed on 6 January 2012)]. Treatment of Female Genital Erosive Lichen Planus(GELP) with Hexaminolevulinate PDT. NLM Identifier: NCT01282515. Available online: http://clinicaltrials.gov/show/NCT01282515. [Google Scholar]
- 73.Patel H., Mick R., Finlay J.C., Zhu T.C., Rickter E., Cengel K.A., Malkowicz S.B., Hahn S.M., Busch T.M. Motexafin lutetium-photodynamic therapy of prostate cancer: Short- and long-term effects of prostate-specific antigen. Clin. Cancer Res. 2008;14:4869–4876. doi: 10.1158/1078-0432.CCR-08-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.University of Pittsburgh . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda, MD, USA: [(accessed on 5 January 2013)]. Photodynamic Therapy using Lutetium Texaphyrin in Treating Patients with Cervical Intraepithelial Neoplasia. NLM Identifier: NCT00005808. Available online: http://clinicaltrials.gov/show/NCT00005808. [Google Scholar]
- 75.Josefsen L.B., Boyle R.W. Photodynamic therapy and the development of metal-based photosensitizers. Met. Based Drugs. 2008:1–24. doi: 10.1155/2008/276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mody T.D., Sessler J.L. Texaphyrins: A new approach to drug development. J. Porphyr. Phthalocyanines. 2001;5:134–142. doi: 10.1002/jpp.326. [DOI] [Google Scholar]
- 77.Rousset N., Bourre L., Thibaud S. Sensitizers in Photodynamic Therapy. In: Patrice T., editor. Photodynamic Therapy. Royal Society of Chemistry; Cambridge, UK: 2003. [Google Scholar]
- 78.Triesscheijn M., Ruevekamp M., Aalders M., Baas P., Stewart F.A. Outcome of mTHPC mediated photodynamic therapy is primarily determined by the vascular response. Photochem. Photobiol. 2005;81:1161–1167. doi: 10.1562/2005-04-04-RA-474. [DOI] [PubMed] [Google Scholar]
- 79.Stapleton M., Rhodes L.E. Photosensitizers for photodynamic therapy of cutaneous disease. J. Dermatol. Treat. 2003;14:107–112. doi: 10.1080/09546630310012127. [DOI] [PubMed] [Google Scholar]
- 80.Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995;24:19–33. doi: 10.1039/cs9952400019. [DOI] [Google Scholar]
- 81.Santoro O., Bandieramonte G., Melloni E., Marchesini R., Zunino F., Lepera P., de Palo G. Photodynamic therapy by topical meso-tetraphenylporphinesulfonate tetrasodium salt administration in superficial basal cell carcinoma. Cancer Res. 1990;50:4501–4503. [PubMed] [Google Scholar]
- 82.Usuda J., Kato H., Okunaka T., Furukawa K., Tsutsi H., Yamada K., Suga Y., Honda H., Nagatsuka Y., Ohira T., et al. Photodynamic therapy (PDT) for lung cancers. J. Thorac. Oncol. 2006;1:489–493. doi: 10.1016/S1556-0864(15)31616-6. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi W., Liu Q., Nakagawa H., Sakaki H., Teh B., Matsumiya T., Yoshida H., Imaizumi T., Satoh K., Kimura H. Photodynamic therapy with mono-L-aspartyl chlorin e6 can cause necrosis of squamous cell carcinoma of tongue: Experimental study on an animal model of nude mouse. Oral Oncol. 2006;42:46–50. doi: 10.1016/j.oraloncology.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Lobel J., MacDonald I.J., Ciesielski M.J., Barone T., Potter W.R., Pollina J., Plunkett R.J., Fenstermaker R.A., Dougherty T.J. 2-[1-Hexyloxyethyl]-2-Devinyl Pyropheophorbide-a (HPPH) in a nude rat glioma model: Implications for photodynamic therapy. Lasers Surg. Med. 2001;29:397–405. doi: 10.1002/lsm.10001. [DOI] [PubMed] [Google Scholar]
- 85.Roswell Park Cancer Institute . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda, MD, USA: [(accessed on 5 January 2013)]. Photodynamic Therapy using HPPH in Treating Patients with Recurrent Dysplasia, Carcinoma in Situ, or Stage I Oral Cavity Cancer. NLM Identifier: NCT00670397. Available online: http://clinicaltrials.gov/show/NCT00670397. [Google Scholar]
- 86.Chen Y., Zheng W., Li Y., Zhong J., Ji J., Shen P. Apoptosis induced by methylene-blue-mediated photodynamic therapy in melanolmas and the involvement of mitochondrial dysfunction revealed by proteomics. Cancer Sci. 2008;99:2019–2027. doi: 10.1111/j.1349-7006.2008.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu Y., Jiao R., Chen X., Zhong J., Ji J., Shen P. Methylene blue-mediated photodynamic therapy induces mitochondria-dependent apoptosis in HeLa cell. J. Cell. Biochem. 2008;105:1451–1460. doi: 10.1002/jcb.21965. [DOI] [PubMed] [Google Scholar]
- 88.Brun P.H., DeGroot J.L., Dickson E.F.G., Farahani M., Pottier R.H. Determination of the in vivo pharmacokinetics of palladium-bacteriopheophorbide (WST09) in EMT6 tumor-bearing Balb/c mice using graphite furnace atomic absorption spectroscopy. Photochem. Photobiol. Sci. 2004;3:1006–1010. doi: 10.1039/b403534h. [DOI] [PubMed] [Google Scholar]
- 89.Zimcik P., Miletin M. Photodynamic Therapy. In: Lang A.R., editor. Dyes and Pigments: New Research. Nova Science Publishers; New York, NY, USA: 2008. [Google Scholar]
- 90.Waranyoupalin R., Wongnawa S., Wongnawa M., Pakawatchai C., Panichayupakaranant P., Sherdshoopongse P. Studies on complex formation between curcumin and Hg(II) ion by spectrophotometric method: A new approach to overcome peak overlap. Cent. Eur. J. Chem. 2009;7:388–394. doi: 10.2478/s11532-009-0037-8. [DOI] [Google Scholar]
- 91.Araujo N.C., Fontana C.R., Gerbi M.E.M., Bagnato V.S. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed. Laser Surg. 2012;30:96–101. doi: 10.1089/pho.2011.3053. [DOI] [PubMed] [Google Scholar]
- 92.Mousavi S.H., Tavakkol-Afshari J., Brook A., Jafari-Anarkooli I. Direct toxicity of rose Bengal in MCF-7 cell line: Roloe of apoptosis. Food Chem. Toxicol. 2009;47:855–859. doi: 10.1016/j.fct.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 93.Guimond M., Balassy A., Barrette M., Brochu S., Perreault C., Roy D.C. P-glycoprotein targeting: A unique strategy to selectively eliminate immunoreactive T cells. Blood. 2002;100:375–382. doi: 10.1182/blood-2001-12-0353. [DOI] [PubMed] [Google Scholar]
- 94.Sanovic R., Krammer B., Grumboeck S., Verwanger T. Time-resolved gene expression profiling of human squamous cell carcinoma cells during the apoptosis process induced by photodynamic treatment with hypericin. Int. J. Oncol. 2009;35:921–939. doi: 10.3892/ijo_00000407. [DOI] [PubMed] [Google Scholar]
- 95.Kacerovska D., Pizinger K., Majer F., Smid F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract-a pilot study. Photochem. Photobiol. 2008;84:779–785. doi: 10.1111/j.1751-1097.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 96.Erola O., Uyana I., Hatipa M., Yilmaza C., Tekinaya A., Gulera M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomedicine. 2018;14:2433–2454. doi: 10.1016/j.nano.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 97.Mehra N.K., Palakurthi S. Interactions between carbon nanotubes and bioactives: A drug delivery perspective. Drug Discov. Today. 2016;21:585–597. doi: 10.1016/j.drudis.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Singh B., Baburao C., Pispati V., Pathipati H., Muthy N., Prassana S., Rathode B.G. Carbon nanotubes: A novel drug delivery system. Int. J. Res. Pharm. Chem. 2012;2:523–532. [Google Scholar]
- 99.He H., Pham-Huy L.A., Dramou P., Xiao D., Zuo P., Pham-Huy C. Carbon nanotubes: Applications in pharmacy and medicine. Biomed Res. Int. 2013 doi: 10.1155/2013/578290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donaldson K., Aitken R., Tran L., Stone V., Duffin R., Forrest G., Alexander A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 101.Hou P., Bai S., Yang Q., Liu C., Cheng H. Multi-step purification of carbon nanotubes. Carbon. 2002;40:81–85. doi: 10.1016/S0008-6223(01)00075-6. [DOI] [Google Scholar]
- 102.Lei W., Jinjin S., Ruiyuan L., Yan L., Jing Z., Xiaoyuan Y., Jun G., Chaofeng Z., Zhenzhong Z. Photodynamic effect of functionalized singlewalled carbon nanotubes: A potential sensitizer for photodynamic therapy. Nanoscale. 2014;6:4642–4652. doi: 10.1039/c3nr06835h. [DOI] [PubMed] [Google Scholar]
- 103.Marangon I., Ménard-Moyon C., Silva A.K.A., Bianco A., Luciani N., Gazeau F. Synergic mechanisms of photothermal and photodynamic therapies mediated by photosensitizer/carbon nanotube complexes. Carbon. 2016;97:110–123. doi: 10.1016/j.carbon.2015.08.023. [DOI] [Google Scholar]
- 104.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., Firsov A.A. Electric Field Effect in Atomically Thin Carbon Films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y., Li Z., Wang J., Li J., Lin Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011;29:205–212. doi: 10.1016/j.tibtech.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalluru P., Vankayala R., Chiang C.S., Hwang K.C. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. 2016;95:1–10. doi: 10.1016/j.biomaterials.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 107.Shi S., Chen F., Ehlerding E.B., Cai W. Surface engineering of graphene-based nanomaterials for biomedical applications. Bioconjug. Chem. 2014;25:1609–1619. doi: 10.1021/bc500332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen R.J., Zhang Y., Wang D., Dai H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001;123:3838–3839. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- 109.Lee D.J., Park S.Y., Oh Y.T., Oh N.M., Oh K.T., Youn Y.S. Preparation of chlorine e6-conjugated single walled carbon nanotube for photodynamic therapy. Macromol Res. 2011;19:848–852. doi: 10.1007/s13233-011-0816-x. [DOI] [Google Scholar]
- 110.Bilalis P., Katsigiannopoulos D., Avgeropoulos A., Sakellario G. Noncovalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014;4:2911–2934. doi: 10.1039/C3RA44906H. [DOI] [Google Scholar]
- 111.Besteman K., Lee J.O., Wiertz F.G., Heering H.A., Dekker C. Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Lett. 2003;3:727–730. doi: 10.1021/nl034139u. [DOI] [Google Scholar]
- 112.Siu K.S., Chen D., Zheng X., Zhang X., Johnston N., Liu Y., Yuan K., Koropatnick J., Gillies E.R., Min W.P. Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials. 2014;35:3435–3442. doi: 10.1016/j.biomaterials.2013.12.079. [DOI] [PubMed] [Google Scholar]
- 113.Paloniemi H., Ääritalo T., Laih T., Like H., Kocharova N., Haapakka K., Terzi F., Seeber R., Lukkari J. Water-soluble full-length single-wall carbon nanotube polyelectrolytes: Preparation and characterization. J. Phys. Chem. B. 2005;109:8634–8642. doi: 10.1021/jp0443097. [DOI] [PubMed] [Google Scholar]
- 114.Ogbodu R.O., Antunes E., Nyokong T. Physicochemical properties of zinc monoaminophthalocyanine conjugated to folic acid and single walled carbon nanotubes. Polyhedron. 2013;60:59–67. doi: 10.1016/j.poly.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Wu C., Li D., Wang L., Guan X., Tian Y., Yang H., Li S., Liu Y. Single wavelength light-mediated synergistic bimodal cancer photoablation and amplified photothermal performance by graphene/gold nanostar/photosensitizer theranostics. Acta Biomater. 2017;53:631–642. doi: 10.1016/j.actbio.2017.01.078. [DOI] [PubMed] [Google Scholar]
- 116.Xie L., Wang G., Zhou H., Zhang F., Guo Z., Liu C., Zhang X., Zhu L. Functional long circulating single walled carbon nanotubes for Fluorescent/photoacoustic imaging-guided enhanced phototherapy. Biomaterials. 2016;103:219–228. doi: 10.1016/j.biomaterials.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 117.Sahoo N.G., Cheng H.K.F., Cai J., Li L., Chan S.H., Zhao J., Yo S. Improvement of mechanical and thermal properties of carbon nanotube composites through nanotube functionalization and processing methods. Mater. Chem. Phys. 2009;117:313–320. doi: 10.1016/j.matchemphys.2009.06.007. [DOI] [Google Scholar]
- 118.Park H., Zhao J., Lu J.P. Effects of Sidewall Functionalization on Conducting Properties of Single Wall Carbon Nanotubes. Nano Lett. 2006;5:916–919. doi: 10.1021/nl052488d. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Z.Q., Liu B., Chen Y.L., Jiang H., Hwang K.C., Huang Y. Mechanical properties of functionalized carbon nanotubes. Nanotechnology. 2008;19:395702. doi: 10.1088/0957-4484/19/39/395702. [DOI] [PubMed] [Google Scholar]
- 120.Banerjee S., Hemraj-Benny T., Wong S.S. Covalent surface chemistry of single walled carbon nanotubes. Adv. Mater. 2005;17:17–29. doi: 10.1002/adma.200401340. [DOI] [Google Scholar]
- 121.Carrillo A., Swartz J.A., Gamba J.M., Kane R.S. Noncovalent Functionalization of Graphite and Carbon Nanotubes with Polymer Multilayers and Gold Nanoparticles. Nano Lett. 2003;3:1437–1440. doi: 10.1021/nl034376x. [DOI] [Google Scholar]
- 122.Xie J., Lee S., Chen X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang W., Gu Z., Huang D., Liu Z., Guo X., Zhong H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene Oxide. Biomaterials. 2011;32:8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 124.Wang C., Chiou S., Chou C., Chen Y., Huang Y., Peng C. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomed. Nanotechnol. Biol. Med. 2011;7:69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 125.Murakami T., Nakatsuji H., Inada M., Matoba Y., Umeyama T., Tsujimoto M., Isoda S., Hashida M., Imahori H. Photodynamic and Photothermal Effects of Semiconducting and Metallic-Enriched Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2012;134:17862–17865. doi: 10.1021/ja3079972. [DOI] [PubMed] [Google Scholar]
- 126.Ogbodu R.O., Limson J.L., Prinsloo E., Nyokong T. Photophysical properties and photodynamic therapy effect of zinc phthalocyanine-spermine-single walled carbon nanotube conjugate on MCF-7 breast cancer cell line. Synth. Metals. 2015;204:122–132. doi: 10.1016/j.synthmet.2015.03.011. [DOI] [Google Scholar]
- 127.Xiao H., Zhu B., Wang D., Pang Y., He L., Ma X., Wang R., Jin C., Chen Y., Zhu X. Photodynamic effects of chlorin e6 attached to single wall carbon nanotubes through noncovalent interactions. Carbon. 2012;50:1681–1689. doi: 10.1016/j.carbon.2011.12.013. [DOI] [Google Scholar]
- 128.Hou L., Yuan Y., Ren J., Zhang Y., Wang Y., Shan X., Liu Q., Zhang Z. In vitro and in vivo comparative study of the phototherapy anticancer activity of hyaluronic acid-modified single-walled carbon nanotubes, graphene oxide, and fullerene. J. Nanopart. Res. 2017;19:286. doi: 10.1007/s11051-017-3977-5. [DOI] [Google Scholar]
- 129.Ogbodu R.O., Ndhundhuma I., Karsten A., Nyokong T. Photodynamic therapy effect of zinc monoaminophthalocyanine–folic acid conjugate adsorbed on single walled carbon nanotubes on melanoma cells. Spectrochim. Acta A. 2015;137:1120–1125. doi: 10.1016/j.saa.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 130.Wang L., Zhang M., Zhang N., Shi J., Zhang H., Li M., Lu C., Zhang Z. Synergistic enhancement of cancer therapy using a combination of docetaxel and photothermal ablation induced by single-walled carbon nanotubes. Int. J. Nanomed. 2011:2642–2652. doi: 10.2147/IJN.S24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neelgund G.M., Oki Aderemi R. Influence of carbon nanotubes and graphene nanosheets on photothermal effect of hydroxyapatite. J. Colloid Interface Sci. 2016;484:135–145. doi: 10.1016/j.jcis.2016.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan M.S., Pandey S., Bhaisare M.L., Gedda G., Talib A., Wu H.F. Graphene oxide @gold nanorods for chemo-photothermal treatment and controlled release of doxorubicin in mice Tumor. Colloids Surf. B Biointerfaces. 2017;160:543–552. doi: 10.1016/j.colsurfb.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 133.Lima-Sousa R., de Melo-Diogo D., Alves C.G., Costa E.C., Ferreira P., Louro R.O., Correia I.J. Hyaluronic acid functionalized green reduced graphene oxide for targeted cancer photothermal therapy. Carbohydr. Polym. 2018;200:93–99. doi: 10.1016/j.carbpol.2018.07.066. [DOI] [PubMed] [Google Scholar]
- 134.Thapa R.K., Soe Z.C., Ou W., Poudel K., Jeong J.-H., Jin S.G., Kwang Ku S., Choi H.-G., Lee Y.M., Yong C.S., et al. Palladium nanoparticle-decorated 2-D graphene oxide for effective photodynamic and photothermal therapy of prostate solid tumors. Collides Surf. B Biointerfaces. 2018;169:429–437. doi: 10.1016/j.colsurfb.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 135.Li Q., Hong L., Li H., Liu C. Graphene oxide-fullerene C60 (GO-C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light. Biosens. Bioelectron. 2017;89:477–482. doi: 10.1016/j.bios.2016.03.072. [DOI] [PubMed] [Google Scholar]
- 136.Zhang D., Wen L., Huang R., Wang H., Hu X.L., Xing D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials. 2018;153:14–26. doi: 10.1016/j.biomaterials.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 137.Jung H.S., Kong W.H., Sung D.K., Lee M.Y., Beack S.E., Keumdo H., Kim K.S., Yun S.H., Hahn S.K. Nanographene oxide–hyaluronic acid conjugate for photothermal ablation therapy of skin cancer. ACS Nano. 2014;8:260–268. doi: 10.1021/nn405383a. [DOI] [PubMed] [Google Scholar]
- 138.Mauro N., Scialabba C., Agnello S., Cavallaro G., Giammona G. Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery. Mater. Sci. Eng. C. 2020;107:110201. doi: 10.1016/j.msec.2019.110201. [DOI] [PubMed] [Google Scholar]
- 139.Sundaram P., Abrahamse H. Effective photodynamic therapy for colon cancer cells using chlorin e6 coated hyaluronic acid-based carbon nanotubes. Int. J. Mol. Sci. 2020;21:4745. doi: 10.3390/ijms21134745. [DOI] [PMC free article] [PubMed] [Google Scholar]