Abstract
Background:
An understanding of acceptability among potential intervention participants is critical to the design successful real-world financial incentive (FI) programs. The purpose of this qualitative study was to explore adolescent and parent perspectives on the acceptability of using FI to promote engagement in diabetes self-care in adolescents with type 1 diabetes (T1D).
Methods:
Focus groups with 46 adolescents with T1D (12–17 years old) and 39 parents of adolescents with T1D were conducted in the Seattle metropolitan area. Semi-structured questions addressed participants’ current use of incentives to promote change in diabetes self-care and receptivity to a theoretical incentive program administered by a third-party. Qualitative data were analyzed and emergent themes identified.
Results:
Three thematic categories informed participant views about the acceptability of FI programs: 1) the extent to which using FIs in the context of diabetes management fit comfortably into a family’s value system, 2) the urgent need for improved self-care due to the threat of diabetes-related health complications, and 3) the perceived effectiveness for FIs to promote improved diabetes self-care. These factors together led most parents and adolescents to be open to FI program participation.
Conclusions:
The results from this qualitative study suggest that well-designed FI programs to support diabetes management are acceptable to families with adolescents with T1D. Additionally, the use of FIs may have the potential to support adolescents with T1D in developing strong self-care habits and ease the often-turbulent transition to independent self-care.
Keywords: Behavioral economics, qualitative, financial incentives, Adolescents, Type 1 Diabetes
INTRODUCTION
Approximately 80% of adolescents with type 1 diabetes mellitus (T1D) fall short of glycemic targets1 placing them at increased risk for acute complications such as diabetic ketoacidosis, as well as chronic complications such as blindness and kidney failure.2,3 Most adolescents struggle with diabetes self-care, including consistent glucose monitoring and timely, appropriate insulin administration.4,5 Blood glucose levels and, in turn, health outcomes, can be improved by increased engagement in recommended diabetes self-care practices.6
Financial incentive (FI) programs are a potentially promising strategy for improving self-care practices and health outcomes in adolescents. Adolescents face converging barriers to adherence, such as social pressure, inclination toward risk-taking, depression, anxiety, and fatigue from chronic disease management.7–10 Therefore, adolescents are likely to benefit from programs that empower them to improve diabetes self-care engagement.5,11
The effectiveness of incentive strategies in improving both engagement in diabetes self-care, such as blood glucose monitoring, and glycemic control has been demonstrated in adults with T1D.12 Given these encouraging outcomes in adult populations, there is momentum towards exploring FI in adolescent patient groups. While pilot studies in adolescent populations are limited in both participant numbers and duration of follow-up, initial results hold promise. FI programs for adolescents with T1D led to improvements in the frequency of incentivized self-care behaviors.13,14 Further, in some, but not all, studies, increased engagement in self-care persisted after the discontinuation of incentives and was associated with durable reductions in HbA1c.15,16 This suggests that FI not only has the potential to improve clinical outcomes but may lead to long-term cost savings due to the relatively low cost of incentives as well as the preventative value of improved glycemic control.
Despite the potential benefits of incentive programs, there is concern regarding their use, particularly in adolescent populations. Parents may have ethical concerns about FI programs, such as worries about coercion, unfairness, and undermining the autonomy of adolescent decision-making (“undue inducement).17–20 While evidence regarding the long-term behavioral impacts of FI is limited, some predict that providing tangible rewards for health behaviors will erode adolescents’ intrinsic motivation.21–23 Finally, some parents may view the transfer of incentives within the context of a healthcare provider-patient relationship as an unacceptable violation of cultural or familial norms.24,25
Given that acceptability is a necessary condition for program uptake outside of randomized controlled trials, an understanding of acceptability among potential intervention participants is critical to design successful FI interventions.26,27 At this time, there is insufficient information characterizing family perspectives on using FI to support adolescents struggling with T1D management. Our primary aim was to assess intervention acceptability by exploring adolescent and caregiver perspectives on using incentives to promote self-care in adolescents with T1D.
METHODS
Study design and research personnel
A qualitative study using focus groups (FG) with adolescents with T1D and parents of adolescents with T1D was conducted in 2018. The qualitative study is part of a larger project to develop and execute a discrete choice experiment to systematically assess patient preferences for financial incentives to promote improvement in adolescents’ diabetes self-care.28 This report conforms to the Standards for Reporting Qualitative Research.29
The research team included a pediatric endocrinologist and health services researcher (FM), a medical anthropologist (KS), a behavioral economist and health services researcher (DW), an ethicist (SS), a research health psychologist (JYF), a senior pediatric endocrinologist (CP), and two clinical research associates (CL, KC). Focus group co-facilitators (FM, KS) have extensive experience working with adolescents.
Data tools
Facilitator guides for adolescent and parent FGs were developed according the study goals and adjusted as necessary throughout data collection as per standard qualitative methodology30. Questions addressed adolescents’ self-care tasks, challenges to completing self-care, incentivizing behaviors and outcomes, receptivity to financial incentives, incentive structures and pressure around self-care (Appendix A & B). FG participants also completed short written demographic questionnaires (Appendix C & D).
Focus Groups
A purposive sample of adolescents and parents of adolescents (hereafter parents) from the Seattle metropolitan area of Washington State was recruited from the Diabetes Clinic at Seattle Children’s Hospital. A group of 799 English-speaking adolescents (ages 12–17 years) with a T1D duration of over one year were identified as potential participants from the medical record data. This group was approached via mail with letters describing the study. Letters allowed interested parties to opt-in to recruitment phone calls from study staff. During recruitment calls, parents were asked if the parent primarily responsible for the child’s diabetes care would like to participate in a FG in addition to the child participating. The sample was intentionally drawn from four geographic sub-regions of the Seattle metropolitan area that differed economically and ethnically. Participants were compensated $50 for FG participation. The Seattle Children’s Research Institute Institutional Review Board approved the study procedures. Parents provided written consent and youth participants provided written assent for participants under 18.
Eighty-five participants participated in 12 FGs that included 5–10 participants each. Two-hour FGs were held on evenings or weekends in private meeting rooms that were familiar and convenient to the participants (e.g., hospital, neighborhood community center).
The initial sample estimates were 12 FGs consisting of 8 adolescent FGs and 4 parent FGs. Midway through data collection, the team shifted the ratio to an equal number of parent and adolescent FGs (6 adolescent, 6 parent) in order to increase geographic (proxy for economic) and ethnic diversity and ensure saturation would be achieved for both parent and adolescent samples.31
Analysis
FGs were digitally recorded, professionally transcribed verbatim, and reviewed by an investigator (DW) to ensure data integrity. Data were uploaded into Dedoose Version 7.0.23 (Sociocultural Research Consultants, Los Angeles, CA) for coding and thematic content analysis32–34 following the procedures outlined by Braun and Clark.35 A hierarchically-organized codebook was developed based on our research goals, discussion guide topic areas, and an initial data review. Steps to codebook development were as follows: initial codes were derived from study goals and instrument questions; codes were adapted and augmented by a reading of two transcripts; codes were tested on three additional transcripts by both coders; the codebook was edited as appropriate until an exhaustive but manageable code list was reached.
Transcripts were open-coded by both FG facilitators using the final version of the codebook (Appendix E). Coders were blind to each other’s coding and all differences were resolved by discussion until 100% agreement was reached. When necessary, the codebook was modified to accommodate new codes or definitions. During synthesis, coded excerpts were summarized into theme domains and subdomains with associated quotes. Demographic data were compiled and tabulated in Stata (version 14) (Tables 1 & 2).
Table 1:
Demographic data (adolescents)
| Adolescent Characteristics (n = 46) | n (%) |
|---|---|
| Racial background | |
| White, non-Hispanic | 32 (69.6) |
| Non-white, non-Hispanic | 10 (21.7) |
| Hispanic | 3 (6.5) |
| Prefer not to answer | 1 (2.2) |
| Age | |
| 12–14 years | 18 (39.1) |
| 15–17 years | 28 (60.9) |
| Gender | |
| Female | 25 (54.4) |
| Male | 20 (43.5) |
| Other | 1 (2.2) |
| Living with | |
| Both caregivers | 39 (84.8) |
| Alternating between caregivers | 4 (8.7) |
| Other | 2 (4.35) |
| Number of children aged < 18 living in household (mean, standard deviation) | 1.71, 0.79 |
| Insurance | |
| Private | 13 (28.3) |
| Public | 9 (19.6) |
| Other/Don’t know | 24 (52.2) |
| Most recent HbA1c (mean, standard deviation) | 8.57, 1.71 |
| Years since diagnosis (mean, standard deviation) | 7.09, 3.86 |
| Primary caregiver | |
| Mother | 25 (54.4) |
| Father | 9 (19.6) |
| Both | 11 (23.91) |
| Other | 1 (2.2) |
| Annual Household income | |
| $50,000 - $74,999 | 3 (6.5) |
| $75,000 - $125,999 | 2 (4.4) |
| $125,000+ | 3 (6.5) |
| Don’t know | 38 (82.6) |
Table 2:
Demographic data (caregivers)
| Caregiver Characteristics (n = 39) | n (%) |
|---|---|
| Racial background | |
| White, non-Hispanic | 29 (74.4) |
| Non-white, non-Hispanic | 5 (12.8) |
| Hispanic | 4 (10.2) |
| Prefer not to answer | 1 (2.6) |
| Age | |
| 35–49 years | 26 (66.7) |
| 15–17 years | 13 (33.3) |
| Gender | |
| Female | 35 (89.7) |
| Male | 3 (7.7) |
| Other | 1 (2.6) |
| Education level | |
| High school or less | 5 (12.8) |
| Vocational school/some college | 11 (28.2) |
| College/graduate/professional degree | 23 (59.0) |
| Number of children aged < 18 living in household (mean, standard deviation) | 1.63, 0.81 |
| Insurance | |
| Private | 29 (72.5) |
| Public | 10 (25.0) |
| Other | 1 (2.5) |
| Annual household income | |
| <30,000 - $49,999 | 7 (17.9) |
| $50,000 - $74,999 | 18 (46.2) |
| $125,000+ | 9 (23.1) |
| Adolescent’s most recent HbA1c (mean, standard deviation) | 8.94, 2.00 |
| Years since adolescent’s diagnosis (mean, standard deviation) | 6.25, 0.07 |
| Adolescent living with | |
| Both caregivers | 31 (77.5) |
| Alternating between caregivers | 3 (7.5) |
| Only once caregiver | 5 (12.5) |
| Other | 1 (2.5) |
| Adolescent gender | |
| Female | 22 (55.0) |
| Male | 18 (45.0) |
| Adolescent age | |
| 12 – 14 years | 16 (40.0) |
| 15 – 17 years | 24 (60.0) |
RESULTS
Three interconnected thematic concepts influenced whether parents and adolescents struggling with diabetes self-care viewed FI program participation as acceptable: 1) the extent to which using FIs in the context of diabetes management fit comfortably into a family’s value system, 2) the perceived effectiveness for FIs to promote improved diabetes self-care, and 3) the urgent need for improved self-care due to the threat of diabetes-related health complications. These three factors informed participants’ conclusions about their willingness to participate in an incentive program in an intertwined manner. For example, while some families viewed providing monetary rewards for behavior as generally incongruent with their family values, the anticipated effectiveness for FIs to address the threat of inadequate diabetes self-care outweighed some families’ concerns about FI program participation (Figure 1).
Figure 1:
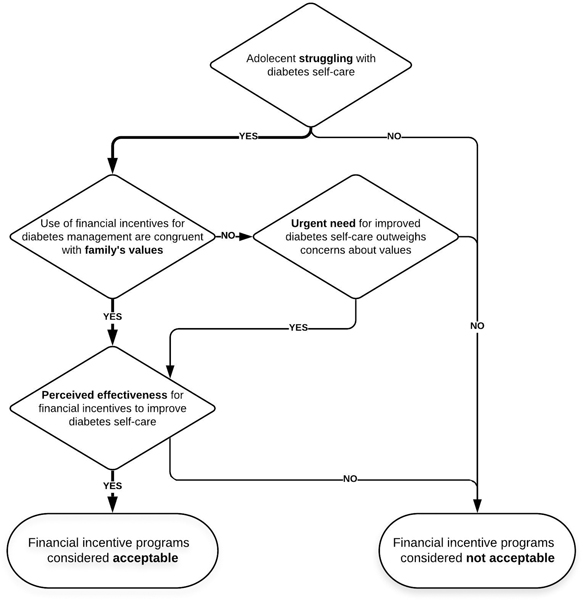
Factors influencing adolescent and parent views on the acceptability of financial incentive programs
Comfort with FI Use for Diabetes Management
When discussing their experiential context for considering the acceptability of FI to promote diabetes self-care, the majority of participants indicated that their families widely embraced rewarding non-diabetes behaviors including chores, school work, oral health, and reading. Many participants had also already tried to incentivize diabetes self-care behaviors and outcomes. One adolescent noted that her parent offered prizes for lowered A1C numbers, which she often earned, though her sibling did not. Family-generated incentive schemes included diverse rewards and sometimes complex incentive behaviors and outcomes (Table 3).
Table 3.
Examples of behavior and outcomes already incentivized with corresponding incentives
| BEHAVIOR | REWARD |
|---|---|
| Checking blood glucose level | $100/month Driving privileges Sleepovers away from home Special meal |
| Administering basal insulin | Ear piercing |
| Administering mealtime insulin independently | Play date/sleepover |
| Administering insulin for correction of hyperglycemia | Continuous glucose monitor |
| Using continuous glucose monitoring system | Driving privileges iPhone |
| Calibrating continuous glucose monitor sensor | TV show |
| Changing insulin pump site | Spontaneous small gifts |
| OUTCOME ONLY | REWARD |
| “Good” blood glucose level | Enthusiastic high-five from parent Video game CD Break from wearing continuous glucose monitor |
| “Good” management | Permission to attend summer camp solo |
| Lower A1c | Special dinner Driving lessons |
| Blood sugars “straight and perfect” for “a whole entire week” | Video Game |
| BEHAVIORS AND OUTCOMES | REWARD |
| Consistent glucose checks, changing insulin pump sites without being prompted, and majority of glucose levels being in target range for one week | Cash payment with bonuses (loosely structured system) |
| Check blood glucose level as recommended and be within target range most of the time | Driving privileges |
| A1c within goals of therapy and maintaining supplies | Permission to attend out-of-state college |
| “Continuous” self-care for 3 months including checking glucose levels and administering insulin reliably | iPhone (for initial 3 months of self-care) and minutes for phone/data use (for continuing self-care) |
The widespread experiences with various incentives suggested that for most participants’ families, the use of FIs to promote diabetes self-care fit comfortably into the family’s value system. One parent explained that she viewed FI as acceptable because it functionally aligned with societal concepts of paid work: “We said, ‘Look, your father gets paid for doing what is expected of him. How is [paying for diabetes self-care] any different? We compensate people for doing what we expect’” [Parent FG3].
All participants were asked what parents would think of their child being rewarded for diabetes self-care. While the majority of participants discussed their family’s openness to FI, a few parents and adolescents talked about FIs being incongruent with their family’s philosophy. These parents saw diabetes self-care as a given obligation and good health as the only acceptable reward; for example one parent said: “You’re being rewarded hopefully with good health and everything. I think, unfortunately, [it’s] their burden to carry and just giving money just because you try to live a healthy life…it’s not worth [a financial reward]” [Parent FG6].
Urgent Need for Improved Self-Care
Parental desire, and in some cases desperation, for novel strategies to improve their child’s diabetes self-care was a contextual factor that pushed even parents who were generally uncomfortable with FI to further consider its potential value. Parents talked about desperation growing out of a lived experience that made them intimately familiar with the immediate threat of suboptimal self-care: “This is a serious disease. He was in diabetic ketoacidosis last January, it was the most terrifying thing. I mean, it was horrible to see him like that. So sick, he was throwing up, dry heaving, it was awful” [Parent FG5]. Even participants who had not experienced acute complications were consistently focused on managing the daily threat: “My biggest fear, and I’m sure everyone can concur, is the low [glucose level]” [Parent FG11].
Perhaps even more challenging than avoiding short-term complications was acknowledging and navigating the threat of long-term health consequences: “They can’t see the big picture. They can’t see the end result. They just think they’re invincible. They don’t know they’re gonna be blind [later]” [Parent FG12], explained one parent. Teens reported parents used “bigger scary stories” to describe acute complications that could result from poor management of the daily threat: “They’ve told me stories about people with diabetes who don’t take care of themselves, lose fingers and toes and sometimes legs” [Adolescent FG2]. Immediate adolescent priorities undermined parental attempts to instill a focus on long-term health in adolescents: “I was so heartbroken and wanted to cry. I was like, ‘Oh my gosh, you are going to ruin all of your organs because you are embarrassed about beeps [from your continuous glucose monitor]’…I felt so sad about that” [Parent FG8].
This understanding of consequential short- and long-term outcomes of inadequate diabetes self-care led some parents to want to exhaust all options that they perceived might be effective in promoting improved self-care: “We’ve taken him to the nutritionist. We have done counseling. We’ve done all these things but he just... doesn’t want to face the fact that this is what his life is and what he has to do” [Parent FG6]. Thus, for some parents, desperation for an effective tool to promote diabetes self-care outweighed any discomfort with using FIs as a means to motivate their adolescent with T1D.
Perceived Effectiveness for FI Programs to Promote Improved Diabetes Self-Care
Participant’s beliefs about the potential of FI programs to positively impact self-care behaviors was a key driver in making their conclusions about the acceptability of FI programs. While families’ prior attempts to generate their own incentive programs gave context to perceived effectiveness, they also considered how a third-party incentive administrator would impact the likely success of a FI program achieving the desired outcome of improved self-care.
Anticipated Impact of FI Programs on Short- and Long-Term Self-Care Engagement
The vast majority of adolescents and parents believed that FI had the potential to promote increased self-care behavior during the short-term incentive period. Both adolescents and parents shared that FI have a unique motivational value for adolescents given limited earning opportunities.
Adolescents discussed how FI could have an impact because it conveys an immediate benefit whereas routine engagement in diabetes self-care does not usually convey a perceptible immediate effect: “[FI] keeps you more motivated because you’re actually getting a reward, you’re not just working for nothing” [Adolescent FG7] Participants also considered whether new FI-inspired habits would deteriorate after the incentive period, and if so, whether that would that negate the benefit of using FI. A substantial proportion felt that developing the habit would override the tendency to backslide once rewards disappeared, because “once it becomes routine, it’s just routine” [Adolescent FG10].
Participants viewed the short-term FI period as the catalyst that would make self-care consistent enough for adolescents to experience a “new normal.” A parent explained how they anticipated the interconnected elements of self-efficacy, feeling better, and perceived health benefits leading to long-term proper self-care; “Over the six-month period [in an FI program, my child would] realize… ‘Wow, I can do this.’ And after a while, the financial piece will fade away. ‘Cause they’ll see, ‘I feel good. My numbers are better, I’m healthier, and Dad or Mom don’t have to remind me all the time to do something.’ So, I think there’s more benefits to it in the long run” [Parent FG11]. One teen was adamant that FI-inspired behavior change coupled with feeling better would promote permanent change: “That would have changed my life as a [younger] kid. If I would’ve realized that, like, with money changing this habit, I feel better. I would never have gone back to the way things were. That would have been my life” [Adolescent FG9].
While the vast majority of participants saw the potential success of an FI program for themselves or their children, a few expressed concerns that not all teens would develop positive habits, and some would become reliant on the reward. As one adolescent expressed, “Once I’m on my own later off, I might not actually do it on my own because I’m expecting to get paid or something like that, but I’m not” [Adolescent FG10]. Several adolescents were also wary due to the belief that FI was not sufficient to promote behavioral change, even in the short term, due to other barriers; “I think regardless of whether you get rewards or not, you’re going to run into issues” [Adolescent FG10]. One parent was adamant that her child would earn the FI but would consciously not change his behavior permanently nor develop positive habits.
Anticipated Impact of Third-Party Administration on FI Effectiveness
Many participants who anticipated that FI programs would be effective in promoting diabetes self-care had previous experience with the failure of family generated incentive schemes. Although family generated incentives often did not result in the desired goal of changing their child’s behavior long-term, most parents conceded that the failure of their personal use of FI was likely due to poor implementation. Some acknowledged that selecting a behavior or outcome to incentivize based on the parent’s expectations rather than considering the difficulty of the task from the adolescent’s perspective had set the family up for failure; “I always focused on paying or rewarding the behavior that was causing us the most erratic blood sugar management…I see now that possibly, I picked the worst thing because it was a major piece for him to get into place…maybe if I’d started with something that wasn’t, as you might call it, high pressure” [Parent FG3].
Parents who recognized flaws in their family’s incentive programs often anticipated that FI administered in a structured, third-party program would overcome these shortcomings. One parent shared that their adolescent sees the endocrinologist as a more credible expert than the parent, implying that adults associated with an incentive program might be similarly credible. Another observed, “It has more value if somebody else gives it to them” [Parent FG3]. Several parents said that adolescents would be more honest if a third party was paying them. Parents also reflected that they had previous experience with third-party administered incentive programs succeeding with their children, such as with their orthodontist for oral self-care and with the local library for promoting summer reading. An adolescent explained that she liked the idea that her parent would not have to finance the incentives stating that she already felt guilty about the cost of her disease knowing “that diabetes especially siphons a lot of resources out of my family” [Adolescent FG10].
Participants were also excited about how third-party administrators would provide outside accountability for diabetes self-care, addressing a point of tension in their current systems created by relying on adolescents for accurate self-reports. Participants were optimistic that this feature of FI programs would de-escalating existing power struggles between adolescents and parents: “I wanna get away from all of that. I wanna have a good relationship with him, not be the diabetes police and mom’s a b**ch you know?” [Parent FG11]. For one busy parent, the program could not happen fast enough: “I got kids in college, I got all the stuff going on, and you come to me with an organized plan: ‘Okay, this is what we have. If you do this, you get this. Here [are] all the boundaries.’ Done” [Parent FG3].
DISCUSSION
The qualitative results of our study highlight that most potential FI program participants, adolescents struggling with T1D management, and parents of adolescents with T1D viewed a FI program designed to support diabetes self-care as acceptable. Many families already attempt to use incentives and believe that systematic and consistent FI could be leveraged when adolescents are struggling to engage in recommended T1D self-management with increased independence. These findings, combined with the fact that meeting glycemic targets is challenging for most adolescents, suggest that while the use of FI is not a comprehensive strategy that would be utilized by all families, it is a possible lever to increase adolescent engagement in T1D self-care that should be rigorously explored.
Our FG participants’ multifaceted analysis of acceptability maps on to theoretical frameworks outlining the components contributing to the perceived acceptability of health interventions. As described by Sekhon et al, the potential intervention participants’ concept of acceptability was assembled by weighing the perceived effectiveness and fit of the intervention with their value system.27 While our FG participants focused on Sekhon’s constructs of perceived effectiveness and ethical acceptability, we anticipate that cognitive and emotional responses would evolve throughout FI program participation. Thus, temporal assessment of intervention acceptability should be considered both during and after intervention participation in order to identify ways to continually optimize program uptake and engagement.
The results of pilot FI programs for adolescents with T1D are well aligned with our FG participants’ predictions of increased frequency of self-care behavior.13,36. This may be linked to increased program engagement in older teens with more financial independence.36 However, these studies had small samples, some did not include control groups, most had weak theoretical underpinning for the value and structure of incentives, and incentives rarely resulted in sustained changes in health behaviors. Thus, there remains a need to test different structures for a T1D FI program to determine the ideal value and structure of incentives to promote meaningful behavior change in a cost-effective manner. Discrete choice experiments offer a methodology in which adolescents can compare the motivational value of dozens of possible incentive structures, thus strengthening the economic theory behind the design of future FI programs.37,38
Adolescents and parents both articulated an anticipated trajectory for adolescents’ positive behavior change and increased understanding of the link between consistent self-care and “feeling better”, resulting in intrinsic motivation beyond the incentive period. This is reinforced by the perspectives of adolescents in pilot FI programs stating that extrinsic financial rewards aided them in realizing the intrinsic value of self-care.13 While some trial programs incorporating FI have led to sustained improvements in participant HbA1c15,16 others had non-significant effects on glycemic control.13,36 This heterogeneity highlights the need for future work to optimize the structure of FI to appeal to adolescent preferences and address the multifactorial nature of glycemic control. Future FI trials should include randomized controlled trials that utilize these optimized incentive structures and include long-term monitoring of glycemic control, measures of intrinsic motivation.39 The inclusion of a non-incentivized control groups with goal setting support would help to distinguish the effect of the value of incentives from the effect of engagement with a program itself.
Beyond the potential of FI for mitigating the immediate and long-term health threat of not meeting glycemic targets, participants also highlighted the appeal of FI programs as a tool to assist in the often-turbulent transition to independent self-care. While families are encouraged to increase the responsibility that adolescents assume for their care as they mature, there is scarce support to facilitate this increase in independence.40–42 Information regarding the impact of FI on family dynamics is of particular interest due to concordance between high rates of conflict in parents-child dyads and worse glycemic control.43 Exploration of change in diabetes distress and family conflict should be considered in the evaluation of future FI pilot programs.44,45
This study has limitations. Due to the qualitative nature of our data, the present work simply supports the hypothesis that FI may be able to support improved self-care and that families may be open to participating in an FI program, but does not test effectiveness of specific attributes of such a program. A rigorous quantitative study in a socioeconomically diverse sample could help determine which incentive designs could promote meaningful engagement in adolescent diabetes self-care. Also, while our FGs were racially representative of the adolescent population served by the Seattle Children’s Hospital Diabetes Clinic and our parental focus groups were socioeconomically representative, there were gaps in self-reported socioeconomic information from adolescent participants and our findings may not be generalizable. While we did not observe a difference between mothers and fathers in our FGs, self-identified primary caregivers were mostly mothers and it is possible that fathers would hold different opinions. However, since mothers do more childrearing than fathers, their perspective may better represent desired incentives.46,47
FI programs that couple appropriately valued incentives with meaningful diabetes self-care behaviors offer an acceptable potential strategy to promote optimal glycemic control and thus, improved health outcomes. Additionally, the use of FIs may help adolescents with T1D develop strong self-care habits, easing the often-turbulent transition to independent self-care. Future research is needed to determine the ideal value and structure of FI in order to design programs in which the upfront costs of providing FI will be offset by the reduction of extreme and costly health events over an individual’s lifetime.2,3,16,48 Our qualitative results indicate that it is prudent to continue work to identify effective ways to deploy FI as a strategy to support adolescents in achieving self-care goals for diabetes management.
Supplementary Material
Acknowledgements:
Funding: This work was supported by the American Diabetes Association (#1–18-ICTS-100) F.S.M’s time was supported in part by a K23 Career Development Award from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK119465).
Footnotes
Conflict of Interest:
None.
REFERENCES
- 1.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes care. 2015;38(6):971–978. [DOI] [PubMed] [Google Scholar]
- 2.Levine B-S, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LM. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. The Journal of pediatrics. 2001;139(2):197–203. [DOI] [PubMed] [Google Scholar]
- 3.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: a longitudinal cohort study. Diabetes care. 2001;24(9):1536–1540. [DOI] [PubMed] [Google Scholar]
- 4.Rausch JR, Hood KK, Delamater A, et al. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes care. 2012;35(6):1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datye KA, Moore DJ, Russell WE, Jaser SS. A review of adolescent adherence in type 1 diabetes and the untapped potential of diabetes providers to improve outcomes. Current Diabetes Reports. 2015;15(8):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group DCaCTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England journal of medicine. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 7.Taddeo D, Egedy M, Frappier J-Y. Adherence to treatment in adolescents. Paediatrics & child health. 2008;13(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palladino DK, Helgeson VS. Friends or foes? A review of peer influence on self-care and glycemic control in adolescents with type 1 diabetes. Journal of pediatric psychology. 2012;37(5):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale DR, Viner RM. The correlates and course of multiple health risk behaviour in adolescence. BMC public health. 2016;16(1):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipping R, Campbell RM, MacArthur G, Gunnell D, Hickman M. Multiple risk behaviour in adolescence. Journal of Public Health. 2012;34(suppl_1):i1–i2. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan A, While A, Coyne I. The experiences and impact of transition from child to adult healthcare services for young people with Type 1 diabetes: a systematic review. Diabetic Medicine. 2015;32(4):440–458. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes R, Chinn CC, Li D, et al. Financial Incentives for Medicaid Beneficiaries With Diabetes: Lessons Learned From HI-PRAISE, an Observational Study and Randomized Controlled Trial. American Journal of Health Promotion. 2018;32(7):1498–1501. [DOI] [PubMed] [Google Scholar]
- 13.Wong CA, Miller VA, Murphy K, et al. Effect of financial incentives on glucose monitoring adherence and glycemic control among adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA pediatrics. 2017;171(12):1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner JA, Petry NM, Weyman K, et al. Glucose management for rewards: A randomized trial to improve glucose monitoring and associated self‐management behaviors in adolescents with type 1 diabetes. Pediatric diabetes. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanger C, Lansing AH, Scherer E, Budney A, Christiano AS, Casella SJ. A Web-Delivered Multicomponent Intervention for Adolescents with Poorly Controlled Type 1 Diabetes: A Pilot Randomized Controlled Trial. Annals of Behavioral Medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petry NM, Cengiz E, Wagner JA, Weyman K, Tichy E, Tamborlane WV. Testing for rewards: a pilot study to improve type 1 diabetes management in adolescents. Diabetes Care. 2015;38(10):1952–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy J, Hope R, Bhabha J, Eyal N. Paying for antiretroviral adherence: is it unethical when the patient is an adolescent? Journal of medical ethics. 2017;43(3):145–149. [DOI] [PubMed] [Google Scholar]
- 18.London AJ, Borasky DA Jr, Bhan A, Network EWGotHPT. Improving ethical review of research involving incentives for health promotion. PLoS medicine. 2012;9(3):e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner AD, Shah SK, Njuguna IN, et al. Financial Incentives to Motivate Pediatric HIV Testing—Assessing the Potential for Coercion, Inducement, and Voluntariness. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2018;78(3):e15–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah S, Malik F, Kirsten S, et al. Ethics of using financial incentives to promote self-care among adolescents with Type 1 diabetes. Pediatric Academic Societies; 2019. [Google Scholar]
- 21.Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? Comparing behaviors studied in psychological and economic literatures. Health Psychology. 2013;32(9):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychological bulletin. 1999;125(6):627. [DOI] [PubMed] [Google Scholar]
- 23.Frey BS, Oberholzer-Gee F. The cost of price incentives: An empirical analysis of motivation crowding-out. The American economic review. 1997;87(4):746–755. [Google Scholar]
- 24.Promberger M, Brown RC, Ashcroft RE, Marteau TM. Acceptability of financial incentives to improve health outcomes in UK and US samples. Journal of medical ethics. 2011;37(11):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoddinott P, Morgan H, MacLennan G, et al. Public acceptability of financial incentives for smoking cessation in pregnancy and breast feeding: a survey of the British public. BMJ open. 2014;4(7):e005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bigsby E, Seitz HH, Halpern SD, Volpp K, Cappella JN. Estimating acceptability of financial health incentives. Health Education & Behavior. 2017;44(4):513–518. [DOI] [PubMed] [Google Scholar]
- 27.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC health services research. 2017;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vass C, Rigby D, Payne K. The role of qualitative research methods in discrete choice experiments: a systematic review and survey of authors. Medical Decision Making. 2017;37(3):298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Academic Medicine. 2014;89(9):1245–1251. [DOI] [PubMed] [Google Scholar]
- 30.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. International journal for quality in health care. 2007;19(6):349–357. [DOI] [PubMed] [Google Scholar]
- 31.Morse JM. The significance of saturation. Sage Publications Sage CA: Thousand Oaks, CA; 1995. [Google Scholar]
- 32.Bazeley P Qualitative data analysis: Practical strategies. Sage; 2013. [Google Scholar]
- 33.Hennink M, Hutter I, Bailey A. Qualitative research methods. Sage; 2010. [Google Scholar]
- 34.Pope C, Ziebland S, Mays N. Qualitative research in health care: analysing qualitative data. BMJ: British Medical Journal. 2000;320(7227):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. [Google Scholar]
- 36.Raiff BR, Barrry VB, Ridenour TA, Jitnarin N. Internet-based incentives increase blood glucose testing with a non-adherent, diverse sample of teens with type 1 diabetes mellitus: a randomized controlled Trial. Translational behavioral medicine. 2016;6(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. PharmacoEconomics. 2019;37(2):201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright DR, Saelens BE, Fontes A, Lavelle TA. Assessment of Parents’ Preferences for Incentives to Promote Engagement in Family-Based Childhood Obesity Treatment. JAMA network open. 2019;2(3):e191490-e191490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greening L, Stoppelbein L, Moll G, Palardy N, Hocking M. Intrinsic motivation and glycemic control in adolescents with type 1 diabetes. Diabetes care. 2004;27(6):1517–1517. [DOI] [PubMed] [Google Scholar]
- 40.Peters A, Laffel L, Group ADATW. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes care. 2011;34(11):2477–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: Links to health outcomes. Journal of Pediatric Psychology. 2007;33(5):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comeaux SJ, Jaser SS. Autonomy and insulin in adolescents with type 1 diabetes. Pediatric diabetes. 2010;11(7):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vesco AT, Feldman MA, Evans MA, Weissberg-Benchell J. Parent–adolescent dyadic diabetes distress: Associations with A1c and diabetes-related strengths. Families, Systems, & Health. 2018;36(3):357. [DOI] [PubMed] [Google Scholar]
- 44.Weissberg‐Benchell J, Antisdel‐Lomaglio J. Diabetes‐specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes‐teen version. Pediatric diabetes. 2011;12(4pt1):341–344. [DOI] [PubMed] [Google Scholar]
- 45.De Wit M, Delemarre-van de Waal HA, Pouwer F, Gemke RJ, Snoek FJ. Monitoring health related quality of life in adolescents with diabetes: a review of measures. Archives of disease in childhood. 2007;92(5):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacob-Files E, Powell J, Wright DR. Exploring parent attitudes around using incentives to promote engagement in family-based weight management programs. Preventive medicine reports. 2018;10:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchi SM, Robinson JP, Milke MA. The changing rhythms of American family life. Russell Sage Foundation; 2006. [Google Scholar]
- 48.Stanger C, Ryan SR, Delhey LM, et al. A multicomponent motivational intervention to improve adherence among adolescents with poorly controlled type 1 diabetes: a pilot study. Journal of pediatric psychology. 2013;38(6):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


