Abstract
The inverted repeat (IR) lacking clade (IRLC) is a monophyletic group within the Papilionoideae subfamily of Fabaceae where plastid genomes (plastomes) do not contain the large IR typical of land plants. Recently, an IRLC legume, Medicago minima, was found to have regrown a ~9 kb IR that contained a number of canonical IR genes, and closely related M. lupulina contained an incomplete IR of ~425 bp. Complete plastomes were generated for seven additional species, putative members of the M. minima clade. Polymerase chain reaction was employed to investigate the presence of the IR across M. minima and M. lupulina including individuals of nine and eight Eurasian and North African accessions and 15 and 14 Texas populations, respectively. While no sequence similar to the ~9 kb IR was detected among the seven newly sequenced plastomes, all Eurasian and North African accessions of M. minima contained the IR. Variation in IR extent was detected within and between the Texas populations. Expansions of 13 bp and 11 bp occurred at the boundaries of both IR/small single‐copy regions, and populations had one or the other expansion, but not both. Expansion of the IR was not detected in the accessions from Eurasia and North Africa suggesting recent mutations yielded at least two additional plastid haplotypes in M. minima.
Keywords: alfalfa, gene conversion, homologous recombination, inverted repeat expansion, medicago, plastid haplotype variation, plastome evolution
A recent study revealed the reemergence of the plastome inverted repeat (IR) in Medicago minima, which belongs to a clade of legumes defined by its absence. Seven putatively related plastomes were sequenced, and accessions/populations of M. minima from Europe, Asia, Africa, and Texas were assayed for presence of the IR and variation in its boundaries. While “Old World” populations of M. minima all contained identical IRs to the accession that was previously sequenced, variation in the IR boundaries was detected within and between Texas field‐collected populations. Range expansion, haplotype distribution, and IR expansion mechanisms are discussed.

1. INTRODUCTION
A plastid genome (plastome) containing large and small single‐copy regions (LSC and SSC, respectively) separated by a large inverted repeat (IR) is widely acknowledged to represent the ancestral form of the plastome monomer of land plants. The typical plastome IR is on the order of 25–30 kb with most size variation driven by expansion and contraction of IR boundaries (Jansen & Ruhlman, 2012; Ruhlman & Jansen, 2014). The ancestral form is not, however, ubiquitous among photosynthetic plants. In disparate lineages, the IR has massively expanded, contracted, and been entirely lost (Ruhlman & Jansen, 2018; Zhu et al., 2016).
Major IR expansions identified among Pelargonium species yielded the largest plastomes, which contain IRs that ranged up to ~88 kb (Weng et al., 2017). Likewise, some of the smallest plastomes among autotrophic plants lack the IR entirely (Blazier et al., 2016; Ruhlman et al., 2017; Sabir et al., 2014; Sanderson et al., 2015), or contain highly reduced remnants of the ancestral repeat (Guisinger et al., 2011). In at least two cases, IR reemergence has been documented within lineages where the IR was lost. The highly unusual long‐branch clade (LBC) species of Erodium exhibit novel IR structures ranging from 25 kb to more than 47 kb concomitant with plastome expansion (Blazier et al., 2016). While it is somewhat obscure whether there were two independent IR losses, or a shared loss with reemergence exclusively in the LBC for the return of the Erodium IR, the case in one clade of legumes is clear.
Loss of the IR is the predominant character that defines the IR lacking clade (IRLC; Wojciechowski et al., 2004) of papilionoid legumes. Since the earliest example of IR loss was uncovered (Kolodner & Tewari, 1979), direct DNA sequencing and more recently next generation, high‐throughput sequencing have facilitated analyses that supported the monophyly of the IRLC (McMahon & Sanderson, 2006; Schwarz et al., 2017; Wojciechowski et al., 2000). The genus Medicago contains about 87 described species (Small, 2011) and belongs to the tribe Trifolieae, which is nested within the IRLC (Cardoso et al., 2015; The Legume Phylogeny Working Group, 2017). The most well recognized Medicago species is the food and forage crop alfalfa (M. sativa. ssp. sativa). Also notable is the plant research model M. truncatula, with high reproductive rates and amenability to genetic manipulation. Medicago were not considered highly rearranged (Cai et al., 2008; Sveinsson & Cronk, 2016); however, this assumption was based on a single plastome, that of M. truncatula. Expanded sampling included 19 species representing all the major clades in the genus and found only modest divergence among congeners with regard to structural organization overall (Choi et al., 2019). However, one clade contained unique variation in repeat structure including an incomplete IR in M. lupulina and the presence of a novel IR of ~9 kb in M. minima. The sequences that flank the novel IR differ from the canonical boundary sequences (Mower & Vickrey, 2018; Yamada, 1991), yet the IR of M. minima contains a portion of the ribosomal operon (rrn23 … trnN‐GUU), with the remaining IR core genes (trnV‐GAC…trnA‐UGC) and other common IR sequences lying upstream of IRb (Choi et al., 2019).
The previous study included single individuals for just three taxa in the M. minima clade. To examine the evolution of IR reappearance in the clade, plastomes for seven taxa suggested to be close relatives in phylogenetic studies (see Small, 2011) were completed. Additionally, populations of M. minima and M. lupulina from across the native range, Northern Africa, and Central Asia, as well as field‐collected populations from across Texas were assayed for the presence of the novel IR and variation in its extent. Questions about the role of the IR in plastome recombination and replication have persisted since its discovery. Investigating variation in IR presence and extent within and between populations and closely related species may illuminate our understanding of the role of the IR and the mechanisms of plastome maintenance.
2. MATERIALS AND METHODS
2.1. Additional taxon selection and plastome sequencing
Phylogenetic studies suggested that additional taxa could be included in the monophyletic group that includes M. minima that were not included in the previous analyses (Small, 2011). Accessions of seven taxa were acquired from USDA‐GRIN (U.S. Department of Agriculture Germplasm Resources Information Network) (Table S1). Seeds were germinated and grown in the greenhouse at UT Austin and emergent leaves were collected in liquid nitrogen from single individuals. All DNA protocols followed Choi et al. (2019), including isolation, sequencing, assembly, and annotation of plastomes. Completed plastome sequences were submitted to NCBI and GenBank accession numbers are given in Table S1. Repeat content of newly sequenced plastomes was estimated according to Choi et al., 2019. Gene sequences were extracted from the new taxa and combined with the phylogenetic data set from the previous analysis (Choi et al., 2019). All shared protein‐coding sequences (69; see Table S2, (Choi et al., 2019) for 27 Medicago, and eight outgroups were used to infer relationships. All phylogenetic methods followed Choi et al. (2019).
2.2. PCR screen of Medicago minima and M. lupulina accessions
Nine accessions of Medicago minima and eight of M. lupulina were acquired from USDA‐GRIN (Table S2) to represent populations from Asia, Europe (Eurasia), and North Africa. Seeds were germinated and grown in the UT greenhouse. Emergent leaves were collected in liquid nitrogen, and DNA was isolated using the NucleoSpin Plant II (Macherey–Nagel, Düren, Germany). Texas field collections included 15 populations of M. minima and 14 of M. lupulina. Young leaves were harvested from a minimum of 10 individuals in each population and frozen in liquid nitrogen for DNA isolation. See Table S3 for accessions, location, and voucher information.
Individuals representing all included accessions, both from USDA and field collected, were vouchered and deposited in the TEX‐LL.
A PCR screen was applied to all accessions and employed primers utilized by Choi et al. (2019) to amplify IR junction sites in M. minima and to assess a small inverted repeat of interest identified previously in M. lupulina (Choi et al., 2019). Initial screening of USDA accessions and Texas populations of M. minima included three individuals, while single individuals were screened from each accession of M. lupulina. Four reactions were conducted for M. minima to amplify IR boundary regions (boundaries of IRA with large and small single‐copy sequences are abbreviated as J LA and J SA, respectively; the boundaries for IRB follow the same convention, that is, J LB and J SB) and two reactions amplified the repeat region in M. lupulina. Subsequent reactions amplified two polymorphic sites detected in M. minima (J SA, J SB) and were conducted with additional seven individuals from each Texas population (Tables S2 and S3). Sanger sequencing of all amplicons was carried out at the University of Texas Genomic Sequencing and Analysis Facility. Amplicon sequences were aligned to corresponding plastome loci in complete plastomes of M. minima (MK460499) and M. lupulina (MK460497) with MAFFT v.7.017 (Katoh & Standley, 2013), implemented in Geneious, using the default settings. Genbank accession numbers for sequenced amplification products are provided in Table S4.
3. RESULTS
3.1. Sequencing and phylogenetic inference for seven new Medicago plastomes
Complete plastomes were assembled for seven Medicago. Sequencing statistics and overall plastome characteristics are reported in Table 1, while Table S1 contains USDA‐GRIN, NCBI, and voucher accession numbers. Phylogenetic inference based on 69 shared plastid genes across 27 species placed three of the seven new Medicago in the M. minima clade. Medicago tenoreana and M. lupulina formed a clade sister to M. disciformis. These three together were sister to a clade that included M. coronata with M. minima. The other four taxa, M. arabica, M. sauvagei, M. secundiflora, and M. praecox were distributed within a large clade that was sister to the M. minima clade (Figure 1). None of the newly sequenced plastomes contained a structure resembling the IR of M. minima. The inclusion of M. secundiflora extended the size range of Medicago IR lacking plastomes, reported here as ~120 to ~126 kb (Table 1; Choi et al., 2019).
Table 1.
Plastome sequencing statistics for seven Medicago
| Taxon | Reads | Unit length (bp) | Plastome coverage | GC (%) | Repeat content (%) |
|---|---|---|---|---|---|
| M. praecox | 48,984,174 | 122,724 | 6,743 | 34.0 | 4.27 |
| M. secundiflora | 48,641,318 | 120,166 | 4,920 | 34.7 | 5.06 |
| M. sauvagei | 44,530,096 | 124,743 | 5,239 | 33.9 | 4.65 |
| M. arabica | 44,443,436 | 125,028 | 5,081 | 34.4 | 5.72 |
| M. disciformis | 49,327,710 | 121,039 | 5,768 | 34.2 | 4.46 |
| M. coronata | 49,364,864 | 123,864 | 5,784 | 34.2 | 5.99 |
| M. tenoreana | 47,121,870 | 120,857 | 8,310 | 34.2 | 5.74 |
Figure 1.
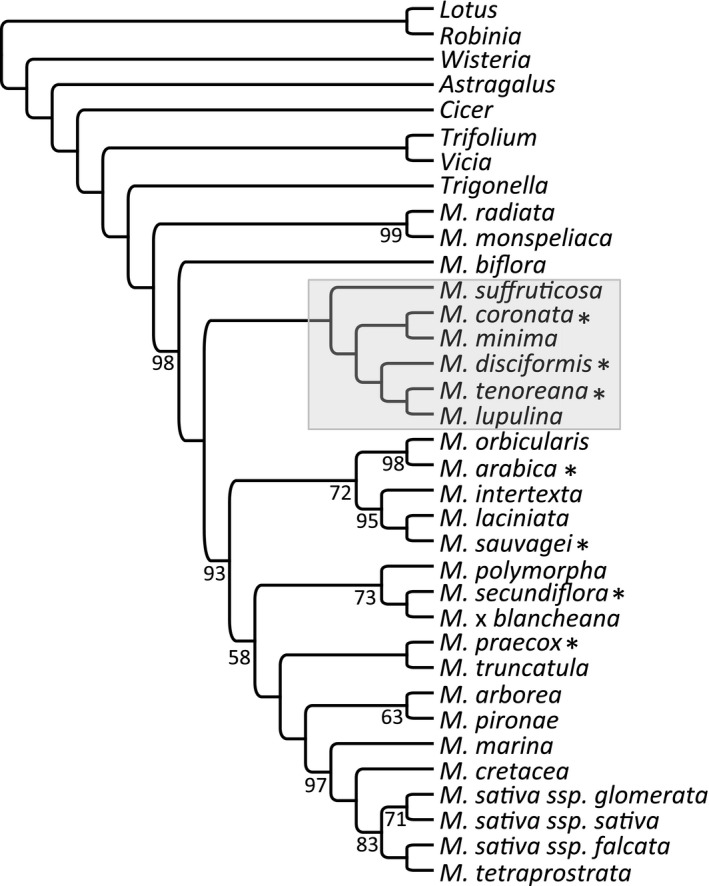
Relationships among 27 Medicago. The cladogram depicting relationships of Medicago (M) and outgroups was based on maximum likelihood phylogenetic inference using 69 protein‐coding genes shared across all taxa. Bootstrap values less than 100 are shown at the nodes. Newly sequenced plastomes are indicated by asterisks, and the M. minima clade is boxed in gray. ssp., subspecies
3.2. PCR screening for IR distribution and extent
Single individuals from USDA accessions and accessions collected from field sites in Texas were screened for variable sites of interest. For M. lupulina, PCR primers were designed to amplify repeat regions with possible analogy to the M. minima IR (Choi et al., 2019). Each of the two repeat units (aligned length two copies, 425 bp) were amplified for each individual. Sequenced amplicons from 22 M. lupulina individuals (eight from USDA; 14 from Texas; Tables S2 and S3) revealed no variation in the length or of boundary positions of the repeats relative to the original accession sequenced by Choi et al. (2019).
In all, 177 individuals of M. minima were screened for variation in IR/SC boundaries. Initial screens included three individuals of the ten sampled from each population. Accessions acquired from USDA, representing Eurasian and North African populations (Figure 2, Table S2), all contained the ~9 kb IR identified in the original sequenced plastome (Choi et al., 2019) and were identical with respect to the IR/SC boundaries. While no variation was detected in the position of the IR/LSC junction, initial screens of the Texas populations did reveal variation in the junction of the IR and the SSC region (Table S3). The remaining seven individuals from each Texas population were screened using primers to amplify the variable junctions at both IRA/SSC (J SA) and IRB/SSC (J SB).
Figure 2.
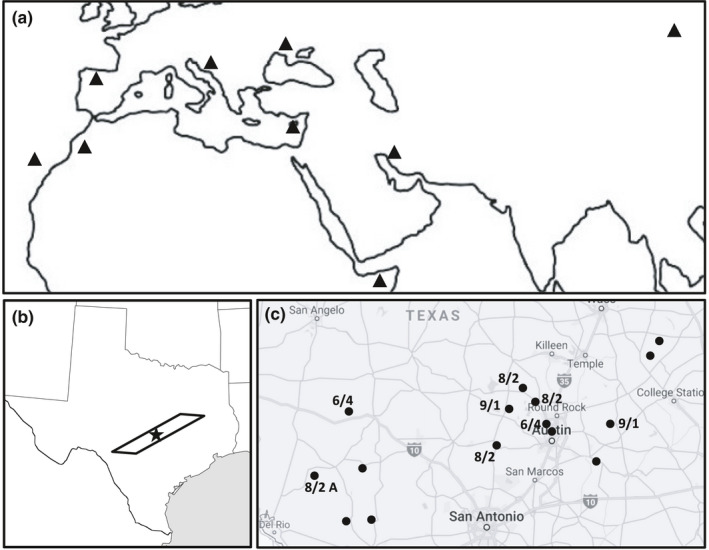
Accession origins of Medicago minima. Accessions of M. minima were acquired from USDA GRIN and collected from field sites in Texas. (a) Triangles indicate approximate origin of accessions from Eurasia and northern Africa (Table S2). An overview of transect (polygon) that included collections sites for all Texas accessions (b) along with specific locations, including haplotype distribution (c). Black star in (b) indicates the location of the state capitol, Austin TX. In (c), dots indicate individual sites. The values at each site give the O haplotype/B haplotype, except for one site (8/2 A), which indicates O/A haplotype distribution. Populations where only the O haplotype was detected lack an associated value (Table S3)
The sequences at J SA and J SB were polymorphic both within and between the Texas populations. Eight of the 15 sampled populations were polymorphic for expansions at either J SA or J SB, but not for both yielding three plastome IR haplotypes: Type O, representing the unexpanded IR identified in the originally sequenced plastome of M. minima (Choi et al., 2019); type A, derived from expansion that initiated at J SB; and type B, derived from expansion that initiated at J SA (Figure 3). Haplotype designation of “A” or “B” indicates the IR junction at which single‐copy sequence was overwritten resulting in expansion of the IR. Figure 2 and Table S3 summarize the geographic distribution of IR boundary variation among the Texas populations. While both polymorphisms extended the IR into the SSC, the less common was expansion to yield the A haplotype. This expansion, which was identified in a single population, appeared to include an additional 13 bp of single‐copy sequence in the IR. The expansion includes 10 bp upstream of J SB that were single copy in M. minima plastomes that lack the expansion. Five base pairs were inserted at J SA (TTTAT), and five base pairs of formerly single‐copy sequence have likely undergone gene conversion (ATAGA ➔ TATGA) homogenizing the two repeats. Coincidentally, extension of the IR places both SSC junctions adjacent to an existing three‐base sequence (TGG) that is present near both ends of the SSC in M. minima plastomes that contain unexpanded IRs. This three‐base sequence does not appear to have been duplicated through IR expansion, but given its identical sequence at both junctions may be considered as included in the IR of the A haplotype. The polymorphic expansion yielding haplotype B included 11 bp in the M. minima IR relative to the unexpanded O haplotype. This expansion appeared less complex, with the sequence formerly found in the SSC adjacent to J SB overwritten by gene conversion (Figure 3).
Figure 3.
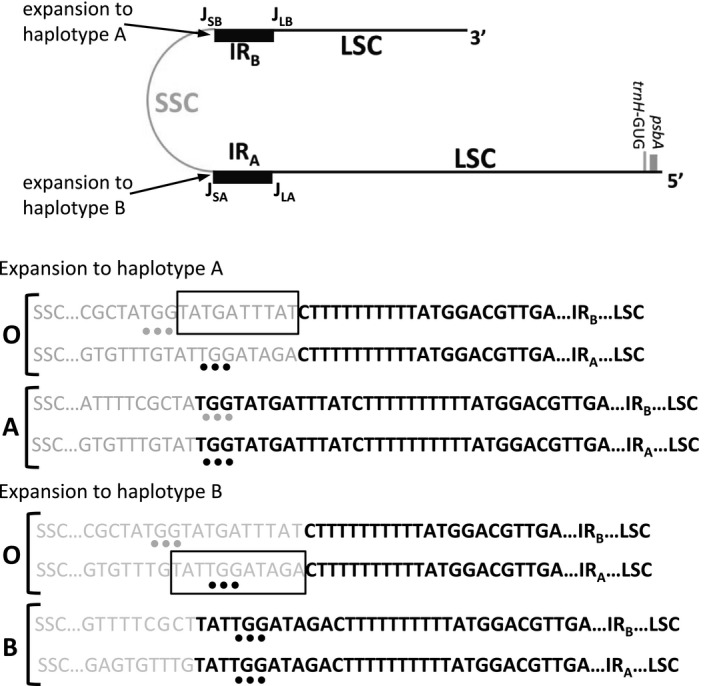
Inverted repeat length variation in Medicago minima. Both polymorphisms extended the IR into the small single‐copy region (SSC). A schematic representing a single unit of the M. minima plastome is presented for context. Inverted repeat expansion to the A haplotype (A) included an additional 13 bp of single‐copy sequence (gray) in the IR. The expansion includes 10 bp upstream of JSB (boxed) that were single copy in M. minima plastomes that lack the expansion (O). Five base pairs were inserted at JSA (TTTAT), and five base pairs of formerly single‐copy sequence have likely undergone gene conversion (ATAGA ➔ TATGA) homogenizing the two repeats. The extension of the IR places both junctions adjacent to an existing three‐base sequence (TGG, gray, and black dots) that is present in near both ends of the SSC in M. minima plastomes that contain unexpanded IRs. The polymorphic expansion yielding haplotype B (B) included 11 bp in the M. minima IR relative to the unexpanded O haplotype. Gene conversion templates are boxed. bp; base pairs. LSC; large single‐copy region. SSC; small single‐copy region. IR; inverted repeat. JSB and JLB; boundary of IRB with SSC and LSC, respectively. JSA and JLA; boundary of IRA with SSC and LSC, respectively
4. DISCUSSION
The phylogeny inferred in Choi et al. (2019) indicated that M. minima and M. lupulina were sister species, suggesting that repeat‐related phenomena were shared between them. The inclusion of three of the new plastomes in the M. minima clade parsed M. minima from M. lupulina (Figure 1). The lack of any structure that resembled the novel 9 kb IR or possible precursor sequences identified in the previous study (Choi et al., 2019) in the new taxa, together with the finding that M. minima and M. lupulina are not sister taxa support independence between the 9 kb IR of M. minima and the two repeat units of M. lupulina (aligned length two copies, 425 bp).
Within species variation in plastome sequence and structure has been reported for a few groups of angiosperms and is often associated with geographical isolation of breeding populations (Barnard‐Kubow et al., 2014), experimental crosses or among cultivars (Gurdon & Maliga, 2014). Less commonly reported however is plastome variation among individuals within natural populations. Plastome transmission is primarily uniparental and predominantly maternal in most groups providing for the assumption that plastomes will be uniform among individuals due to the lack of sexual recombination (Birky, 2001; Greiner et al., 2015). In biparental lineages, those that inherit their plastomes from both the pollen and seed parent, the potential for plastome haplotype diversity is greatly increased.
Several studies have investigated the mode of organelle inheritance in different species of Medicago. Many efforts have been dedicated to characterizing alfalfa (Medicago sativa ssp. sativa) and the closely allied taxa of the M. sativa complex, which contains both tetraploid (2n = 4x = 32) and diploid (2n = 2x = 16) genotypes (Quiros & Bauchan, 1988). The members of the complex appear to be interfertile, as hybrids have been achieved both within and between ploidy levels (Masoud et al., 1990; Quiros & Bauchan, 1988; Schumann & Hancock, 1989). Both the diploids and the tetraploids of the complex are allogamous and employ varying degrees of self‐incompatibility, lending to their value as agricultural crops as allogamy often results in larger individuals and increases amenability to hybrid production. Inheritance modes have been identified as predominantly uniparental paternal, biparental, and uniparental maternal, depending on a number of variables (Lee et al., 1988; Nagata et al., 1999; Schumann & Hancock, 1989; Smith, 1989; Zhu et al., 1993).
The diploid annuals of Medicago have been less investigated. These diminutive species have carried only passing interest as a source of germplasm to improve the crop species M. sativa, due in part to their recalcitrance to hybridization. The annual species are autogamous, with both male and female reproductive structures completely contained within the minute flowers. Only rare instances of hybridization have been reported, and only under experimental conditions, where abnormalities ranged from early embryo abortion to chlorophyll deficiencies in hybrid progeny (Quiros & Bauchan, 1988 and references therein). Among the diploid annuals, M. truncatula has received the most attention as a genetic model system. Mechanical cross‐pollination experiments designed to examine plastome inheritance have employed ecotypes that contain polymorphic sites so that the parental origin of the sequence marker can be followed in “hybrid” progeny (i.e., Matsushima et al., 2008). While both seed and pollen parental markers were detected in cotyledons of F1 progeny, leaves evaluated later in development tended to retain only one or the other marker. Populations of F2 progeny raised from the seed of a single mother uniformly showed the markers of either parent supporting the notion that vegetative segregation (sorting out) resolves rapidly in this system (Birky, 1978; Johnson & Palmer, 1989).
The annual diploid Medicago minima is a predominantly self‐pollinated inbreeder that yields highly uniform progeny and is naturalized the world over (Small, 2011). Although omitted from an 1891 list of Medicago reported in a flora of Western Texas (Coulter, 1891), M. minima was recorded in North America (Alabama and Louisiana) in a 1901 publication from the US Department of Agriculture (Mohr, 1901). A USDA publication from 1913 discusses M. minima, along with several other nonperennial Medicago that were introduced to study their agronomic value (McKee & Ricker, 1913). By 1914, a herbarium specimen of M. minima was collected in Travis County, Texas (M. S. Young s.n., TEX00340358, TEX‐LL). Later reports include mentions of the species in Virginia (Fernald, 1941), Oklahoma (Hopkins et al., 1943), Arizona, and California (Howell, 1949). Today, M. minima can be found on six continents and is particularly common in disturbed habitats (Small, 2011). Most likely, multiple introductions of M. minima throughout North and South America in association with livestock imports have contributed to the present day populations in the Americas. Its burr‐like appendages on the seed capsules allow attachment to livestock as well as wildlife further contributing to range expansion.
Among the diploid Medicago, M. minima has received more recent notice for its potential as a forage particularly in the arid regions of south‐central Texas (Muir et al., 2005; Smith et al., 2008; Ueckert et al., 2003; http://aggieclover.tamu.edu/files/2010/06/ForageLegumesTexas.pdf). Recognized by its common name, little burr medic, M. minima was suggested to have been introduced to Texas sometime in the early twentieth century (Diggs et al., 1999) in approximate agreement with the herbarium record from 1914. A number of ecotypes/cultivars were collected from several locations in Texas for evaluation of tolerance to the colder and/or drier regions of the state (Ocumpaugh, 2001) by researchers from Texas A&M University. One cultivar collected from a grass pasture near Devine, TX showed particular promise on the poor soils in the southwestern part of the state. The unimproved cultivar “Devine” was released in 2005 (Smith et al., 2008) and registered to Texas A&M AgriLife foundation seed (Ocumpaugh et al., 2007). Currently, little burr medic “Devine” is produced and distributed under license by Pogue Agri Partners of Kenedy, TX, and sold throughout Texas and Oklahoma.
Mixed plastome haplotypes were identified within and between Texas collected populations of M. minima, while a single haplotype was detected among 10 populations across Eurasia and northern Africa. All individuals from all accessions contained at minimum the ~9 kb IR identified by Choi et al. (2019). This suggests that events leading to the reemergence of the IR in M. minima initiated prior to range expansion in Eurasia and northern Africa, and subsequent introduction to the Americas. Further expansion of IR boundaries is more obscure with respect to timing. A quick search of the US National Germplasm System site delivers 365 accessions across Eurasia and northern Africa, with sampling that includes just 10 accessions of progenitor populations containing either expansion may have been missed. Alternatively, either of the two IR expansions detected in the Texas populations could have arisen since introduction of the species to the Americas, the definitive timing of which remains uncertain as there were likely multiple and unintentional introductions. Regardless, the detection of populations with variable IR structure exclusively in the Texas populations suggests the mutations are recent.
Whether the frequencies of the detected plastome variations will change in the Texas populations will likely be independent of the parameters affecting breeding populations as outcrossing is rare in M. minima. In a predominantly or entirely selfing species, mode of inheritance would be unlikely to influence organelle haplotype diversity as the two gametes are inherited from the same parent, increasing homozygosity and reducing haploid and organelle gene flow. Reports of biparental inheritance of organelle genomes in Medicago have relied on either outcrossing taxa of the M. sativa complex or artificially fertilized diploid taxa. The presence of plastid haplotype variation in the Texas populations was more likely a result of intentional or unintentional mixture of seeds from different source populations. All collections sites for this study were current or former ranch lands, or current recreational hunting sites, which are commonly seeded with forage mixtures of Medicago species.
The more commonly observed variant haplotype included an additional 11 bp in the IR, while the less common variant included 13 additional bases, both extending the IR into the SSC. Branch migration of a Holliday junction across either IR/SSC boundary can result in heteroduplex formation prior to stalling of the replication fork. Resolution of the heteroduplex restarts replication with copy correction (gene conversion) yielding the parental situation or migration of the IR boundary depending on the template sequence. In both variants, no IR adjacent gene was disrupted by the gene conversion that homogenizes the IR, allowing the expansion to be retained. This mechanism has been invoked to explain small migrations in IR/SC boundaries in many land plants (Goulding et al., 1996; Wang et al., 2008; Zhu et al., 2016).
Incorporation of single‐copy sequence by branch migration across IR boundaries is unlikely to significantly expand the novel ~9 kb IR in M. minima. The extent of noncoding sequence flanking the IR should limit small scale expansion caused by Holliday junction migration across the IR boundaries. There remain 4 bp and 39 bp of noncoding sequences flanking the LSC boundaries with IRA and IRB, respectively, whereas 254 bp and 96 bp separate the IRA and IRB boundaries from SSC coding regions, respectively. These regions of noncoding sequence may be incorporated into the IR piecemeal; however, the need to maintain coding regions will require a mechanism capable to duplicate larger expanses of sequence. The recombination‐based mechanisms hypothesized to have played a role in reemergence of the IR in an IRLC legume could continue to include genes presently situated adjacent in the large (i.e., rpl20, ycf1) or small (i.e., psbB, trnA‐UGC) single‐copy regions. All of the genes contained the canonical IR are currently situated upstream of IRb in the SSC and remain in the same order those in typical IR containing plants.
Denser sampling in the M. minima clade failed to identify examples of IR expansion in closely related taxa, but instead revealed variation in the extent of the novel 9 kb IR across different naturalized populations of M. minima in Texas relative to germplasm accessions from Europe, Africa, and Asia. The presence of the IR in all accessions of M. minima along with the two unique IR boundary movements could suggest relaxed control of IR extent in species, if in fact such mechanisms exist. Small‐ scale movements of IR boundaries via gene conversion are thought to be random (Goulding et al., 1996) but are likely influenced by controls affecting homologous recombination.
Expanded sampling of M. minima populations throughout the Americas may help to elucidate source germplasm for the plastome haplotypes that were identified in central Texas, supporting a clearer chronology for the observed IR changes. Similar population level surveys focused on outcrossing taxa that display unusual IRs may provide a population genetics system to evaluate the differential fitness of plastome/IR haplotypes in a given environment. Such a study could illuminate long‐standing questions about the role of the plastome IR.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
In‐Su Choi: Conceptualization (equal); data curation (lead); formal analysis (lead); funding acquisition (equal); investigation (equal); methodology (equal); project administration (supporting); software (lead); supervision (equal); validation (equal); visualization (supporting); writing–original draft (supporting); writing–review and editing (supporting). Robert Jansen: Conceptualization (equal); data curation (supporting); formal analysis (supporting); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); writing–review and editing (supporting). Tracey A. Ruhlman: Conceptualization (equal); formal analysis (supporting); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); visualization (lead); writing–original draft (lead); writing–review and editing (lead).
Supporting information
Appendix S1–S4
ACKNOWLEDGMENT
This work was supported by grants from Texas Ecological Laboratory Program to R.K.J, T.A.R, and I.C., the National Science Foundation (DEB‐1853024) to R.K.J. and T.A.R and the Sidney F. and Doris Blake Professorship in Systematic Botany to R.K.J. The authors thank TEX‐LL for serving as a repository for voucher specimens and the United States Department of Agriculture Germplasm Resources Information Network for providing seed.
Choi I‐S, Jansen R, Ruhlman T. Caught in the Act: Variation in plastid genome inverted repeat expansion within and between populations of Medicago minima . Ecol. Evol. 2020;10:12129–12137. 10.1002/ece3.6839
In‐Su Choi, Robert Jansen and Tracey Ruhlman contributed equally to the work.
DATA AVAILABILITY STATEMENT
DNA sequences were deposited in GenBank. GenBank and USDA‐GRIN accession numbers, sample voucher information, and collection locations are provided in Appendix S1.
REFERENCES
- Barnard‐Kubow, K. B. , Sloan, D. B. , & Galloway, L. F. (2014). Correlation between sequence divergence and polymorphism reveals similar evolutionary mechanisms acting across multiple timescales in a rapidly evolving plastid genome. BMC Evolutionary Biology, 14 10.1186/s12862-014-0268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky, C. W. (1978). Transmission genetics of mitochondria and chloroplasts. Annual Review of Genetics, 12, 471–512. 10.1146/annurev.ge.12.120178.002351 [DOI] [PubMed] [Google Scholar]
- Birky, C. W. (2001). The inheritance of genes in mitochondria and chloroplasts: Laws, mechanisms, and models. Annual Review of Genetics, 35, 125–148. 10.1146/annurev.genet.35.102401.090231 [DOI] [PubMed] [Google Scholar]
- Blazier, J. C. , Jansen, R. K. , Mower, J. P. , Govindu, M. , Zhang, J. , Weng, M.‐L. , & Ruhlman, T. A. (2016). Variable presence of the inverted repeat and plastome stability in Erodium . Annals of Botany, 117, 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Z. , Guisinger, M. , Kim, H.‐G. , Ruck, E. , Blazier, J. C. , McMurtry, V. , Kuehl, J. V. , Boore, J. , & Jansen, R. K. (2008). Extensive reorganization of the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous repeated sequences and novel DNA insertions. Journal of Molecular Evolution, 67, 696–704. 10.1007/s00239-008-9180-7 [DOI] [PubMed] [Google Scholar]
- Cardoso, D. , São‐Mateus, W. M. B. , da Cruz, D. T. , Zartman, C. E. , Komura, D. L. , Kite, G. , Prenner, G. , Wieringa, J. J. , Clark, A. , Lewis, G. , Pennington, R. T. , & de Queiroz, L. P. (2015). Filling in the gaps of the papilionoid legume phylogeny: The enigmatic Amazonian genus Petaladenium is a new branch of the early‐diverging Amburaneae clade. Molecular Phylogenetics and Evolution, 84, 112–124. 10.1016/j.ympev.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Choi, I.‐S. , Jansen, R. , & Ruhlman, T. (2019). Lost and Found: Return of the inverted repeat in the legume clade defined by its absence. Genome Biology and Evolution, 11, 1321–1333. 10.1093/gbe/evz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter, J. M. (1891). Manual of the phanerogams and pteridophytes of western Texas: Polypetalae. Contrivutions from the United States National Herbarium, 2, 1–152. [Google Scholar]
- Diggs, G. M., Jr. & Lipscomb, B. L. , O'Kennon, B. , Mahler, W. F. , & Shinners, L. H. , (1999). Shinners & Mahler's illustrated flora of north central Texas. Botanical Research Institute of Texas. [Google Scholar]
- Fernald, M. L. (1941). Another century of additions to the flora of Virginia. Contributions from the Gray Herbarium of Harvard University, 139, 485–665. [Google Scholar]
- Goulding, S. E. , Wolfe, K. H. , Olmstead, R. G. , & Morden, C. W. (1996). Ebb and flow of the chloroplast inverted repeat. MGG Molecular & General Genetics, 252, 195–206. 10.1007/BF02173220 [DOI] [PubMed] [Google Scholar]
- Greiner, S. , Sobanski, J. , & Bock, R. (2015). Why are most organelle genomes transmitted maternally? BioEssays, 37, 80–94. 10.1002/bies.201400110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisinger, M. M. , Kuehl, J. V. , Boore, J. L. , & Jansen, R. K. (2011). Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: Rearrangements, repeats, and codon usage. Molecular Biology and Evolution, 28, 583–600. 10.1093/molbev/msq229 [DOI] [PubMed] [Google Scholar]
- Gurdon, C. , & Maliga, P. (2014). Two distinct plastid genome configurations and unprecedented intraspecies length variation in the accD coding tegion in Medicago truncatula . DNA Research, 21, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, M. , & Waterfall, U. T. , (1943). Notes on Oklahoma plants. Rhodora, 45, 113–117. [Google Scholar]
- Howell, J. T. (1949). Medicago minima in California and Arizona. Leaflets of Western Botany 5. [Google Scholar]
- Jansen, R. K. , & Ruhlman, T. A. (2012). Plastid Genomes of Seed Plants In Bock R., & Knoop V. (Eds.), Genomics of chloroplasts and mitochondria, advances in photosynthesis and respiration (pp. 103–126). Dordrecht, Switzerland: Springer. [Google Scholar]
- Johnson, L. B. , & Palmer, J. D. (1989). Heteroplasmy of chloroplast DNA in Medicago . Plant Molecular Biology, 12, 3–11. 10.1007/BF00017442 [DOI] [PubMed] [Google Scholar]
- Katoh, K. , & Standley, D. M. (2013). MAFFT: Multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner, R. , & Tewari, K. K. (1979). Inverted repeats in chloroplast DNA from higher plants. Proceedings of the National Academy of Sciences of the United States of America, 76, 41–45. 10.1073/pnas.76.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. J. , Blake, T. K. , & Smith, S. E. (1988). Biparental inheritance of chloroplast DNA and the existence of heteroplasmic cells in alfalfa. Theoretical and Applied Genetics, 76, 545–549. 10.1007/BF00260905 [DOI] [PubMed] [Google Scholar]
- Masoud, S. A. , Johnson, L. B. , & Sorensen, E. L. (1990). High transmission of paternal plastid DNA in alfalfa plants demonstrated by restriction fragment polymorphic analysis. Theoretical and Applied Genetics, 79, 49–55. 10.1007/BF00223786 [DOI] [PubMed] [Google Scholar]
- Matsushima, R. , Hu, Y. , Toyoda, K. , Sodmergen , & Sakamoto, W. (2008). The model plant Medicago truncatula exhibits biparental plastid inheritance. Plant Cell Physiology, 49, 81–91. 10.1093/pcp/pcm170 [DOI] [PubMed] [Google Scholar]
- McKee, R. , & Ricker, P. L. (1913). Nonperennial Medicagos: The agronomic value and botanical relationship of the species (p. 267). Sacramento CA: U.S. Department of Agriclutre, Bureau of Plant Industry. [Google Scholar]
- McMahon, M. M. , & Sanderson, M. J. (2006). Phylogenetic supermatrix analysis of GenBank sequences from 2228 papilionoid legumes. Systematic Biology, 55, 818–836. 10.1080/10635150600999150 [DOI] [PubMed] [Google Scholar]
- Mohr, C. (1901). Plant life of Alabama. VI. Contributions from the U.S. National Herbarium. [Google Scholar]
- Mower, J. P. , & Vickrey, T. L. (2018). Structural diversity among plastid genomes of land plants In Chaw S.‐M., & Jansen R. K. (Eds.), Advances in botanical research, plastid genome evolution (pp. 263–292). Academic Press. [Google Scholar]
- Muir, J. P. , Ocumpaugh, W. R. , & Butler, T. J. (2005). Trade‐offs in forage and seed parameters of annual Medicago and Trifolium species in north‐central Texas as affected by harvest intensity. Agronomy Journal, 97, 118–124. [Google Scholar]
- Nagata, N. , Saito, C. , Sakai, A. , Kuroiwa, H. , & Kuroiwa, T. (1999). The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis one controls cytoplasmic inheritance. Planta, 209, 53–65. 10.1007/s004250050606 [DOI] [PubMed] [Google Scholar]
- Ocumpaugh, W. R. (2001). Develping annual medics for Texas In Proceedings of the 56th Southern Pasture and Forage Crop Improvement Conference. [Google Scholar]
- Ocumpaugh, W. R. , Ueckert, D. N. , Muir, J. P. , Butler, T. K. , & Reed, R. L. (2007). Registration of ‘Devine’ Little Burr Medic. Journal of Plant Registrations, 1, 31–32. 10.3198/jpr2006.05.0338crc [DOI] [Google Scholar]
- Quiros, C. F. , & Bauchan, G. R. (1988). The genus Medicago and the origin of the Medicago sativa complex. Agronomy (USA), 29, 93–124. [Google Scholar]
- Ruhlman, T. A. , & Jansen, R. K. (2014). The plastid genomes of flowering plants In Maliga P. (Ed.), Chloroplast Biotechnology: Methods and Protocols (pp. 3–38) : New York, NY: Springer Science and Business Media LLC. [Google Scholar]
- Ruhlman, T. A. , & Jansen, R. K. (2018). Aberration or Analogy? The atypical plastomes of Geraniaceae In Chaw S.‐M., & Jansen R. K. (Eds.), Advances in Botanical Research, Plastid Genome Evolution (pp. 223–262). Academic Press. [Google Scholar]
- Ruhlman, T. A. , Zhang, J. , Blazier, J. C. , Sabir, J. S. M. , & Jansen, R. K. (2017). Recombination‐dependent replication and gene conversion homogenize repeat sequences and diversify plastid genome structure. American Journal of Botany, 104, 559–572. 10.3732/ajb.1600453 [DOI] [PubMed] [Google Scholar]
- Sabir, J. , Schwarz, E. , Ellison, N. , Zhang, J. , Baeshen, N. A. , Mutwakil, M. , Jansen, R. , & Ruhlman, T. (2014). Evolutionary and biotechnology implications of plastid genome variation in the inverted‐repeat‐lacking clade of legumes. Plant Biotechnology Journal, 12, 743–754. 10.1111/pbi.12179 [DOI] [PubMed] [Google Scholar]
- Sanderson, M. J. , Copetti, D. , Burquez, A. , Bustamante, E. , Charboneau, J. L. M. , Eguiarte, L. E. , Kumar, S. , Lee, H. O. , Lee, J. , McMahon, M. , Steele, K. , Wing, R. , Yang, T.‐J. , Zwickl, D. , & Wojciechowski, M. F. (2015). Exceptional reduction of the plastid genome of saguaro cactus (Carnegiea gigantea): Loss of the ndh gene suite and inverted repeat. American Journal of Botany, 102, 1115–1127. 10.3732/ajb.1500184 [DOI] [PubMed] [Google Scholar]
- Schumann, C. M. , & Hancock, J. F. (1989). Paternal inheritance of plastids in Medicago sativa . Theoretical and Applied Genetics, 78, 863–866. 10.1007/BF00266672 [DOI] [PubMed] [Google Scholar]
- Schwarz, E. N. , Ruhlman, T. A. , Weng, M.‐L. , Khiyami, M. A. , Sabir, J. S. M. , Hajarah, N. H. , Alharbi, N. S. , Rabah, S. O. , & Jansen, R. K. (2017). Plastome‐wide nucleotide substitution rates reveal accelerated rates in Papilionoideae and correlations with genome features across legume subfamilies. Journal of Molecular Evolution, 84, 187–203. 10.1007/s00239-017-9792-x [DOI] [PubMed] [Google Scholar]
- Small, E. (2011). Alfalfa and relatives: Evolution and Classification of Medicago. NRC Research Press. [Google Scholar]
- Smith, G. R. , Evers, G. W. , Ocumpaugh, W. R. , & Rouquette, F. M. (2008). Forage legumes for Texas. Agrilife Research ‐ Texas A&M System, 17–26. [Google Scholar]
- Smith, S. E. (1989). Influence of parental genotype on plastid inheritance in Medicago sativa . The Journal of Heredity, 80, 214–217. 10.1093/oxfordjournals.jhered.a110838 [DOI] [PubMed] [Google Scholar]
- Sveinsson, S. , & Cronk, Q. C. B. (2016). Conserved gene clusters in the scrambled plastomes of IRLC legumes (Fabaceae: Trifolieae and Fabeae). bioRxiv. 10.1101/040188. [DOI] [Google Scholar]
- The Legume Phylogeny Working Group (2017). A new subfamily classification of the leguminosae based on a taxonomically comprehensive phylogeny. Taxon, 66, 44–77. [Google Scholar]
- Ueckert, D. N. , Petersen, J. L. , & Ocumpaugh, W. R. (2003). Production and natural regeneration of annual medics in west‐central Texas. The Texas Journal of Agriculture and Natural Resources, 16, 86–92. [Google Scholar]
- Wang, R.‐J. , Cheng, C.‐L. , Chang, C.‐C. , Wu, C.‐L. , Su, T.‐M. , & Chaw, S.‐M. (2008). Dynamics and evolution of the inverted repeat‐large single‐copy junctions in the chloroplast genomes of monocots. BMC Evolutionary Biology, 8, 10.1186/1471-2148-8-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, M.‐L. , Ruhlman, T. A. , & Jansen, R. K. (2017). Expansion of inverted repeat does not decrease substitution rates in Pelargonium plastid genomes. New Phytologist, 214, 842–851. [DOI] [PubMed] [Google Scholar]
- Wojciechowski, M. F. , Lavin, M. , & Sanderson, M. J. (2004). A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well‐supported subclades within the family. American Journal of Botany, 91, 1846–1862. [DOI] [PubMed] [Google Scholar]
- Wojciechowski, M. , Sanderson, M. J. , Steele, K. , & Liston, A. (2000). Molecular phylogeny of the “Temperate Herbaceous Tribes” of papilionoid legumes: A supertree approach In Herendeen P. S., & Bruneau A. (Eds.), Advances in Legume Systematics 9 (pp. 277–298). Royal Botanic Gardens, Kew. [Google Scholar]
- Yamada, T. (1991). Repetitive sequence‐mediated rearrangements in Chlorella ellipsoidea chloroplast DNA: Completion of nucleotide sequence of the large inverted repeat. Current Genetics, 19, 139–147. [DOI] [PubMed] [Google Scholar]
- Zhu, A. , Guo, W. , Gupta, S. , Fan, W. , & Mower, J. P. (2016). Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytologist, 209, 1747–1756. 10.1111/nph.13743 [DOI] [PubMed] [Google Scholar]
- Zhu, T. , Mogensen, H. L. , & Smith, S. E. (1993). Quantitative, three‐dimensional analysis of alfalfa egg cells in two genotypes: Implications for biparental plastid inheritance. Planta, 190, 143–150. 10.1007/BF00196605 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1–S4
Data Availability Statement
DNA sequences were deposited in GenBank. GenBank and USDA‐GRIN accession numbers, sample voucher information, and collection locations are provided in Appendix S1.


