Abstract
Cellular communications play pivotal roles in multi-cellular species, but they do so also in uni-cellular species. Moreover, cells communicate with each other not only within the same individual, but also with cells in other individuals belonging to the same or other species. These communications occur between two unicellular species, two multicellular species, or between unicellular and multicellular species. The molecular mechanisms involved exhibit diversity and specificity, but they share common basic features, which allow common pathways of communication between different species, often phylogenetically very distant. These interactions are possible by the high degree of conservation of the basic molecular mechanisms of interaction of many ligand–receptor pairs in evolutionary remote species. These inter-species cellular communications played crucial roles during Evolution and must have been positively selected, particularly when collectively beneficial in hostile environments. It is likely that communications between cells did not arise after their emergence, but were part of the very nature of the first cells. Synchronization of populations of non-living protocells through chemical communications may have been a mandatory step towards their emergence as populations of living cells and explain the large commonality of cell communication mechanisms among microorganisms, plants, and animals.
Keywords: hormone, quorum sensing, receptor, bacteria, fungi, metazoa, plants, microbiota, evolution, origin of life
1. Introduction
The cell is the structural and functional unit of all living organisms. Unicellular organisms such as bacteria, archeas, yeasts, or protists consist of a single cell. In contrast, multicellular organisms such as sponges, nematodes, trees, or vertebrates can comprise from a few hundred to several billion or even trillion of cells. In such complex multicellular species, the cells exhibit many differentiated phenotypes playing highly-specialized functions and are often associated within individualized organs. The cellular activities are coordinated at the level of each organ as well as between organs, in order to allow adaptation of the living organism as a whole to its environment. This coordination rests on the traffic of information between all cell types and constitutes in animals the endocrine, nervous, and immune systems.
Most intercellular mediators are either small, simple molecules, such as nitrogen oxide (NO), ethylene, nucleotides (ATP, AMP), and amino acid derivatives (serotonin, melatonin, auxin, thyroxine, homoserine-lactones, etc.), or amino acid polymers (peptide, protein and glycoprotein hormones, cytokines, growth factors, etc.), lipid derivatives (steroid hormones, prostaglandins, jasmonate, etc.), and various other molecules.
In unicellular species, all cells seem identical and independent, suggesting that it is the survival and reproduction of each of them that ensures the sustainability of these species. Nevertheless, communications do indeed exist between these cells, hence allowing some coordinated common responses as well as specialized roles for sub-populations. These coordinations optimize the development and survival of unicellular species populations.
Moreover, information exchanges are not limited to cells inside an organism, not even to cells belonging to one given species. They also exist between cells from different species, whether unicellular or multicellular.
Unicellular species have been prosperous for approximately 3.5 billion years and still represent the vast majority of living species. They can communicate indirectly through soluble mediators to regulate their growth and/or phenotype according to the available food resources. In spite of this success, multicellular species relying on direct cellular adhesiveness and specialization also emerged several hundred million years ago. The main innovation in plants and metazoan is the formation of highly specialized cell populations, requiring proper communications inside these organisms to ensure their development, survival, and reproduction.
All intercellular communications rely on intercellular messengers (mediators) and their cognate receptors in their target cells. The receptors play pivotal roles as they connect intercellular communications to downstream intracellular signaling. Despite the considerable diversity of communications among living species, the number of receptor types and transduction mechanisms is somewhat limited, suggesting their conservation during the Evolution.
Cell communications occur not only inside multicellular species, but also in unicellular species, as well as between different species, whether unicellular and/or multicellular.
2. Cell Communications and Communitarianism in Unicellular Species
By definition, unicellular species do not possess specialized differentiated cells and organs. Nevertheless, communications exist between cells of unicellulars, inside or between different species.
2.1. Bacteria
The membrane receptors in bacteria often directly respond to nutrients [1], or to quorum sensing (QS) signaling molecules [2], or to various other molecules in the environment. Bacteria also directly interact with each other, in particular in the soils [3] or in animal guts or at the level of plant roots.
Many bacteria communities develop in synchronized fashion to face changing environments [4] and form biofilms in which they share nutrients and protection [5].
The establishment of a biofilm requires a sufficient number of bacteria, and many QS signaling molecules (autoinducers) and cognate receptors exist in various bacteria [6,7]. Four main general types of autoinducers (AI) have been described: (1) AI-1, mainly present in Gram-negative bacteria, are N-acylated homoserine-lactones (AHLs) with a core homoserine-lactone ring and a 4- to 18-carbon acyl chain with eventual modifications [8]. The AHLs bind to specific LuxR-type cytoplasmic receptors [9], which control transcription of numerous virulence genes, and to LuxN-type membrane receptors. (2) AI-2, present in both Gram-positive and Gram-negative bacteria, are furanosyl borate diesters, considered as universal signal involved in unicellular interspecies communications [10,11]. (3) AI-3, mainly in Gram-positive systems, typically use secreted oligopeptides [12,13] and two-component systems (TCSs), consisting of membrane-bound sensor kinase receptors (QseC) and cytoplasmic transcription factors (QseB) that direct alterations in gene expression [2,14,15,16]. (4) PQS (pseudomonas quinolone signal) makes use of 2-heptyl-3-hydroxy-4(1H)-quinolone [17], which binds to its specific LysR-type transcriptional regulator receptor (PqsR) to control the synthesis of a rhamnolipid, which is a critical surfactant for biofilm formation [18].
Bacteria pump ions across their membranes, and several recent papers have reported spikes of electrical activity in bacteria, which suggest that, like neurons, bacteria use potassium ions to propagate electrical signals [19]. This electrical communication is considerably quicker and more extensive than QS.
Viruses and other capsidless replicons such as plasmids, transposons, and viroids promote an enormous amount of exchanges among bacteria. Bacteriophages are present everywhere bacteria are located and kill many of them, but never all of them, without the risk of disappearing themselves. In a way, bacteriophages are kind of QS mediators, allowing bacteria to sense how many of them have already succumbed. For example, the coordination of the lysis-lysogeny decision of phages in Bacillus is based on the release of phage-specific communication hexapeptides. These peptides are imported in bacteria by the oligopeptide permease transporter (OPP) and bind to their intracellular receptors, which then no longer activate the expression of an inhibitor of lysogeny [20,21,22].
2.2. Yeasts and Fungi
Direct communications between yeasts occur through membrane proteins such as flocculins, which are lectins recognizing their partners’ polysaccharide chains to form a solid mass (veil, biofilm) [23,24]. Yeasts also communicate via soluble molecules that can diffuse and affect the community’s organization in the long term by quorum sensing. QS molecules identified in fungi include peptide pheromones, oxylipins, aromatic alcohols (such as tyrosol and farnesol [25]), α1–3 glucans [26], and pantothenic acid [23,27,28]. A number of fungi among Arthoniomycetes also participate in the formation of lichens, and thus do communicate with algaes (see 1.4).
2.3. Large Unicellular Eukaryotic Microorganisms (Formerly Called “Protists”)
Amoebas, paramecia, or trypanosomes present, in the case of limited resources, phases with several different phenotypes. In the amoeba, D. discoideum cyclic AMP acts as an intercellular mediator. Its export co-occurs with its intracellular synthesis by adenylate cyclase, which contains a general structure similar to ATP binding cassette (ABC) transporters [29], known to export anionic cargoes like cAMP. ABC transporter inhibitors disrupt amoeba development in a manner consistent with a lack of cAMP export, indicating that cAMP plays an intercellular messenger role during D. discoideum development [30]. When the density of bacterial prey becomes low compared with the amoeba population, the concentration of the pre-starvation factor (PSF) sensor protein decreases, leading to activation of protein kinase YakA, which relieves the inhibition of expression of a cAMP-dependent protein kinase. The latter stimulates the expression of several genes, including a cAMP membrane receptor responsible for the aggregation in spores through a positive cAMP-dependent activation loop [30,31]. Amoebas also use quorum sensing-like communication systems based on the complex dipeptide glorin [32] to coordinate the periodic transition from uni- to multi-cellularity.
Among trypanosomes, the cross-species interactions between QS systems have important implications for their virulence, transmission, competition, and evolution [33]. The parasites exploit oligopeptide signals generated by released peptidases to monitor cell density. Then, a transporter protein takes up combinations of small oligopeptides to control trypanosome differentiation either towards the actively dividing slender form or towards the stumpy non-dividing form [34].
2.4. Lichens
Lichens are long-term intimate symbiotic partnerships between mushrooms (Arthoniomycetes) and photosynthetic algaes occupying nutrient-poor niches [35]. Inter-species communications are thus crucial for the initial steps of symbiosis in lichen formation and development [36]. Recognition of compatible algal cells is performed by specific lectins produced and secreted by the potential mycobiont. For example, the lectin of Peltigera canina recruits both algal cells (chlorobionts) and cyanobacteria (cyanobionts), forming high-affinity bonds with different galactose units in the poly-α-1,4-galactoside side chain of their wall. Free non-motile cells of the cyanobacterium that bind the lectin are recruited and move toward the lectin source [37,38]. Upon reaching the maximal concentration of the gradient, the cells become desensitized, and lectin binding promotes stable cell aggregation between the two partners [39]. Bacterial communities (particularly cyanobacteria) also participate in the constitution of these inter-species associations [40]. Some of these bacteria possess genes allowing the synthesis of auxins, and can thus attract their eukaryote partners (mushrooms and/or algaes).
A number of bacteria and archaea live in even harsher environments and, in a similar way, associate with other species to withstand extreme temperature, pressure, dryness, salinity, pH, or other chemical conditions. These associations require intra- and inter-species cellular communications, not simply mutual metabolic complementation [41,42,43].
3. Cell Communications in Multicellular Species
Multicellularity has only emerged and succeeded in fungi, algae, plants, and animals [44]. All multicellular organisms are eukaryotes, and the nuclear chromatin structure in each cell controls its specific fate through the expression of homeotic genes. This development in animals is controlled and coordinated only by a few extracellular ligands (such as Hh, Wnt, FGF, BMP, and some others) providing complex structuring information via their identities, concentrations, combinations, and dynamics [45], as well as via the constitution of niches, particularly for stem cells [46,47]. Intercellular communications through soluble mediators already existed in unicellular organisms, probably for 3.5 billion years. The specialization of various cell populations in multicellular species emerged much more recently, approximately 600 million years ago [48]. In these species, both direct and indirect cellular communication mechanisms co-exist.
Intercellular communications in multicellular organisms arise via four different molecular mechanisms: (1) cytoplasmic bridges, (2) exosomes and ectosomes, (3) direct interactions between membrane proteins of adjacent cells, and (4) soluble messenger molecules (mediators) controlling more or less distant target cells.
3.1. Communications through Direct Cytoplasmic or Membrane Contacts
There are several types of cytoplasmic bridges, including gap junctions [49,50], plasmodesmata [51,52], tunneling nanotubes [53,54], and others formed by incomplete cytokinesis [55,56,57,58,59,60]. Exosomes and ectosomes are two distinct kinds of extracellular vesicles (EVs) generated by all types of cells [61], particularly at the tip of primary cilia [62]. Their cargoes include proteins, lipids, and nucleic acids, which are imported into the target cells’ cytoplasm to affect the activity of transcription factors; signaling proteins; and many enzymes in animals, plants, and microorganisms [61,63,64,65].
Many membrane multidomain proteins [66] allow cell–cell adhesiveness and communications. Most of these proteins exhibit repeated stable conformations [67,68,69,70,71,72,73]. In animals, cells are also in contact through the extracellular matrix (ECM), kind of an extracellular “glue”, which includes proteins such as collagens, fibronectins, or laminins [74,75,76], as well as oligosaccharides [77] interacting with cell membrane cadherins, integrins, CAM (cell adhesion molecules), selectins, and so on [78,79,80]. In plants, there is a thick polysaccharide wall around most cells, which prevents the plasma membranes of neighboring cells from coming into contact, so that germ cells require a particular structure, the pollen tube, to come in contact [81].
3.2. Communications through Soluble Mediators
Almost all cells, in uni- and multicellular organisms, produce soluble messenger molecules (mediators), capable of influencing distant cells. In animals, these mediators include hormones, neuromediators, cytokines, growth factors, morphogens, and so on. Their target cells can contact the mediator-emitting cell (post-synaptic cells, immune cells) or be at a considerable distance in another individual (target cells for pheromones). In plants, many different hormones also exist and act at a very short distance, such as ethylene, or at distance such as auxin, gibberellins, florigen, and various phyto-œstrogens.
The mediators’ receptors are located at the functional interface between intercellular communications and intracellular signaling. They belong to two prominent families: (1) membrane receptors with their binding site at the external surface of cells, and (2) intracellular receptors acting at the level of DNA (nuclear receptors in eukaryotes). The receptors of the first group bind to mediators that do not penetrate the cell, whereas those of the second group perceive only ligands capable of penetrating the cells.
The mediators’ receptors in plants and animals are either soluble intracellular transcription factors or proteins inserted in the plasma membrane (Table 1). The nuclear receptors exist in both animals and plants, but, whereas they form a large family of related transcription factors in animals [82], in plants, diverse proteins serve as intermediaries in the genomic actions of the hormones [83]. These binding proteins in plants are structurally very diverse, in contrast to the kinship of animals’ nuclear receptors. Nevertheless, the general mechanisms in animals and plants appear to share many similarities [84].
Table 1.
Intracellular and membrane receptor families in microorganisms, plants, and animals. GPCR, G protein-coupled receptor; AHL, N-Acyl homoserine lactone; NO, nitrogen oxide; TLR, toll-like receptor; QS, quorum sensing; PQS, pseudomonas quinolone signal; LPS, lipopolysaccharide.
| Receptors | Mechanisms | Ligands | ||
|---|---|---|---|---|
| Intracellular, Ligand-Regulated, Transcription Factors | LuxR (LasR, TraR) | Transcription | QS autoinducers (various AHL) | Bacteria |
| LysR (PqsR) | Transcription | PQS (various Quinolones) | Bacteria | |
| Nuclear Receptors | Transcription | oleate ergosterol | Fungi | |
| Transcription | Florigen (PEBP) | Plants | ||
| Transcription | brassinosteroids, gibberellins, jasmonates, salicylates | Plants | ||
| Transcription | Steroid and thyroid hormones, VitD, RA, prostaglandins | Animals | ||
| Other, Intracellular, Ligand-Regulated Targets | Ubiquitin-ligase | Protein degradation | auxin | Plants |
| Monomeric G protein (Ste2–3p) | ? | farnesol tyrosol tryptophol | Fungi | |
| NO sensing protein | Two-step His/Asp transfer | Nitric Oxide | Bacteria | |
| soluble guanylate cyclase | cGMP increase | Nitric Oxide | Animals | |
| His kinases | Two-step His/Asp transfer | Ethylene, brassinosteroids | Plants | |
| His kinases | Two-step His/Asp transfer | arabinose, Mg++ | Fungi | |
| Di-guanylate cyclase | di-cGMP increase | environment signals | Bacteria | |
| Tyr kinases (RTK) | IRS, Shc Tyr phosphorylation | IGF, insulin, EGF | Animals | |
| Ser/Thr kinases | SMAD S/T phosphorylation | TGFβ, BMP, Activin, Inhibin | Animals | |
| guanylate-cyclase | cGMP increase | ANF | Animals | |
| Tyr-phosphatase | Tyr dephosphorylation | Proteoglycans or unknowns | Animals | |
| Plasma Membrane, Non-Enzyme, Receptors | ionotropic R | Ion entry | glutamate acetylcholine, amino acids?, mechano-stress, sterols | Animals, Plants, Bacteria |
| Notch | Transcription domain liberation by proteolysis | Cell membranes proteins (Delta Jagged Serrate) | Animals | |
| Cytokine R | Kinase recruitment (JAK) | GH, Prl, interleukins, | Animals | |
| BcR, TcR, FcR | Kinase recruitment (lck, lyn) | MHC, antigens, immunoglobulins | Animals | |
| TNFR | TRADD TRAF RIP caspases recruitment | TNF | Animals | |
| Integrins | SFK Talin Kindin Vinculin recruitment (cytoskeleton organization) | Extracellular matrix components | Animals | |
| Toll, TLR | Myd88 recruitment | LPS, bact DNA, flagelin | Animals | |
| 7TMR (GPCR) | Trimeric G-protein recruitment | alpha mating factor | Yeast | |
| 7TMR (GPCR) | Trimeric G-protein recruitment | hormones neuromediators, pheromones | Animals | |
| 7TMR (mGluR), 7TMR (Frizzled) | Homer recruitment, Dishevelled recruitment | Glutamate, Wnt | Animals | |
In brief, as shown in Figure 1, the plasma membrane receptors of animals and plants are either
receptors acting by direct catalysis (receptors with intrinsic enzymatic activity, i.e., protein kinase activity [87,88], or phosphatase activity [89], or guanylate cyclase activity [90,91]);
receptors acting through recruitment of various downstream intracellular effectors (G proteins [92,93,94,95,96,97,98,99], adenylate cyclases [100,101,102], phospholipases C [103,104,105], soluble protein kinases [106,107,108,109,110,111], methyltransferases [112], proteases [113,114,115,116], and so on).
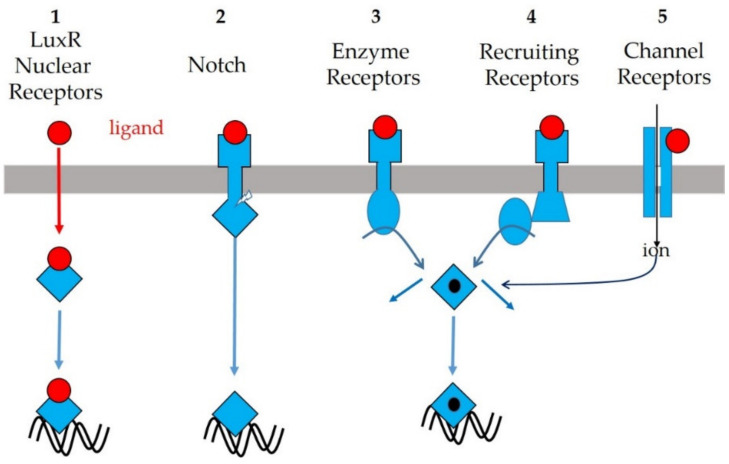
Figure 1.
General view of intercellular messengers’ receptors and their downstream signaling pathways. (1) Intracellular ligand-regulated transcription factor. (2)–(5) Plasma membrane receptors: (2) protease-cleavable receptor with intracellular domain exhibiting transcriptional activity; (3) enzyme receptors (Tyr, Ser/Thr, His kinases, GMPcyclase, phosphatase); (4) non-enzymatic receptors recruiting cytoplasmic partners (kinases, G proteins, scaffolding proteins); and (5) channel receptors (ionotropic). For details, see also Table 1.
These receptors possess (a) either a very short or huge extracellular domain (ECD), (b) one or several membrane-spanning sequences (seven in G protein-coupled receptor (GPCR)), and (c) an intracellular domain (ICD) comprising one or several peptide sequences. The ECD permits ligand binding, whereas the ICD either possesses enzymatic activity like the insulin receptor (tyr-kinase) or TGFβ receptor (ser/thr kinase) (Figure 1); or recruits cytoplasmic soluble enzyme(s), like the growth hormone and cytokine receptors (Jak and Tyk kinases); or recruits G-proteins, like the numerous GPCRs (Figure 1); or is clipped off as a transcription factor to perform intracellular signaling, like the Notch receptor (Figure 1). Membrane receptors and downstream partners are generally concentrated at the level of lipid rafts [117] or primary cilium in metazoan [62,118]. The downstream signaling pathways and their evolution have been thoroughly described in a recent comprehensive review [119].
4. Cell Communications between Uni- and Multi-Cellular Organisms (Microbiotas)
Microbiotas are sets of commensal microorganisms of animals or plants occupying a favorable environment and exchanging numerous advantages to their shared host. It is most likely that these associations existed right from the origin of plant and metazoan lineages.
4.1. In Animals
Animals host microbiotas at different locations in their body. In vertebrates, microbes mainly settle in their intestine as a complex community (10–100 times the number of the host’s own cells), comprising aerobic and anaerobic bacteria, archeas, viruses, fungi, and other microbial eukaryotes, playing critical roles in the host physiology. Most insect guts contain relatively few microbial species compared with mammals, but some harbor large communities of specialized bacteria. The microbiota in animals have significant influences on endocrine, nervous, and immune systems.
The communications of microbiota bacteria with animal host cells affect not only local intestinal functions [120,121], but also general integrated functions. The best-known bacterial recognition patterns are lipopolysaccharide (LPS) and peptidoglycan (PG), which act through binding with the host’s pattern recognition receptors (PRRs), including Toll (Drosophila), Toll-like receptors (TLRs) (vertebrates), and C-type lectin receptors (CLRs) (vertebrates). In vertebrates, membrane TLRs of the epithelial and lymphoid cells of the small intestine, which are responsible for innate intestinal immunity, differentiate microbiota microorganisms and promote immunological tolerance towards them [122]. Moreover, chemical mimicry of animals’ signaling molecules such as neuromediators or hormones can be present in commensal bacteria. For example, N-acyl amide synthase in commensal bacteria produce lipids, such as endocannabinoids, which interact with GPCRs involved in gastrointestinal tract physiology [123]. The mammalian gut microbiota affects not only the host’s immune system, but also its autonomous [124,125] and central [126,127] nervous systems, in particular, via the vagus nerve from the intestine towards the hypothalamus, where it controls the host’s appetite [128] and other behaviors [129].
Fungal microbiota secretes substances such as pyrazines, which play very important roles in animals’ physiology and behavior. Some fungal pyrazines are used as a guide for individuals to find their way back to the anthill. In the model species Drosophila, behavioral responses to 25 fungal pyrazines vary widely despite their chemical similarity, ranging from strong, attractive responses to no response at all. Two olfactory receptors in Drosophila, Or33b and Or59a, yield remarkably long-lasting responses to certain pyrazines [130].
4.2. In Plants
The conquest of land by terrestrial plants occurred thanks to their interactions with fungi, and the survival of animals and plants is dependent on their respective microbiota. It is thus interesting to consider which molecular communication tools are present in unicellular and/or multicellular species, and how these mechanisms ensure optimal cell interactions favoring their respective survival and joint expansion.
The rhizobiota (root microbiota) is the set of the soil organisms (bacteria, mushrooms, virus, and so on) interacting with plant roots and thus supplying them with many compounds that they do not synthesize. Plants contain a significant number of pattern recognition receptors (PRRs) that share remarkable structural and functional similarity with the Drosophila Toll receptor and mammalian Toll-like receptors (TLRs) recognizing various pathogens or mediators. The plant PPRs are either membrane receptors with intrinsic intracytoplasmic kinase activity (RLKs) or non-kinase membrane receptors (RLPs) able to recruit RLKs and cytoplasmic kinases [131,132]. Plants are also able to cope with their microbiota through the emission of extracellular vesicles [64] and complex cross-talks through their respective secretomes to constitute functional holobionts [43,133].
Mycorrhiza (symbiosis of fungi with plant roots) is considered to be at the origin of the colonization of dry land by water plants, approximately 450 million years ago. More than 90% of the living land plants can form a mycorrhizal symbiosis, and non-mycorrhizal status is an exception. Besides, the mycorrhizal network allows communications between plants of the same species or different species. There are two types of mycorrhization: arbuscular mycorrhizae (AM) and ectomycorrhizae (EM).
The arbuscular mycorrhizal (AM) fungi have developed a symbiotic relationship with most land plants, facilitating the uptake of minerals and water from the soil by plants [134,135] and providing carbon sources to fungi. Hormones from the plants play a prominent role in AM establishment [136]. In AM, fungal hyphae penetrate the cortical tissues of roots, between the cell wall and plasma membrane, thus coming into close contact with plant cells. Although AM interactions are physically restricted to the roots, they influence the whole-plant performance [137].
The ectomycorrhizae (EM) develops a fungal system close to the roots with a mantle surrounding short roots and a network (called Hartig net) that penetrates between the roots’ cortical cells.
The establishment of AM or EM requires complex dialogue between the two partners, including the perception by roots’ His-kinase receptors of specific lipo-chitooligosaccharides (LCOs), called Myc-LCO, secreted by fungi. These receptors share a high degree of similarity with receptors for plant hormones (ethylene and cytokinin) and must have played an essential role in their interaction with plants, leading to their joint conquest of land.
Endophytic bacteria represent a major part of the plant microbiota, which exert growth-promoting activities by increasing the availability of limiting plant nutrients, such as nitrogen, iron, and phosphorus [138,139], and providing photosynthetic carbon to bacteria through the formation of nodules [140]. The first signal comes from the roots and is a cocktail of flavonoids, which stimulates the synthesis of nodulation (Nod) factors by the bacteria, which cause the formation of nodules around them by roots. Nod factors are lipo-chitooligosaccharides (LCOs) that act via the hetero-dimerization of root membrane receptor-kinases and a calcium- and calmodulin-dependent kinase (CCaMK) [141,142]. Ethylene signaling is not required for Nod factor signaling, infection development, or nodule organogenesis, but it is for initiation of nitrogen fixation and negative regulation of nodulation [143,144,145]. In return, ethylene production by the plant is modulated by Nod factors [146]. Gibberellins exert both positive and negative effects on nodulation because they act as suppressors of infection, but also as promoters of nodule organogenesis. Gibberellins and ethylene act through independent pathways in nodule development [147]. Cytokinins also interact with the previous phytohormones in different ways to impact nodulation [148].
5. Cell Communications between Different Multi-Cellular Organisms
5.1. Cell Communications between Animals
In the present paper, we consider cell communications as integrated networks leading to a mutually beneficial equilibrium for two communicating species. Therefore, the detection of their hosts by parasites cannot be considered as communication. Pheromones are very important volatile mediators inside each animal species, and have been introduced before. Although involved in inter-species detection, kairomones cannot be considered as communication molecules as they benefit only parasites for detecting potential hosts, and not for establishing a mutual favorable interaction.
5.2. Cell Communications between Plants
Plant volatile organic compounds (VOCs) such as sesquiterpenes, emitted particularly under salicylic and jasmonic acid control [149,150], are major vehicles of alarm information between plants in response to herbivore damage. VOCs may either directly affect cell membranes’ potentials and induce endogenous signal transduction cascades, or enter the cell and directly bind to co-repressors of stress-responsive genes.
Plants can integrate multiple volatile cues into specific adaptive defense responses [151]. It is interesting to point out that these communication channels can be private for one plant species or even one genotype inside a species. By contrast, others are open channels, allowing information sharing with different species [152]. This sharing may be favorable despite the cost of alarm signaling toward potential competitor species [153]. Indeed, it allows cooperation in herbivore insect exclusion from their common neighborhood through herbivore-induced plant volatile molecules acting via epigenetic mechanisms that sustain the memorization of the defense response [154].
5.3. Cell Communications between Animals and Plants
Plants broadcast signals either to attract or to repel animals (pollinating and herbivore animals, respectively). For pollination, most of the visitor species interact with only one or very few plant species. Mutualistic interactions imply sophisticated interplay of floral stimuli (scent, color) and the sensory systems of pollinators (bees, hawkmoths, geckos, and so on), among which vision and temperature play significant roles. Information transfer between plant species reduces the attraction of bee pollinators to herbivore-attacked plants or warned plants relative to undamaged/unwarned plants [155]. When wounded by herbivore insects, some plants, like tomatoes, produce the 18aa peptide systemin, which binds to its receptor and stimulates the jasmonic acid cascade to produce bad-tasting chemicals and escape further consumption [156].
Cell communications from animals to plants are not a very usual research field. Nevertheless, it has been recently reported that bees bite plants’ leaves to induce their flowering to get access to pollen and chemicals in the insects’ saliva may be involved [157]. For the time being, the precise molecular and cellular mechanisms are not known.
6. Origin and Evolution of Cell Communications
The origin and evolution of cell communications obviously cannot be directly studied experimentally. Only a global view of current cell communications can guide us to speculate about their origin and evolution.
6.1. Origin
The origin of inter-cellular communications must be intricated with that of the cells themselves. Life did not emerge directly as cellular organisms, but initially as simple genetic or chemical replicators [158,159,160,161]. The dynamic kinetic stability hypothesis that bridges abiogenesis to life [162] would require sustained communications to maintain the required far-from-equilibrium state during this process, whatever it was precisely. Even if life could have begun as a system of such RNA and polypeptide replicators, chemically occurring membranes must have played a primary role in the very first steps of life on Earth. Indeed, compartmentalization by membranes has led to cellularity [163] that is today the hallmark of all living beings.
Primordial ancestral membranes likely were single-chain amphiphiles that formed vesicles and protocells, and gradually evolved into phospholipids during the emergence of cells [164]. Membranes not only enclose a limited volume where genetic and metabolic reactions can occur much more efficiently, but also provide the site for chemical potential energy in the form of the proton-driven ATP synthase motor [165,166].
It is highly challenging to trace back the unique history of life, approximately 3.8 billion years ago [167]. A wide range of theoretical and experimental methods have been developed for constructing and testing possible evolutionary scenarios of prebiotic evolution, towards the emergence of the first living cell(s) [168,169]. It is generally accepted that multicellular organisms emerged from unicellular organisms [170] and that communications became mandatory only a long time after cell emergence. However, cyanobacteria, one of the earliest types of bacteria, dating back to between 3.4 and 2.8 billion years ago, formed huge colonies (stromatolites) [171]. It is thus likely that life actually began much earlier, perhaps as early as 3.8 billion years ago, and most likely under the form of synchronized communicating cell populations.
Taking this into consideration, the protocells and first living cells must have never existed in a truly isolated state. Chemical communications might have already existed among protocells and were probably essential for the emergence of living cells, as suggested by the dynamic kinetic stability hypothesis [162]. If true, this hypothesis implies that the first living cells were not isolated and emerged from inanimate protocell communities without rupture of communications between them.
Compartmentalization of the initial chemical “primordial soup” by membranes might have favored the interactions between polynucleotides and polypeptides to initiate the very first steps leading to metabolism and heredity. Cross-communications between the proto-cells must have played a favorable role in the emergence of genuine cells (prokaryotes and eukaryotes).
The hypothesis of synchronized protocell populations is difficult to prove (or disprove), but would make sense. For example, to avoid extinction by dilution, the protocells must out-compete other vesicles either by having a more rapid cycle, thereby generating more progeny during division, or by surviving destructive processes more efficiently [172]. Synchronization of populations of protocells, requiring chemical communications, should have given them an advantage in terms of number and then facilitated their emergence into living cell populations. These first cell populations, retaining chemical communications, would have offered a more protective and stable milieu for the emergence of life. The emergence of prokaryote as well as eukaryote cells from a common (proto-karyotic) syncytial ancestral root [173] is a stimulating view in this respect (Figure 2). Nevertheless, it is much more generally accepted that eukaryogenesis occurred only around 1.7 Gy ago by endosymbiosis involving archaea and bacteria [174,175], most likely with no arrest in their previous communication networks and, therefore, is the very beginning of communications between prokaryote and eukaryote cells.
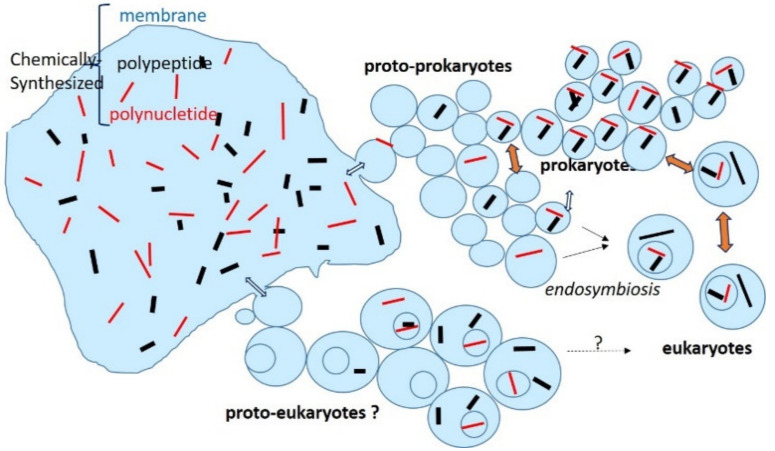
Figure 2.
Possible early origin of communications between proto-prokaryotes, prokaryotes, eukaryotes, and hypothetical proto-eukaryotes.
The fact that living cells are non-equilibrium systems suggests that life can emerge only from non-equilibrium chemical systems, thus needing energy input. The energy from the sun is the ultimate source of energy for sustaining life on Earth. From an astrobiological standpoint, non-equilibrium chemical systems can arise naturally when solar irradiation strikes rotating surfaces of planets [176]. It is tempting to imagine that solar photoperiodism might have synchronized protocells’ chemical functioning, and then kept synchronizing biological metabolism and divisions in the newly-formed cell populations. Their synchronization should have favored the functional cohesion between cells through circadian light exposure. Interestingly, cell communications are still synchronized by light, circadian rhythms in a vast number of prokaryote, plant, and animal species.
6.2. Evolution
Intercellular mediators emerged during Evolution in conjunction with the appearance of their cognate receptors. In some cases, one of them (ligand or receptor) appeared first and “found” its counterpart later, but, more often, they appeared “simultaneously” at the phylogenetic time scale [177]. It is likely that molecules with no partner (i.e., with no role) are not conserved for long in the following generations. When a ligand or receptor appeared a long time before its current partner, it likely had another interacting partner (i.e., another role) in the meantime.
The most ancient intercellular messengers were probably either intracellular metabolites like ATP and other nucleotides, released by damaged cells, or naturally excreted metabolites. In the receiving cells, their initial targets were probably the same proteins as those recognizing their proper internal molecule. To discriminate the incoming messenger molecules from their own intracellular ligands, part of these intracellular proteins must have evolved to become located at the surface of the cells. The plasma membrane is the most favorable place for such receptors to discriminate outside messengers from their own metabolites. De novo protein domains emerging from non-coding thymine-rich DNA sequences must have played advantageous roles in this evolutive process. Indeed, such DNA sequences exhibit a high potential to be translated into transmembrane domains [178]. These domains next to the coding sequences of copies of the intracellular protein have led to this new location at the plasma membrane. Because these cells belong to the same species, this initial phenomenon corresponded to an autocrine mechanism.
From this simple autocrine communication, diversification of cell phenotypes has led to more and more complex and specific communication networks in microorganisms, plants, and animals.
In prokaryote and eukaryote microorganisms, quorum sensing represents the most general example of distant intercellular communications. In plants, specialized molecules play specific roles in communications between their various parts for coordinating their development and functions. In animals, an even higher integration occurs through their endocrine, neuronal, and immune systems. The neuronal system is unique to animals, but the ion channels and proteins involved in synapses originated long before their emergence. Moreover, the animals with a nervous system do not form a monophyletic group. Therefore, the extension and disappearance of membrane ion-channel genes during Evolution can explain the differences in the functioning of the nervous systems in these various species [179].
Cell communications generally require highly specific ligand–receptor high-affinity binding. High specificity necessitates that the receptors are strictly specific, and do not bind molecules with structures close to that of their ligands. For example, steroid hormone receptors discriminate each hormone, and so do the glycoprotein hormone receptors, despite that these hormones share a common alpha-subunit, which plays an essential role in receptor binding [180]. The “negative specificity” hypothesis that we have previously introduced [181] postulates that high affinity and high specificity can be brought by different parts in the ligand–receptor interfaces. During Evolution, more and more numerous and specific ligand–receptor pairs have appeared, requiring that each receptor of course recognizes its own ligand, but also cannot be activated by a molecule akin to it. Therefore, each receptor during Evolution was shaped to bind its own ligand and to reject structurally-related molecules, particularly ligands for other receptors. It is interesting to point out that the estrogenic receptor that does not bind other steroid hormones (cortisol, corticosterone, progesterone, testosterone) is activated, although weakly, by a myriad of organic molecules from industrial origin that do not resemble estradiol (bisphenols, phtalates, and so on) and are known as endocrine disrupter compounds. No specific receptor-binding inhibition against these molecules could be selected during evolution as they did not exist [182].
Cell communications are based on molecules and mechanisms that appeared at the very early steps of life emergence, thus probably at the same time as cells themselves. During Evolution, a huge diversification of ligand–receptor pairs occurs with high conservation to retain high affinity and modifications of details to gain specificity through inhibition of binding of the wrong ligands.
6.3. Interplays
Cell communications show considerable diversification, allowing outstanding cell interactions inside multicellular species, but also between different species, either uni- and/or multi-cellular. In animals, various physiological systems have emerged (endocrine, neural, immune), which share common molecular mechanisms that ensure their close functional integration in terms of biological and behavioral responses [183,184,185,186]. In the most “primitive” metazoa, the distinction between neuronal, endocrine, and immune systems is not clear-cut. For example, in cnidarians (medusa, corals), there is no endocrine gland, but the diffuse neurons secrete neuromediators and molecules that are chemically related to vertebrate hormones such as steroids, melatonin, or GnRH [187]. During Evolution, the endocrine system has gained in complexity in all invertebrates, but even more in vertebrates because of the dual duplication of the entire genome at the root of vertebrates’ radiation [188]. This has permitted the emergence of the pituitary as a major integrator of endocrine and neuro-endocrine functions [189,190,191]. In individual plants, the physiological integration relies on less complex morphological and functional integration than in animals. However, because of their immobility, plants establish numerous stable interactions with soil fungi and bacteria. During Evolution, the root–nodule symbiosis concerned more numerous species than today [192], indicating its loss in multiple clades. Conserved co-regulated genes found within legumes paved the way for nodule formation and nitrogen fixation [193]. The ability of bacteria to decrease plant ethylene levels by the expression of the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase or via the production of rhizobitoxine was found to be essential for leguminous plants to nodulate [194]. Mycorrhization preceded bacterial symbioses during the conquest of land by terrestrial plants [195], thus showing tripartite interactions in this process during Evolution. Through their microbiota, individual plants can communicate with others, of the same or other species, leading to even higher integration [196] thanks to the shared molecular language of plants, fungi, and bacteria..
7. Conclusions
Cellular communications play pivotal roles in all uni- and multi-cellular species, leading to an outstanding variety of essential biological processes not only inside each species, but also for numerous favorable interactions between uni- and multi-cellular species. Inter-species communications were selected during Evolution when mutually beneficial. They have been made possible by the high degree of conservation of the basic mechanisms of ligand–receptor pairs in evolutionary remote species.
Acknowledgments
The authors thank the University of Tours (France) for the invitation to T.M.D.N. for a two-month stay at the INRA laboratory in Nouzilly. We also thank the University of Quy Nhon (Viet Nam) for authorizing T.M.D.N. to leave her teaching duties during that period of time.
Abbreviations
| ABC | ATP binding cassette |
| AHL | N-Acyl homoserine lactones |
| AI-2 | autoinducer-2 |
| AI-3 | autoinducer-3 |
| AM | arbuscular mycorrhizae |
| AMP(1) | antimicrobial peptide |
| AMP(2) | 5’-adenosine mono-phosphate |
| ATP | 5’-adenosine tri-phosphate |
| BMP | bone morphogenic protein |
| CAM | cell adhesion molecule |
| cAMP | 3’5’cyclic-AMP |
| CBP | CREB binding protein |
| CLR | C-type lectin receptor |
| CREB | cAMP-responsive element binding protein |
| CRP | cyclic-AMP receptor protein |
| DAMP | damage-associated molecular pattern |
| ECD | extracellular domain |
| eDNA | extracellular DNA |
| EM | ectomycorrhizae |
| EV | extracellular vesicle |
| FGF | fibroblast growth factor |
| FSH | follicle-stimulating hormone |
| GnRH | gonadotropin-releasing hormone |
| GPCR | G protein-coupled receptor |
| Hh | hedgehog |
| HSL | homoserine lactone |
| ICD | intracellular domain |
| IMD | immune deficiency pathway |
| JAK | janus kinase |
| LCO | lipochitooligosaccharide |
| LH | luteinizing hormone |
| LRR | leucine-rich repeat |
| LysM-LK | lysin-motif receptor-like kinase |
| MAPK | Mitogen-activated protein kinases |
| MAPKK | Mitogen-activated protein kinases |
| MAPKKK | Mitogen-activated protein kinases |
| NO | nitrogen oxide |
| Nod | nodulation factor |
| PGN | peptidoglycan |
| PGRP | peptidoglycan-binding receptor protein |
| PRR | pattern recognition receptor |
| PTS | phosphotransferase system |
| QS | quorum sensing |
| RLK | receptor-like kinase |
| RLP | receptor-like protein |
| TCS | receptor-histidine-kinase two-component system |
| TNT | tunneling nanotubes |
| TLR | toll-like receptor |
| TNF | tumor-necrosis factor |
| VOC | volatile organic compound |
| Wnt | vertebrate homolog of drosophila Wingless |
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wojtowicz H., Prochnicka-Chalufour A., de Amorim G.C., Roudenko O., Simenel C., Malki I., Pehau-Arnaudet G., Gubellini F., Koutsioubas A., Perez J., et al. Structural basis of the signalling through a bacterial membrane receptor HasR deciphered by an integrative approach. Biochem. J. 2016;473:2239–2248. doi: 10.1042/BCJ20160131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigel W.A., Demuth D.R. QseBC, a two-component bacterial adrenergic receptor and global regulator of virulence in Enterobacteriaceae and Pasteurellaceae. Mol. Oral Microbiol. 2016;31:379–397. doi: 10.1111/omi.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tecon R., Ebrahimi A., Kleyer H., Levi S.E., Or D. Cell-to-cell bacterial interactions promoted by drier conditions on soil surfaces. Proc. Natl. Acad. Sci. USA. 2018;115:9791–9796. doi: 10.1073/pnas.1808274115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee S., Bassler B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019;17:371–382. doi: 10.1038/s41579-019-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mhatre E., Monterrosa R.G., Kovacs A.T. From environmental signals to regulators: Modulation of biofilm development in Gram-positive bacteria. J. Basic Microbiol. 2014;54:616–632. doi: 10.1002/jobm.201400175. [DOI] [PubMed] [Google Scholar]
- 6.Welsh M.A., Blackwell H.E. Chemical probes of quorum sensing: From compound development to biological discovery. FEMS Microbiol. Rev. 2016;40:774–794. doi: 10.1093/femsre/fuw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talagrand-Reboul E., Jumas-Bilak E., Lamy B. The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems. Front. Microbiol. 2017;8:37. doi: 10.3389/fmicb.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papenfort K., Bassler B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCready A.R., Paczkowski J.E., Henke B.R., Bassler B.L. Structural determinants driving homoserine lactone ligand selection in the Pseudomonas aeruginosa LasR quorum-sensing receptor. Proc. Natl. Acad. Sci. USA. 2019;116:245–254. doi: 10.1073/pnas.1817239116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naser I.B., Hoque M.M., Faruque S.N., Kamruzzaman M., Yamasaki S., Faruque S.M. Vibrio cholerae strains with inactivated cqsS gene overproduce autoinducer-2 which enhances resuscitation of dormant environmental V. cholerae. PLoS ONE. 2019;14:e0223226. doi: 10.1371/journal.pone.0223226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tourneroche A., Lami R., Hubas C., Blanchet E., Vallet M., Escoubeyrou K., Paris A., Prado S. Bacterial-Fungal Interactions in the Kelp Endomicrobiota Drive Autoinducer-2 Quorum Sensing. Front. Microbiol. 2019;10:1693. doi: 10.3389/fmicb.2019.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeke F., De Craemer S., Debunne N., Janssens Y., Wynendaele E., Van de Wiele C., De Spiegeleer B. Peptides as Quorum Sensing Molecules: Measurement Techniques and Obtained Levels In vitro and In vivo. Front. Neurosci. 2017;11:183. doi: 10.3389/fnins.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krypotou E., Scortti M., Grundstrom C., Oelker M., Luisi B.F., Sauer-Eriksson A.E., Vazquez-Boland J. Control of Bacterial Virulence through the Peptide Signature of the Habitat. Cell Rep. 2019;26:1815–1827.e5. doi: 10.1016/j.celrep.2019.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L., Dai K., Wen X., Ding L., Cao S., Huang X., Wu R., Zhao Q., Huang Y., Yan Q., et al. QseC Mediates Osmotic Stress Resistance and Biofilm Formation in Haemophilus parasuis. Front. Microbiol. 2018;9:212. doi: 10.3389/fmicb.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak E.A., Shao H., Daep C.A., Demuth D.R. Autoinducer-2 and QseC control biofilm formation and in vivo virulence of Aggregatibacter actinomycetemcomitans. Infect. Immun. 2010;78:2919–2926. doi: 10.1128/IAI.01376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K., Meng J., Huang Y.C., Ye L.H., Li G.J., Huang J., Chen H.M. The role of the QseC quorum-sensing sensor kinase in epinephrine-enhanced motility and biofilm formation by Escherichia coli. Cell Biochem. Biophys. 2014;70:391–398. doi: 10.1007/s12013-014-9924-5. [DOI] [PubMed] [Google Scholar]
- 17.Van Kessel J.C. PQS Signaling for More than a Quorum: The Collective Stress Response Protects Healthy Pseudomonas aeruginosa Populations. J. Bacteriol. 2019;201 doi: 10.1128/JB.00568-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm A., Vikstrom E. Quorum sensing communication between bacteria and human cells: Signals, targets, and functions. Front. Plant Sci. 2014;5:309. doi: 10.3389/fpls.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prindle A., Liu J., Asally M., Ly S., Garcia-Ojalvo J., Suel G.M. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erez Z., Steinberger-Levy I., Shamir M., Doron S., Stokar-Avihail A., Peleg Y., Melamed S., Leavitt A., Savidor A., Albeck S., et al. Communication between viruses guides lysis-lysogeny decisions. Nature. 2017;541:488–493. doi: 10.1038/nature21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokar-Avihail A., Tal N., Erez Z., Lopatina A., Sorek R. Widespread Utilization of Peptide Communication in Phages Infecting Soil and Pathogenic Bacteria. Cell Host Microbe. 2019;25:746–755.e5. doi: 10.1016/j.chom.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laganenka L., Sander T., Lagonenko A., Chen Y., Link H., Sourjik V. Quorum Sensing and Metabolic State of the Host Control Lysogeny-Lysis Switch of Bacteriophage T1. MBio. 2019;10 doi: 10.1128/mBio.01884-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow J., Dionne H.M., Prabhakar A., Mehrotra A., Somboonthum J., Gonzalez B., Edgerton M., Cullen P.J. Aggregate Filamentous Growth Responses in Yeast. Msphere. 2019;4 doi: 10.1128/mSphere.00702-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veelders M., Bruckner S., Ott D., Unverzagt C., Mosch H.U., Essen L.O. Structural basis of flocculin-mediated social behavior in yeast. Proc. Natl. Acad. Sci. USA. 2010;107:22511–22516. doi: 10.1073/pnas.1013210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebaa S., Boucherit-Otmani Z., Courtois P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol. Med. Rep. 2019;19:3201–3209. doi: 10.3892/mmr.2019.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padder S.A., Prasad R., Shah A.H. Quorum sensing: A less known mode of communication among fungi. Microbiol. Res. 2018;210:51–58. doi: 10.1016/j.micres.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Albuquerque P., Nicola A.M., Nieves E., Paes H.C., Williamson P.R., Silva-Pereira I., Casadevall A. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. MBio. 2013;5:e00986-e00913. doi: 10.1128/mBio.00986-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivas W., Leonhardt I., Hunniger K., Hader A., Marolda A., Kurzai O. Multiple Signaling Pathways Involved in Human Dendritic Cell Maturation Are Affected by the Fungal Quorum-Sensing Molecule Farnesol. J. Immunol. 2019;203:2959–2969. doi: 10.4049/jimmunol.1900431. [DOI] [PubMed] [Google Scholar]
- 29.Miranda E.R., Nam E.A., Kuspa A., Shaulsky G. The ABC transporter, AbcB3, mediates cAMP export in D. discoideum development. Dev. Biol. 2015;397:203–211. doi: 10.1016/j.ydbio.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldbeter A. Oscillations and waves of cyclic AMP in Dictyostelium: A prototype for spatio-temporal organization and pulsatile intercellular communication. Bull. Math. Biol. 2006;68:1095–1109. doi: 10.1007/s11538-006-9090-z. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y., Schaap P. Cyclic AMP induction of Dictyostelium prespore gene expression requires autophagy. Dev. Biol. 2019;452:114–126. doi: 10.1016/j.ydbio.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghar A., Groth M., Siol O., Gaube F., Enzensperger C., Glockner G., Winckler T. Developmental gene regulation by an ancient intercellular communication system in social amoebae. Protist. 2012;163:25–37. doi: 10.1016/j.protis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Silvester E., Young J., Ivens A., Matthews K.R. Interspecies quorum sensing in co-infections can manipulate trypanosome transmission potential. Nat. Microbiol. 2017;2:1471–1479. doi: 10.1038/s41564-017-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas F., Matthews K.R. Quorum sensing in African trypanosomes. Curr. Opin. Microbiol. 2019;52:124–129. doi: 10.1016/j.mib.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Armaleo D., Muller O., Lutzoni F., Andresson O.S., Blanc G., Bode H.B., Collart F.R., Dal Grande F., Dietrich F., Grigoriev I.V., et al. The lichen symbiosis re-viewed through the genomes of Cladonia grayi and its algal partner Asterochloris glomerata. BMC Genom. 2019;20:605. doi: 10.1186/s12864-019-5629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joneson S., Armaleo D., Lutzoni F. Fungal and algal gene expression in early developmental stages of lichen-symbiosis. Mycologia. 2011;103:291–306. doi: 10.3852/10-064. [DOI] [PubMed] [Google Scholar]
- 37.Chekanov K., Feoktistov A., Lobakova E. Spatial organization of the three-component lichen Peltigera aphthosa in functional terms. Physiol. Plant. 2017;160:328–338. doi: 10.1111/ppl.12552. [DOI] [PubMed] [Google Scholar]
- 38.Manoharan S.S., Miao V.P., Andresson O.S. LEC-2, a highly variable lectin in the lichen Peltigera membranacea. Symbiosis. 2012;58:91–98. doi: 10.1007/s13199-012-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg N., Zeng Y., Edlund A., Melnik A.V., Sanchez L.M., Mohimani H., Gurevich A., Miao V., Schiffler S., Lim Y.W., et al. Spatial Molecular Architecture of the Microbial Community of a Peltigera Lichen. Msystems. 2016;1 doi: 10.1128/mSystems.00139-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigurbjornsdottir M.A., Andresson O.S., Vilhelmsson O. Nutrient scavenging activity and antagonistic factors of non-photobiont lichen-associated bacteria: A review. World J. Microbiol. Biotechnol. 2016;32:68. doi: 10.1007/s11274-016-2019-2. [DOI] [PubMed] [Google Scholar]
- 41.Gelsinger D.R., Uritskiy G., Reddy R., Munn A., Farney K., DiRuggiero J. Regulatory Noncoding Small RNAs Are Diverse and Abundant in an Extremophilic Microbial Community. Msystems. 2020;5 doi: 10.1128/mSystems.00584-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., Wang M., Shi X., Yang J., Qian C., Liu Q., Zong L., Liu X., Zhu Z., Tang D., et al. Investigation into archaeal extremophilic lifestyles through comparative proteogenomic analysis. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1808531. [DOI] [PubMed] [Google Scholar]
- 43.Cullings K., Stott M.B., Marinkovich N., DeSimone J., Bhardwaj S. Phylum-level diversity of the microbiome of the extremophilic basidiomycete fungus Pisolithus arhizus (Scop.) Rauschert: An island of biodiversity in a thermal soil desert. Microbiologyopen. 2020;9:e1062. doi: 10.1002/mbo3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babonis L.S., Martindale M.Q. Phylogenetic evidence for the modular evolution of metazoan signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2015.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li P., Elowitz M.B. Communication codes in developmental signaling pathways. Development. 2019;146 doi: 10.1242/dev.170977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh A., Yadav C.B., Tabassum N., Bajpeyee A.K., Verma V. Stem cell niche: Dynamic neighbor of stem cells. Eur. J. Cell Biol. 2019;98:65–73. doi: 10.1016/j.ejcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Pastula A., Marcinkiewicz J. Cellular Interactions in the Intestinal Stem Cell Niche. Arch. Immunol. Ther. Exp. 2019;67:19–26. doi: 10.1007/s00005-018-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodsky V.Y. Direct cell-cell communications and social behavior of cells in mammals, protists, and bacteria. Possible causes of multicellularity. Russ. J. Dev. Biol. 2009;40:69–82. doi: 10.1134/S1062360409020027. [DOI] [PubMed] [Google Scholar]
- 49.Beyer E.C., Berthoud V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta Biomembr. 2018;1860:5–8. doi: 10.1016/j.bbamem.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez A., Castro C., Flores D.L., Gutierrez E., Baldi P. Gap Junction Channels of Innexins and Connexins: Relations and Computational Perspectives. Int. J. Mol. Sci. 2019;20:2476. doi: 10.3390/ijms20102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han X., Huang L.J., Feng D., Jiang W., Miu W., Li N. Plasmodesmata-Related Structural and Functional Proteins: The Long Sought-After Secrets of a Cytoplasmic Channel in Plant Cell Walls. Int. J. Mol. Sci. 2019;20:2946. doi: 10.3390/ijms20122946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganusova E.E., Burch-Smith T.M. Review: Plant-pathogen interactions through the plasmodesma prism. Plant. Sci. 2019;279:70–80. doi: 10.1016/j.plantsci.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Patel H., Bhartiya D. Direct action of FSH on testicular stem cells. Stem Cell Res. Ther. 2019;10:261. doi: 10.1186/s13287-019-1390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodman S., Naphade S., Khan M., Sharma J., Cherqui S. Macrophage polarization impacts tunneling nanotube formation and intercellular organelle trafficking. Sci. Rep. 2019;9:14529. doi: 10.1038/s41598-019-50971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baidya A.K., Bhattacharya S., Dubey G.P., Mamou G., Ben-Yehuda S. Bacterial nanotubes: A conduit for intercellular molecular trade. Curr. Opin. Microbiol. 2018;42:1–6. doi: 10.1016/j.mib.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Haglund K., Nezis I.P., Stenmark H. Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun. Integr. Biol. 2011;4:1–9. doi: 10.4161/cib.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niedenberger B.A., Cook K., Baena V., Serra N.D., Velte E.K., Agno J.E., Litwa K.A., Terasaki M., Hermann B.P., Matzuk M.M., et al. Dynamic cytoplasmic projections connect mammalian spermatogonia in vivo. Development. 2018;145 doi: 10.1242/dev.161323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel H., Bhartiya D., Parte S. Further characterization of adult sheep ovarian stem cells and their involvement in neo-oogenesis and follicle assembly. J. Ovarian Res. 2018;11:3. doi: 10.1186/s13048-017-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urbisz A.Z., Chajec L., Ito M., Ito K. The ovary organization in the marine limnodriloidin Thalassodrilides cf. briani (Annelida: Clitellata: Naididae) resembles the ovary of freshwater tubificins. Zoology. 2018;128:16–26. doi: 10.1016/j.zool.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Wang C.Y., Li X., Wu Q.F., Wang X.Y. Cytoplasmic channels and their association with plastids in male meiocytes of tobacco, onion and lily. Cell Biol. Int. 2006;30:406–411. doi: 10.1016/j.cellbi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 62.Nachury M.V., Mick D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019;20:389–405. doi: 10.1038/s41580-019-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui Y., Gao J., He Y., Jiang L. Plant extracellular vesicles. Protoplasma. 2019 doi: 10.1007/s00709-019-01435-6. [DOI] [PubMed] [Google Scholar]
- 64.Spinler J.K., Karri V., Hirschi K.D. Planting the Microbiome. Trends Microbiol. 2019;27:90–93. doi: 10.1016/j.tim.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Gill S., Catchpole R., Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019;43:273–303. doi: 10.1093/femsre/fuy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han J.H., Batey S., Nickson A.A., Teichmann S.A., Clarke J. The folding and evolution of multidomain proteins. Nat. Rev. Mol. Cell Biol. 2007;8:319–330. doi: 10.1038/nrm2144. [DOI] [PubMed] [Google Scholar]
- 67.Ko J. The leucine-rich repeat superfamily of synaptic adhesion molecules: LRRTMs and Slitrks. Mol. Cells. 2012;34:335–340. doi: 10.1007/s10059-012-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pellenc D., Schmitt E., Gallet O. Purification of a plant cell wall fibronectin-like adhesion protein involved in plant response to salt stress. Protein Expr. Purif. 2004;34:208–214. doi: 10.1016/j.pep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 69.O’Farrell T.J., Pourmotabbed T. Identification of structural elements important for matrix metalloproteinase type V collagenolytic activity as revealed by chimeric enzymes. Role of fibronectin-like domain and active site of gelatinase B. J. Biol. Chem. 2000;275:27964–27972. doi: 10.1074/jbc.M003936200. [DOI] [PubMed] [Google Scholar]
- 70.Chen J., Wang B., Wu Y. Structural Characterization and Function Prediction of Immunoglobulin-like Fold in Cell Adhesion and Cell Signaling. J. Chem. Inf. Model. 2018;58:532–542. doi: 10.1021/acs.jcim.7b00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cota E., Steward A., Fowler S.B., Clarke J. The folding nucleus of a fibronectin type III domain is composed of core residues of the immunoglobulin-like fold. J. Mol. Biol. 2001;305:1185–1194. doi: 10.1006/jmbi.2000.4378. [DOI] [PubMed] [Google Scholar]
- 72.Muller R., Jenny A., Stanley P. The EGF repeat-specific O-GlcNAc-transferase Eogt interacts with notch signaling and pyrimidine metabolism pathways in Drosophila. PLoS ONE. 2013;8:e62835. doi: 10.1371/journal.pone.0062835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ensslin M.A., Shur B.D. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–417. doi: 10.1016/S0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 74.Wang H., Feng Z., Xu B. Intercellular Instructed-Assembly Mimics Protein Dynamics To Induce Cell Spheroids. J. Am. Chem. Soc. 2019;141:7271–7274. doi: 10.1021/jacs.9b03346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young B.M., Shankar K., Tho C.K., Pellegrino A.R., Heise R.L. Laminin-driven Epac/Rap1 regulation of epithelial barriers on decellularized matrix. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z., Cai J., Li Y., He Y., Dong Z., Dai J., Lu F. External Volume Expansion Adjusted Adipose Stem Cell by Shifting the Ratio of Fibronectin to Laminin. Tissue Eng. Part A. 2019 doi: 10.1089/ten.tea.2019.0095. [DOI] [PubMed] [Google Scholar]
- 77.Buckeridge M.S. The evolution of the Glycomic Codes of extracellular matrices. Biosystems. 2018;164:112–120. doi: 10.1016/j.biosystems.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Polisetti N., Zenkel M., Menzel-Severing J., Kruse F.E., Schlotzer-Schrehardt U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells. 2016;34:203–219. doi: 10.1002/stem.2191. [DOI] [PubMed] [Google Scholar]
- 79.Andreadis D., Epivatianos A., Poulopoulos A., Nomikos A., Christidis K., Papazoglou G., Antoniades D., Barbatis C. Immunohistochemical detection of the expression of the cell adhesion molecules E-cadherin, desmoglein-2, beta4-integrin, ICAM-1 and HCAM (CD44s) in Warthin’s tumour of the parotid gland. Oral Oncol. 2005;41:799–805. doi: 10.1016/j.oraloncology.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Woods A., Wang G., Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J. Cell Physiol. 2007;213:1–8. doi: 10.1002/jcp.21110. [DOI] [PubMed] [Google Scholar]
- 81.Mizuta Y., Higashiyama T. Chemical signaling for pollen tube guidance at a glance. J. Cell Sci. 2018;131 doi: 10.1242/jcs.208447. [DOI] [PubMed] [Google Scholar]
- 82.Cotnoir-White D., Laperriere D., Mader S. Evolution of the repertoire of nuclear receptor binding sites in genomes. Mol. Cell Endocrinol. 2011;334:76–82. doi: 10.1016/j.mce.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Wu Y., Zhang D., Chu J.Y., Boyle P., Wang Y., Brindle I.D., De Luca V., Despres C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012;1:639–647. doi: 10.1016/j.celrep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 84.Zhai Q., Li L., An C., Li C. Conserved function of mediator in regulating nuclear hormone receptor activation between plants and animals. Plant Signal. Behav. 2018;13:e1403709. doi: 10.1080/15592324.2017.1403709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reeves D.C., Lummis S.C. The molecular basis of the structure and function of the 5-HT3 receptor: A model ligand-gated ion channel (review) Mol. Membr. Biol. 2002;19:11–26. doi: 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- 86.Olsen R.W. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology. 2018;136:10–22. doi: 10.1016/j.neuropharm.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diwanji D., Thaker T., Jura N. More than the sum of the parts: Toward full-length receptor tyrosine kinase structures. IUBMB Life. 2019;71:706–720. doi: 10.1002/iub.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Josso N., di Clemente N. Serine/threonine kinase receptors and ligands. Curr. Opin. Genet. Dev. 1997;7:371–377. doi: 10.1016/S0959-437X(97)80151-7. [DOI] [PubMed] [Google Scholar]
- 89.Kostas M., Haugsten E.M., Zhen Y., Sorensen V., Szybowska P., Fiorito E., Lorenz S., Jones N., de Souza G.A., Wiedlocha A., et al. Protein Tyrosine Phosphatase Receptor Type G (PTPRG) Controls Fibroblast Growth Factor Receptor (FGFR) 1 Activity and Influences Sensitivity to FGFR Kinase Inhibitors. Mol. Cell Proteom. 2018;17:850–870. doi: 10.1074/mcp.RA117.000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Misono K.S., Philo J.S., Arakawa T., Ogata C.M., Qiu Y., Ogawa H., Young H.S. Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase. FEBS J. 2011;278:1818–1829. doi: 10.1111/j.1742-4658.2011.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duda T., Pertzev A., Sharma R.K. Atrial natriuretic factor receptor guanylate cyclase, ANF-RGC, transduces two independent signals, ANF and Ca(2+) Front. Mol. Neurosci. 2014;7:17. doi: 10.3389/fnmol.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lobingier B.T., von Zastrow M. When trafficking and signaling mix: How subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic. 2019;20:130–136. doi: 10.1111/tra.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamp M.E., Liu Y., Kortholt A. Function and Regulation of Heterotrimeric G Proteins during Chemotaxis. Int. J. Mol. Sci. 2016;17:90. doi: 10.3390/ijms17010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schappi J.M., Krbanjevic A., Rasenick M.M. Tubulin, actin and heterotrimeric G proteins: Coordination of signaling and structure. Biochim. Biophys. Acta. 2014;1838:674–681. doi: 10.1016/j.bbamem.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmed S.M., Angers S. Emerging non-canonical functions for heterotrimeric G proteins in cellular signaling. J. Recept. Signal Transduct. Res. 2013;33:177–183. doi: 10.3109/10799893.2013.795972. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y., Li Y., Shi G. The regulating function of heterotrimeric G proteins in the immune system. Arch. Immunol. Ther. Exp. 2013;61:309–319. doi: 10.1007/s00005-013-0230-5. [DOI] [PubMed] [Google Scholar]
- 97.Baltoumas F.A., Theodoropoulou M.C., Hamodrakas S.J. Interactions of the alpha-subunits of heterotrimeric G-proteins with GPCRs, effectors and RGS proteins: A critical review and analysis of interacting surfaces, conformational shifts, structural diversity and electrostatic potentials. J. Struct. Biol. 2013;182:209–218. doi: 10.1016/j.jsb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Tall G.G. Ric-8 regulation of heterotrimeric G proteins. J. Recept. Signal Transduct. Res. 2013;33:139–143. doi: 10.3109/10799893.2013.763828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naor Z., Huhtaniemi I. Interactions of the GnRH receptor with heterotrimeric G proteins. Front. Neuroendocr. 2013;34:88–94. doi: 10.1016/j.yfrne.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Cooper D.M., Tabbasum V.G. Adenylate cyclase-centred microdomains. Biochem. J. 2014;462:199–213. doi: 10.1042/BJ20140560. [DOI] [PubMed] [Google Scholar]
- 101.Jakobsen E., Lange S.C., Bak L.K. Soluble adenylyl cyclase-mediated cAMP signaling and the putative role of PKA and EPAC in cerebral mitochondrial function. J. Neurosci. Res. 2019;97:1018–1038. doi: 10.1002/jnr.24477. [DOI] [PubMed] [Google Scholar]
- 102.Baldwin T.A., Dessauer C.W. Function of Adenylyl Cyclase in Heart: The AKAP Connection. J. Cardiovasc. Dev. Dis. 2018;5:2. doi: 10.3390/jcdd5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jang H.J., Suh P.G., Lee Y.J., Shin K.J., Cocco L., Chae Y.C. PLCgamma1: Potential arbitrator of cancer progression. Adv. Biol. Regul. 2018;67:179–189. doi: 10.1016/j.jbior.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Nomikos M., Kashir J., Lai F.A. The role and mechanism of action of sperm PLC-zeta in mammalian fertilisation. Biochem. J. 2017;474:3659–3673. doi: 10.1042/BCJ20160521. [DOI] [PubMed] [Google Scholar]
- 105.Nakamura Y., Fukami K. Regulation and physiological functions of mammalian phospholipase C. J. Biochem. 2017;161:315–321. doi: 10.1093/jb/mvw094. [DOI] [PubMed] [Google Scholar]
- 106.Jho E.H. Hippo signaling: Special issue of BMB Reports in 2018. BMB Rep. 2018;51:105. doi: 10.5483/BMBRep.2018.51.3.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hubbard S.R. Mechanistic Insights into Regulation of JAK2 Tyrosine Kinase. Front. Endocrinol. 2017;8:361. doi: 10.3389/fendo.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bousoik E., Aliabadi H.M. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018;8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hammaren H.M., Virtanen A.T., Raivola J., Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 110.Satterthwaite A.B. Bruton’s Tyrosine Kinase, a Component of B Cell Signaling Pathways, Has Multiple Roles in the Pathogenesis of Lupus. Front. Immunol. 2017;8:1986. doi: 10.3389/fimmu.2017.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blas-Rus N., Bustos-Moran E., Martin-Cofreces N.B., Sanchez-Madrid F. Aurora-A shines on T cell activation through the regulation of Lck. Bioessays. 2017;39 doi: 10.1002/bies.201600156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salah Ud-Din A.I.M., Roujeinikova A. Methyl-accepting chemotaxis proteins: A core sensing element in prokaryotes and archaea. Cell. Mol. Life Sci. 2017;74:3293–3303. doi: 10.1007/s00018-017-2514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Merilahti J.A.M., Elenius K. Gamma-secretase-dependent signaling of receptor tyrosine kinases. Oncogene. 2019;38:151–163. doi: 10.1038/s41388-018-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Opdenbosch N., Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Songane M., Khair M., Saleh M. An updated view on the functions of caspases in inflammation and immunity. Semin. Cell Dev. Biol. 2018;82:137–149. doi: 10.1016/j.semcdb.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 116.Herr A.B. Evolution of an allosteric “off switch” in apoptotic caspases. J. Biol. Chem. 2018;293:5462–5463. doi: 10.1074/jbc.H118.002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varshney P., Yadav V., Saini N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology. 2016;149:13–24. doi: 10.1111/imm.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Resh M.D. Covalent lipid modifications of proteins. Curr. Biol. 2013;23:R431–R435. doi: 10.1016/j.cub.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nair A., Chauhan P., Saha B., Kubatzky K.F. Conceptual Evolution of Cell Signaling. Int. J. Mol. Sci. 2019;20:3292. doi: 10.3390/ijms20133292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kleino A., Myllymaki H., Kallio J., Vanha-aho L.M., Oksanen K., Ulvila J., Hultmark D., Valanne S., Ramet M. Pirk is a negative regulator of the Drosophila Imd pathway. J. Immunol. 2008;180:5413–5422. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 121.Bosco-Drayon V., Poidevin M., Boneca I.G., Narbonne-Reveau K., Royet J., Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe. 2012;12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 122.Lazar V., Ditu L.M., Pircalabioru G.G., Gheorghe I., Curutiu C., Holban A.M., Picu A., Petcu L., Chifiriuc M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cohen L.J., Esterhazy D., Kim S.H., Lemetre C., Aguilar R.R., Gordon E.A., Pickard A.J., Cross J.R., Emiliano A.B., Han S.M., et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruning J., Chapp A., Kaurala G.A., Wang R., Techtmann S., Chen Q.H. Gut Microbiota and Short Chain Fatty Acids: Influence on the Autonomic Nervous System. Neurosci. Bull. 2019 doi: 10.1007/s12264-019-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Toral M., Robles-Vera I., de la Visitacion N., Romero M., Yang T., Sanchez M., Gomez-Guzman M., Jimenez R., Raizada M.K., Duarte J. Critical Role of the Interaction Gut Microbiota—Sympathetic Nervous System in the Regulation of Blood Pressure. Front. Physiol. 2019;10:231. doi: 10.3389/fphys.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heiss C.N., Olofsson L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocr. 2019;31:e12684. doi: 10.1111/jne.12684. [DOI] [PubMed] [Google Scholar]
- 127.Van de Wouw M., Stilling R.M., Peterson V.L., Ryan F.J., Hoban A.E., Shanahan F., Clarke G., Claesson M.J., Dinan T.G., Cryan J.F., et al. Host Microbiota Regulates Central Nervous System Serotonin Receptor 2C Editing in Rodents. ACS Chem. Neurosci. 2019;10:3953–3960. doi: 10.1021/acschemneuro.9b00414. [DOI] [PubMed] [Google Scholar]
- 128.Ma Q., Xing C., Long W., Wang H.Y., Liu Q., Wang R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019;16:53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Whittaker D.J., Slowinski S.P., Greenberg J.M., Alian O., Winters A.D., Ahmad M.M., Burrell M.J.E., Soini H.A., Novotny M.V., Ketterson E.D., et al. Experimental evidence that symbiotic bacteria produce chemical cues in a songbird. J. Exp. Biol. 2019;222:jeb202978. doi: 10.1242/jeb.202978. [DOI] [PubMed] [Google Scholar]
- 130.Chen Y.E., Fischbach M.A., Belkaid Y. Skin microbiota-host interactions. Nature. 2018;553:427–436. doi: 10.1038/nature25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saijo Y., Loo E.P., Yasuda S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018;93:592–613. doi: 10.1111/tpj.13808. [DOI] [PubMed] [Google Scholar]
- 132.Tang D., Wang G., Zhou J.M. Receptor Kinases in Plant-Pathogen Interactions: More Than Pattern Recognition. Plant Cell. 2017;29:618–637. doi: 10.1105/tpc.16.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vincent D., Rafiqi M., Job D. The Multiple Facets of Plant–Fungal Interactions Revealed Through Plant and Fungal Secretomics. Front. Plant Sci. 2020;10 doi: 10.3389/fpls.2019.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parniske M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 135.Markmann K., Giczey G., Parniske M. Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biol. 2008;6:e68. doi: 10.1371/journal.pbio.0060068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bedini A., Mercy L., Schneider C., Franken P., Lucic-Mercy E. Unraveling the Initial Plant Hormone Signaling, Metabolic Mechanisms and Plant Defense Triggering the Endomycorrhizal Symbiosis Behavior. Front. Plant. Sci. 2018;9:1800. doi: 10.3389/fpls.2018.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang M., Schafer M., Li D., Halitschke R., Dong C., McGale E., Paetz C., Song Y., Li S., Dong J., et al. Blumenols as shoot markers of root symbiosis with arbuscular mycorrhizal fungi. Elife. 2018;7 doi: 10.7554/eLife.37093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Afzal I., Shinwari Z.K., Sikandar S., Shahzad S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 139.Deicke M., Mohr J.F., Roy S., Herzsprung P., Bellenger J.P., Wichard T. Metallophore profiling of nitrogen-fixing Frankia spp. to understand metal management in the rhizosphere of actinorhizal plants. Metallomics. 2019;11:810–821. doi: 10.1039/C8MT00344K. [DOI] [PubMed] [Google Scholar]
- 140.Desbrosses G.J., Stougaard J. Root nodulation: A paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe. 2011;10:348–358. doi: 10.1016/j.chom.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 141.Morieri G., Martinez E.A., Jarynowski A., Driguez H., Morris R., Oldroyd G.E., Downie J.A. Host-specific Nod-factors associated with Medicago truncatula nodule infection differentially induce calcium influx and calcium spiking in root hairs. New Phytol. 2013;200:656–662. doi: 10.1111/nph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Svistoonoff S., Hocher V., Gherbi H. Actinorhizal root nodule symbioses: What is signalling telling on the origins of nodulation? Curr. Opin. Plant Biol. 2014;20:11–18. doi: 10.1016/j.pbi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 143.Kawaharada Y., James E.K., Kelly S., Sandal N., Stougaard J. The Ethylene Responsive Factor Required for Nodulation 1 (ERN1) Transcription Factor Is Required for Infection-Thread Formation in Lotus japonicus. Mol. Plant Microbe Interact. 2017;30:194–204. doi: 10.1094/MPMI-11-16-0237-R. [DOI] [PubMed] [Google Scholar]
- 144.Sun J., Cardoza V., Mitchell D.M., Bright L., Oldroyd G., Harris J.M. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 2006;46:961–970. doi: 10.1111/j.1365-313X.2006.02751.x. [DOI] [PubMed] [Google Scholar]
- 145.Oldroyd G.E., Engstrom E.M., Long S.R. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001;13:1835–1849. doi: 10.1105/TPC.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reid D., Liu H., Kelly S., Kawaharada Y., Mun T., Andersen S.U., Desbrosses G., Stougaard J. Dynamics of Ethylene Production in Response to Compatible Nod Factor. Plant Physiol. 2018;176:1764–1772. doi: 10.1104/pp.17.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McAdam E.L., Reid J.B., Foo E. Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. J. Exp. Bot. 2018;69:2117–2130. doi: 10.1093/jxb/ery046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fonouni-Farde C., Kisiala A., Brault M., Emery R.J.N., Diet A., Frugier F. DELLA1-Mediated Gibberellin Signaling Regulates Cytokinin-Dependent Symbiotic Nodulation. Plant Physiol. 2017;175:1795–1806. doi: 10.1104/pp.17.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu Y.H., Liao Y.C., Zhang Z., Liu J., Sun P.W., Gao Z.H., Sui C., Wei J.H. Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci. Rep. 2016;6:21843. doi: 10.1038/srep21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lv Z., Guo Z., Zhang L., Zhang F., Jiang W., Shen Q., Fu X., Yan T., Shi P., Hao X., et al. Interaction of bZIP transcription factor TGA6 with salicylic acid signaling modulates artemisinin biosynthesis in Artemisia annua. J. Exp. Bot. 2019;70:3969–3979. doi: 10.1093/jxb/erz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu L., Ye M., Erb M. Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance. Plant Cell Environ. 2019;42:959–971. doi: 10.1111/pce.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kalske A., Shiojiri K., Uesugi A., Sakata Y., Morrell K., Kessler A. Insect Herbivory Selects for Volatile-Mediated Plant-Plant Communication. Curr. Biol. 2019;29:3128–3133.e3. doi: 10.1016/j.cub.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 153.De Vries J., Evers J.B., Dicke M., Poelman E.H. Ecological interactions shape the adaptive value of plant defence: Herbivore attack versus competition for light. Funct. Ecol. 2019;33:129–138. doi: 10.1111/1365-2435.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ali M., Sugimoto K., Ramadan A., Arimura G. Memory of plant communications for priming anti-herbivore responses. Sci. Rep. 2013;3:1872. doi: 10.1038/srep01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Glaum P., Kessler A. Functional reduction in pollination through herbivore-induced pollinator limitation and its potential in mutualist communities. Nat. Commun. 2017;8:2031. doi: 10.1038/s41467-017-02072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L., Einig E., Almeida-Trapp M., Albert M., Fliegmann J., Mithofer A., Kalbacher H., Felix G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants. 2018;4:152–156. doi: 10.1038/s41477-018-0106-0. [DOI] [PubMed] [Google Scholar]
- 157.Pashalidou F.G., Lambert H., Peybernes T., Mescher M.C., De Moraes C.M. Bumble bees damage plant leaves and accelerate flower production when pollen is scarce. Science. 2020;368:881–884. doi: 10.1126/science.aay0496. [DOI] [PubMed] [Google Scholar]
- 158.Higgs P.G., Lehman N. The RNA World: Molecular cooperation at the origins of life. Nat. Rev. Genet. 2015;16:7–17. doi: 10.1038/nrg3841. [DOI] [PubMed] [Google Scholar]
- 159.Goldman A.D., Beatty J.T., Landweber L.F. The TIM Barrel Architecture Facilitated the Early Evolution of Protein-Mediated Metabolism. J. Mol. Evol. 2016;82:17–26. doi: 10.1007/s00239-015-9722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Danchin A. The origin of life revisited: From metabolites to cells. Med. Sci. 2018;34:984–989. doi: 10.1051/medsci/2018234. [DOI] [PubMed] [Google Scholar]
- 161.Danchin A. From chemical metabolism to life: The origin of the genetic coding process. Beilstein J. Org. Chem. 2017;13:1119–1135. doi: 10.3762/bjoc.13.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pross A., Pascal R. The origin of life: What we know, what we can know and what we will never know. Open Biol. 2013;3:120190. doi: 10.1098/rsob.120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takagi Y.A., Nguyen D.H., Wexler T.B., Goldman A.D. The Coevolution of Cellularity and Metabolism Following the Origin of Life. J. Mol. Evol. 2020;88:598–617. doi: 10.1007/s00239-020-09961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hanczyc M.M., Monnard P.A. Primordial membranes: More than simple container boundaries. Curr. Opin. Chem. Biol. 2017;40:78–86. doi: 10.1016/j.cbpa.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 165.Martin W., Russell M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. Lond. 2003;358:59–83. doi: 10.1098/rstb.2002.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lane N., Martin W.F. The origin of membrane bioenergetics. Cell. 2012;151:1406–1416. doi: 10.1016/j.cell.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 167.Pearce B.K.D., Tupper A.S., Pudritz R.E., Higgs P.G. Constraining the Time Interval for the Origin of Life on Earth. Astrobiology. 2018;18:343–364. doi: 10.1089/ast.2017.1674. [DOI] [PubMed] [Google Scholar]
- 168.Czaran T., Konnyu B., Szathmary E. Metabolically Coupled Replicator Systems: Overview of an RNA-world model concept of prebiotic evolution on mineral surfaces. J. Theor. Biol. 2015;381:39–54. doi: 10.1016/j.jtbi.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 169.Lopez A., Fiore M. Investigating Prebiotic Protocells for A Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life. 2019;9:49. doi: 10.3390/life9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Staps M., van Gestel J., Tarnita C.E. Emergence of diverse life cycles and life histories at the origin of multicellularity. Nat. Ecol. Evol. 2019;3:1197–1205. doi: 10.1038/s41559-019-0940-0. [DOI] [PubMed] [Google Scholar]
- 171.Allen J.F. A Proposal for Formation of Archaean Stromatolites before the Advent of Oxygenic Photosynthesis. Front. Microbiol. 2016;7:1784. doi: 10.3389/fmicb.2016.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schrum J.P., Zhu T.F., Szostak J.W. The origins of cellular life. Cold Spring Harb. Perspect. Biol. 2010;2:a002212. doi: 10.1101/cshperspect.a002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Egel R. Primal eukaryogenesis: On the communal nature of precellular States, ancestral to modern life. Life. 2012;2:170–212. doi: 10.3390/life2010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Archibald J.M. Endosymbiosis and Eukaryotic Cell Evolution. Curr. Biol. 2015;25:R911–R921. doi: 10.1016/j.cub.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 175.Lopez-Garcia P., Eme L., Moreira D. Symbiosis in eukaryotic evolution. J. Theor. Biol. 2017;434:20–33. doi: 10.1016/j.jtbi.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Spitzer J. Emergence of life from multicomponent mixtures of chemicals: The case for experiments with cycling physicochemical gradients. Astrobiology. 2013;13:404–413. doi: 10.1089/ast.2012.0924. [DOI] [PubMed] [Google Scholar]
- 177.Grandchamp A., Monget P. Synchronous birth is a dominant pattern in receptor-ligand evolution. BMC Genom. 2018;19:611. doi: 10.1186/s12864-018-4977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vakirlis N., Acar O., Hsu B., Castilho Coelho N., Van Oss S.B., Wacholder A., Medetgul-Ernar K., Bowman R.W., Hines C.P., Iannotta J., et al. De novo emergence of adaptive membrane proteins from thymine-rich genomic sequences. Nat. Commun. 2020;11:781. doi: 10.1038/s41467-020-14500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liebeskind B.J., Hillis D.M., Zakon H.H. Convergence of ion channel genome content in early animal evolution. Proc. Natl. Acad. Sci. USA. 2015;112:E846–E851. doi: 10.1073/pnas.1501195112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Combarnous Y. Molecular basis of the specificity of binding of glycoprotein hormones to their receptors. Endocr. Rev. 1992;13:670–691. doi: 10.1210/edrv-13-4-670. [DOI] [PubMed] [Google Scholar]
- 181.Combarnous Y., Henge M.H. Equine follicle-stimulating hormone. Purification, acid dissociation, and binding to equine testicular tissue. J. Biol. Chem. 1981;256:9567–9572. [PubMed] [Google Scholar]
- 182.Combarnous Y., Nguyen T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics. 2019;7:5. doi: 10.3390/toxics7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Besedovsky H.O., del Rey A. Immune-neuro-endocrine interactions: Facts and hypotheses. Endocr. Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 184.Liu Z., Wang L., Zhou Z., Sun Y., Wang M., Wang H., Hou Z., Gao D., Gao Q., Song L. The simple neuroendocrine-immune regulatory network in oyster Crassostrea gigas mediates complex functions. Sci. Rep. 2016;6:26396. doi: 10.1038/srep26396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Del Rey A., Besedovsky H.O. Immune-Neuro-Endocrine Reflexes, Circuits, and Networks: Physiologic and Evolutionary Implications. Endocr. Immunol. 2017;48:1–18. doi: 10.1159/000452902. [DOI] [PubMed] [Google Scholar]
- 186.Procaccini C., Pucino V., De Rosa V., Marone G., Matarese G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 2014;5:143. doi: 10.3389/fimmu.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tarrant A.M. Endocrine-like Signaling in Cnidarians: Current Understanding and Implications for Ecophysiology. Integr. Comp. Biol. 2005;45:201–214. doi: 10.1093/icb/45.1.201. [DOI] [PubMed] [Google Scholar]
- 188.MacKintosh C., Ferrier D.E.K. Recent advances in understanding the roles of whole genome duplications in evolution. F1000Research. 2017;6:1623. doi: 10.12688/f1000research.11792.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uchida K., Moriyama S., Chiba H., Shimotani T., Honda K., Miki M., Takahashi A., Sower S.A., Nozaki M. Evolutionary origin of a functional gonadotropin in the pituitary of the most primitive vertebrate, hagfish. Proc. Natl. Acad. Sci. USA. 2010;107:15832–15837. doi: 10.1073/pnas.1002208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dufour S., Querat B., Tostivint H., Pasqualini C., Vaudry H., Rousseau K. Origin and Evolution of the Neuroendocrine Control of Reproduction in Vertebrates, With Special Focus on Genome and Gene Duplications. Physiol. Rev. 2020;100:869–943. doi: 10.1152/physrev.00009.2019. [DOI] [PubMed] [Google Scholar]
- 191.Neuman H., Debelius J.W., Knight R., Koren O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015;39:509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 192.Griesmann M., Chang Y., Liu X., Song Y., Haberer G., Crook M.B., Billault-Penneteau B., Lauressergues D., Keller J., Imanishi L., et al. Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science. 2018;361 doi: 10.1126/science.aat1743. [DOI] [PubMed] [Google Scholar]
- 193.Wu Z., Wang M., Yang S., Chen S., Chen X., Liu C., Wang S., Wang H., Zhang B., Liu H., et al. A global coexpression network of soybean genes gives insights into the evolution of nodulation in nonlegumes and legumes. New Phytol. 2019;223:2104–2119. doi: 10.1111/nph.15845. [DOI] [PubMed] [Google Scholar]
- 194.Nascimento F.X., Tavares M.J., Rossi M.J., Glick B.R. The modulation of leguminous plant ethylene levels by symbiotic rhizobia played a role in the evolution of the nodulation process. Heliyon. 2018;4:e01068. doi: 10.1016/j.heliyon.2018.e01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jiang Y., Wang W., Xie Q., Liu N., Liu L., Wang D., Zhang X., Yang C., Chen X., Tang D., et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172–1175. doi: 10.1126/science.aam9970. [DOI] [PubMed] [Google Scholar]
- 196.Song Y.Y., Zeng R.S., Xu J.F., Li J., Shen X., Yihdego W.G. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE. 2010;5:e13324. doi: 10.1371/journal.pone.0013324. [DOI] [PMC free article] [PubMed] [Google Scholar]