Highlights
-
•
GABA+ concentrations were reduced in mPFC of hypothyroid patients.
-
•
Reduced GABA+ correlated with depressive and memory state in hypothyroidism.
-
•
Levothyroxine reverses GABA+ and neuropsychological impairment in hypothyroidism.
Keywords: GABA, Hypothyroidism, Levothyroxine, MRS
Abstract
Objective
Increasing evidence indicates the involvement of the GABAergic system in the pathophysiology of hypothyroidism. We aimed to investigate longitudinal changes of brain GABA in primary hypothyroidism before and after levothyroxine (L-T4) treatment.
Material and methods
In 18 patients with hypothyroidism, we used the MEGA-PRESS (Mescher-Garwood point-resolved spectroscopy) editing sequence to measure brain GABA levels from medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) at baseline and after 6-months of L-T4 treatment. Sex- and age-matched healthy controls (n = 18) were scanned at baseline. Thyroid function and neuropsychological tests were also performed.
Results
GABA signals were successfully quantified from all participants with fitting errors lower than 15%. GABA signal was labeled as GABA+ due to contamination from co-edited macromoleculars and homocarnosine. In hypothyroid patients, mean GABA+ was significantly lower in the mPFC region compared with controls (p = 0.031), and the mPFC GABA+ measurements were significantly correlated with depressive symptoms and memory function (r = −0.558, p = 0.016; r = 0.522, p = 0.026, respectively). After adequate L-T4 treatment, the mPFC GABA+ in hypothyroid patients increased to normal level, along with relieved neuropsychological impairments.
Conclusion
The study suggested the decrease of GABA+ may be an important neurobiological factor in the pathophysiology of hypothyroidism. Treatment of L-T4 may reverse the abnormal GABA+ and hypothyroidism-induced neuropsychiatric impairments, indicating the action mode of L-T4 in adjunctive treatment of affective disorders.
1. Introduction
Hypothyroidism (Chaker et al., 2017) is a common clinical condition which is characterized by elevated levels of the thyroid stimulating hormone (TSH), and additional low levels of triiodothyronine (T3) or thyroxin (T4). Deficiency of brain thyroid hormones (THs) may disturb normal brain function and influence mood and cognition (Leyhe and Mussig, 2014, Bauer et al., 2008, Samuels, 2014). Advances in brain imaging techniques enabled us to explore the structural (Cooke et al., 2014), functional (He et al., 2011, Singh et al., 2015), and neurochemical deficits (Singh et al., 2016, Modi et al., 2008) contributing to various neuropsychiatric symptoms in hypothyroid patients. However, the underlying mechanisms still have not been fully identified.
γ-Aminobutyric acid (GABA), the main inhibitory neurotransmitter in human brain, plays important roles in modulating mood and cognition. Dysregulation of GABAergic system has been implicated in the pathophysiology of many neurological and psychiatric disorders (Schur et al., 2016). Evidence suggests a reciprocal regulation of THs and the GABAergic system (Wiens and Trudeau, 2006, Ahmed et al., 2008). In vivo and in vitro studies on animals indicate that THs have effects on multiple components of the GABAergic system (Wiens and Trudeau, 2006, Ahmed et al., 2010), including the rate-limiting synthesizing enzyme glutamic acid decarboxylase (GAD), levels of GABA and glutamate (Glu), and GABA receptor function. Reciprocally, GABA also affects thyroid system function and shows therapeutic potential in hypothyroidism (Yang et al., 2019). Thus, further investigation of brain GABA alterations may provide insights to better understand the neurobiological mechanisms in hypothyroidism-related symptoms.
Many studies have focused on GABAergic neurotransmission in hypothyroidism. Experimental results revealed hypothyroidism may impair GABAergic synaptic transmission in the developing brain (Gilbert and Paczkowski, 2003, Koromilas et al., 2010). Electrophysiological recordings revealed reduced GABAergic synaptic transmission in the anterior cingulate cortex (ACC) of hypothyroid mice (Yi et al., 2014). In terms of brain GABA content, early preclinical studies showed increased GABA in the whole brain, cortex and hypothalamus of adult rats with hypothyroidism (Chapa et al., 1995, Upadhyaya and Agrawal, 1993), but decreased GABA content in the hippocampus (Abd Allah et al., 2014). Our previous proton magnetic resonance spectroscopy (1H-MRS) study (Liu et al., 2017) has found that GABA levels were decreased in the medial prefrontal cortex (mPFC) region of untreated primary hypothyroidism patients. These preliminary results suggested that alteration of GABAergic neurotransmission may play an important role in the pathophysiology of hypothyroidism. Levothyroxine therapy is the standard care for correcting hypothyroidism (Jonklaas et al., 2014). The neuropsychiatric impairments accompanying dysfunction of the thyroid are usually reversed rapidly following return to euthyroid hormone status (Alzoubi et al., 2009, Chaalal et al., 2019). Several studies also have shown the reversible effects of L-T4 in restoring brain blood flow velocity (Utku et al., 2011), inhibitory synaptic transmission (Yi et al., 2014), brain activation (He et al., 2011), and cortical excitability (Rizzo et al., 2008) using functional magnetic resonance imaging (MRI). Brain metabolic changes may also be reversible after supplementation of L-T4 (Singh et al., 2016).
1H-MRS provides a non-invasive method for quantification of brain metabolites in discrete regions of the human brain. GABA is present at low millimolar concentrations and is difficult to resolve from overlapping signals of other more concentrated metabolites in conventional 1H-MRS at 3.0 T. Spectral editing techniques such as Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) sequence have been established and successfully applied in a number of clinical studies (Puts and Edden, 2012). However, 1H-MRS derived GABA signals may reflect the entire GABA content of the selected voxel. The interpretation of the MRS-detected GABA measures may have uncertainties (Stagg et al., 2011), because it cannot distinguish among intracellular or synaptic or extracellular GABA compartments.
In this study, we conducted a longitudinal proton MRS study investigating alterations of brain GABA in primary hypothyroid patients before and after L-T4 treatment (6 months). It was hypothesized that GABA concentrations, together with mood and cognition changes will normalize after L-T4 treatment. The mPFC and posterior cingulate cortex (PCC) were selected as volume of interest (VOI). The mPFC region is mainly located in the anterior middle cingulate cortex (MCC), which has extensive connections with cognitive areas of cortex (e.g. orbitofrontal cortex). The mPFC region was suggested to be associated with mood and cognition regulation (Stan et al., 2014, Schulz et al., 2011) and also have been implicated in previous neuroimaging studies of hypothyroidism (He et al., 2011, Bauer et al., 2009). Imbalance of excitation and inhibition (E/I ratio) within the PFC specifically may be a possible mechanism leading to dysregulation of cognition (Bicks et al., 2015). The PCC was chosen as a control region.
2. Material and methods
2.1. Participants
The protocol was approved by the local institutional review board. Written informed consent for participation was obtained from each subject and his/her family. 18 hypothyroidism patients (12 female, age 43.06 ± 7.04) and 18 age-, weight- and sex-matched healthy controls (13 female, age 44.5 ± 6.13) voluntarily participated in this study. All hypothyroid patients were newly diagnosed with elevated serum thyroid-stimulating hormone (TSH) and lowered free thyroxine (fT4) and free triiodothyronine (fT3), recruited as outpatients at Shandong Provincial Hospital Affiliated to Shandong First Medical University from April 2015 to October 2018. All controls were recruited from the local community via fliers. Each participant was given a Structured Clinical Interview for DSM-IV (SCID) for Axis I disorders. Exclusion criteria for participants included: current or past psychiatric disorders; dementia; head injury; diabetes; stroke; oral contraceptives; alcohol or drug dependence; or other psychotropic medication or cigarettes in the 6 months before enrolment. No female participant was postmenopausal.
We conducted a longitudinal study that included two identical sessions of MRI and neuropsychological evaluation for each patient. The first session was performed at baseline when the hypothyroidism diagnosis was made. Then all patients were treated with L-T4 immediately and became euthyroid after 4–6 weeks. After that, they were treated continuously to maintain the euthyroid states. The second session was performed after 6 months of L-T4 replacement therapy to investigate a stabilized treating effect. The control group only participated in one session. All participants completed neuropsychological tests and MR scanning within 2 days of administration of thyroid function tests. For female subjects, MRI scanning was performed in the follicular phase (1–7 days following the end of menses). Ovulation was assessed with a urinary luteinizing hormone kit (BOSON, Xiamen, China).
2.2. Laboratory test
Thyroid function tests were carried out for all patients and controls. Serum TSH, fT3 and fT4 were measured with chemiluminescent enzyme immunoassay methods (ADVIA Centaur, Siemens Healthcare Diagnostics Inc., NY, USA). The normal reference ranges were 0.55–4.78 mIU/L for TSH, 3.5–6.5 pmol/L for fT3 and 11.5–22.7 pmol/L for fT4.
2.3. Neuropsychological evaluation
The Montreal Cognitive Assessment (MoCA) is a brief cognitive screening instrument developed to screen milder forms of cognitive impairment. MoCA was performed to assess global cognitive function of all participants. Memory decline is a common complaint in hypothyroid patients. Wechsler Memory Scale-Chinese Revision (WMS-CR) was used to assess the specific memory states of each participant by providing an overall memory quotient (MQ) (Gong et al., 1989). The 21-item self-rated Beck Depression Inventory-II (BDI-II) was used to assess depressive symptoms in all participants. The same qualified neuropsychologist with 8-year work experiences conducted all the neuropsychological testing.
2.4. MRI scanning protocol
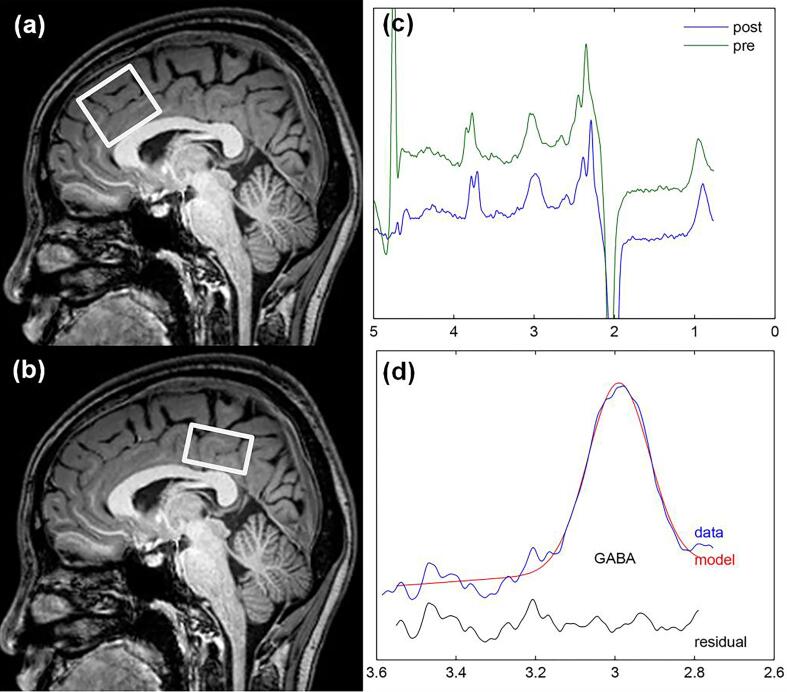
All MRI procedures were carried out on a 3.0 T scanner (Philips Achieva TX, Best, The Netherlands), equipped with an eight-channel phased-array head coil. T2-FLAIR images were first acquired to rule out neurological abnormalities in all participants. For structural scan, a T1-weighted three-dimensional turbo field echo (TFE) scan was acquired for MRS voxel placement and brain tissue segmentation. The scanning parameters were as follows: repetition time (TR) = 8.2 ms; echo time (TE) = 3.7 ms; slice thickness = 1 mm; matrix = 256 × 256; field of view = 24 × 24 cm2; and flip angle = 8°. For MRS scan, the MEGA-PRESS sequence was performed to detect GABA signals from two volumes of interest (VOI) (as shown in Fig. 1): (1) the mPFC encompassing portions of MCC (3 × 3 × 3 cm3); and (2) the PCC (3 × 3 × 2 cm3). The mPFC and PCC voxels were positioned with the inferior edge of the voxels parallel to the anterior and posterior descending surfaces of the truncus corporis callosi, respectively, and were located on the medial aspect of the axial plane with maximum inclusion of grey matter. The same one technician performed all the voxel placements. The scanning parameters were as follows: TR = 2000 ms, TE = 68 ms, acquisition bandwidth = 2000 Hz, 256 averages and scan duration 8 min 48 s. For quantification, a shorter measurement (4 averages) of the unsuppressed water signal was obtained. FASTMAP shimming of the voxels was performed automatically before each acquisition, yielding water signal line widths of 6 to 9 Hz.
Fig. 1.
Mescher-Garwood point resolved spectroscopy (MEGA-PRESS) voxel position and representative spectra. T1-weighted sagittal TFE images show voxel placements of the median prefrontal cortex (mPFC) (a) and the posterior cingulate cortex (PCC) (b). (c) Representative GABA+ spectra (pre- and post-alignment). (d) GABA+ peak fitting by Gannet; the red line in the panel is the result of the Gaussian line-shape curve-fitting, the blue line shows the processed GABA+ spectrum, and the black line is the residual difference between the processed and fitted spectra. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. MRI data processing
As the detected GABA signal at 3.02 ppm is also expected to contain contribution from co-edited macromoleculars (MMs) and homocarnosine (Puts and Edden, 2012), this signal is labeled GABA+ in the rest of this manuscript for indication of potential influence from the co-edited compounds. The MEGA-PRESS data were processed using “Gannet” (GABA-MRS Analysis Tool) in Matlab 2010b (Mathworks) with Gaussian curve fitting of the GABA+ peaks (Edden et al., 2014). Gannet comprised two main modules: GannetLoad, which is used to process the raw data, including application of an exponential line broadening of 3 Hz, and frequency and phase correction for individual spectrum using Spectral Registration; and GannetFit, which uses a single-Gaussian model to fit the edited GABA+ peak, and a Gaussian-Lorentzian model for water spectrum. A representative GABA+ spectrum is shown in Fig. 1.
The ratios of the integrals of GABA+ and water signals, making corrections for T1 and T2 relaxations and tissue composition, were used to calculate water-scaled GABA+ levels in institutional units (IU) as [GABA+] using the formula (Mullins et al., 2014, Gasparovic et al., 2006):
where IG and IW are the fitting integrals of GABA+ (G) and water (W) as determined by AMARES, [H2O] is the pure water concentration (55,550 mmol/L), VISw is water visibility of MR (0.65), and fGM, fWM, fCSF are the fractions of water attributable to GM, WM, and CSF respectively (Gasparovic et al., 2006). The relaxation attenuation factors are given by the equation RW_y = exp[−TE/T2W_y](1 − exp[−TR/T1W_y)), where T1W_y and T2W_y are the T1 and T2 relaxation times of water in compartment y (GM, WM, or CSF). Similarly, RG is the relaxation attenuation factor for GABA. The relaxation times used were as follows (Gao et al., 2015): GM water: T1 = 1331 ms, T2 = 110 ms; WM water: T1 = 832 ms, T2 = 79.6 ms; CSF: T1 = 3817 ms, T2 = 503 ms. GABA: T1 = 1310 ms, T2 = 88 ms. MMcor is a macromolecular correction factor given by the fraction of GABA+ peak that is thought to be the GABA (0.45) (Mullins et al., 2014). Gannet provides normalized residual fitting errors of GABA+. To further ensure the robustness of our findings, only edited spectra with a GABA+ fitting error of less than 15% were included in final analyses.
To account for partial volume effects, the structural TFE images were tissue-segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) subvolumes using FSL FAST (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST) (Zhang et al., 2001). VOIs were co-registered to the anatomical images using the “Re-creation of VOI” Matlab tool.
2.6. Statistical analysis
For demographic and clinical variables (age, education years, sex, neuropsychiatric scores, and tissue fraction), two-tailed independent-samples t-tests were used to compare continuous variables and categorical data were analyzed using the χ2 test. A paired t-test was applied separately for the patient group to compare the longitudinal changes in GABA+ level and neuropsychiatric scores. Relationships between [GABA+] and clinical variables (THs levels and neuropsychiatric scores) in patient group were examined by Pearson correlation. P values < 0.05 were considered statistically significant. Results were given as mean ± SD. Statistical analyses were conducted using SPSS 16.0 (Chicago, IL, USA).
3. Results
3.1. Participant characteristics
Demographic and clinical characteristics are listed in Table 1. There were no group differences in age (p = 0.608), sex (p = 0.717), BMI (p = 0.499), and education background (p = 0.661). Before L-T4 treatment, patients with hypothyroidism showed significantly higher self-rating scales of depressive symptoms (BDI-II) (p < 0.001). The patient group also showed worse MoCA (p < 0.001) and MQ scores (p < 0.001) than healthy controls. After L-T4 treatment, the BDI-II (p < 0.001) and MQ (p < 0.001) scores were improved for the patients with hypothyroidism.
Table 1.
Demographics and clinical characteristics of all subjects.
| Healthy Controls (n = 18) | Hypothyroid (n = 18) |
||
|---|---|---|---|
| Before L-T4 | After L-T4 | ||
| Age (year) | 44.50 ± 6.13 | 43.06 ± 7.04 | |
| Sex (female/male) | 12/6 | 13/5 | |
| BMI | 23.46 ± 1.26 | 23.72 ± 1.02 | |
| Education (year) | 13.00 ± 1.49 | 13.22 ± 1.52 | |
| TSH (mIU/l) | 2.48 ± 0.84 | 95.3 ± 34.51 | 2.44 ± 0.79 |
| fT3 (pmol/l) | 4.52 ± 0.92 | 1.98 ± 0.63 | 4.74 ± 0.58 |
| fT4 (pmol/l) | 15.7 ± 3.19 | 5.96 ± 1.59 | 17.4 ± 2.09 |
| MoCA | 26.01 ± 1.60 | 23.68 ± 1.61¶ | 25.6 ± 1.91¢ |
| WMS-CR (MQ) | 124 ± 7.46 | 104 ± 11.67¶ | 122 ± 10.88§ |
| BDI-II | 3.78 ± 1.83 | 11.0 ± 2.29¶ | 3.06 ± 0.94§ |
BMI, body mass index; TSH, thyroid stimulating hormone; fT3, free triiodothyronine; fT4, free thyroxine; MoCA, Montreal Cognitive Assessment; WMS-CR (MQ), Wechsler Memory Scale-Chinese Revision (memory quotient); BDI-II, Beck Depression Inventory-II; L-T4, levothyroxine. Significant differences revealed by independent t-test between patients and controls, ¶P < 0.001; Significant differences revealed by paired t-test between patients before and after L-T4 treatment, ¢P < 0.05, §P < 0.001.
3.2. Segmentation results
The segmentation results are listed in Table 2. No significant differences were found between the patients with hypothyroidism and the healthy controls in terms of GM%, WM%, CSF% in either region (all p values > 0.27). We found no significant tissue composition changes before and after 6-month treatment in the patient group (all p values > 0.36).
Table 2.
GABA+ quantification and voxel segmentation results.
| Healthy Controls | Hypothyroid |
||
|---|---|---|---|
| Before L-T4 | After L-T4 | ||
| mPFC | |||
| [GABA+] (IU) | 1.41 ± 0.07 | 1.34 ± 0.09 | 1.40 ± 0.05 |
| Fitting error (%) | 5.43 ± 0.95 | 5.92 ± 1.20 | 5.73 ± 1.17 |
| GM% | 45.5 ± 1.68 | 45.9 ± 1.89 | 46.3 ± 1.72 |
| WM% | 40.2 ± 2.21 | 39.4 ± 2.34 | 40.6 ± 2.31 |
| CSF% | 14.3 ± 2.33 | 14.7 ± 2.19 | 13.1 ± 2.35 |
| PCC | |||
| [GABA+] (IU) | 1.16 ± 0.09 | 1.12 ± 0.10 | 1.13 ± 0.08 |
| Fitting error (%) | 6.51 ± 1.06 | 6.18 ± 1.25 | 5.98 ± 0.91 |
| GM% | 49.1 ± 2.51 | 49.1 ± 2.56 | 48.5 ± 2.32 |
| WM% | 41.3 ± 2.96 | 40.8 ± 2.72 | 40.5 ± 2.13 |
| CSF% | 9.60 ± 2.18 | 10.1 ± 2.59 | 11.0 ± 2.65 |
GABA+, γ-Aminobutyric acid; mPFC, medial prefrontal cortex; PCC, posterior cingulated cortex; GM, grey matter; WM, white matter; CSF, cerebral spinal fluid.
3.3. GABA+ concentrations
GABA+ concentrations were successfully quantified from the MEGA-PRESS spectra of all participants with fitting errors lower than 15% (Table 2). There were no significant differences in fitting error among three MRI sessions (two patient sessions and one control session) in two regions (mPFC, p = 0.181; PCC, p = 0.409).
In the mPFC, patients with hypothyroidism (1.34 ± 0.09 IU) demonstrated significantly decreased GABA+ levels compared to healthy controls (1.41 ± 0.07 IU) (P = 0.031) (Table 2; Fig. 2), whereas the PCC GABA+ levels were not significantly different (P = 0.269) in patients with hypothyroidism (1.12 ± 0.10 IU) compared to the control group (1.16 ± 0.09 IU).
Fig. 2.
Box and whisker plots show absolute GABA+ levels [GABA+] in institutional unit (IU) of (a) medial prefrontal cortex (mPFC) and (b) posterior cingulated cortex (PCC) in hypothyroid patients (before and after L-T4 treatment) and control subjects. The within-box horizontal lines represent median values. The box extremities correspond to the 25th and 75th percentiles. ‘●’ symbols represent the outliers.
We observed a mean 5.0% increase in mPFC GABA+ levels after L-T4 treatment compared with baseline (1.40 ± 0.05 IU vs. 1.34 ± 0.09 IU; p = 0.008; Fig. 2), such that after treatment, GABA levels did not differ from healthy controls (p = 0.84). There was no significant change in PCC GABA+ before and after L-T4 treatment (1.13 ± 0.08 vs. 1.12 ± 0.10; p = 0.89).
3.4. Correlation analyses
The mPFC GABA+ concentrations in patients with hypothyroidism were negatively correlated with depressive symptoms (BDI-II) (r = −0.558, p = 0.016), and positively correlated with memory function (MQ) (r = 0.522, p = 0.026) at baseline (Fig. 3). No significant correlation was found between GABA+ levels and serum TSH, fT3, or fT4 levels (all p values > 0.05).
Fig. 3.
Relationships between [GABA+] levels and BDI-II scores (a), and between [GABA+] levels and MQ values (b) in medial prefrontal cortex (mPFC) of hypothyroid patients before L-T4 treatment.
4. Discussion
The main findings of the present study are reduced GABA+ concentrations in the mPFC region among hypothyroid patients and an increase in GABA+ concentrations after 6-month treatment of L-T4. Exploratory analyses revealed that mPFC GABA+ correlated negatively with depressive symptoms rated by BDI-II scores, and correlated positively with memory function rated by WMC-CR scores.
The finding of reduced mPFC GABA+ replicates our previous study (Liu et al., 2017) in a slightly larger cohort. An animal study has also demonstrated reduced GABA levels in hypothyroid male rats, though in the hippocampus area (Abd Allah et al., 2014). Functional MRI studies showed diminished task-induced deactivation (TID) in the mPFC area (He et al., 2011), and rCBF aberrations (Schraml et al., 2006) in frontal areas in hypothyroid patients. Since high regional GABA is associated with enhanced negative TID (Hu et al., 2013, Northoff et al., 2007) and CBF-weighted ASL signals (negative correlation) (Donahue et al., 2010), our results are consistent with, and may explain these findings. Moreover, the reduced GABA+ also colocalized with abnormal brain metabolism in the PFC region in a PET imaging study (Bauer et al., 2009). Disturbance in THs-mediated GABAergic function may be partially related to these brain dysfunctions in hypothyroidism. Further multimodal imaging studies may be informative to uncover the linkage between MRS-derived neurotransmitter and brain dysfunction.
Reduced GABA+ levels may be due to loss of GABAergic interneurons or impairment of GABA production or transport. Preclinical study showed hypothyroidism could reduce the number of inhibitory GABAergic neurons in adult rats (Wiens and Trudeau, 2006). THs deficiency may cause disturbances in GABA synthesis enzyme GAD (Sawano et al., 2013) or in the glutamate/GABA-glutamine cycle between astrocytes and neurons (Wiens and Trudeau, 2006), resulting in decreased GABA levels. Supplementation of L-T4 recovers normal thyroid hormone levels, and our study suggests this reverses the impact on the GABA system. The effect of L-T4 in restoring GABA levels may further demonstrate the modulatory effect of THs on the inhibitory GABAergic system. The reversible effect of L-T4 in brain metabolites was also in line with other forms of neuroimaging studies (He et al., 2011, Bauer et al., 2009).
However, it should be cautious to draw the conclusion that decreased GABA+ was due to THs deficiency, since we did not find associations between brain GABA+ levels and serum fT3, fT4 or TSH levels. The small participant sample with only relatively mild symptoms may be one factor. Another possible reason was that THs levels measured in the blood may not reflect their levels in the brain tissue. Deiodinase II activity in the brain is increased in condition of hypothyroidism so that conversion of T4 to T3 is higher (Serrano-Lozano et al., 1993). The relationship between brain GABA and brain THs merits further study. Given that the relationship between GABA+ and depressive or cognitive symptoms, we concede that the observed decrease in GABA+ may also be a consequence of depressive or cognitive symptoms rather than hypothyroidism per se.
The neurotransmitter GABA is present at approximately one third of all synapses in the central nervous system and shapes neural network dynamics via GABAergic interneurons (Mohler, 2007). GABA-mediated inhibition mainly includes two forms (Wlodarczyk et al., 2013): phasic inhibition generated by the transient activation of synaptic GABA-A receptors by presynaptic GABA release, and tonic inhibition generated by the persistent activation of extrasynaptic GABA-A receptors by ambient or extracellular GABA. Study has shown that the MRS-detected GABA signals most likely represent pools of extracellular GABA providing the inhibitory tone of a brain area rather than rather than synaptic GABA involved in phasic synaptic neurotransmission (Dyke et al., 2017). The decreased GABA+ in this study may reflect the decreased extracellular GABA content. The tonic inhibition as well as phasic inhibition both participates in control of neuron excitability (Farrant and Nusser, 2005, Lee and Maguire, 2014), plays critical roles in cognitive functions under physiological and pathological conditions (Woo et al., 2018). Decreased GABA+ means abnormal GABAergic inhibition, which may disturb normal neurophysiological processes and result in dysregulation of mood and memory function in hypothyroidism. Furthermore, GABA has been proved to regulate the MPFC BOLD signal and further participate in emotion processing (Stan et al., 2014). MRS studies at 7 Tesla found associations between decreased GABA and mild cognition impairment (MCI) (Oeltzschner et al., 2019).
In the present study, we found correlations between GABA+ levels and depressive symptoms, and between GABA+ and memory defects (MQ) in patients with hypothyroidism. GABAergic deficit could be a consequence of low thyroid hormones, and contribute to mood and cognition changes in hypothyroid patients. Furthermore, abnormal GABA levels were also found in other psychiatric diseases accompanying with mood and cognition changes (Schur et al., 2016). In this point of view, GABA reduction may be a biological marker across psychiatric disorders.
Brain GABA+ was increased to a normal level following L-T4 treatment. At the same time-point, depressive symptoms and memory impairment were relieved in euthyroid patients. The reversible effects of L-T4 replacement were also reported in other studies (Alzoubi et al., 2009). More importantly, L-T4 has long been used as adjunctive treatment in patients with affective disorders (e.g. major depression disorder) (Bauer et al., 2003). The mechanisms of action were unclear. Clinical evidence showed an increased vulnerability to affective disorders associated with hypothyroid state (Bauer et al., 2008). Augmentation of THs-mediated GABAergic inhibition following treatment with supraphysiological doses of L-T4 may be a potential mechanism in treatment of other affective disorders, since decreased GABA is broadly implicated across affective disorders (Schur et al., 2016). However, thyroid hormones also interact with other neurotransmitter systems (e.g. serotonin, norepinephrine, and dopamine) (Bauer et al., 2003, Tousson et al., 2012), which needs to be investigated intensively.
The present study has several limitations. First, our participant sample was small with only relatively mild symptoms. Take BDI-II scores for example, hypothyroid patients showed minimal to mild severity of depressive symptoms (11.0 ± 2.29), and did not reach clinically significant borderline score (usually > 16). This might be one of the factors for the lack of correlations between GABA+ levels and neuropsychological or THs levels. Thus, a large sample of patients with severe symptoms, together with detailed neuropsychological testing tools, will be included to replicate and confirm the preliminary results. Second, GABA is mainly found in three major pools, cytoplasm (intracellular), presynaptic vesicular (synaptic), and ambient (extracellular) (Stagg et al., 2011). Only vesicular GABA plays an immediate role in inhibitory synaptic neurotransmission. MRS-detected GABA signals cannot distinguish among these functional pools of GABA. In the future, novel imaging approach like [11C]Ro15-4513 PET study (Stokes et al., 2014) with 1H-MRS in the same subjects, and potentially during the same scanning session on combined PET-MR platforms, might investigate changes in synaptic GABA concentrations. Third, the detected GABA+ signals contain a significant contribution (~50%) from co-edited macromolecules (MMs) and homocarnosine. New methods for MMs suppression are being developed which can detect “pure GABA” (Edden et al., 2012). Fourth, GABA-MRS voxel is relatively large, which improves signal-noise ratio at the cost of regional specificity. Smaller voxels for more precise brain regions (e.g. hippocampus) may be investigated in future to provide more information.
In conclusion, this is the first longitudinal study to report brain GABA+ changes among patients with hypothyroidism. Dysregulation of GABAergic inhibition may be a related neurobiological mechanism underlying hypothyroidism-related cognitive deficits and depressive symptoms. The normalization of brain GABA+ after L-T4 therapy suggests a reversible condition, indicating the action mode of L-T4 in adjunctive treatment of affective disorders.
CRediT authorship contribution statement
Bo Liu: Conceptualization, Writing - original draft, Funding acquisition. Zhensong Wang: Resources, Visualization. Liangjie Lin: Visualization, Writing - review & editing. Huan Yang: Data curation. Fei Gao: Methodology. Tao Gong: Formal analysis. Richard A.E. Edden: Software, Writing - review & editing. Guangbin Wang: Supervision.
Acknowledgments
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation (project ZR2018BH029), and by Shandong Provincial Key Research and Development Program (project 2017GSF218010). This study applies tools developed under NIH grants R01 EB016089, R01 EB023963 and P41 EB015909; RAEE also receives salary support from these grants.
Disclosure
None of the authors have any conflicts of interest to declare.
References
- Abd Allah E.S., Gomaa A.M., Sayed M.M. The effect of omega-3 on cognition in hypothyroid adult male rats. Acta Physiol. Hung. 2014;101(3):362–376. doi: 10.1556/APhysiol.101.2014.3.11. [DOI] [PubMed] [Google Scholar]
- Ahmed O.M. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. 2008;26(2):147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Ahmed O.M., Abd El-Tawab S.M., Ahmed R.G. Effects of experimentally induced maternal hypothyroidism and hyperthyroidism on the development of rat offspring: I. The development of the thyroid hormones-neurotransmitters and adenosinergic system interactions. Int. J. Dev. Neurosci. 2010;28(6):437–454. doi: 10.1016/j.ijdevneu.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Alzoubi K.H. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19(1):66–78. doi: 10.1002/hipo.20476. [DOI] [PubMed] [Google Scholar]
- Bauer M. Thyroid, brain and mood modulation in affective disorder: insights from molecular research and functional brain imaging. Pharmacopsychiatry. 2003;36(Suppl 3):S215–S221. doi: 10.1055/s-2003-45133. [DOI] [PubMed] [Google Scholar]
- Bauer M. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008;20(10):1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- Bauer M. Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J. Clin. Endocrinol. Metab. 2009;94(8):2922–2929. doi: 10.1210/jc.2008-2235. [DOI] [PubMed] [Google Scholar]
- Bicks L.K. Prefrontal cortex and social cognition in mouse and man. Front. Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaalal A. Thyroid hormone supplementation restores spatial memory, hippocampal markers of neuroinflammation, plasticity-related signaling molecules, and beta-amyloid peptide load in hypothyroid rats. Mol. Neurobiol. 2019;56(1):722–735. doi: 10.1007/s12035-018-1111-z. [DOI] [PubMed] [Google Scholar]
- Chaker L. Hypothyroidism. Lancet. 2017;390(10101):1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapa F. Adult-onset hypothyroidism and the cerebral metabolism of (1,2–13C2) acetate as detected by 13C nuclear magnetic resonance. Endocrinology. 1995;136(1):296–305. doi: 10.1210/endo.136.1.7828544. [DOI] [PubMed] [Google Scholar]
- Cooke G.E. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid. 2014;24(3):433–440. doi: 10.1089/thy.2013.0058. [DOI] [PubMed] [Google Scholar]
- Donahue M.J. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53(2):392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Dyke K. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage. 2017;152:360–370. doi: 10.1016/j.neuroimage.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden R.A. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging. 2014;40(6):1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden R.A., Puts N.A., Barker P.B. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn. Reson. Med. 2012;68(3):657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M., Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Gao F. Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. Neuroimage. 2015;106:311–316. doi: 10.1016/j.neuroimage.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Gilbert M.E., Paczkowski C. Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Brain Res. Dev. Brain Res. 2003;145(1):19–29. doi: 10.1016/s0165-3806(03)00191-3. [DOI] [PubMed] [Google Scholar]
- Gong, Y., Wang, D,. D.J., 1989. Handbook of Wechsler MemoryScale–Revised. Changsha, China: Bulletin of Human Medical.
- He X.S. Functional magnetic resource imaging assessment of altered brain function in hypothyroidism during working memory processing. Eur. J. Endocrinol. 2011;164(6):951–959. doi: 10.1530/EJE-11-0046. [DOI] [PubMed] [Google Scholar]
- Hu Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 2013;33(47):18566–18573. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonklaas J. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas C. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab. Brain Dis. 2010;25(3):339–354. doi: 10.1007/s11011-010-9208-8. [DOI] [PubMed] [Google Scholar]
- Lee V., Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circuits. 2014;8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T., Mussig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav. Immun. 2014;41:261–266. doi: 10.1016/j.bbi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Liu B. Investigation of brain GABA+ in primary hypothyroidism using edited proton MR spectroscopy. Clin. Endocrinol. (Oxf.) 2017;86(2):256–262. doi: 10.1111/cen.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S. Assessment of the metabolic profile in Type 2 diabetes mellitus and hypothyroidism through proton MR spectroscopy. Magn. Reson. Imaging. 2008;26(3):420–425. doi: 10.1016/j.mri.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Mohler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J. Neurochem. 2007;102(1):1–12. doi: 10.1111/j.1471-4159.2007.04454.x. [DOI] [PubMed] [Google Scholar]
- Mullins P.G. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Oeltzschner G. Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla. Neurobiol. Aging. 2019;73:211–218. doi: 10.1016/j.neurobiolaging.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts N.A., Edden R.A. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V. Neural response to transcranial magnetic stimulation in adult hypothyroidism and effect of replacement treatment. J. Neurol. Sci. 2008;266(1–2):38–43. doi: 10.1016/j.jns.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Samuels M.H. Psychiatric and cognitive manifestations of hypothyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21(5):377–383. doi: 10.1097/MED.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawano E. Thyroid hormone-dependent development of the GABAergic pre- and post-synaptic components in the rat hippocampus. Int. J. Dev. Neurosci. 2013;31(8):751–761. doi: 10.1016/j.ijdevneu.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Schraml F.V. Cerebral accumulation of Tc-99m ethyl cysteinate dimer (ECD) in severe, transient hypothyroidism. J. Cereb. Blood Flow Metab. 2006;26(3):321–329. doi: 10.1038/sj.jcbfm.9600191. [DOI] [PubMed] [Google Scholar]
- Schulz K.P. Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. Neuroimage. 2011;57(1):242–250. doi: 10.1016/j.neuroimage.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur R.R. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016;37(9):3337–3352. doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Lozano A. 5' Deiodinase activity in brain regions of adult rats: modifications in different situations of experimental hypothyroidism. Brain Res. Bull. 1993;30(5–6):611–616. doi: 10.1016/0361-9230(93)90090-x. [DOI] [PubMed] [Google Scholar]
- Singh S. Alterations of functional connectivity among resting-state networks in hypothyroidism. J. Neuroendocrinol. 2015;27(7):609–615. doi: 10.1111/jne.12282. [DOI] [PubMed] [Google Scholar]
- Singh S. Hippocampal neurometabolite changes in hypothyroidism: an in vivo (1) H magnetic resonance spectroscopy study before and after thyroxine treatment. J. Neuroendocrinol. 2016;28(9) doi: 10.1111/jne.12399. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun. Integr. Biol. 2011;4(5):573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan A.D. Glutamate and GABA contributions to medial prefrontal cortical activity to emotion: implications for mood disorders. Psychiatry Res. 2014;223(3):253–260. doi: 10.1016/j.pscychresns.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Stokes P.R. Acute increases in synaptic GABA detectable in the living human brain: a [(1)(1)C]Ro15-4513 PET study. Neuroimage. 2014;99:158–165. doi: 10.1016/j.neuroimage.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Tousson E. Monoamine concentrations changes in the PTU-induced hypothyroid rat brain and the ameliorating role of folic acid. Hum. Exp. Toxicol. 2012;31(3):282–289. doi: 10.1177/0960327111405863. [DOI] [PubMed] [Google Scholar]
- Upadhyaya L., Agrawal J.K. Effect of L-thyroxine and carbimazole on brain biogenic amines and amino acids in rats. Endocr. Res. 1993;19(2–3):87–99. doi: 10.3109/07435809309033016. [DOI] [PubMed] [Google Scholar]
- Utku U., Gokce M., Ozkaya M. Changes in cerebral blood flow velocity in patients with hypothyroidism. Eur. J. Endocrinol. 2011;165(3):465–468. doi: 10.1530/EJE-11-0254. [DOI] [PubMed] [Google Scholar]
- Wiens S.C., Trudeau V.L. Thyroid hormone and gamma-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2006;144(3):332–344. doi: 10.1016/j.cbpa.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk A.I. GABA-independent GABAA receptor openings maintain tonic currents. J. Neurosci. 2013;33(9):3905–3914. doi: 10.1523/JNEUROSCI.4193-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc. Natl. Acad. Sci. U.S.A. 2018;115(19):5004–5009. doi: 10.1073/pnas.1721187115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Analysis of the protective effects of gamma-aminobutyric acid during fluoride-induced hypothyroidism in male Kunming mice. Pharm. Biol. 2019;57(1):29–37. doi: 10.1080/13880209.2018.1563621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J. Decreased pain threshold and enhanced synaptic transmission in the anterior cingulate cortex of experimental hypothyroidism mice. Mol. Pain. 2014;10:38. doi: 10.1186/1744-8069-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]