Abstract
In December 2019, a new severe acute respiratory syndrome coronavirus (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), emerged in Wuhan, China. Despite containment measures, SARS-CoV-2 spread in Asia, Southern Europe, then in America and currently in Africa. Identifying effective antiviral drugs is urgently needed. An efficient approach to drug discovery is to evaluate whether existing approved drugs can be efficient against SARS-CoV-2. Doxycycline, which is a second-generation tetracycline with broad-spectrum antimicrobial, antimalarial and anti-inflammatory activities, showed in vitro activity on Vero E6 cells infected with a clinically isolated SARS-CoV-2 strain (IHUMI-3) with median effective concentration (EC50) of 4.5 ± 2.9 µM, compatible with oral uptake and intravenous administrations. Doxycycline interacted both on SARS-CoV-2 entry and in replication after virus entry. Besides its in vitro antiviral activity against SARS-CoV-2, doxycycline has anti-inflammatory effects by decreasing the expression of various pro-inflammatory cytokines and could prevent co-infections and superinfections due to broad-spectrum antimicrobial activity. Therefore, doxycycline could be a potential partner of COVID-19 therapies. However, these results must be taken with caution regarding the potential use in SARS-CoV-2-infected patients: it is difficult to translate in vitro study results to actual clinical treatment in patients. In vivo evaluation in animal experimental models is required to confirm the antiviral effects of doxycycline on SARS-CoV-2 and more trials of high-risk patients with moderate to severe COVID-19 infections must be initiated.
Keywords: COVID-19, SARS-CoV-2, doxycycline, treatment, prophylaxis, antiviral, anti-inflammatory
1. Introduction
In December 2019, a new severe acute respiratory syndrome coronavirus (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), emerged in Wuhan, China [1]. Despite containment measures, SARS-CoV-2 spread in Asia, Southern Europe, then in America and in Africa. As of 9 October 2020, according to the treatment guideline of the National Institutes of Health (NIH), there is no Food and Drug Administration-approved antiviral treatment against SARS-CoV-2 [2]. Several drugs are tested in the context of the DisCoVeRy trial [3] and a controversial early treatment that associates a combination of hydroxychloroquine and azithromycin has been used worldwide with a reduced risk of hospitalization, a reduced risk of death and a shorter duration of viral shedding [4,5,6,7,8,9,10]. Conversely, some studies showed that treatment of mild-to-moderate or mild-to-severe COVID-19 with hydroxychloroquine alone or in combination with azithromycin did not improve clinical status or duration of viral shedding in comparison with standard care [11,12,13,14,15]. Identifying effective, low cost antiviral drugs with limited side effects which are affordable immediately, especially for emerging countries, is urgently needed. An efficient approach to drug discovery is drug repurposing that consists of evaluating whether existing approved drugs can be efficient against SARS-CoV-2.
Doxycycline is a second-generation tetracycline with broad-spectrum antimicrobial [16] and anti-inflammatory activities [17]. Additionally, doxycycline was approved as prophylaxis against malaria by the Food and Drug Administration in 1994 and has been used since 2006 at the dose of 100 mg/day by the French military forces deployed in malaria-endemic areas [18].
Doxycycline also shows antiviral activity in vitro. This tetracycline derivative significantly inhibited the replication of the vesicular stomatis virus in vitro [19] and that of the dengue virus by inhibition of NS2B-NS3 serine protease [20,21,22]. Doxycycline showed inhibition of entry and replication of Chikungunya virus in Vero cell at 11 µM [23]. Using the in-silico method, doxycycline might be a potential inhibitor of Crimean-Congo hemorrhagic fever virus nucleoprotein, an essential protein in virus replication [24]. Additionally, doxycycline inhibited the early-stage replication of the porcine reproductive and respiratory syndrome virus, which causes respiratory disease, with EC50 (median effective concentration) of 0.25 µg/mL (about 0.5 µM) [25]. The current study evaluated the antiviral effect of doxycycline against SARS-CoV-2.
Doxycycline could be an attractive option for the treatment of COVID-19 [26]. Therefore, the activity of doxycycline was assessed in vitro against a clinically isolated SARS-CoV-2 strain and was compared with the activity of chloroquine.
2. Results
The cytotoxicity evaluation of doxycycline and chloroquine showed that the CC50 values were >100 µM for 48 h. The CC50 value of chloroquine is consistent with those previously described [27,28]. The antiviral effects of doxycycline against the clinically isolated SARS-CoV-2 strain (IHUMI-3) were concentration-dependent.
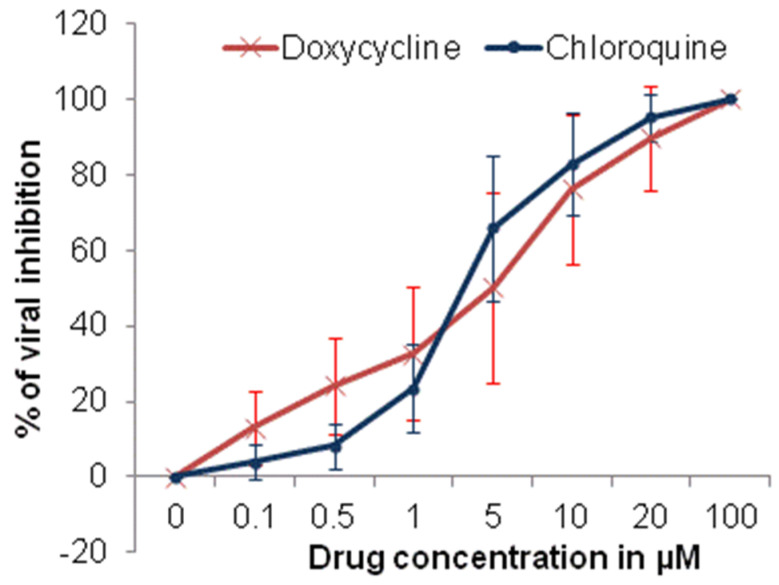
The median effective concentration (EC50) and 90% effective concentration (EC90) for doxycycline were 4.5 ± 2.9 µM and 23.5 ± 16.5 µM, respectively (Figure 1). The EC50 and EC90 for chloroquine were 3.2 ± 1.8 µM and 13.9 ± 6.4 µM, respectively (Figure 2). The EC50 value for chloroquine is consistent with previous results on Vero E6 cells at MOI of 0.2 to 0.25 [27,29].
Figure 1.
Comparative antiviral efficacy of doxycycline and chloroquine against SARS-CoV-2 infection in vitro (error bar represents standard deviation, 10 experiments).
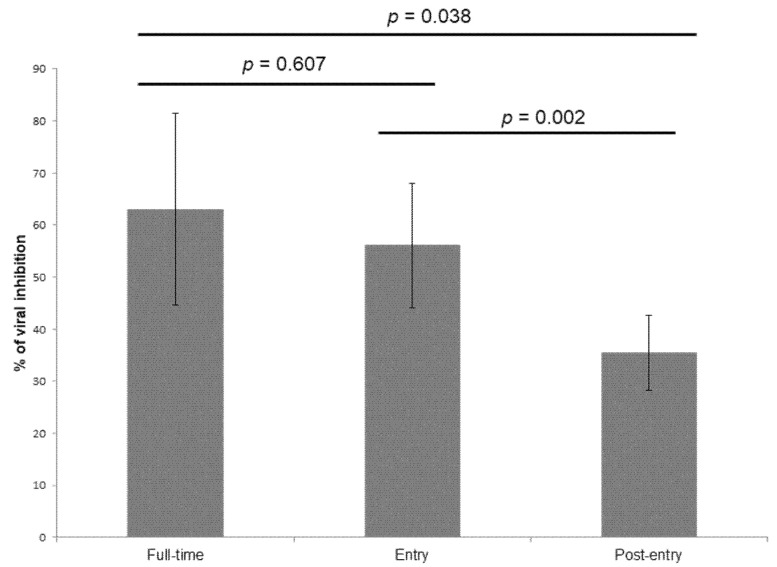
Figure 2.
Antiviral activities of doxycycline at 5 µM against the SARS-CoV-2 IHUMI-003 strain in vitro. For full-time treatment, Vero E6 cells were pre-treated with doxycycline for 4 h and virus was then added for 48 h. For “entry” treatment, doxycycline was added to Vero E6 cells 4 h before viral infection and the virus-doxycycline mixture was replaced with fresh medium after 2 h post infection and was maintained for 46 h. For “post-entry” treatment, doxycycline was added 2 h post infection and was maintained for 46 h. Error bars represent the standard deviation of 10 experiments.
The ratios Cmax/EC50 and Cmax/EC90 in blood for doxycycline were estimated at 0.75 and 0.07, respectively after an oral administration of doxycycline 100 mg, at 2.21 and 0.41 after an oral administration of doxycycline 200 mg and at 1.14 and 0.21 after intravenous administration of 100 mg of doxycycline.
Our results demonstrated that doxycycline interacted at both entry and post-entry stages of the SARS-CoV-2 infection in Vero E6 cells (Figure 2).
3. Discussion
The in vitro activity of doxycycline against the SARS-CoV-2 (EC50 = 4.5 µM) was consistent with those reported with hydroxychloroquine [27,29,30,31], with antimalarial drugs like chloroquine [27,28,29,32], amodiaquine [29,31,32], ferroquine [29], or mefloquine [29,31,33], antiviral agents like remdesivir [28,32,34] or lopinavir [32], and macrolides like azithromycin [34,35].
A daily oral uptake of 100 mg or 200 mg of doxycycline in healthy volunteers led to a Cmax (maximum blood concentration) value of 1.7 and 5 µg/mL [36,37]. An intravenous doxycycline dose of 100 mg showed a Cmax of 2.6 µg/mL [38]. The ratios Cmax/EC50 and Cmax/EC90 for doxycycline in plasma ranged from 0.75 to 2.21 and 0.07 to 0.41, respectively. The ratios Cmax/EC50 and Cmax/EC90 in plasma seem low to reach effective concentrations to inhibit SARS-CoV-2 in humans. However, in lungs, doxycycline were 2- to 4-fold higher than in plasma [39,40]. A daily oral uptake of 100 or 200 mg of doxycycline led to a Cmax value from 3.4 to 20 µg/g of lungs. The ratios Cmax/EC50 and Cmax/EC90 for doxycycline in lungs ranged from 1.5 to 8.8 and 0.15 to 1.6, respectively.
Doxycycline interacted at both entry and post-entry stages of the SARS-CoV-2 infection in Vero E6 cells. Doxycycline inhibited in vitro both dengue virus replication by interaction with the dengue virus serine protease (DENV2 NS2B-NS3pro) and dengue viral entry by inhibition of the E2 envelope glycoprotein involved in virus entry [20,22]. Doxycycline interfered particularly with the adsorption and entry of the Chikungunya virus by interaction with the E2 envelope glycoprotein, but also inhibited viral replication in a lower manner [23]. However, doxycycline had no effect in vitro on the adsorption and entry of the vesicular stomatitis virus (VSV) and inhibited only viral replication [19]. Moreover, doxycycline enhanced the adsorption of the porcine reproductive and respiratory syndrome virus (PRRSV) and inhibited its replication after viral entry [25]. The inhibition of both entry and viral replication after SARS-CoV-2 entry is consistent with the results from combinatorial computational approaches. Docking analysis showed that doxycycline could strongly bind the spike protein (S) of SARS-CoV-2 [41]. The spike viral protein of SARS-CoV-2 used the ACE-2 receptor for entry [42]. Therefore, blocking these interactions offers a potential target for drug development. Moreover, doxycycline and more generally tetracyclines could bind the main protease (Mpro) of SARS-CoV-2 [43,44]. This protein, also called 3C-like protease, is essential to conduct the replication cycle of SARS-CoV-2 by leading to the formation of non-structural proteins (NSPs) [45,46]. Moreover, Alexpandi et al. showed that doxycycline inhibited the RNA-directed 5′-3′ polymerase activity of SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) (Alexpandi et al., personal data), a key enzyme for the replication of the SARS-CoV-2 [47], like remdesivir [48,49].
However, these results must be taken with caution regarding the potential use of antimalarial drugs in SARS-CoV-2-infected patients: it is difficult to translate in vitro study results into actual clinical treatment in patients. For instance, experts agree on the in vitro activity of chloroquine or hydroxychloroquine against SARS-CoV-2 but disagree on the hydroxychloroquine efficacy in COVID-19 treatments, which remains controversial [50,51]. In vivo evaluation in animal experimental models is required to confirm the antiviral effects of doxycycline on SARS-CoV-2.
Besides its antiviral activity, doxycycline has anti-inflammatory effects by decreasing the expression of various pro-inflammatory cytokines including interleukins 1 (IL-1), 6 (IL-6), and 8 (Il-8) and tumor necrosis factor-alpha (TNF-α) by macrophages [17,52] and chemokines including monocyte chemotactic protein 1, macrophage inflammatory protein 1α and 1β [53]. The immunomodulatory activity of doxycycline improved survival of septic mice with pulmonary inflammation [54]. Moreover, doxycycline is effective for the treatment of several chronic inflammatory airway diseases, including acute respiratory distress syndrome [55]. Doxycycline was the more effective tetracycline in the reduction of IL-6 and TNF-α in patients with dengue fever [56]. Doxycycline ameliorated pulmonary inflammation in a murine polymicrobial sepsis model by decreasing levels of IL-1β, IL-6 and TNFα in plasma and lungs [54]. Doxycycline improved the lung function and quality of life in chronic obstructive pulmonary disease (COPD) [57]. Levels of IL-6, IL-8 and TNFα were reduced in doxycycline-treated COPD patients. Moreover, doxycycline reduced neuroinflammation and preserved oxidative balance in traumatic brain injury-induced cognitive/motor impairments in rats due to its anti-inflammatory and free scavenging mechanisms [58]. Doxycycline could be a potential partner of COVID-19 therapies due to its anti-inflammatory effects.
Moreover, doxycycline is a bacteriostatic antibiotic that is active against the bacterial causes of community-acquired pneumonia, including Streptococcus pneumoniae, Haemophilus influenzae, and atypical pathogens such as Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella spp [59]. Doxycycline is used commonly for lower respiratory tract infection [60]. The use of doxycycline as antimalarial prophylaxis is associated with a reduced risk of Influenza-like illness among travelers [61]. Even without antiviral effects, doxycycline could be a potential partner of COVID-19 therapies due to anti-inflammatory effects and antibiotic effects that could prevent co-infections and superinfections.
Early treatment with doxycycline of 100 mg a day for 7 days (per os or intravenous) in 89 high-risk patients with moderate to severe COVID-19 infections was associated with early clinical recovery, decreased hospitalization and reduced mortality [62]. Another observational study on 54 high-risk patients in 3 long term care facilities in New York revealed that the use of a combination of 100 mg of doxycycline twice a day for 7 days and 400 mg of hydroxychloroquine twice a day on the first day and daily for the next 6 days was associated with a decrease in transfer to hospital and reduced mortality [63]. Results were compared and analyzed against the data observed in a long term care facility in Washington. However, these results must be confirmed by rigorous studies. Many clinical trials on doxycycline alone or in combination to treat COVID-19 are in progress [64,65,66].
4. Materials and Methods
4.1. Agent, Virus, and Cells
Stock solution of doxycycline hyclate (Sigma, Saint Louis, MO, USA) was prepared in methanol and diluted in Minimum Essential Media (MEM, Gibco, ThermoFischer, Waltham, MA, USA) in order to have 7 final concentrations ranging from 0.1 µM to 100 µM. Chloroquine diphosphate (Sigma) was used as comparator. The clinically isolated SARS-CoV-2 strain (IHUMI-3) [4] was maintained in production in Vero E6 cells (American type culture collection ATCC® CRL-1586™) in MEM with 4% of fetal bovine serum and 1% glutamine (complete medium).
4.2. Cytotoxicity Assay
In vitro cell viability evaluation on the VERO E6 cell line was performed according to the method described by Mosmann with slight modifications [67]. Briefly, 105 cells in 200 µL of complete medium were added to each well of 96-well plates and incubated at 37 °C in a humidified 5% CO2. After 24 h incubation, 25 µL of complete medium and 25 µL of each concentration of methylene blue, hydroxychloroquine or azithromycin were added and the plates were incubated for 48 h at 37 °C. After removal of the surpernatant, 100 µL of MTT (3-(4,5-dimethyl-2-thiazolyl) -2,5-diphenyl-2H-tetrazolium bromide, Sigma Aldrich, St Quentin Fallavier, France) solution (0.5 mg/mL in MEM without FBS) was then added to each well. Cells were incubated for 2 h at 37 °C. After incubation, the MTT solution was removed and 100 µL of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. Then, plates were shaken at 700 rpm for 10 min at 37 °C. The absorbance was measured at 570 nm using a TECAN Infinite F200 Microplate Reader. DMSO was used as blank. The 50% cytotoxicity concentration (CC50) was calculated with the inhibitory sigmoid Emax model, which estimated the CC50 through nonlinear regression by using a standard function of the R software (ICEstimator version 1.2) [68]. CC50 value resulted in the mean of 5 different experimentations.
4.3. Antiviral Activity Assay
Briefly, 96-well plates were prepared with 5.105 cells/mL of Vero E6 (200 µL per well), as previously described. Doxycycline and chloroquine concentrations were added 4 h before infection. Vero E Cells were infected with IHUMI-3 strain at an MOI of 0.25. After 48 h post-infection, the replication was estimated by RT-PCR using the Superscrit III platinum one step with Rox kit (Invitrogene) after extraction with the BIoExtract SuperBall kit (Biosellal, Dardilly, France). The primers used were previously described [69]. The percentage of inhibition of SARS-CoV-2 replication was estimated for each drug concentration as following: (mean CTdrug concentration − mean CTcontrol 0%)/(mean CTcontrol 100% − mean CTcontrol 0%) × 100. EC50 (median effective concentration) and EC90 (90% effective concentration) were calculated with the inhibitory sigmoid Emax model, which estimated the EC50 and EC90 through nonlinear regression by using a standard function of the R software (ICEstimator version 1.2). EC50 and EC90 values resulted in the mean of 10 different experimentations.
4.4. Determination of the Inhibition Stage
Determining in vitro at what stage doxycycline is acting against the SARS-CoV-2 IHUMI-003 strain was assessed at a concentration of at 5 µM. For “full-time treatment”, Vero E6 cells were pre-treated with doxycycline for 4 h and virus was then added for 48 h. For “entry” treatment, doxycycline was added to Vero E6 cells 4 h before viral infection and the virus-doxycycline mixture was replaced with fresh medium after 2 h post infection and was maintained for 46 h. For “post-entry” treatment, doxycycline was added 2 h after post infection and was maintained for 46 h. The percentage of inhibition of SARS-CoV-2 replication by 5 µM of doxycycline was estimated for each drug concentration as following: (mean CTdrug concentration − mean CTcontrol 0%)/(mean CTcontrol 100% − mean CTcontrol 0%) × 100. The result was the mean of 10 different experiments.
4.5. Data Analysis and Interpretation
Results were estimated as a mean and standard deviation of 5 to 10 experiments. Selectivity index (SI) as a ratio of CC50/EC50 was estimated for doxycycline. The expected maximum blood concentration (Cmax) was estimated from the literature for doxycycline at doses commonly administered in oral or intravenous treatment. The ratios Cmax/EC50 and Cmax/EC90 were estimated to find out if the effective concentration in plasma to cure SARS-CoV-2 is achievable in humans. The ratios Clung/EC50 and Clung/EC90 were estimated from the data on doxycycline accumulation into the lung.
5. Conclusions
In conclusion, doxycycline showed a high in vitro antiviral effective activity against SARS-CoV-2 with IC50 (4.5 µM) compatible with oral uptake and intravenous administrations. Doxycycline interacted both on SARS-CoV-2 entry and in replication after virus entry. Besides its in vitro antiviral activity against SARS-CoV-2, doxycycline has anti-inflammatory effects by decreasing the expression of various pro-inflammatory cytokines and could prevent co-infections and superinfections due to broad-spectrum antimicrobial activity. Therefore, doxycycline could be a potential partner of COVID-19 therapies. However, these results must be taken with caution regarding their potential use in SARS-CoV-2-infected patients: it is difficult to translate in vitro study results into actual clinical treatment in patients. In vivo evaluation in animal experimental models is required to confirm the antiviral effects of doxycycline on SARS-CoV-2 and more trials of high-risk patients with moderate to severe COVID-19 infections must be initiated.
Author Contributions
Conceptualization, M.G., B.L.S. and B.P.; validation, M.G., S.H., H.B. and B.P.; formal analysis, B.P.; investigation, M.G., J.A., O.D., M.B., J.M. and I.F.; resources, S.H., P.J., M.L.B., I.D. and C.R.; writing—original draft preparation, M.G., B.L.S. and B.P.; writing—review and editing, J.A., and S.H.; supervision, B.L.S. and B.P.; project administration, B.P.; funding acquisition, B.L.S. and B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Agency, program “Investissement d’avenir”grant number ANR-10-IAHU-03” and Institut Hospitalo-Universiare (IHU) Méditerranée Infection grant number COVID-19. Manon Boxberger received e PhD grant supported by L’Occitane Society.
Conflicts of Interest
The authors have no conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability: Samples of the compounds are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:365–369. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Treatment Guidelines. [(accessed on 9 October 2020)]; Available online: https://www.covid19treatmentguidelines.nih.gov/introduction/
- 3.Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) [(accessed on 16 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04315948.
- 4.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Esteves Vieira V., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: An observational study. Trav. Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Million M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Trav. Med. Infect. Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagier J.C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A., Honoré S., Gaubert J.Y., Fournier P.E., Tissot-Dupont H., et al. Outcomes of 3737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Trav. Med. Infect. Dis. 2020;36:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wanf D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., McKinnon J.E., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong K.S., Jang J.G., Hur J., Le J.H., Kim H.N., Lee W., Ahn J.H. Early hydroxychloroquine administration for rapid severe acute respiratory syndrome coronavirus 2 eradication. Infect. Chemother. 2020;52:e43. doi: 10.3947/ic.2020.52.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue H., Liu Y., Luo P., Liu X., Qiu L., Liu D., Li J. Hydroxychloroquine treatment in COVID-19: A descriptive observational analysis of 30 cases from a single center in Wuhan, China. J. Med. Virol. 2020;92:2523–2527. doi: 10.1002/jmv.26193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dorado L., Lisboa T., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez A., Duclos G., Patene B., Bezulier K., Guilhaumou R., Solas C., Zieleskiewicz L., Leone M. Effects of hydroxychloroquine on Covid-19 in intensive care unit patients: Preliminary results. Int. J. Antimicrob. Agents. 2020;56:106136. doi: 10.1016/j.ijantimicag.2020.106136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paccoud O., Tubach F., Baptiste A., Bleibtreu A., Hajage D., Monsel G., Tebano G., Boutolleau D., Klement E., Godefroy N., et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa791. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitja O., Corbacho-Monné M., Ubals M., Tebe C., Penafiel J., Tobias A., Ballana E., Alemany A., Riera-Marti N., Perez C.A., et al. Hydroxychloroquine for early treatment of adults with mild-Covid-19: A randomized-controlled trial. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abd-Elsalam S., Esmail E.S., Khalaf M., Abdo E.F., Medhat M.A., Abd El Ghafar M.S., Ahmed O.A., Soliman S., Serangawy G.N., Alboraie M. Hydroxychloroquine in the treatment of COVID-19: A multicenter randomized controlled study. Am. J. Trop. Med. Hyg. 2020;103:1635–1639. doi: 10.4269/ajtmh.20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Michalopoulos A. A clinical and laboratory study of doxycycline (‘Vibramycin’): A broad-spectrum antibiotic. Curr. Med. Res. Opin. 1973;1:445–455. doi: 10.1185/03007997309111706. [DOI] [PubMed] [Google Scholar]
- 17.Cazalis J., Bodet C., Gagnon G., Grenier D. Doxycycline reduces lipopolysaccharide-induced inflammatory mediator secretion in macrophage and ex vivo human whole blood models. J. Periodontol. 2008;79:1762–1768. doi: 10.1902/jop.2008.080051. [DOI] [PubMed] [Google Scholar]
- 18.Gaillard T., Madamet M., Pradines B. Tetracyclines in malaria. Malar. J. 2015;14:445. doi: 10.1186/s12936-015-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z.C., Wang X., Wei J.C., Li B.B., Shao D.H., Li Y.M., Liu K., Shi Y.Y., Zhou B., Qiu Y.F., et al. Antiviral activity of doxycycline against vesicular stomatis virus in vitro. FEMS Microbiol. Lett. 2015;362:fnv195. doi: 10.1093/femsle/fnv195. [DOI] [PubMed] [Google Scholar]
- 20.Yang J.M., Chen Y.F., Tu Y.Y., Yen K.R., Yang Y.L. Combinatorial computational approaches to identify tetracycline derivatives as flavivirus inhibitors. PLoS ONE. 2007;2:e428. doi: 10.1371/journal.pone.0000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothan H.A., Buckle M.J., Ammar Y.A., Mohammadjavad P., Shatrah O., Noorsaadah A.R., Rohana Y. Study the antiviral activity of some derivatives of tetracycline and non-steroid anti inflammatory drugs towards dengue virus. Trop. Biomed. 2013;30:681–690. [PubMed] [Google Scholar]
- 22.Rothan H.A., Mohamed Z., Paydar M., Rahman N.A., Yusof R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch. Virol. 2014;159:711–718. doi: 10.1007/s00705-013-1880-7. [DOI] [PubMed] [Google Scholar]
- 23.Rothan H.A., Bahrani H., Mohamed Z., Teoh T.C., Shankar E.M., Rahman N.A., Yusof R. A combination of doxycycline and ribavirin alleviated Chikungunya infection. PLoS ONE. 2015;10:e0126360. doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharifi A., Amanlou A., Moosavi-Movahedi F., Golestanian S., Amanlou M. Tetracyclines as a potential antiviral therapy against Crimean Congo hemorrhagic fever virus: Docking and molecular dynamic studies. Comput. Biol. Chem. 2017;70:1–6. doi: 10.1016/j.compbiolchem.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Wu Z., Liu K., Qi P., Xu J., Wei J., Li B., Shao D., Shi Y., Qiu Y., et al. Doxycycline enhances adsorption and inhibits early-stage replication of porcine reproductive and respiratory syndrome virus in vitro. FEMS Microbiol. Lett. 2017;364:fnx170. doi: 10.1093/femsle/fnx170. [DOI] [PubMed] [Google Scholar]
- 26.Malek A.E., Granwehr B.P., Kontoyiannis D.P. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21:e00864. doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCov) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendrot M., Andreani J., Boxberger M., Jardot P., Fonta I., Le Bideau M., Duflot I., Mosnier J., Rolland C., Bogreau H., et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Trav. Med. Infect. Dis. 2020;37:101873. doi: 10.1016/j.tmaid.2020.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maisonnasse P., Guedji J., Contreras V., Behillil S., Solas C., Marlin R., Naninck T., Pizzorno A., Lemaitre J., Gonçalves A., et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 31.Weston S., Coleman C.M., Haupt R., Logue J., Mattheuws K., Li Y., Reyes H.M., Weiss S.R., Frieman M.B. Broad anti-coronal activity of FDA approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J. Virol. 2020 doi: 10.1128/JVI.01218-20. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzorno A., Padey B., Dubois J., Julien T., Traversier A., Dulière V., Brun P., Lina B., Rosa-Calatrava M., Terrier O. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antivir. Res. 2020;181:104878. doi: 10.1016/j.antiviral.2020.104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q., Song L.H., Tong Y.G. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chin. Med. J. 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Touret F., Gilles M., Barral K., Nougairède A., van Helden J., Decroly E., de Lamballerie X., Coutard B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020;10:13093. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.M., Colson P., La Scola B., et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welling P.G., Koch P.A., Lau C.C., Craig W.A. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrob. Agents Chemother. 1977;11:462–469. doi: 10.1128/AAC.11.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gschwend M.H., Martin W., Erenmemisoglu A., Scherm M., Dilger C., Tamur U., Kanzik I., Hincal A.A. Pharmacokinetics and bioequivalence study of doxycycline capsules in healthy male subjects. Arzneimittelforschung. 2007;57:347–351. doi: 10.1055/s-0031-1296629. [DOI] [PubMed] [Google Scholar]
- 38.Beringer P.M., Owens H., Nguyen A., Benitez D., Rao A., D’Argenio D.Z. Pharmacokinetics of doxycycline in adults with cystic fibrosis. Antimicrob. Agents Chemother. 2012;56:70–74. doi: 10.1128/AAC.05710-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gengenbacher M., Zimmerman M.D., Sarathy J.P., Kaya F., Wang H., Mina M., Carter C., Hossen M.A., Su H., Trujillo C., et al. Tissue distribution of doxycycline in animal models of tuberculosis. Antimicrob. Agents Chemother. 2020;64:e02479-19. doi: 10.1128/AAC.02479-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchard P., Rudhardt M., Fabre J. Behaviour of doxycycline in the tissues. Chemotherapy. 1975;21:8–18. doi: 10.1159/000221886. [DOI] [PubMed] [Google Scholar]
- 41.Sachdeva C., Wadhwa A., Kumari A., Hussain F., Jha P., Kaushik N.K. In silico potential of approved antimalarial drugs for repurposing against COVID-19. OMICS. 2020;24:568–580. doi: 10.1089/omi.2020.0071. [DOI] [PubMed] [Google Scholar]
- 42.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bharadwaj S., Lee K.E., Dwivedi V.D., Kang S.G. Computational insights into tetracyclines as inhibitors against SARS-CoV-2 Mpro via combinatorial molecular simulation calculations. Life Sci. 2020;257:118080. doi: 10.1016/j.lfs.2020.118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sencanski M., Perovic V., Pajovic S.B., Adzic M., Paessler S., Glisic S. Drug repurposing for candidate SARS-CoV-2 main protease inhibitors by a novel in silico methods. Molecules. 2020;25:3830. doi: 10.3390/molecules25173830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C., Liu Y., Yand Y., Zhang P., Zhuong W., Wang Y., Wang Q., Xu Y., Li M., Li X., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzini S., Musso L., Dallavalle S., Artali R. Putative SARS-CoV-2 Mpro inhibitors from an in-house library of natural and nature-inspired products: A virtual screening and molecular docking study. Molecules. 2020;25:3745. doi: 10.3390/molecules25163745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziebuhr J. The coronavirus replicase. Curr. Top. Microbiol. Immunol. 2005;287:57–94. doi: 10.1007/3-540-26765-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368:1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cairoli E., Espinosa G. Hydroxychloroquine in the treatment of COVID-19: How to use it waiting for conclusive scientific evidence. Med. Clin. 2020;155:134–135. doi: 10.1016/j.medcli.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acharya Y., Sayed A. Chloroquine and hydroxychloroquine as a repurposed agent against COVID-19: A narrative review. Ther. Adv. Infect. Dis. 2020 doi: 10.1177/2049936120947517. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Caprio R., Lembo S., Di Costanzo L., Balato A., Monfrecola G. Anti-inflammatory properties of low and high doxycycline doses: An in vitro study. Mediat. Inflamm. 2015;2015:329418. doi: 10.1155/2015/329418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krakauer T., Buckley M. Doxycycline is anti-inflammatory and inhibits staphylococcal exotin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 2003;47:3630–3633. doi: 10.1128/AAC.47.11.3630-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel A., Khande H., Periasamy H., Mokale S. Immunomodulatory effect of doxycycline ameliorates systemic and pulmonary inflammation in a murine polymicrobial sepsis model. Inflammation. 2020;43:1035–1043. doi: 10.1007/s10753-020-01188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rempe S., Hayden J.M., Robbins R.A., Hoyt J.C. Tetracyclines and pulmonary inflammation. Endocr. Metab. Immune Disord. Drug Targets. 2007;7:232–236. doi: 10.2174/187153007782794344. [DOI] [PubMed] [Google Scholar]
- 56.Fredeking T.M., Castro J.E.Z., Vado-Solis I., Perez-Osorio C. Modulation of cytokine and cytokine receptor/antagonist by treatment with doxycycline and tetracycline in patients with dengue fever. Clin. Dev. Immunol. 2011;2011:370872. doi: 10.1155/2011/370872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh B., Ghosh N., Saha D., Sarkar S., Bhattacharyya P., Chaudhurry K. Effect of doxycycline in chronic obstructive pulmonary disease—An exploratory study. Pulm. Pharmacol. Ther. 2019;58:101831. doi: 10.1016/j.pupt.2019.101831. [DOI] [PubMed] [Google Scholar]
- 58.Rana A., Singh S., Deshmukh R., Kumar A. Pharmacological potential of tocopherol and doxycycline against traumatic brain injury-induced cognitive/motor impairment in rats. Brain Inj. 2020;34:1039–1050. doi: 10.1080/02699052.2020.1772508. [DOI] [PubMed] [Google Scholar]
- 59.Plouffe J.F. Importance of atypical pathogens of community-acquired pneumonia. Clin. Infect. Dis. 2000;31:S35–S39. doi: 10.1086/314058. [DOI] [PubMed] [Google Scholar]
- 60.Marra F., McCabe M., Sharma P., Zhao B., Mill C., Leung V., Chong M., Patrick D.M. Utilization of antibiotics in long-term care facilities in British Columbia, Canada. JAMDA. 2017;18:1098.e1–1098.e11. doi: 10.1016/j.jamda.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Lago K., Telu K., Tribble D., Ganessan A., Kunz A., Geist C., Fraser J., Mitra I., Lalani T., Yun H. Impact of doxycycline as malaria prophylaxis on risk of Influenza-like illness among international travelers. Am. J. Trop. Med. Hyg. 2020;102:821–826. doi: 10.4269/ajtmh.19-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alam M.M., Mahmud S., Rahman M.M., Simpson J.A., Aggarwal S., Ahmed Z. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020;12:e9658. doi: 10.7759/cureus.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad I., Alam M., Saadi R., Mahmud S., Saadi E. Doxycycline and hydroxychloroquine as treatment for high-risk COVID-19 patients: Experience from case series of 54 patients in long-term care facilities. MedRxiv. 2020 doi: 10.1101/2020.05.18.20066902. under review. [DOI] [Google Scholar]
- 64.RepurpoSing Old Drugd to SuppRess a Modern Threat: COVID-19 STORM (STORM) [(accessed on 10 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04433078?term=doxycycline&cond=Covid19&draw=2.
- 65.Dynamic Study (DoxycYcliNe AMbulatoire COVID-19 (DYNAMIC) [(accessed on 10 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04371952?term=doxycycline&cond=Covid19&draw=2&rank=2.
- 66.The Fleming [FMTVDM] Directed CoVid-19 Treatment Protocol (FMTVDM) [(accessed on 10 September 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04349410?term=doxycycline&cond=Covid19&draw=2&rank=10.
- 67.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 68.ICEstimator Version 1.2. [(accessed on 10 September 2020)]; Available online: http://www.antimalarial-icestimator.net.
- 69.Amrane S., Tissot-Dupont H., Doudier B., Eldin C., Hocquart M., Mailhe M., Dudouet P., Ormières E., Ailhaud P., Lager J.C., et al. Rapid viral diagnosis and ambulatory management of suspected COVID-19 cases presenting at the infection diseases referral hospital in Marseille, France, -January 31st to March 1st, 2020: A respiratory virus snapshot. Travel. Med. Infect. Dis. 2020;36:101632. doi: 10.1016/j.tmaid.2020.101632. [DOI] [PMC free article] [PubMed] [Google Scholar]