Abstract
Depressive disorders and alcohol use disorders are widespread among the general population and are significant public health and economic burdens. Alcohol use disorders often co-occur with other psychiatric conditions and this dual diagnosis is called comorbidity. Depressive disorders invariably contribute to the development and worsening of alcohol use disorders, and vice versa. The mechanisms underlying these disorders and their comorbidities remain unclear. Recently, interest in the lateral habenula, a small epithalamic brain structure, has increased because it becomes hyperactive in depression and alcohol use disorders, and can inhibit dopamine and serotonin neurons in the midbrain reward center, the hypofunction of which is believed to be a critical contributor to the etiology of depressive disorders and alcohol use disorders as well as their comorbidities. Additionally, calcium/calmodulin-dependent protein kinase II (CaMKII) in the lateral habenula has emerged as a critical player in the etiology of these comorbidities. This review analyzes the interplay of CaMKII signaling in the lateral habenula associated with depressive disorders and alcohol use disorders, in addition to the often-comorbid nature of these disorders. Although most of the CaMKII signaling pathway’s core components have been discovered, much remains to be learned about the biochemical events that propagate and link between depression and alcohol abuse. As the field rapidly advances, it is expected that further understanding of the pathology involved will allow for targeted treatments.
Keywords: lateral habenula, CaMKII, addiction, drugs of abuse, alcohol, depression, comorbidity
1. Introduction
Alcohol use disorders (AUDs) is the medical diagnosis given to individuals who are suffering from severe problem drinking. AUDs are chronic relapsing brain disorders characterized by an impaired ability to stop or control alcohol use despite adverse social, occupational, or health consequences. AUDs are a severe problem in the United States. According to the National Institute of Health Alcohol Facts and Statistics, in 2018, in the United States, AUDs affect an estimated 15 million people, 5.8 percent or 14.4 million adults (ages 18 and older). This includes 9.2 million men and 5.3 million women. AUDs also affect an estimated 401,000 adolescents between the ages of 12 and 17 (National Institute on Alcohol Abuse and Alcoholism (NIAAA): Understanding the impact of alcohol on human health and well-being).
In 2018, 26.45 percent and 6.6 percent of adults engaged in binge drinking or heavy drinking, respectively. An estimated 88,000 people (62,000 men and 26,000 women) die from alcohol-related causes each year, making alcohol the third leading preventable cause of death in the United States (Centers for Disease Control and Prevention. Alcohol and Public Health: Alcohol-Related Disease Impact). In 2014, 9967 deaths (31 percent of overall driving fatalities) were due to alcohol-impaired driving (Traffic Safety Facts CrashStats. Report No. DOT HS 812 219, Washington, DC: National Highway Traffic Safety Administration, 2015). AUDs are a severe economic burden on society. In 2010, alcohol misuse cost the United States $249 billion [1]. Three-quarters of the total cost of alcohol misuse is related to binge drinking [1]. AUDs seriously affect family life. According to a 2012 study, more than 10 percent of children in the U.S. live with a parent with alcohol struggles.
AUDs are also a severe problem worldwide. According to the World Health Organization (WHO), in 2012, the harmful use of alcohol caused 3.3 million deaths globally, or 5.9 percent of all global deaths (7.6 percent for men and 4.0 percent for women) (WHO Global status report on alcohol and health, 2018). In 2014, the WHO reported that alcohol contributed to more than 200 diseases and injury-related health conditions, most notably, Diagnostic and Statistical Manual of Mental Disorders-4 (DSM-IV) alcohol dependence, liver cirrhosis, cancers, and injuries. In 2012, 5.1 percent of the burden of disease and injury worldwide (139 million disability-adjusted life years) was attributable to alcohol consumption (WHO Global status report on alcohol and health, 2018). Globally, alcohol misuse was the fifth leading risk factor for premature death and disability in 2010. It was the primary risk factor among people between the ages of 15 and 49 (WHO Global status report on alcohol and health, 2018). In the age group of 20–39 years, 25 percent of the total deaths were attributable to alcohol consumption (WHO Global status report on alcohol and health, 2018).
In the same vein, depressive disorders (DDs), including major depressive disorders, are among the most prevalent neuropsychiatric disorders that can interfere with patients’ functioning [2,3]. DDs are mood disorders characterized by sadness severe enough or persistent enough to interfere with function and often decreased interest or pleasure in activities. Individuals who suffer from DDs may have trouble doing normal day-to-day activities, think their lives are meaningless, and even engage in suicide ideations or behaviors. The exact cause of DDs is unknown, but it may involve a combination of heredity, changes in neurotransmitter levels, altered neuroendocrine function, and psychosocial factors.
AUDs often occur with other psychiatric conditions and this dual diagnosis is called comorbidity. This pattern of comorbidity adversely affects the prognosis, course, and treatment of both DDs and AUDs. High severity in one of these disorders is associated with high severity in another condition. Alcohol dependence prolongs the course of depression and increases the risk of suicidal symptoms and behaviors. DDs invariably contribute to the development and worsening of AUDs [4]. Patients with depression and AUDs are at increased risk of relapse to heavy drinking. However, the mechanisms underlying this association are not fully understood. This gap in our knowledge prevents us to find out a better treatment strategy for these comorbidities. Professionals working with patients suffering from these comorbidities face unique and challenging dilemmas about providing the best treatment to address both conditions. Despite the growing interest in this issue, relatively few clinical studies have tested treatments for this patient population [5]. This highlights the need to understand the etiology of the disorders and develop an effective treatment regimen.
Although many factors can contribute to the comorbidity of AUDs and DDs [6], the molecular interplay will be discussed in depth. Recently, the lateral habenula (LHb) has emerged as a crucial brain region in the pathophysiology of DDs and AUDs. Fortunately, a detailed outline of the calcium/calmodulin-dependent protein kinase II (CaMKII) signaling pathway has emerged. The target of its signaling cascade in the LHb has proven beneficial for treating AUDs, especially for those with comorbid DDs [7].
2. The Role of the Lateral Habenula in Depressive Disorders and Alcohol Use Disorders
Before diving into CaMKII’s prominent role in AUDs and DDs, it is essential to review the role of the habenula, a small structure in the brain located posterior to the thalamus and adjacent to the third ventricle [8,9,10]. The habenula is divided into two distinct regions: the medial habenula and the LHb. The LHb has been neglected in the scientific literature because of its small size [11]. However, interest and research in the LHb have flourished in recent years due to the revelation that it becomes pathologically hyperactive in major depressive disorders [12,13,14,15,16,17,18,19]. The LHb receives relay information from the limbic forebrain and basal ganglia structures to basically all midbrain monoaminergic centers, including the serotonergic raphe and dopaminergic midbrain nuclei [8,20,21,22], controlling mood and emotions and playing a critical part in the brain’s response to reward (Figure 1). Thus, the LHb is thought to have a role in the progression of depression and drug abuse [7,13,23,24].
Figure 1.
The brain network of the lateral habenula (LHb) in alcohol use disorders (AUDs) and its comorbidities. The LHb gets major inputs from the habenula-projecting globus pallidus (GPh), the prefrontal cortex (PFC), and the lateral hypothalamus (LH) and sends projections to dopamine neurons in the ventral tegmental area (VTA)/substantia nigra compacta (SNc) and serotonin neurons in the raphe directly or indirectly through the rostromedial tegmental nucleus (RMTg). The LHb also receives projections from the raphe. LHb’s spike output and glutamatergic transmission are increased in alcohol withdrawal rats and in animal models of depression, suggesting that the LHb plays a key role in AUDs and depressive disorders (DDs) and their comorbidities.
The ability to seek and predict reward and pleasure and avoid confrontations is crucial for an animal’s survival and well-being. The measure of the discrepancy between reward prediction and reward outcome is referred to as reward prediction error (RPE). It serves as a powerful incentive that guides approach or avoidance (go or no-go) behavior [23,25]. Positive RPE is when the actual reward exceeds the expected one and facilitates behaviors associated with reward (approach or go). Conversely, suppressed actions related to reward (avoidance or no-go) occur when the actual reward is smaller than expected, known as negative RPE [26].
Information about the reward state is transmitted via the outer region of the fasciculus retroflexus axon bundle that originates in the LHb to midbrain structures, particularly the rostromedial tegmental nucleus (RMTg). The RMTg is a small nucleus that consists of gamma-aminobutyric acid (GABA) cells [27], also known as the GABAergic tail of the ventral tegmental area [28]. The fasciculus retroflexus governs the release of glutamate onto GABAergic cells in the RMTg. The resulting GABA release from RMTg neurons inhibits dopamine cells in the ventral tegmental area (VTA)/substantia nigra compacta. This process allows the LHb to control dopamine levels in their target areas, such as the medial frontal cortex and nucleus accumbens (Figure 1). These structures have essential roles in a broad range of motivated behaviors and neuropsychiatric disorders [10,20,29,30,31,32,33,34,35,36,37,38]. Thus, positive RPE has been associated with the inhibition of LHb neurons and, therefore, increases in the firing of dopamine neurons. Since dopamine produces feelings of reward, drugs that induce increased dopamine levels have abuse potential [39,40,41,42,43]. In addition, correlating with a reward prediction error (the difference between expected and actual rewards), VTA dopamine cells can also signal aversion, saliency, uncertainty, and novelty [44]. In contrast, negative RPE is associated with LHb neurons’ activation and decreases in dopamine neuron firing [45].
Additionally, the LHb projects to the midbrain serotoninergic system. This system plays a significant role in the motivation process [46], and its dysregulation contributes to drug addiction [47] and the etiology of mood disorders [48]. Like the projections to the midbrain dopamine neurons, LHb neurons project to the raphe serotonergic neurons either directly or indirectly through the RMTg [20,27,49,50]. LHb neurons projecting to the raphe are mostly in the medial part of the LHb, and the medial raphe (MRN) receives more LHb projections than the dorsal raphe (DRN), as shown by tracing studies [49,50]. Studies using the rabies-based viral strategy and Cre driver mouse lines show that LHb neurons project to DRN/MRN serotonergic neurons and DRN GABA neurons [51,52]. Remarkably, the raphe also sends axons to the LHb. Studies using phalloidin anterograde labeling tracing techniques show that serotonergic fibers from the MRN and DRN are throughout the whole LHb [53,54]. Studies using transgenic mice expressing Cre recombinase in serotonin-positive raphe neurons show that DRN serotonin cells project to the lateral portion of the LHb [55,56], consistent with the evidence that serotonin and its transporter are in the presynaptic side in the LHb [57,58,59,60]. For an overview on how the LHb integrates serotonergic signals, please read the recent review by Tchenio and colleagues [61].
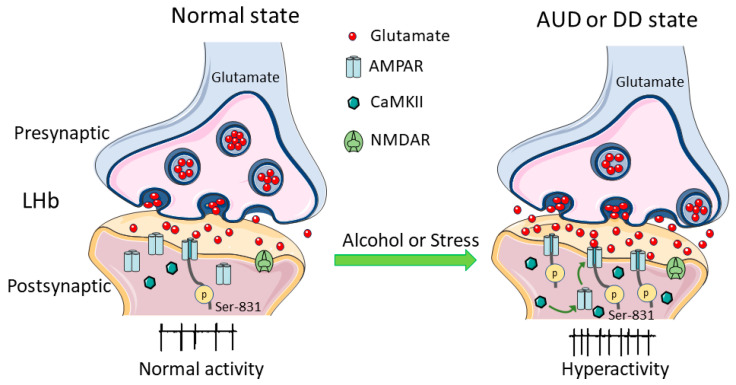
Neurons in the LHb are almost uniformly glutamatergic [62,63]. The LHb inhibits the brain’s reward centers, including the dopaminergic VTA [21,38,64] and the serotonergic DRN [20], either via the direct projection to local interneurons within the VTA and DRN [7,27,36,38,65,66,67] or indirectly via the GABAergic relay in the RMTg [27,28,58,68,69,70] (Figure 1). The tight connection between the LHb and monoaminergic neurotransmitter systems suggests a crucial role of the LHb in neuropsychiatric diseases including DDs [18,19,34,37] and AUDs [71,72]. Accumulating evidence in rodents and humans shows that the LHb exhibits hyperactivity during depressive states [7,13,19,73,74,75,76] and alcohol withdrawal [7,71,72,77]. Conversely, a reduction of LHb hyperactivity by pharmacological or chemogenetic approaches, and deep brain stimulation or lesioning the LHb ameliorates depressive symptoms, including those in rats withdrawn from chronic alcohol [7,12,13,67,78,79,80]. Furthermore, LHb hyperactivity is involved in increased presynaptic glutamate release and upregulation of βCaMKII, which enhances the function of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) [13,75]. The role of CaMKII will be discussed in more detail in the later parts of this review.
Since it is a critical crossroad that influences brain responses to and encoding of aversive stimuli, such as stress, pain, anxiety, or pleasurable stimuli such as reward [23], the LHb has been correlated with major depressive disorders [13,18,26,75]. When rats and mice were treated with negative stimuli, LHb neurons were activated immediately [72,81,82]. Similarly, in vivo electrophysiological recording in monkeys showed that unexpected punitive signals such as an air puff in the eye, in addition to the omission of an expected reward, strongly excited LHb neurons [38]. Conversely, inhibiting LHb neurons suppresses depressive-like behaviors, as exhibited in animal models [7,17,71]. In addition to directly facilitating neuron activity, stress can alter the plasticity of LHb neurons, possibly contributing to long-term changes in the LHb under chronic stress, which can lead to depressive-like behaviors [26,83]. Though studies have shown enhanced synaptic activity and spike output of LHb neurons in animal models of depression, the precise molecular mechanisms by which depression leads to these changes are not yet understood [13,19,75]. Glutamate is the predominant excitatory neurotransmitter in the LHb. Potentiation of glutamate transmission causes LHb hyperactivity, as occurs in AUDs and DDs [7,13,26,71,77,84] (Figure 1 and Figure 2).
Figure 2.
Changes of calcium/calmodulin-dependent protein kinase II (CaMKII) signaling in the LHb glutamatergic neurons in alcohol use disorders (AUDs) and depressive disorders (DDs). Withdrawal from chronic alcohol administration and stress enhance glutamatergic transmission to and the spike output of LHb neurons. This is due in part through phosphorylating serine 831 (Ser831) on the glutamate A1 (GluA1) subunit and activating the CaMKII.
Through a series of experiments and human genome-wide studies, the LHb is linked to addiction to drugs, such as cocaine, morphine, and alcohol [23]. Drugs of abuse cause addiction because they can increase the activity of VTA dopamine neurons and dopamine levels in the target areas, including the prefrontal cortex, nucleus accumbens (NAc), and striatum [40,85,86,87]. Akin to withdrawal from cocaine [88], withdrawal from chronic alcohol increases the firing of and the glutamate transmission to LHb neurons (Figure 1 and Figure 2).
It is well accepted that the dopamine system, including the VTA, is involved in alcohol-seeking behavior and relapse [87,89,90]. Like other drugs of abuse, alcohol’s reinforcing properties involve enhanced dopamine neuron activity. A large body of evidence suggests that transition from recreational to compulsive drinking, characteristic of AUDs, involves neuroadaptations in the mesolimbic reward system [90,91]. Although alcohol acutely activates mesolimbic dopamine neurotransmission, withdrawal from chronic alcohol leads to substantial decrements in VTA dopamine neuron activity [92,93,94] and extracellular dopamine levels in the NAc [95,96]. It is believed that this dopamine hypofunction leads to a negative emotional state that drives drug-seeking behavior to restore dopamine to normal, drug-naive levels [96,97,98]. However, the mechanisms causing this dopamine hypofunction remain unclear. Recent evidence from serious electrophysiological, immunohistochemical, molecular, and behavioral experiments points to a significant role played by the LHb. LHb neurons’ hyperactivity during alcohol withdrawal may inhibit VTA dopamine neurons and lead to dopamine hypofunction, causing depressive symptoms such as anhedonia and anxiety [7,58,69,71,77,97,98]. These results provide strong support for a key role of the LHb in the connection between DDs, AUDs, and their comorbidities (Figure 1 and Figure 2). However, our understanding of the mechanisms by which chronic alcohol administration and withdrawal as well as depression increase LHb spike output and glutamatergic transmission is incomplete.
3. CaMKII Structure and Regulation
Calcium ion (Ca2+) is a ubiquitous second messenger in cellular signaling [99,100]. The chief intracellular receptor and modulator of Ca2+ is calmodulin (CaM), a highly conserved Ca2+ sensor, and its primary targets are protein kinases [101]. Saturation of CaM with Ca2+ induces a conformational change that allows the protein to interact with and activate a wide variety of downstream target enzymes [102]. One of the primary downstream targets of CaM is a family of enzymes known as calmodulin-dependent kinases (CaM-kinases), which are vital for proper cellular function. Members of the CaM-kinase family are classified as serine/threonine kinases. They all have either a serine or threonine on their substrate P-sites, the targeted locale for phosphorylation. CaMKII, in particular, is among the most well-studied members of the CaM-kinase family [102]. CaMKII is composed of 12 subunits that are arranged into two rings, each containing six subunits, allowing for regulating and modifying the function and localization of the kinase [103]. Four isoforms of CaMKII (α, β, γ, and δ) exist in mammals, each one expressed from a separate gene [102], and the α and β isoforms are the most prevalent in the brain. The abundance of CaMKII in the nervous system makes it an unusual enzyme since catalytic molecules are typically found in lesser amounts [104]. Overall, CaMKII plays a significant role in regulating neurotransmitter secretion, receptor function, axonal transport, and structural modifications of the cytoskeleton [105,106].
CaMKII activation is dependent on calcium influx and the binding of Ca2+/CaM [102]. This complex binds to the regulatory region of CaMKII, producing a conformational change that not only leads to phosphorylation of target proteins, but also autophosphorylation, preventing the enzyme from reverting to its inactive conformation and decreasing the dissociation rate of bound CaM [103]. Thus, phosphorylated CaMKII acquires autonomous and Ca2+-independent activity and can become independent of Ca2+/CaM even after intracellular Ca2+ levels subside [107,108,109]. Functional alterations from autophosphorylation result, allowing Ca2+ to be translated into proper cellular responses. Autophosphorylation ramifications underlie the enzyme’s complex autoregulatory behavior, enabling it to activate at various frequencies of calcium spikes, detect calcium spikes, and behave as a molecular switch in learning and memory, a readout of synaptic activity [99]. Due to its switch-like regulatory properties, CaMKII is highly touted as a sophisticated control over so many disparate cellular functions and has been proposed to be a primary molecular component in the etiology of AUDs and DDs [7,75].
4. The Role of CaMKII in Alcohol Use Disorders
The role of CaMKII in AUDs has not been extensively studied. However, some evidence indicates that CaMKII contributes to several AUD-related behaviors (Table 1). Alcohol self-administration increases phosphorylation of GluA1 at the CaMKIIα recognition site (pGluA1- Ser831) in the central amygdala (CeA) of selectively bred alcohol-preferring P-rats as compared to behavior-matched (non-drug) sucrose controls. Intra-CeA injection of the AMPAR-positive modulator aniracetam or the cell-permeable CaMKII peptide inhibitor myristolated autocamtide-2-related inhibitory peptide (m-AIP), respectively, facilitated or inhibited alcohol self-administration [110]. Interestingly, CaMKII plays a different role in the aberrant behaviors induced by different doses of alcohol. In αCaMKII autophosphorylation-deficient αCaMKII-T286A mice, acute and subchronic administration of a low dose of alcohol (2 g/kg, intraperitoneal injection, i.p.) failed to induce locomotion, but a high dose (3.5 g/kg, i.p.) caused sedation. In αCaMKII-T286A mice, acute or subchronic alcohol administration did not change dopamine (DA) levels, measured by in vivo microdialysis, in the NAc, but enhanced serotonin (5-HT) responses in the prefrontal cortex were observed. Thus, αCaMKII autophosphorylation and the change in the DA–5-HT balance contribute to the establishment of alcohol-drinking behavior [111]. CaMKII may also act as a primary molecular mechanism that regulates relapse in alcohol addiction and cue-induced reinstatement of alcohol-seeking behavior, a hallmark behavioral pathology of addiction. In male C57BL/6J mice, reinstatement was associated with increased pCaMKII-T286 immunofluorescence reactive in specific reward- and memory-related brain regions, including the amygdala, NAc, lateral septum, mediodorsal thalamus, and piriform cortex, as compared with extinction control [112].
Table 1.
Evidence on CaMKII in alcohol use disorders and its comorbidities.
| Brain Region | Alcohol Treatment | Tested In | CaMKII Levels | Findings | Reference |
|---|---|---|---|---|---|
| Global | 2 g/kg, i.p. | pCaMKIIα-deficient, heterozygous, and WT mice | N/A | ↑ EtOH’s negative reinforcing action in pCaMKIIα-deficient mice | [114] |
| Three-bottle free choice | Onset of EtOH consumption delayed in pCaMKIIα-deficient mice | [111] | |||
| 2 g/kg, i.p. | pCaMKII is crucial to hippocampal DG neuron activation after EtOH exposure | [115] | |||
| PFC | Operant self-admin | C57BL/6J mice | CaMKIIα (↑) | ↓ mPFC CaMKII ↑ the positive reinforcing effects of sweetened EtOH | [116] |
| CIE | L-E rats (M) | CaMKIIα (↑) | pCaMKII in dorsal mPFC is associated with EtOH-induced cognitive inflexibility | [117] | |
| Three-bottle free choice | Mice | CaMKIIα (↓) | PFC CaMkIIα gene is linked to EtOH consumption | [118] | |
| mPFC and NAc Sh | Self-admin (20%) (28 d) | S-D rats (M) | pCaMKII (↑) at 6 h deprivation | CaMKII mediates EtOH-WD-induced anxiety and neurochemical adaptations in mPFC and NAc | [119] |
| NAc Sh | CIE | Adult Wistar rats (M) | pCaMKII (↓) in high responders | ↓ pCaMKII is involved in EtOH-seeking behaviors | [120] |
| Self-admin (6%) (28 d) | S-D rats (M) | pCaMKII CIE (↓) 24 h WD (↑) |
Activation of NMDAR1–CaMKII contributes to EtOH drinking and negative emotional states | [121] | |
| Drink in the dark (14 d) | Adult and adolescent C57BL/6J mice (M) | CaMKII at 24 h WD Adult (↑) Adolescent (↔) |
CaMKII is positively linked to negative affective symptoms in EtOH-WD adults | [122] | |
| BNST | 0.8 g/kg, i.p. + CIE | C57BL/6J mice (M) | CaMKIIα (↓) | ↓ CaMKIIα in EtOH-exposed vBNST contributes to changes in synaptic NMDAR kinetics | [123] |
| Amygdala, NAc, septum, thalamus, piriform cortex | Self-admin | C57BL/6J mice (M) | pCaMKII-T286 (↑) | Cue-induced reinstatement of EtOH-seeking is associated with pCaMKII-T286 in reward- and memory-related brain regions | [112] |
| LHb | Two-bottle free choice (8–12 w) | S-D and L-E rats (M) | CaMKII (↑) at 24 h WD | ↓ CaMKII in the LHb ↓ EtOH intake and depressive symptoms | [7,124] |
| Amygdala | Self-admin | Adult P rats (M) | pCaMKII-T286 and CaMKIIα (↑) | ↓ Acb CaMKII ↓ EtOH self-administration | [110] |
| Operant self-admin (15%) | Adult C57BL/6J mice (M) | pCaMKII (↑) | EtOH drinking ↑ CaMKII in the amygdala that regulates EtOH’s positive reinforcing effects | [125] | |
| Home-cage drink and operant self-admin | Adolescent and adult C57BL/6J mice (M) | pCaMKIIα-T286 Adolescent (↓) Adults (↔) |
Differential CaMKIIα-dependent AMPAR activation underlies age-related escalation of binge drinking | [126] | |
| Basolateral amygdala | CIE | S-D rats (M) | pCaMKII-T286 CIE (↑), WD (↔) |
CIE- and WD-induced changes in CaMKII activity contribute to ↑ GluA1R phosphorylation/trafficking | [127] |
| pCaMKII-T305 CIE (↔), WD (↑) | |||||
| Hippocampal CA1 and DG |
Self-admin (20%) (28 d) | S-D rats (M) | pCaMKII-T286 CIE (↓) WD (↑) |
CaMKII activation in hippocampal subregions contributes to EtOH dependence | [128] |
| Cerebral cortex | Self-admin (10%) | Wistar rats | CaMKIIα (↑) | Pre- and post-natal EtOH exposure ↑ CaMKII levels in membrane and cytosolic fractions | [129] |
↑ indicate increase, ↓ decrease, ↔ no change. AMPAR, AMPA receptor; CA1, hippocampal cornu ammonis 1; CIE, chronic intermittent ethanol exposure; DG, dentate gyrus; EtOH-WD, alcohol withdrawal; PFC, prefrontal cortex; i.p., intraperitoneal injection; L-E, Long–Evans rats; M, males; mPFC, medial prefrontal cortex; NAc Sh, nucleus accumbens shell; NMDAR, NMDA receptor; pCaMKIIα, alpha CaMKII autophosphorylation; P rats, alcohol-preferring rats; S-D, Sprague-Dawley; Self-admin, self-administration; vBNST, ventral bed nucleus of the stria terminalis; WD, withdrawal; WT, wild type; the concentrations of alcohol (EtOH), and the duration of alcohol treatment are in parenthesis.
Importantly, CaMKII in the LHb plays a significant role in aversive behaviors during alcohol withdrawal [7,58,110]. The protein level of phosphorylated AMPAR GluA1 subunit at a CaMKII locus (pGluA1-Ser831) was higher in the LHb of Long–Evans rats withdrawn from chronic intermittent alcohol drinking compared with alcohol-naive rats. These alcohol-withdrawn rats showed clear depressive-like behaviors, measured by forced swimming test and sucrose-preferring test [7,58,77], which were mitigated by intra-LHb injection of the AMPAR antagonist quinoxalinediones 6, 7-dinitroquinoxaline-2, 3-Dione (DNQX) or the CaMKII antagonist 1-[N,O-bis-(5-isoquinolinesulphonyl)-N-methyl-L-tyrosy]-4-phenylpiperazine (KN-62), or inhibiting LHb activity with a chemogenetic tool or deep brain stimulation [7,113]. Conversely, in alcohol-naive rats, activation of LHb AMPARs induced depressive-like behaviors [7].
CaMKII also takes part in pain hypersensitivity that often occurs in alcoholics and AUD animal models, especially during abstinence [77,124,130]. Chronic pain is a significant contributor in DD etiology [131,132,133]. In rats, the 5-HT receptors (5-HTRs) expressed on LHb neurons [58,134,135,136] and at the glutamatergic synapses on LHb neurons [137] contribute to nociception. Intra-LHb injection of meta-chlorophenylpiperazine (mCPP, a 5-HT2CR agonist) and 2,5-dimethoxy-4-iodoamphetamine (DOI, a 5-HT2A/2CR agonist) increased nociceptive sensitivity in alcohol naive rats, while antagonists SB200646 (a 5-HT2CR antagonist) and ritanserin (a 5-HT2A/2CR antagonist), 5-HT reuptake blocker citalopram, or CaMKII antagonist KN-62 mitigated the elevated nociceptive sensitivity in alcohol withdrawn rats [58].
An important facet to consider in understanding AUDs is glutamate, the primary excitatory neurotransmitter in the central nervous system. The N-methyl-D-aspartate receptors (NMDARs) and AMPARs are the two major subtypes of ionotropic glutamate receptors and are critical targets of alcohol. The NMDAR is a well-characterized target of ethanol that has been associated with both drinking behavior [138] and withdrawal [139]. Acute alcohol application inhibits NMDAR function in several preparations including recombinant heteromeric NMDA channels, NMDARs expressed in the oocytes [140,141], hippocampal neurons [142,143], cortical neurons [144], and neurons in the ventral bed nucleus of the stria terminalis (BNST) [145]. In contrast, chronic alcohol exposure leads to an upregulation of NMDAR function in several brain regions related to reward and memory [146,147], such as the BNST [123] and the dorsal striatum [138]. Chronic alcohol exposure, both in vivo and in vitro, increases the gene expression of NMDAR subunits NR1 and NR2B and their polypeptide levels [148]. Enhanced NMDAR activity significantly increases the amount of calcium that enters nerve cells. Ca2+ influx through NMDARs and subsequent activation of CaMKII are critical events in the induction of long-term potentiation, learning, and memory [149,150,151,152,153].
Similarly, the AMPARs also play an essential role in regulating synaptic strength [154] and AUDs [7,155]. The glutamate A1 (GluA1), one of the four subunits (GluA1–4) in the AMPARs, plays a particularly important role in AUDs [7,110,125,156]. The LHb receives robust glutamatergic inputs and expresses the GluA1 subunit predominantly [157]. It is worth mentioning the significant role of AMPAR activation in removing the voltage-dependent block by Mg2+ of NMDARs. During bouts of synaptic activity, AMPAR-mediated depolarization of the postsynaptic membrane facilitates the activation of NMDARs [158].
In Long–Evans rats, activation of CaMKII enhances glutamatergic transmission by phosphorylating serine 831 (Ser831) on the GluA1 subunit and by promoting AMPAR insertion into the synapse [7] (Figure 2). This enables AMPARs to play a critical role in regulating synaptic strength [159]. Site-specific phosphorylation has been studied extensively and is likely to be upregulated to increase AMPAR surface expression, thereby enhancing glutamatergic transmission. This is the key to understanding synaptic plasticity and addictive disorders, including AUDs [7,160]. The activation of AMPARs in LHb neurons promotes drug-taking episodes and vulnerability to relapse [7,159].
Chronic alcohol exposure increases surface concentrations of GluA1 in the striatum, promoting alcohol self-administration by modifying reinforcement processes [156]. Additionally, phosphorylation of GluA1 at Ser831 is significantly increased in the LHb of rats undergoing alcohol withdrawal, consistent with the findings that phosphorylation at this site increases AMPAR activity, thus reinforcing alcohol-drinking and alcohol-seeking behaviors [7]. On the other hand, local pharmacological inhibition of LHb AMPAR activity or blocking CaMKII signaling alleviated the depressive-like behaviors and alcohol-drinking behavior (Figure 2) [7]. However, insights about the input-specific expression and subcellular localization of these receptors are still lacking and require further investigation. The combinatorial use of genetic tools, optogenetic strategies, and electrophysiology will be necessary to refine our understanding of the LHb connectivity.
As mentioned, electrophysiological and behavioral evidence from rats also shows that adaptation in the serotonin (5-HT) 2 receptor-CaMKII signaling pathway contributes to the hyper-glutamatergic state, the hyperactivity of LHb neurons, and the higher nociceptive sensitivity in rats withdrawing from chronic alcohol consumption [124]. Through a combination of behavioral and molecular approaches, a study in the selectively bred alcohol-preferring (P) and alcohol-non-preferring (NP) lines of rats found that compared with NP rats, the P-rats had a higher sensitivity to mechanical stimuli and displayed depressive-like behaviors, as well as a higher level of CaMKII in the LHb, all of which were ameliorated by alcohol drinking [161]. CaMKII in the LHb plays a significant role not only in AUDs, but also in the other commonly abused drugs, such as morphine, as shown in a recent mouse study in which chronic morphine treatment resulted in CaMKII overexpression in the LHb [162].
5. The Role of CaMKII in Depressive Disorders
Depressive disorders (DDs) are one of the most widespread and incapacitating mental disorders, resulting in the loss of motivation and interest, feelings of despair, and the inability to feel pleasure [75]. Since LHb was recently discovered as the key brain region in the pathophysiology of depression, a surge in interest over CaMKII has uncovered its role in regulating several signal transduction pathways associated with learning and memory. Since it can remain activated beyond the timeframe of Ca2+ infiltration that initially stimulated molecule, CaMKII is often dubbed a “memory molecule” [102]. Stress-induced elevations in bCaMKII signaling, in particular, are related to depression because they influence the formation and retention of aversive memories [31].
Through a combination of electrophysiological, behavioral, and molecular approaches, the bCaMKII was identified as the key contributor for habenular hyperactivity and, thus, depressive-like behaviors. Recent studies have shown that amplification of bCaMKII function mediates increased depolarization of LHb neurons. When tested in rats and mice, overexpression of bCaMKII, but not αCaMKII, in the LHb, using viral vectors (adeno-associated virus 2 (AAV2)) strongly enhanced the synaptic efficacy and spike output of LHb neurons and produced profound depressive symptoms, including anhedonia and behavioral despair, which were reversed by downregulation of βCaMKII levels, blocking its activity or its target molecule glutamate A1 (GluR1) [75]. In contrast to chronic stress, acute stress has been shown to increase both isoforms, αCaMKII and bCaMKII [83]. Furthermore, an increase of bCaMKII enhanced the synaptic efficacy of LHb neurons, producing profound depressive-like symptoms. This could be because of elevated bCaMKII, strengthening AMPAR-mediated synaptic transmission and hyperactivity of the LHb. On the other hand, blocking CaMKII activity, or its target molecule, GluA1, reversed depressive-like symptoms [75].
Additionally, bCaMKII may regulate other channels of LHb neurons that enhance spike output. An important question that has yet to be addressed is the feature that renders βCaMKII sensitive to depressive stimuli and antidepressants [75]. This leads to speculation that βCaMKII plays a significant role in LHb neuronal function and is a key determinant of depression [75]. That facilitation of glutamate transmission in the LHb may lead to depressive symptoms [7,18,19,71,84,163] (Figure 1 and Figure 2). Further support of this idea is the finding that ketamine, an NMDAR antagonist, suppresses the depressive-like behaviors in rodent models of depression [18]. The discovery of ketamine’s rapid antidepressant effects is perhaps the most critical advance in the psychiatry field in recent history [164]. To investigate the underlying mechanisms, in a recent study, ketamine was applied into the LHb in rats, which quickly rescued depression-like behaviors. In vivo and ex vivo, electrophysiological recording found that burst firing, which was increased in the LHb of depressive rodent models, was suppressed by ketamine [18].
6. The Comorbidity of Depressive Disorders and Alcohol Use Disorders
Alcoholics have extremely high rates of co-occurring psychological disorders, including depressive disorders (DDs) [165,166,167,168]. Compared to non-alcoholics, alcohol-dependent Americans have 1.7–3.6 times the risk for major depression, and 7.1 times for other drug addictions [165,167]. Although the reasons for the comorbidity of AUDs and DDs remain unknown, DDs are almost invariably involved in developing AUDs, thereby weakening the effects of treatment and increasing the risk of relapse [7,169]. Nearly one-third of patients who present with the major depressive disorder also suffer from AUDs [170]. Studies in the general population have shown that people with depressive symptoms have a 2–3-fold increased risk of developing AUDs [171]. It is essential to understand the overlapping neural mechanisms behind these comorbidities.
An important facet to consider in understanding depression that is concomitant with AUDs is the AMPARs. Enhanced AMPAR signaling via CaMKII interaction with the GluA1 may be one of the pathophysiological changes that influence drug-taking and drug-seeking behaviors and produce a phenotype akin to that of an alcoholic [159]. Building on this idea, in a study in alcohol-withdrawing rats, which showed clear depressive-like symptoms, AMPARs and CaMKII in the LHb were increased. Inhibition of LHb AMPARs, CaMKII activity, and LHb neuronal activity significantly mitigated depressive-like behaviors and alcohol-drinking and alcohol-seeking behaviors. Conversely, activation of LHb AMPARs induced depressive-like symptoms [7]. These results suggest that the LHb CaMKII–AMPAR signaling pathway may be a potential target and provide a new therapeutic approach not only to relieve symptoms of depression, but also to alleviate the desire for alcohol consumption [7]. Thus, LHb hyperactivity and enhanced glutamatergic transmissions during both DD and AUD states may be the cellular and molecular mechanisms underlying LHb’s role in the connection between depression and addiction. Building on the finding of the significant antidepressant effect of the NMDAR antagonist ketamine, interest in the effect of ketamine on AUDs is growing and has shown promising results. In humans, ketamine reduced harmful drinking by pharmacologically rewriting drinking memories [172]. In adult Wistar male rats, both ketamine and 2,3-Dihydroxy-6-nitro-7-sulphamoylbenzo(f)-quinoxaline, 6-Nitro-7-sulphamoylbenzo(f)-quinoxaline-2,3-dione (NBQX), (daily intraperitoneal injections of ketamine (2.5 mg/kg) and NBQX (5 mg/kg), alone or in combination) attenuated alcohol-withdrawal induced depression, measured by forced swim test [173]. These data provide further support for the connection between AUDs and DDs. Future studies on the effect of LHb application of ketamine and other NMDAR antagonists on the comorbidities of AUDs and DDs will further reveal the role of LHb in the connection between DDs and AUDs (Figure 2).
7. Conclusions
Depressive disorders concomitant with alcohol use disorders reinforce each other. Together, they are often challenging to treat, as relapse in one quickly leads to relapse in the other, and the symptoms overlap. As reviewed in this article, both depressive disorders and alcohol use disorders can cause LHb hyperactivity, which inhibits the activity of dopamine neurons and serotonin neurons in the midbrain reward center. The hypofunction of these midbrain neurons is believed to be a significant cause of psychological disorders. Both alcohol use disorders and depressive disorders increase glutamate transmission to the LHb neurons and, consequently, the activation of AMPARs and NMDARs, among others. The increased calcium influx through NMDARs activates CaMKII, a key molecule in the pathophysiology of both alcohol use disorders and depressive disorders. Since CaMKII in the LHb plays a prominent role in depression and addiction, it is expected that further study of the pathophysiology involved will allow for the development of targeted therapies to address the comorbidities.
Funding
This research is made possible by grants from National Institutes of Health, US to J.-H.Y.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sacks J.J., Gonzales K.R., Bouchery E.E., Tomedi L.E., Brewer R.D. 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 2015;49:e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Ripke S., Wray N.R., Lewis C.M., Hamilton S.P., Weissman M.M., Breen G., Byrne E.M., Blackwood D.H.R., Boomsma D.I., Cichon S., et al. Major Depressive Disorder Working Group of the Psychiatric, G.C. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassano P., Fava M. Depression and public health, and overview. J.Psychosom. Res. 2002;53:849–857. doi: 10.1016/S0022-3999(02)00304-5. [DOI] [PubMed] [Google Scholar]
- 4.Burns L., Teesson M. Alcohol use disorders comorbid with anxiety, depression and drug use disorders. Findings from the Australian National Survey of Mental Health and Well Being. Drug Alcohol Depend. 2002;68:299–307. doi: 10.1016/S0376-8716(02)00220-X. [DOI] [PubMed] [Google Scholar]
- 5.Petrakis I.L., Gonzalez G., Rosenheck R., Krystal J.H. Comorbidity of alcoholism and psychiatric disorders: An overview. Alcohol Res. Health. 2002;26:81–89. [Google Scholar]
- 6.Akbar M., Egli M., Cho Y.E., Song B.J., Noronha A. Medications for alcohol use disorders: An overview. Pharmacy. 2018;185:64–85. doi: 10.1016/j.pharmthera.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Kang S., Fu R., Wu L., Wu W., Liu H., Gregor D., Zuo W., Bekker A., Ye J.H. Inhibition of AMPA receptor and CaMKII activity in the lateral habenula reduces depressive-like behavior and alcohol intake in rats. Neuropharmacology. 2017;126:108–120. doi: 10.1016/j.neuropharm.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nat. Rev. Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aizawa H., Amo R., Okamoto H. Phylogeny and ontogeny of the habenular structure. Front. Neurosci. 2011;5:138. doi: 10.3389/fnins.2011.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namboodiri V.M., Rodriguez-Romaguera J., Stuber G.D. The habenula. Curr. Biol. 2016;26:R873–R877. doi: 10.1016/j.cub.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 11.Geisler S., Trimble M. The lateral habenula: No longer neglected. CNS Spectr. 2008;13:484–489. doi: 10.1017/S1092852900016710. [DOI] [PubMed] [Google Scholar]
- 12.Sartorius A., Kiening K.L., Kirsch P., von Gall C.C., Haberkorn U., Unterberg A.W., Henn F.A., Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Li B., Piriz J., Mirrione M., Chung C., Proulx C.D., Schulz D., Henn F., Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shabel S.J., Proulx C.D., Piriz J., Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecca S., Pelosi A., Tchenio A., Moutkine I., Lujan R., Hervé D., Mameli M. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 16.Tchenio A., Lecca S., Valentinova K., Mameli M. Limiting habenular hyperactivity ameliorates maternal separation-driven depressive-like symptoms. Nat. Commun. 2017;8:1135. doi: 10.1038/s41467-017-01192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y., Yang Y., Ni Z., Dong Y., Cai G., Foncelle A., Ma S., Sang K., Tang S., Li Y., et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554:323–327. doi: 10.1038/nature25752. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y., Cui Y., Sang K., Dong Y., Ni Z., Ma S., Hu H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Wang H., Hu J., Hu H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 2018;48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Wang R.Y., Aghajanian G.K. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197:89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- 21.Christoph G.R., Leonzio R.J., Wilcox K.S. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J. Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga V., Kocsis B., Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur. J. Neurosci. 2003;17:280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- 23.Velasquez K., Molfese D., Salas R. The role of the habenula in drug addiction. Front. Hum. Neurosci. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., Zhang B.-L., Yang S.-J., Rusak B. The role of lateral habenula–dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav. Brain Res. 2015;277:89–98. doi: 10.1016/j.bbr.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Keiflin R., Janak P.H. Dopamine prediction errors in reward learning and addiction: From theory to neural circuitry. Neuron. 2015;88:247–263. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu H., Cui Y., Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020;21:277–295. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- 27.Jhou T.C., Fields H.L., Baxter M.G., Saper C.B., Holland P.C. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufling J., Veinante P., Pawlowski S.A., Freund-Mercier M.J., Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J. Comp. Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 29.Lecourtier L., Defrancesco A., Moghaddam B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur. J. Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammel S., Lim B.K., Ran C., Huang K.W., Betley M.J., Tye K.M., Deisseroth K., Malenka R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browne C.A., Hammack R., Lucki I. Dysregulation of the lateral habenula in major depressive disorder. Front. Synaptic Neurosci. 2018;10:46. doi: 10.3389/fnsyn.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omelchenko N., Bell R., Sesack S.R. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur. J. Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proulx C.D., Aronson S., Milivojevic D., Molina C., Loi A., Monk B., Shabel S.J., Malinow R. A neural pathway controlling motivation to exert effort. Proc. Natl. Acad. Sci. USA. 2018;115:5792–5797. doi: 10.1073/pnas.1801837115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proulx C.D., Hikosaka O., Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Root D.H., Melendez R.I., Zaborszky L., Napier T.C. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog. Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatakis A.M., Stuber G.D. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecca S., Meye F.J., Mameli M. The lateral habenula in addiction and depression: An anatomical, synaptic and behavioral overview. Eur. J. Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto M., Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 39.Volkow N.D., Fowler J.S., Wang G.-J., Swanson J.M., Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch. Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 40.Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wise R.A., Robble M.A. Dopamine and addiction. Annu. Rev. Psychol. 2020;71:79–106. doi: 10.1146/annurev-psych-010418-103337. [DOI] [PubMed] [Google Scholar]
- 42.You C., Vandegrift B., Brodie M.S. Ethanol actions on the ventral tegmental area: Novel potential targets on reward pathway neurons. Psychopharmacol. 2018;235:1711–1726. doi: 10.1007/s00213-018-4875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise R.A., Rompre P.-P. Brain dopamine and reward. Annu. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 44.Pignatelli M., Bonci A. Role of dopamine neurons in reward and aversion: A synaptic plasticity perspective. Neuron. 2015;86:1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Graziane N.M., Neumann P.A., Dong Y. A Focus on reward prediction and the lateral habenula: Functional alterations and the behavioral outcomes induced by drugs of abuse. Front. Synaptic Neurosci. 2018;10:12. doi: 10.3389/fnsyn.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faulkner P., Deakin J.F. The role of serotonin in reward, punishment and behavioural inhibition in humans: Insights from studies with acute tryptophan depletion. Neurosci. Biobehav. Rev. 2014;46:365–378. doi: 10.1016/j.neubiorev.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Müller C.P., Homberg J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015;277:146–192. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Cannon D.M., Ichise M., Rollis D., Klaver J.M., Gandhi S.K., Charney D.S., Manji H.K., Drevets W.C. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C] DASB.; Comparison with bipolar disorder. Biol. Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Bernard R., Veh R.W. Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J. Comp. Neurol. 2012;520:2545–2558. doi: 10.1002/cne.23080. [DOI] [PubMed] [Google Scholar]
- 50.Quina L.A., Tempest L., Ng L., Harris J.A., Ferguson S., Jhou T.C., Turner E.E. Efferent pathways of the mouse lateral habenula. J. Comp. Neurol. 2015;523:32–60. doi: 10.1002/cne.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollak Dorocic I., Fürth D., Xuan Y., Johansson Y., Pozzi L., Silberberg G., Carlén M., Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Weissbourd B., Ren J., DeLoach K.E., Guenthner C.J., Miyamichi K., Luo L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron. 2014;83:645–662. doi: 10.1016/j.neuron.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vertes R.P., Fortin W.J., Crane A.M. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. doi: 10.1002/(SICI)1096-9861(19990517)407:4<555::AID-CNE7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 54.Vertes R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 55.Morin L.P., Meyer-Bernstein E.L. The ascending serotonergic system in the hamster: Comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/S0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- 56.Muzerelle A., Scotto-Lomassese S., Bernard J.F., Soiza-Reilly M., Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct. Funct. 2016;221:535–561. doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiyasova V., Fernandez S.P., Laine J., Stankovski L., Muzerelle A., Doly S., Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu R., Zuo W., Shiwalkar N., Mei Q., Fan Q., Chen X., Li J., Bekker A., Ye J.H. Alcohol withdrawal drives depressive behaviors by activating neurons in the rostromedial tegmental nucleus. Neuropsychopharmacology. 2019;44:1464–1475. doi: 10.1038/s41386-019-0378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geisler S., Andres K.H., Veh R.W. Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. J. Comp. Neurol. 2003;458:78–97. doi: 10.1002/cne.10566. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Hernández V.S., Vázquez-Juárez E., Chay F.K., Barrio R.A. Thirst is associated with suppression of habenula output and active stress coping: Is there a role for a non-canonical vasopressin-glutamate pathway? Front. Neural Circuits. 2016;10:13. doi: 10.3389/fncir.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tchenio A., Valentinova K., Mameli M. Can the lateral habenula crack the serotonin code? Front. Synaptic Neurosci. 2016;8:34. doi: 10.3389/fnsyn.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aizawa H., Kobayashi M., Tanaka S., Fukai T., Okamoto H. Molecular characterization of the subnuclei in rat habenula. J. Comp. Neurol. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 63.Aizawa H., Bianco I.H., Hamaoka T., Miyashita T., Uemura O., Concha M.L., Russell C., Wilson S.W., Okamoto H. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr. Biol. 2005;15:238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji H., Shepard P.D. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J. Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian J., Uchida N. Habenula lesions reveal that multiple mechanisms underlie dopamine prediction errors. Neuron. 2015;87:1304–1316. doi: 10.1016/j.neuron.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L., Liu M.-Z., Li Q., Deng J., Mu D., Sun Y.-G. Organization of functional long-range circuits controlling the activity of serotonergic neurons in the dorsal raphe nucleus. Cell Rep. 2017;18:3018–3032. doi: 10.1016/j.celrep.2017.02.077. [DOI] [PubMed] [Google Scholar]
- 67.Kang S., Li J., Zuo W., Fu R., Gregor D., Krnjevic K., Bekker A., Ye J.-H. Ethanol withdrawal drives anxiety-related behaviors by reducing m-type potassium channel activity in the lateral habenula. Neuropsychopharmacology. 2017;42:1813–1824. doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonçalves L., Sego C., Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. J. Comp. Neurol. 2012;520:1278–1300. doi: 10.1002/cne.22787. [DOI] [PubMed] [Google Scholar]
- 69.Sego C., Gonçalves L., Lima L., Furigo I.C., Donato J., Jr., Metzger M. Lateral habenula and the rostromedial tegmental nucleus innervate neurochemically distinct subdivisions of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 2014;522:1454–1484. doi: 10.1002/cne.23533. [DOI] [PubMed] [Google Scholar]
- 70.Sun Y., Cao J., Xu C., Liu X., Wang Z., Zhao H. Rostromedial tegmental nucleus-substantia nigra pars compacta circuit mediates aversive and despair behavior in mice. Exp. Neurol. 2020;333:113433. doi: 10.1016/j.expneurol.2020.113433. [DOI] [PubMed] [Google Scholar]
- 71.Kang S., Li J., Bekker A., Ye J.H. Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology. 2018;129:47–56. doi: 10.1016/j.neuropharm.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo W., Fu R., Hopf F.W., Xie G., Krnjević K., Li J., Ye J.H. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict. Biol. 2017;22:103–116. doi: 10.1111/adb.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris J., Smith K., Cowen P., Friston K., Dolan R.J. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 74.Shumake J., Edwards E., Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/S0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 75.Li K., Zhou T., Liao L., Yang Z., Wong C., Henn F., Malinow R., Yates J.R., III, Hu H. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawson R.P., Drevets W.C., Roiser J.P. Defining the habenula in human neuroimaging studies. Neuroimage. 2013;64:722–727. doi: 10.1016/j.neuroimage.2012.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang S., Li J., Zuo W., Chen P., Gregor D., Fu R., Han X., Bekker A., Ye J.H. Downregulation of M-channels in lateral habenula mediates hyperalgesia during alcohol withdrawal in rats. Sci. Rep. 2019;9:2714. doi: 10.1038/s41598-018-38393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang L.-M., Hu B., Xia Y.-H., Zhang B.-L., Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav. Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 79.Meng H., Wang Y., Huang M., Lin W., Wang S., Zhang B. Chronic deep brain stimulation of the lateral habenula nucleus in a rat model of depression. Brain Res. 2011;1422:32–38. doi: 10.1016/j.brainres.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 80.Winter C., Vollmayr B., Djodari-Irani A., Klein J., Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav. Brain Res. 2011;216:463–465. doi: 10.1016/j.bbr.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 81.Shabel S.J., Wang C., Monk B., Aronson S., Malinow R. Stress transforms lateral habenula reward responses into punishment signals. Proc. Natl. Acad. Sci. USA. 2019;116:12488–12493. doi: 10.1073/pnas.1903334116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wirtshafter D., Asin K.E., Pitzer M.R. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 83.Park H., Rhee J., Park K., Han J.-S., Malinow R., Chung C. Exposure to stressors facilitates long-term synaptic potentiation in the lateral habenula. J. Neurosci. 2017;37:6021–6030. doi: 10.1523/JNEUROSCI.2281-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gregor D.M., Zuo W., Fu R., Bekker A., Ye J.H. Elevation of transient receptor potential vanilloid 1 function in the lateral habenula mediates aversive behaviors in alcohol-withdrawn rats. Anesthesiology. 2019;130:592–608. doi: 10.1097/ALN.0000000000002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volkow N.D., Fowler J.S., Wang G.J., Swanson J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 86.Volkow N.D., Fowler J.S., Wang G.J., Baler R., Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol. Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 88.Jhou T.C., Good C.H., Rowley C.S., Xu S.P., Wang H., Burnham N.W., Hoffman A.F., Lupica C.R., Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J. Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nowak K.L., McBride W.J., Lumeng L., Li T.K., Murphy J.M. Involvement of dopamine D2 autoreceptors in the ventral tegmental area on alcohol and saccharin intake of the alcohol-preferring P rat. Alcohol Clin. Exp. Res. 2000;24:476–483. doi: 10.1111/j.1530-0277.2000.tb02014.x. [DOI] [PubMed] [Google Scholar]
- 90.Gonzales R.A., Job M.O., Doyon W.M. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol. Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diana M., Pistis M., Muntoni A., Rossetti Z.L., Gessa G. Marked decrease of A10 dopamine neuronal firing during ethanol withdrawal syndrome in rats. Eur. J. Pharmacol. 1992;221:403–404. doi: 10.1016/0014-2999(92)90734-L. [DOI] [PubMed] [Google Scholar]
- 93.Diana M., Pistis M., Carboni S., Gessa G.L., Rossetti Z.L. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: Electrophysiological and biochemical evidence. Proc. Natl. Acad. Sci. USA. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen R.Y. Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J. Pharm. Exp. 2003;307:566–572. doi: 10.1124/jpet.103.053371. [DOI] [PubMed] [Google Scholar]
- 95.Weiss F., Parsons L.H., Schulteis G., Hyytiä P., Lorang M.T., Bloom F.E., Koob G.F. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barak S., Carnicella S., Yowell Q.V., Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: Implications for alcohol reward and seeking. J. Neurosci. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szczypiński J.J., Gola M. Dopamine dysregulation hypothesis: The common basis for motivational anhedonia in major depressive disorder and schizophrenia? Rev. Neurosci. 2018;29:727–744. doi: 10.1515/revneuro-2017-0091. [DOI] [PubMed] [Google Scholar]
- 98.Belujon P., Grace A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20:1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hudmon A., Schulman H. Structure–function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/bj20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 101.Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II—Discovery, progress in a quarter of a century, and perspective: Implication for learning and memory. Biol. Pharm. Bull. 2005;28:1342–1354. doi: 10.1248/bpb.28.1342. [DOI] [PubMed] [Google Scholar]
- 102.Swulius M., Waxham M. Ca2+/Calmodulin-dependent Protein Kinases. Cell. Mol. Life Sci. 2008;65:2637–2657. doi: 10.1007/s00018-008-8086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zalcman G., Federman N., Romano A. CaMKII isoforms in learning and memory: Localization and function. Front. Mol. Neurosci. 2018;11:445. doi: 10.3389/fnmol.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Griffith L.C. Calcium/calmodulin-dependent protein kinase II: An unforgettable kinase. J. Neurosci. 2004;24:8391–8393. doi: 10.1523/JNEUROSCI.2888-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soderling T.R., Stull J.T. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem. Rev. 2001;101:2341–2352. doi: 10.1021/cr0002386. [DOI] [PubMed] [Google Scholar]
- 106.Colbran R.J. Protein phosphatases and calcium/calmodulin-dependent protein kinase ii-dependent synaptic plasticity. J. Neurosci. 2004;24:8404–8409. doi: 10.1523/JNEUROSCI.3602-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Braun A.P., Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annu. Rev. Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 108.Colbran R.J., Brown A.M. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Coultrap S.J., Buard I., Kulbe J.R., Dell’Acqua M.L., Bayer K.U. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J. Biol. Chem. 2010;285:17930–17937. doi: 10.1074/jbc.M109.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cannady R., Fisher K.R., Graham C., Crayle J., Besheer J., Hodge C.W. Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. Addict. Biol. 2017;22:652–664. doi: 10.1111/adb.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Easton A.C., Lucchesi W., Lourdusamy A., Lenz B., Solati J., Golub Y., Lewczuk P., Fernandes C., Desrivieres S., Dawirs R.R., et al. αCaMKII autophosphorylation controls the establishment of alcohol drinking behavior. Neuropsychopharmacology. 2013;38:1636–1647. doi: 10.1038/npp.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salling M.C., Hodge C.J., Psilos K.E., Eastman V.R., Faccidomo S.P., Hodge C.W. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased CaMKII T286 phosphorylation in the reward pathway of mice. Pharm. Biochem. Behav. 2017;163:20–29. doi: 10.1016/j.pbb.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J., Zuo W., Fu R., Xie G., Kaur A., Bekker A., Ye J.H. High Frequency electrical stimulation of lateral habenula reduces voluntary ethanol consumption in rats. Int. J. Neuropsychopharmacol. 2016;19:10. doi: 10.1093/ijnp/pyw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Easton A.C., Lucchesi W., Mizuno K., Fernandes C., Schumann G., Giese K.P., Muller C.P. alphaCaMKII autophosphorylation controls the establishment of alcohol-induced conditioned place preference in mice. Behav. Brain. Res. 2013;252:72–76. doi: 10.1016/j.bbr.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 115.Schopf I., Easton A.C., Solati J., Golub Y., Kornhuber J., Giese K.P., Muller C.P. alphaCaMKII autophosphorylation mediates neuronal activation in the hippocampal dentate gyrus after alcohol and cocaine in mice. Neurosci. Lett. 2015;591:65–68. doi: 10.1016/j.neulet.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 116.Faccidomo S., Reid G.T., Agoglia A.E., Ademola S.A., Hodge C.W. CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav. Brain Res. 2016;298:286–290. doi: 10.1016/j.bbr.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Natividad L.A., Steinman M.Q., Laredo S.A., Irimia C., Polis I.Y., Lintz R., Buczynski M.W., Martin-Fardon R., Roberto M., Parsons L.H. Phosphorylation of calcium/calmodulin-dependent protein kinase II in the rat dorsal medial prefrontal cortex is associated with alcohol-induced cognitive inflexibility. Addict. Biol. 2018;23:1117–1129. doi: 10.1111/adb.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Paiva Lima C., da Silva E.S.D.A., Damasceno S., Ribeiro A.F., Rocha C.S., Berenguer de Matos A.H., Correia D., Boerngen-Lacerda R., Brunialti Godard A.L. Loss of control over the ethanol consumption: Differential transcriptional regulation in prefrontal cortex. J. Neurogenet. 2017;31:170–177. doi: 10.1080/01677063.2017.1349121. [DOI] [PubMed] [Google Scholar]
- 119.Yuanyuan J., Junyan Z., Cuola D., Jingjing C., Yuhui S., Dan X., Wei D., Yongsheng Z. Memantine attenuated alcohol withdrawal-induced anxiety-like behaviors through down-regulating NR1-CaMKII-ERK signaling pathway. Neurosci. Lett. 2018;686:133–139. doi: 10.1016/j.neulet.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 120.Somkuwar S.S., Mandyam C.D. Individual differences in ethanol drinking and seeking behaviors in rats exposed to chronic intermittent ethanol vapor exposure is associated with altered CaMKII autophosphorylation in the nucleus accumbens shell. Brain Sci. 2019;9:367. doi: 10.3390/brainsci9120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao B., Wang Y., Li Y., Qiao X., Yan P., Zhu Y., Lai J. Differential phosphorylation of NMDAR1-CaMKII-MAPKs in the rat nucleus accumbens following chronic ethanol exposure. Neurosci. Lett. 2015;597:60–65. doi: 10.1016/j.neulet.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 122.Lee K.M., Coelho M.A., McGregor H.A., Solton N.R., Cohen M., Szumlinski K.K. adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front. Cell Neurosci. 2016;10:265. doi: 10.3389/fncel.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kash T.L., Baucum A.J., Conrad K.L., Colbran R.J., Winder D.G. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zuo W., Wu L., Mei Q., Zuo Q., Zhou Z., Fu R., Li W., Wu W., Matthew L., Ye J.H. Adaptation in 5-HT(2) receptors-CaMKII signaling in lateral habenula underlies increased nociceptive-sensitivity in ethanol-withdrawn rats. Neuropharmacology. 2019;158:107747. doi: 10.1016/j.neuropharm.2019.107747. [DOI] [PubMed] [Google Scholar]
- 125.Salling M.C., Faccidomo S.P., Li C., Psilos K., Galunas C., Spanos M., Agoglia A.E., Kash T.L., Hodge C.W. Moderate alcohol drinking and the amygdala proteome: Identification and validation of calcium/calmodulin dependent kinase II and AMPA receptor activity as novel molecular mechanisms of the positive reinforcing effects of alcohol. Biol. Psychiatry. 2016;79:430–442. doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Agoglia A.E., Holstein S.E., Reid G., Hodge C.W. CaMKIIalpha-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcohol Clin. Exp. Res. 2015;39:1680–1690. doi: 10.1111/acer.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christian D.T., Alexander N.J., Diaz M.R., Robinson S., McCool B.A. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y., Cui H., Wang W., Zhao B., Lai J. The region-specific activation of Ca2+/calmodulin dependent protein kinase II and extracellular signal-regulated kinases in hippocampus following chronic alcohol exposure. Brain Res. Bull. 2012;89:191–196. doi: 10.1016/j.brainresbull.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 129.Mahadev K., Chetty C.S., Vemuri M.C. Effect of prenatal and postnatal ethanol exposure on Ca2+ /calmodulin-dependent protein kinase II in rat cerebral cortex. Alcohol. 2001;23:183–188. doi: 10.1016/S0741-8329(01)00133-1. [DOI] [PubMed] [Google Scholar]
- 130.Fu R., Gregor D., Peng Z., Li J., Bekker A., Ye J. Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2015;7:136–144. [PMC free article] [PubMed] [Google Scholar]
- 131.Li J., Li Y., Zhang B., Shen X., Zhao H. Why depression and pain often coexist and mutually reinforce: Role of the lateral habenula. Exp. Neurol. 2016;284:106–113. doi: 10.1016/j.expneurol.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 132.Li Y., Wang Y., Xuan C., Li Y., Piao L., Li J., Zhao H. Role of the lateral habenula in pain-associated depression. Front. Behav. Neurosci. 2017;11:31. doi: 10.3389/fnbeh.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sheng J., Liu S., Wang Y., Cui R., Zhang X. The link between depression and chronic pain: Neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi: 10.1155/2017/9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Delicata F., Bombardi C., Pierucci M., Di Maio R., De Deurwaerdère P., Di Giovanni G. Preferential modulation of the lateral habenula activity by serotonin-2A rather than -2C receptors: Electrophysiological and neuroanatomical evidence. CNS Neurosci. Ther. 2018;24:721–733. doi: 10.1111/cns.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo Y., Zhang L., Zhang J., Du C.-X., Lv S.-X., Wang T., Wang H.-S., Xie W., Liu J. Activation and blockade of serotonin4 receptors in the lateral habenula improve working memory in unilateral 6-hydroxydopamine-lesioned Parkinson’s rats. Neurol. Res. 2019;41:585–593. doi: 10.1080/01616412.2019.1596055. [DOI] [PubMed] [Google Scholar]
- 136.Shabel S.J., Proulx C.D., Trias A., Murphy R.T., Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie G., Zuo W., Wu L., Li W., Wu W., Bekker A., Ye J.-H. Serotonin modulates glutamatergic transmission to neurons in the lateral habenula. Sci. Rep. 2016;6:23798. doi: 10.1038/srep23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang J., Carnicella S., Phamluong K., Jeanblanc J., Ronesi J.A., Chaudhri N., Janak P.H., Lovinger D.M., Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: Implications for alcohol drinking behavior. J. Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoffman P.L., Tabakoff B. The role of the NMDA receptor in ethanol withdrawal. Exs. 1994;71:61–70. doi: 10.1007/978-3-0348-7330-7_7. [DOI] [PubMed] [Google Scholar]
- 140.Mirshahi T., Woodward J.J. Ethanol sensitivity of heteromeric NMDA receptors: Effects of subunit assembly, glycine and NMDAR1 Mg2+-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-L. [DOI] [PubMed] [Google Scholar]
- 141.Chu B., Anantharam V., Treistman S.N. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J. Neurochem. 1995;65:140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- 142.Lovinger D.M., White G., Weight F.F. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 143.Lovinger D.M., White G., Weight F.F. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J. Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kalluri H.S., Ticku M.K. Effect of ethanol on phosphorylation of the NMDAR2B subunit in mouse cortical neurons. Mol. Brain Res. 1999;68:159–168. doi: 10.1016/S0169-328X(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 145.Kash T.L., Matthews R.T., Winder D.G. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2008;33:1379–1390. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kalluri H.S., Mehta A.K., Ticku M.K. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res. Mol. Brain Res. 1998;58:221–224. doi: 10.1016/S0169-328X(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 147.Carpenter-Hyland E.P., Chandler L.J. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur. J. Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- 148.Chandrasekar R. Alcohol and NMDA receptor: Current research and future direction. Front. Mol. Neurosci. 2013;6:14. doi: 10.3389/fnmol.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hell J.W. CaMKII: Claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fukunaga K., Stoppini L., Miyamoto E., Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- 151.Kasahara J., Fukunaga K., Miyamoto E. Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J. Biol. Chem. 2001;276:24044–24050. doi: 10.1074/jbc.M100247200. [DOI] [PubMed] [Google Scholar]
- 152.Fukunaga K., Muller D., Miyamoto E. Increased phosphorylation of Ca2+/calmodulin-dependent protein kinase II and its endogenous substrates in the induction of long-term potentiation. J. Biol. Chem. 1995;270:6119–6124. doi: 10.1074/jbc.270.11.6119. [DOI] [PubMed] [Google Scholar]
- 153.Fukunaga K., Muller D., Miyamoto E. CaM kinase II in long-term potentiation. Neurochem. Int. 1996;28:343–358. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 154.Bredt D.S., Nicoll R.A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/S0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 155.Kryger R., Wilce P. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167:361–371. doi: 10.1016/j.neuroscience.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 156.Wang J., Hamida S.B., Darcq E., Zhu W., Gibb S.L., Lanfranco M.F., Carnicella S., Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: Implications for alcohol drinking behavior. J. Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meye F.J., Lecca S., Valentinova K., Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Front. Hum. Neurosci. 2013;7:860. doi: 10.3389/fnhum.2013.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rao V.R., Finkbeiner S. NMDA and AMPA receptors: Old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 159.Cannady R., Fisher K.R., Durant B., Besheer J., Hodge C.W. Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict. Biol. 2013;18:54–65. doi: 10.1111/adb.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mao L.M., Guo M.L., Jin D.Z., Fibuch E.E., Choe E.S., Wang J.Q. Post-translational modification biology of glutamate receptors and drug addiction. Front. Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han X., Bian E., Li J., Zuo W., Mei Q., Fu R., Shiwalkar N., Fan Q., Gajewski M., Ye J.-H. Pain, anxiety- and depression-like behaviors in alcohol-preferring and -non-preferring rats. Neurol. Neurobiol. 2020;3:1–8. [Google Scholar]
- 162.Wang J., Li M., Wang P., Zha Y., He Z., Li Z. Inhibition of the lateral habenular CaMKII abolishes naloxone-precipitated conditioned place aversion in morphine-dependent mice. Neurosci. Lett. 2017;653:64–70. doi: 10.1016/j.neulet.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 163.Shah A., Zuo W., Kang S., Li J., Fu R., Zhang H., Bekker A., Ye J.H. The lateral habenula and alcohol: Role of glutamate and M-type potassium channels. Pharm. Biochem. Behav. 2017;162:94–102. doi: 10.1016/j.pbb.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 164.Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 165.Regier D.A., Farmer M.E., Rae D.S., Locke B.Z., Keith S.J., Judd L.L., Goodwin F.K. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264:2511–2518. doi: 10.1001/jama.1990.03450190043026. [DOI] [PubMed] [Google Scholar]
- 166.Kessler R.C., Nelson C.B., McGonagle K.A., Edlund M.J., Frank R.G., Leaf P.J. The epidemiology of co-occurring addictive and mental disorders: Implications for prevention and service utilization. Am. J. Orthopsychiatry. 1996;66:17–31. doi: 10.1037/h0080151. [DOI] [PubMed] [Google Scholar]
- 167.Grant B.F., Harford T.C. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 168.Vaillant G.E. Is alcoholism more often the cause or the result of depression? Harv Rev. Psychiatry. 1993;1:94–99. doi: 10.3109/10673229309017064. [DOI] [PubMed] [Google Scholar]
- 169.Pettinati H.M. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol. Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 170.Davis L., Uezato A., Newell J.M., Frazier E. Major depression and comorbid substance use disorders. Curr. Opin. Psychiatry. 2008;21:14–18. doi: 10.1097/YCO.0b013e3282f32408. [DOI] [PubMed] [Google Scholar]
- 171.Hasin D.S., Stinson F.S., Ogburn E., Grant B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 172.Das R.K., Gale G., Walsh K., Hennessy V.E., Iskandar G., Mordecai L.A., Brandner B., Kindt M., Curran H.V., Kamboj S.K. Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nat. Commun. 2019;10:5187. doi: 10.1038/s41467-019-13162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Getachew B., Tizabi Y. Both ketamine and NBQX attenuate alcohol-withdrawal induced depression in male rats. J. Drug Alcohol Res. 2019;8:236069. doi: 10.4303/jdar/236069. [DOI] [PMC free article] [PubMed] [Google Scholar]