Abstract
Genetic variants contribute to the risk of chronic pancreatitis (CP) in adults and children. The risk variant CEL-HYB1, a recombinant hybrid allele of CEL and its neighboring pseudogene (CELP), encodes a pathogenic variant of the pancreatic digestive enzyme carboxyl ester lipase (CEL). We previously identified combinations of two non-synonymous SNPs, c.1463T>C (p. Ile488Thr) and c.1643C>T (p. Thr548Ile), in the break point region of CEL-HYB1. Herein, we tested whether these missense variants alter CP risk and their impact on functional properties of the CEL-HYB1 protein. Examination of CEL-HYB1 haplotypes in European patients and controls revealed that the combinationThr488-Ile548 was present only in cases (p ≤ .001). The lipase activity of purified recombinant CEL-HYB1 variants showed normal or near normal activity. CEL-HYB variants expressed in HEK293T cells all had decreased secretion compared with CEL, formed intracellular protein aggregates, and triggered endoplasmic reticulum stress. Thus, we propose that the presence of missense variants in CEL-HYB increases the pathogenicity of CEL-HYB1 through misfolding and gain-of-function proteotoxicity. Interestingly, Thr488-Ile548 and Thr488-Thr548 were equally pathogenic in the functional assays even though only the Thr488-Ile548 haplotype was significantly enriched in cases. The explanation for the mismatch between genetic and functional data requires further investigation.
Keywords: chronic pancreatitis, genetic variants, lipase, protein misfolding, single nucleotide polymorphisms
1 |. INTRODUCTION
Chronic pancreatitis (CP) results from the progressive destruction of the pancreas by continuing or relapsing inflammation. Multiple studies have demonstrated that variants in genes expressed in pancreatic exocrine cells contribute to the risk of developing CP in 50% of adults and 75% of children (Kumar et al., 2016; Yadav, O’Connell, & Papachristou, 2012). The earliest identified genetic risk factors for CP either resided in components of the protease and antiprotease systems of the exocrine pancreas or perturbed these functions indirectly (Hegyi & Sahin-Toth, 2017). The recent observations that variants of two pancreatic lipase genes, pancreatic triglyceride lipase and carboxyl ester lipase (CEL), associate with increased risk for CP provide a unique opportunity to study the pathophysiology of CP apart from protease-dependent pathways (Behar et al., 2014; Fjeld et al., 2015; Lasher et al., 2019; Szabo et al., 2015).
CEL (EC 3.1.1.13) is expressed in the pancreas and the lactating mammary gland (Hui & Howles, 2002; Johansson et al., 2018). CEL is one of the most abundant proteins in pancreatic acinar cells (Danielsson et al., 2014; Lugea et al., 2017). The protein has two domains, an N-terminal globular domain and a heavily O-glycosylated C-terminal domain with a variable number of proline-rich tandem repeats (VNTR; El Jellas et al., 2018; Moore et al., 2001). The number of VNTR repeats varies in humans from 3 to 23 repeats with 16 repeats being the most common (Dalva et al., 2017; Fjeld et al., 2016). The human CEL gene and a tandemly arranged pseudogene, CELP, reside on chromosome 9q34.13. CELP lacks exons 2–7 of CEL, contains a stop codon in its second exon and has a short VNTR (Kumar et al., 1992; Lidberg et al., 1992; Madeyski, Lidberg, Bjursell, & Nilsson, 1998).
One-base pair deletions in the CEL VNTR cause an autosomal dominant syndrome of pancreatic exocrine dysfunction and diabetes classified as maturity-onset diabetes of the young, Type 8 (MODY8; MIM# 609812; Raeder et al., 2006; Raeder et al., 2014). Subsequently, a copy number variant of CEL was found to be five-fold more frequent in Northern Europeans with idiopathic CP than in controls (Fjeld et al., 2015). This risk allele likely resulted from a recombination event between CEL and CELP that joined the first 10 exons of CEL with exon 11 of CELP, producing a hybrid variant of CEL (CEL-HYB1) with only three VNTR repeats (Fjeld et al., 2015). Expression of the CEL-HYB1 protein in cultured cells revealed decreased secretion and intracellular accumulation of the fusion protein compared with normal CEL (Dalva et al., 2020; Fjeld et al., 2015). However, protein misfolding and activation of ER stress were not directly assessed.
Two follow-up reports tested for an association of the CEL-HYB1 allele with CP in different populations. The first study screened for CEL-HYB1 in three Asian populations and did not identify the allele in any subjects (Zou et al., 2016). Instead, an alternative hybrid allele, CEL-HYB2, was found. CEL-HYB2 did not associate with CP most likely because a premature stop codon in this allele prevents protein expression. The second study screened Polish children with CP and healthy controls (Oracz et al., 2019). Although more cases (4.8%) than controls (2.4%) carried the CEL-HYB1 allele, the difference was not statistically significant. Notably, the 2.4% detection rate in controls was considerably higher than in German (0.7–1.0%), French (0.7%), and Norwegian (0.5%) populations (Fjeld et al., 2015). Taken together, the observations suggest that other genetic or environmental factors interact with the CEL-HYB1 allele to increase the risk for CP.
Mapping of the breakpoint region of CEL-HYB1 may provide a clue to a possible modifying factor (Fjeld et al., 2015). Two non-synonymous single nucleotide polymorphisms (SNPs) are present in the CEL-CELP junction, rs77696629 and rs750991274, corresponding to missense mutations, c.1463T>C (p. Ile488Thr) and c.1643C>T (p. Thr548Ile), respectively. Herein, we propose that these SNPs may explain the varying susceptibility to CP in subjects carrying the CEL-HYB1 allele. We test this hypothesis by analyzing whether the two amino acid substitutions influence secretion and misfolding of the CEL-HYB1 protein (hereafter referred to as CEL-HYB).
2 |. MATERIALS AND METHODS
2.1 |. Study population and SNPs
We re-evaluated DNA sequence data from previously reported subjects carrying the CEL-HYB allele (Fjeld et al., 2015). These samples were CP patients and controls from Germany, France, and Norway. The allele distribution of rs77696629 and rs750991274 was assessed. SNP rs750991274 has only recently been named. It corresponds to the most distal C/T variant in the CEL-HYB exon 10-exon 11 junction as depicted in Figure S2 of Fjeld et al. (2015).
2.2 |. CEL variant plasmid constructs
The full-length human CEL complementary DNA (cDNA; clone ID 5187959; BC042510.1) was purchased from OpenBiosystems. The cDNA codes for a 14-repeat VNTR CEL protein, and site-directed mutagenesis was performed to correct a three-base pair in-frame deletion resulting in p. Glu365del (Xiao et al., 2016, 2017). The EcoRI/XhoI fragment containing the entire CEL cDNA coding region was subcloned into yeast (pPICZA) and mammalian (pcDNA3) protein expression vectors (Xiao et al., 2016). The XmnI/XhoI cDNA fragment containing a 16-repeat VNTR was synthesized by Genscript. The synthetic DNA fragment was used to replace the XmnI/XhoI part in pPICZA/CEL-14R to create pPICZA/CEL-16R. The EcoRI/XhoI fragment encoding CEL-16R was excised from pPICZA/CEL-16R and subcloned into pcDNA3 to create pcDNA3/CEL-16R. The XmnI/XhoI cDNA containing CEL-HYB sequence was generated by overlapping PCR. pPICZA/CEL-HYB and pcDNA3/CEL-HYB were generated following the same strategy described above for that of CEL-16R. The desired SNP variants were introduced by site-directed mutagenesis using customized primers. The constructs included Ile488-Thr548, Thr488-Ile548, Ile488-Thr548, and Ile488-Ile548 in both CEL-HYB and CEL-16R backbones (Figure 1). All final DNA constructs were confirmed by Sanger sequencing.
FIGURE 1.
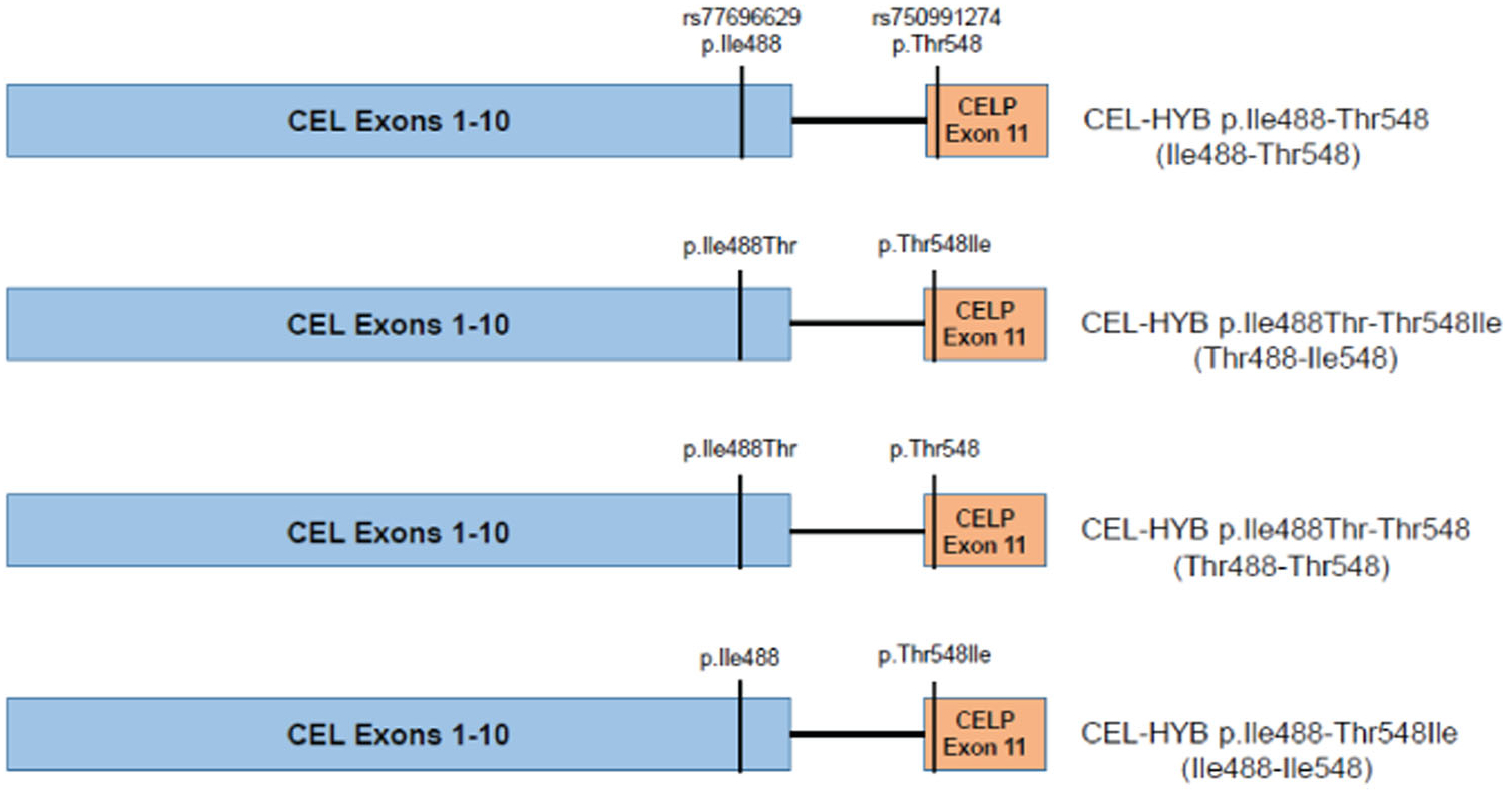
Schematic drawing of the four hybrid variant of carboxyl ester lipase (CEL-HYB) haplotypes. The portion of CEL-HYB encoded by exons 1–10 of carboxyl ester lipase (CEL) is depicted in light blue. The portion encoded by exon 11 of CELP is depicted in orange. The location of rs77696629 and rs750991274 is marked by vertical lines. The amino acid at each site is listed above the vertical line using the three letter code. Throughout the manuscript the haplotypes will be referred to by the abbreviated nomenclature given in parenthesis in the figure, that is, Ile488-Thr548
2.3 |. Production, purification, and characterization of CEL variant proteins
P. pastoris GS115 yeast cells were transformed to produce five recombinant human CEL protein variants as secretory proteins under methanol induction. They were CEL-16R as the CEL wild type reference protein and the four CEL-HYB variants, Ile488-Thr548, Thr488-Ile548, Thr488-Thr548 and Ile488-Ile548. The detailed procedure for CEL recombinant protein expression, production, and purification was described previously (Xiao, Mukherjee, Ross, & Lowe, 2011). CEL variant proteins were purified by HiTrap Heparin HP Affinity Column (GE Healthcare Life Sciences) controlled by an ÄKTA pure protein purification system. Five micrograms of each purified protein was resolved by 4–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with GelCode Blue stain reagent (Pierce) to assess molecular mass, homogeneity, and integrity. Functional characterization of recombinant CEL variant proteins was carried out by measuring lipase activity in the presence of 12 mM sodium cholate using a pH-stat (Radiometer Analytical) at pH 8.0. For each assay, 5 μg of purified CEL protein was added to emulsions of varying chain-length triglycerides: tributyrate, trioctanoate, and triolein (Xiao et al., 2011). The lipolytic activities were expressed in international lipase units per mg of enzyme (1 unit corresponds to 1 μmol of fatty acid released per min). The functional activity was expressed as relative specific activity after being normalized by the molecular mass of each CEL variant.
2.4 |. Cell culture and transfection of HEK293T cells
HEK293T cells were purchased from American Type Culture Collection and maintained at 37°C at 5% CO2 in a humidified incubator. Cells were routinely maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 100 units/ml penicillin and streptomycin (Gibco). Total, 1 × 106 cells in 2 ml medium per well were seeded in six-well plates. Plasmid CEL constructs and pcDNA3 empty vector were transiently transfected into HEK293T cells following the FuGENE-6 protocol (Promega; Xiao et al., 2016). Forty-eight hours posttransfection, conditioned media were collected and exchanged with 1.2 ml Reduced-Serum Opti-MEM media (Gibco). Twenty hours post-media exchange, culture media, and cells were harvested.
2.5 |. Collection of culture media and cells
Unless stated otherwise, the harvesting and processing of samples were carried out either on ice or at 4°C to minimize protein degradation. Conditioned or reduced serum media were centrifuged at 200g for 5 min to obtain cell-free media. The cells were washed off the plates using 2 ml ice-cold phosphate-buffered saline (PBS) and centrifuged at 200g for 5 min. The pelleted cells were re-suspended in 1.5 ml ice-cold PBS, followed by another round of centrifugation. Whole cell lysates were extracted using 500 μl radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) supplemented with ethylenediaminete-traacetic acid-free complete protease inhibitor mixture (Roche). Three rounds of brief sonication (5–10 s) were employed to ensure complete cell lysis. The resultant whole cell lysates were further processed into detergent soluble and insoluble fractions by centrifugation at 16,000g for 20 min. The supernatants were transferred into another 1.5-ml microcentrifuge tube and designated as soluble fractions. The pellets were washed twice with ice-cold PBS followed by centrifugation at 16,000g for 5 minutes. The final pellets were re-suspended in 150 μl of 1x sample Buffer (1:1 mixture of 2x SDS sample buffer [Bio-Rad] with RIPA) at room temperature. The resuspensions were sonicated 3–5 × 10 s until no visible particles were present. The resulting resuspensions were designated as insoluble fractions.
2.6 |. Lipase activity assay of medium samples
Lipase activity of conditioned media was measured with a pH-stat as described above. For each assay, 500 μl of medium sample was added to the emulsification mixture of 14.25 ml 12 mM sodium cholate and 250 μl trioctanoate. The activity was expressed as relative activity to either CEL-16R or CEL-HYB in an equal volume of culture medium.
2.7 |. Protein immunoblot
Aliquots of conditioned media, whole cell lysate, and detergent soluble and insoluble fractions were processed by mixing with 2x SDS Sample Buffer (Bio-Rad) supplemented with 2-mercaptoethanol, followed by boiling at 95°C for 5 min. Twenty micro liters of processed samples were resolved on 4–15% Mini-PROTEAN TGX pre-cast gels (Bio-Rad) and transferred onto Immobilon polyvinylidene difluoride FL membranes (Millipore). Blots were blocked in 10 ml Odyssey Blocking Buffer (LI-COR) for one hour, and then incubated with primary antibody for one hour at room temperature or at 4°C overnight. Afterwards, blots were incubated with a corresponding secondary antibody at room temperature for 30 min. Blots were imaged using Odyssey Infrared Imager, and densitometry of bands were assessed using ImageStudio (LI-COR).
Antibodies used in this study were as follows: a rabbit polyclonal antibody against human CEL generated in our lab (1:10,000; Xiao et al., 2016); a mouse monoclonal antibody against α-tubulin (1:5,000; 3873S) and a rabbit polyclonal antibody against BiP (1:1,000; 3183S) from Cell Signaling Technology; an IRDye 800 goat anti-rabbit IgG (926–32,221; 1:10,000) and an IRDye 680 goat anti-mouse IgG (926–68,070; 1:10,000) from LI-COR.
2.8 |. Data analysis
Two-tailed Fisher exact test was used to assess the difference in variant SNPs in subjects carrying the CEL-HYB allele. Data on lipase activity and protein abundance in western blot analysis were first analyzed by one-way analysis of variance, followed by Holms-Sidak post hoc pairwise multiple comparison. p < .05 was considered statistically significant. In the figures, bars with different letters (a, b, c, or d) have significant differences in means by Holms-Sidak post-hoc pairwise multiple comparison.
3 |. RESULTS
3.1 |. Presence of missense variants in CEL-HYB alleles
To explore whether genetic data might support a phenotypic effect of the two missense variants, we re-examined sequence information obtained during the initial identification of the CEL-HYB allele (Fjeld et al., 2015). We retrieved data for 75 sequenced CEL-HYB alleles; 55 from non-related subjects with CP and 20 from control subjects. Two non-synonymous SNPs were observed in the CEL-CELP recombination region, rs77696629 (p. Ile488Thr) and rs750991274 (p. Thr548Ile). The SNPs defined four haplotypes (Figure 1). Differences in frequencies of the SNP variants were assessed (Table 1). The C nucleotide of rs77696629 encoding p. Ile488Thr was found in almost all sequenced CEL-HYB alleles (carrier frequency = 0.96 among 75 subjects) with no significant difference between cases and controls (p = .172). Intriguingly, the T nucleotide of rs750991274 encoding p. Thr548Ile was only observed in cases (carrier frequency = 0.267 among 75 subjects), resulting in a significant distribution difference (p ≤ .001) between cases and controls.
TABLE 1.
Carrier frequencies for SNP variants present in subjects with the CEL-HYB allele
| Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|
| SNP | A1a | A2a | A2/total | A2 frequency | A2/total | A2 frequency | P valueb |
| rs77696629 (p. Ile488Thr)c | T | C | 54/55 | 0.982 | 18/20 | 0.900 | 0.172 |
| rs750991274 (p. Thr548Ile)c | C | T | 20/55 | 0.363 | 0/20 | 0.000 | <.001 |
Abbreviations: CEL-HYB, hybrid variant of carboxyl ester lipase; SNP, single nucleotide polymorphism.
A1 and A2 are the reference and alternative alleles, respectively.
Two-tailed Fisher exact test.
Three-letter amino acid code is used.
The skewed distribution of SNP variant frequencies was reflected in the haplotypes observed for cases and controls. Thus, only cases exhibited the Thr488-Ile548 haplotype, and the Ile488-Ile548 haplotype was not detected at all (Table 2). In summary, our analysis demonstrates that the vast majority of CEL-HYB alleles contain threonine at position 488. Moreover, the data strongly suggest that the CEL-HYB allele with both missense variants, Thr488-Ile548, increases the risk for CP above that of other CEL-HYB variants.
TABLE 2.
Haplotype counts in subjects with the CEL-HYB allele
3.2 |. Recombinant CEL-16R and CEL-HYB variants expressed in P. pastoris have similar lipase activity
To determine if the haplotype influences the lipase activity of CEL-HYB, we utilized P. pastoris to express CEL-16R and four haplotypes of CEL-HYB (Figure 1). Each protein was purified to near homo-geneity as verified by SDS-PAGE (Figure 2a). The amount of protein in the major bands was not significantly different as determined by densitometry based on three independent experiments.
FIGURE 2.

Lipase activity of CEL-16R and CEL-HYB variants expressed in P. pastoris and purified by column chromatography. (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel of purified CEL-16R and CEL-HYB variants stained with GelCode Blue. (b) Relative activity of the five variants against three different triglyceride substrates (n = 3). The specific activity (U/mg) was adjusted for the differences in molecular weights between CEL-16R and the CEL-HYB variants. By one-way analysis of variance (ANOVA), there is a statistically significant difference in the mean values for tributyrin activity (F(4,10) = 13.5, p ≤ .001), for trioctanoate activity (F(4,10) = 15.7, p ≤ .001), and for triolein activity by one-way (F(4,10) = 10.6, p = .001). Bars with different letters have significant differences in means by the Holms-Sidak post-hoc pairwise multiple comparison method. CEL-HYB, hybrid variant of carboxyl ester lipase
The specific activity of each lipase was then measured using three different substrates, tributyrate, trioctanoate, and triolein. CEL-16R, and CEL-HYB variants Ile488-Thr548, Thr488-Ile548, and Thr488-Thr548 had statistically indistinguishable activity against all three substrates (Figure 2b). CEL-HYB variant Ile488-Ile548 had significantly lower activity than the other four variants against each substrate except when compared with the activity of CEL-16R against triolein. Overall, the results showed that the variable amino acids of CEL-HYB had no major impact on lipase activity as residual activity exhibited by the Ile488-Ile548 variant was still 70–90% of CEL-16R’s activity.
3.3 |. Together the missense variants decrease secretion and increase misfolding of CEL-HYB
Since the missense variants had normal or near normal lipase activity, we next sought to determine if they could increase risk for CP through CEL-HYB protein misfolding. We first compared the secretion and intracellular location of the CEL-HYB haplotype found only in patients, Thr488-Ile548, to the properties of CEL-16R and CEL-HYB without the variants, Ile488-Thr548. Each construct was expressed in HEK293T cells, a cell line utilized in multiple studies of pancreatic zymogens including CEL (Fjeld et al., 2015; Hegyi & Sahin-Toth, 2019; Johansson et al., 2011; Lasher et al., 2019; Szabo et al., 2015; Torsvik et al., 2014; Xiao et al., 2016). Sixty-eight hours after transfection, aliquots of medium were analyzed by lipase activity and by protein immunoblot (Figure 3a,b). No lipase activity or protein was detected in cells transfected with empty vector (mock cells). Relative to the medium from cells expressing CEL-16R, the lipase activity in the medium was significantly lower for both CEL-HYB variants. Steady-state levels in the medium were 20% lower for Ile488-Thr548 (p = .003) and 75% lower for Thr488-Ile548 (p < .001; Figure 3a). The determination of CEL protein abundance in the medium mirrored the activity measurements (Figure 3b,c). Consistent with these findings, the intracellular amounts of the two CEL-HYB proteins were significantly greater than the amount of intracellular CEL-16R (p < .001 for both; Figure 3b,c). When we partitioned the intracellular proteins into detergent-soluble and detergent-insoluble fractions, essentially all of the intracellular CEL-16R was soluble whereas the majority of both CEL-HYB proteins partitioned into the insoluble fraction (p < .001) (Figure 3b,c). These results confirm that CEL-HYB has decreased secretion and increased intracellular aggregation compared with CEL-16R (Fjeld et al., 2015). Importantly, we found that the missense SNP variants decrease secretion significantly more than CEL-HYB without these variants.
FIGURE 3.

The short variable number of proline-rich tandem repeat (VNTR) and the missense variants decrease secretion and increase misfolding of CEL-HYB relative to CEL-16R. CEL-16R, and CEL-HYB variants Ile488-Thr548 and Thr488-Ile548 were expressed in HEK293T cells. (a) Lipase activity in the medium 68 h after transfection. CEL-16R (n = 4); Ile488-Thr548 (n = 3) and Thr488-Ile548 (n = 4). There is a statistically significant difference in the mean values of the different groups by one-way ANOVA (F(2,8) = 374, p ≤ .001). Bars with different letters have significant differences in means by the Holms-Sidak post-hoc pairwise multiple comparison method. 16R is significantly different from Thr488-Ile548 (p < .001) and Ile488-Thr548 (p = .003); CEL-HYB Ile488-Thr548 is significantly different from Thr488-Ile548 (p < .001). (b) Immunoblot of medium, total cells and detergent-soluble and -insoluble fractions. Green = anti-CEL; Red = anti-tubulin. (c) Estimation of CEL abundance by densitometry of immunoblots. CEL-16R (n = 4); Ile488-Thr548 (n = 3) and Thr488-Ile548 (n = 4). There is a statistically significant difference in the mean values of the different groups by one-way ANOVA for medium: (F(2,8) = 295, p < .001), whole cell: (F(2,9) = 26, p < .001), soluble: (F(2,9) = 166, p < .001), and insoluble: (F(2,9) = 221, p < .001). Bars with different letters have significant differences in means by the Holms-Sidak post hoc pairwise multiple comparison method. ANOVA, analysis of variance; CEL-HYB, hybrid variant of carboxyl ester lipase
3.4 |. Each missense variant individually decreases secretion and increases misfolding of CEL-HYB
To determine if one SNP had greater effect on protein folding than the other, we expressed CEL-HYB variants Thr488-Thr548 and Ile488-Ile548 in HEK293T cells and compared their secretion and aggregation with that of variants Ile488-Thr548 and Thr488-Ile548 (Figure 4). We determined steady-state secretion levels of each variant by lipase activity and immunoblot analysis. When measured by lipase activity, there was a graded decrease in secretion compared with Ile488-Thr548 of Ile488-Ile548 (67%, p < .001), Thr488-Thr548 (27%, p < .001), and Thr488-Ile548 (19%, p < .001; Figure 4a). The secretion of Thr488-Ile548 was also significantly lower than that of Thr488-Thr548 (p < .001) and Ile488-Ile548 (p < .001). Estimation of the abundance of each CEL-HYB variant in the medium by protein immunoblotting gave results that were qualitatively similar to the analysis by lipase activity, although in this case the difference between Thr488-Ile548 and Thr488-Thr548 was not statistically significant (Figure 4b,c). Analysis of extracts from the whole cell revealed no differences in the intracellular levels of the four CEL variants. In the detergent-soluble fraction, both Thr488-Ile548 (p = .006) and Thr488-Thr548 (p = .005) were present at lower levels than Ile488-Thr548. In the detergent-insoluble fractions, the amounts of aggregated Thr488-Thr548 (p = .005) and Ile488-Ile548 (p = .028) were significantly increased compared with Ile488-Thr548; the amounts of Thr488-Ile548 did not differ from the other three variants. Although both SNPs decrease secretion and increase aggregation of CEL-HYB, p. Ile488Thr appears to account for most of the detrimental effects on both processes when expressed in cultured cells.
FIGURE 4.
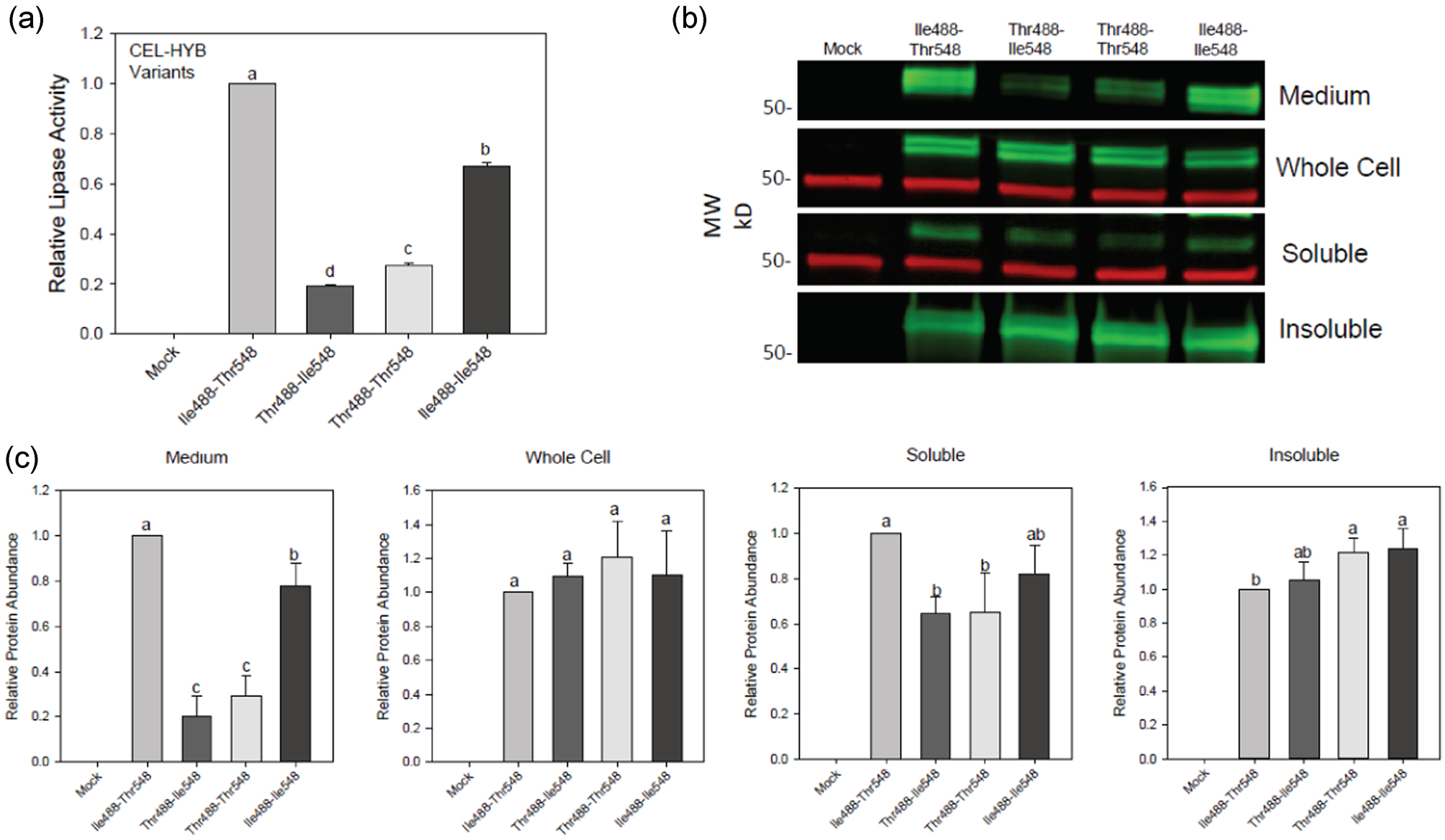
Role of p. Ile488Thr and p. Thr548Ile missense variants in the secretion and misfolding of CEL-HYB. CEL-HYB variants Ile488-Thr548, Thr488-Ile548, Thr488-Thr548 or Ile488-Ile548 were expressed in HEK293T cells. (a) Lipase activity in the medium 68 h after transfection (n = 3 for all variants). There is a statistically significant difference in the mean values of the different groups by one-way ANOVA (F(3,12) = 5665, p ≤ .001). Bars with different letters have significant differences in means by the Holms-Sidak post-hoc pairwise multiple comparison method. (b) Immunoblot of medium, total cells and detergent-soluble and -insoluble fractions. Green = anti-CEL; Red = anti-tubulin. (c) Estimation of CEL abundance by densitometry of immunoblots (n = 4 for all variants). By one-way ANOVA, there is no significant difference for the whole cell samples (F(3/12) = 0.94, p = .45), and there is a statistically significant difference in the mean values of the different groups for medium: (F(3,12) = 90, p < .001), soluble: (F(3,12) = 8.5, p = .003), and insoluble: (F(3,12) = 6.7, p = .007). Bars with different letters have significant differences in means by the Holms-Sidak post hoc pairwise multiple comparison method. ANOVA, analysis of variance; CEL-HYB, hybrid variant of carboxyl ester lipase
3.5 |. CEL-HYB missense variants decrease secretion and increase misfolding of CEL-16R
Because of the effect of the missense variants on CEL-HYB folding, we asked whether either variant also would affect the folding of the normal CEL-16R protein, which includes p. Ile488 and p. Thr548 in the reference sequence. To this end, we expressed CEL-16R and CEL variants Thr488-Ile548, Thr488-Thr548, and Ile488-Ile548- in HEK293T cells (Figure 5). The introduction of each missense variant alone or together into CEL-16R decreased secretion as determined by lipase activity (Figure 5a). Compared with the activity in medium containing CEL-16R, there was less lipase activity in the medium containing Ile488-Ile548 (68%, p < .001), Thr488-Thr548 (34%, p < .001) or Thr488-Ile548 (25%, p < .001). As found with the CEL-HYB variants, the secretion of Thr488-Ile548 was significantly lower than that of Thr488-Thr548 (p = .016) for the CEL-16R proteins. Determination of protein abundance in the medium by immunoblot confirmed the detrimental effects of the missense variants on secretion (Figure 5b,c). Total intracellular amounts of the CEL-16R variants were not statistically different from that of normal CEL-16R. After fractionation, the amount of Thr488-Ile548 and Thr488-Thr548 were significantly greater than CEL-16R in the soluble lysate (p = .017 and .03, respectively). Very little CEL 16R was present in the insoluble fraction for all four variants, and here the amount of Thr488-Ile548 and Thr488-Thr548 were significantly greater than CEL-16R (p = .014 and <.001, respectively). Thus, the missense variants of CEL-HYB have adverse effects on secretion and aggregation of CEL-16R with p. Ile488Thr accounting for most of the effect.
FIGURE 5.

The effect of p. Ile488Thr and p. Thr548Ile on the secretion and misfolding of CEL-16R expressed in HEK293T cells. CEL-16R and CEL variants Thr488-Ile548, Thr488-Thr548 or Ile488-Ile548 were expressed in HEK293T cells. (a) The relative lipase activity in the medium 68 h after transfection (n = 3). There is a statistically significant difference in the mean values of the different groups by one-way ANOVA (F(3,8) = 300, p < .001). Bars with different letters have significant differences in means by the Holms-Sidak post hoc pairwise multiple comparison method. (b) Immunoblot of expressed CEL variants in the medium, whole cell and the detergent soluble and insoluble intracellular fractions. Green = anti-CEL; Red = anti-tubulin. (c) Estimation of CEL abundance by densitometry of immunoblots (n = 3). By one-way ANOVA, there is a statistically significant difference in the mean values of the different groups for medium: (F(3,18) = 182, p < .001), whole cell: (F(3,8) = 4.3, p = 0.045), soluble: (F(3,8) = 2.7, p = .011), and insoluble: (F(3,8) = 49, p < .001). Bars with different letters have significant differences in means by the Holms-Sidak post hoc pairwise multiple comparison method. ANOVA, analysis of variance; CEL, carboxyl ester lipase
3.6 |. CEL-HYB missense variants trigger an ER stress response
Because we found that the CEL-HYB and CEL-16R missense variants misfold and form intracellular aggregates, we hypothesized that they would trigger the ER stress response. We tested this hypothesis by measuring the levels of BiP in HEK293T cells expressing each variant (Figure 6). Although cells expressing CEL-16R had higher levels of BiP than did cells transfected with an empty vector, the difference was not significant (p = .071). In contrast, BiP levels in cells expressing one of the four CEL-HYB constructs were significantly higher than the levels in the mock cells (p < .001) with Thr488-Ile548 and Thr488-Thr548 showing the highest levels of BiP. Also, cells expressing CEL-16R variants showed increased BiP levels (Figure 6c,d). BiP levels in cells expressing CEL-16R were not significantly increased compared with mock cells (p = .33) whereas cells expressing CEL Thr488-Ile548 and Thr488-Thr548 had the highest levels of BiP. Cells expressing CEL containing the Thr488-Ile548 or Thr488-Thr548 substitutions showed significantly higher levels of BiP than found in cells expressing CEL-16R. Taken together, these results show that the substitution of one or both missense variants into CEL-HYB or CEL-16R increases ER stress more than CEL-HYB or CEL-16R without the variants.
FIGURE 6.

The alternative amino acids, p. Ile488Thr and p. Thr548Ile upregulate ER stress when present in CEL-HYB or CEL-16R as evidenced by increased BiP. (a) Immunoblots of BiP levels in HEK293T cells transfected with empty vector (Mock) or vectors expressing CEL-16R or CEL-HYB variants Ile488-Thr548, Thr488-Ile548, Thr488-Thr548, or Ile488-Ile548. Green = anti-BiP; Red = anti-tubulin. (b) Estimation of BiP abundance by densitometry of immunoblots from (A; n = 4). There is a statistically significant difference in the mean values of the different groups by one-way ANOVA (F(5,26) = 24, p = < .001). Bars with different letters have significant differences in means by the Holms-Sidak post hoc pairwise multiple comparison method. (c) Immunoblots of BiP levels in HEK293T cells transfected with empty vector (Mock) or vectors expressing CEL-16R or CEL variants Thr488-Ile548, Thr488-Thr548 or Ile488-Ile548. Green = anti-BiP; Red = anti-tubulin. (d) Estimation of BiP abundance by densitometry of immunoblots from (c; n = 3). There is a statistically significant difference in the mean values of the different groups by one-way ANOVA (F(4,10) = 12, p = < .001). Bars with different letters have significant differences in means by the Holms-Sidak post hoc pairwise multiple comparison method. ANOVA, analysis of variance; CEL-HYB, hybrid variant of carboxyl ester lipase
4 |. DISCUSSION
With few exceptions, genetic risk in CP is not inherited in a Mendelian pattern (Kleeff et al., 2017; Whitcomb, 2016). Instead, most genetic risk variants modify the likelihood of developing CP. In these instances, other factors, genetic or environmental, also contribute to the risk of CP and it is the combination of risk factors that causes disease. The CEL-HYB allele is no exception. Although the pedigrees of some families with CEL-HYB positive members suggested an autosomal-dominant pattern of inheritance, the CEL-HYB allele did not always co-segregate with CP (Fjeld et al., 2015; Oracz et al., 2019). In this study, we sought to examine how the risk for CP conferred by the CEL-HYB allele might be influenced by two previously identified missense SNPs in the CEL-CELP recombination region (Fjeld et al., 2015).
We initially re-examined sequence data of 75 subjects included in previous report describing CEL-HYB to determine if the two missense SNPs segregate with CP (Fjeld et al., 2015). One, rs77696629 (p. Ile488Thr), was present in almost all cases and controls who carried the CEL-HYB allele. The other SNP, rs750991274 (p. Thr548Ile), was only present in cases. These findings suggest that the variants p. Ile488Thr and p. Thr548Ile when linked together increase the risk for CP associated with CEL-HYB to a greater degree than either missense variant alone.
Notably, p. Ile488 and p. Thr548 are the variants of the CEL reference sequences. The alternative p. Ile488Thr allele is listed with a frequency of 0.0181 in dbSNP (www.ncbi.nlm.nih.gov/snp). This prevalence could reflect a combination of CEL-HYB-positive samples and samples where the variant is present within true CEL sequence. On the other hand, the alternative p. Thr548Ile allele seems to be almost completely specific for the CELP pseudogene with a listed allele frequency in CEL of only 0.000012. However, the VNTR region of the CEL gene is notoriously difficult to sequence and duplicated CEL/CELP alleles are relatively frequently encountered (Fjeld et al., 2020). Thus, deep sequencing data and SNP frequencies in the CEL exon 10–11 region should be treated with caution.
Since missense mutations can affect protein function, we tested several possible mechanisms whereby the two SNPs might alter the properties of CEL. First, we tested the hypothesis that increased or decreased lipase activity might contribute to CP onset. Our analysis of purified CEL (CEL-16R) and the four possible CEL-HYB haplotype showed that each CEL-HYB variant except Ile488-Ile548 had the same activity against various triglycerides as did CEL-16R. The lower activity of Ile488-Ile548 was intriguing since Thr488-Ile548 had normal lipase activity. If the p. Thr548Ile substitution accounted for the decreased activity, the substitution should have resulted in lower activity in Thr488-Ile548 as well. The explanation for this incongruity is not readily apparent. As assessed by SDS-PAGE and protein staining, the purity and amounts of the four CEL-HYB variants were similar. It is possible that the presence of the p. Thr548Ile variant alone affects protein structure or stability through changes that are balanced by the presence of p. Ile488Thr. Still, the activity of CEL-HYB Ile488-Ile548 is relatively preserved at 70–90% of CEL-16R activity. Thus, the presence of the two SNP variants in CEL-HYB has minimal effect on lipase activity and loss- or gain-of-lipase function is unlikely to contribute to the mechanism of CP onset. This conclusion is in accordance with the lack of a pancreatic phenotype in the Cel knockout mouse (Vesterhus et al., 2010).
The original description of CEL-HYB showed that CEL-HYB containing Thr488-Ile548 had lower activity than CEL (Fjeld et al., 2015). Any of several factors could account for the differences between that finding and ours. In the Fjeld paper, activity was determined on conditioned medium from transfected HEK293 cells using a 4-nitrophenyl valerate rather than a triglyceride as a substrate. Furthermore, the lipase was not purified and was modified with a V5/His tag. Either the purity of the protein, the modification, or the nature of the substrate alone or in combination could have affected the activity of CEL-HYB. Additionally, the protein amount was determined by immunoblot and densitometry of cell media in the earlier study and by spectrometry on purified protein in the current study. Inaccuracies when determining the amount of each variant could therefore also account for the observed difference in activities.
Next, we determined if the two amino acid substitutions might increase misfolding of the CEL-HYB variants and confer gain-of-function proteotoxicity. Multiple studies implicate protein misfolding in the pathophysiology of genetic variants associated with CP (Sahin-Toth, 2017). The genetic variants increase the amount of misfolded or aggregated protein, resulting in intracellular retention and decreased secretion. The result is maladaptive ER stress and activation of cell death and inflammatory pathways. This mechanism has also been proposed for the pathogenicity of both CEL-MODY and CEL-HYB (Johansson et al., 2011; Xiao et al., 2016). Although the Fjeld study provided indirect evidence for misfolding of CEL-HYB Thr488-Ile548, evidence for intracellular aggregation and activation of the ER stress response was not provided.
Expression of CEL-HYB with and without the two missense variants revealed that secretion of CEL-HYB with Thr488-Ile548 was significantly decreased compared with CEL-HYB with Ile488-Thr548 and CEL-16R. However, both CEL-HYB with Ile488-Thr548 or Thr488-Ile548 had increased intracellular levels compared with CEL-16R, and the majority of the intracellular protein was present as detergent-insoluble aggregates. Further analysis showed that the presence of both missense variants in CEL-HYB decreased secretion the most although the p. Ile488Thr variant accounted for much of the effect. Similarly, we found that the two variants affected secretion and intracellular aggregation of CEL-16R. Thus, our results suggest that the missense variants cause misfolding of both CEL-16R and CEL-HYB. In support of this conclusion, we found that the presence of either or both missense variants in CEL-16R or CEL-HYB significantly increased BiP, a marker of ER stress, over that found in cells expressing CEL-HYB Ile488-Thr548 or CEL-16R. Taken together, these results demonstrate that the missense variants of CEL-HYB increase misfolding of CEL independent of the composition and length of the VNTR.
Interestingly, there is a mismatch between our genetic and functional analysis in that the Thr488-Ile548 haplotype was found only in subjects with CP, and Thr488-Thr548 was observed in both cases and controls. Yet, Thr488-Thr548 was as functionally impaired in most assays as Thr488-Ile548. Only the lower secretion rate of Thr488-Ile548 suggests that this variant may be more proteotoxic than Thr488-Thr548. The large effect of one variant, p. Ile488Thr, in decreasing secretion and increasing intracellular aggregation of Thr488-Thr548 suggests that this variant might also be proteotoxic and raises the question of why Thr488-Ile548 was more enriched in cases than was Thr488-Thr548. There are several potential explanations for the differences. First, the evaluation of the variants was done in transfected cultured cells and overexpression may increase misfolding of the recombinant proteins and obscure differences in misfolding that may be present in vivo. Second, the in vivo expression levels of the different haplotypes could vary significantly if the SNP or other variants influence messenger RNA expression. Low expressing haplotypes would not be as proteotoxic and not trigger disease. Third, there may be differences in the flux through degradation pathways between the variants such that Thr488-Thr548 is degraded more efficiently than Thr488-Ile548 and, therefore, less likely to cause proteotoxicity. Testing the two haplotypes in mouse models may provide further insight.
In summary, our study showed that the presence of two amino acid substitutions in CEL-HYB, Thr488-Ile548, were only observed in CP cases. Thus, we conclude that the CEL-HYB variant Thr488-Ile548 increases the risk for CP. The variant Thr488-Thr548 did not show a statistically significant difference between cases and controls suggesting it may not modify the risk of the CEL-HYB allele for CP as strongly as Thr488-Ile548. This observation may help with further sub-dividing patients with CEL-HYB into different risk categories. Finally, our evaluation of the CEL-HYB variants in transfected cells suggests that the mechanism for increasing risk is linked to proteotoxic protein-misfolding, thereby, increasing the evidence that at least a subset of patients with CEL-HYB associated CP have a protein misfolding disease.
ACKNOWLEDGMENTS
Financial support was provided by Western Norway Regional Health Authority (Helse Vest, no. 912057) to Anders Molven, the Research Council of Norway (FRIMEDBIO, no. 289534) to Anders Molven, The National Pancreas Association Western PA Chapter to Xunjun Xiao, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital to Xunjun Xiao (MI-II-2018-750-2), and National Institutes of Diabetes and Digestive and Kidney Disease (DK124415) to Mark E. Lowe. The authors are indebted to the physicians and laboratories that have provided CEL-HYB positive samples for sequencing.
Funding information
National Institutes of Diabetes and Digestive and Kidney Disease, Grant/Award Number: DK124415; Research Council of Norway, Grant/Award Numbers: FRIMEDBIO, 289534; National Pancreas Association: CEL-HYB (Western PA); Western Norway Regional Health Authority, Grant/Award Numbers: Helse Vest, 912057; The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, Grant/Award Number: MI-II-2018-750-2
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
The data that support the conclusions of this study are available from the corresponding author on reasonable request.
REFERENCES
- Behar DM, Basel-Vanagaite L, Glaser F, Kaplan M, Tzur S, Magal N, … Zeharia A (2014). Identification of a novel mutation in the PNLIP gene in two brothers with congenital pancreatic lipase deficiency. Journal of Lipid Research, 55(2), 307–312. 10.1194/jlr.P041103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva M, El Jellas K, Steine SJ, Johansson BB, Ringdal M, Torsvik J, … Molven A (2017). Copy number variants and VNTR length polymorphisms of the carboxyl-ester lipase (CEL) gene as risk factors in pancreatic cancer. Pancreatology, 17(1), 83–88. 10.1016/j.pan.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Dalva M, Lavik IK, El Jellas K, Gravdal A, Lugea A, Pandol SJ, … Molven A (2020). Pathogenic carboxyl ester lipase (CEL) variants interact with the normal CEL protein in pancreatic cells. Cells, 9(1). 10.3390/cells9010244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A, Ponten F, Fagerberg L, Hallstrom BM, Schwenk JM, Uhlen M, … Lindskog C (2014). The human pancreas proteome defined by transcriptomics and antibody-based profiling. PLoS One, 9(12), e115421 10.1371/journal.pone.0115421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeld K, Beer S, Johnstone M, Zimmer C, Mossner J, Ruffert C, … Rosendahl J (2016). Length of variable numbers of tandem repeats in the carboxyl ester lipase (CEL) gene may confer susceptibility to alcoholic liver cirrhosis but not alcoholic chronic pancreatitis. PLOS One, 11(11), e0165567 10.1371/journal.pone.0165567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeld K, Masson E, Lin JH, Michl P, Stokowy T, Gravdal A, … Molven A (2020). Characterization of CEL-DUP2: Complete duplication of the carboxyl ester lipase gene is unlikely to influence risk of chronic pancreatitis. Pancreatology, 20(3), 377–384. 10.1016/j.pan.2020.01.011 [DOI] [PubMed] [Google Scholar]
- Fjeld K, Weiss FU, Lasher D, Rosendahl J, Chen JM, Johansson BB, … Molven A (2015). A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nature Genetics, 47(5), 518–522. 10.1038/ng.3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi E, & Sahin-Toth M (2017). Genetic risk in chronic pancreatitis: The trypsin-dependent pathway. Digestive Diseases and Sciences, 62(7), 1692–1701. 10.1007/s10620-017-4601-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi E, & Sahin-Toth M (2019). Human CPA1 mutation causes digestive enzyme misfolding and chronic pancreatitis in mice. Gut, 68(2), 301–312. 10.1136/gutjnl-2018-315994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DY, & Howles PN (2002). Carboxyl ester lipase: Structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. Journal of Lipid Research, 43(12), 2017–2030. 10.1194/jlr.r200013-jlr200 [DOI] [PubMed] [Google Scholar]
- El Jellas K, Johansson BB, Fjeld K, Antonopoulos A, Immervoll H, Choi MH, … Molven A (2018). The mucinous domain of pancreatic carboxyl-ester lipase (CEL) contains core 1/core 2 O-glycans that can be modified by ABO blood group determinants. Journal of Biological Chemistry, 293(50), 19476–19491. 10.1074/jbc.RA118.001934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, Fjeld K, El Jellas K, Gravdal A, Dalva M, Tjora E, … Molven A (2018). The role of the carboxyl ester lipase (CEL) gene in pancreatic disease. Pancreatology, 18(1), 12–19. 10.1016/j.pan.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Johansson BB, Torsvik J, Bjorkhaug L, Vesterhus M, Ragvin A, Tjora E, … Njolstad PR (2011). Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): A protein misfolding disease. Journal of Biological Chemistry, 286(40), 34593–34605. 10.1074/jbc.M111.222679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, … Neoptolemos JP (2017). Chronic pancreatitis. Nat Rev Dis Primers, 3, 17060 10.1038/nrdp.2017.60 [DOI] [PubMed] [Google Scholar]
- Kumar BV, Aleman-Gomez JA, Colwell N, Lopez-Candales A, Bosner MS, Spilburg CA, … Lange LG (1992). Structure of the human pancreatic cholesterol esterase gene. Biochemistry, 31(26), 6077–6081. 10.1021/bi00141a017 [DOI] [PubMed] [Google Scholar]
- Kumar S, Ooi CY, Werlin S, Abu-El-Haija M, Barth B, Bellin MD, … Uc A (2016). Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr, 170(6), 562–569. 10.1001/jamapediatrics.2015.4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasher D, Szabo A, Masamune A, Chen JM, Xiao X, Whitcomb DC, … Sahin-Toth M (2019). Protease-sensitive pancreatic lipase variants are associated with early onset chronic pancreatitis. American Journal of Gastroenterology, 114(6), 974–983. 10.14309/ajg.0000000000000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidberg U, Nilsson J, Stromberg K, Stenman G, Sahlin P, Enerback S, & Bjursell G (1992). Genomic organization, sequence analysis, and chromosomal localization of the human carboxyl ester lipase (CEL) gene and a CEL-like (CELL) gene. Genomics, 13(3), 630–640. 10.1016/0888-7543(92)90134-e [DOI] [PubMed] [Google Scholar]
- Lugea A, Waldron RT, Mareninova OA, Shalbueva N, Deng N, Su HY, … Pandol SJ (2017). Human pancreatic acinar cells: proteomic characterization, physiologic responses, and organellar disorders in ex vivo pancreatitis. American Journal of Pathology, 187(12), 2726–2743. 10.1016/j.ajpath.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeyski K, Lidberg U, Bjursell G, & Nilsson J (1998). Structure and organization of the human carboxyl ester lipase locus. Mammalian Genome, 9(4), 334–338. 10.1007/s003359900762 [DOI] [PubMed] [Google Scholar]
- Moore SA, Kingston RL, Loomes KM, Hernell O, Blackberg L, Baker HM, & Baker EN (2001). The structure of truncated recombinant human bile salt-stimulated lipase reveals bile salt-independent conformational flexibility at the active-site loop and provides insights into heparin binding. Journal of Molecular Biology, 312(3), 511–523. 10.1006/jmbi.2001.4979 [DOI] [PubMed] [Google Scholar]
- Oracz G, Kujko AA, Fjeld K, Wertheim-Tysarowska K, Adamus-Białek W, Steine SJ, … Rygiel AM (2019). The hybrid allele 1 of carboxyl-ester lipase (CEL-HYB1) in Polish pediatric patients with chronic pancreatitis. Pancreatology, 19(4), 531–534. [DOI] [PubMed] [Google Scholar]
- Raeder H, Johansson S, Holm PI, Haldorsen IS, Mas E, Sbarra V, … Njolstad PR (2006). Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nature Genetics, 38(1), 54–62. 10.1038/ng1708 [DOI] [PubMed] [Google Scholar]
- Raeder H, McAllister FE, Tjora E, Bhatt S, Haldorsen I, Hu J, … Kulkarni RN (2014). Carboxyl-ester lipase maturity-onset diabetes of the young is associated with development of pancreatic cysts and upregulated MAPK signaling in secretin-stimulated duodenal fluid. Diabetes, 63(1), 259–269. 10.2337/db13-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin-Toth M (2017). Genetic risk in chronic pancreatitis: The misfolding-dependent pathway. Current Opinions in Gastroenterology, 33(5), 390–395. 10.1097/MOG.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Xiao X, Haughney M, Spector A, Sahin-Toth M, & Lowe ME (2015). A novel mutation in PNLIP causes pancreatic triglyceride lipase deficiency through protein misfolding. Biochimica et Biophysica Acta, 1852(7), 1372–1379. 10.1016/j.bbadis.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvik J, Johansson BB, Dalva M, Marie M, Fjeld K, Johansson S, … Molven A (2014). Endocytosis of secreted carboxyl ester lipase in a syndrome of diabetes and pancreatic exocrine dysfunction. Journal of Biological Chemistry, 289(42), 29097–29111. 10.1074/jbc.M114.574244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterhus M, Raeder H, Kurpad AJ, Kawamori D, Molven A, Kulkarni RN, … Njolstad PR (2010). Pancreatic function in carboxyl-ester lipase knockout mice. Pancreatology, 10(4), 467–476. 10.1159/000266284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC (2016). Peering into the “Black Box” of the complex chronic pancreatitis syndrome. Pancreas, 45(10), 1361–1364. 10.1097/MPA.0000000000000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Jones G, Sevilla WA, Stolz DB, Magee KE, Haughney M, … Lowe ME (2016). A carboxyl ester lipase (CEL) mutant causes chronic pancreatitis by forming intracellular aggregates that activate apoptosis. Journal of Biological Chemistry, 291(44), 23224–23236. 10.1074/jbc.M116.734384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Jones G, Sevilla WA, Stolz DB, Magee KE, Haughney M, … Lowe ME (2017). A carboxyl ester lipase (CEL) mutant causes chronic pancreatitis by forming intracellular aggregates that activate apoptosis. Journal of Biological Chemistry, 292(19), 7744 10.1074/jbc.A116.734384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mukherjee A, Ross LE, & Lowe ME (2011). Pancreatic lipase-related protein-2 (PLRP2) can contribute to dietary fat digestion in human newborns. Journal of Biological Chemistry, 286(30), 26353–26363. 10.1074/jbc.M111.249813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, O’Connell M, & Papachristou GI (2012). Natural history following the first attack of acute pancreatitis. American Journal of Gastroenterology, 107(7), 1096–1103. 10.1038/ajg.2012.126 [DOI] [PubMed] [Google Scholar]
- Zou WB, Boulling A, Masamune A, Issarapu P, Masson E, Wu H, … Liao Z (2016). No association between CEL-HYB hybrid allele and chronic pancreatitis in Asian populations. Gastroenterology, 150(7), 1558–1560. 10.1053/j.gastro.2016.02.071 [DOI] [PMC free article] [PubMed] [Google Scholar]


