Abstract
Paecilomyces, a common saprobic filamentous fungus, not only plays an important role in biological control, but also has applications in medicine, food, and environmental protection. In this paper, 223 secondary metabolites and their bioactivities from 13 known species and various unidentified strains of Paecilomyces are reviewed. Their structures can be described as polyketide, terpenoid, peptide, alkaloid, quinone, pyrone, sterol, and fatty acid. They have been demonstrated varying biological activities, including antimicrobial, antitumor, insecticidal, antiplasmodial, antimalarial, nematicidal, herbicidal, and enzyme-inhibiting. This review provides a comprehensive overview of secondary metabolites and their biological activities from strains of Paecilomyces.
Keywords: paecilomyces, fungi, metabolites, bioactivities, structures
1. Introduction
Paecilomyces is a common saprobic filamentous fungus. It is found in a wide range of habitats, including soils, forests, grassland, deserts, sediments, and even sewage sludge [1]. Paecilomyces belongs to the phylum Ascomycota, and the order Eurotiales, which has septate, branching hyphae, bearing long chains of conidia from the tips of conidiophores, and flask- to oval-shaped or subglobose phialide. Colonies of Paecilomyces are at first floccose and white, then become different colors. Paecilomyces strains do not harm to health in general and are in occasion opportunistic in humans and mammals.
Many species of Paecilomyces are important entomopathogenic fungi, which refer to a class that can infect or parasitize living host organisms and are an ecologically highly specialized group of micro-organisms. Entomopathogenic fungi are well known for their ability to produce various bioactive compounds during infection and proliferation in insects, and are considered as potential sources of novel bioactive compounds. The entomopathogenic fungi belonging to the genus Paecilomyces have been extensively studied as potential biological control agents against insects. Besides, Paecilomyces species have been used as Chinese traditional medicine to treat impotence, sedation, analgesia, backache, cancer, memory loss, and also as a tonic to nourish the lungs and kidneys [2]. Moreover, strains of Paecilimyces can survive in a wide range of temperatures and pH, which allows them to grow in a variety of substrates and makes them a rich source of biologically active natural products [3].
Paecilomyces is closely related to all aspects of human life and it plays an irreplaceable role in biological control, and has an important role in medicine and health. This review presents 223 secondary metabolites and their biological activities isolated from the 13 known species and various unidentified strains of Paecilomyces. The review covers reports from 1972 until the present. The structures of all compounds are summarized in Figures 1–3, and the active metabolites are concluded in Table 1.
2. Secondary Metabolites from Paecilomyces
2.1. Metabolites Derived from Paecilomyces with Antimicrobial Activity
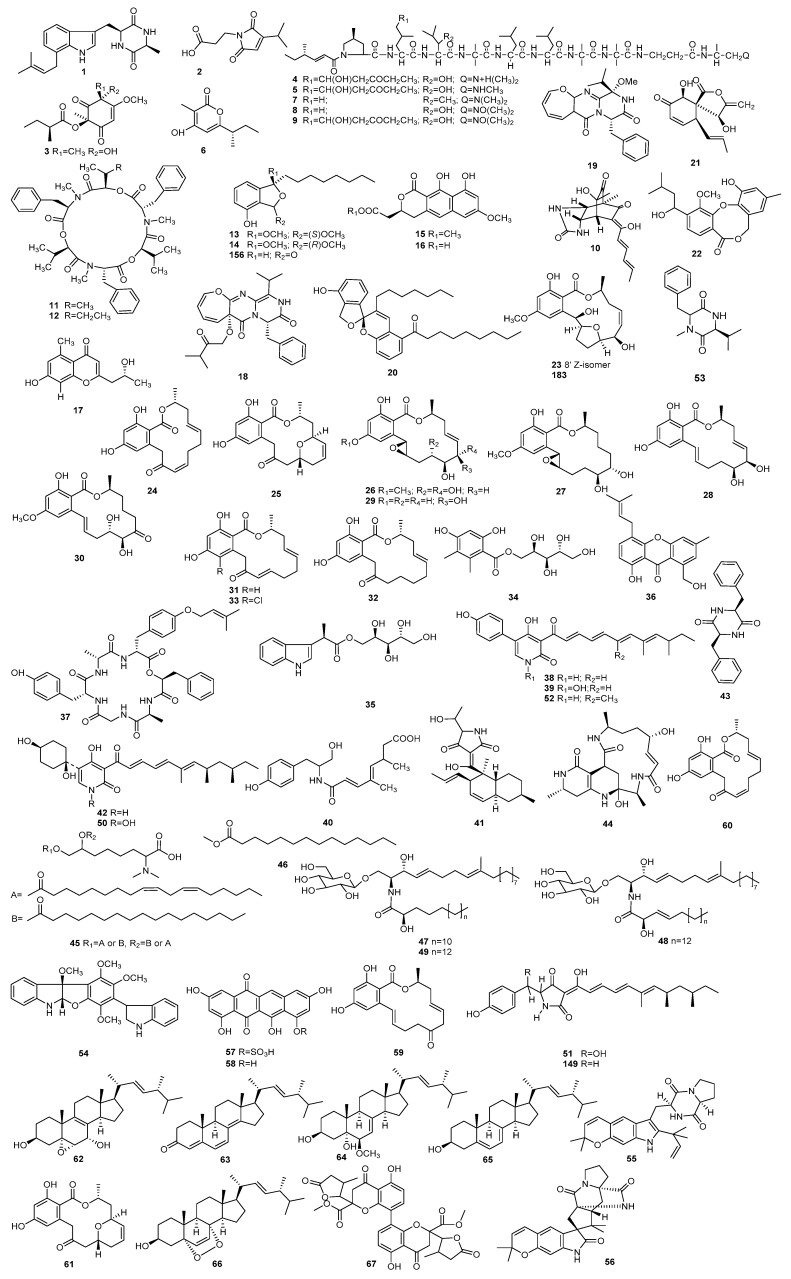
Diketopiperazine terezine D (1) (Figure 1 and Table 1) was isolated from P. cinnamomeus BCC 9616, which was firstly found from the coprophilous fungus Spwormiella teretispora. It demonstrated activity against Sordaria fimicola (NRRL6459), causing a 50% reduction in the radial growth rate at 200 μg/disk [4,5]. A maleimide-bearing compound, farinomalein (2), was isolated from P. farinosus HF599, which showed potent activity against the plant pathogen Phytophthora sojae P6497 at 5 µg/disk [6]. α-Pyrone analogue phomaligol A (3) was identified from a strain of P. lilacinus derived from the marine sponge Petrosia sp. [7]. In addition, phomaligol A (3) was also obtained from the marine derived fungus Aspergillus flavus [8] and the blackleg fungus Leptosphaeria maculans [9], and exhibited antibacterial activity against Staphylococcus aureus, methicillin-resistant S. aureus, and multidrug-resistant S. aureus with minimum inhibitory concentration (MIC) values at 31.2–62.5 μg/mL [8]. The complex antibiotic, leucinostatin, was isolated from P. lilacinus A-267 [10], before being separated into leucinostatin A (4) and leucinostatin B (5). They demonstrated antimicrobial activity against bacteria and fungi [11,12]. The compound phomapyrone C (6) was identified from a strain of P. lilacinus [7] and Aspergillus sp. SCSIO 41024 isolated from deep-sea [13]. It had weak antibacterial activity against Acinetobacter baumannii with the MIC value of 250 μg/mL [13].
Figure 1.
The structures of metabolites produced by Paecilomyces (1).
Table 1.
The active metabolites derived from Paecilomyces.
| Metabolites | Paecilomyces Strain | Biological Activities | References |
|---|---|---|---|
| terezine D (1) | P. cinnamomeus BCC 9616 | antifungal | [4] |
| farinomalein (2) | P. farinosus HF599 | antifungal | [6] |
| phomaligol A (3) | P. lilacinus | antibacterial | [7,8] |
| leucinostatin A (4) | P. lilacinus A-267 | antimicrobial, antitumor, uncoupling effect on rat liver mitochondrial function | [10,11,12] |
| leucinostatin B (5) | P. lilacinus A-267 | antimicrobial, antitumor, uncoupling effect on rat liver mitochondrial function | [10,11,12] |
| phomapyrone C (6) | P. lilacinus | antibacterial | [7,13] |
| leucinostatin D (7) | P. marquandii | antimicrobial, cytotoxic, phytotoxicity | [15] |
| leucinostatin H (8) | P. marquandii | antimicrobial | [16] |
| leucinostatin K (9) | P. marquandii | antimicrobial | [16] |
| sorbicillinoid (10) | P. marquandii | antibacterial | [17] |
| beauvericin (11) | P. tenuipes BCC 1614 | antimicrobial, cytotoxic, insecticidal | [18] |
| beauvericin A (12) | P. tenuipes BCC 1614 | antimicrobial, cytotoxic, insecticidal | [18] |
| paecilocin B (13) | P. variotii | antibacterial | [23] |
| paecilocin C (14) | P. variotii | antibacterial | [23] |
| semi-viriditoxin (15) | P. varioti | antibacterial | [24] |
| semi-viriditoxic acid (16) | P. varioti | antibacterial | [24] |
| lawsozaheer (17) | P. varioti | antibacterial | [25] |
| varioloid A (18) | P. variotii EN-291 | antifungal | [26] |
| varioloid B (19) | P. variotii EN-291 | Antifungal, cytotoxic | [26] |
| paeciloketal A (20) | P. variotii J08NF-1 | antibacterial | [27] |
| paecilospirone (21) | Paecilomyces sp. | antibacterial | [28] |
| paeciloxocin A (22) | Paecilomyces sp. | antifungal, cytotoxic | [29] |
| paecilomycin M (23) | Paecilomyces sp. SC0924 | antifungal | [31] |
| monocillin VI (24) | Paecilomyces sp. SC0924 | antifungal | [32] |
| monocillin VII (25) | Paecilomyces sp. SC0924 | antifungal | [32] |
| aigilomycin B (26) | Paecilomyces sp. SC0924 | antifungal | [30] |
| aigilomycin C (27) | Paecilomyces sp. SC0924 | antifungal | [30] |
| aigilomycin D (28) | Paecilomyces sp. SC0924 | antifungal | [30] |
| 1′,2′-epoxy aigialomycin D (29) | Paecilomyces sp. SC0924 | antifungal | [30] |
| LL-Z1640-1 (30) | Paecilomyces sp. SC0924 | antifungal | [30] |
| monocillin II (31) | Paecilomyces sp. SC0924 | antibacterial | [33] |
| monocillin IV (32) | Paecilomyces sp. SC0924 | antibacterial | [33] |
| monorden D (33) | Paecilomyces sp. SC0924 | antibacterial | [33] |
| paeciloside A (34) | Paecilomyces sp. CAFT156 | antibacterial, cytotoxic | [38] |
| acremoauxin A (35) | Paecilomyces sp. CAFT156 | antibacterial, cytotoxic | [38] |
| paeciloxanthone (36) | Paecilomyces sp. (tree 1–7) | antimicrobial, cytotoxic, enzyme inhibition | [39] |
| paecilodepsipeptide A (37) | P. cinnamomeus BCC 9616 | cytotoxic, enzyme inhibition, antimalarial | [40] |
| farinosone A (38) | P. farinosus RCEF 0101 | induce neurite outgrowth in the PC-12 cell line | [41] |
| farinosone B (39) | P. farinosus RCEF 0101 | cytotoxic | [41,42] |
| farinosone C (40) | P. farinosus RCEF 0101 | induce neurite outgrowth in the PC-12 cell line | [41] |
| paecilosetin (41) | P. farinosus | cytotoxic | [42] |
| (+)-N-deoxymilitarinone A (42) | P. farinosus RCEF 0097 | cytotoxic, induce neurite sprouting in PC-12 cell line | [43] |
| (3S,6S)-3,6-dibenzylpiperazine-2,5-dione (43) | P. formous 17D47-2 | cytotoxic | [44] |
| gunnilactam A (44) | P. gunnii | cytotoxic | [45] |
| 1,2-dilinolylglycero-O-4′-(N,N,N-trimethyl) homoserine (45) | P. lilacinus ZBY-1 | cytotoxic | [46] |
| methyl myristate (46) | P. lilacinus ZBY-1 | cytotoxic | [46] |
| cerebroside B (47) | P. lilacinus ZBY-1 | cytotoxic | [47] |
| cerebroside C (48) | P. lilacinus ZBY-1 | cytotoxic | [47] |
| cerebroside D (49) | P. lilacinus ZBY-1 | cytotoxic | [47] |
| militarinone A (50) | P. militaris | neurotrophic effect in PC-12 cells | [49] |
| militarinone B (51) |
P. militaris P. farinosus RCEF 0097 |
cytotoxic | [43,48] |
| militarinone D (52) |
P. militaris P. farinosus RCEF 0097 |
cytotoxic | [43,48] |
| (3S)-6-phenethyl-3-isopropyl-1-methyl-2,5-diketopiperazine (53) | P. tenuipes | cytotoxic | [50] |
| indolyl-6,10b-dihydro-5aH-[1]benzofuro[2,3-b]indole derivative (54) | P. variotii EN-291 | cytotoxic | [51] |
| dihydrocarneamide A (55) | P. variotii EN-291 | cytotoxic | [52] |
| iso-notoamide B (56) | P. variotii EN-291 | cytotoxic | [52] |
| UCE1022 (57) | Paecilomyces sp. | cytotoxic | [53] |
| saintopin (58) | Paecilomyces sp. | cytotoxic | [54] |
| paecilomycin P (59) | Paecilomyces sp. SC0924 | cytotoxic | [32] |
| monocillin VI (60) | Paecilomyces sp. SC0924 | cytotoxic | [32] |
| monocillin VII (61) | Paecilomyces sp. SC0924 | cytotoxic | [32] |
| 5α,6α-epoxy-(22E,24R)-ergosta-8,22-diene-3β,7α-diol (62) | Paecilomyces sp. J300 | cytotoxic | [55] |
| ergosta-4,6,8(14),22-tetraene-3-one (63) | Paecilomyces sp. J300 | cytotoxic | [55] |
| 3β,5α-dihydroxy-6β-methoxyer-gosta-7,22-diene (64) | Paecilomyces sp. J300 | cytotoxic | [55] |
| ergosterol (65) | Paecilomyces sp. J300 | cytotoxic | [55] |
| ergosterol endoperoxide (66) | Paecilomyces sp. J300 | cytotoxic | [55] |
| paecilin A (67) | Paecilomyces sp. (tree 1–7) | cytotoxic | [56] |
| secalonic acid D (68) | Paecilomyces sp. (tree 1–7) | cytotoxic, enzyme inhibition | [57] |
| secalonic acid A (69) | Paecilomyces sp. (tree 1–7) | cytotoxic | [58] |
| tenellic acid A (70) | Paecilomyces sp. (tree 1–7) | cytotoxic | [58] |
| tetracenomycin D (71) | Paecilomyces sp. (tree 1–7) | cytotoxic | [59] |
| physcioin (72) | Paecilomyces sp. (tree 1–7) | cytotoxic | [59] |
| emodin (73) | Paecilomyces sp. (tree 1–7) | cytotoxic | [59] |
| chrysophanol (74) | Paecilomyces sp. (tree 1–7) | cytotoxic | [59] |
| 1,4-dihydroxy-2-methy anthraquinone (75) | Paecilomyces sp. (tree 1–7) | cytotoxic | [59] |
| paecilopeptin (76) | P. carneus | inhibiting human cathepsin S | [60] |
| paeciloquinone A (77) | P. carneus P-177 | enzyme inhibition | [61] |
| paeciloquinone C (78) | P. carneus P-177 | enzyme inhibition | [61] |
| paeciloquinone D (79) | P. carneus P-177 | enzyme inhibition | [63] |
| YW3548 (80) | P. formosus LHL10 | enzyme inhibition | [64] |
| paecilomycone A (81) | P. gunnii | enzyme inhibition | [65] |
| paecilomycone B (82) | P. gunnii | enzyme inhibition | [65] |
| paecilomycone C (83) | P. gunnii | enzyme inhibition | [65] |
| paecilomide (84) | P. lilacinus | enzyme inhibition | [66] |
| Sphingofungin E (85) | P. variotii ATCC 74097 | enzyme inhibition | [67] |
| Sphingofungin F (86) | P. variotii ATCC 74097 | enzyme inhibition | [67] |
| verticilatin (87) | P. verticillatus | enzyme inhibition | [68] |
| 3α-hydroxy-3,5-dihydro ML-236C (88) | P. viridis L-68 | enzyme inhibition | [69] |
| 12-hydroxyalbrassitriol (89) | Paecilomyces sp. TE-540 | enzyme inhibition | [70] |
| 2-hydroxyalbrassitriol (90) | Paecilomyces sp. TE-540 | enzyme inhibition | [70] |
| phenopicolinic acid (91) | Paecilomyces sp. AF2562 | enzyme inhibition | [71] |
| kurasoin A (92) | Paecilomyces sp. FO-3684 | enzyme inhibition | [72] |
| kurasoin B (93) | Paecilomyces sp. FO-3684 | enzyme inhibition | [72] |
| catenioblin C (94) | P. cateniobliquus YMF1.01799 | promoted the growth of the larvae of cotton bollworm | [73] |
| phomalactone (95) | P. cateniobliquus YMF1.01799 | inhibition cotton bollworm | [73] |
| cerebrosides A (96) | P. lilacinus ZBY-1 | nematicidal | [74] |
| 4-(4′-carboxy-2′-ethyl-hydroxypentyl)-5,6-dihydro-6-methyl-cyclobut[b]pyridine-3,6-dicarboxylic acid (97) | Paecilomyces sp. YMF1.01761 | nematicidal | [75] |
| paeciloxazine (98) | Paecilomyces sp. BAUA3058 | Nematicidal, insecticidal | [76] |
| harzialactone A (99) | Paecilomyces sp. 7A22 | insecticidal | [77] |
| paecilomycin E (100) | Paecilomyces sp. SC0924 | antiplasmodial | [78] |
| paecilomycin F (101) | Paecilomyces sp. SC0924 | antiplasmodial | [78] |
| aigilomycin B (102) | Paecilomyces sp. SC0924 | antiplasmodial | [78] |
| aigialomycin F (103) | Paecilomyces sp. SC0924 | antiplasmodial | [78] |
| pyrenocine I (104) | Paecilomyces sp. FKI-3573 | antitrypanosomal | [79] |
| pyrenocine A (105) | Paecilomyces sp. FKI-3573 | antitrypanosomal | [79] |
| pyrenocine B (106) | Paecilomyces sp. FKI-3573 | antitrypanosomal | [79] |
| citreoviridin (107) | Paecilomyces sp. FKI-3573 | antitrypanosomal | [79] |
| spirotenuipesine A (108) | P. tenuipes | activity in neurotrophic factor biosynthesis in glial cells | [81] |
| spirotenuipesine B (109) | P. tenuipes | activity in neurotrophic factor biosynthesis in glial cells | [81] |
| paecilomycine A (110) | P. tenuipes | activity in neurotrophic factor biosynthesis in glial cells | [80] |
| formoxazine (111) | P. formosus | radical-scavenging activity | [82] |
| terreusinone (112) | P. formosus | UV-A absorbing activity | [83] |
| 3-[(2Z)-1-oxo-2-buten-1-yl]oxazolidin-2-one (113) | P. formosus | radical-scavenging activity | [82] |
| 14-hydroxycornexistin (114) | P. variotii | herbicidal | [84] |
| cornexistin (115) | P. variotii SANK 21086 | herbicidal | [85,86] |
A series of peptidic antibiotics: leucinostatin A (4), D (7), H (8), and K (9), were identified from P. marquandii [14,15,16]. Leucinostatin D (7) showed biological activities against Gram-positive bacteria and several fungi, for example, Bacillus subtilis ICI, Micrococcus luteus ISS, Streptococcus pneumonia, S. haemolyticus, and S. aureus [15]. Leucinostatin H (8) and K (9) also exhibited activities against Gram-positive bacteria and fungi, but the antibacterial and the antimycotic activity reduce significantly upon N-oxidation [16]. A Diels-Alder product of sorbicillinoid (10) with a urea group was isolated from P. marquandii, a intertidal marine strain, which had antibacterial activity against B. subtilis ATCC 6633 and E. coli ATCC 25922 [17].
Cyclodepsipeptides beauvericin (11) and beauvericin A (12) were isolated from P. tenuipes BCC 1614. The two compounds displayed antimicrobial activities [18]. Beauvericin (11) can also be obtained from several fungi, including Beauveria bassiana, Polyporus sulphureus, and Fusarium spp. [19,20,21,22].
Two polyketides paecilocin B (13) and C (14) were identified from P. variotii derived from the jellyfish Nemopilema nomurai, which showed moderate antibacterial activity against S. aureus SG 511 and MRSA 3089 with MIC values ranging from 5 to 40 μg/mL [23]. Two metabolites, semi-viriditoxin (15) and semi-viriditoxic acid (16), were produced by a strain of P. variotii, isolated from the larvae of Dendroctonus ponderosa, and the two metabolites (15, 16) showed weak antibacterial activity against a number of bacteria [24]. One chromone, lawsozaheer (17) was isolated from the broth of Paecilomyces variotii. It demonstrated highly selective activity against S. aureus (NCTC 6571) with 84.26% inhibition at 150 μg/mL [25]. Two oxepine-containing diketopiperazine-type alkaloids, varioloid A (18) and B (19), were identified from the marine alga-derived P. variotii EN-291, exhibiting potent activity against the plant pathogenic fungus Fusarium graminearum with MIC values of 8 and 4 mg/mL, respectively [26]. A benzannulated spiroketal derivative, paeciloketal A (20), was obtained from P. variotii J08NF-1, a jellyfish-derived strain, which showed antibacterial activity with a MIC value of 40 μg/mL against the marine pathogen Vibrio ichthyoenteri [27].
A metabolite paecilospirone (21), was reported from Paecilomyces sp., with a MIC value of 5 μg/mL against B. subtilis at 25 °C; however, at 37 °C, it did not show any antimicrobial activity [28]. Paeciloxocin A (22) was isolated from Paecilomyces sp., and it inhibited the growth of Curvularia lunata and Candida albicans ATCC 10231 with inhibition zones of 12 and 10 mm, respectively [29]. Paecilomycin M (23), monocillin VI (24) and VII (25), aigilomycin B–D (26–28), 1′,2′-epoxy aigialomycin D (29), LL-Z1640-1 (30), monocillin II (31), monocillin IV (32), and monorden D (33) were produced by Paecilomyces sp. SC0924. Compounds 23–30 exhibited weak antifungal activity against Peronophythora litchi [30,31,32]. Metabolites 31–33 can be separated from Pochonia chlamydosporia and demonstrated modest activity against Xanthomonas campestris, with a MIC value of 25.6 μg/mL [33]. Aigialomycin-type compound was also reported to be derived from Aigialus parvus [34,35]. LL-Z1640-1 (30) was firstly isolated from an unidentified fungus [36] and was also obtained from the gorgonian derived fungus Cochliobolus lunatus [37].
The antimicrobial and cytotoxic polyketide paeciloside A (34) and the compound acremoauxin A (35) were identified from a strain of Paecilomyces sp. CAFT156 [38]. Compounds 34 and 35 displayed inhibitory effects on two bacteria B. subtilis and S. aureus at 40 μg/disk [38]. A metabolite, paeciloxanthone (36), was obtained from Paecilomyces sp. (tree 1–7), a strain isolated from an estuarine mangrove from the Taiwan Strait. Metabolite 36 is active against C. lunata, E. coli., and C. albicans at 40 μg/disk, producing inhibitory zones of 6, 12, and 10 mm, respectively [39].
2.2. Cytotoxic Metabolites Derived from Paecilomyces
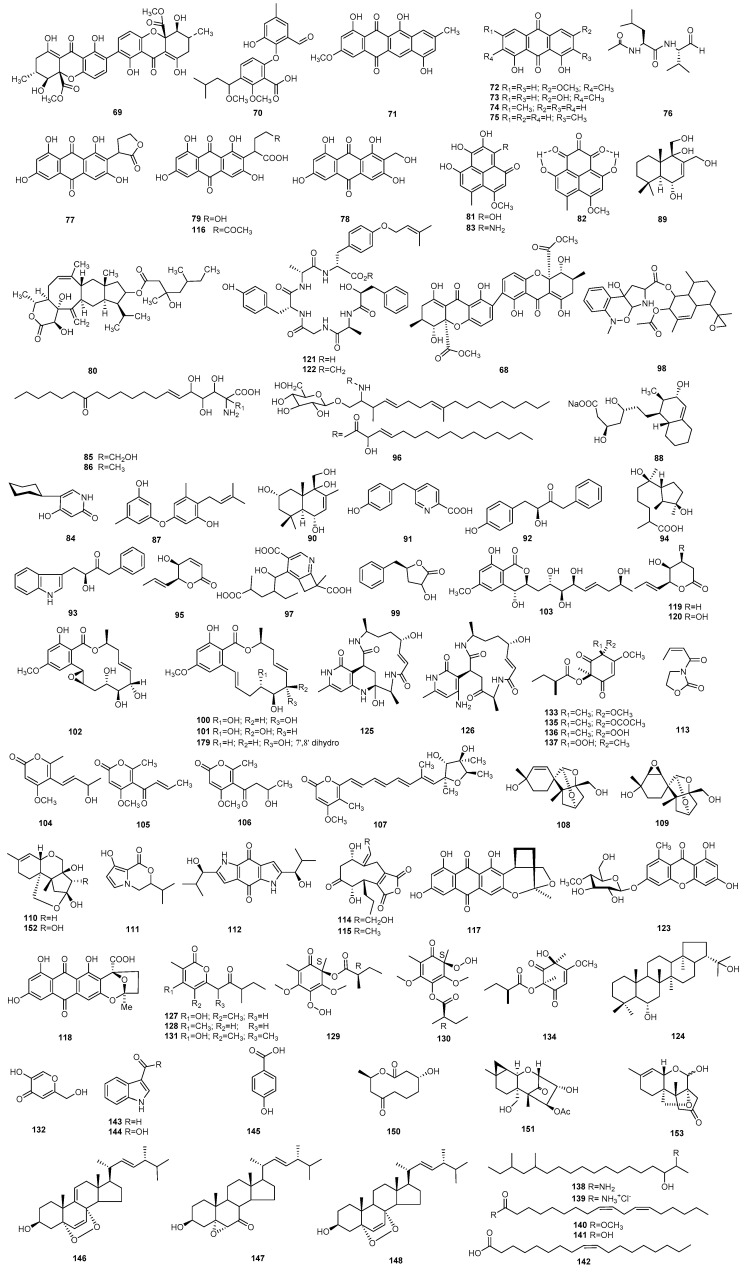
An antitumor cyclohexadepsipeptide, paecilodepsipeptide A (37), was derived from P. cinnamomeus BCC 9616. Paecilodepsipeptide A (37) exhibits cytotoxicity against cancer cell lines, KB and BC, with IC50 (the half maximal inhibitory concentration) values of 5.9 and 6.6 µM, respectively [40].
Farinosone A–C (38–40), three neurotrophic alkaloidal metabolites produced by P. farinosus RCEF 0101. Farinosone A (38) and C (40) can induce neurite outgrowth in the PC-12 cell line at concentrations of 50 µM, while farinosone B is inactive [41]. A tetramic acid derivative, paecilosetin (41), along with farinosone B (39), was isolated from P. farinosus. The two metabolites showed activity against the P388 cell line with IC50 values of 3.1 and 1.1 µg/mL, respectively [42]. A pyridone alkaloid, (+)-N-deoxymilitarinone A (42), was obtained from a strain of P. farinosus RCEF 0097. Compound 42 induced neurite sprouting in the PC-12 cell line when tested at concentrations of 33 and 100 µM and a cytotoxic effect was observed in human neurons (IMR-32) at 100 µM [43]. The metabolite (3S,6S)-3,6-dibenzylpiperazine-2,5-dione (43) was isolated from a culture extract of marine-derived P. formous 17D47-2; it showed selective cytotoxic activity in human pancreatic carcinoma PANC-1 cells adapted to glucose-starved conditions, with an IC50 value of 28 µM, whereas no effect against PANC-1 cells under general culture conditions up to 1000 µM [44].
A novel macrocyclic, tetralactams gunnilactam A (44), isolated from P. gunnii, exhibited cytotoxic activity against human prostate cancer C42B cells with an IC50 value of 5.4 μM [45].
A series of metaboilites, including 1,2-dilinolylglycero-O-4′-(N,N,N-trimethyl) homoserine (45), methyl myristate (46) [46] and cerebroside B–D (47–49) [47], were isolated from marine-derived P. lilacinus ZBY-1. The metabolites 45 and 46 inhibited the human cancer K562, MCF-7, HL-60, and BGC-823 cells lines with the IC50 values ranging from 1.12 to 8.63 μmol/L [46]. The compounds cerebroside B–D (47–49) inhibited K562, MCF-7, HL-60, and BGC-823 cells with IC50 values ranging from 9.5 to 59.6 mg/L [47]. Leucinostatin A (4) and B (5), derived from P. lilacinus A-267, as well as having antimicrobial activity, also showed antitumor activity and an uncoupling effect on rat liver mitochondrial function [11,12].
Three novel pyridone alkaloids, militarinone A (50), B (51), and D (52), were isolated from the mycelium of P. militaris [48,49]. Militarinone A (50) had a pronounced neurotrophic effect in the PC-12 cells at concentration of 10 µM [48]. Militarinone D (52) showed significant cytotoxicity against PC-12 cells with 74.0% and 30.7% at concentrations of 100 and 33 µM, respectively, and militarinone B (51) was weakly cytotoxic at 100 µM (16.8%) [49]. In addition, militarinone B (51) and D (52) can also be obtained from a strain of P. farinosus RCEF 0097 [43].
A peptidic antibiotic, leucinostatin D (7), was obtained from P. marquandii. The phytotoxicity test on tomato cuttings proved positive at 2 μg/mL, and in vitro cytotoxic activity assays showed that it inhibited HeLa, KB, and P388/S with ID50 values of 850, 0.95, and l.00 ng/mL [15].
The novel metabolite (3S)-6-phenethyl-3-isopropyl-1-methyl-2,5-diketopiperazine (53) was obtained from P. tenuipes and showed cytotoxicity against 22RV1 and DU-145 prostate cancer cells with inhibition rates of 37.8% and 38.6% at 5 µm/L [50]. Cyclodepsipeptide beauvericin (11) and beauvericin A (12) derived from P. tenuipes BCC 1614 also showed cytotoxic activity [18].
A series of compounds, including a indolyl-6,10b-dihydro-5aH-[1]benzofuro[2,3-b]indole derivative (54), a diketopiperazine-type alkaloid varioloid B (19), and two prenylated indole alkaloids dihydrocarneamide A (55), and iso-notoamide B (56), were identified from the marine alga-derived P. variotii EN-291 [26,51,52]. Compounds 19 and 54 exhibited cytotoxicity against A549, HCT116, and HepG2 cell lines, with IC50 values from 2.6 to 8.2 µg/mL [51]. Dihydrocarneamide A (55) and iso-notoamide B (56) showed cytotoxic activities against NCI-H460 with IC50 values of 69.3 and 55.9 mmol/L, respectively [52].
Three metabolites, UCE1022 (57), saintopin (58), and paeciloxocin A (22), were identified from an unidentified species of Paecilomyces. UCE1022 (57) displayed in vitro cytotoxic activity against HeLa S3 at IC50 6.1 μM [53]. Saintopin (58) shows in vitro cytotoxic activity against HeLa S3 at IC50 0.35 μg/mL, and further demonstrated in vivo antitumor activity against murine leukemia P388 (ip) [54]. Paeciloxocin A (22) exhibited significant cytotoxicity against hepG2 with an IC50 value of 1 μg/mL [29]. A β-resorcylic acid lactone, paecilomycin P (59), and two radicicol-type metabolites, monocillin VI and VII (60, 61) were produced by a strain of Paecilomyces sp. SC0924 [32]. The three compounds (59–61) exhibited cytotoxicity against MCF-7, A549, and HeLa cells [32]. The metabolites paeciloside A (34) and acremoauxin A (35) were identified from Paecilomyces sp. CAFT156. The two compounds displayed moderate cytotoxicity towards Artemia salina [38]. The cytotoxic ergosterols, including 5α,6α-epoxy-(22E,24R)-ergosta-8,22-diene-3β,7α-diol (62), ergosta-4,6,8(14),22-tetraene-3-one (63), 3β,5α-dihydroxy-6β-methoxyer-gosta-7,22-diene (64), ergosterol (65), and ergosterol endoperoxide (66), were produced by Paecilomyces sp. J300 [55]. These compounds showed moderate cytotoxicity against A549, SK-OV-3, SK-MEL-2, XF498 (CNS), and HCT15 cells [55]. A sequence of metabolites, including paeciloxanthone (36) [39], paecilin A (67), secalonic acid D (68), secalonic acid A (69), tenellic acid A (70), and five anthraquinone derivates, tetracenomycin D (71), physcioin (72), emodin (73), chrysophanol (74), 1,4-dihydroxy-2-methy anthraquinone (75) were obtained from Paecilomyces sp. (tree 1–7) [39,56,57,58]. Paeciloxanthone (36) exhibited in vitro cytotoxicity against hepG2 with an IC50 value of 1.08 μg/mL [39]. Paecilin A (67) showed inhibiting activity against KB and KBv cells with IC50 values of 40 and 50 nmol/mL, respectively [56]. Secalonic acid D (68) showed cytotoxicity towards KB cells with an value of IC50 < 1 µg/mL and inhibited human topoisomerase I with an IC50 value of 0.16 µmol/mL [57]. Secalonic acid A (69) (Figure 2) and tenellic acid A (70) inhibited the growth of the human hepatoma cell line HepG2, with IC50 values of 62.1 and 2.0 μg/mL, respectively [58]. Compounds 71–75 showed anticancer activity against KB and KBv, with IC50 values of 11, 20, 8, 15, and 18 μmg/mL and 17, 30, 10, 20, and 25 μmg/mL, respectively [59].
Figure 2.
The structures of metabolites produced by Paecilomyces (2).
2.3. Metabolites with Enzyme Inhibitory Activity from Paecilomyces
Paecilopeptin (76) is a novel cathepsin S inhibitor produced by P. carneus, which inhibits human cathepsin S in vitro with an IC50 value of 2.1 nM [60]. A series of inhibitors of the protein tyrosine kinases paeciloquinone A (77), C (78), and D (79) were obtained from P. carneus P-177 [61,62]. Paeciloquinone A (77) and C (78) are potent and selective inhibitors of the v-abl protein tyrosine kinase with an IC50 value of 0.4 μM [61]. Paeciloquinone D (79) is a protein kinase C inhibitor with an IC50 value around 6 μM [63].
Two metabolites, sester-terpenoid YW3548 (80) and a cyclic peptide paecilodepsipeptide A (37), were isolated from endophytic P. formosus LHL10 [64]. The two compounds exhibited remarkable inhibitory rates against α-glucosidase and urease, with IC50 values of 61.80 ± 5.7 and 75.68 ± 6.2, and 74.25 ± 4.3 and 190.5 ± 10.31 µg/g, respectively [64], which were also obtained from P. cinnamomeus [40]. Paecilomycone A–C (81–83) were identified from P. gunnii with IC50 values of 0.11, 0.17, and 0.14 mM on Tyrosinase, respectively [65]. A pyridone alkaloid, paecilomide (84), derived from P. lilacinus, demonstrated an acetylcholinesterase inhibition of 57.5 ± 5.50% [66].
Sphingofungins E (85) and F (86) are novel structures in the sphingofungin family which can inhibit serinepalmitoyl transferase at nanomolar levels; the estimated IC50 values were 7.2 and 57 nM, respectively, which were obtained from a strain of P. variotii ATCC 74097 [67]. A oxybis cresol, verticilatin (87), was identified from cultures of P. verticillatus. Verticilatin (87) exhibited significant inhibitory activity against CDC25B, cathepsin B, MEG2, and SHP2 enzyme, with IC50 values of 11.5, 3.5, 7.8, and 15 μg/mL, respectively [68]. A metabolite of the compactin family, 3α-hydroxy-3,5-dihydro ML-236C (88), was isolated from P. viridis L-68, and the in vitro activity of HMG-CoA reductase was inhibited by approximately 50% by this compound 88 [69].
The cadinane-type sesquiterpenoid analogs, 12-hydroxyalbrassitriol (89) and 2-hydroxyalbrassitriol (90), were obtained from the endophytic fungus Paecilomyces sp. TE-540. The two compounds showed moderate activities against acetylcholinesterase (AChE), with IC50 values of 43.02 ± 6.01 and 35.97 ± 2.12 μM, respectively [70]. Phenopicolinic acid (91), a potent inhibitor of dopamine β-hydroxylase, was found in culture filtrates of Paecilomyces sp. AF2562. The LD50 (median lethal dose or concentration) of phenopicolinic acid (91) for mice was about 350 mg/kg through intraperitoneal injection [71].
Two novel protein farnesyltransferase (PFTase) inhibitors, kurasoin A (92) and B (93), were derived from the cultured broth of Paecilomyces sp. FO-3684 [72]. The two metabolites inhibited PFTase in a dose-dependent, with IC50 values of 59.0 and 58.7 μM, respectively [72]. The metabolites paeciloxanthone (36) and secalonic acid D (68) were isolated from Paecilomyces sp. (tree 1–7). Paeciloxanthone (36) exhibited in vitro AChE inhibition with an IC50 value of 2.25 μg/mL [39], and secalonic acid D (68) inhibited human topoisomerase I with an IC50 value of 0.16 µmol/mL [57].
2.4. Insecticidal, Nematicidal, Antiplasmodial, and Antimalarial Metabolites Derived from Paecilomyces
Catenioblin C (94) and phomalactone (95) were identified from P. cateniobliquus YMF1.01799 [73]. The polyketide-derived phomalactone (95) had a significant inhibitory effect on the growth of the cotton bollworm Helicoverpa armigera, while the terpenoid derived metabolite catenioblin C (94) promoted the growth of the larvae [73]. Beauvericin (11) and beauvericin A (12) with diversiform bioactivities obtained from P. tenuipes BCC 1614, also demonstrated promising insecticidal activity [18]. The metabolite cerebrosides A (96) was isolated from marine-derived P. lilacinus ZBY-1, and its nematicidal activity against Bursaphelenchus xylophilus was investigated. The result showed that the average mortality of B. xylophilus treated with cerebroside A (96) at the mass concentrations of 1000, 100, and 10 μg/mL were 100%, 100%, and 11.1%, respectively [74].
A nematicidal metabolite 4-(4′-carboxy-2′-ethyl-hydroxypentyl)-5,6-dihydro-6-methyl- cyclobut[b]pyridine-3,6-dicarboxylic acid (97), was produced by Paecilomyces sp.YMF1.01761. Within 24 h, the LD50 value was 50.86 mg/L against Panagrellus redivivus, 47.1 mg/L against Meloidogyne incognita, and 167.7 mg/L against B. xylophilus [75]. Paeciloxazine (98) was isolated from Paecilomyces sp. BAUA3058, demonstrating moderate nematicidal activity against Rhabditis pseudoelongata and weak activity against some insects [76].
The metabolite paecilodepsipeptide A (37) was obtained from P. cinnamomeus BCC 9616. It possesses three D-amino acid residues and can act against the malarial parasite Plasmodium falciparum K1, with an IC50 value of 4.9 µM [4,40]. The compound harzialactone A (99) was isolated from the marine-derived fungus Paecilomyces sp. 7A22. It exhibited significant activity against Leishmania amazonensis with an IC50 value of 5.25 mg/mL and a moderate activity against intracellular amastigotes with an IC50 value of 18.18 mg/mL [77].
Two novel β-resorcylic acid lactones, paecilomycin E, F (100, 101), along with aigilomycin B (102) and aigialomycin F (103) were isolated from a strain of Paecilomyces sp. SC0924 [78]. Paecilomycin E (100) and aigialomycin F (103) exhibited antiplasmodial activity against the Plasmodium falciparum line 3D7 with IC50 values of 20.0 and 10.9 nM, respectively, and paecilomycin E, F (100, 101) and aigilomycin B (102) showed moderate activity against the P. falciparum line Dd2 [78]. Four metabolites containing pyrenocine I (104), pyrenocine A, B (105, 106) and citreoviridin (107) were produced by Paecilomyces sp. FKI-3573 [79]. These compounds exhibit in vitro antitrypanosomal activity, and pyrenocine A (105) showed the most potent activity with an IC50 value of 0.12 mg/mL [79].
2.5. Other Active Metabolites Derived from Paecilomyces
The metabolites spirotenuipesine A, B (108, 109) and paecilomycine A (110) were obtained from P. tenuipes. The three compounds showed potent activity in neurotrophic factor biosynthesis in glial cells [80,81]. A pyrrolooxazine, formoxazine (111), a dipyrroloquinone derivative, terreusinone (112), and a 2-oxazolidinone analogue, 3-[(2Z)-1-oxo-2-buten-1-yl]oxazolidin-2-one (113) were isolated from the marine-derived P. formosus [82]. The compounds 111 and 113 displayed potent radical-scavenging activity against DPPH, with IC50 values of 0.1 and 10 μM [82]. Terreusinone (112) exhibited a UV-A absorbing activity with an ED50 value of 70 µg/mL [83]. Phytotoxin 14-hydroxycornexistin (114), a member of the nonadride family, was obtained from P. variotii, exhibiting a potent activity against broadleaf weeds and a selectivity to corn [84]. A herbicidal antibiotic, cornexistin (115), was isolated from P. variotii SANK 21086, which shows non-selective, broad spectrum herbicidal activity against annual plants including mono- and dicotyledonous weeds and may be useful for postemergence weed control with selective protection of corn [85,86]. In addition, cornexistin (115) was also isolated from P. tenuipes [25].
2.6. Metabolites with Unknown Activity Derived from Paecilomyces
The metabolites paeciloquinone B (116) and paeciloquinone E, F (117, 118) were obtained from P. carneus P-177 [61,62]. Two compounds, catenioblin A, B (119, 120) were identified from P. cateniobliquus YMF1.01799 for the first time [73]. The metabolites paecilodepsipeptide B and C (121, 122), a xanthone glycoside, norlichexanthone-6-O-(4-O-methylglucopyranoside) (123), and hopane triterpene zeorin (124) were produced by P. cinnamomeus BCC 9616 [4,40]. Two novel macrocyclic tetralactams, gunnilactam B, C (125, 126) were produced by P. gunnii [45].
A series of compounds, including two α-pyrones, paecilopyrone A, B (127, 128); two cyclohexenones, phomaligol B, C (129, 130); and the analogues, phomapyrone B (131) and C (6), kojic acid (132), phomaligol A (3), methylphomaligol A (133), phomaligol A1 (134), acetylphomaligol A (135), phomaligol A hydroperoxide (136), and phomaligol A1 hydroperoxide (137), were identified from a strain of P. lilacinus derived from the marine sponge Petrosia sp. The compounds kojic acid (132), phomaligol A (3), and methylphomaligol A (133) were evaluated for their cytotoxicity against a small panel of human solid tumor cell lines and were found to be inactive up to a concentration of 30 µg/mL [7]. In addition, phomaligol A1 (134) can also be obtained from the blackleg fungus L. maculans [9]. Eleven metabolites, including paecilaminol (138), paecilaminol hydrochlorate (139), methyl linoleate (140), linoleate (141), oleinic acid (142), indole-3-carboxaldehyde (143), indolyl-3-carboxylic acid (144), 4-hydroxybenzoic acid (145), 9(11)-dehydroergosterol peroxide (146), (22E,24R)-5α,6α-epoxy-3β-hydroxyergosta-22-ene-7-one (147), and ergosterol peroxiden (148) were isolated from marine derived P. lilacinus ZBY-1 [46,47].
A novel pyridone alkaloid, militarinone C (149), was obtained from the mycelium of P. militaris [49]. Six secondary metabolites were obtained from P. tenuipes, which include (4S,10R)-4-hydroxy-8-oxygen-10-methyl solactone (150), tenuipesine A (151), paecilomycines B, C (152, 153), cepharosporolide C (154) (Figure 3) and E (155) [50,80,87]. Paecilocin A (156) and D (157), 4-(2-hydroxyethyl) phenol (158), stigmasta 4,6,8(14),22-tetraen-3-one, β-sitosterol (159) and stigmasterol (160) were reported from P. variotii [23,25]. Two bicyclic fatty acids, paecilonic acid A (161) and B (162), together with two benzannulated spiroketal derivatives, paeciloketal B (163) and 1-epi-paeciloketal B (164) were obtained from jellyfish-derived strain of P. variotii J08NF-1 [27,88]. The metabolites 5-methylresorcinol (165) and 2,4-dihydroxy-3,6-dimethylbenzaldehyde (166) were isolated from cultures of P. verticillatus [68].
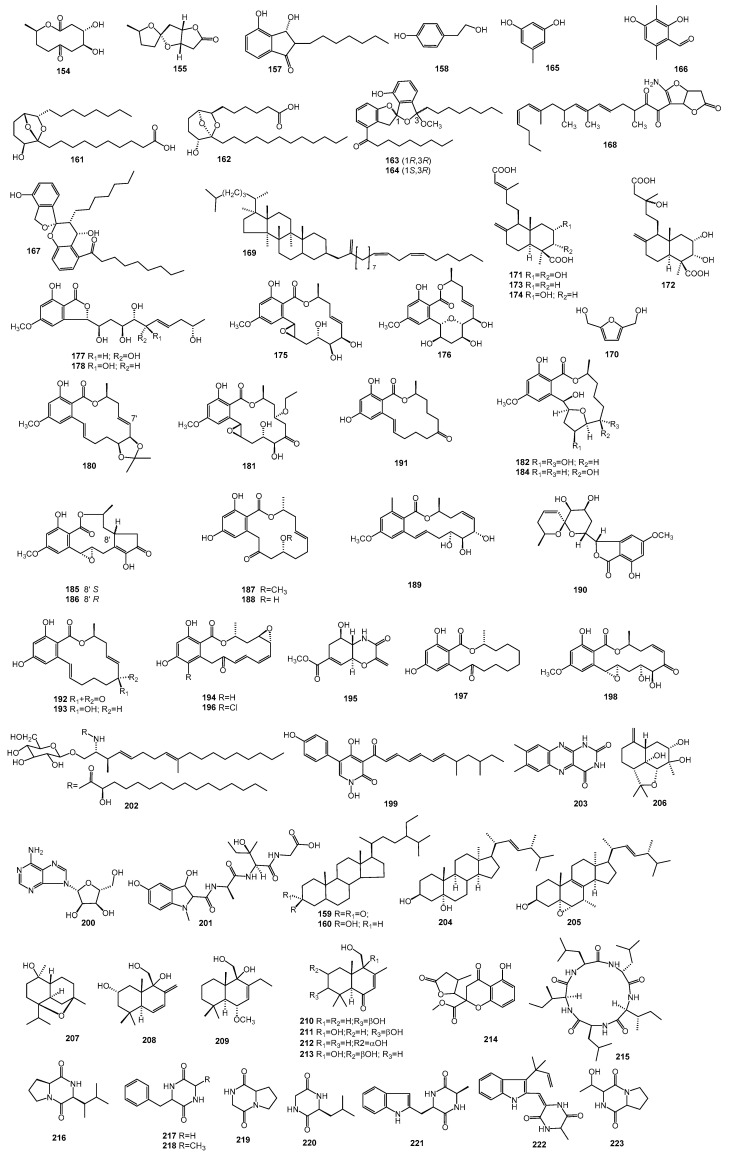
Figure 3.
The structures of metabolites produced by Paecilomyces (3).
Two novel unique spiro[chroman-2,1′(3′H)-isobenzofuran] derivative (167), (3R*,5E, 7E,9R*,11E,13Z)-1-((3′aS*,6′aR*)-2-amino-5-oxo-3′a,5′,6′,6′α-tetrahydrofu-ro[3′,2-b]furan-3-yl)-3,7,9,11-tetramethylheptadeca-5,7,11,13-tetraene-1,2-dione (168), together with cholesteryl linoleate (169), and 2,5-furandimethanol (170) were isolated from marine-derived strains of Paecilomyces [3,89].
A diterpenoid, paecilomycine B (171), with a five-membered lactone ring, and three labdane diterpenoids, botryosphaerin E (172), agathic acid (173), and rel-(1R,3S,4aS,5R,8aS)-5-[(3E)-4- carboxy-3-methylbut-3-en-1-yl]decahydro-3-hydrxy-1,4a-dimethyl-6-methylidenenaphthalene-1-carboxylic acid (174) were identified from the solid culture of Paecilomyces sp. ACCC 37762 [90]. A number of β-resorcylic acid lactones paecilomycin A–D (175–178), paecilomycin G–L (179–184), paecilomycin N, O (185,186), 4′-hydroxymonocillin IV (187), 4′-methoxymonocillin IV (188), zeaenol (189), aigialospirol (190), zearalenone (191), 7′-dehydrozearalenone (192), trans-7′,8′- dehydrozearalenol (193), monocillin I (194), monocillin Ⅲ (195), radicicol (196), lasicicol (197), and hypothemycin (198) were produced by a strain of Paecilomyces sp. SC0924 [30,31,32,78]. Furthermore, the compound 7′-dehydrozearalenone (192) was firstly isolated from Gibberella zeae [91].
The metabolites 1,5-dideoxy-3-C-methyl-arabitol (199) and adenosine (200) were identified from a strain of Paecilomyces sp. CAFT156 [38]. Several compounds, including a indolinepeptide, 3β,5-dihydroxy-l-N-methyl-indoline-2β-carbonyl amino-d-alanyl-erythro-β-hydoxyisoleucinyl-glycine (201), (4E, 8E, 2S, 2′R, 3R)-N-2′-hydroxy-hexadecanoyl-l-O-β-d-glucopyranosyl-9-methyl-4, 8-sphingadienin (202), alloxazine (203), along with the ergosterol derivatives, 3β,5α-dihydroxy-ergosta-7,22-diene (204), 5α,6α-epoxy-(22E,24R)-ergosta-8(14),22-diene-3β,7α-diol (205), were isolated from Paecilomyces sp. J300 [2,55]. Two cadinane-type sesquiterpenoids, paecilacadinol A and B (206, 207), two drimane-type sesquiterpenoids, ustusol D (208) and ustusol E (209), and the four analogs, deoxyuvidin B (210), 3β,9α,11-trihydroxy-6-oxodrim-7-ene (211), 2α,11-dihydroxy-6-ox-odrim-7-ene (212), and ustusol B (213) were obtained from the endophytic fungus Paecilomyces sp. TE-540 [70]. The metabolite, paecilin B (214) [57], and nine cyclic peptides, viscumamide (215), cyclo(Pro-Iso) (216), cyclo(Phe-Gly) (217), cyclo(Phe-Ana) (218), cyclo(Gly-Pro) (219), cyclo(Gly-Leu) (220), cyclo(Trp-Ana) (221), necoeshinulin A (222), and cyclo(Pro-Thr) (223) were identified from Paecilomyces sp. (tree 1–7) [92].
3. Conclusions
Since Paecilomyces were first described, many have been proven to be insect pathogens. As a result of the hardiness, wide adaptability, and ease of culture of most species of Paecilomyces, they play an important role in pest control, medicine, functional foods, environmental pollution control, and genetic engineering. Furthermore, Paecilomyces species are a source of bioactive natural products. At present, more than two hundred metabolites have been isolated and identified from Paecilomyces. In this paper, 223 metabolites produced from 13 species and various unidentified species of Paecilomyces were reviewed.
The structures of metabolites from Paecilomyces vary and have been reported ranging from polyketide, terpenoid, peptide, alkaloid, quinone, pyrone, sterol, fatty acid, xanthone, macrocyclic, pyrenocine analog, to radicicol-type forms. The representative secondary metabolites are the highly toxic linear peptides known as leucinostatins, the tyrosine kinase inhibitors paeciloquinones, the tetramic acid derivative, paecilosetin, and a series of trichothecanes. These metabolites have diverse biological activities, such as antimicrobial, antiviral, antitumor, herbicidal, insecticidal, antiplasmodial, antitrypanosomal, nematicidal, cytotoxic, enzyme inhibitors, phytotoxicity, and radical scavenging. The control effect of Paecilomyces is mainly the result of insecticidal activity of its metabolites. Many Paecilomyces metabolites not only directly cause disease in insects, but also have indirect insecticidal effect. For example, the fermentation filtrate of P. lilacinus showed obvious avoidance of soybean cyst nematode larvae and noticeably inhibited the infection of nematodes in roots [93].
In summary, Paecilomyces is a type of fungi with huge potential for development in various applications. With further study, Paecilomyces will play an increasingly important role in biological control, medicine and environmental protection.
Author Contributions
Investigation and collection references, D.Z.B., W.X., and L.G.H.; Writing, D.Z.B., W.X., and L.G.H.; Funding acquisition, W.X. and L.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (31860015, 31760024) and by the Applied Basic Research Foundation of Yunnan Province (202001BB050061, 2018FA006, 2018FB024).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mountfort D.O., Rhodes L.L. Anaerobic growth and fermentation characteristics of Paecilomyces lilacinus isolated from mullet gut. Appl. Environ. Microbiol. 1991;57:1963–1968. doi: 10.1128/AEM.57.7.1963-1968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon H.C., Kim K.R., Sang D.Z., Cho S.Y., Kang R.L. A new indolinepeptide from Paecilomyces sp. J 300. Arch. Pharm. Res. 2004;27:604–609. doi: 10.1007/BF02980157. [DOI] [PubMed] [Google Scholar]
- 3.Mosadeghzad Z., Zuriati Z., Asmat A., Gires U., Wickneswari R., Pittayakhajonwut P., Farahani G.H.N. Chemical components and bioactivity of the marine-derived fungus Paecilomyces sp. collected from Tinggi Island, Malaysia. Chem. Nat. Compd. 2013;49:621–625. doi: 10.1007/s10600-013-0693-y. [DOI] [Google Scholar]
- 4.Isaka M., Palasarn S., Kocharin K., Hywel-Jones N.L. Comparison of the bioactive secondary metabolites from the scale insect pathogens, anamorph Paecilomyces cinnamomeus, and teleomorph Torrubiella luteorostrata. J. Antibiot. 2007;60:577–581. doi: 10.1038/ja.2007.73. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Gloer J.B., Scott J.A., Malloch D. Terezines A-D: New amino acid-derived bioactive metabolites from the coprophilous fungus Sporormiella teretispora. J. Nat. Prod. 1995;58:93–99. doi: 10.1021/np50115a011. [DOI] [PubMed] [Google Scholar]
- 6.Putri S.P., Kinoshita H., Ihara F., Igarashi Y., Nihira T. Farinomalein, a maleimide-bearing compound from the entomopathogenic fungus Paecilomyces farinosus. J. Nat. Prod. 2009;72:1544–1546. doi: 10.1021/np9002806. [DOI] [PubMed] [Google Scholar]
- 7.Elbandy M., Shinde P.B., Hong J., Bae K.S., Kim M.A., Lee S.M., Jung J.H. α-Pyrones and yellow pigments from the sponge-derived fungus Paecilomyces lilacinus. Bull. Korean Chem. Soc. 2009;30:188–192. doi: 10.1002/chin.200929198. [DOI] [Google Scholar]
- 8.Yang G.H., Sandjo L., Yun K., Leutou A.S., Kim G.D., Hong D.C., Kang J.S., Hong J., Son B.W. Flavusides A and B, antibacterial cerebrosides from the marine-derived fungus Aspergillus flavus. Chem. Pharm. Bull. 2011;59:1174–1177. doi: 10.1248/cpb.59.1174. [DOI] [PubMed] [Google Scholar]
- 9.Pedras M.S.C., Morales V.M., Taylor J.L. Phomaligols and phomaligadiones: New metabolites from the blackleg fungus. Tetrahedron. 1993;49:8317–8322. doi: 10.1016/S0040-4020(01)81915-3. [DOI] [Google Scholar]
- 10.Arai T., Mikami Y., Fukushima K., Utsumi T., Yazawa K. A new antibiotic, leucinostatin, derived from Penicillium lilacinum. J. Antibiot. 1973;26:157–161. doi: 10.7164/antibiotics.26.157. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima K., Arai T., Mori Y.J., Tsuboi M., Suzuki M. Studies on peptide antibiotics, leucinostatins. I: Separation, physico-chemical properties and biological activities of leucinostatins A and B. J. Antibiot. 1983;36:1606–1612. doi: 10.7164/antibiotics.36.1606. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima K., Arai T., Mori Y.J., Tsuboi M., Suzuki M. Studies on peptide antibiotics, leucinostatins. II: The structures of leucinostatins A and B. J. Antibiot. 1983;36:1613–1630. doi: 10.7164/antibiotics.36.1613. [DOI] [PubMed] [Google Scholar]
- 13.Chen W.H., Liu H.Y., Long J.Y., Tao H.M., Lin X.P., Liao S.R., Yang B., Zhou X.F., Liu Y.H., Wang J.F. Asperpentenone A, A novel polyketide isolated from the deep-sea derived fungus Aspergillus sp. SCSIO 41024. Phytochem. Lett. 2020;35:99–102. doi: 10.1016/j.phytol.2019.11.009. [DOI] [Google Scholar]
- 14.Dosio F., Ricci M., Brusa P., Rossi C., Cattel L. Antibody-targeted leucinostatin A. J. Control Release. 1994:37–44. doi: 10.1016/0168-3659(94)90223-2. [DOI] [Google Scholar]
- 15.Rossi C., Tuttobello L., Ricci M., Casinovi C.G., Radios L. Leucinostatin D, a novel peptide antibiotic from Paecilomyces marquandii. J. Antibiot. 1987;40:130–133. doi: 10.7164/antibiotics.40.130. [DOI] [PubMed] [Google Scholar]
- 16.Radios L., Kajtar-Peredy M., Casinovi C.G., Rossi C., Ricci M., Tuttobello L. Leucinostatins H and K, two novel peptide antibiotics with tertiary amine-oxide terminal group from Paecilomyces marquandii isolation, structure and biological activity. J. Antibiot. 1987;40:714–716. doi: 10.7164/antibiotics.40.714. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera G.M., Butler M., Rodriguez M.A., Godeas A., Haddad R., Eberlin M.N. A sorbicillinoid urea from an intertidal Paecilomyces marquandii. J. Nat. Prod. 2006;69:1806–1808. doi: 10.1021/np060315d. [DOI] [PubMed] [Google Scholar]
- 18.Nilanonta C., Isaka M., Kittakoop P., Palittapongarnpim P., Kamchonwongpaisan S., Pittayakhajonwut D., Tanticharoen M., Thebtaranonth Y. Antimycobacterial and antiplasmodial cyclodepsipeptides from the insect pathogenic fungus Paecilomyces tenuipes BCC 1614. Planta Med. 2000;66:756–758. doi: 10.1055/s-2000-9776. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S., Krasnoff S.B., Underwood N.L., Renwick J.A.A., Roberts D.W. Isolation of beauvericin as an insect toxin from Fusarium semitectum and Fusarium moniliforme var. subglutinans. Mycopathologia. 1991;115:185–189. doi: 10.1007/BF00462223. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S., Montllor C., Hwang Y.S. Isolation of novel beauvericin analogues from the fungus Beauveria bassiana. J. Nat. Prod. 1995;58:733–738. doi: 10.1021/np50119a012. [DOI] [Google Scholar]
- 21.Hamill R.L., Higgens G.E., Boaz H.E., Gorman M. The structure op beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969;10:4255–4258. doi: 10.1016/S0040-4039(01)88668-8. [DOI] [Google Scholar]
- 22.Deol B.S., Ridley D.D., Singh P. Isolation of cyclodepsipeptides from plant pathogenic fungi. Aust. J. Chem. 1978;31:1397–1399. doi: 10.1071/CH9781397. [DOI] [Google Scholar]
- 23.Liu J., Li F., Kim E.L., Li J.L., Hong J., Bae K.S., Chung H.Y., Kim H.S., Jung J.H. Antibacterial polyketides from the jellyfish-derived fungus Paecilomyces variotii. J. Nat. Prod. 2011;74:1826–1829. doi: 10.1021/np200350b. [DOI] [PubMed] [Google Scholar]
- 24.Ayer W.A., Craw P.A., Nozawa K. Two 1H-naphtho(2,3-c)pyran-1-one metabolites from the fungus Paecilomyces variotii. Can. J. Chem. 1991;69:189–191. doi: 10.1139/v91-030. [DOI] [Google Scholar]
- 25.Abbas Z., Siddiqui B.S., Shahzad S., Sattar S., Begum S., Batool A., Choudhary M.I. Lawsozaheer, a new chromone produced by an endophytic fungus Paecilomyces variotii isolated from Lawsonia Alba Lam. inhibits the growth of Staphylococcus aureus. Nat. Prod. Res. 2020 doi: 10.1080/14786419.2020.1729148. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P., Li X.M., Wang J.N., Wang B.G. Oxepine-containing diketopiperazine alkaloids from the algal-derived endophytic fungus Paecilomyces variotii EN-291. Helv. Chim. Acta. 2015;98:800–804. doi: 10.1002/hlca.201400328. [DOI] [Google Scholar]
- 27.Wang H., Hong J., Yin J., Moon H.R., Liu Y.H., Wei X.Y., Oh D.C., Jung J.H. Dimeric octaketide spiroketals from the Jellyfish-derived fungus Paecilomyces variotii J08NF-1. J. Nat. Prod. 2015;11:2832. doi: 10.1021/acs.jnatprod.5b00594. [DOI] [PubMed] [Google Scholar]
- 28.Hirota A., Nakagawa M., Hirota H. Structure of paecilospirone, a new antibiotic from Paecilomyces. Agric. Biol. Chem. 1991;55:1187–1188. doi: 10.1271/bbb1961.55.1187. [DOI] [Google Scholar]
- 29.Wen L., Chen G., She Z., Yan C., Cai J., Mu L. Two new paeciloxocins from a mangrove endophytic fungus Paecilomyces sp. Russ. Chem. Bull. 2010;59:1656–1659. doi: 10.1007/s11172-010-0290-1. [DOI] [Google Scholar]
- 30.Xu L.X., Xue J.H., Zou Y., He S.J., Wei X.Y. Three new β-resorcylic acid lactones from Paecilomyces sp. SC0924. Chin. J. Chem. 2012;30:1273–1277. doi: 10.1002/cjoc.201200406. [DOI] [Google Scholar]
- 31.Xu L.X., Wu P., Wei H.H., Xue J.H., Hu X.P., Wei X.Y. Paecilomycins J–M, four new β-resorcylic acid lactones from Paecilomyces sp. SC0924. Tetrahedron Lett. 2013;54:2648–2650. doi: 10.1016/j.tetlet.2013.03.036. [DOI] [Google Scholar]
- 32.Xu L.X., Wu P., Xue J.H., Molnar I., Wei X.Y. Antifungal and cytotoxic β-resorcylic acid lactones from a Paecilomyces species. J. Nat. Prod. 2017;80:2215–2223. doi: 10.1021/acs.jnatprod.7b00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin F., Li Y., Lin R., Zhang X., Xie B. Antibacterial Radicicol Analogues from Pochonia chlamydosporia and Their Biosynthetic Gene Cluster. J. Agric. Food Chem. 2019;67:7266–7273. doi: 10.1021/acs.jafc.9b01977. [DOI] [PubMed] [Google Scholar]
- 34.Isaka M., Suyamsestakom C., Tanticharoen M. Aigialomycins A-E, new resorcylic macrolides from the marine mangrove fungus Aigialus parvus. J. Org. Chem. 2002;67:1561–1566. doi: 10.1021/jo010930g. [DOI] [PubMed] [Google Scholar]
- 35.Isaka M., Yangchum A., Intamas S., Kocharin K., Prabpai S. Aigialomycins and related polyketide metabolites from the mangrove fungus Aigialus parvus BCC 5311. Tetrahedron. 2009;65:4396–4403. doi: 10.1016/j.tet.2009.03.050. [DOI] [Google Scholar]
- 36.Ellestad G.A., Lovell F.M., Perkinson N.A., Hargreaves R.T., Mcgahren W.J. New zearalenone related macrolides and isocoumarins from an unidentified fungus. J. Org. Chem. 1978;43:2339–2343. doi: 10.1021/jo00406a007. [DOI] [Google Scholar]
- 37.Shao C.L., Wu H.X., Wang C.Y., Liu Q.A., Xu Y., Wei M.Y., Qian P.Y., Gu Y.C., Zheng C.J., She Z.G. Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. J. Nat. Prod. 2011;74:629–633. doi: 10.1021/np100641b. [DOI] [PubMed] [Google Scholar]
- 38.Talontsi F.M., Kenla T.N., Dittrich B., Douanla-Meli C., Laatsch H. Paeciloside A, a new antimicrobial and cytotoxic polyketide from Paecilomyces sp. strain CAFT156. Planta Med. 2012;78:1020–1023. doi: 10.1055/s-0031-1298622. [DOI] [PubMed] [Google Scholar]
- 39.Wen L., Lin Y.C., She Z.G., Du D.S., Chan W.L., Zheng Z.H. Paeciloxanthone, a new cytotoxic xanthone from the marine mangrove fungus Paecilomyces sp. (Tree1-7) J. Asian. Nat. Prod. Res. 2008;10:133–137. doi: 10.1080/10286020701273783. [DOI] [PubMed] [Google Scholar]
- 40.Isaka M., Palasarn S., Lapanun S., Sriklung K. Paecilodepsipeptide A, an antimalarial and antitumor cyclohexadepsipeptide from the insect pathogenic fungus Paecilomyces cinnamomeus BCC 9616. J. Nat. Prod. 2007;70:675–678. doi: 10.1021/np060602h. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y.X., Schneider B., Riese U., Schubert B., Li Z., Hamburger M. Farinosones A−C, neurotrophic alkaloidal metabolites from the entomogenous deuteromycete Paecilomyces farinosus. J. Nat. Prod. 2004;67:1854–1858. doi: 10.1021/np049761w. [DOI] [PubMed] [Google Scholar]
- 42.Lang G., Blunt J.W., Cummings N.J., Cole A.L.J., Munro M.H.G. Paecilosetin, a new bioactive fungal metabolite from a new zealand isolate of Paecilomyces farinosus. J. Nat. Prod. 2005;68:810–811. doi: 10.1021/np0500979. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y.X., Schneider B., Riese U., Schubert B., Hamburger M. (+)-N-Deoxymilitarinone A, a neuritogenic pyridone alkaloid from the insect pathogenic fungus Paecilomyces farinosus. J. Nat. Prod. 2006;69:436–438. doi: 10.1021/np050418g. [DOI] [PubMed] [Google Scholar]
- 44.Tang R., Zhou D., Kimishima A., Setiawan A., Arai M. Selective cytotoxicity of marine-derived fungal metabolite (3S,6S)-3,6-dibenzylpiperazine-2,5-dione against cancer cells adapted to nutrient starvation. J. Antibiot. 2020 doi: 10.1038/s41429-020-0340-3. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Y.B., Zhang J.Y., Wei L.F., Shi M.M., Wang J.F.S. Gunnilactams A–C, macrocyclic tetralactams from the mycelial culture of the entomogenous fungus Paecilomyces gunnii. J. Nat. Prod. 2017;80:1935–1938. doi: 10.1021/acs.jnatprod.7b00060. [DOI] [PubMed] [Google Scholar]
- 46.Cui X., Li C.W., Wu C.J., Hua W., Cui C.B., Zhu T.J., Gu Q.Q. Secondary metabolites of Paecilomyces lilacinus ZBY-1 and their antitumor activity. J. Int. Pharm. Res. 2013;40:765–771. doi: 10.13220/j.cnki.jipr.2013.06.032. [DOI] [Google Scholar]
- 47.Cui X., Li C.W., Wu C.J., Hua W., Cui C.B., Zhu T.J., Gu Q.Q. Metabolites of Paecilomyces lilacinus ZBY-1 from deep-sea water and their antitumor activity. J. Int. Pharm. Res. 2013;40:177–186. doi: 10.13220/j.cnki.jipr.2013.02.005. [DOI] [Google Scholar]
- 48.Schmidt K., Günther W., Stoyanova S., Schubert B., Li Z.Z., Hamburger M. Militarinone A, a neurotrophic pyridone alkaloid from Paecilomyces militaris. Org. Lett. 2002;4:197–199. doi: 10.1021/ol016920j. [DOI] [PubMed] [Google Scholar]
- 49.Karen S., Ulrike R., Li Z.Z., Hamburger M. Novel tetramic acids and pyridone alkaloids, militarinones B, C, and D, from the insect pathogenic fungus Paecilomyces militaris. J. Nat. Prod. 2003;66:378–383. doi: 10.1021/np020430y. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y.B., Pang H.Y., Wang J.F., Chen D.X., Shi G.W., Huang J.Z. Novel diketopiperazine and ten-membered macrolides from the entomogenous fungus Paecilomyces tenuipes. Chem. J. Chin. Univ. 2014;35:1665–1669. doi: 10.7503/cjcu20140080. [DOI] [Google Scholar]
- 51.Zhang P., Li X.M., Mao X.X., Mandi A., Kurtan T., Wang B.G. Varioloid A, a new indolyl-6,10b-dihydro-5aH-1 benzofuro 2,3-b indole derivative from the marine alga-derived endophytic fungus Paecilomyces variotii EN-291. Beilstein J. Org. Chem. 2016;12:2012–2018. doi: 10.3762/bjoc.12.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang P., Li X.M., Wang J.N., Li X., Wang B.G. Prenylated indole alkaloids from the marine-derived fungus Paecilomyces variotii. Chin. Chem. Lett. 2015;26:313–316. doi: 10.1016/j.cclet.2014.11.020. [DOI] [Google Scholar]
- 53.Fujii N., Yamashita Y., Ando K., Agatsuma T., Saitoh Y., Gomi K., Nishiie Y., Nakano H. UCE1022, a new antitumor antibiotic with topoisomerase I mediated DNA cleavage activity, from Paecilomyces. J. Antibiot. 1994;47:949–951. doi: 10.7164/antibiotics.47.949. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita Y., Saitoh Y., Ando K., Takahashi K., Ohno H., Nakano H. Saintopin, a new antitumor antibiotic with topoisomerase II dependent DNA cleavage activity, from Paecilomyces. J. Antibiot. 1990;43:1344–1346. doi: 10.7164/antibiotics.43.1344. [DOI] [PubMed] [Google Scholar]
- 55.Kwon H.C., Sang D.Z., Cho S.Y., Sang U.C., Kang R.L. Cytotoxic ergosterols from Paecilomyces sp. J300. Arch. Pharm. Res. 2002;25:851–855. doi: 10.1007/BF02977003. [DOI] [PubMed] [Google Scholar]
- 56.Wen L., Guo Z.Y., Liu F., Wan Q., She Z.G., Lin Y.C., Fu L.W. Studies on the secondary metabolites and bioactivity of mangrove endophytic fungus Paecilomyces sp.(tree1-7) Chem. Res. Appl. 2009;21:198–202. [Google Scholar]
- 57.Guo Z.Y., She Z.G., Shao C.L., Wen L., Liu F., Zheng Z.H., Lin Y.C. 1H and 13C NMR signal assignments of Paecilin A and B, two new chromone derivatives from mangrove endophytic fungus Paecilomyces sp. (tree 1–7) Magn. Reson. Chem. 2007;47:777–780. doi: 10.1002/mrc.2035. [DOI] [PubMed] [Google Scholar]
- 58.Cai X.L., Gao J.P., Li Q., Wen L., Lin Y.C. Cytotoxicity of the secondary metabolites of marine mangrove fungus Paecilomyces sp. tree 1-7 on human hepatoma cell line HepG2. China J. Chin. Mater. Med. 2008;31:864–865. doi: 10.13863/j.issn:1001-4454.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 59.Li X.L., XU Y., Pan D.R., Zhang L., Guo Z.Y., Zou K. Five anticancer anthraquinone derivates from mangrove endophytic fungus Paecilomyces sp. (tree1-7) J. China Three Gorges Univ. 2010;32:101–105. doi: 10.3969/j.issn.1672-948X.2010.04.026. [DOI] [Google Scholar]
- 60.Shindo K., Suzuki H., Okuda T. Paecilopeptin, a new cathepsin S inhibitor produced by Paecilomyces carneus. Biosci. Biotechnol. Biochem. 2002;66:2444–2448. doi: 10.1271/bbb.66.2444. [DOI] [PubMed] [Google Scholar]
- 61.Petersen F., Fredenhagen A., Mett H., Lydon N.B., Delmendo R., Jenny H.B., Peter H.H. Paeciloquinones A, B, C, D, E and F: New potent inhibitors of protein tyrosine kinase produced by Paecilomyces carneus. I. Taxonomy, Fermentation, Isolation and Biological Activity. J. Antibiot. 1995;48:191–198. doi: 10.7164/antibiotics.48.191. [DOI] [PubMed] [Google Scholar]
- 62.Fredenhagen A., Hug P., Sauter H., Peter H.H. Paeciloquinones A, B, C, D, E and F: New potent inhibitors of protein tyrosine kinase produced by Paecilomyces carneus. II. Characterization and structure determination. J. Antibiot. 1995;48:199–204. doi: 10.7164/antibiotics.48.199. [DOI] [PubMed] [Google Scholar]
- 63.Fredenhagen A., Mett H., Meyer T., Buchdunger E., Regenass U., Roggo B.E., Petersen F. Protein tyrosine kinase and protein kinase C inhibition by fungal anthraquinones related to emodin. J. Antibiot. 1995;48:1355–1358. doi: 10.7164/antibiotics.48.1355. [DOI] [PubMed] [Google Scholar]
- 64.Saqib B., Ali L., Khan A.L., Shahzad R., Asaf S.J., Imran M., Kang S.M., Kim S.K., Lee I.J. Endophytic fungus Paecilomyces formosus LHL 10 produces sester-terpenoid YW3548 and cyclic peptide that inhibit urease and α-glucosidase enzyme activities. Arch. Microbiol. 2018 doi: 10.1007/s00203-018-1562-7. [DOI] [PubMed] [Google Scholar]
- 65.Lu R.L., Liu X.X., Gao S., Zhang W.C., Peng F., Hu F.L., Huang B., Chen L.Y., Bao G.H., Li C.R. New tyrosinase inhibitors from Paecilomyces gunnii. J. Agric. Food Chem. 2014;62:11917–11923. doi: 10.1021/jf504128c. [DOI] [PubMed] [Google Scholar]
- 66.Teles A.P.C., Takahashi J.A. Paecilomide, a new acetylcholinesterase inhibitor from Paecilomyces lilacinus. Microbiol. Res. 2013;168:204–210. doi: 10.1016/j.micres.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Horn W.S., Smith J.L., Bills G.F., Raghoobar S.L., Mandala S.M., Helms G.L., Kurtz M.B. Sphingofungins E and F: Novel serinepalmitoyl transferase inhibitors from Paecilomyces variotii. J. Antibiot. 1992;45:1692–1696. doi: 10.7164/antibiotics.45.1692. [DOI] [PubMed] [Google Scholar]
- 68.Wei P.Y., Li L., Yang C., Luo D.Q., Zheng Z.H., Lu X.H., Shi B.Z. A novel oxybis cresol verticilatin with highly varying degrees of biological activities from the insect pathogenic fungus Paecilomyces verticillatus. J. Asian. Nat. Prod. Res. 2014;16:1153–1157. doi: 10.1080/10286020.2014.959438. [DOI] [PubMed] [Google Scholar]
- 69.Murakawa S., Sakai K., Endo A. Isolation of 3α-hydroxy-3, 5-dihydro ML-236C (Sodium Salt) from Paecilomyces viridis L-68. J. Antibiot. 1994;47:108–109. doi: 10.7164/antibiotics.47.108. [DOI] [PubMed] [Google Scholar]
- 70.Xu K., Zhou Q., Li X.Q., Luo T., Yuan X.L., Zhang Z.F., Zhang P. Cadinane- and drimane-type sesquiterpenoids produced by Paecilomyces sp. TE-540, an endophyte from Nicotiana tabacum L., are acetylcholinesterase inhibitors. Bioorg. Chem. 2020;104:104252. doi: 10.1016/j.bioorg.2020.104252. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura T., Yasuda H., Obayashi A., Tanabe O., Matsumura S., Ueda F., Ohata K. Phenopicolinic acid, a new microbial product inhibiting dopamine beta-hydroxylase. J. Antibiot. 1975;28:477–478. doi: 10.7164/antibiotics.28.477. [DOI] [PubMed] [Google Scholar]
- 72.Uchida R., Shiomi K., Sunazuka T., Inokoshi J., Nishizawa A., Hirose T., Tanaka H., Iwai Y., Omura S. Kurasoins A and B, new protein farnesyltransferase inhibitors produced by Paecilomyces sp. FO-3684. II. Structure elucidation and total synthesis. J. Antibiot. 1996;49:932–934. doi: 10.7164/antibiotics.49.932. [DOI] [PubMed] [Google Scholar]
- 73.Wu H.Y., Wang Y.L., Tan J.L., Zhu C.Y., Li D.X., Huang R., Zhang K.Q., Niu X.M. Regulation of the growth of cotton bollworms by metabolites from an entomopathogenic fungus Paecilomyces cateniobliquus. J. Agric. Food Chem. 2012;60:5604–5608. doi: 10.1021/jf302054b. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y.G., Yuan W.P., Xia X.K., Liu X., Meng X.M., Wang X.J., Zhang M.S., Liu C.H. Isolation and identification of the nematicidal secondary metabolites from one strain of entomogenous fungi. Chin. J. Pestic. Sci. 2010;12:225–228. [Google Scholar]
- 75.Liu Y.J., Zhai C.Y., Liu Y., Zhang K.Q. Nematicidal activity of Paecilomyces spp. and isolation of a novel active compound. J. Microbiol. 2009;47:248–252. doi: 10.1007/s12275-009-0012-2. [DOI] [PubMed] [Google Scholar]
- 76.Kanai Y., Fujimaki T., Kochi S.I., Konno H., Kanazawa S., Tokumasu S. Paeciloxazine, a novel nematicidal antibiotic from Paecilomyces sp. J. Antibiot. 2004;57:24–28. doi: 10.7164/antibiotics.57.24. [DOI] [PubMed] [Google Scholar]
- 77.Braun G.H., Ramos H.P., Candido A.C.B.B., Pedroso R.C.N., Pietro R.C.L.R. Evaluation of antileishmanial activity of harzialactone A isolated from the marine-derived fungus Paecilomyces sp. Nat. Prod. Res. 2019:1–4. doi: 10.1080/14786419.2019.1619725. [DOI] [PubMed] [Google Scholar]
- 78.Xu L.X., He Z.X., Xue J.H., Chen X.P., Wei X.Y. β-Resorcylic acid lactones from a Paecilomyces fungus. J. Nat. Prod. 2010;73:885–889. doi: 10.1021/np900853n. [DOI] [PubMed] [Google Scholar]
- 79.Hashida J., Niitsuma M., Iwatsuki M., Mori M., Ishiyama A., Namatame M., Nishihara-Tsukashima A., Nonaka K., Ui H., Masuma R. Pyrenocine I, a new pyrenocine analog produced by Paecilomyces sp. FKI-3573. J. Antibiot. 2010;63:559–561. doi: 10.1038/ja.2010.65. [DOI] [PubMed] [Google Scholar]
- 80.Kikuchi H., Miyagawa Y., Sahashi Y., Inatomi S., Haganuma A., Nakahata N., Oshima Y. Novel trichothecanes, paecilomycine A, B, and C, isolated from entomopathogenic fungus, Paecilomyces tenuipes. Tetrahedron Lett. 2004;45:6225–6228. doi: 10.1016/j.tetlet.2004.06.107. [DOI] [Google Scholar]
- 81.Kikuchi H., Miyagawa Y., Sahashi Y., Inatomi S., Haganuma A., Nakahata N., Oshima Y. Novel spirocyclic trichothecanes, spirotenuipesine A and B, isolated from entomopathogenic fungus, Paecilomyces tenuipes. J. Org. Chem. 2004;69:352–356. doi: 10.1021/jo035137x. [DOI] [PubMed] [Google Scholar]
- 82.Yun K., Leutou A.S., Rho J.R., Son B.W. Formoxazine, a new pyrrolooxazine, and two amines from the Marine–Mudflat-derived fungus Paecilomyces formosus. Bull. Korean Chem. Soc. 2016;37:103–104. doi: 10.1002/bkcs.10615. [DOI] [Google Scholar]
- 83.Lee S.M., Li X.F., Jiang H., Cheng J.G., Son B.W. Terreusinone, a novel UV-A protecting dipyrroloquinone from the marine algicolous fungus Aspergillus terreus. Cheminform. 2004;44:7707–7710. doi: 10.1002/chin.200405212. [DOI] [Google Scholar]
- 84.Fields S.C., Mireles-Lo L., Gerwick B.C. Hydroxycornexistin: A new phytotoxin from Paecilomyces variotii. J. Nat. Prod. 1996;59:698–700. doi: 10.1021/np960205e. [DOI] [Google Scholar]
- 85.Takahashi S., Nakajima M., Kinoshita T., Haruyama H., Sugai S., Honma T., Sato S., Haneishi T. Hydantocidin and cornexistin-new herbicidal antibiotics. ACS Symp. Ser. 1993;551:74–84. doi: 10.1021/bk-1994-0551.ch006. [DOI] [Google Scholar]
- 86.Nakajima M., Itoi K., Takamatsu Y., Sato S., Furukawa Y., Furuya K., Honma T., Kadotani J., Kozasa M., Haneishi T. Cornexistin: A new fungal metabolite with herbicidal activity. J. Antibiot. 1991;44:1065–1072. doi: 10.7164/antibiotics.44.1065. [DOI] [PubMed] [Google Scholar]
- 87.Kikuchi H., Miyagawa Y., Nakamura K., Sahashi Y., Inatomi S., Oshima Y. A novel carbon skeletal trichothecane, tenuipesine A, isolated from an entomopathogenic fungus, Paecilomyces tenuipes. Org. Lett. 2004;6:4531–4533. doi: 10.1021/ol048141j. [DOI] [PubMed] [Google Scholar]
- 88.Wang H.B., Hong J., Yin J., Liu J., Liu Y.H., Choi J.S., Jung J.H. Paecilonic acids A and B, bicyclic fatty acids from the jellyfish-derived fungus Paecilomyces variotii J08NF-1. Bioorganic Med. Chem. Lett. 2016;26:2220–2223. doi: 10.1016/j.bmcl.2016.03.057. [DOI] [PubMed] [Google Scholar]
- 89.Namikoshi M., Kobayashi H., Yoshimoto T., Meguro S. Paecilospirone, a unique spiro chroman-2,1 ‘(3’H)-isobenzofuran derivative isolated from tropical marine fungus Paecilomyces sp. Chem. Lett. 2000:308–309. doi: 10.1246/cl.2000.308. [DOI] [Google Scholar]
- 90.Zhou K., Zhao X.L., Han L.P., Cao M.M., Chen C., Shi B.Z., Luo D.Q. Paecilomycines A and B, novel diterpenoids, isolated from insect-pathogenic fungi Paecilomyces sp. ACCC 37762. Helv. Chim. Acta. 2015;98:642–649. doi: 10.1002/hlca.201400272. [DOI] [Google Scholar]
- 91.Bolliger G., Tamm C. Vier neue Metabolite von Giberella zeae: 5-Formyl-zearalenon, 7-Dehydrozearalenon, 8-Hydroxy- und 8-epi-Hydroxy-zearalenon Helv. Chim. Acta. 1972;55:3030–3048. doi: 10.1002/hlca.19720550835. [DOI] [Google Scholar]
- 92.Guo Z.Y., Huang Z.J., Wen L., Wan Q., Liu F., She Z.G., Lin Y.C., Zhou S.N. The metabolites of cyclic peptides from three endophytic mangrove fungi. China J. Chin. Mater. Med. 2007;30:1526–1529. doi: 10.13863/j.issn1001-4454.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 93.Sun M.H., Liu X.Z. Effects of Paecilomyces lilacinus M-14 fermentation filtrate on the affinity between soybean cyst nematode and soybean root. Acta Phytopathol. Sin. 2004;34:376–379. doi: 10.13926/j.cnki.apps.2004.04.015. [DOI] [Google Scholar]