Abstract
A general regioselective one-pot synthesis of 1,2-benzothiazine 1,1-dioxides from 2-iodo benzenesulfonamide moieties and allenylstannanes is described using a domino Stille-like/Azacyclization reaction. The conditions developed also opened a novel access to β-carbolinones, indolopyranones, thienopyranones and pyrano-imidazopyridines.
Keywords: regioselective synthesis; 1,2-benzothiazine 1,1-dioxides; β-carbolinones; indolo[2,3-c] and [3,2-c]pyrane-1-one derivatives; Stille/Heterocyclization reaction
1. Introduction
With the development of efficient cross-coupling catalysts, the heterocyclic synthesis of compounds of various interests has been facilitated in terms of numerous parameters such as temperature, catalytic charge, selectivity or efficiency. Several methodologies have been set up to create carbon-carbon or carbon-heteroatom bonds and applied to intramolecular cyclization and heterocyclization reactions. The development of palladium cross-coupling processes in particular has enabled easy access to various cores with oxygen, nitrogen or sulfur as heteroelement.
In this paper we focus on 1,2-benzothiazine 1,1-dioxide also known as benzosultam (1), β-carbolinone (2), pyranoindole (3,4), thienopyranone (5) and pyrano-imidazopyridine (6) cores (Figure 1).
Figure 1.
General structures of synthesized cores.
Molecules possessing the moiety 3 are known to inhibit the hepatitis C virus NS5B polymerase [1,2] while 1,2-benzothiazine 1,1-dioxide derivatives possess versatile biological activities [3,4]. For example the 1 moiety constitutes the heterocyclic core of oxicam (e.g., Ampiroxicam A and meloxicam B, Figure 2), a class of non-steroidal anti-inflammatory drugs [3]. In addition, benzothiazine dioxide derivatives exhibit strong inhibitory properties against HIV integrand [5], and Calpain 1 [6]. Compounds 3 and 4 may constitute interesting precursors to β- and γ-carbolinone alkaloids respectively. The β-carbolin-1-ones skeleton 2 is found in the structure of numerous natural products of which some derivatives act on the central nervous system [7,8] and are recognized as HeLa and HLE cancer cell line inhibitors [9]. β-carbolin-1-ones are also important intermediates for the synthesis of many alkaloids [10,11], such as Bauerine C [12] and Secofascaplysin A [13] (Figure 2), and they are effective as anti-diabetics ATAD25 agents [14] and inhibitors [15]. Pyrano [3’,4’:4,5]imidazo[1,2-a]pyridin-1-one derivatives are known for their interesting pharmacological activities particularly as antitumoral agents [16].
Figure 2.
Biologically active benzothiazine dioxides and β-carbolin-1-ones.
Numerous ways to access these moieties are depicted in the literature. The most popular route used is metal catalyzed bond formation [17,18,19,20,21,22,23,24]. For example, β-carbolinones are accessed via Heck [15], gold cycloisomerization [25] or copper catalyzed C-N bond formation [26]. Other synthetic approaches for β-carbolinones have been developed via phase-transfer catalyzed intramolecular cyclization of 3-alkynylindole-2-carboxamides [27], intramolecular Diels-Alder reaction [28] and cyclization of pyridone ring [8,29]. Recently, Xia et al. described the synthesis of β-carbolinones via Pd/Cu catalyzed tandem C-H aminocarbonylation and dehydrogenation of tryptamines [30]. Although the 1,2-benzothiazine 1,1-dioxide core is widely accessed via Heck coupling [31], other metal catalyzed methods like domino Sonogashira-azacyclization [18], silver assisted aza-cyclization of enyne [19], gold-catalyzed cycloisomerization of terminal alkene [32], gold(I)-catalyzed ammonium formation strategy [33], Rh(III)-ctalyzed strategy by the ortho C-H activation [34] or C-H bond alkynylation of aryl sulfonamides [21] have been published. Moreover, 1,2-benzothiazine 1,1-dioxides has been achieved by a palladium-catalyzed tandem-cyclization of ynamides [35]. Recently, Volla et al. [36] reported a cobalt-catalyzed C-H activation of arylsulfonamides and their intermolecular heteroannulation reaction with allenes for the synthesis in a highly regioselective manner of aryl fused sultams. Due to their various pharmacological importances, the development of a novel and simple synthetic method for the synthesis of benzothiazine dioxide and derivatives would be highly desirable. Metal-catalyzed transition carbon-carbon bond formation has attracted much attention over the last three decades. The palladium-catalyzed cross-coupling reaction is one of the most efficient methods for the construction of C-C bonds. These reactions are frequently employed to promote the synthesis of numerous natural products or bio-active molecules. Among them the value of the Stille cross-coupling reaction [37] is commonly recognized in the scientific community and the reactivity of aromatic halide is widely known using that methodology. We previously published an easy and mild palladium-catalyzed process for rapid access to α-pyrones, α-pyridones and isocoumarins by one-pot approaches involving the intramolecular addition of carboxylic acid derivatives to allenyl moiety (Scheme 1). The reactions proceeded as a tandem coupling heterocyclization sequence in the presence of palladium catalyst and an alkaline carbonate.
Scheme 1.
Convergent Stille coupling/heterocyclization reaction of β-iodo-α,β-unsaturated carboxylic acid or carboxamide systems.
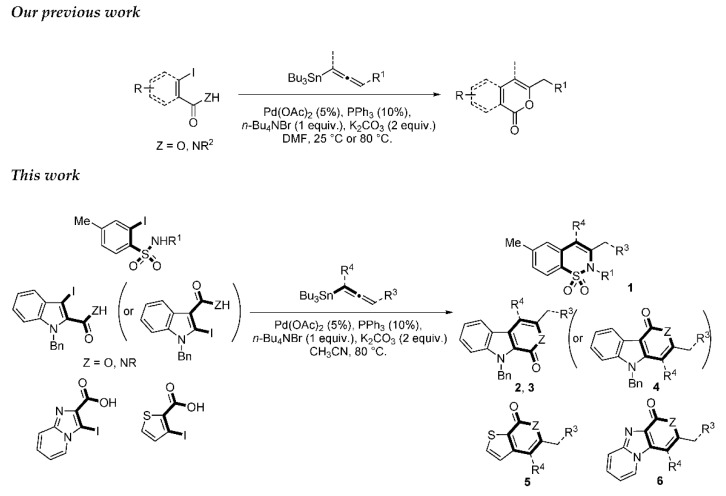
We report here a valuable synthetic extension of this method onto aromatic and heteroaromatic substrates such as o-iodo arylsulfonamide, indole, thiophene and imidazo[1,2-a]pyridine bearing a β-iodo-α,β-unsaturated carboxylic acid or carboxamide system in order to produce 1, 2, 3, 4, 5 and 6 cores (Scheme 1). To the best of our knowledge, no cross-coupling using allenyltin reagent has been reported to date to access to these compounds.
2. Results and Discussion
Our investigations began with assays on aromatic sulfonamides (7) for the synthesis of 1,2-benzothiazine 1,1-dioxide derivatives 1. The required 7 were prepared from the corresponding sulfonyl chloride by treatment with alkylamines, followed by a reaction with n-BuLi then elemental iodine according to the procedure reported in the literature (Scheme 2)[38,39].
Scheme 2.
Synthesis of 2-iodo benzenesulfonamide derivatives.
A series of experiments on the same basis as in our previous work was carried out to optimise the reaction conditions and establish the minimum requirements for this process. It was found that for good performance one needs at least 0.05 equiv. of palladium acetate, 0.1 equiv. of triphenylphosphine, 1 equiv. of tetrabutylammonium bromide and 2 equiv. of potassium carbonate in MeCN. Surprisingly, compared to our previous work on aryl bearing a β-iodo α,β-unsaturated carboxylic moiety, assays in dimethyl formamide ended-up with extremely poor yield. No evidence for the moment has been found for the moment to explain that observation. The use of a phosphine ligated palladium (0) catalyst such as tetrakis(triphenylphosphine)-palladium(0) is also suitable for the transformation, giving very similar yields. As expected, in the absence of Pd, no reaction occurred. The reaction requires temperatures of at least 80 °C to proceed. Below that temperature, no reaction was observed and starting materials were fully recovered. Compared to terminal alkynes used in Sonogashira like reactions, allenyltin offers a major advantage in terms of regioselectivity (see Scheme 3). Published experiments using a Pd catalyzed tandem Sonogashira/azacyclization or Ag catalyzed intramolecular Csp-azacyclization resulted in a mixture of 5-exo dig and 6-endo dig cyclization products [40,41]. This is because alkynes offer two attack areas resulting in two possible cyclization products (see Scheme 3, path a). Unlike the latter, the allene structure has a well-defined electrophilic area located on the digonal carbon (see Scheme 3, path b). This provides a regiospecific outcome to the reaction and therefore an undeniable advantage in terms of selectivity in comparison to alkyne cyclizations. In addition, allenyltin reagents offer an important feature as they can be used to transfer small volatile fragments such as C3 hydrocarbon because of the heavy weight of the trialkyltin group.
Scheme 3.
Difference between alkyne and allenyltin reagent in the synthetic paths of 2H-1,2-benzothiazine 1,1-dioxide derivatives.
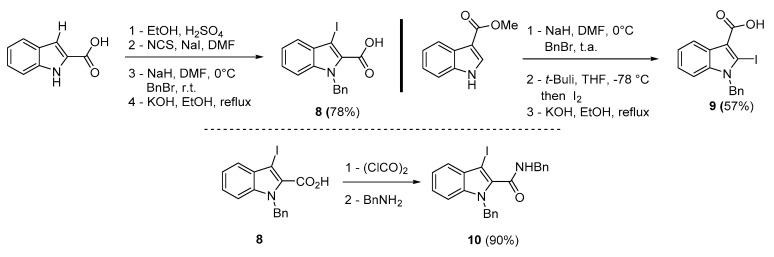
To broaden the scope of the use of allenyltin reagents, we extended our investigations to indole derivatives bearing a β-iodo α,β-unsaturated carboxylic or carboxamide moiety (8, 9 and 10). Compounds 8, 9 and 10 were synthesized in good yields starting from the corresponding commercial indoles (Scheme 4). Compound 8 was obtained in four steps from (1H)indole-2-carboxylic acid [42,43]. After esterification of the starting indole and halogenation of the ester with N-chlorosuccinimide/sodium iodide (NCS/NaI) in DMF, the resulting indole was reacted with benzylbromide and saponified into 8 in good yield. It was impossible to obtain indole 9 in the same way. Methyl indole-3-carboxylate was benzylated prior to halogenation and saponification. A treatment with t-BuLi and molecular iodine led to the synthesis of 9 in good yield [42], while the use of n-BuLi or s-BuLi led to moderate to poor yields. Compound 10 was obtained in good yield by treatment of 8 with oxalyl chloride followed by a reaction with benzylamine [27]. Having the starting materials, we subjected them to react with allenyltin derivatives (Table 1). No significant difference in the behavior of the transformation between aromatic and heteroaromatic substrates was found, except for the fact that DMF proved to be surprisingly inefficient and led to poor yields.
Scheme 4.
Synthesis of 3 (or 2)-iodoindole-2(or 3)-carboxylic acid 8, 9 and 3-iodoindole-2-carboxamide 10.
Table 1.
Synthesis of 1, 2, 3, 4, 5 and 6 via tandem Stille coupling/heterocyclization reaction.
| Entry | R1 | Z | Allenylstannane | Product | N° | Yield (%) a |
|---|---|---|---|---|---|---|
| 1 | Bn |

|

|
1a | 71 | |
| 2 | Bn |

|
|
1b | 80 | |
| 3 | Bn |

|

|
1c | 77 | |
| 4 | CH(CH3)Ph |

|

|
1d | 76 | |
| 5 | Allyl |

|

|
1e | 73 | |
| 6 | N-Bn |

|

|
2 | 73 | |
| 7 | O |

|

|
3a | 56 | |
| 8 | O |

|

|
3b | 62 | |
| 9 | O |

|

|
3c | 76 | |
| 10 | O |

|

|
4a | 57 | |
| 11 | O |

|

|
4b | 60 | |
| 12 | O |

|

|
5 | 72 | |
| 13 | O |

|

|
6 | 68 |
a Isolated yield.
As expected, 10 led to the synthesis of β-carbolinone 2 (entry 6), showing an efficient route to that important class of alkaloids. Note that in the case of amide 10, the replacement of the benzyl group with the acetyl group proved to be ineffective, as only the formation of a few traces of cyclization product was observed, indicating that the nucleophilicity of the amine is an essential parameter in this heterocyclization reaction. In the same way, 8 and 9 afforded indolo[2,3-c]pyran-1-ones 3a–c and indolo[3,2-c]pyran-1-ones 4a,b respectively with reasonable to good yields. A certain number of methods to access these indolopyranones have been reported in the literature. For example, compounds 3 and 4 can be accessed from γ-ketoester cyclization [44], anhydride rearrangement [45,46], metal-catalyzed enyne cyclization [47,48], or metal-catalyzed coupling [49,50]. We also published recently a convenient Cu-catalyzed domino route to these type of molecules. However, the present study shows that although the copper catalyzed process is cheaper in term of catalyst, the tandem Stille coupling/heterocyclization using allenyltin reagents offers the possibility of accessing a wide variety of new heterocyclic compounds, and the reaction requires a lower temperature than in the case of Cu-catalyzed cyclization. Likewise, this strategy has been successfully extended to 3-iodothiophene-2-carboxylic acid and 3-iodoimidazo[1,2-a]pyridin-2-carboxylic acid to lead, with good yields, to thieno[2,3-c] pyran-7(7H)-one 5 and pyrano[3’,4’:4,5]imidazo[1,2-a]pyridin-1-one 6, respectively (entries 12 and 13). Note that few synthesis of this type of compound has been reported to date [51,52].
3. Materials and Methods
3.1. General Protocol for Synthesis of 1,2-Benzothiazine 1,1-dioxides 1
To a Schlenk tube, under argon, containing 3.1 mmol of sulfonamide 6 in CH3CN (10 mL), potassium carbonate (860 mg, 2 equiv.), tetrabutylammonium bromide (1 g, 1 equiv.), triphenylphosphine (80 mg, 10 mol %) and Pd(OAc)2 (35 mg, 5 mol %) were added successively. The mixture was degassed, placed under nitrogen and well-stirred during 10 min. Allenyltributyltin (5.9 mmol, 1 equiv.) was then added. After 4 h at reflux, the mixture was hydrolyzed with water, and the organic phases were extracted with ethyl acetate. The combined organic extracts were dried over MgSO4, concentrated under vacuum, and the resulting residue was purified by silica gel column chromatography (petroleum ether/diethyl ether = 90/10) to afford the desired product 1.
2-Benzyl-3,7-dmethyl-1,2-benzothiazine 1,1-dioxide (1a), 71%; yellow solid; mp: 119–121 °C; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.83 (1H, d, J = 8.1 Hz), 7.30–7.22 (m, 4H), 7.14–7.12 (m, 3H), 6.13 (s, 1H), 5.05 (s, 2H), 2.47 (s, 3H), 2.17 (s, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 142.8, 139.8, 137.1, 133.3, 129.1 (2C), 128.8, 128.3, 127.9, 127.0 (2C), 126.9, 121.9, 110.0, 48.7, 22.0, 21.2. LR-MS (EI, 70 eV): m/z (%): 299 [M]+ HR-MS (ESI): Anal. Calcd for C17H17NO2S [M + H]+ 300.0980, found 300.0995.
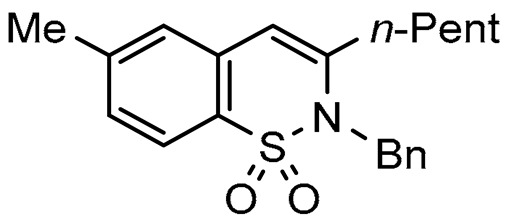
2-Benzyl-7-methyl-3-pentyl-1,2-benzothiazine 1,1-dioxide (1b), 80%; yellow solid; mp: 122–124 °C. 1H NMR (300 MHz, CDCl3, ppm) δ: 7.83 (d, J = 8.0 Hz, 1H). 7.30–7.03 (m, 7Har), 6.23 (s, 1H), 4.99 (s, 2H), 2.46 (s, 3H), 2.38 (t, J = 7.7 Hz, 2H), 1.67–1.25 (m, 6H), 0.94 (t, J = 6.7 Hz, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 143.8, 142.8, 137.1, 133.2, 129.0 (2C), 128.6, 128.5, 127.9, 127.3, 127.2 (2C), 121.8, 111.0, 49.2, 33.9, 31.2, 27.1, 22.0, 21.2, 14.2,. HRMS (ESI) m/z calcd for C21H25NO2S [M + H]+ 356.1606, found 356.1622.
2-Benzyl-3-ethyl-7-methyl-1.2-benzothiazine 1,1-dioxide (1c), 77%; orange solid; mp: 119–121 °C. 1H NMR (300 MHz, CDCl3, ppm) δ: 7.81 (d, J = 8.0 Hz, 1H), 7.31–7.05 (m, 7Har), 6.24 (s, 1H), 5.01 (s, 2H), 2.47 (s, 3H), 2.42 (q, J = 7.3 Hz, 2H), 1.23 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 144.6, 142.3, 136.7, 132.8, 129.1, 128.5 (2C), 128.1, 127.4, 126.9, 126.7 (2C), 121.5, 109.3, 48.5, 26.5, 21.6, 11.7. HRMS (ESI) m/z calcd for C18H19NO2S [M + H]+ 314.1215, found 314.1205.
3,7-Dimethyl-2-(1-Phenyl-ethyl)-1.2-benzothiazine 1,1-dioxide (1d), 76% yield, white solid; mp: 160–162 °C. 1H NMR (300 MHz, CDCl3, ppm) δ: 7.81 (d, J = 8.0 Hz, 1H), 7.33–7.28 (m, 6Har), 7.12 (s, 1H), 6.27 (s, 1H), 5.68 (q, J = 7.1 Hz, 1H), 2.47 (s, 3H), 1.88 (s, 3H), 1.68 (d, J = 7.1 Hz, 3H), 13C NMR (75 MHz, CDCl3, ppm) δ: 142.4, 140.7, 139.7, 132.9, 129.6, 128.4, 128.2 (2C), 127.3 (2C), 126.7, 126.6, 121.6, 113.8, 55.7, 22.5, 21.6, 19.4. HRMS (ESI) m/z calcd for C18H20NO2S [M + H]+ 314.1215, found 314.1211.
2-Allyl-3,7-dimethyl-1.2-benzothiazine 1,1-dioxide (1e), 73%; yellow oil; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.83 (d, J = 8.1 Hz, 1H), 7.30-7.24 (m, 1H), 7.13 (s, 1H), 6.21 (s, 1H), 5.88–5.69 (m, 1H), 5.17–5.12 (m, 2H), 4.34 (dt, J = 5.0 Hz, J = 1.6 Hz, 2H), 2.46 (s, 3H), 2.26 (s, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 142.2, 139.3, 133.0, 132.7, 128.1, 127.7, 126.2, 121.2, 117.1, 109.1, 46.9, 21.4, 20.3. HRMS (ESI) m/z calcd for C13H16NO2S [M + H]+ 250.0896, found 250.0895.
3.2. General Protocol for Synthesis of 2,9-Dihydro-1H-pyrido[3,4-b]indol-1-one Derivated 2–6
Potassium carbonate (860 mg, 2 equiv.), tetrabutylammonium bromide (1 g, 1 equiv.), triphenylphosphine (80 mg, 10 mol %) and Pd(OAc)2 (35 mg, 5 mol %) were added successively to a Schlenk tube, under argon, containing the indole derivative 6, 7 or 8 (3.1 mmol) in CH3CN (10 mL). The mixture was degassed, placed under nitrogen and well-stirred during 10 min. Allenyltributyltin (5.9 mmol, 1 equiv.) was then added. After 8h at reflux, the mixture was hydrolyzed with water and the organic phases were extracted with ethyl acetate. The combined organic extracts were dried over MgSO4, concentrated under vacuum and the resulting residue was purified by silica gel column chromatography (petroleum ether/diethyl ether = 80/20) to afford the desired product.
2,9-Dibenzyl-3-methyl-2,9-dihydro-1H-pyrido[3,4-b]indol-1-one (2), 73%; beige solid; mp: 119–121 °C. 1H NMR (300 MHz, CDCl3, ppm) δ: 7.95 (d, J = 7.8 Hz, 1H), 7.44 (d, J = 6.0 Hz, 2H), 7.30–7.11 (m, 11H), 6.83 (s, 1H), 6.13 (s, 2H), 5.51 (s, 2H), 2.43 (s, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 156.7, 140.3, 138.2, 136.9, 135.8, 129.1, 128.3 (2C), 128.0 (2C), 126.6 (2C), 126.4 (2C), 125.7, 125.1, 124.5, 121.1, 120.7, 119.5, 100.5, 100.2, 47.3, 46.3, 20.5. (1H NMR and 13C NMR of compounds 1a–e, 2, 3a–c, 4a,b are in Supplementary Materials). HRMS (ESI) m/z calcd for C26H23N2O [M + H]+ 379.1805, found 379.1804.
9-Benzyl-3-butylpyrano[3,4-b]indol-1(9H)-one (3a), 56%; orange solid; mp: 90–91 °C; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.86 (d, J = 8.0 Hz, 1H), 7.48–7.41 (m, 2H), 7.31–7.10 (m, 6H), 6.72 (s, 1H), 5.93 (s, 2H), 2.65 (t, J = 7.5 Hz, 2H), 1.77–1.73 (m, 2H), 1.46–1.42 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 157.8, 157.5, 141.0, 137.7, 128.7, 128.0, 127.6, 127.2, 126.5, 121.7, 121.3, 120.9, 120.2, 111.4, 98.2, 48.0, 33.4, 29.6, 22.3, 13.9. HRMS (ESI) m/z calcd for C22H21NO2 [M + H]+ 332.1645, found 332.1650.
9-Benzyl-3-hexylpyrano[3,4-b]indol-1(9H)-one (3b), 62%; yellow oil; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.90 (d, J = 8.0 Hz, 1 H), 7.48–7.46 (m, 2 H), 7.31–7.23 (m, 6 H), 6.72 (s, 1 H), 5.94 (s, 2 H), 2.65 (t, J = 7.5 Hz, 2 H), 1.78–1.76 (m, 2 H), 1.44–1.34 (m, 6 H), 0.92 (t, J = 6.9 Hz, 3 H). 13C NMR (75 MHz, CDCl3, ppm) δ: 157.7, 157.4, 140.9, 137.6, 128.6 (2 C), 127.9, 127.5, 127.1 (2 C), 126.4, 121.6, 121.2, 120.8, 120.1, 111.3, 98.0, 47.9, 33.6, 31.6, 28.8, 27.4, 22.6, 14.1. HRMS (ESI) m/z calcd for C24H26NO2 [M + H]+ 360.1885, found 360.1889.
3-Hexyl-9-methylpyrano[3,4-b]indol-1(9H)-one (3c), 76%; white solid; mp: 88–90 °C; 1H NMR (300 MHz, CDCl3, ppm) δ: 7.88 (d, J = 8.1 Hz, 1 H), 7.55 (dd, J = 8.5, 6.8, 1.1 Hz, 1 H), 7.47 (d, J = 8.5 Hz, 1 H), 7.27 (dd, J = 8.1, 6.8, 1.1 Hz, 1 H), 6.70 (s, 1 H), 4.22 (s, 3 H), 2.64 (t, J = 7.5 Hz, 2 H), 1.76 (qt, J = 7.5 Hz, 2 H), 1.42–1.27 (m, 6 H), 0.91 (t, J = 7.0 Hz, 3 H). 13C NMR (75 MHz, CDCl3, ppm) δ: 157.9, 157.0, 141.3, 127.7, 125.8, 121.6, 120.8, 120.6 (2 C), 110.5, 98.1, 33.6, 31.6, 31.2, 28.7, 27.5, 22.5, 14.0. HRMS (ESI) m/z calcd for C18H22NO2 [M + H]+ 284.1572, found 284.1576.
5-Benzyl-3-methylpyrano[4,3-b]indol-1(5H)-one (4a), 57%; yellow solid; mp: 115–117 °C; 1H NMR (300 MHz, CDCl3, ppm) δ: 8.28–8.21 (m, 1H), 7.38-7.22 (m, 6H), 7.25–7.23 (m, 2H), 6.33 (q, J = 0.8 Hz, 1H), 5.42 (s, 2H), 2.39 (d, J = 0.8 Hz, 3H). 13C NMR (75 MHz, CDCl3, ppm) δ: 160.6, 160.1, 146.7, 138.4, 135.7, 129.2 (2C), 128.2, 126.3 (2C), 124.6, 124.5, 122.9, 121.4, 109.9, 99.5, 93.5, 47.3, 20.8. HRMS (ESI) m/z calcd for C19H16NO2 [M + H]+ 290.1175, found 290.1173.
5-Benzyl-3-pentylpyrano[4,3-b]indol-1(5H)-one (4b), 60%; colorless solid; mp: 123–125 °C. 1H NMR (300 MHz, CDCl3, ppm) δ: 8.03–7.97 (m, 1H), 7.35–7.27 (m, 6H), 7.12–7.03 (m, 3H), 6.29 (s, 1H), 5.38 (s, 2H), 2.57 (t, J = 7.9 Hz, 2H,), 1.80–1.65 (m, 2H), 1.35–1.27 (m, 4H), 0.88 (t, J = 6.4 Hz, 3H,). 13C NMR (75 MHz, CDCl3, ppm) δ: 164.5, 160.2, 146.7, 138.4, 135.7, 129.2 (2C), 128.2, 126.3 (2C), 124.5, 122.8, 122.2, 121.4, 109.9, 99.6, 92.7, 47.7, 34.6, 31.3, 27.1, 22.5, 14.1. HRMS (ESI) m/z calcd for C23H23NO2 [M + H]+ 346.1729, found 346.1736.
5-Pentyl-7H-thieno[2,3-c]pyran-7(7H)-one (5) [42], 72%, Yellow gum. The data of the spectroscopic analyzes (1H NMR and 13C NMR) of product 5 are in agreement with those described in the literature [42].
3-Hexyl pyrano[3’,4’:4,5] imidazo[1,2-a]pyridin-1-one (6) [53], 68%, Yellow solid, mp: 142–144 °C. The data of the spectroscopic analyzes (1H NMR and 13C NMR) of product 6 are in agreement with those described in the literature [53].
4. Conclusion
In summary, we have developed a general and convenient one step regioselective route for the preparation of 1,1-dioxide 1,2-benzothiazines, β-carbolinones and pyranoindoles via Stille coupling of aromatic or heteroaromatic halide derivatives and allenyltributyltins reagents. The transformation proceeded selectively and provided good to excellent yields of a variety of potentially bioactive activities of the targeted cores. The results obtained may lead to the use of allenyltin reagent as an excellent alternative to previously published methodologies for the scientists involved in the field.
Acknowledgments
The authors thank the ‘‘Département d’analyses Chimiques et Médicales’’ (Tours, France) for chemical analyses.
Supplementary Materials
The following are available online. 1H-NMR and 13C-NMR of compounds 1a–e, 2, 3a–c, 4a,b.
Author Contributions
B.J., and K.C. performed the experiments; C.M. and S.I.N. wrote the paper and M.A. designed and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gopalsamy A., Lim K., Ciszewski G., Park K., Ellingboe J.W., Bloom J., Insaf S., Upeslacis J., Mansour T.S., Krishnamurthy G., et al. Discovery of Pyrano[3,4-b]indoles as Potent and Selective HCV NS5B Polymerase Inhibitors. J. Med. Chem. 2004;47:6603–6608. doi: 10.1021/jm0401255. [DOI] [PubMed] [Google Scholar]
- 2.LaPorte M.G., Draper T.L., Miller L.E., Blackledge C.W., Leister L.K., Amparo E., Hussey A.R., Young D.C., Chunduru S.K., Benetatos C.A., et al. The discovery and structure–activity relationships of pyrano[3, 4-b]indole based inhibitors of hepatitis C virus NS5B polymerase. Bioorg. Med. Chem. Lett. 2010;20:2968–2973. doi: 10.1016/j.bmcl.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Lombardino J.G., Wiseman E.H., Mclamore W. Synthesis and antiinflammatory activity of some 3-carboxamides of 2-alkyl-4-hydroxy-2H-1, 2-benzothiazine 1, 1-dioxide. J. Med. Chem. 1971;14:1171–1175. doi: 10.1021/jm00294a008. [DOI] [PubMed] [Google Scholar]
- 4.Lombardino J.G., Wiseman E.H. Piroxicam and other anti-inflammatory oxicams. Med. Res. Rev. 1982;2:127–152. doi: 10.1002/med.2610020202. [DOI] [Google Scholar]
- 5.Brzozowski F., Saczewski F., Neamati N. Synthesis and anti-HIV-1 activity of a novel series of 1, 4, 2-benzodithiazine-dioxides. Bioorg. Med. Chem. Lett. 2006;16:5298. doi: 10.1016/j.bmcl.2006.07.089. [DOI] [PubMed] [Google Scholar]
- 6.Wells G.J., Tao M., Josef K.A., Bihovsky R. 1, 2-benzothiazine 1, 1-dioxide P2− P3 peptide mimetic aldehyde calpain I inhibitors. J. Med. Chem. 2001;44:3488–3503. doi: 10.1021/jm010178b. [DOI] [PubMed] [Google Scholar]
- 7.Mali R.S., Manekar-Tilve A. Useful Syntheses of Pyrano-and Pyridoindoles. Org. Prep. Proced. Int. 1994;26:573–577. doi: 10.1080/00304949409458060. [DOI] [Google Scholar]
- 8.Shi Z., Cui Y., Jiao N. Synthesis of β-and γ-Carbolinones via Pd-Catalyzed Direct Dehydrogenative Annulation (DDA) of Indole-carboxamides with Alkynes Using Air as the Oxidant. Org. Lett. 2010;12:2908–2911. doi: 10.1021/ol1007839. [DOI] [PubMed] [Google Scholar]
- 9.Veale C.A., Damewood J.R., Steelman G.B., Bryant C., Gomes B., Williams J. Non-peptidic inhibitors of human leukocyte elastase. 4. design, synthesis, and in vitro and in vivo activity of a series of. beta.-carbolinone-containing trifluoromethyl ketones. J. Med. Chem. 1995;38:86–97. doi: 10.1021/jm00001a014. [DOI] [PubMed] [Google Scholar]
- 10.Bracher F., Hildebrand D. 1,9-Dimetalated ß-carbolines. Versatile building blocks for the total synthesis of Alkaloids. Tetrahedron. 1994;50:12329–12336. doi: 10.1016/S0040-4020(01)89542-9. [DOI] [Google Scholar]
- 11.Nicolaou K.C., Kiappes J.L., Tian W., Gondi V.B., Becker J. Synthesis of the carboline disaccharide domain of shishijimicin A. Org. Lett. 2011;13:3924–3927. doi: 10.1021/ol201444t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen L.K., Moore R.E., Patterson G.M.L. β-Carbolines from the Blue-green Alga Dichothrix Baueriana. J. Nat. Prod. 1994;57:419–421. doi: 10.1021/np50105a018. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez C., Quiñoa E., Adamczeski M., Hunter L.M., Crews P. Novel Sponge-Derived Amino Acids. 12. Tryptophan-Derived Pigments and Accompanying Sesterterpenes from Fascaplysinopis Reticulata. J. Org. Chem. 1991;56:3403–3410. doi: 10.1021/jo00010a041. [DOI] [Google Scholar]
- 14.Morris L.C., Nance K.D., Gentry P.R., Days E.L., Weaver C.D., Niswender C.M., Thompson A.D., Jones C.K., Locuson C.W., Morrison R.D., et al. Discovery of (S)-2-Cyclopentyl-N-((1-isopropylpyrrolidin2-yl)-9-methyl-1-oxo-2,9-dihydro-1H-pyrrido[3,4-b]indole -4-carboxamide (VU0453379): A Novel, CNS Penetrant Glucagon-Like Peptide 1 Receptor (GLP-1R) Positive Allosteric Modulator (PAM) J. Med. Chem. 2014;57:10192–10197. doi: 10.1021/jm501375c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fillery S.M., Gregson C.L., Guérot C.M. Expeditious Access to Functionalized Tricyclic Pyrrolo-Pyridones via Tandem or Sequential C−N/C−C Bond Formations. Org. Lett. 2019;21:9128–9132. doi: 10.1021/acs.orglett.9b03514. [DOI] [PubMed] [Google Scholar]
- 16.Cao R., Peng W., Wang Z., XU A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007;14:479. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 17.Barrange D.K., Batchu V.R., Gorja D., Pattabiraman V.R., Tatini L.K., Babu J.M., Pal M. Regioselective construction of six-membered fused heterocyclic rings via Pd/C-mediated C–C coupling followed by iodocyclization strategy: A new entry to 2H-1, 2-benzothiazine-1, 1-dioxides. Tetrahedron. 2007;63:1775–1789. doi: 10.1016/j.tet.2006.12.040. [DOI] [Google Scholar]
- 18.Debnath S., Mondal S. One-Pot Sonogashira Coupling-Cyclization toward regioselective Synthesis of Benzosultams. J. Org. Chem. 2015;80:3940–3948. doi: 10.1021/acs.joc.5b00274. [DOI] [PubMed] [Google Scholar]
- 19.Rambabu D., Murthy P.V.N.S., Prasad K.R.S., Kandale A., Deora G.S., Rao M.V.B., Pal M. AgNO3 mediated C–N bond forming reaction: Synthesis of 3-substituted benzothiazines as potential COX inhibitors. Tetrahedron Lett. 2012;53:6577–6583. doi: 10.1016/j.tetlet.2012.09.102. [DOI] [Google Scholar]
- 20.Siva Reddy A., Siva Kumari A.L., Saha S., Kumara Swamy K.C. Palladium-Catalyzed Tandem-Cyclization of Functionalized Ynamides: An Approach to Benzosultams. Adv. Synth. Cat. 2016;358:1625–1638. doi: 10.1002/adsc.201500854. [DOI] [Google Scholar]
- 21.Hou H., Zhao Y., Pu S., Chen J. Rhodium-catalyzed direct C–H bond alkynylation of aryl sulfonamides with bromoalkynes. Org. Biomol. Chem. 2019;17:2948–2953. doi: 10.1039/C9OB00061E. [DOI] [PubMed] [Google Scholar]
- 22.Zhou K., Xia H., Wu J. Generation of benzosultams via a radical process with the insertion of sulfur dioxide. Org. Chem. Front. 2017;4:1121–1124. doi: 10.1039/C7QO00127D. [DOI] [Google Scholar]
- 23.Barange D.K., Nishad T.C., Swamy N.K., Bandameedi V., Kumar D., Sreekanth B.R., Vyas S.K., Pal M. A remarkable accelerating effect of Ag-salt on intramolecular cyclization of o-(1-alkynyl) benzenesulfonamides. J. Org. Chem. 2007;72:8547–8550. doi: 10.1021/jo701470h. [DOI] [PubMed] [Google Scholar]
- 24.Harris J.M., Padwa A. A New α-Carbolinone Synthesis Using a Rh(II)-Promoted [3+2]-Cycloaddition and Pd(0) Cross-Coupling/Heck Cyclization Chemistry. Org. Lett. 2003;5:4195–4197. doi: 10.1021/ol0356338. [DOI] [PubMed] [Google Scholar]
- 25.England D.B., Padwa A. Gold-catalyzed cycloisomerization of N-propargylindole-2-carboxamides: Application toward the synthesis of lavendamycin analogues. Org. Lett. 2008;10:3631–3634. doi: 10.1021/ol801385h. [DOI] [PubMed] [Google Scholar]
- 26.Worayuthakarn R., Nealmongkol P., Ruchirawat S., Thasana N. Synthesis of benzoindoloquinolizines via a Cu (I)-mediated C–N bond formation. Tetrahedron. 2012;68:2864–2875. doi: 10.1016/j.tet.2012.01.094. [DOI] [Google Scholar]
- 27.Tulichala R.N.P., Swamy K.C.K. Reactivity of Alkynylindole-2-carboxamides in [Pd]-Catalysed C-H Activation and Phase Transfer Catalysis: Formation of Pyrrolo-diindolones vs. β-Carbolinones. Org. Biomol. Chem. 2016;14:4519–4533. doi: 10.1039/C6OB00583G. [DOI] [PubMed] [Google Scholar]
- 28.Tahri A., Buysens K.J., Eycken E.V.V., Vandenberghe D.M., Hoornaert G.J. Synthesis of α-carbolines and β-carbolinones via intramolecular Diels-Alder reactions of 2 (1H)-pyrazinones. Tetrahedron. 1998;54:13211–13226. doi: 10.1016/S0040-4020(98)00802-3. [DOI] [Google Scholar]
- 29.Beccalli E.M., Broggini G., Marchesini A., Rossi E. Intramolecular Heck Reaction of 2- And 3-Iodoindole Derivatives for the Synthesis of β- and γ-Carbolinones. Tetrahedron. 2002;58:6673–6678. doi: 10.1016/S0040-4020(02)00688-9. [DOI] [Google Scholar]
- 30.Han H., Yang S., Xia J. Pd/Cu Cocatalyzed Oxidative Tandem C−H Aminocarbonylation and Dehydrogenation of Tryptamines: Synthesis of Carbolinones. J. Org. Chem. 2019;84:3357–3369. doi: 10.1021/acs.joc.8b03266. [DOI] [PubMed] [Google Scholar]
- 31.Rayabarapu D.K., Zhou A., Jeon K.O., Samarakoon T., Rolfe A., Siddiqui H., Hanson P.R. α-Haloarylsulfonamides: Multiple cyclization pathways to skeletally diverse benzofused sultams. Tetrahedron. 2009;65:3180–3188. doi: 10.1016/j.tet.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X.Y., Li C.H., Che C.M. Phosphine gold (I)-catalyzed hydroamination of alkenes under thermal and microwave-assisted conditions. Org. Lett. 2006;8:2707–2710. doi: 10.1021/ol060719x. [DOI] [PubMed] [Google Scholar]
- 33.Pertschi R., Weibel J.M., Pale P., Blanc A. Benzosultam synthesis by gold (I)-catalyzed ammonium formation/nucleophilic substitution. Org. Lett. 2019;21:5616–5620. doi: 10.1021/acs.orglett.9b01962. [DOI] [PubMed] [Google Scholar]
- 34.Pham M.V., Ye B., Cramer N. Access to Sultams by Rhodium (III)-Catalyzed Directed C-H Activation. Angew. Chem. Int. Ed. 2012;51:10610–10614. doi: 10.1002/anie.201206191. [DOI] [PubMed] [Google Scholar]
- 35.Reddy A.S., Kumari A.L.S., Saha S., Swamy K.C.K. Palladium-Catalyzed Tandem-Cyclization of FunctionalizedYnamides:AnApproach to Benzosultams. Adv. Synth. Catal. 2016;358:1625–2638. doi: 10.1002/adsc.201500854. [DOI] [Google Scholar]
- 36.Thrimurtulu N., Nallagonda R., Volla C.M.R. Cobalt-catalyzed aryl C–H activation and highly regioselective intermolecular annulation of sulfonamides with allenes. Chem. Commun. 2017;53:1872–1875. doi: 10.1039/C6CC08622E. [DOI] [PubMed] [Google Scholar]
- 37.Stille J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles [new synthetic methods (58)] Angew. Chem. Int. Ed. Engl. 1986;25:508–524. doi: 10.1002/anie.198605081. [DOI] [Google Scholar]
- 38.Lane C., Snieckus V. Combined Directed ortho Metalation-Grubbs Metathesis Tactics. Synthesis of Benzazepine, Benzazocine, and Benzannulated 7-, 8-, 9-, and 15-Membered Ring Sulfonamide Heterocycles. Synlett. 2000;9:1294–1296. doi: 10.1002/chin.200049158. [DOI] [Google Scholar]
- 39.MacNeil S., Familoni O.B., Sniekus V. Selective ortho and benzylic functionalization of secondary and tertiary p-tolylsulfonamides. Ipso-bromo desilylation and Suzuki cross-coupling reactions. J. Org. Chem. 2001;66:3662–3670. doi: 10.1021/jo001402s. [DOI] [PubMed] [Google Scholar]
- 40.Harmata M., Rayanil K.O., Gomes M.G., Zheng P., Calkins N.L., Kim S.-Y., Fan Y., Bumbu V., Lee D.R., Wacharasindhu S., et al. New synthesis of benzothiazines and benzoisothiazoles containing a sulfoximine functional group. Org. Lett. 2005;7:143–145. doi: 10.1021/ol047781j. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Q., Sheng J., Chen Z., Wu J. Generation of 4-((trifluoromethyl) thio)-2 H-benzo[e][1,2]thiazine 1,1-dioxides via a reaction of trifluoromethanesulfanylamide with 2-(2-alkynyl) benzenesulfonamide. Chem. Commun. 2013;49:8647–8649. doi: 10.1039/c3cc44263b. [DOI] [PubMed] [Google Scholar]
- 42.Inack Ngi S., Guilloteau V., Abarbri M., Thibonnet J. Regioselective Copper-Mediated Synthesis of Thieno[2,3-c]pyran-7-one, Indolo[2,3-c]pyran-1-one, and Indolo[3,2-c]pyran-1-one. J. Org. Chem. 2011;76:8347–8354. doi: 10.1021/jo201528u. [DOI] [PubMed] [Google Scholar]
- 43.Rama Suresh R., Kumara Swamy K.C. Palladium-Catalyzed Annulation of Allenes with Indole-2-carboxylic Acid Derivatives: Synthesis of Indolo[2,3-c]pyrane-1-ones via Ar–I Reactivity or C–H Functionalization. J. Org. Chem. 2012;77:6959–6969. doi: 10.1021/jo301149s. [DOI] [PubMed] [Google Scholar]
- 44.Freter K. Synthesis and reactions of 3-indolyl. beta. ketones. J. Org. Chem. 1972;37:2010–2015. doi: 10.1021/jo00977a032. [DOI] [Google Scholar]
- 45.Mashelkar U.C., Usgaonkar R.N. Synthesis of 2-alkyl-, 2-allyl-, 2-phenyl- and 2-pyridyl-3-methyl-β-carbolinones. Indian J. Chem. Sect. B. 1979;17B:407. [Google Scholar]
- 46.Kita Y., Mohri S., Tsugoshi T., Maeda H., Tamura Y. Reaction of heteroaromatic analogs of homophthalic anhydride: Synthesis of hetero analogs of peri-hydroxy polycyclic aromatic compounds, isocoumarins, isoquinolinones, and related compounds. Chem. Pharm. Bull. 1985;33:4723–4731. doi: 10.1248/cpb.33.4723. [DOI] [Google Scholar]
- 47.Yao T., Larock R.C. Synthesis of isocoumarins and α-pyrones via electrophilic cyclization. J. Org. Chem. 2003;68:5936–5942. doi: 10.1021/jo034308v. [DOI] [PubMed] [Google Scholar]
- 48.Hellal M., Bourguignon J.-J., Bihel F.J.J. 6-endo-dig Cyclization of heteroarylesters to alkynes promoted by Lewis acid catalyst in the presence of Brønsted acid. Tetrahedron Lett. 2008;49:62–65. doi: 10.1016/j.tetlet.2007.11.020. [DOI] [Google Scholar]
- 49.Zhang H., Larock R.C. Synthesis of annulated γ-carbolines and heteropolycycles by the palladium-catalyzed intramolecular annulation of alkynes. J. Org. Chem. 2003;68:5132–5138. doi: 10.1021/jo0343228. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu M., Hirano K., Satoh T., Miura M. Waste-free synthesis of condensed heterocyclic compounds by rhodium-catalyzed oxidative coupling of substituted arene or heteroarene carboxylic acids with alkynes. J. Org. Chem. 2009;74:3478–3483. doi: 10.1021/jo900396z. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Xintong W.Y., Cong X., Zhen Q., Li D., Zhang-Negrerie Y., Du K., Zhao K. Lactonization of 2-Alkynylbenzoates for the Assembly ofIsochromenones Mediated by BF3·Et2O. J. Org. Chem. 2019;84:10402–10411. doi: 10.1021/acs.joc.9b01601. [DOI] [PubMed] [Google Scholar]
- 52.Dong G., Li C., Liu H. Rh (III)-Catalyzed Annulation of Boc-Protected Benzamides with Diazo Compounds: Approach to Isocoumarins. Molecules. 2019;24:937. doi: 10.3390/molecules24050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bahlaouan Z., Abarbri M., Duchene A., Thibonnet J., Henry N., Enguehard-Gueiffier C., Gueiffier A. Copper(I)-mediated preparation of new pyrano[3’,4’:4,5]imidazo[1,2-a]pyridine-1-one compounds under mild palladium-free conditions. Org. Biomol. Chem. 2011;9:1212–1218. doi: 10.1039/C0OB00622J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.