Abstract
GLOBOCAN 2018 identified lung cancer as the leading oncological pathology in terms of incidence and mortality rates. Angiogenesis is a key adaptive mechanism of numerous malignancies that promotes metastatic spread in view of the dependency of cancer cells on nutrients and oxygen, favoring invasion. Limitation of the angiogenic process could significantly hamper the disease advancement through starvation of the primary tumor and impairment of metastatic spread. This review explores the basic molecular mechanisms of non-small cell lung cancer (NSCLC) angiogenesis, and discusses the influences of the key proangiogenic factors—the vascular endothelial growth factor-A (VEGF-A), basic fibroblast growth factor (FGF2), several matrix metalloproteinases (MMPs—MMP-2, MMP-7, MMP-9) and hypoxia—and the therapeutic implications of microRNAs (miRNAs, miRs) throughout the entire process, while also providing critical reviews of a number of microRNAs, with a focus on miR-126, miR-182, miR-155, miR-21 and let-7b. Finally, current conventional NSCLC anti-angiogenics—bevacizumab, ramucirumab and nintedanib—are briefly summarized through the lens of evidence-based medicine.
Keywords: microRNA, miRNA, ncRNA, lung cancer, NSCLC, angiogenesis, cancer therapy
1. Introduction
Lung cancer remains the most common diagnosed malignancy in the world. In 2018, there were 2.094 million new cases of lung cancer diagnosed worldwide, and 1.761 million deaths caused by this malignancy [1]. Lung cancer is classified according to the histological type. Small cell lung carcinoma (SCLC) accounts for approximately 15% of lung cancers and NSCLC for the remaining 85% [2]. NSCLC is further classified into three histological subtypes: lung adenocarcinoma (AC, LUAD), which accounts for approximately 40%; squamous-cell carcinoma (SCC, LUSC) with 30% of the cases; and large-cell carcinoma (LCC), which represents 10% [3]. Adenocarcinoma is considered to be the most common type of lung cancer, comprising more than 50% of all NSCLC diagnoses [4].
NSCLC progression relies on the formation of new blood vessels that provide oxygen and nutrients to cells within the tumor microenvironment (TME), and also enhance the metastatic potential. Angiogenesis is a hallmark of cancer [5] and is largely implicated in the proliferative process and tumorigenesis, and in the physiological growth of blood vessels. Progression of NSCLC to advanced stages is closely linked to the angiogenic process. Angiogenesis is driven by proangiogenic factors such as VEGF, FGF2 and PDGF which bind to specific receptors in order to initiate signaling cascades with a net final effect of new blood vessel formation. Thus, the neoangiogenic process represents an obvious target in cancer therapy by limiting cancer progression. In today’s world, alteration of the neoangiogenic process in oncological patients can be achieved through the inhibition of various pathways by antibodies directed against VEGF or VEGF receptors (VEGFRs) or tyrosine kinase inhibitors (TKIs).
Non-coding RNAs (ncRNAs) such as miRNAs have a great therapeutic potential, as multiple ncRNAs are able to target a number of angiogenesis-related factors. In the current review we discuss a palette of miRNAs that we deem important therapeutic candidates for future advanced therapies, along with the mechanistic implications of these miRNAs.
2. Major Angiogenic Factors Are Regulated by microRNAs
Angiogenesis is a common feature for tumor growth beyond 2 to 3 mm3 [6]. The newly formed blood vessels allow the diffusion of nutrients and oxygen to depleted peripheral tumor cells, with concomitant elimination of waste products, thereby facilitating tumor cell survival and tumor growth. These tumor vessels are morphologically and functionally different from normal vasculature, with reduced blood flow, abnormal EC junctions and increased leakiness, which allows tumor cell intravasation [7]. The angiogenic process is regulated at various levels, starting with factors within the TME (e.g., hypoxia [8,9] and extracellular matrix (ECM)), followed by modifications in signaling pathways containing key regulatory molecules (e.g., VEGF expression) and fine-tuning modulation through alterations in the ncRNA profile, including miRNA alterations. MiRNAs are small single-stranded non-coding RNAs with total lengths of 19–25 nucleotides [10] that are able to regulate gene expression through translational repression, cleavage of the mRNA and mRNA decay by rapid-deadenylation [11]. A large number of studies found that translational repression, mRNA deadenylation and the decapping process are the result of the binding of the miRNA to the 3′-UTR region of the target mRNA [12,13]. In the last few decades, numerous studies found that miRNAs are largely implicated in cancers as either tumor suppressors or oncogenic factors, from the onset of the uncontrolled, chaotic growth of the first tumor cells to the metastatic and angiogenic processes, mainly by modulating the pathways implicated in the neoplasm. Recent literature suggests that miRNAs could represent a new approach to cancer therapy [14]. The selective approach generated by miRNA therapy would make them the ultimate therapeutic agents in targeting NSCLC angiogenesis. Figure 1 illustrates an integrative overview of the mechanism of angiogenesis, along with the main proangiogenic factors involved and the corresponding modulatory miRNAs.
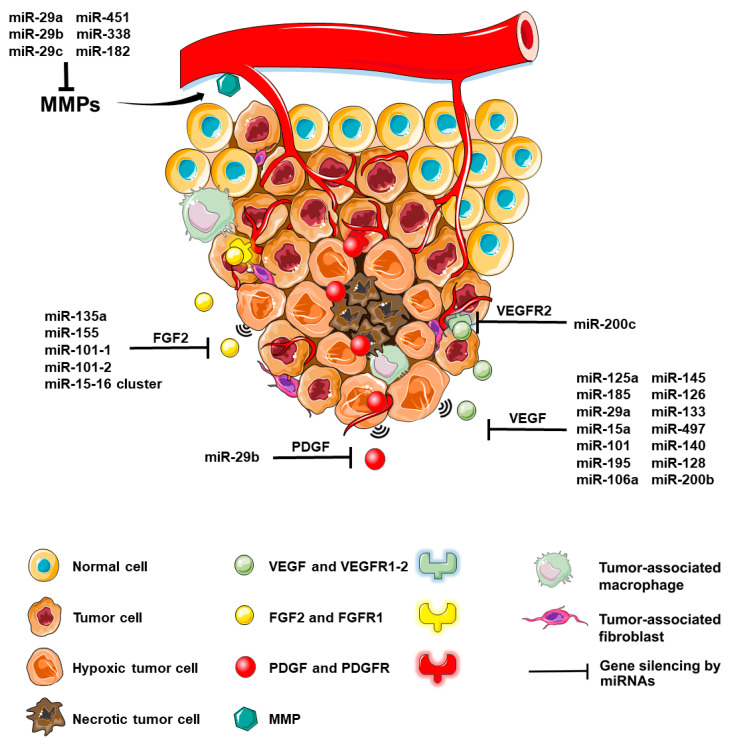
Figure 1.
A brief summary of the mechanisms of angiogenesis and modulatory microRNAs. The tumor microenvironment (TME) presented here is composed of tumor cells, hypoxic tumor cells, tumor-associated macrophages, fibroblasts and extracellular matrix. The angiogenic process interests TME-incorporated hypoxic tumor cells situated beyond the Folkman limit, at a distance of 2–3 mm from the nearest arterial blood vessel, which release proangiogenic signaling molecules. The main player, VEGF, is stimulated by these low oxygen levels through the hypoxia inducible factor-1α (HIF-1α) [9], further binding to its receptors, VEGFR1-2. Other key proangiogenic players represented include FGF2 and its receptor, FGFR1 and the PDGF-PDGFR duo. MMPs, along with FGF2, degrade the ECM in order to promote angiogenesis through various mechanisms, including the proteolytic release of angiogenic factors sequestered within the ECM. For integrative purposes, Figure 1 also highlights a selection of miRNAs that target these proangiogenic factors (VEGF, VEGFR2, FGF2, PDGF, MMPs) with silencing effects.
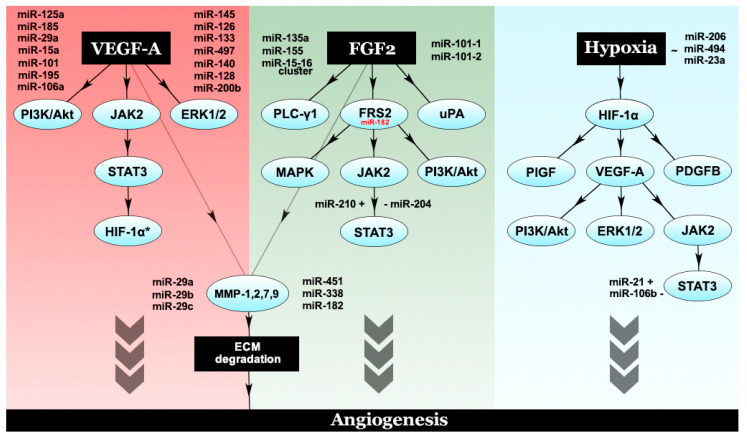
Moreover, neoangiogenesis is a complex process driven by the angiogenic switch, an imbalance between proangiogenic factors and anti-angiogenic factors. The most important proangiogenic factors, and suitable therapeutic targets, along with their anti-angiogenic counterparts, are represented in Table 1, along with miRNAs that target these factors, obtained through bioinformatic means. Angiogenesis is driven by the overexpression of these factors rather than the underexpression of the anti-angiogenic factors. The interplay between proangiogenic factors, their corresponding pathways and modulatory miRNAs are illustrated in Figure 2.
Table 1.
The main proangiogenic and anti-angiogenic factors, along with a selection of miRNAs that target their genes. In general terms, miRNAs compiled in this table which target the proangiogenic factors are downregulated in the context of angiogenic non-small cell lung cancer (NSCLC), whilst miRNAs that target anti-angiogenic factors are upregulated. The factors were compiled after [15,16,17]. The gene mutation frequency was obtained through TCGA analysis. miRNAs were selected through a clear methodology: (1) The factors were searched in the miRTargetLink Human tool (Saarland University) which returned a number of miRNAs; only miRNAs with strong evidence were taken into consideration; (2) these miRNAs were inserted into the OncoMir Cancer Database from the Masonic Cancer Center, University of Minnesota which uses TCGA-based data; (3) the tissue type that was taken into consideration consisted of LUAD and LUSC; (4) the resulting heatmaps were used to evaluate the implications of the aforementioned miRNAs.
| Gene | Gene Mutation Frequency 1 | miRNAs Regulating the Gene 2 | References 5 |
|---|---|---|---|
| Proangiogenic | |||
| VEGFA | <0.1% | miR-125a, miR-185, miR-29a, miR-101, miR-15a, miR-195, miR-140, miR-145, miR-126, miR-133 | [18,19,20,21,22,23] |
| FGF2 | No mutations identified | No strong evidence miRNAs identified in the database searchmiR-101-1 3, miR-101-2 3 | NR |
| PDGFB | 0.3% | miR-29b 4 | NR |
| EGF | No mutations identified | No strong evidence miRNAs identified in the database searchmiR-3188 3 | NR |
| TGFB1 | miR-144, miR-29b 4 | NR | |
| IL8 | miR-520b | NR | |
| MMP2 | miR-451, miR-29a, miR-29b 4, miR-29c, miR-338, miR-451 | [24,25,26] | |
| Anti-angiogenic | |||
| THBS1 | No mutations identified | miR-182 | NR |
| TIMP1 | miR-1293 | [27] | |
| IL1A | No strong evidence miRNAs identified in the database searchmiR-181b-2 3 | [28] | |
| COL18A1 | No strong evidence miRNAs identified in the database searchmiR-450a-1 3, miR-450a-2 3, miR-1292 3, miR-877 3 | NR | |
1 TCGA analysis on 1144 NSCLC samples; 2 marked as strong evidence on the miRTargetLink Human tool (Saarland University). 3 Weak support, warrants further study. 4 miR-29b levels can be either increased or decreased. 5 miRTargetLink Human–OncoMir analysis along with literature references. NR = no relevant references identified in the literature, but miRNAs identified in the analysis.
Figure 2.
The interplay between different proangiogenic factors and their corresponding modulatory microRNAs. VEGF-A, the chief proangiogenic entity, activates PI3K/Akt, ERK1/2 and JAK2/STAT3. Phosphorylated STAT3 leads to HIF-1α activation, which further increases VEGF-A levels through an autocrine loop. FGF2 activates PLC-γ1, uPA and FRS2. Downstream effectors of FRS2 include the MAPK pathway PI3K/Akt and STAT3. Both VEGF-A and FGF2 are capable of activating a number of MMPs, such as MMP-1,2,7,9, which degrade the ECM and promote angiogenesis. Another major player in angiogenesis is considered to be hypoxia, which exerts its molecular effects through HIF-1α, the hypoxic master regulator. HIF-1α promotes the expression of a number of proangiogenic factors—PlGF, PDGFB and VEGF-A.
2.1. VEGF as a Central Player and Its miRNA Regulation
VEGF is the main regulator of the physiological and pathological growth of any type of blood vessel in the human body, acting through vasculogenic and angiogenic processes [29,30]. The VEGF family consists of several members, including the placental growth factor (PLGF); VEGF-A, B, C, D; and the viral VEGF-Es. VEGF-A is the chief proangiogenic factor from the VEGF family, with six isoforms that are able to bind to two tyrosine kinase receptors: VEGFR1 (Flt-1) and VEGFR2 (KDR/Flk1) [31,32]. VEGFR3 regulates lymphangiogenesis [32] and not angiogenesis, whilst VEGFR2 mediates the majority of effects attributed to VEGF through paracrine and autocrine mechanisms, including growth and permeability actions [33].
As such, VEGF production is upregulated in multiple malignancies, including lung cancers [34]. Moreover, the upregulation of VEGF is a marker of poor prognosis in NSCLC [35] and is associated with an enhancement of the angiogenic process. Mechanistically, in NSCLC, VEGF activates several pathways, including PI3K/Akt, extracellular signal-regulated kinase 1/2 (ERK 1/2) and STAT3, which lead to increasing amounts of VEGF through an autocrine loop [36]. The implications of the PI3K/Akt and ERK1/2 pathways have also been highlighted by Ma et al. in a 2019 study, suggesting their vast implications in the modulation of angiogenesis [37]. Moreover, an in vitro study by Xie et al. found that hypoxia is able to induce PI3k/Akt activation in A549 cells; treatment with the PI3K/Akt inhibitor LY294002 had a suppressive effect on VEGF production within the A549 cells in both normoxic and hypoxic conditions, although the inhibition was more potent under normoxic conditions. However, this suppression was not complete, suggesting that other signaling pathways are involved in VEGF expression [38]. Zhao et al. underlined the importance of the JAK2/STAT3 pathway in tumor angiogenesis by upregulating VEGF and FGF2 expression in lung cancer cells. Furthermore, the same Zhao study found a correlation between pSTAT3 expression and VEGF/FGF2 expression, and a JAK2/STAT3 positive association with NSCLC stage and overall survival (OS) [39]. Intrinsically, the vast pathway activation has further stimulatory roles upon neoplastic cells in secreting VEGF [36], creating a regulatory loop. In a study by Shi et al., increased expression of miR-200c led to the inhibition of Akt in A549 cell line, through its primary target, the VEGFR2 [40]. Another study by Dodd et al. showed that in the hypoxic TME, phosphorylated STAT3 is able to activate the hypoxia mediator HIF-1α by driving its mRNA transcription [41]. The activated HIF-1α elevates the expression of angiogenic VEGF [9,42]. As such, the aforementioned regulatory loop enhances angiogenesis and induces cancer progression. It is abundantly clear that the intricate mechanisms of angiogenesis can be regulated at various levels by various miRNA entities.
MiR-126 acts on multiple target genes involved in lung cancer angiogenesis, such as VEGFA and epidermal growth factor-like domain 7 (EGFL7) [43,44,45]. It is one of the most differentially expressed miRNA in lung cancer [46], with an overall reduced expression. MiR-126 directly targets the 3′-UTR of the VEGF-A gene. Liu et al. compared lung cancer tissue with normal lung tissue and found miR-126 expression to be downregulated in the neoplastic tissue, with increased VEGF-A expression [23]. Moreover, transfection experiments by Liu et al. and Zhu et al. confirmed this finding [23]: miR-126 suppresses VEGF-A expression and inhibits cancer cell growth [45]. Furthermore, Zhu et al. reported the first association between miR-126 expression and cancer chemotherapy; the team found that enhanced miR-126 expression increases the sensitivity of NSCLC cells to therapy through negative regulation of the VEGF-A/PI3K/Akt/multidrug resistance protein 1 (MRP1) pathway. Moreover, the activity of miR-126 on the PI3K-Akt pathway was confirmed in a study by Yang et al. where the team demonstrated that miR-126 overexpression in NSCLC cells reduces cell growth in vitro and also reduces tumor proliferation in a nude mouse xenograft model. The authors identified the 3′-UTR region of the PI3K regulatory beta subunit (PI3KR2) mRNA as a target of miR-126 [47]. A study by Shi et al. concluded that overexpression of miR-126 downregulates VEGF-A, and the expression of the VEGFR2, by inactivating the VEGF-A/VEGFR2/ERK signaling pathway [48], proving its importance in regulating angiogenesis.
Furthermore, studies have shown high expression of miR-21, a well-known oncomiR, in the plasma [49] and also in the tissues [50] of NSCLC patients compared to healthy donors. Liu et al. demonstrated that a STAT3 knockdown reduced miR-21 levels in HBE (human bronchial epithelial)-derived exosomes—exosomes are small membrane vesicles secreted by most cell types that are involved in intercellular communication—with consequent inhibition of angiogenesis [51,52]. Exosome-derived miR-21 induces STAT3 activation, increasing VEGF levels in recipient cells and activating angiogenesis. Furthermore, Liu et al. identified a dose-response dependency between miR-21-exosomes and VEGF levels in recipient cells. Inhibition of exosomal miR-21 led to decreased VEGF expression within the recipient cells, proving the modulatory effects of miR-21 in angiogenesis [51]. Recent work by Dong et al. found that enhanced expression of miR-21 might be correlated with the development of brain metastases in patients with NSCLC, as miR-21 had increased expression in the cohort associated with brain metastatic spots. The same authors showed that a knockdown of miR-21 might restrain cell proliferation, migration, invasion and angiogenic properties and might induce tumor cell apoptosis. Moreover, an in vitro experiment found that A549 cells in the miR-21 inhibition group showed almost no tube formation, contrarily to the negative control group and the mock group. Further analysis of tube length difference showed that the inhibition group had statistically significant shorter tube length, and a lower number of junction points compared to the negative control (p < 0.05). As such, Dong et al. indicated miR-21’s potential in angiogenic modulation, and its potential as a biomarker for the development of brain metastases in NSCLC [53].
Gu et al. demonstrated the implication of miR-497 in modulating VEGF-A expression. The authors showed that miR-497 overexpression in lung cancer cell lines reduced VEGF-A expression by binding to the 3′-UTR of VEGF-A mRNA, thereby inhibiting its translation, and subsequently, angiogenesis. Furthermore, their results showed that miR-497 can inhibit NSCLC cell growth and invasion, as miR-497 depletion had the opposite effect, increasing cell invasiveness and growth [54].
Another study by Jusufovic et al. reported a significantly decreased expression of let-7b and a higher degree of MVD in tumor tissue compared to non-tumor tissue from NSCLC patients. The expression of let-7b has been negatively correlated with MVD and low let-7b expression was significantly correlated with the prognosis, as patients with low let-7b showed shorter progression-free survival (PFS) and OS [22]. LIN28B—a RNA-binding protein that regulates let-7 microRNAs—has been shown to be associated with tumor initiation, progression and resistance to therapy in lung cancer. Furthermore, Meder et al. reported that LIN28B increased VEGF-A expression in a KRAS-mutant lung AC [55]. Its expression is associated with a poor prognosis in several solid cancers, including lung cancer, as it enhances the tumor’s angiogenic properties [55,56].
2.2. FGF2—A Multifaceted Proangiogenic Factor and Its miRNA Modulation
FGF2 is a member of the fibroblast growth factor family and is frequently found upregulated in different malignancies. Takanami et al. were the first to report the expression of FGF2 in NSCLC [57,58,59]; further studies suggested that increased expression of FGF2 correlates with poor prognosis [57]. Moreover, FGF2 presents a neurotrophic and mitogenic effect [60], whilst also being a potent inducer of the angiogenic process. This mitogenic effect, along with the chemotactic response, requires activation of ERK1/2 and protein kinase C (PKC) pathways [61]. FGF2 plays autocrine roles in ECs, which predominantly express FGFR1, a type of tyrosine kinase receptor that binds the factor. Interactions of these entities induce EC proliferation, migration and angiogenesis. Mechanistically, FGF2 binds FGFR1 with the help of a stabilizing partner such as the heparan sulfate proteoglycan (HSPG) syndecan, leading to signal transmission to two key intracellular substrates—phospholipase C-γ1 (PLC-γ1, FRS1) and FGFR substrate 2 (FRS2). Subsequent phosphorylation of FRS2 activates the Ras-mitogen-activated protein kinase (MAPK), and the PI3K/Akt signaling pathways in cancer cells and ECs, promoting angiogenic processes [61]. Another pathway that is largely involved in FGF2-induced cancer angiogenesis is JAK2/STAT3; a study by Zhao et al. showed that JAK2/STAT3 activation increased FGF2 expression, inducing angiogenesis. Furthermore, treatment of A549 and NCI-H292 cells with AG490, a JAK2 inhibitor, reduced FGF2 expression, proving that the JAK2/STAT3 pathway is associated with FGF2 modulation in lung cancer cells [39]. Additionally, FGF2 induces MMP and urokinase-type plasminogen activator (uPA) synthesis in ECs, further stimulating the angiogenic process by breaking down the extracellular matrix, allowing ECs to migrate [61].
Moreover, FGF2 and FGFRs may also play an essential role in anti-angiogenic therapy resistance in cancer. By treating the patient with anti-VEGF agents, the VEGF-dependent vessels will be targeted and disrupted, whilst the tumor vessels will be increasingly covered by pericytes; these pericytes have been shown to overexpress FGF2, thereby switching the angiogenic pathway dependency towards FGF2 [62]. Mechanistically, in late stage tumors, as the resistance to VEGFR2 blockade develops, the malignancy promotes a VEGF-independent angiogenic process that relies on other proangiogenic factors, including FGF2, highlighting the extensive crosstalk between FGF2, VEGF and their receptors [63].
A study by Zhou et al. found that miR-135a is able to regulate several proangiogenic factors, including FGF2. The team found that miR-135a levels were lower in NSCLC tissues compared to normal adjacent tissue. Contrarily, IGF-1, PI3K and Akt mRNA levels were higher in the lung cancer tissues. The same authors conducted a series of in vitro experiments on A549 cancer cells that further proved the implication of miR-135a in the regulation of different proangiogenic factors, with an emphasis on FGF2. Accordingly, the authors concluded that miR-135a is able to inhibit angiogenesis by decreasing VEGF, FGF2 and IL-8 levels in an IGF-1-dependent mechanism [60].
MiR-182, frequently upregulated in cancers, is mostly viewed as an oncogene, its activity being correlated with the modulation of multiple targets [64], including the anti-angiogenic factor Tsp1. However, contrarily, miR-182 targets FRS2, a downstream member of the FGF pathway [44,65], which is supposedly an inducer of tumor progression in NSCLC through the activation of angiogenesis. Older studies showed that FRS2 plays a role in the transmission of signals from the FGFR to the Ras/MAPK signaling pathway [66]. Moreover, the Ras/MAPK pathway is involved in multiple aberrant cell functions featured in malignancies, including neoplastic angiogenesis. Additionally, activated FRS2 is involved in PI3K/Akt signaling, a well-known modulatory pathway of angiogenesis [61]. We have detailed the interaction of FGF2–FGFR and the involvement of FRS2 in NSCLC angiogenesis in the FGF2-centered mechanistic section above. The overall effect of FRS2-targeting miR-182 is an indirect inhibition of angiogenesis. Stenvold et al. identified a rather weak, but significant correlation between miR-182 and FGF2 [65], further supporting that miR-182 can regulate angiogenesis by its connection with this proangiogenic factor.
Various studies have found miR-155 to be overexpressed in NSCLC [67,68,69,70]. Two of the studies mentioned beforehand have investigated the prognostic impact of miR-155 in NSCLC, high miR-155 expression being correlated with a poor OS in lung AC and SCC [71,72]. Donnem et al. investigated the correlation between miR-155 and different angiogenic markers in NSCLC, and found miR-155 to be significantly correlated with FGF2 in the studied cohort (r = 0.17, p = 0.002). Consequently, miR-155 may be able to modulate angiogenesis through the alteration of FGF2 and its downstream pathways [44]. The mechanism behind FGF2’s action includes the phosphorylation of FRS2 which activates the MAPK and PI3K/Akt signaling pathways in both cancer cells and ECs [61]. Additionally, the JAK2/STAT3 pathway is involved in the FGF2-dependent angiogenic modulation, and MMP secretion [39]. Moreover, Voortman et al. found miR-155 to have no significant prognostic impact in the studied cohort in a qRT-PCR study of formalin-fixed paraffin-embedded NSCLC tumor specimens from 639 resected NSCLC patients who participated in the International Adjuvant Lung Cancer Trial (IALT) [73]. Contrarily, a study by Donnem et al. investigating the prognostic impacts of miR-155 in NSCLC on 335 patients with stage I to IIIA NSCLC found that miR-155 expression had no significant prognostic impact in the total cohort (p = 0.43), but depended on the histological subtype. In the same study, high miR-155 expression in ACs had a negative prognostic effect on survival in univariate analysis (p = 0.086) and was found to be an independent prognostic factor in multivariate analysis (HR 1.87, CI 95% 1.01–3.48, p = 0.047). Moreover, in SCC patients with lymph node metastasis, miR-155 was found to have a positive prognostic impact on survival in both univariate analysis (p = 0.034) and multivariate analysis (HR 0.45, CI 95% 0.21–0.96, p = 0.039) [72].
Another study by Fan et al. identified miR-210 as a proangiogenic microRNA. In their experiment, Fan et al. showed that exosomes containing miR-210 stimulated angiogenesis through a cancer-associated fibroblasts (CAF)-dependent mechanism. In addition, an in-depth analysis found that miR-210 was able to increase the expression of several proangiogenic factors, including FGF2, VEGF-A and MMP9, by activating a number of pathways involved in the angiogenic switch, such as JAK2/STAT3 and ten-eleven translocation 2 (TET2). Concomitantly, the same study found that miR-210 was overexpressed in serum exosomes of patients with untreated NSCLC, further proving the implication of this microRNA in lung cancer angiogenesis [74].
2.3. The Extracellular Matrix: Support for Angiogenesis
The TME is an important consideration in the molecular biology of cancer. It comprises, among others, cancer cells, a large palette of non-cancer cells, blood vessels and ECM entities [75]. Degradation of the ECM entities which support the TME allows ECs to migrate, positively influencing the angiogenic process. In lung cancer, the hypoxic TME enhances the transcription of a number of proangiogenic factors, growth factors and MMPs [76]. Furthermore, the activation of MMPs can be induced by a number of angiogenic factors—VEGF, FGF2, TGF-α,β and angiogenin [77]. These MMPs poses angiogenic activity by degrading the surrounding ECM.
An old study by Itoh et al. found that mice who are MMP-2 deficient show reduced tumor angiogenic properties [78]. Moreover, an in vitro study by Chetty et al. showed that there was a connection between MMP-2 and lung cancer cell tube formation of ECs—MMP-2 siRNA inhibited tube formation—whilst recombinant human-MMP-2 induced angiogenic properties [79]. Mechanistically, MMP-2 inhibition decreased HIF-1α levels and disrupted PI3K-dependent VEGF expression. The authors concluded that the transcriptional suppression of MMP-2 may be able to decrease neoangiogenesis by downregulating VEGF-A [79]. Another study by Chetty et al. found that MMP-2 inhibition in lung tumor cells leads to an inhibition of VEGF-related activation of AKT in ECs, followed by increased ERK activation and an inductive effect on TIMP-3 [80]. TIMPs (TIMP1-4) play an essential role in the regulation of ECM by their inhibitory action on MMPs. Specifically, TIMPs hamper neoangiogenesis by inhibiting MMPs [77] with a consecutive sequestration of ECs in the ECM, further proving the intricate mechanisms of ECM in the regulation of angiogenesis.
In addition, a study by Blanco-Prieto et al. researched serum MMPs (MMP-1, MMP-2, MMP-7, MMP-9, MMP-10) and TIMP-1 levels by multiplexed immunoassays in a study cohort of 19 NSCLC cases and 19 healthy controls. The authors found that MMP-1, MMP-7 and MMP-9 serum levels were slightly elevated in NSCLC patients, prompting the team to rely on larger cohorts for the evaluation of these MMPs as biomarkers for NSCLC. MMP-2 and MMP-10 were discarded from the study because of the low AUC levels, showing poor diagnostic capacity, in spite of their importance in angiogenesis. Blanco-Prieto et al. found that although MMP-1 serum levels were higher than control, the discrimination was poor, highlighted by an AUC value of 0.538; further reference studies suggest that MMP-1 may be implicated in the late stages of the neoplastic disease [81,82]. The team also studied MMP-7 levels in a larger population, showing that MMP-7 serum levels were significantly increased in NSCLC patients with a moderate discrimination level (AUC value of 0.604). Last but not least, MMP-9 serum levels were found to be significantly elevated in NSCLC patients compared to both healthy controls and patients with benign pulmonary pathologies. Blanco-Prieto et al. concluded that MMP-9 serum levels offered the best diagnostic capacity for NSCLC (AUC value of 0.739) of the studied entities [81]. Other studies support the importance of high MMP-9 levels in NSCLC [83,84].
Moreover, Stenvold et al. identified a correlation between miR-182 and MMP-7 in a NSCLC cohort. The examination of coexpression of the miR-182 and MMP-7 resulted in patients with high miR-182 and high MMP-7 expression having independently better survival compared to those with low miR-182 and low MMP-7 expression (hazard ratio (HR) = 0.49, p = 0.015). SCC patients with high/high expression levels have far better prognoses compared to those in the rest of the groups (HR = 0.26, p = 0.012) [65].
2.4. Hypoxia Modulates the Angiogenic Process in Lung Cancer
As mentioned beforehand, hypoxia is a key factor in the modulation of angiogenesis [8,9]. Hypoxia induces vast effects in the TME due to the increased oxygen consumption and requirement caused by the proliferative process.
The majority of hypoxia-derived effects are orchestrated by HIF-1, the hypoxic master regulator; HIF-1, through HIF-1α and HIF-2α, binds to the hypoxia responsive element (HRE) located in the promoter region of the chief proangiogenic factor, VEGF [9], activating the downstream PI3K/Akt, ERK1/2 and JAK2/STAT3 pathways and promoting angiogenesis [37,39]. We have previously shown that the autocrine loop created further stimulates VEGF production. Low oxygen levels can also stimulate a number of other proangiogenic entities, such as FGF and HGF, which promote EC proliferation and enhance migration [85], Angpt2 in a HIF-2α-dependent manner, PLGF, PDGF-β and others. Several studies showed that HIF-1α and HIF-2α have completing effects in the angiogenic setting—the first drives blood vessel growth while the latter induces maturation [86,87]. Tumor hypoxia combined with high serum levels of angiogenesis markers have previously been associated with poor prognosis in NSCLC [88]. The mechanistic interrelation between angiogenesis and hypoxia has been extensively characterized in a previous paper by our group [9].
In a study by Xue et al., miR-206 was able to decrease the angiogenic ability in NSCLC by inhibiting a specific pathway: 14-3-3ζ/STAT3/HIF-1α/VEGF. Precisely, the team showed that miR-206 targets 14-3-3ζ and thus inhibits the downstream STAT3/HIF-1α/VEGF pathway, impeding the angiogenic process [42]. Furthermore, another study conducted by Mao et al. identified miR-494 as an angiogenic promoter in human vascular endothelial cells (HUVECs), and in the A549 NSCLC cell line. MiR-494 effectively targets PTEN and thus the consecutive activation of Akt/eNOS pathway. The same team found that hypoxia induces miR-494 expression, perhaps via a HIF-1α-dependent mechanism [89].
Moreover, a study by Hsu et al. exemplified the intricate mechanisms behind hypoxia-dependent miRNA regulation of angiogenesis. In the Hsu study, the hypoxic lung cancer cell-derived exosomes containing miR-23a reduced PHD2 expression by directly targeting the 3′-UTR of PHD2 in HUVECs, leading to enhanced HIF-1α activity. Concomitantly, miR-23a increased HIF-1α transcription under both hypoxic and normoxic conditions, with a subsequent activation of angiogenesis. The Hsu study found that the hypoxic lung cancer cell-derived exosomes containing miR-23a disrupted the endothelial barrier by targeting ZO-1 and increased the number of tumor vessels, further proving its implication in lung cancer angiogenesis [90].
2.5. miRNAs with Regulatory Potential in Lung Cancer
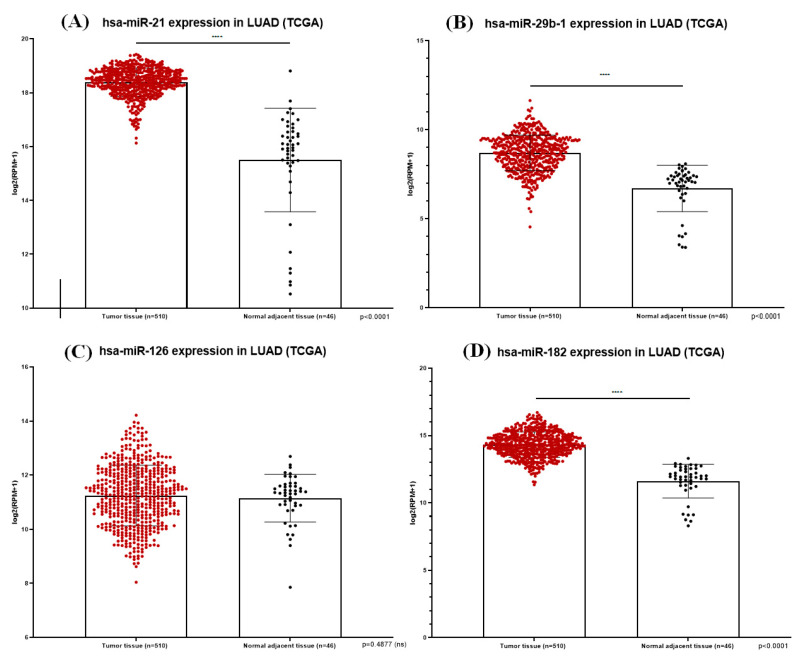
Table 2 presents a short description of a selection of miRNAs that drive angiogenesis in lung cancer, with information varying from miRNA expression and the target gene, to the study type and overall action: miR-106a, miR-141, miR-378, miR-15-16 cluster, miR-106b, miR-128, miR-200b, miR-497 and miR-29b. For integrative and coordination purposes, Table 2 also summarizes the palette of miRNAs discussed in the sections above. Figure 3 illustrates the expression of miR-21, miR-29b-1, miR-126 and miR-182 in LUAD tissue samples from TCGA database, with an obvious dysregulation.
Table 2.
miRNAs with regulatory potential in lung cancer.
| miRNA | Expression | Study Type | Target | Overall Action | References |
|---|---|---|---|---|---|
| miR-106a | ▲ | [44]—ex vivo (tissue) [67]—ex vivo (tissue) [91]—in vitro [92]—clinical/ex vivo |
Predicted: VEGF, FGFR2, STAT3 | Upregulated during hypoxia in breast and colon cancer. Augmented expression in NSCLC. | [44,67,91,92] |
| miR-141 | ▲ in [93] | [93]—in vitro, ex vivo (tissue) [94]—in vitro, in vivo, ex vivo (tissue) |
KLF6, NRP1, GAB1, CXCL12β, TGFβ2, GATA6 | In a lung adenocarcinoma model, overexpression of miR-141 inhibited KLF6 and consecutively increased VEGF-A levels, thus promoting angiogenesis. Opposite results were obtained by Dong et al. who found that miR-141 overexpression exhibits anti-angiogenic properties by suppressing endothelial cell proliferation, migration and tube formation. These effects are the result of miR-141 acting on NRP1, GAB1, CXCL12β, TGFβ2 and GATA6. However, Dong et al. proposed that the effects may vary in different tumor environments. |
[93,94] |
| miR-155 | ▲ | [44]—ex vivo (tissue) [67]—ex vivo (tissue) |
Predicted: FGF2 | MiR-155 has been significantly correlated with FGF2 and with a poor OS in lung AC and SCC. | [44,67,71] |
| miR-182 | ▲ | [44]—ex vivo (tissue) [64]—in vitro, ex vivo (tissue) [65]—ex vivo (tissue) |
FRS2, Tsp1 | The action of miR-182 is intricated, acting on at least 2 targets with antagonizing effect. However, miR-182 is mostly viewed as an oncogene by suppressing the antiangiogenic Tsp1 and thus promoting angiogenesis. | [44,64,65] |
| miR-21 | ▲ | [51]—in vitro, ex vivo (serum) [53]—in vitro, ex vivo (blood) |
HIF-1α, PTEN, PDCD4, hMSH2 | MiR-21 is a well-known oncomiR with various implications in lung cancer. A study by Liu et al. showed that exosome-derived miR-21 induces STAT3 activation, increasing VEGF levels and thus activating angiogenesis. Furthermore, the X study showed that A549 cells within the miR-21 inhibition group showed almost no tube formation when compared to the control group and the mock group. | [51,53,95] |
| miR-210 | ▲ | [74]—in vitro, in vivo
[96]—in vitro |
Succinate dehydrogenase complex subunit D (SDHD), E2F3 | MiR-210 exhibits its proangiogenic effects through a CAF-associated mechanism. A study by Fan et al. showed that miR-210 was able to increase the expression of FGF2, VEGFA and MMP9 by activating JAK2/STAT3 and TET2 pathways. | [74,96] |
| miR-221/222 cluster | ▲ | [97]—in vitro, in vivo
[98]—in vitro, in vivo, ex vivo (tissue) |
TIMP-3 PTEN |
MiR-221/222 are over-expressed in NSCLC cells. The cluster suppresses PTEN and TIMP-3 expression, inducing migration and invasiveness. Janssen et al. proved that TIMP-3 knockdown tumors had a higher level of vascularization versus control. |
[97,98] |
| miR-23a | ▲ | [90]—in vitro, in vivo, ex vivo (tissue) | PHD2, ZO-1 | The Hsu study found that miR-23a increases tumor angiogenesis in both hypoxic and normoxic environment. MiR-23a is able to directly target the 3′-UTR of PHD2 in HUVECs, leading to enhanced HIF-1α activity with proangiogenic features. Furthermore, Hsu et al. showed that miR-23a can target ZO-1, disrupting the endothelial barrier and promoting angiogenesis. | [90] |
| miR-378 | ▲ | [99]—in vitro, in vivo, ex vivo (tissue) [100]—in vitro, in vivo, ex vivo (tissue) [62]—in vitro, in vivo |
RBX1, CRKL | MiR-378 is upregulated in highly invasive lung cancer sub-cell lines. MiR-378 promotes invasion, metastasis through EMT (RBX1, CRKL) and angiogenesis in vivo. A study by Ho et al. showed that RBX1 intervenes in HIF-1α pathway to produce VEGF with a subsequent inductive effect on angiogenesis. Accumulating evidence suggests that miR-378 could act as both oncogene and tumor suppressor. |
[62,99,100] |
| miR-494 | ▲ | [89]—in vitro, in vivo | PTEN | MiR-494 promotes angiogenesis in HUVECs/A549 cells and effectively targets PTEN with the consequent inhibition of the Akt/eNOS pathway. | [89] |
| miR-15-16 cluster | ▼ | [101]—in vitro, in vivo, ex vivo (tissue) | FGF2 | In a study conducted by Xue et al., hypoxia repressed the miR-15-16 cluster, with a loss of restriction of its target gene, FGF2. This action promoted tumor angiogenesis and metastasis. | [101] |
| miR-106b | ▼ | [102]—in vitro, in vivo
[103]—in vitro |
STAT3 | MiR-106b exhibits anti-angiogenic effects by inhibiting STAT3 in ECs. Niu et al. found that VEGF expression correlated positively with STAT3 activity in different human cancer cell lines. |
[102,103] |
| miR-128 | ▼ | [104]—in vitro, in vivo, ex vivo (tissue) | VEGF-A, VEGF-C, VEGFR-2, VEGFR-3 | MiR-128 was significantly downregulated in NSCLC tissues and cancer cells and was correlated with NSCLC differentiation, stage and metastasis to lymph nodes. Overexpression of miR-128 in NSCLC cells decreased expression of VEGF-A, VEGFR-2, VEGFR-3, in in vitro and in vivo experiments. |
[104] |
| miR-200b | ▼ | [105]—in vitro | VEGFA, FLT/VEGFR1, KDR/VEGFR2, Ets1 | MiR-200b binds to the 3′-UTR of Ets-1 mRNA to induce translational repression. Ets-1 is a key transcription factor known for its role in promoting angiogenesis. Physiological levels of miR-200b have an inhibitory effect on angiogenesis. In hypoxic conditions, miR-200b downregulation cancels Ets-1 repression, thus promoting angiogenesis. MiR-200b also targets VEGF and its receptors. | [105] |
| miR-206 | ▼ | [42]—in vitro, in vivo, ex vivo (tissue) [106]—in vitro, ex vivo (tissue) |
SOX9, 14-3-3 ζ | A study by Zhang et al. found downregulated levels of miR-206 and concluded that miR-206 may act as a tumor suppressor partly by targeting SOX9. In another study by Xue et al., miR-206 decreased the angiogenic ability in NSCLC by inhibiting the 14-3-3 ζ/STAT3/HIF-1α/VEGF pathway. | [42,106] |
| miR-497 | ▼ | [107]—in vitro, in vivo, ex vivo (tissue) | HDGF, FGF2 | MiR-497 is downregulated in NSCLC tumors and cell lines. Ectopic expression inhibited cell proliferation and angiogenesis in a SCID mouse xenograft model. | [107,108] |
| miR-126 | ▼ | [23]—in vitro, in vivo
[43]—in vitro, in vivo [46]—in vitro, ex vivo (tissue) [47]—in vitro, in vivo, ex vivo (tissue) [48]—in vitro, ex vivo (tissue) |
VEGFA, EGFL7, PI3KR2 | MiR-126 is one of the most differentially expressed miRNA in lung cancer, with an overall reduced expression. A number of studies found that miR-126 targets the VEGFA with a silencing effect. Enhanced miR-126 expression increases the sensitivity of NSCLC cells to chemotherapy through the VEGFA/PI3K/Akt/MRP1 pathway. Furthermore, two studies showed that miR-126 may target PI3KR2 and that by targeting VEGFA it inactivates the VEGFA/VEGFR2/ERK signaling pathway. | [23,43,46,47,48] |
| miR-135a | ▼ | [60]—in vitro, ex vivo (tissue) | IGF-1 | The Zhou study identified IGF-1 as a direct target of miR-135a. Zhou et al. showed that miR-135a decreased the angiogenic factors VEGF, FGF2 and IL-8 in the A549 cell line by IGF-1 inhibition. | [60] |
| miR-29b | ▼/▲ (TCGA analysis) | [24]—in vitro, ex vivo (tissue) | MMP-2, PTEN, PDGFB, TGF-β1 |
MMP-2 is a known promoter of angiogenesis. In a paper by Wang et al., bioinformatics analysis combined with a polymerase chain reaction study suggested that MMP-2 and PTEN may represent important targets of miR-29b. The same authors concluded that miR-29b behaves as a tumor metastasis suppressor through MMP-2 inhibition. Our analysis found that PDGFB and TGF-β1 may also be targets of miR-29b, marking it as a therapeutic candidate. | [24] |
| miR-204 | ▼ (TCGA analysis) | [109]—in vitro, in vivo | Predicted: JAK2/STAT3 | MiR-204 functions as a tumor suppressor in LUAD; in the Liu study, miR-204 promoted cancer cell apoptosis and inhibited cell migration and proliferation in vitro, and tumor growth in the in vivo model. Conditioned media from A549 cancer cell line with overexpression of miR-204 hampered tube formation and migration of HUVECs. The same authors found decreased levels of HIF-1α, VEGF, PDGF in the A549 cells transfected with miR-204 mimics. Liu et al. concluded that miR-204 inhibits angiogenesis in LUAD, probably via the JAK2/STAT3 pathway. | [109] |
▲—upregulated/▼—downregulated.
Figure 3.
MiR-21 (A), miR-29b-1 (B), miR-126 (C) and miR-182 (D) expression in LUAD tissue samples from TCGA database; the plots show statistically significant overexpression of miR-21, miR-29b-1 and miR-182 with p < 0.0001
3. Current and Future Anti-Angiogenic Therapeutic Options in NSCLC
Several anti-angiogenic drugs have been approved for use in NSCLC, such as monoclonal antibodies directed against VEGF-A/VEGFRs and small molecule tyrosine kinase inhibitors (TKIs). New prospects include the use of ncRNAs that interfere either directly with factors implicated in angiogenesis, or indirectly with intermediary factors in various pathways linked to the angiogenic process. Our exhaustive search from within the literature has not identified any angiogenesis-related miRNAs in use or in clinical trials, and no combinations of standard anti-angiogenic therapy with miRNA-based therapy.
3.1. Conventional Anti-Angiogenics
A summary of the conventional anti-angiogenics used in the treatment of NSCLC is presented within Table 3.
Table 3.
Therapeutic agents that target the angiogenic process in lung cancer. Status by the FDA refers to approval in NSCLC.
| Therapeutic Agent | Type | Target | Mechanism of Action | Status by the FDA | References |
|---|---|---|---|---|---|
| Bevacizumab | Monoclonal antibody | VEGF-A | Recombinant humanized monoclonal antibody directed against VEGF-A. | Approved in combination with carboplatin and paclitaxel chemotherapy for first-line treatment of unresectable, locally advanced, recurrent or metastatic non-squamous NSCLC. | [110] |
| Ramucirumab | Monoclonal antibody | VEGFR2 | Fully humanized monoclonal antibody that specifically binds to VEGFR2, inhibiting angiogenesis. | Approved in combination with docetaxel for metastatic NSCLC with progression after platinum-based chemotherapy. Approved by FDA in May 2020 in combination with erlotinib for first-line treatment of metastatic NSCLC with EGFR exon 19 deletions or exon 21 (L858R) mutations in accordance to the RELAY trial (ClinicalTrials.gov identifier: NCT02411448). |
[111] |
| Nintedanib | Small-molecule, multi-targeted TKI | VEGFR1-3; FGFR1-3; PDGFR-α, β; Src family |
Nintedanib inhibits downstream signaling by binding to the adenosine triphosphate (ATP) sites of proangiogenic receptors. | Approved in combination with docetaxel for the treatment of locally advanced, recurrent or metastatic lung adenocarcinoma 1. | [112,113] |
1 regulated by EMA and not by FDA.
3.2. MiRNAs as Therapeutic Agents: Prospects
In the past few years, the potential of miRNAs as therapeutic agents increased significantly, which is reflected in the number of publications. However, there are no clinical trials that use miRNAs to target angiogenesis in non-small cell lung cancer at the time of submitting this manuscript. To our knowledge, only two phase I clinical trials evaluated the safety of miRNA-based drugs that included NSCLC patients—the MRX34 clinical trial (clinicaltrials.gov identifier: NCT01829971) and the MesomiR 1 trial (clinicaltrials.gov identifier: NCT02369198). These trials represent a starting point for future research regarding the therapeutic and translational potential of these ncRNAs. Recent results from MRX34 and MesomiR 1 suggest that miRNA-based therapy is effective in a selected pool of patients, taking into consideration the inflammatory adverse effects that may lead to severe complications, as exemplified in the MRX34 study [114].
Considering the aforementioned, we find that the modulation of miR-126 and miR-21 presents great potential as a therapeutic strategy. A valuable argument is that both these miRNAs target multiple pathways involved in NSCLC progression and angiogenesis, with a suggestive increased overall effect.
4. Conclusions
The silent progression of NSCLC is one of the crucial factors that shape this malignancy. Folkman’s hypothesis led to the development of a new therapeutic idea in cancers, creating a new game plan in the treatment of NSCLC. To this day, inhibition of angiogenesis represents a viable option in the therapeutic approach of NSCLC.
The identification of the main factors that drive the angiogenic process, along with the corresponding pathways, allowed researchers to build new therapeutic strategies. Essentially, current anti-angiogenic therapy targets either key proangiogenic factors such as VEGF and VEGFR1-2, or intermediaries in the signal transduction cascade. Key proangiogenic factors include VEGF, FGF2, PDGF and others. Current approved anti-angiogenic therapies used in NSCLC consist of either monoclonal antibodies directed against VEGF-A (bevacizumab) and VEGFR2 (ramucirumab), or small-molecule tyrosine kinase inhibitors that bind proangiogenic factors’ receptors, such as the EMA-approved nintedanib which has a multi-targeted profile. To date, small-molecule TKIs have failed to demonstrate any significant improvement in overall survival, with the exception of the EMA-approved nintedanib. A number of studies proved that the efficacy of some anti-angiogenics plus standard chemotherapy is greater than chemotherapy only, rendering their importance in treating NSCLC.
A rather new but not fully explored path in anti-angiogenic therapy is represented by ncRNAs. The paramount role of the miRNAs in the angiogenic process would make them the ultimate therapeutic agents. In this review, we have critically summarized the specific miRNA palette that regulate the angiogenic process in NSCLC and that may present a therapeutic starting point for future research related to anti-angiogenic therapeutics. For instance, miR-126 is able to downregulate VEGF-A levels and thus suppress the angiogenic process [23]; on the other hand, miR-21 is an oncomiR that can be targeted. We suggest that miR-126 and miR-21 present a great therapeutic potential in NSCLC angiogenesis. At the time of submitting this manuscript, there are no clinical trials that use miRNAs in order to target the angiogenic process in NSCLC. The MRX34 and MesomiR 1 clinical trials proved that miRNA therapeutics may benefit carefully selected patients; these clinical trials represent the starting point for future research in this domain. As such, the therapeutic potential of miRNAs in the inhibition of angiogenesis is of great importance and should be further exploited in order to improve patient survival.
Acknowledgments
The figures within this manuscript were generated with the help of the Servier Medical ART tool and processed with Adobe Photoshop. Information from within Table 1 was identified with the help of the miRTargetLink Human tool (Saarland University) and the OncoMir Cancer Database from the Masonic Cancer Center, University of Minnesota.
Author Contributions
Conceptualization, A.T., D.G., L.A.P., P.C. and I.B.-N.; writing—original draft preparation, A.T., D.G. and G.R.T.; writing—review and editing, all authors; image conceptualization, A.T., D.G., A.N., A.I. and I.B.-N.; image design, A.T., D.G. and A.N.; supervision, I.B.-N.; project administration, I.B.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Competitivity Operational Program, 2014–2020, entitled “Clinical and economical impact of personalized targeted anti-microRNA therapies in reconverting lung cancer chemoresistance”—CANTEMIR, number 35/01.09.2016, MySMIS 103375; project PNCDI III 2015–2020 entitled “Increasing the performance of scientific research and technology transfer in translational medicine through the formation of a new generation of young researchers”—ECHITAS, number 29PFE/18.10.2018; project H2020-MSCA-RISE-2019, entitled “DevelOpmeNt of Cancer RNA TherapEutics”—cONCReTE; project CNFIS-FDI-2020-0436, entitled “Development of entrepreneurial skills among UMF Cluj-Napoca students through the development of experiential educational modules.”
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sher T., Dy G.K., Adjei A.A. Small cell lung cancer. Mayo Clin. Proc. 2008;83:355–367. doi: 10.4065/83.3.355. [DOI] [PubMed] [Google Scholar]
- 3.Zappa C., Mousa S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revannasiddaiah S., Thakur P., Bhardwaj B., Susheela S.P., Madabhavi I. Pulmonary adenocarcinoma: Implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification. J. Thorac. Dis. 2014;6:S502–S525. doi: 10.3978/j.issn.2072-1439.2014.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 7.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. CMLS. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braicu C., Gulei D., Cojocneanu R., Raduly L., Jurj A., Knutsen E., Calin G.A., Berindan-Neagoe I. miR-181a/b therapy in lung cancer: Reality or myth? Mol. Oncol. 2019;13:9–25. doi: 10.1002/1878-0261.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tirpe A.A., Gulei D., Ciortea S.M., Crivii C., Berindan-Neagoe I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019;20:6140. doi: 10.3390/ijms20246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braicu C., Buiga R., Cojocneanu R., Buse M., Raduly L., Pop L.A., Chira S., Budisan L., Jurj A., Ciocan C., et al. Connecting the dots between different networks: miRNAs associated with bladder cancer risk and progression. J. Exp. Clin. Cancer Res. 2019;38:433. doi: 10.1186/s13046-019-1406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B., Pan X., Cobb G.P., Anderson T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Ipsaro J.J., Joshua-Tor L. From guide to target: Molecular insights into eukaryotic RNA-interference machinery. Nat. Struct. Mol. Biol. 2015;22:20–28. doi: 10.1038/nsmb.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berindan-Neagoe C.B.I., Gulei D., Tomuleasa C., Calin G.A. Noncoding RNAs in Lung Cancer Angiogenesis. In: Simionescu D., editor. Physiologic and Pathologic Angiogenesis—Signaling Mechanisms and Targeted Therapy. IntechOpen; Rijeka, Croatia: 2017. [Google Scholar]
- 15.Marech I., Leporini C., Ammendola M., Porcelli M., Gadaleta C.D., Russo E., De Sarro G., Ranieri G. Classical and non-classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett. 2016;380:216–226. doi: 10.1016/j.canlet.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Rajabi M., Mousa S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines. 2017;5:34. doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremnes R.M., Camps C., Sirera R. Angiogenesis in non-small cell lung cancer: The prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L., Huang Q., Zhang S., Zhang Q., Chang J., Qiu X., Wang E. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010;10:318. doi: 10.1186/1471-2407-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X., Qiu J., Kang H., Wang Y., Qian J. miR-125a-5p suppresses colorectal cancer progression by targeting VEGFA. Cancer Manag. Res. 2018;10:5839–5853. doi: 10.2147/CMAR.S161990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Chen Y., Li Y., Li C., Qin T., Bai M., Zhang Z., Jia R., Su Y., Wang C. miR195 suppresses metastasis and angiogenesis of squamous cell lung cancer by inhibiting the expression of VEGF. Mol. Med. Rep. 2019;20:2625–2632. doi: 10.3892/mmr.2019.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P., Xiong J., Zuo L., Liu K., Zhang H. miR1405p regulates cell migration and invasion of nonsmall cell lung cancer cells through targeting VEGFA. Mol. Med. Rep. 2018;18:2866–2872. doi: 10.3892/mmr.2018.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jusufovic E., Rijavec M., Keser D., Korosec P., Sodja E., Iljazovic E., Radojevic Z., Kosnik M. Let-7b and miR-126 are down-regulated in tumor tissue and correlate with microvessel density and survival outcomes in non-small-cell lung cancer. PLoS ONE. 2012;7:e45577. doi: 10.1371/journal.pone.0045577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B., Peng X.C., Zheng X.L., Wang J., Qin Y.W. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Guan X., Tu Y., Zheng S., Long J., Li S., Qi C., Xie X., Zhang H., Zhang Y. MicroRNA-29b attenuates non-small cell lung cancer metastasis by targeting matrix metalloproteinase 2 and PTEN. J. Exp. Clin. Cancer Res. 2015;34:59. doi: 10.1186/s13046-015-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J.Y., Zhang Q., Wang D.D., Yan W., Sha H.H., Zhao J.H., Yang S.J., Zhang H.D., Hou J.C., Xu H.Z., et al. MiR-29a: A potential therapeutic target and promising biomarker in tumors. Biosci. Rep. 2018:38. doi: 10.1042/BSR20171265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Zhu Y., Zhao M., Wu C., Zhang P., Tang L., Zhang H., Chen X., Yang Y., Liu G. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2) PLoS ONE. 2013;8:e70192. doi: 10.1371/journal.pone.0070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P., Ma Y., Wang Y., Chen T., Wang H., Chu H., Zhao G., Zhang G. Identification of miR-1293 potential target gene: TIMP-1. Mol. Cell. Biochem. 2013;384:1–6. doi: 10.1007/s11010-013-1775-7. [DOI] [PubMed] [Google Scholar]
- 28.Nurul-Syakima A.M., Yoke-Kqueen C., Sabariah A.R., Shiran M.S., Singh A., Learn-Han L. Differential microRNA expression and identification of putative miRNA targets and pathways in head and neck cancers. Int. J. Mol. Med. 2011;28:327–336. doi: 10.3892/ijmm.2011.714. [DOI] [PubMed] [Google Scholar]
- 29.Xu X., Zong Y., Gao Y., Sun X., Zhao H., Luo W., Jia S. VEGF Induce Vasculogenic Mimicry of Choroidal Melanoma through the PI3k Signal Pathway. BioMed Res. Int. 2019;2019:3909102. doi: 10.1155/2019/3909102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altorki N.K., Markowitz G.J., Gao D., Port J.L., Saxena A., Stiles B., McGraw T., Mittal V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer. 2019;19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin S., Yi M., Jiao D., Li A., Wu K. Distinct Roles of VEGFA and ANGPT2 in Lung Adenocarcinoma and Squamous Cell Carcinoma. J. Cancer. 2020;11:153–167. doi: 10.7150/jca.34693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian L., Li X.L., Xu M.D., Li X.M., Wu M.Y., Zhang Y., Tao M., Li W., Shen X.M., Zhou C., et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19:183. doi: 10.1186/s12885-019-5322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu G., Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets. 2010;11:1000–1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Q., Guo L., Lin G., Chen Z., Chen T., Lin J., Zhang B., Gu X. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015;39:539–544. doi: 10.1016/j.canep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Frezzetti D., Gallo M., Roma C., D’Alessio A., Maiello M.R., Bevilacqua S., Normanno N., De Luca A. Vascular Endothelial Growth Factor A Regulates the Secretion of Different Angiogenic Factors in Lung Cancer Cells. J. Cell. Physiol. 2016;231:1514–1521. doi: 10.1002/jcp.25243. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y., Xiu Z., Zhou Z., Huang B., Liu J., Wu X., Li S., Tang X. Cytochalasin H Inhibits Angiogenesis via the Suppression of HIF-1alpha Protein Accumulation and VEGF Expression through PI3K/AKT/P70S6K and ERK1/2 Signaling Pathways in Non-Small Cell Lung Cancer Cells. J. Cancer. 2019;10:1997–2005. doi: 10.7150/jca.29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y., Qi Y., Zhang Y., Chen J., Wu T., Gu Y. Regulation of angiogenic factors by the PI3K/Akt pathway in A549 lung cancer cells under hypoxic conditions. Oncol. Lett. 2017;13:2909–2914. doi: 10.3892/ol.2017.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao M., Gao F.H., Wang J.Y., Liu F., Yuan H.H., Zhang W.Y., Jiang B. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer. 2011;73:366–374. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Shi L., Zhang S., Wu H., Zhang L., Dai X., Hu J., Xue J., Liu T., Liang Y., Wu G. MiR-200c increases the radiosensitivity of non-small-cell lung cancer cell line A549 by targeting VEGF-VEGFR2 pathway. PLoS ONE. 2013;8:e78344. doi: 10.1371/journal.pone.0078344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodd K.M., Yang J., Shen M.H., Sampson J.R., Tee A.R. mTORC1 drives HIF-1alpha and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34:2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue D., Yang Y., Liu Y., Wang P., Dai Y., Liu Q., Chen L., Shen J., Ju H., Li Y., et al. MicroRNA-206 attenuates the growth and angiogenesis in non-small cell lung cancer cells by blocking the 14-3-3zeta/STAT3/HIF-1alpha/VEGF signaling. Oncotarget. 2016;7:79805–79813. doi: 10.18632/oncotarget.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y., Bai Y., Zhang F., Wang Y., Guo Y., Guo L. miR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem. Biophys. Res. Commun. 2010;391:1483–1489. doi: 10.1016/j.bbrc.2009.12.098. [DOI] [PubMed] [Google Scholar]
- 44.Donnem T., Fenton C.G., Lonvik K., Berg T., Eklo K., Andersen S., Stenvold H., Al-Shibli K., Al-Saad S., Bremnes R.M., et al. MicroRNA signatures in tumor tissue related to angiogenesis in non-small cell lung cancer. PLoS ONE. 2012;7:e29671. doi: 10.1371/journal.pone.0029671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu X., Li H., Long L., Hui L., Chen H., Wang X., Shen H., Xu W. miR-126 enhances the sensitivity of non-small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A. Acta Biochim. Biophys. Sin. 2012;44:519–526. doi: 10.1093/abbs/gms026. [DOI] [PubMed] [Google Scholar]
- 46.Cho W.C., Chow A.S., Au J.S. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur. J. Cancer. 2009;45:2197–2206. doi: 10.1016/j.ejca.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Yang J., Lan H., Huang X., Liu B., Tong Y. MicroRNA-126 inhibits tumor cell growth and its expression level correlates with poor survival in non-small cell lung cancer patients. PLoS ONE. 2012;7:e42978. doi: 10.1371/journal.pone.0042978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi H., Bi H., Sun X., Dong H., Jiang Y., Mu H., Liu G., Kong W., Gao R., Su J. Antitumor effects of Tubeimoside-1 in NCI-H1299 cells are mediated by microRNA-126-5p-induced inactivation of VEGF-A/VEGFR-2/ERK signaling pathway. Mol. Med. Rep. 2018;17:4327–4336. doi: 10.3892/mmr.2018.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markou A., Sourvinou I., Vorkas P.A., Yousef G.M., Lianidou E. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer. 2013;81:388–396. doi: 10.1016/j.lungcan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Jiang M., Zhang P., Hu G., Xiao Z., Xu F., Zhong T., Huang F., Kuang H., Zhang W. Relative expressions of miR-205-5p, miR-205-3p, and miR-21 in tissues and serum of non-small cell lung cancer patients. Mol. Cell. Biochem. 2013;383:67–75. doi: 10.1007/s11010-013-1755-y. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Luo F., Wang B., Li H., Xu Y., Liu X., Shi L., Lu X., Xu W., Lu L., et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370:125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Gulei D., Berindan-Neagoe I. Activation of Necroptosis by Engineered Self Tumor-Derived Exosomes Loaded with CRISPR/Cas9. Mol. Ther. Nucleic Acids. 2019;17:448–451. doi: 10.1016/j.omtn.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong J., Zhang Z., Gu T., Xu S.F., Dong L.X., Li X., Fu B.H., Fu Z.Z. The role of microRNA-21 in predicting brain metastases from non-small cell lung cancer. Oncotargets Ther. 2017;10:185–194. doi: 10.2147/OTT.S116619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu A., Lu J., Wang W., Shi C., Han B., Yao M. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2016;70:118–125. doi: 10.1016/j.biocel.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Meder L., Konig K., Dietlein F., Macheleidt I., Florin A., Ercanoglu M.S., Rommerscheidt-Fuss U., Koker M., Schon G., Odenthal M., et al. LIN28B enhanced tumorigenesis in an autochthonous KRAS(G12V)-driven lung carcinoma mouse model. Oncogene. 2018;37:2746–2756. doi: 10.1038/s41388-018-0158-7. [DOI] [PubMed] [Google Scholar]
- 56.Sato H., Shien K., Tomida S., Okayasu K., Suzawa K., Hashida S., Torigoe H., Watanabe M., Yamamoto H., Soh J., et al. Targeting the miR-200c/LIN28B axis in acquired EGFR-TKI resistance non-small cell lung cancer cells harboring EMT features. Sci. Rep. 2017;7:40847. doi: 10.1038/srep40847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu M., Hu Y., He J., Li B. Prognostic Value of Basic Fibroblast Growth Factor (bFGF) in Lung Cancer: A Systematic Review with Meta-Analysis. PLoS ONE. 2016;11:e0147374. doi: 10.1371/journal.pone.0147374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L., Zhang S., Wei L., Wang Z., Ma W., Liu F., Qian Y. FGF2 and FGFR2 in patients with idiopathic pulmonary fibrosis and lung cancer. Oncol. Lett. 2018;16:2490–2494. doi: 10.3892/ol.2018.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takanami I., Tanaka F., Hashizume T., Kodaira S. Pulmonary adenocarcinoma angiogenesis. Int. J. Oncol. 1997;10:101–106. doi: 10.3892/ijo.10.1.101. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Y., Li S., Li J., Wang D., Li Q. Effect of microRNA-135a on Cell Proliferation, Migration, Invasion, Apoptosis and Tumor Angiogenesis Through the IGF-1/PI3K/Akt Signaling Pathway in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2017;42:1431–1446. doi: 10.1159/000479207. [DOI] [PubMed] [Google Scholar]
- 61.Akl M.R., Nagpal P., Ayoub N.M., Tai B., Prabhu S.A., Capac C.M., Gliksman M., Goy A., Suh K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2/bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget. 2016;7:44735–44762. doi: 10.18632/oncotarget.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho C.S., Noor S.M., Nagoor N.H. MiR-378 and MiR-1827 Regulate Tumor Invasion, Migration and Angiogenesis in Human Lung Adenocarcinoma by Targeting RBX1 and CRKL, Respectively. J. Cancer. 2018;9:331–345. doi: 10.7150/jca.18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casanovas O., Hicklin D.J., Bergers G., Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Zhang H., Li Y., Zhao C., Fan Y., Liu J., Li X., Liu H., Chen J. MiR-182 inhibits the epithelial to mesenchymal transition and metastasis of lung cancer cells by targeting the Met gene. Mol. Carcinog. 2018;57:125–136. doi: 10.1002/mc.22741. [DOI] [PubMed] [Google Scholar]
- 65.Stenvold H., Donnem T., Andersen S., Al-Saad S., Busund L.T., Bremnes R.M. Stage and tissue-specific prognostic impact of miR-182 in NSCLC. BMC Cancer. 2014;14:138. doi: 10.1186/1471-2407-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ong S.H., Guy G.R., Hadari Y.R., Laks S., Gotoh N., Schlessinger J., Lax I. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 2000;20:979–989. doi: 10.1128/MCB.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raponi M., Dossey L., Jatkoe T., Wu X., Chen G., Fan H., Beer D.G. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 2009;69:5776–5783. doi: 10.1158/0008-5472.CAN-09-0587. [DOI] [PubMed] [Google Scholar]
- 68.Landi M.T., Zhao Y., Rotunno M., Koshiol J., Liu H., Bergen A.W., Rubagotti M., Goldstein A.M., Linnoila I., Marincola F.M., et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin. Cancer Res. 2010;16:430–441. doi: 10.1158/1078-0432.CCR-09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanaihara N., Caplen N., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R.M., Okamoto A., Yokota J., Tanaka T., et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 70.Gulei D., Raduly L., Broseghini E., Ferracin M., Berindan-Neagoe I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol. Asp. Med. 2019;70:33–56. doi: 10.1016/j.mam.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Olson P., Lu J., Zhang H., Shai A., Chun M.G., Wang Y., Libutti S.K., Nakakura E.K., Golub T.R., Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donnem T., Eklo K., Berg T., Sorbye S.W., Lonvik K., Al-Saad S., Al-Shibli K., Andersen S., Stenvold H., Bremnes R.M., et al. Prognostic impact of MiR-155 in non-small cell lung cancer evaluated by in situ hybridization. J. Transl. Med. 2011;9:6. doi: 10.1186/1479-5876-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voortman J., Goto A., Mendiboure J., Sohn J.J., Schetter A.J., Saito M., Dunant A., Pham T.C., Petrini I., Lee A., et al. MicroRNA expression and clinical outcomes in patients treated with adjuvant chemotherapy after complete resection of non-small cell lung carcinoma. Cancer Res. 2010;70:8288–8298. doi: 10.1158/0008-5472.CAN-10-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan J., Xu G., Chang Z., Zhu L., Yao J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin. Sci. 2020;134:807–825. doi: 10.1042/CS20200039. [DOI] [PubMed] [Google Scholar]
- 75.Lv Y., Zhao X., Zhu L., Li S., Xiao Q., He W., Yin L. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics. 2018;8:2830–2845. doi: 10.7150/thno.23209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.John A., Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. POR. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 77.Quintero-Fabian S., Arreola R., Becerril-Villanueva E., Torres-Romero J.C., Arana-Argaez V., Lara-Riegos J., Ramirez-Camacho M.A., Alvarez-Sanchez M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Itoh T., Tanioka M., Yoshida H., Yoshioka T., Nishimoto H., Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 79.Chetty C., Lakka S.S., Bhoopathi P., Rao J.S. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int. J. Cancer. 2010;127:1081–1095. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chetty C., Lakka S.S., Bhoopathi P., Kunigal S., Geiss R., Rao J.S. Tissue inhibitor of metalloproteinase 3 suppresses tumor angiogenesis in matrix metalloproteinase 2-down-regulated lung cancer. Cancer Res. 2008;68:4736–4745. doi: 10.1158/0008-5472.CAN-07-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blanco-Prieto S., Barcia-Castro L., Paez de la Cadena M., Rodriguez-Berrocal F.J., Vazquez-Iglesias L., Botana-Rial M.I., Fernandez-Villar A., De Chiara L. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer. 2017;17:823. doi: 10.1186/s12885-017-3842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M., Xiao T., Zhang Y., Feng L., Lin D., Liu Y., Mao Y., Guo S., Han N., Di X., et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer. 2010;69:341–347. doi: 10.1016/j.lungcan.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Fiorelli A., Rizzo A., Messina G., Izzo A., Vicidomini G., Pannone G., Santini M., Di Domenico M. Correlation between matrix metalloproteinase 9 and 18F-2-fluoro-2-deoxyglucose-positron emission tomography as diagnostic markers of lung cancer. Eur. J. Cardio-Thorac. Surg. 2012;41:852–860. doi: 10.1093/ejcts/ezr117. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y., Wu J.Z., Zhang J.Y., Xue J., Ma R., Cao H.X., Feng J.F. Detection of circulating vascular endothelial growth factor and matrix metalloproteinase-9 in non-small cell lung cancer using Luminex multiplex technology. Oncol. Lett. 2014;7:499–506. doi: 10.3892/ol.2013.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu H., Zhang S. Hypoxia inducible factor-1alpha/vascular endothelial growth factor signaling activation correlates with response to radiotherapy and its inhibition reduces hypoxia-induced angiogenesis in lung cancer. J. Cell. Biochem. 2018;119:7707–7718. doi: 10.1002/jcb.27120. [DOI] [PubMed] [Google Scholar]
- 86.Skuli N., Liu L., Runge A., Wang T., Yuan L., Patel S., Iruela-Arispe L., Simon M.C., Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang N., Wang L., Esko J., Giordano F.J., Huang Y., Gerber H.P., Ferrara N., Johnson R.S. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 88.Goudar R.K., Vlahovic G. Hypoxia, angiogenesis, and lung cancer. Curr. Oncol. Rep. 2008;10:277–282. doi: 10.1007/s11912-008-0043-6. [DOI] [PubMed] [Google Scholar]
- 89.Mao G., Liu Y., Fang X., Liu Y., Fang L., Lin L., Liu X., Wang N. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis. 2015;18:373–382. doi: 10.1007/s10456-015-9474-5. [DOI] [PubMed] [Google Scholar]
- 90.Hsu Y.L., Hung J.Y., Chang W.A., Lin Y.S., Pan Y.C., Tsai P.H., Wu C.Y., Kuo P.L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 91.Hua Z., Lv Q., Ye W., Wong C.K., Cai G., Gu D., Ji Y., Zhao C., Wang J., Yang B.B., et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Espinosa-Parrilla Y., Munoz X., Bonet C., Garcia N., Vencesla A., Yiannakouris N., Naccarati A., Sieri S., Panico S., Huerta J.M., et al. Genetic association of gastric cancer with miRNA clusters including the cancer-related genes MIR29, MIR25, MIR93 and MIR106: Results from the EPIC-EURGAST study. Int. J. Cancer. 2014;135:2065–2076. doi: 10.1002/ijc.28850. [DOI] [PubMed] [Google Scholar]
- 93.Tejero R., Navarro A., Campayo M., Vinolas N., Marrades R.M., Cordeiro A., Ruiz-Martinez M., Santasusagna S., Molins L., Ramirez J., et al. miR-141 and miR-200c as markers of overall survival in early stage non-small cell lung cancer adenocarcinoma. PLoS ONE. 2014;9:e101899. doi: 10.1371/journal.pone.0101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong H., Weng C., Bai R., Sheng J., Gao X., Li L., Xu Z. The regulatory network of miR-141 in the inhibition of angiogenesis. Angiogenesis. 2018 doi: 10.1007/s10456-018-9654-1. [DOI] [PubMed] [Google Scholar]
- 95.Bica-Pop C., Cojocneanu-Petric R., Magdo L., Raduly L., Gulei D., Berindan-Neagoe I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell. Mol. Life Sci. 2018;75:3539–3551. doi: 10.1007/s00018-018-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Puissegur M.P., Mazure N.M., Bertero T., Pradelli L., Grosso S., Robbe-Sermesant K., Maurin T., Lebrigand K., Cardinaud B., Hofman V., et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janssen A., Hoellenriegel J., Fogarasi M., Schrewe H., Seeliger M., Tamm E., Ohlmann A., May C.A., Weber B.H., Stohr H. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Investig. Ophthalmol. Vis. Sci. 2008;49:2812–2822. doi: 10.1167/iovs.07-1444. [DOI] [PubMed] [Google Scholar]
- 98.Garofalo M., Di Leva G., Romano G., Nuovo G., Suh S.S., Ngankeu A., Taccioli C., Pichiorri F., Alder H., Secchiero P., et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Chen L.T., Xu S.D., Xu H., Zhang J.F., Ning J.F., Wang S.F. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med. Oncol. 2012;29:1673–1680. doi: 10.1007/s12032-011-0083-x. [DOI] [PubMed] [Google Scholar]
- 100.Skrzypek K., Tertil M., Golda S., Ciesla M., Weglarczyk K., Collet G., Guichard A., Kozakowska M., Boczkowski J., Was H., et al. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal. 2013;19:644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue G., Yan H.L., Zhang Y., Hao L.Q., Zhu X.T., Mei Q., Sun S.H. c-Myc-mediated repression of miR-15-16 in hypoxia is induced by increased HIF-2alpha and promotes tumor angiogenesis and metastasis by upregulating FGF2. Oncogene. 2015;34:1393–1406. doi: 10.1038/onc.2014.82. [DOI] [PubMed] [Google Scholar]
- 102.Niu G., Wright K.L., Huang M., Song L., Haura E., Turkson J., Zhang S., Wang T., Sinibaldi D., Coppola D., et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 103.Maimaiti A., Maimaiti A., Yang Y., Ma Y. MiR-106b exhibits an anti-angiogenic function by inhibiting STAT3 expression in endothelial cells. Lipids Health Dis. 2016;15:51. doi: 10.1186/s12944-016-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu J., Cheng Y., Li Y., Jin Z., Pan Y., Liu G., Fu S., Zhang Y., Feng K., Feng Y. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur. J. Cancer. 2014;50:2336–2350. doi: 10.1016/j.ejca.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 105.Choi Y.C., Yoon S., Jeong Y., Yoon J., Baek K. Regulation of vascular endothelial growth factor signaling by miR-200b. Mol. Cells. 2011;32:77–82. doi: 10.1007/s10059-011-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y.J., Xu F., Zhang Y.J., Li H.B., Han J.C., Li L. miR-206 inhibits non small cell lung cancer cell proliferation and invasion by targeting SOX9. Int. J. Clin. Exp. Med. 2015;8:9107–9113. [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao W.Y., Wang Y., An Z.J., Shi C.G., Zhu G.A., Wang B., Lu M.Y., Pan C.K., Chen P. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013;435:466–471. doi: 10.1016/j.bbrc.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 108.Huang X., Wang L., Liu W., Li F. MicroRNA-497-5p inhibits proliferation and invasion of non-small cell lung cancer by regulating FGF2. Oncol. Lett. 2019;17:3425–3431. doi: 10.3892/ol.2019.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X., Gao X., Zhang W., Zhu T., Bi W., Zhang Y. MicroRNA-204 deregulation in lung adenocarcinoma controls the biological behaviors of endothelial cells potentially by modulating Janus kinase 2-signal transducer and activator of transcription 3 pathway. IUBMB Life. 2018;70:81–91. doi: 10.1002/iub.1706. [DOI] [PubMed] [Google Scholar]
- 110.Russo A.E., Priolo D., Antonelli G., Libra M., McCubrey J.A., Ferrau F. Bevacizumab in the treatment of NSCLC: Patient selection and perspectives. Lung Cancer. 2017;8:259–269. doi: 10.2147/LCTT.S110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arrieta O., Zatarain-Barron Z.L., Cardona A.F., Carmona A., Lopez-Mejia M. Ramucirumab in the treatment of non-small cell lung cancer. Expert Opin. Drug Saf. 2017;16:637–644. doi: 10.1080/14740338.2017.1313226. [DOI] [PubMed] [Google Scholar]
- 112.Rolfo C., Raez L.E., Bronte G., Santos E.S., Papadimitriou K., Buffoni L., van Meerbeeck J.P., Russo A. BIBF 1120/nintedanib: A new triple angiokinase inhibitor-directed therapy in patients with non-small cell lung cancer. Expert Opin. Investig. Drugs. 2013;22:1081–1088. doi: 10.1517/13543784.2013.812630. [DOI] [PubMed] [Google Scholar]
- 113.Bronte G., Passiglia F., Galvano A., Barraco N., Listi A., Castiglia M., Rizzo S., Fiorentino E., Bazan V., Russo A. Nintedanib in NSCLC: Evidence to date and place in therapy. Ther. Adv. Med. Oncol. 2016;8:188–197. doi: 10.1177/1758834016630976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hong D.S., Kang Y.K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.L., Kim T.Y., et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]