Abstract
Dendrobium officinale Kimura et Migo, a rare and traditional medicinal plant, contains many nutrients such as polysaccharides, alkaloids, amino acids and so on. Different growth environment and intraspecific hybridization of different germplasm resources lead to large differences in the yield, quality and medicinal value of D. officinale. Here, the volatile compounds of D. officinale from four producing regions (Zhejiang, Fujian, Yunnan and Jiangxi) were analyzed to provide a certain reference value for the selection of a specific medicinal component in D. officinale breeding. Fresh stems of D. officinale germplasm resources were collected, and the chemical constituents were determined by gas chromatography-mass spectrometry. A total of 101 volatile compounds were identified, of which esters and alcohols accounted for 23 and 22. Hexacosane is the highest relative content of all volatile components. The highest content of hexacosane was observed in YA1 from Yunnan was 34.41%, and the lowest (23.41%) in JA1 from Jiangxi. Moreover, 5-10 unique substances were determined in different regions. A total of 17 medicinal components were detected, and three unique medicinal components were detected only in YA1, revealing that YA1 can provide raw materials for the application of specific medicinal substances extraction. A total of four toxic components were detected, but no toxic components were detected in JA1 from Jiangxi, suggested that the germplasm resources from Jiangxi could be exploited efficiently for breeding superior D. officinale specimens. The results provide a theoretical basis for the collection, protection and utilization of D. officinale germplasm resources in different regions.
Keywords: Dendrobium officinale, volatile components, different regions, GC-MS
1. Introduction
Dendrobium is a perennial herb in the family Orchidacea (Dendrobium Sw.), widely distributed in Australasia, Oceania and other tropical and subtropical areas. In China, there are 74 Dendrobium species and two varieties, and nearly 50 of these species are used in medicine, among which Dendrobium officinale Kimura et Migo is commercially valuable. Dendrobium officinale Kimura et Migo is a rare and traditional medicinal plant in China, containing many nutrients such as polysaccharides, alkaloids, amino acids, stilbenes, flavonoids, trace elements and so on [1]. Polysaccharides of D. officinale exhibit anti-aging, immune-enhancing, anti-inflammatory, liver-protective and anti-tumor activities [2]. The biological activities of alkaloid have been proved anti-oxidation, treat cell damage, anti-inflammation, liver-protection, anti-neurodegeneration and so on [3,4]. The medicinal value of D. officinale is recognized by the public and market demand has been steadily on the increase, but wild D. officinale usually grows on nutrient-poor cliffs or tree trunks on mountains close to one kilometer above sea level and prefers to grow on wet rocks, indicating that it has very strict requirements for its habitats, and mainly distributed in Southwest Anhui, Eastern Zhejiang, Jiangxi, Western Fujian, Northwest Guangxi, Sichuan and Southeast Yunnan [5]. Meanwhile, over-exploitation led to the depletion of its wild resources and is now listed as an IUCN critically endangered plant [6,7].
In the long-term evolution process, the self-incompatibility mechanism of reproductive isolation was formed, which showed that the pistils and stamens develop normally and mature at the same time and the seeds can’t be produced after self-pollination or cross pollination with the same genotype [8]. The fine varieties of D. officinale can only be preserved by intraspecific hybridization or asexual propagation. At present, most of the D. officinale in the market are generated through intraspecific hybridization of different categories, results in different quality and medicinal value of D. officinale in different regions [9]. In addition, different distribution regions and growth environment may lead to a large gap in the yield and quality of D. officinale [10]. Therefore, it is of great significance to find, collect and cultivate of D. officinale germplasm resources with different qualities for its industrialization.
Here, we collected fresh stem of D. officinale germplasm resources from different regions (Zhejiang, Fujian, Yunnan and Jiangxi), the chemical constituents of D. officinale from different regions were determined by a gas chromatography-mass spectrometry (GC-MS)-based metabolomic approach. The medicinal components and toxic substances of D. officinale from the four producing regions were analyzed to provide a certain reference value for the selection of a specific medicinal component in D. officinale breeding. The results should provide theoretical basis for the collection, protection and utilization of D. officinale germplasm resources in different regions.
2. Results
2.1. Metabolic Profiling of D. officinale in Different Regions
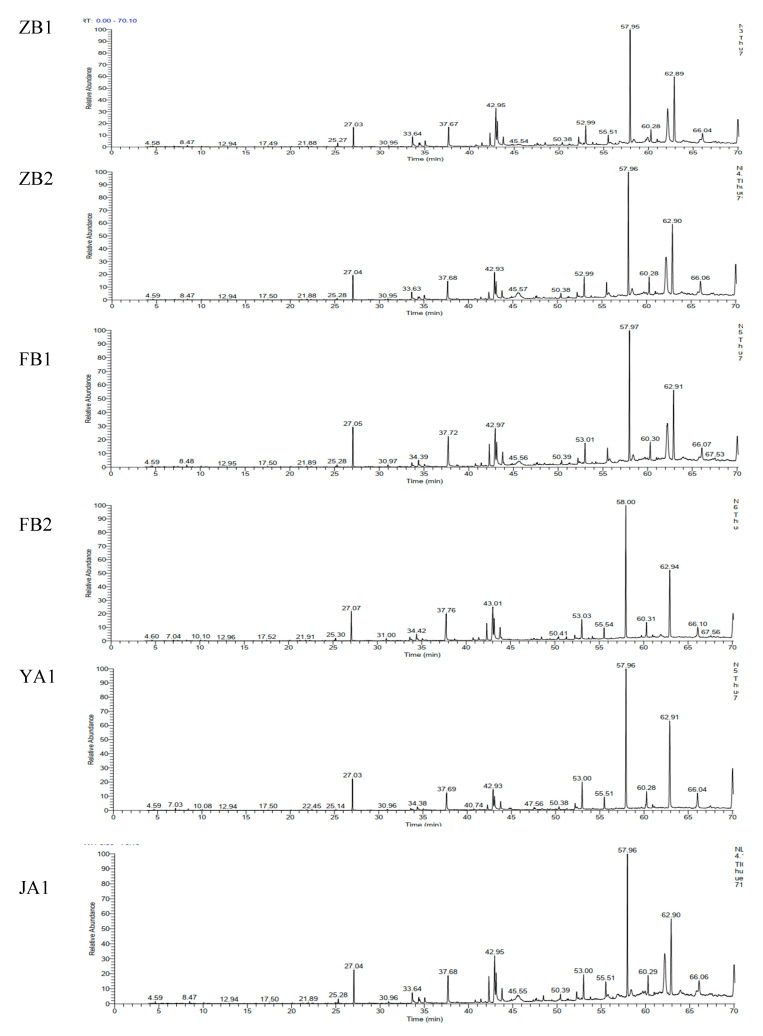
To investigate whether the chemical composition of D. officinale is associated with its origin, we analyzed six samples of D. officinale from different territory, ZB1 (purple stems, Zhejiang), ZB2 (green stems, Zhejiang), FB1 (purple stems, Fujian), FB2 (green stems, Fujian), YA1 (purple stems, Yunnan) and JA1 (purple stems, Jiangxi). We analyzed these stems using a GC-MS-based metabolomics approach, and the total ion chromatographic (TIC) diagram is shown in Figure 1. The TIC for ZB1 and ZB2 are basically the same, and FB1 was similar to FB2. There was a significant difference in the intensity of compound peaks in the TIC for the samples from different regions, especially the peaks with retention time between 45–70 min (Figure 1).
Figure 1.
The total ion chromatograms of volatile compounds determined by GC-MS in the D. officinale of ZB1, ZB2, FB1, FB2, YA1 and JA1.
Among the 101 identified metabolites from the six samples, three were identified as aldehydes, 22 were alcohols, 23 were esters, one was a terpene, four were ketones, four were phenols, nine were organic acids, six were olefins, 17 were alkanes and their derivatives and 12 were other compounds (Table 1). In ZB1 and ZB2, the most volatile compounds were alcohols, and there were 11 volatile compounds in both groups (Table 1). The varieties of other constituent categories except alcohols were also similar. In FB1 and FB2, esters were the most volatile compounds, and the kinds of other composition categories were similar.
Table 1.
Categories and relative contents of volatile components in Dendrobium officinale from different regions.
| Place of Origin | Sample Number | Relative Content % (the Numbers of Volatile Components) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde | Alcohols | Esters | Terpenes | Ketones | Phenols | Organic Acids | Olefins | Alkanes and Their Derivatives | Other | ||
| Zhejiang | ZB1 | 0.41(1a) | 14.25(11ab) | 10.00(8c) | 0.62(1a) | 0.51(3a) | 0.54(2a) | 17.28(8a) | 0.88(4a) | 52.64(10a) | 3.11(4ab) |
| ZB2 | 0.83(1a) | 16.57(11ab) | 8.53(7c) | 0.55(1a) | 0.28(1a) | 2.83(1a) | 12.32(8a) | 0.48(2ab) | 56.74(10a) | 0.86(4ab) | |
| Fujian | FB1 | 0.46(1a) | 17.92(12a) | 9.59(14a) | 0.43(1a) | 0.50(2a) | 1.89(2a) | 18.26(8a) | 0.28(1b) | 49.86(8ab) | 0.46(2b) |
| FB2 | 0.91(1a) | 5.18(6d) | 10.57(16a) | 0.46(1a) | 0.57(1a) | 0.63(3a) | 21.49(8a) | 0.55(3ab) | 58.17(7b) | 1.50(5a) | |
| Yunnan | YA1 | 0.82(1a) | 2.44(7cd) | 8.39(11b) | 0.66(1a) | 0.54(3a) | 0.39(2a) | 11.25(8a) | 0.51(1b) | 72.40(9ab) | 1.30(5a) |
| Jiangxi | JA1 | 0.60(2a) | 16.74(9bc) | 10.40(8c) | 0(0a) | 0.41(2a) | 1.64(1a) | 17.1(8a) | 1.06(3ab) | 50.32(9ab) | 0.75(2b) |
Note: Different letters indicate significant differences at p < 0.05.
Among the volatile components of YA1, esters were the highest, but in JA1, alcohols were the highest. The results showed that there was no significant difference in the composition categories of volatile components in the stems of D. officinale with different colors in the same region, but there were significant differences in the composition categories of volatile components in different regions, such as esters.
2.2. Analysis of Relative Content of Volatile Components in D. officinale
The volatile components and their relative contents in the stem of D. officinale are listed in the Supplementary Materials (Table S1). The substances with the largest proportion of volatile components were alkanes and their derivatives, which were 52.64%, 56.74%, 49.86%, 58.17%, 50.32% and 72.40% respectively in ZB1, ZB2, FB1, FB2, YA1 and JA2 (Table S1). Hexacosane shows the highest relative content of all volatile component, the highest content being 34.41% in YA1 and the lowest 23.41% in JA1 (Table S1). In addition to hexacosane, there were five kinds of volatile substances with a relative content of more than 2%, which were triacontane (highest level 27.42% in YA1, and the lowest 17.29 in FB1), tetratriacontane (highest 8.42% in YA1, and lowest 2.55% in FB2), phosphoric acid, dibutyl 1,1-dimethyl-2,2,3,3-tetrafluoro propyl ester (the highest level was 5.02% in YA1, and the lowest was 2.87% in ZB1), 9-octadecynoic acid (the highest content 7.32% in ZB1, and the lowest was 4.06% in YA1), palmitic acid (the highest in FB1 (6.09%), and the lowest in ZB1 (3.21)). Interestingly, D. officinale from different regions has 5–10 unique substances, accounting for no more than 5.5% of the total, that revealed that the categories and relative contents of volatile components from different regions vary greatly.
2.3. Medicinal Components of D. officinale from Different Regions
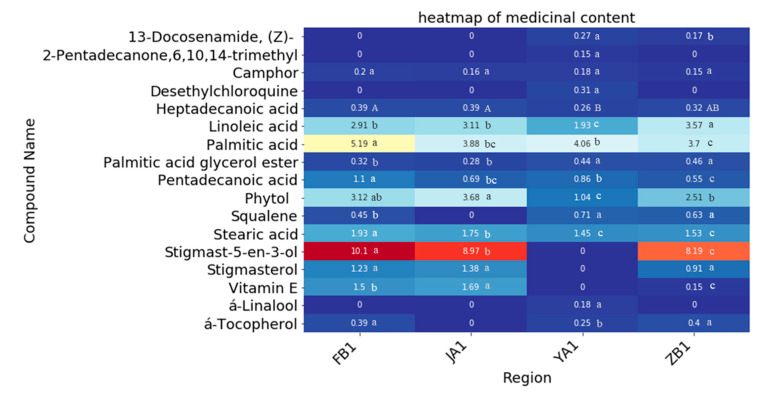
Xiang [11] found that D. officinale contains many medicinal components, such as vitamin E, phytol, hexadecanoic acid and other bioactive components. As shown in Figure 2, a total of 17 medicinal components were detected in the volatile substances, including α-linalool, camphor, pentadecanoic acid, 6,10,14-trimethyl-2-pentadecanone, palmitic acid, heptadecanoic acid, phytol, linoleic acid, stearic acid, desethylchloroquine, vitamin E, stigmasterol, stigmast-5-en-3-ol, palmitic acid glycerol ester, (Z)-13-docosenamide, squalene and α-tocopherol. The relative content of stigmast-5-en-3-ol was the highest among these 17 medicinal components. Moreover, the highest content of stigmast-5-en-3-ol in FB1 was 10.05%, which was significantly higher than that in ZB1, FB1 and JA1 (p < 0.01). Unexpectedly, stigmast-5-en-3-ol was not detected in YA1 from Yunnan. The contents of palmitic acid in YA1 were significantly higher than that in the other three regions, but the content of phytol and linoleic acid in YA1 was the lowest. The content of vitamin E in the Jiangxi sample was higher than that in ZB1 and FB1, and it was not detected in YA1. (Z)-13-Docosenamide was found in the ZB1 and YA1 samples, and α-linalool, 6,10,14-trimethyl-2-pentadecanone and desethylchloroquine were detected only in YA1.
Figure 2.
The relative levels of medicinal components differed in D. officinale in four different regions from GC-MS data (ZB1, FB1, YA1 and JA1). Capital letters A and B indicate significant differences at p < 0.01, and lowercase letters a, b, and c indicate extremely significant differences at p < 0.05.
2.4. Toxic Components of D. officinale from Different Regions
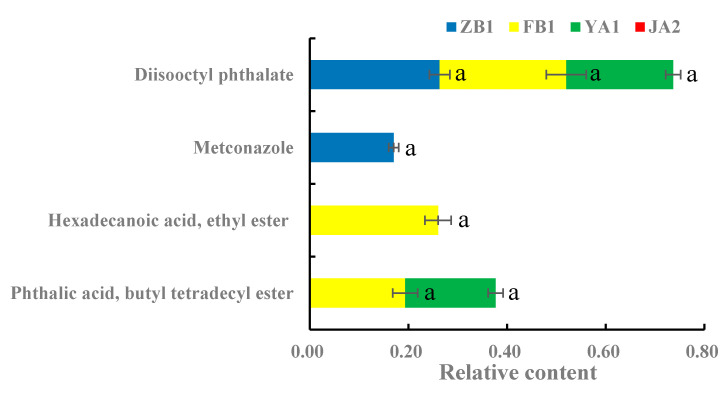
As shown in Figure 3, the categories and relative contents of toxic rational components of D. officinale in different regions were different. A total of two components in ZB1 are considered to be toxic, with a total relative content of 0.43%. The relative content of diisooctyl phthalate was the highest (0.26%) and that of metconazole was the lowest (0.17%), which was significantly higher than that of FB1, JA1 and YA1 (p < 0.05). A total of three toxic components were detected in FB1, with a total relative content of 0.71%, which was the highest among the four groups. Among them, the relative contents of diisooctyl phthalate and hexadecanoic acid ethyl ester were the highest, both 0.26%. The relative content of phthalic acid butyl tetradecyl ester with the lowest relative content was only 0.19%, which was significantly higher than that of ZB1 and JA1 (p < 0.05). A total of two components in YA1 were considered to be toxic, with a total relative content of 0.40%. The relative content of diisooctyl phthalate was the highest (0.22%) and that of phthalic acid butyl tetradecyl ester was the lowest, which was not significantly different from that of FB1, but significantly higher than that of ZB1 and JA1 (p < 0.05). Interestingly, no toxic components were detected in JA1.
Figure 3.
The relative content of toxic components in D. officinale in four different regions from GC-MS data (ZB1, FB1, YA1 and JA1). The letters a and b indicate significant difference at p < 0.05. Since the sample data is 0, b was not indicated in the figure.
3. Discussion
Previous studies have shown that the growth and chemical components of D. officinale are significantly different under different growth conditions, light, growth years and harvest ways [12,13,14]. Bai [15] reported that there were remarkable differences in morphology including stem height and diameter, amount of lateral bud, and texture of stem etc. from five regions, and the content of polysaccharides was also significantly different. However, few studies have focused on the chemical composition differences of D. officinale from different regions. In this study, the categories and contents of volatile compounds in D. officinale from different regions were different, with the samples from Zhejiang Province (ZB1 and ZB2) containing the most, and those from Fujian Province (FB1 and FB2) the least (Table 1). Cheng [16] found that short light/dark cycle can increase the biomass and polysaccharide yield of D. officinale. Different growth condition and symbiotic bacteria in the roots of D. officinale may also lead to differences in volatile components [17], so the categories and contents of volatile compounds in D. officinale from different regions may be caused by the different temperature, humidity, soil conditions or sunshine duration in the different regions.
The volatile components of plants mainly contain terpenoids, which have strong bactericidal, anti-inflammatory, analgesic and anticancer effects and are an important component of medicinal plants [18]. A total of 17 medicinal components were detected in the volatile substances from the four different regions, and the content of stigmast-5-en-3-ol was the highest except in sample YA1 from Yunnan, where it was not detected (Figure 2). Stigmast-5-en-3-ol belongs to the phytosterols. Phytosterols, triterpene compounds with a cyclopentane polyhydrophenanthrene as the main structure, are an important part of plant cell biofilm and widely exist in all kinds of plants [19]. The molecular structure of phytosterols is similar to that of cholesterol, which can inhibit the intestinal absorption of cholesterol, thereby reducing the level of cholesterol in the blood and reducing the risk of cardiovascular disease [20]. Moreover, the content of organic acids in FB1 from Fujian Province was highest (Figure 2.). Linoleic acid is an essential fatty acid for human body and has obvious anticancer effects under the joint action of other compounds. It can also reduce cholesterol and triglycerides in the blood, reduce blood viscosity, enhance the body’s defense system functions, etc. [21]. Palmitic acid and stearic acid can reduce the content of cholesterol in serum. Therefore, it has been suggested that replacing dietary lauric acid and myristic acid with palmitic acid and oleic acid may be beneficial for the treatment of thrombosis [22]. In addition, some studies have shown that pentadecanoic acid can improve hyperinsulinemia, protect islet cells and improve the inflammatory state of diabetic mice [23]. Heptadecanoic acid is an effective drug against NSCLC cells in vitro [24].
Interestingly, many unique medicinal ingredients including α-linalool, 6,10,14-trimethyl-2-pentadecanone and desethylchloroquine were detected only in sample YA1 from Yunnan Province (Figure 2.). α-Linalool is a natural flavor and fragrance that exists in many medicinal plants. It has medical and health care functions and can be used against dental caries, for deworming and an insecticide [25]. 6,10,14-Trimethyl-2-pentadecanone is an intermediate in the production of vitamin E acetate. The results provide a theoretical basis for the development of medicinal value of D. officinale in different regions and the utilization of D. officinale resources with specific efficacy.
At present, most of the D. officinale on the market is artificially cultivated, and thus may contain pesticide residues or other toxic ingredients. A total of four toxic components were detected, including phthalic acid butyl tetradecyl ester, hexadecanoic acid ethyl ester, metconazole and diisooctyl phthalate. There were three categories of toxic components in FB1 from Fujian, followed by ZB1 from Zhejiang and YA1 from Yunnan (Figure 3). Diisooctyl phthalate was detected in samples from all regions except in JA1 from Jiangxi. Diisooctyl phthalate, identified as the fourth category of toxic chemicals, is a kind of plasticizer and widely used in plastic products, building materials, food packaging, cosmetics and household materials [26]. Human intake of high doses of plasticizers not only harms the reproductive system, but also causes cardiovascular disease and may even cause cancer [27]. Metconazole is a fungicide that interferes with the synthesis of fungal cell membranes [28]. As mentioned, unexpectedly, no toxic components were detected in JA1 from Jiangxi. In addition, the categories of volatile compounds and the content of medicinal components in JA1 from Jiangxi were also high. Xie [29] showed that the origin of market-collected individuals was derived from Zhejiang and the germplasm resources from Jiangxi were well preserved, so the germplasm resources from Jiangxi should be exploited efficiently for breeding superior D. officinale individuals.
4. Material and Methods
4.1. Plant Materials
Our previous results showed that genetic diversity of D. officinale varies between different regions, most of the D. officinale in the market were derived from Zhejiang and the germplasm resources from Jiangxi were well preserved [29], so D. officinale germplasm resources from Zhejiang, Yunnan, Jiangxi and Fujian were collected as experimental materials. All the experimental materials were cultivated in the greenhouse. The seedlings were transplanted into pots and cultivated in the growth chamber with a light: dark cycle of 12 h each at 15 to 28 °C and a relative humidity of 70 to 80%. Pine bark was used as the substrate. Biennial materials were collected in February 2018.
Zhu [30] found that the stems of D. officinale can have two colors (purple and green) and different morphology. In addition, the morphological characteristics of D. officinale are correlated with the contents of polysaccharides and total alkaloids. In order to explore whether the color of the stem is related to the content of volatile substances, D. officinale samples from the same area were divided into two categories according to the color of the stem. D. officinale with purple stem from Zhejiang was named ZB1. Correspondingly, D. officinale with green stem from Zhejiang was named ZB2. Similarly, D. officinale with purple stem from Fujian was named FB1, while D. officinale with green stem from Fujian was named FB2. Since no D. officinale with green stem was found in Yunnan and Jiangxi, the two groups were named YA1 and JA1, respectively.
4.2. Sample Preparation for GC–MS Analysis
The samples with uniform thickness and no insect eye and mildew were selected, defoliated and cut off. The samples of fresh stem of D. officinale were dried in an oven at 50 °C for 15 days. The dried stems were ground to powder. One hundred g of powder from each group was placed in a round bottom flask with 500 mL of n-hexane (analytical purity) and stirred evenly. After extracting twice by the condensation reflux method, the liquid supernatant was obtained by decompression filtration. The n-hexane was removed by rotary evaporation and the paste can be used as a backup. Each extraction was repeated three times.
4.3. GC–MS Analysis
Volatile compounds of D. officinale were extracted with an n-hexane reflux method [31], then transferred into a glass bottle for GC-MS analysis (Trace1300/ISQ, Thermo Fisher Scientific, Waltham, MA, USA). Each group (ZB1, FB1, YA1, and JA1) contains three biological replicate times. On microliter of each sample was injected into the GC-MS at 250 °C in a shunt mode (20:1) with carrier gas (>99.999% helium) at a flow rate of 1 mL/min, and separated by a HP-5 MS (30 m × 0.25 mm, 0.25 μm) capillary column. The temperature was held isothermally for 3 min at 80 °C, then raised to 260 °C at a rate of 3 °C per minute, and held for 10 min. The transmission line temperature was set to 280 °C, and the ion source temperature was set to 280 °C. The mass range analyzed was from m/z 50 to 650 [32].
4.4. Data Processing and Multivariate Statistical Analysis
The obtained GC-MS data was processed by the Xcalibur 2.2 SP1 (Thermo Fisher Scientific, Waltham, MA, USA). The compounds in D. officinale stems were identified by matching the MS mass spectrogram with the mass spectrogram in the NIST library and referring to the retention time and molecular formula in the relevant literature. Then, using the Xcalibur chromatography workstation data processing system, the relative content of each compound was calculated according to the peak regions normalization method. SPSS23.0 (International Business Machines Corporation, Armonk, NY, USA) was used for one-way ANOVA, and Tukey B and the least significant difference method (LSD) were used for significance testing to analyze the significant differences (p value of <0.01) of drugs or toxic components among the four groups.
5. Conclusions
In this study, the categories and contents of volatile compounds in D. officinale from different regions are variety, and the categories from Zhejiang Province (ZB1 and ZB2) are the most, and those from Fujian Province (FB1 and FB2) are the least. There are unique substances in D. officinale from different regions, showing that we can selectively cultivate D. officinale according to the different unique substances desired A total of 17 medicinal components were detected. The highest content of stigmast-5-en-3-ol (10.05%) was observed in FB1 from Fujian, which was significantly higher than that in ZB1, FB1 and JA1 (p < 0.01), revealing that FB1 can provide high quality raw materials for applications using stigmast-5-en-3-ol. Interestingly, no toxic components were detected in JA1 from Jiangxi, suggested that the germplasm resources from Jiangxi should be exploited efficiently for breeding superior D. officinale individuals.
Supplementary Materials
The following are available online, Table S1: Categories and relative contents of volatile components in Dendrobium officinale from different regions.
Author Contributions
J.H. and W.H.: Investigation, Methodology, Formal analysis, Writing—original draft. F.Z.: Methodology, Supervision. X.L.: Resources, Methodology. Y.C.: Conceptualization, Resources, Methodology, Writing-review & editing, Supervision, Project administration. J.X.: Conceptualization, Resources, Methodology, Writing-review & editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was Supported by Major Projects of Key Research Programs in Jiangxi Province (Grant No: 20192ACB60013; 20171ACF60017), awarded to J.X.; the Major Projects of the Natural Science Foundation of China (Grant Nos. 31800640) and Young Talents of Jiangxi Normal University, awarded to Y.C.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Sample Availability: Samples of the compounds are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang K.W., Li Y.R., Tao S.C., Wei G., Huang Y.C., Chen D.F., Wu C.F. Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules. 2016;21:701. doi: 10.3390/molecules21060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S.Y., Chen F., Cheng H., Huang G.L. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020;157:385–393. doi: 10.1016/j.ijbiomac.2020.04.141. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Wang Y.Z., Lyu P., Chen L.P., Shen C.G., Sun C.B. Comparative transcriptomic analysis reveal the regulation mechanism underlying MeJA-induced accumulation of alkaloids in Dendrobium officinale. J. Plant Res. 2019;132:419–429. doi: 10.1007/s10265-019-01099-6. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Zhu T., Niu Q.Q., Yang X.X., Suo H., Zhang H. Dendrobium nobile alkaloids protects against H2O2-induced neuronal injury by suppressing JAK-STATs pathway activation in N2A cells. Biol. Pharm. Bull. 2020;43:716–724. doi: 10.1248/bpb.b19-01083. [DOI] [PubMed] [Google Scholar]
- 5.Shiau Y.J., Nalawade S.M., Hsia C.N., Mulabagal V., Tsay H.S. In vitro propagation of the Chinese medicinal plant, Dendrobium officinale Wall. Ex Lindl., from axenic nodal segments. In Vitro Cell. Dev. Biol. Plant. 2005;41:666–670. doi: 10.1079/IVP2005685. [DOI] [Google Scholar]
- 6.Fu L.K., Jin J.M. China Plant Red Data Book-Rare and Endangered Plants. Volume 1. Science Press; Beijing, China: 1992. [Google Scholar]
- 7.Zhang Y.J., Song J., Cheng X.J. The complete chloroplast genome sequence of an endangered traditional Chinese medicine plant Dendrobium officinale (Orchidaceae) Conser. Genet. Resour. 2018;10:9–11. doi: 10.1007/s12686-017-0750-0. [DOI] [Google Scholar]
- 8.Johansen B. Incompatibility in Dendrobium (Orchidaceae) Bot. J. Linnean Soc. 1990;103:165–196. doi: 10.1111/j.1095-8339.1990.tb00183.x. [DOI] [Google Scholar]
- 9.Wu Y., Si J. Discussion on the present situation and sustainable development of Dendrobium industry. Chin. J. Tradit. Chin. Med. 2010;35:2033–2037. doi: 10.4268/cjcmm20101525. [DOI] [PubMed] [Google Scholar]
- 10.Kuang M.T., Li J.Y., Yang X.B., Yang L., Xu J.Y., Yan S., Lv Y.F., Ren F.C., Hu J.M., Zhou J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohyd. Polym. 2020;241:116326. doi: 10.1016/j.carbpol.2020.116326. [DOI] [PubMed] [Google Scholar]
- 11.Xiang D., Gao X.Y., Liu L., Chen L.L., Liu Q.M., Zhang D.Q. Function-specific volatiles and volatilization characteristics of Dendrobium officinale. J. King Saud Univ. Sci. 2020;32:2020–2028. [Google Scholar]
- 12.Lin Y., Li J., Li B., He T., Chun Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. 2011;105:329–335. doi: 10.1007/s11240-010-9871-9. [DOI] [Google Scholar]
- 13.Wang Y., Zhu Y., Si J.P., Liu J.J., Zhu Y.Q., Liu X.J. Comparison of different harvest ways of Dendrobium officinale. China J. Chin. Mater. Med. 2015;40:881–884. [PubMed] [Google Scholar]
- 14.Jin Q., Jiao C.Y., Sun S.W., Song C., Cai Y.P., Lin Y., Fan H.H., Zhu Y.F. Metabolic analysis of medicinal Dendrobium officinale and Dendrobium huoshanense during different growth years. PLoS ONE. 2016;11:e0146607. doi: 10.1371/journal.pone.0146607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y., Wang W.Q., Bao Y.H., Sun Z.R., Yan Y.N. The comparative study on morphology and polysaccharides content of Dendrobium loddigesii from different habits. J. Chin. Med. Mater. 2017;30:130–132. [PubMed] [Google Scholar]
- 16.Cheng Y.S., He D.X., He J., Niu G.H., Gao R.F. Effect of light/dark cycle on photosynthetic pathway switching and CO2 absorption in two Dendrobium species. Front. Plant Sci. 2019;10:659. doi: 10.3389/fpls.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y.D., Tang X.G., Jia Z.H., Li C., Ma J.Y., Zhang J.C. The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests. 2020;11:94. doi: 10.3390/f11010094. [DOI] [Google Scholar]
- 18.Gonzalez-Burgos E., Gomez-Serranillos M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012;19:5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 19.Sonia M.C., Esther B., Barredo J.L., Marta R.S. Scale-up of phytosterols bioconversion into Androstenedione. Microb. Steroids. 2017;1654:199–210. doi: 10.1007/978-1-4939-7183-1_14. [DOI] [PubMed] [Google Scholar]
- 20.Ostlund R.E. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004;15:37–41. doi: 10.1097/00041433-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Wang P., Zhang Y., Jiang M. Research progress of polyunsaturated fatty acids. China Fats Oils. 2008;33:42–46. [Google Scholar]
- 22.Ng T.K.W., Hayes K.C., Dewitt G.F., Jegathesan M., Satgunasingam N., Ong A.S.H., Tan D. Dietary palmitic and oleic acids exert similar effects on serum cholesterol and lipoprotein profiles in normocholesterolemic men and women. J. Am. Coll. Nutr. 1992;11:383–390. doi: 10.1080/07315724.1992.10718241. [DOI] [PubMed] [Google Scholar]
- 23.Mao X., Shen X. A population study on the relationship between different dietary fatty acids and type 2 diabetes mellitus. J. Environ. Hyg. 2005;4:236–239. [Google Scholar]
- 24.Xu C.Z., Wu P.F., Gao J.J., Zhang L.L., Ma T.F., Ma B.B., Yang S., Shao G.J., Yu Y., Huang X.D., et al. Heptadecanoic acid inhibits cell proliferation in PC9 non small cell lung cancer cells with acquired gefitinib resistance. Oncol. Rep. 2019;41:3499–3507. doi: 10.3892/or.2019.7130. [DOI] [PubMed] [Google Scholar]
- 25.Chen S., Zhao L., Xu X. Natural Linalool resources and its development and utilization. For. Sci. Technol. Dev. 2013;27:13–17. [Google Scholar]
- 26.Wang M. Toxicity of phthalates (plasticizers) and harm to human health. Jiangsu Prev. Med. 2011;22:68–70. [Google Scholar]
- 27.Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., et al. NTP center for the evaluation of Risks to human reproduction: Phthalates exprt panel report on the reproductive and developmental toxicity of Di (2-ethylhexyl) Phthalate. Reprod. Toxicol. 2002;16:529–653. doi: 10.1016/S0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 28.Aignasse M.F., Prognon P., Stachowics M., Gheyouche R., Pradeau D. A new simple and rapid HPLC method for determination of DEHP in PVC packaging and releasing studies. Int. J. Pharm. 1995;113:241–246. doi: 10.1016/0378-5173(94)00204-I. [DOI] [Google Scholar]
- 29.Xie J.K., Zuo J.H., Huang Y.H., Li C.S., Chen Y.L. The origin and germplasm collection for cultivated Dendrobium officinale k. kimura & migo individuals revealed by EST-SSR markers. Genet Resour. Crop Evol. 2020;67:1209–1219. [Google Scholar]
- 30.Zhu Y., Si J., Guo B., He B., Zhang A. Variation of polysaccharide content of artificially cultivated Dendrobium officinale. Chin. J. Chin. Mater. Med. 2010;4:427–430. [PubMed] [Google Scholar]
- 31.Yang L., Liu S., Hu J., Sun Y. Determination of volatile components in stems of Dendrobium officinale by GC-MS. Chin. Mod. Tradit. Chin. Med. 2013;15:362–364. [Google Scholar]
- 32.Zhang C., Liu S., Yang L., Hu J. Determination of volatile components in flowers of Dendrobium officinale from Yunnan by GC-MS. J. Yunnan Agric. Univ. Nat. Sci. 2017;32:174–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.