Abstract
Cognitive deficits have an important role in the neurodevelopment of schizophrenia and other psychotic disorders. However, there is a continuing debate as to whether cognitive impairments in the psychosis prodrome are stable predictors of eventual psychosis or undergo a decline due to the onset of psychosis. In the present study, to determine how cognition changes as illness emerges, we examined baseline neurocognitive performance in a large sample of helping-seeking youth ranging in clinical state from low-risk for psychosis through individuals at clinical high-risk (CHR) for illness to early first-episode patients (EFEP). At baseline, the MATRICS Cognitive Consensus battery was administered to 322 individuals (205 CHRs, 28 EFEPs, and 89 help-seeking controls, HSC) that were part of the larger Early Detection, Intervention and Prevention of Psychosis Program study. CHR individuals were further divided into those who did (CHR-T; n=12, 6.8%) and did not (CHR-NT, n=163) convert to psychosis over follow-up (Mean=99.20 weeks, SD=21.54). ANCOVAs revealed that there were significant overall group differences (CHR, EFEP, HSC) in processing speed, verbal learning, and overall neurocognition, relative to healthy controls (CNTL). In addition, the CHR-NTs performed similarly to the HSC group, with mild to moderate cognitive deficits relative to the CTRL group. The CHR-Ts mirrored the EFEP group, with large deficits in processing speed, working memory, attention/vigilance, and verbal learning (>1 SD below CNTLs). Interestingly, only verbal learning impairments predicted transition to psychosis, when adjusting for age, education, symptoms, antipsychotic medication, and neurocognitive performance in the other domains. Our findings suggest that large neurocognitive deficits are present prior to illness onset and represent vulnerability markers for psychosis. The results of this study further reinforce that verbal learning should be specifically targeted for preventive intervention for psychosis.
Keywords: Schizophrenia, Early Intervention, Neuropsychology, Neurocognition, Clinical high risk, Prodrome, Early Psychosis
Impaired neurocognition has long been recognized to be a core feature of schizophrenia.(Green, 2006, Nuechterlein et al., 2004) Cognitive deficits in attention, processing speed, working memory, verbal declarative memory, and executive functioning,(Gold, 2004, Heinrichs and Zakzanis, 1998) for example, are not only readily apparent in the established illness,(Harvey et al., 2010) but also prior to the onset of the disorder.(Cannon et al., 2000) In fact, a pattern of cognitive dysfunction generally holds across a range of ages and clinical states, including very early in the pre-psychosis illness state, as extensively documented in individuals at clinical high-risk (CHR)(Brewer et al., 2006, Carrion et al., 2015, Cornblatt et al., 2015, Hawkins et al., 2004, Niendam et al., 2006, Seidman et al., 2010, Woodberry et al., 2010) for developing psychosis. Of particular interest, the early deficit pattern is typically less severe but qualitatively matches the cognitive impairment established for fully affected patients across all phases of psychosis.(Aylward et al., 1984, Reichenberg et al., 2006) Decades of research have focused on the role of cognitive deficits in the processes leading to psychosis and possible prevention via cognitive remediation because of this developmental pattern. Nevertheless, there are a number of unresolved issues limiting progress in the field. Chief among these is whether cognitive impairment acts as a stable risk factor in a largely neurodevelopmental process or follows a neurodegenerative course through the progression of the illness.(Harvey, 2009, Pino et al., 2014) A second, and related issue, is whether cognition as a whole declines after the onset of psychosis or whether deterioration is found only in specific domains. These distinctions have important implications for progress in prevention research. For example, treatment might best be directed to early and specific deficits while these are still moderate in intensity, thereby reducing the disease vulnerability,(Cornblatt et al., 2003, Pukrop et al., 2007) possibly limiting the profound disability that is associated with the illness or improving the neurocognitive functioning itself.(Green and Harvey, 2014)
Recent efforts aimed at reconciling the neurodevelopmental and neurodegenerative perspectives have increasingly focused on the extent of neurocognitive deficits prior to psychosis onset in Clinical High-Risk (CHR) adolescents and young adults(2017) (also referred to as ultra-high-risk, UHR) who display clinical features (e.g., symptoms, behaviors) that place them at heightened risk for developing psychosis. To date, numerous cross-sectional studies have reported small-to-medium impairments across various cognitive domains prior to illness onset (approximately 0.3–0.6SDs below healthy controls) in CHR individuals.(Fusar-Poli et al., 2012, Giuliano et al., 2012, Woodberry, 2010) However, as noted above, these impairments are not as large as those seen upon first-episode of psychosis,(Corigliano et al., 2014, Jahshan et al., 2010, Woodberry et al., 2013, Zhang et al., 2015) typically 1.0–1.5SDs below healthy controls.(Corigliano, 2014, Zhang, 2015)
The aforementioned pattern of impairments suggests that while deficits precede acute psychosis manifestation, the period from CHR to psychosis onset may involve a progressive decline.(Kim et al., 2011) In this case, rather than serving as vulnerability markers, neurocognition would serve as an illness (state) indicator of a worsening clinical state in the context of a neurodegenerative process. Accordingly, most functions would be deteriorating at around the same time as the illness progressed.(Knoll et al., 1998, Seidman et al., 2006)
Alternatively, and consistent with neurodevelopmental models,(Cornblatt, 2003, Lewis and Levitt, 2002, Murray et al., 1992, Walker and Bollini, 2002, Weinberger, 1987, Zubin and Spring, 1977) there is evidence from CHR individuals that neurocognitive impairments are risk factors for psychosis that reflect underlying vulnerabilities of the emerging illness(Carrion, 2015, Cornblatt, 2015, Hawkins et al., 2008, Keefe et al., 2006, Lencz et al., 2006a, Seidman, 2010) and do not decline post-onset.(Carrion, 2015) For example, a recent report from the North American Prodrome Longitudinal Study (NAPLS), a large-scale, prospective study of high-risk youth, found that CHR subjects who transition to psychosis (also referred to as CHR converters) had moderate deficits in attention and working memory and declarative memory (approximately −0.75 SDs below controls) and performed significantly worse on these dimensions than non-converters (Cohen d effect size of 0.28 and 0.48, respectively). Transition to psychosis was best predicted by baseline measures of verbal learning and declarative memory.(Seidman et al., 2016) In a recent report from our group(Carrion, 2015), CHR converters showed large domain-specific impairments at baseline in processing speed, verbal memory, sustained attention, and executive function, compared to CHR non-converters. These impairments were stable and persistent, but showed no further deterioration when retested soon after psychosis onset.(Carrion, 2015)
These findings suggest that comparisons between CHR individuals and first-episode patients on neurocognitive performance are confounded, since, as a group and over a short-term (6–30 months), only approximately 20–35% of at-risk individuals are found to have an acute episode.(Fusar-Poli et al., 2013) As a result, CHRs are expected as a group to be much less severely impaired.(Bang et al., 2015, Liu et al., 2015) The true comparison, therefore, must be with individuals who are tested when they meet CHR criteria and develop psychosis over the course of the study. To date, however, very few studies have directly compared baseline performance of CHR converters to first-episode patients using the same neurocognitive battery.
The current study aimed to examine the baseline neurocognitive performance of three clinical subgroups of adolescents and young adults seeking treatment for psychosis-related symptoms. As part of the Early Detection, Intervention and Prevention of Psychosis Program (EDIPPP(McFarlane, 2012)), baseline performance on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS)(Green and Nuechterlein, 2004, Green et al., 2004b, Kern et al., 2004, Nuechterlein et al., 2008) consensus cognitive battery was collected from help-seeking controls (HSC), CHR individuals, and early first-episode psychosis patients (EFEPs). Help-seeking controls were included as an ecologically valid clinical control group, as they were referred to the prodromal clinic for risk assessment though did not meet strict CHR criteria.(McGlashan et al., 2010) In addition, healthy comparison subjects (CNTL) were included to examine deviation from general population norms.
In the present study we aimed to: (1) Compare the three diagnostic subgroups (HSCs, CHRs, EFEPs) across six MATRICS neurocognitive domains, relative to healthy comparison (CNTL) subjects; (2) Examine differences between CHRs who transitioned to psychosis (CHR-T) to CHRs who did not (CHR-NT) and the EFEPs group; and (3) Determine whether specific neurocognitive impairments predict psychosis conversion among CHR youth. Our hypotheses were three-fold. First, we expected differences in baseline neurocognition across groups, with the largest global impairment in the EFEP group. Second, based on previous findings,(Addington et al., 2017, Cornblatt, 2015, Hauser M., 2017) we expected CHRs who transitioned to psychosis to perform worse than CHRs who did not transition to psychosis, specifically in verbal learning and processing speed. Finally, we expected baseline neurocognition to predict transition status beyond symptoms and other potential confounders, further supporting the role of neurocognition as a vulnerability marker for psychosis onset.
Material and Methods
The data reported here were collected as part of EDIPPP, a large multi-site clinical trial for reducing risk for psychosis among young people funded by the Robert Wood Johnson Foundation (2007–2011).(Lynch et al., 2016, McFarlane et al., 2015) EDIPPP consisted of six participating sites: Portland, ME; Glen Oaks, NY; Ann Arbor, MI; Salem, OR; Sacramento, CA; Albuquerque, NM. Details of the study design, study implementation, assessments, psychosocial and pharmacological treatments, methods, and sample characteristics have been reported elsewhere.(Carrion et al., 2016, McFarlane, 2015) Following standard CHR research, attenuated positive symptom levels were measured using the Scale of Prodromal Symptoms (SOPS) from the Structured Interview for Prodromal Syndromes (SIPS(Miller et al., 2003, 2002, 1999). Allocation to treatment was determined by a clinical cut-off. Scores ≥7 on total attenuated positive symptom levels defined the treatment group (N=250) and <7 defined the control condition (N=87).
In order to compare our findings with the CHR literature, in this study the CHR group was reconfigured to only include subjects meeting the standardly used Criteria of Prodromal Syndromes (COPS) as defined by the SIPS. After the reconfiguration, the original 337 subjects included 210 CHR subjects, 32 EFEPs, and 95 HSCs (please see(Carrion, 2016) for more details). Five CHRs, 4 EFEPs, and 6 HSC did not complete the MATRICS battery at baseline and were therefore removed from the current study, leaving a final sample of 205 CHRs, 28 EFEPs, and 89 HSCs.
CHR inclusion criteria were based on the presence of one or more SOPS rated attenuated positive symptoms (scale of 0–6) CHR subjects met one of the three Criteria of Prodromal Syndromes (COPS) diagnoses based on the SIPS(Miller, 2003, 2002, 1999); 1) Attenuated Positive Symptom Syndrome (APS), presence of attenuated positive symptoms, 2) Genetic Risk and Deterioration Syndrome (GRD), genetic risk for psychosis with deterioration in global functioning, and 3) Brief Intermittent Psychotic Syndrome (BIPS), intermittent, psychotic symptoms that are recent, brief in duration, and not seriously disorganizing or dangerous.
EFEPs included participants with psychotic symptoms of <30 days duration, defined according to the Presence of Psychosis Scale (POPS) criteria: developing any psychotic level intensity positive symptom (SOPS score of 6) that is sustained for at least an hour per day, at an average of 4 days per week over 1 month, or demonstrating seriously disorganized or dangerous behavior. Individuals in the HSC group had sought help at one of the EDIPPP prodromal sites, but did not meet standard COPS criteria(Miller, 2003, 2002, 1999) and had attenuated positive symptoms that were: 1) Long-standing (>1 year) at severity levels of 3–5 (n=38); 2) Did not meet severity criterion (APS<3, n=55); and 3) At severity levels of 0, (n=2). These help-seeking individuals were combined, as post-hoc comparisons revealed no significant differences between the first two subgroups on key demographic (i.e., age, gender ratio, education) and neurocognitive variables (IQ and overall neurocomposite score).
Although published MATRICS normative data are available,(Kern et al., 2008) the present report also includes a healthy comparison (CNTL) group (n=60) that is well-matched to the CHR subjects on key demographic features. CNTL subjects were recruited and enrolled at the RAP Program at Hillside through announcements in local newspapers and within the medical center.
Inclusion criteria required participants to be 12–25 years-old. Exclusion criteria were: 1) Psychotic episode (SOPS score=6) for longer than 30 consecutive days, 2) Prior episode of psychosis or having received antipsychotic medication for ≥30 days at a dosage appropriate to treat a psychotic episode, 3) IQ<70, 4) Permanent residence outside the catchment area, 5) Non-English speaking, 6) Current imprisonment in the criminal justice system, and 7) Psychotic symptoms due to an acute toxic or medical etiology.
Written informed consent (with assent from participants <18 years-old) was obtained from all participants. The research protocol was approved by the IRBs at the six participating sites.
Baseline Clinical and Neurocognitive Assessments
Details of the clinical assessment are reported elsewhere.(McFarlane, 2012) Axis I diagnoses were assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-I/CV).(First et al., 1995) Prodromal symptoms were assessed by the SIPS and the companion SOPS.(Miller, 2003, 2002, 1999)
Neurocognition was assessed with the MATRICS Consensus Cognitive Battery which includes ten neuropsychological tests that assess seven neurocognitive domains(Green et al., 2004a, Nuechterlein, 2004): 1) Speed of Processing (Trail Making Test Part A, Brief Assessment of Cognition in Schizophrenia, Symbol Coding); 2) Attention/Vigilance (Continuous Performance Test: Identical-Pairs); 3) Working Memory (Wechsler Memory Scale Spatial Span, Letter-number Span); 4) Verbal Learning (Hopkins Verbal Learning Test); 5) Visual Learning (Brief Visuospatial Memory Test; BVMT); 6) Reasoning and Problem-solving (Neuropsychological Assessment Battery: Mazes); and 7) Social Cognition (Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions; MSCEIT). Of these domains, the MSCEIT was not included because it was developed specifically for adults and is not considered appropriate for adolescents.
In a partial sample (n=262), estimated full-scale IQ scores were derived from the Vocabulary and Block Design subscales of the Wechsler Intelligence Scale for Children, 3rd Edition (WISC-III)(Wechsler, 1991) for subjects <16 years-old, and the Wechsler Adult Intelligence Scale, Revised (WAIS-R)(Wechsler, 1981) for subjects ≥16 years-old.
Clinical Outcome
Of the 205 CHR subjects, 175 (85.4%) had at least one follow-up assessment to determine CHR transition (or ‘conversion’) status (CHR-T=transition to psychosis; CHR-NT=not transition to psychosis). Of the 175, 12 (6.85%) transitioned to psychosis (defined according to POPS criteria) over the course of the study. Mean follow-up time (to transition or last follow-up) was 99.20 weeks (SD=21.54; median=106.00).
Statistical Analyses
All analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, Illinois). Prior to neurocognitive domain construction, raw test scores were log-transformed to reduce skewness and improve distribution. Test scores were then transformed into standard Z-scores using the age-stratified means and SDs of CNTLs to control for age-related changes in performance (Mean=0, SD=1). When appropriate, tests were reverse-scored so that lower scores reflected worse performance. Domain scores with multiple tests were computed by averaging each subject’s Z-scores on tests assessing the same neurocognitive domain and then re-standardized using the mean and SD of the domain scores of CNTLs. Global neurocognitive performance was calculated by averaging the neurocognitive domains and then re-standardizing using the mean and SD of the global composite of CNTLs. The number of individuals contributing to the analysis of any particular test varied slightly because of subject compliance.
Comparisons of demographic and clinical characteristics were performed with Student’s T-tests for continuous variables, Pearson Chi-Square or Fisher Exact tests for categorical variables, and Kolmogorov-Smirnov Z for one ordinal variable (two-tailed, P<0.05). Individual ANCOVAs were used to examine differences in neurocognitive performance by domain, with group as a between-subject factor, neurocognitive domain scores as dependent variables, and adjustment for age, education level, and anti-psychotic medication usage at testing (Yes/No). Post-hoc comparisons were performed with Bonferroni corrections for multiple comparisons.
The predictive associations between neurocognitive performance and transition to psychosis were examined in two steps. First, six separate Cox proportional hazard models were constructed (enter, LR method, P<0.05) with the neurocognitive domain scores of the CHR-Ts and CHR-NTs as independent variables. The −2 log-likelihood ratio test was used to test the overall significance. Second, a follow-up multivariable model was constructed to determine whether specific neurocognitive domains could predict conversion to psychosis, above and beyond the contributions of key demographic variables, symptom levels, as well as neurocognitive performance in the other domains. This model included all six neurocognitive domain scores, adjusting for age, gender, education level, Total SIPS-Positive Symptoms, and baseline anti-psychotic medication usage at testing. Bootstrapped 95% confidence intervals (B=10,000 bootstrap samples) were used to internally validate the model.(Sauerbrei and Schumacher, 1992)
Results
Demographic and Clinical Characteristics
Table 1 summarizes the baseline demographic and clinical characteristics of the four subgroups.
Table 1.
Demographic and Clinical Characteristics of Participants at Baseline
| Characteristic | Healthy Controls (CNTL) (n=60) | Help-seeking Controls (HSC) (n=89) | Clinical High-Risk (CHR) (n=205) | Early First Episode (EFEP) (n=28) | P Value | Post-hoc |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 17.16 (0.33) | 16.40 (3.13) | 16.46 (3.30) | 18.39 (3.36) | 0.014 | EFEP>HSC, CHR |
| Years of education, mean (SD) | 11.25 (2.38) | 9.84 (2.60) | 9.75 (2.63) | 11.29 (2.43) | 0.000 | CNTL>HSC, CHR; EFEP>HSC, CHR |
| Gender, No. (%) | ||||||
| Male | 31 (51.7) | 59 (66.3) | 120 (58.5) | 17 (60.7) | 0.34 | - |
| Race, No. (%) | ||||||
| White | 35 (58.3) | 27 (31.8) | 126 (64.3) | 13 (48.1) | 0.37 | - |
| Ethnic Origin | ||||||
| Hispanic, No. (%) | 12 (20.0) | 13 (14.9) | 31 (15.7) | 3 (12.0) | 0.78 | - |
| Handedness, Right, No. (%) | 79 (89.8) | 189 (92.2) | 24 (88.9) | |||
| Estimated Current IQ, mean (SD) | 103.32 (13.36) | 106.06 (17.32) | 105.15 (16.65) | 103.33 (14.35) | 0.14 | - |
| Total SOPS score, mean (SD) | ||||||
| Positive | 1.15 (1.82) | 5.53 (3.47) | 12.06 (4.22) | 19.68 (3.47) | 0.000 | * |
| Negative | 1.90 (0.29) | 11.97 (6.67) | 13.98 (5.67) | 14.46 (6.70) | 0.000 | CNTL<HSC, CHR, EFEP; HSC<CHR |
| Disorganized | 0.83 (1.29) | 3.81 (2.63) | 5.77 (3.11) | 9.36 (3.69) | 0.000 | * |
| General | 1.33 (2.00) | 8.72 (4.45) | 10.98 (4.22) | 12.07 (4.85) | 0.000 | CNTL<HSC, CHR, EFEP; HSC<CHR, EFEP |
| GAF, mean (SD) | 82.63 (12.78) | 48.68 (12.95) | 40.28 (12.47) | 24.94 (12.46) | 0.000 | * |
| DSM-IV Diagnoses, No. (%) | ||||||
| Moodb | - | 31 (34.8) | 104 (50.7) | 4 (14.3) | 0.000 | EFEP<HSC, CHR; CHR>HSC |
| Anxietyc | - | 23 (25.8) | 88 (42.9) | 6 (21.4) | 0.005 | CHR>HSC, EFEP |
| Substance Abused | - | 10 (11.2) | 15 (7.3) | 2 (7.1) | 0.521 | - |
| Test Medication, No. (%) | ||||||
| Anti-psychotics | - | 13 (14.6) | 54 (26.3) | 15 (53.6) | 0.000 | * |
| Anti-depressants | - | 13 (14.6) | 52 (25.4) | 4 (14.3) | 0.074 | - |
Note: SOPS=Scale of Prodromal Symptoms; GAF=Global Assessment of Functioning.
Socioeconomic status, Hollingshead index (Hollingshead & Redlich, 1958), where 1–3 “high” and 4 –5 “low”.
DSM-IV defined diagnosis of major depressive disorder, dysthymic disorder, mood disorder NOS, or depressive disorder NOS.
DSM-IV defined diagnosis of panic disorder, posttraumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), generalized anxiety disorder, anxiety disorder NOS, or phobias including simple phobias and social phobia.
DSM-IV defined diagnosis of alcohol, amphetamine, cannabis, cocaine, hallucinogen, nicotine, opioid, or polysubstance related substance abuse disorder.
All Groups Different From Each Other
The four subgroups were fairly well-matched, comparable on gender ratio, race, ethnicity, and handedness. The subgroups were also comparable on estimated current IQ. EFEPs were significantly older than the HSC and CHR groups. In addition, CNTLs and EFEPs had significantly more education than the HSCs and CHRs.
Attenuated positive and disorganized symptoms significantly differed across all between-group comparisons, with increased severity from CNTLs to HSCs to CHRs to EFEPs. Negative symptoms were significantly lower among CNTLs compared to the three clinical groups; and lower among HSCs compared to CHRs. General symptoms were significantly lower among CNTLs compared to HSCs, CHRs and EFEPs; and lower among HSCs compared to CHRs and EFEPs. In addition, GAF scores significantly differed across all between-group comparisons, with decreased global functioning from CNTLs to HSCs to CHRs to EFEPs.
CNTLs did not carry any DSM-IV clinical diagnoses. Rates of mood disorder diagnoses were significantly lower among EFEPs compared to HSCs and CHRs; and higher among CHRs compared to HSCs. Rates of anxiety disorders were higher among CHRs compared to HSCs and EFEPs. Rates of substance abuse were not significantly different among the clinical subgroups.
At the time of neurocognitive test administration, no CNTLs were taking psychotropic medications (anti-psychotics, anti-depressants). Anti-psychotic medication usage significantly differed across all between-group comparisons, with a general pattern of greatest usage among EFEPs, followed by CHRs and then HSCs with the lowest usage. The three clinical subgroups (HSCs, CHRs, EFEPs) were comparable in anti-depressant medication usage.
Baseline Neurocognitive Performance: Comparisons Amongst All Clinical Diagnostic Subgroups
Figure 1 displays the mean performance across the six neurocognitive domains and overall neurocomposite score for the CHR, HSC, and EFEP groups, relative to the CNTL group (CNTL represented as 0). Individual ANCOVAs revealed that there were significant group differences in processing speed, verbal learning, and overall neurocognition. Differences in working memory and attention/vigilance were at trend levels (P<0.10). Moreover, visual learning, as well as reasoning and problem solving, were relatively intact and comparable across all subgroups.
Figure 1.
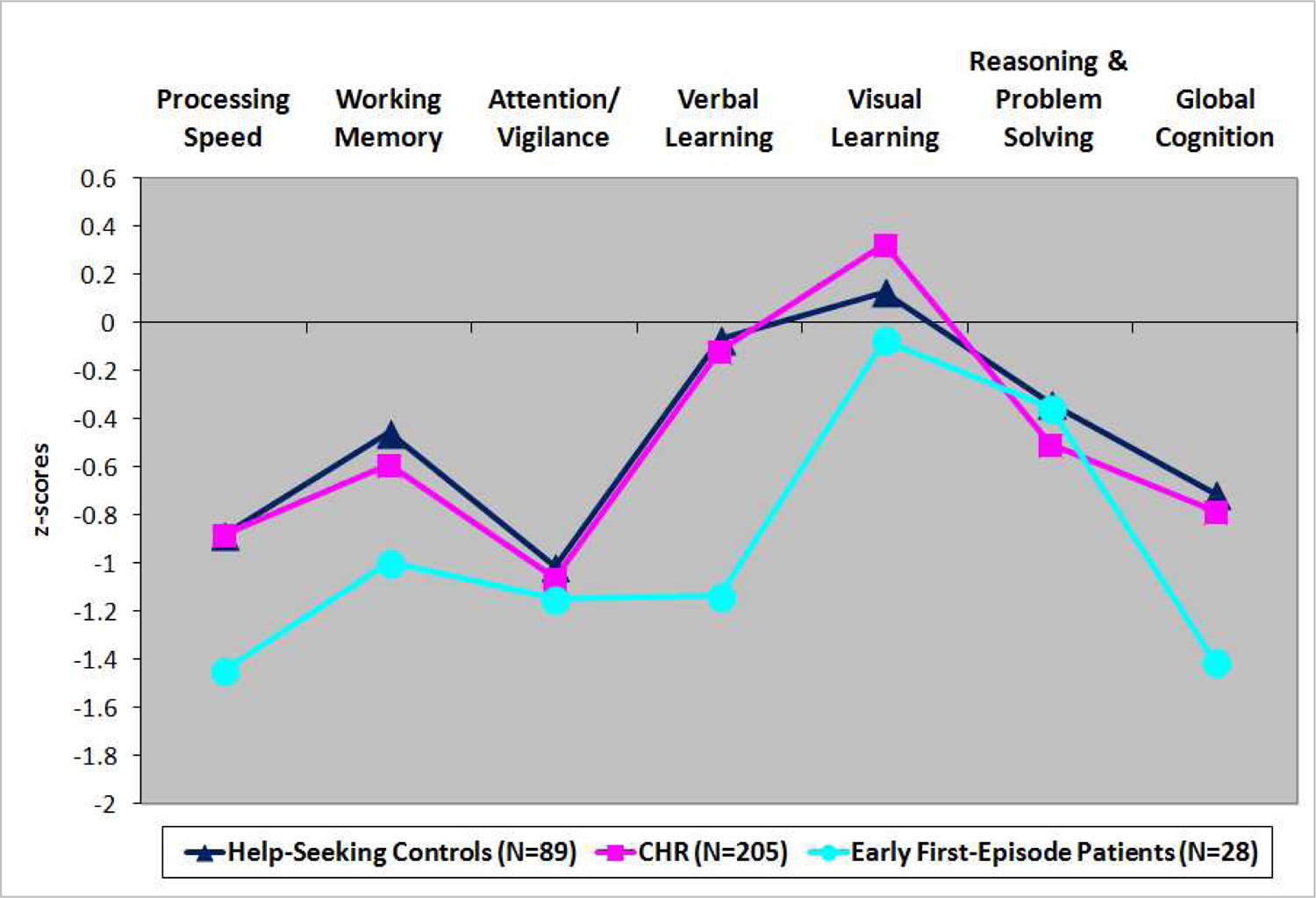
Baseline Neurocognitive Performance Among Help-Seeking Controls (HSC), Clinical High-Risk (CHR), and Early First-Episode Patients (EFEP), relative to Healthy Comparison subjects
As shown in Figure 1, when evaluated as a group, CHR subjects showed moderate impairments in processing speed (d=0.63) and attention/vigilance (d=0.69) relative to CNTLs, although these results did not reach statistical significance. In contrast, EFEPs had large impairments on most domains, with significant differences relative to the CNTL group on processing speed (Cohen’s d=1.09), verbal learning (d=0.72), and overall neurocomposite score (d=0.95) (see Table S1).
Moreover, the overall CHR group closely resembled the HSC group, with the HSC and CHR groups both showing moderate effect size impairments on processing speed (HSC, d=.63; CHR, d=0.63) and attention/vigilance (HSC, d=0.66; CHR, d=0.69; see Table S3) All subgroups had comparable performance on visual learning and reasoning and problem solving (see Table S1 for means, SDs, overall group and post-hoc subgroup comparisons).
Baseline Neurocognitive Performance: CHR-T and EFEP Subgroups
As shown in Figure 2, cognitive results are quite different when the CHR group was divided into individuals who did (CHR-T) and did not transition (CHR-NT) to psychosis over the course of the study. After teasing out the true positive CHR subjects from the overall CHR group, it became clear that the CHR-Ts resemble the EFEPs prior to psychosis onset, with large impairments for both groups (over 1SD) in the domains of processing speed, working memory, attention/vigilance, and verbal learning (see Table S2).
Figure 2.

Baseline Neurocognitive Performance Among CHR Converters (CHR-T), CHR Non-Converters (CHR-NT), and Early First-Episode Patients (EFEP), relative to Healthy Comparison subjects
Individuals who transitioned to psychosis (CHR-T) showed significant differences on processing speed (d=1.10), verbal learning (d=1.12), and overall neurocognitive performance (d=1.21) relative to CNTLs. (see Table S2) Compared to the CHR-NT group, the CHR-T group specifically showed significantly greater impairments on verbal learning (d=.86, p<.008, see Table S3 for more details).
Of interest, neurocognitive performance of CHR individuals who did not transition (CHR-NT) continued to resemble the HSC group (see Figure 2), with relatively small effect size differences (Cohen’s d ranging from 0.00–0.16 across all domains, see Table S3).
Prediction of Transition to Psychosis
Of the six neurocognitive domains, working memory (HR=0.802, 95% CI=0.646–0.994; Wald χ2=1.736, df=1, P=0.044) and verbal learning (HR=0.747, 95% CI=0.615–0.908; Wald χ2=747, df=1, P=0.003) significantly predicted transition to psychosis (see Table S4). As shown in Table 2, only baseline verbal learning remained a significant predictor (P=.007) after adjusting for the contributions of key demographic variables (age, educational levels, gender ratio), SOPS positive symptom total and performance in the other five neurocognitive domains.
Table 2.
Cox Proportional Hazard Models Predicting Transition to Psychosis (CHR-T vs. CHR-NT)
| Predictor Variable | B | SE | Wald | Hazard Ratio | Bootstrapped 95% CIa | P Value |
|---|---|---|---|---|---|---|
| Total SIPS Positive Symptoms | 0.219 | 0.079 | 7.583 | 1.244 | 0.115–1.442 | 0.006 |
| Age | −0.391 | 0.291 | 1.814 | 0.676 | −2.123–0.197 | 0.178 |
| Gender | 0.030 | 0.816 | 0.001 | 1.030 | −2.084–6.104 | 0.971 |
| Education level | 0.759 | 0.360 | 4.449 | 2.137 | −0.002–3.853 | 0.035 |
| Anti-psychotics at testing | −0.351 | 0.920 | 0.146 | 0.704 | −2.292–4.477 | 0.703 |
| Processing Speed | 0.254 | 0.296 | 0.739 | 1.289 | −0.592–2.451 | 0.390 |
| Working Memory | 0.117 | 0.217 | 0.291 | 1.125 | −1.11–1.124 | 0.589 |
| Attention/Vigilance | 0.102 | 0.333 | 0.093 | 1.107 | −0.95–2.157 | 0.760 |
| Verbal Learning | −0.533 | 0.198 | 7.275 | 0.587 | −3.098–(−0.033) | 0.007 |
| Visual Learning | −0.213 | 0.294 | 0.522 | 0.808 | −2.542–0.442 | 0.470 |
| Reasoning and Problem Solving | −0.122 | 0.200 | 0.375 | 0.885 | −0.568–3.821 | 0.540 |
Note: Mean Total SIPS Positive Symptoms (SD) of CHR-T=15.17 (3.24) vs. CHR-NT=11.92 (4.06), p=.008; Mean Age (SD) of CHR-T=18.33 (3.09) vs. CHR-NT=16.43 (3.32), p=.056; Gender Ratio (% Male) of CHR-T=66.7% vs. CHR-NT=57.9%, p=.73; Mean Education level (SD) of CHR-T=11.5 (2.36) vs. CHR-NT=9.68 (2.61), p=.02; Anti-psychotics at testing (%) of CHR-T=33.3% vs. CHR-NT=27.4%, p=.74.
B=10,000 bootstrap samples
Discussion
In the current study, we compared baseline neurocognitive performance of three diagnostic groups of help-seeking youth that included individuals who did not meet CHR criteria (HSC), individuals at CHR for psychosis, and patients in their first-episode of psychosis (EFEP). We found that very early first-episode psychosis patients displayed the largest neurocognitive impairments. These baseline impairments were significantly larger than those among CHRs, which preliminarily suggested there may be a decline from the CHR state to post-psychosis onset. However, when considering CHR transition status, the baseline cognitive impairments among true-positive CHRs (i.e., CHR converters) and EFEPs were comparable. CHR non-converters matched help-seeking controls in performance, with both groups showing moderate-to-no baseline impairments relative to healthy comparison subjects. Moreover, baseline impairments in verbal learning predicted transition to psychosis. Taken together, our findings reinforce the notion that specific cognitive impairments (i.e., verbal learning) during the prodrome represent trait risk markers and may be more effective for predicting future psychosis than a global cognitive deficit.
Consistent with previous studies(Addington and Addington, 2002, Addington et al., 2003, 2014), first-episode patients showed moderate-to-large impairments of around 1.0 SDs below CNTLs in specific areas of neurocognition; namely processing speed, working memory, sustained attention, and verbal learning. These impairments were generally larger than those in the overall CHR group, especially in verbal learning, where the overall CHR group showed performance similar to CNTLs (d=.01). Past studies typically found that CHRs are less cognitively impaired than already affected EFEPs, suggesting a decline in neurocognitive functioning following psychosis onset. However, to the best of our knowledge, only a small number of studies(Jahshan, 2010) have directly compared baseline performance of CHR converters to first-episode patients using the same cognitive test battery. In the present study, CHRs who transitioned to psychosis over follow-up (i.e., converters) showed impairments closely mirroring those observed among early first-episode patients. Impairments in verbal learning and memory have consistently been shown to make an independent contribution to psychosis prediction among CHRs.(Cornblatt, 2015, Lencz et al., 2006b, Metzler et al., 2016, Pukrop, 2007) Our data supports this view, as we found that verbal learning contributed unique variance to the prediction of psychosis conversion above and beyond the contribution of several potential confounders and the neurocognitive performance across other MATRICS domains.
CHR non-converters demonstrated similar performance to the help-seeking control group, with overall neurocognitive performance at around −.4 SDs below healthy controls. This suggests neurocognitive impairments may not be specific to the CHR state; rather, they are most likely directly related to help-seeking behavior that includes receiving treatment for social and role functioning difficulties.(Carrion et al., 2011, 2013, Cotter et al., 2014, Olvet et al., 2015) Future CHR longitudinal studies should consider including help-seeking individuals who do not meet CHR criteria that may serve as psychiatric controls, in order to fully characterize deficits unique to both the overall CHR state and to truly prodromal individuals.
The current report had limitations. First, despite the longitudinal clinical design, the cognitive comparisons are essentially cross–sectional in nature. Future studies need focus on within-individual changes across time, especially within specific neurocognitive domains (as in the current report), and with declining clinical state. Second, heterogeneity (subthreshold positive and negative/functional symptoms) of the help-seeking control groups renders it difficult to determine the relationship between clinical symptoms and neurocognitive impairments. Third, the small sample size of the CHR converters may have reduced our ability to detect subtle group differences by virtue of limited statistical power. Despite the small sample size, however, verbal learning impairments among CHR converters dramatically predicted transition to psychosis. In addition, in order to ensure that the CHR subjects in the present study were representative of those included in standard CHR studies, only participants that met criteria for met one of the three COPS diagnoses based on the SIPS were included.
The present findings further support the notion that impairments in specific domains, rather than a general neurocognitive impairment, may provide insight into the process underlying psychosis.(Chapman and Chapman, 1978) Moreover, our findings have major implications for neurocognitive remediation and training programs. Such programs have effectively targeted overall neurocognition among adult patients.(McGurk et al., 2007, Wykes et al., 2011) In contrast, prodromal training programs for youth have not as yet been as successful.(Lewandowski, 2016) Our results suggest neurocognitive training among prodromal youth may benefit from targeting specific domains, which has recently been implemented in adults with schizophrenia.(Kurtz et al., 2017) This approach may also shorten the intervention time and, in turn, improve feasibility among younger participants. In addition, our findings suggest that neurocognitive interventions in at risk youth should be initiated as early as possible as neurocognitive impairments in ‘true-positive’ participants may be long-standing as opposed to more recently emerging attenuated positive symptoms.
In conclusion, our findings reinforce the utility of neurocognitive indices as markers of psychosis susceptibility, with a specific emphasis on verbal learning. This supports the inclusion of this deficit in the NAPLS2 psychosis risk calculator as reported by Cannon et al.(2016) and replicated by Carrión et al.(2016) In line with a neurodevelopmental perspective,(Cornblatt, 2003, Weinberger, 1987) our findings highlight the important underlying role of cognition prior to the onset of psychosis as vulnerability markers, which may ultimately serve as a prime target for preventive intervention. This may have implications for the future conceptualization of the Attenuated Psychosis Syndrome (APS) diagnosis currently included in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) in Section III under “conditions for further study”. In particular, criteria defining the at-risk syndrome should consider the specific neurocognitive impairments that have consistently predicted impending psychosis.
Supplementary Material
Acknowledgments
Funding: Supported by grants MH61523 and MH081857 from the National Institute of Mental Health (Cornblatt), 5R01MH059883-11 (Carter), R21MH101676 (Taylor), K23MH087708 (Niendam), R01MH105411 (Ragland), and the Zucker Hillside Hospital NIMH Advanced Center for Intervention and Services Research for the Study of Schizophrenia MH074543 (John M Kane, M.D.). Robert Wood Johnson Foundation (#67525) with additional institutional support from the Maine Medical Center Research Institute and the State of Maine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
Drs. Carrión, Walder, Auther, Taylor, Niendam, Adelsheim, Ragland, and Calkins, and Ms. McLaughlin and Zyla report no financial relationships with commercial interests relevant to this work. Dr. Cornblatt was the original developer of the CPT-IP. Dr. McFarlane provides training on request to public and not-for-profit clinical services implementing psychosis early intervention programs.
REFERENCES
- Addington J, Addington D. Cognitive functioning in first-episode schizophrenia. Journal of psychiatry & neuroscience 2002;27:188–92. [PMC free article] [PubMed] [Google Scholar]
- Addington J, Brooks BL, Addington D. Cognitive functioning in first episode psychosis: initial presentation. Schizophr Res 2003;62:59–64. [DOI] [PubMed] [Google Scholar]
- Addington J, Liu L, Perkins DO, Carrion RE, Keefe RS, Woods SW. The Role of Cognition and Social Functioning as Predictors in the Transition to Psychosis for Youth With Attenuated Psychotic Symptoms. Schizophr Bull 2017;43:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984;10:430–59. [DOI] [PubMed] [Google Scholar]
- Bang M, Kim KR, Song YY, Baek S, Lee E, An SK. Neurocognitive impairments in individuals at ultra-high risk for psychosis: Who will really convert? Aust N Z J Psychiatry 2015;49:462–70. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull 2006;32:538–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull 2000;26:379–93. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, et al. An Individualized Risk Calculator for Research in Prodromal Psychosis. Am J Psychiatry 2016;173:980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, Cornblatt BA, Burton CZ, Tso IF, Auther AM, Adelsheim S, et al. Personalized Prediction of Psychosis: External Validation of the NAPLS-2 Psychosis Risk Calculator With the EDIPPP Project. Am J Psychiatry 2016;173:989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry 2011;168:806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, McLaughlin D, Auther AM, Olsen R, Correll CU, Cornblatt BA. The impact of psychosis on the course of cognition: a prospective, nested case-control study in individuals at clinical high-risk for psychosis. Psychol Med 2015;45:3341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry 2013;70:1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res 1978;14:303–11. [DOI] [PubMed] [Google Scholar]
- Corigliano V, De Carolis A, Trovini G, Dehning J, Di Pietro S, Curto M, et al. Neurocognition in schizophrenia: from prodrome to multi-episode illness. Psychiatry Res 2014;220:129–34. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Carrion RE, Auther A, McLaughlin D, Olsen RH, John M, et al. Psychosis Prevention: A Modified Clinical High Risk Perspective From the Recognition and Prevention (RAP) Program. Am J Psychiatry 2015;172:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull 2003;29:633–51. [DOI] [PubMed] [Google Scholar]
- Cotter J, Drake RJ, Bucci S, Firth J, Edge D, Yung AR. What drives poor functioning in the at-risk mental state? A systematic review. Schizophr Res 2014;159:267–77. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute. Biometrics Research 1995.
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry 2013;70:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry 2012;69:562–71. [DOI] [PubMed] [Google Scholar]
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des 2012;18:399–415. [DOI] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res 2004;72:21–8. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 2006;67:e12. [PubMed] [Google Scholar]
- Green MF, Harvey PD. Cognition in schizophrenia: Past, present, and future. Schizophrenia Research: Cognition 2014;1:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 2004a;72:41–51. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res 2004;72:1–3. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 2004b;56:301–7. [DOI] [PubMed] [Google Scholar]
- Harvey PD. When does cognitive decline occur in the period prior to the first episode of schizophrenia? Psychiatry (Edgmont) 2009;6:12–4. [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Reichenberg A, Bowie CR, Patterson TL, Heaton RK. The course of neuropsychological performance and functional capacity in older patients with schizophrenia: influences of previous history of long-term institutional stay. Biol Psychiatry 2010;67:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M, Zhang JP, Sheridan EM, Burdick KE, Mogil R, Kane JM, et al. Neuropsychological Test Performance to Enhance Identification of Subjects at Clinical High Risk for Psychosis and Be Most Promising for Predictive Algorithms for Conversion to Psychosis: A Meta-Analysis. J Clin Psychiatry 2017. [DOI] [PubMed]
- Hawkins KA, Addington J, Keefe RS, Christensen B, Perkins DO, Zipurksy R, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res 2004;67:115–22. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Keefe RSE, Christensen BK, Addington J, Woods SW, Callahan J, et al. Neuropsychological course in the prodrome and first episode of psychosis: Findings from the PRIME North America Double Blind Treatment Study. Schizophr Res 2008;105:1–9. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998;12:426–45. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology 2010;24:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman Ja. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res 2006;88:26–35. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Nuechterlein KH, Deng BH. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res 2004;72:11–9. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry 2008;165:214–20. [DOI] [PubMed] [Google Scholar]
- Kim KR, Park JY, Song DH, Koo HK, An SK. Neurocognitive performance in subjects at ultrahigh risk for schizophrenia: a comparison with first-episode schizophrenia. Compr Psychiatry 2011;52:33–40. [DOI] [PubMed] [Google Scholar]
- Knoll JLt, Garver DL, Ramberg JE, Kingsbury SJ, Croissant D, McDermott B. Heterogeneity of the psychoses: is there a neurodegenerative psychosis? Schizophr Bull 1998;24:365–79. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Trask CL, Rosengard R, Hyman S, Kremen L, Mehta S, et al. Verbal Learning and Memory Enhancement Strategies in Schizophrenia: A Randomized, Controlled Investigation. J Int Neuropsychol Soc 2017:1–6. [DOI] [PubMed]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry 2006a;59:863–71. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological Psychiatry 2006b;59:863–71. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE. Cognitive Remediation for the Treatment of Cognitive Dysfunction in the Early Course of Psychosis. Harv Rev Psychiatry 2016;24:164–72. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia As A Disorder of Neurodevelopment. Annual review of neuroscience 2002;25:409–32. [DOI] [PubMed] [Google Scholar]
- Liu CC, Hua MS, Hwang TJ, Chiu CY, Liu CM, Hsieh MH, et al. Neurocognitive functioning of subjects with putative pre-psychotic states and early psychosis. Schizophr Res 2015;164:40–6. [DOI] [PubMed] [Google Scholar]
- Lynch S, McFarlane WR, Joly B, Adelsheim S, Auther AM, Cornblatt B, et al. Early Detection for the Prevention of Psychosis Program: Community Outreach and Early Identification at Six Sites across the United States. Psychiatric Services. Psychiatric Services 2016. [DOI] [PubMed]
- McFarlane WR, Cook WL, Downing D, Ruff A, Lynch S, Adelsheim S, Calkins R, Carter CS, Cornblatt BA, Milner K. Early Detection, Intervention, and Prevention of Psychosis Program: Rationale, Design, and Sample Description. Adolescent Psychiatry 2012;2:112–24. [Google Scholar]
- McFarlane WR, Levin B, Travis L, Lucas FL, Lynch S, Verdi M, et al. Clinical and functional outcomes after 2 years in the early detection and intervention for the prevention of psychosis multisite effectiveness trial. Schizophr Bull 2015;41:30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Walsh B, Woods S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up: Oxford University Press, USA; 2010. [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry 2007;164:1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler S, Dvorsky D, Wyss C, Nordt C, Walitza S, Heekeren K, et al. Neurocognition in help-seeking individuals at risk for psychosis: Prediction of outcome after 24 months. Psychiatry Res 2016;246:188–94. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003;29:703–15. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 2002;159:863–5. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q 1999;70:273–87. [DOI] [PubMed] [Google Scholar]
- Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull 1992;18:319–32. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O’Brien M, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res 2006;84:100–11. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res 2004;72:29–39. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008;165:203–13. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Carrion RE, Auther AM, Cornblatt BA. Self-awareness of functional impairment in individuals at clinical high-risk for psychosis. Early Interv Psychiatry 2015;9:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino O, Guilera G, Gomez-Benito J, Najas-Garcia A, Rufian S, Rojo E. Neurodevelopment or neurodegeneration: review of theories of schizophrenia. Actas Esp Psiquiatr 2014;42:185–95. [PubMed] [Google Scholar]
- Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkötter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res 2007;92:116–25. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Caspi A, Knobler HY, Lubin G, Harvey PD, et al. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol 2006;28:193–207. [DOI] [PubMed] [Google Scholar]
- Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Statistics in Medicine 1992;11:2093–109. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology 2006;28:225–42. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry 2010;67:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, et al. Association of Neurocognition With Transition to Psychosis: Baseline Functioning in the Second Phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry 2016;73:1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Bollini AM. Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophr Res 2002;54:17–23. [DOI] [PubMed] [Google Scholar]
- Wechsler D Wechsler Adult Intelligence Scale, Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987;44:660–9. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, McFarlane WR, Giuliano AJ, Verdi MB, Cook WL, Faraone SV, et al. Change in neuropsychological functioning over one year in youth at clinical high risk for psychosis. Schizophr Res 2013;146:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res 2010;123:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry 2011;168:472–85. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li H, Stone WS, Woodberry KA, Seidman LJ, Tang Y, et al. Neuropsychological Impairment in Prodromal, First-Episode, and Chronic Psychosis: Assessing RBANS Performance. PLoS One 2015;10:e0125784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability--a new view of schizophrenia. J Abnorm Psychol 1977;86:103–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


