Abstract
Background
Exposure to environmental stressors can lead to shorter leukocyte telomere length and increase the risk of chronic diseases. Preservation of leukocyte telomere length by reducing oxidative stress exposure and reinforcing immunity may be a mechanism by which nutritional factors delay or prevent chronic disease development.
Methods
Healthy pregnant women (aged 18–45 years) at 9–15 weeks of gestation living in Gasabo District, Kigali, Rwanda, were recruited from 10 health centers for a prospective, longitudinal study from September to October 2017 to determine possible associations between nutrition health, infectious disease and leukocyte telomere length. Anthropometric and laboratory measurements were performed using standard procedures; sociodemographic parameters and health histories were assessed via surveys, and leukocyte telomere length was assessed using quantitative PCR expressed as the ratio of a telomeric product to a single-copy gene product (T/S).
Results
Mean gestational age of participants (n = 297) at enrollment was 13.04 ± 3.50 weeks, age was 28.16 ± 6.10 years and leukocyte telomere length was 1.16 ± 0.22 (T/S). Younger age; no schooling vs. primary schooling; and lower levels of ferritin, soluble transferrin receptors and retinol-binding protein were independent predictors of longer telomere length in multivariable models.
Conclusions
Leukocyte telomere length is an indicator of biological aging in pregnant Rwandan women. Maternal micronutrient status, specifically lower ferritin, soluble transferrin receptor levels, and retinol-binding protein levels were associated with longer maternal telomere length in contrast with some studies from North America and Europe. There were no associations between inflammation and infectious disease status and maternal leukocyte telomere length. Further studies are needed to enhance our understanding of the interplay between maternal nutritional status and infectious disease in relation to leukocyte telomere length in developing countries.
Keywords: Nutrition, Infection, Pregnancy, Oxidative stress, Leukocyte Telomere length
Background
Leukocyte telomeres and maternal health
Telomeres are protective nucleoprotein structures that appear at the ends of chromosomes and confer protection against chromosomal damage [1]. They undergo attrition with each cell division due to limitations in the ability of the DNA replication machinery to replicate chromosome ends. Changes in telomeres serve as a biological marker for cellular aging, [2] with critical shortening resulting in cellular senescence [3]. Telomeres shorten throughout the normal aging process, and shorter telomere length has been associated with multiple chronic diseases, including cancer, type II diabetes, and cardiovascular disease [4]. Further, they are particularly sensitive to reactive oxygen species (ROS) damage; exposure to chronic or acute and social and environmental stressors can often lead to increased ROS exposure [3, 5].
Antioxidants, which maintain homeostasis via affecting redox status and/or redox-sensitive signaling pathways and gene expression, may help reduce ROS damage [5]. They play a specific role in boosting immune response and restricting pathological aspects of the cytokine-mediated response [6, 7]. Shorter leukocyte telomere length is reportedly associated with micronutrient deficiencies and inadequate diet attributed to the insufficient intake of iron, vitamin B12 and D, and other vitamins and minerals [8, 9]. During pregnancy, maternal nutritional health is pivotal as deficiencies of certain vitamins or micronutrients and poor dietary intake may affect fetal telomere length, consequently having an adverse effect on the health of the newborn child [9, 10].
Nutrition and infection in pregnancy
Malnutrition in pregnant, lactating women and in women of childbearing age is a common problem in sub-Saharan Africa (SSA) and is attributable to limited weight gain during pregnancy and micronutrient deficiencies [11, 12]. In Rwanda, anemia and low hemoglobin levels are present in 19% women of childbearing age, of which 7% are underweight [13, 14]. In a recent study, during early pregnancy, anemia was found to be present in 33% participants; in addition, participants showed deficiencies in iron levels as measured by ferritin and soluble transferrin receptors (sTfRs) (19.1%) [15]. In the same study, 27.3% and 7.9% participants showed high levels of inflammatory markers; C reactive protein (CRP) and alpha A glycoprotein (AGP) suggestive of infection or other inflammatory processes [15].
Systemic, sexually transmitted or genitourinary infections are common in SSA populations [16, 17]. In Rwanda, hepatitis B virus, co-infection with hepatitis B virus/human immunodeficiency virus (HIV), and syphilis affects 3.5%, 4.1%, and 2% pregnant women, respectively [18]. Other infectious diseases such as malaria affect 5.7% of pregnant women [19]. Overall malaria burden is high with 403 cases per 1,000 in 2016 and annual incidence increasing since 2012 [20]. Maternal systemic inflammation during pregnancy has been associated with diverse adverse outcomes including fetal growth restriction, and preterm birth [1, 21, 22], but little is known about its association with maternal leukocyte telomere length, particularly in SSA, where malnutrition and infection during pregnancy are more common than North American or European contexts.
To the best of our knowledge, no studies have investigated the relationship between maternal nutritional status, infection, and maternal leukocyte telomere length in SSA. In this study, we evaluated the association between nutritional and infectious disease risk factors and leukocyte telomere length during early pregnancy in women from Gasabo District, Rwanda.
Methods
Participants and inclusion criteria
We assessed leukocyte telomere length in healthy pregnant women aged 18–45 years as part of a prospective, cohort study of pregnant women from 10 health centers in Gasabo District, Kigali Province [15]. Participants were enrolled from September to October 2017 as previously described [15] and were in early pregnancy (gestational age 9–15 weeks, as confirmed by ultrasound and self-reported last menstrual period). Inclusion criteria included testing negative for HIV and syphilis, singleton pregnancy, speaking Kinrywanda or English and providing written consent for participation. Out of 420 participants, 300 were included in the telomere substudy based on convenience sampling. Two women were excluded due to unclear labeling of the samples and 1 did not complete the study due to loss to follow-up. Leukocyte length was assessed in 297 participants. The participating health centers were categorised into rural or urban and each contributed 50% of the participants.
Recruitment procedure and ethical approval
Pregnant women were approached by community health workers in charge of maternal and child healthcare in the catchment area under their responsibilities as previously described in detail [15]. Eligible candidates reported to the nearest health center for preliminary screening, and trained enumerators obtained verbal and written informed consent from participants prior to their inclusion in the study.
The Institutional Review Board (IRB) of the University of Rwanda and Committee on Human Research (CHR) at the University of California, San Francisco, approved the study protocol.
Data collection
After participant enrollment, trained phlebotomists collected whole blood samples, and six trained interviewers conducted verbal interviews to collect data on sociodemographic parameters and performed anthropometric measurements. Four on-site laboratory technicians separated blood for micronutrient analyses and processed laboratory specimens.
Infection and nutritional assessment
Serum and whole blood samples were frozen at − 80 °C at the University Teaching Hospital of Kigali before shipping them to VitMin Lab (NutriSurvey) in Germany for analyses of micronutrients and inflammatory markers. Using the combined sandwich enzyme-linked immunosorbent assay technique [23], samples were analyzed for sTfRs, RBP, ferritin, AGP, and CRP. For data interpretation, we used cutoff point values recommended by the World Health Organization (WHO): anemia (hemoglobin < 11 g/dL) [24], low serum ferritin < 12 µg/L, and RBP < 0.83 µmol/L. Further, serum concentrations of > 5 mg/L CRP and > 1 AGP served as acute and chronic markers of inflammation, respectively [25].
Leukocyte telomere length analysis
Whole blood samples were shipped to the Elizabeth Blackburn Laboratory at the University of California, San Francisco, for leukocyte telomere length analysis. DNA was extracted using the QIAamp® DNA Investigator Kit (cat. no. 56,504; QIAGEN), and leukocyte telomere length was determined using quantitative PCR. The assay to measure telomere length was an adaptation of the original method published by Cawthon [26], as presented by Lin et al. [27]. Telomere length is expressed as T/S, representing the ratio of a telomeric product to a single-copy gene product [27]. The average coefficient of variation for these samples were 2.1%.
Screening for genitourinary infections
Vaginal swabs were collected from all participants and examined for genitourinary infections. Cultures were grown and biochemical identification was performed, according to standard procedures [28]. As previously described [15], Trichomonas vaginalis presence was determined using wet mount microscopy [29], while Candida albicans presence was determined by cultures, followed by the germ tube test [30]. Chlamydia trachomatis was detected using CORTEZ One-Step Chlamydia Rapicard™ [31]. The culture method used for detecting Neisseria gonorrhea was not successful.
Urinary tract infection was diagnosed by growing cultures using standard procedures [32], and the final identification process involved using the catalase test and analytical profile index (API 20E) [33].
Demographic, dietary, and anthropometric data
Questionnaires were administered to collect demographic data, including maternal age, education level, residence, socioeconomic status, partnership/marital status, and reproductive history. The questionnaire covered food frequency items necessary to evaluate the Minimum Dietary Diversity for Women [34, 35].
A standardized digital scale and portable stadiometer were used to measure the weight and height, respectively, of participants, and body mass index (BMI) was then calculated. Following the WHO and Centers for Disease Control guidelines [36], BMI was used to categorize women as follows: <18.5 kg/m2 = underweight; 18.5 to < 25 kg/m2 = normal weight; 25.0 to < 30 kg/m2 = overweight; and ≥ 30.0 kg/m2 = obese. The mid-upper arm circumference (MUAC) was recorded, which served as an indirect indicator of nutritional status. MUAC < 23 cm indicated nutritional deficiency, while MUAC > = 23 cm indicated nutritional sufficiency [37].
Statistical analysis
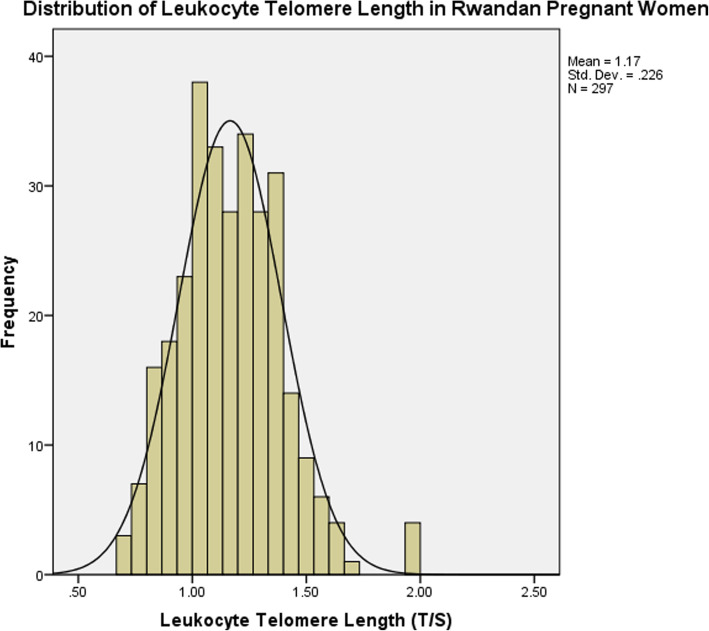
Data were checked for consistency using Excel 16.5 and then coded and exported to SPSS v20 for analyses. Normality of continuous variables was graphically assessed using Quantile-Quantile (QQ), Probability-Probability (PP) plots and histograms. Test indicated that leukocyte telomere length has a normal distribution (Fig. 1). Simple and multiple linear regressions were used to assess possible relationships for all predictors, including maternal nutrition and infectious disease status with maternal leukocyte telomere length. Means and standard deviations were calculated for predictors of interest. All variables with significance at p ≤ 0.15 were included in multivariable models. Backward stepwise regression was used, with significance set at p < 0.05; similarly, multivariable models had significance set at p < 0.05. Variables that were collinear (r > 0.7) were not included in the models concurrently, including presence of Chlamydia trachomatis bacterial infection and other sexually transmitted infections. Results are reported as slope (β) with 95% confidence interval (CI) estimated by final multivariable models using linear multivariable regression and backward stepwise technique.
Fig. 1.
Distribution of Leukocyte Telomere Lenght among pregnant women in Rwanda
Results
Sociodemographic, anthropometric and behavioral data and leukocyte telomere length
Mean gestational age at enrollment was 13.04 ± 3.50 weeks for 297 participants. Mean maternal age was 28.16 ± 6.10 years, and mean leukocyte telomere length was 1.16 ± 0.22 T/S. Most participants (82.2%; n = 244) were between 20 and 35 years, and for this age category, the mean leukocyte telomere length was 1.17 ± 0.22 T/S (Table 1). Maternal age had a significantly negative association with maternal leukocyte telomere length (β = −2.96; p = 0.003). No association was found between residential area within Gasabo District (urban vs. rural), occupation (employed vs. not working), and marital status (living with a partner vs. living alone) and maternal leukocyte telomere length (Table 1). Participants who had secondary schooling or higher had longer telomeres than those who did not (β = 0.17, 95% CI: 0.03–0.14; p = 0.003; Table 1). Primary schooling was associated with shorter leukocyte telomere length as compared with no education (Table 1).
Table 1.
Social demographic/behavioral characteristics and leukocyte telomere length (N = 297)
| Descriptive | Simple linear regression | ||||
|---|---|---|---|---|---|
| Total N (%) or mean +/-SD | Leukoctye telomere length mean+/-SD(T/S) | β | CI | p | |
| Age in years | 28.16 ± 6.10 | 1.16 ± 0.22 | -2.96 | [.010; -0.02] | 0.003** |
| Age | |||||
| <20 | 13 (4.3) | 1.18 ± 0.21 | Ref. | ||
| ≥ 20-35 | 244 (82.2) | 1.17 ± 0.22 | -0.11 | [-021; 0.06] | 0.31 |
| >35 | 40 (13.5) | 1.11 ± 0.22 | -0.14 | [-013; 0.11] | 0.90 |
| Gestational age (weeks) at recruitment | 13.04 ± 3.5 | 0.012 | [0.01; 0.2] | 0.74 | |
| Residence | |||||
| Urban | 104 (35.0) | 1.17 ± 0.21 | Ref. | ||
| Rural | 193 (65.0) | 1.14 ± 0.25 | 0.5 | [-03; 0.08] | 0.32 |
| Occupation | |||||
| Paying Job | 150 (50.5) | 1.19 ± 0.23 | refer | ||
| Unemployed | 147 (49.5) | 1.17 ± 0.22 | 0.8 | [-0.01; 0.05] | 0.14 |
| Living with a partner (na = 296) | |||||
| Yes | 273 (92.2) | 1.17 ± 023 | Ref. | ||
| No | 23 (7.8) | 1.11 ± 0.16 | -0.06 | [-0.15; 0.04] | 0.26 |
| Educational level | |||||
| Never schooled | 146 (49.2) | 1.15 ± 0.23 | Ref. | ||
| Primary | 61 (20.5) | 1.06 ± 0.18 | -0.16 | [-0.16; -0.3] | .005** |
| Secondary or higher | 90 (30.3) | 1.24 ± 0.21 | 0.17 | [0.03; 0.14] | .003* |
| Body Mass Index (BMI) (kg/m2) | 23.09 (3.39) | 1.16 ± 0.22 | -2.11 | [-0.02; 0.01] | 0.03* |
| BMI (category) (na = 293) | |||||
| Underweight | 11 (3.8) | 1.12 ± 0.26 | Ref. | ||
| Normal Weight | 215 (73.4) | 1.18 ± 0.22 | 0.10 | [-0.06;0.17] | 0.39 |
| Overweight | 55 (18.7) | 1.11 ± 0.23 | -0.04 | [-0.15; 0.11] | 0.73 |
| Obese | 12 (4.1) | 1.16 ± 0.25 | 0.31 | [-014; 0.19] | 0.75 |
| Alcohol use (at least 2-4 times per month) | |||||
| No | 228 (76.8) | 1.17 ± 0.25 | Ref. | ||
| Yes | 69 (23.2) | 1.16 ± 0.21 | 0.01 | [-0.05; 0.06] | 0.83 |
| Gender-based Violence | |||||
| Yes | 12 (4.0) | 1.17 ± 0.22 | Ref. | ||
| No | 285 (96.0) | 1.05 ± 016 | 0.10 | [-0.24; 0.01] | 0.08 |
**p < 0.01, *p < 0.05
na Sample size reduced by missing responses among covariates
BMI of most participants was within the normal range (73.4%; n = 215), with the mean value 23.09 ± 3.39 kg/m2. An increase in BMI measured continuously was associated with a decrease in overall leukocyte telomere length (β = −2.11, 95% CI: −0.02 to 0.01; p = 0.03). No association was found between alcohol consumption and maternal leukocyte telomere length, although self-reported gender-based violence trended towards significance with shorter telomere length (β = 0.10, 95% CI: −0.24 to 012; p = 0.08).
Obstetrical history, pregnancy outcomes and telomere length
The majority of the cohort was multiparous (n = 178, 59.9%) and had not experienced stillbirth (n = 289, 96.3%). Most participants had not undergone a cesarean section (n = 261, 90.9%), experienced previous preterm labor (n = 246, 82.8%), or suffered a miscarriage (n = 250, 85.6%). Most participants had a vaginal delivery (n = 239, 80.5%) with the current pregnancy.
In total, 9.1% (n = 27) of participants experienced preterm birth (gestational age < 37 weeks). The mean gestational age of the cohort was 38.8 ± 2.1 weeks, and for participants with preterm neonates, it was 33.6 ± 2.6 weeks. Participants who delivered before 34 weeks of gestation represented 3.7% of the cohort (n = 11), while those who delivered from 34 to 36 weeks of gestation represented 5.4% (n = 16) of the cohort. No statistically significant association was found between maternal obstetrical history or delivery-related variables, including previous preterm birth and gestational age, and maternal leukocyte telomere length (Table 2).
Table 2.
Obstetrical history, pregnancy outcomes and leukocyte telomere length (n=297)
| Descriptive | Simple Linear regression | ||||
|---|---|---|---|---|---|
| Total N (%) or mean +/-SD) | Leukocyte telomere Length ( mean +/-SD T/S) | Β | CI | p | |
| Maternal parity | |||||
| Nullipara | 20 (6.7) | 1.23 ± 0.19 | Ref | ||
| Primipara | 99 (33.3) | 1.15 ± 0.21 | -1.44 | [-0.09; 0.01] | 0.15 |
| Multipara | 178 (60.0) | 1.16 ± 0.23 | -1.14 | [-0.16; 0.04] | 0.25 |
| History of stillbirth | |||||
| No | 286 (96.3) | 1.16 ± 0.22 | Ref | ||
| Yes | 11 (3.7) | 1.13 ± 0.25 | -0.03 | [-0.17; 0.10] | 0.60 |
| Previous miscarriage (na = 292) | |||||
| No | 250 (85.7) | 1.16 ± 0.22 | Ref | ||
| Yes | 42 (14.3) | 1.16 ± 0.21 | -0.01 | [-0.08; 0.06] | 0.81 |
| Previous Caesarian Section (na = 287) | |||||
| No previous C. Section | 261 (90.9) | 1.16 ± 0.22 | Ref | ||
| Previous C. Section | 26 (9.1) | 1.12 ± 0.19 | -0.06 | [-0.13; 0.04] | 0.32 |
| Previous Preterm Labor/ birth | |||||
| No | 246 (82.8) | 1.17 ± 0.22 | Ref | ||
| Yes | 51 (17.2) | 1.16 ± 0.23 | 0.01 | [-0.06; 0.07] | 0.84 |
| Mode of delivery | |||||
| Caesarian section | 58 (19.5) | 1.17 ± 0.25 | Ref | ||
| Vaginal | 239 (80.5) | 1.16 ± 0.21 | 0.03 | [-0.05; 0.08] | 0.61 |
| Gestational age, weeks | 38.81± 2.1 | 1.16 ± 0.22 | 0.04 | [-01; 0.02] | 0.59 |
| Gestational age, weeks (preterm only <37 weeks) (n = 27) | 33.59 ± 2.61 | 1.15 ± 0.15 | 0.09 | [-0.19;0.03] | 0.62 |
| Preterm delivery (<37 weeks) | |||||
| No | 270 (90.9) | 1.16 ± 0.23 | Ref | ||
| Yes | 27 (9.1) | 1.46 ± 0.16 | -0.01 | [-09; 0.08] | 0.84 |
| Preterm delivery (<34 weeks) | |||||
| No | 286 (96.3) | 1.16 ± 0.23 | Ref | ||
| Yes | 11 (3.7) | 1.17 ± 0.14 | 0.01 | [-0.13; 0.14] | 0.98 |
| Late preterm delivery (34-<37 weeks) | |||||
| No | 281 (94.6) | 1.15 ± 0.17 | Ref | ||
| Yes | 16 (5.4) | 1.16 ± 0.23 | -0.02 | [-0.13; 0.09] | 0.79 |
| Birthweight (n = 297) (grams) | 3153.22 ± 544.45 | 1.16 ± 0.22 | -0.08 | [-0.01;0.02] | 0.15 |
| Birthweight preterm (<37 weeks) (n = 27) (grams) | 2063.33 ± 616.85 | 1.15 ± 0.16 | 0.14 | [-0.06; 0.14] | 0.47 |
| Low birth weight (<2500 g) | 1874.80 ± 451.84 | 1.18 ± 0.16 | 0.27 | [-0.05;0.025] | 0.20 |
na Sample size reduced by missing responses among covariates
Inflammation and infections in pregnancy and leukocyte telomere length
Overall, 26% of the cohort (n = 77) had acute inflammation as suggested by elevated CRP levels and 7.4% had chronic inflammation as indicated by elevated AGP level (Table 3). There was no association between maternal leukocyte telomere length and CRP levels, AGP levels, or presence of urinary tract infections (Table 3). Having a sexually transmitted infection during pregnancy and shorter maternal telomere length approached statistical significance (β = −0.104, 95% CI: −0.102 to 0.005; p = 0.07), as did having a bacterial Chlamydia trachomatis infection (β = −0.010, 95% CI: −0.12 to 0.01; p = 0.08; Table 3).
Table 3.
Maternal inflammation, infection and leukocyte telomere length (n=297)
| Descriptive | Simple Linear regression | ||||
|---|---|---|---|---|---|
| Total N (%) or mean ±SD) | Leukocyte telomere Length mean ±SD (T/S) | Β | CI | P | |
| Markers of Acute and Chronic Inflammation | |||||
| C Reactive Protein (CRP mg/l) | 5.08 ± 12.33 | 1.16 ± 0.22 | 0.02 | [-0.01; 0.03] | 0.67 |
| CRP(>5 mg/l) (na = 296) | |||||
| No | 219 (74.0) | 1.16 ± 0.23 | ref | ||
| Yes | 77 (26) | 1.17 ± 0.21 | 0.04 | [-0.04; 0.07] | 0.53 |
| α1-acid glycoprotein (AGPg/dl) | 0.61 ± 0.41 | 1.16 ±0.22 | -0.45 | [-0.07; 0.04] | 0.64 |
| α1-acid glycoprotein (AGP>1 g/l) | |||||
| No | 275 (92.6) | 1.17. ±0.23 | ref | ||
| Yes | 22 (7.4) | 1.13 ± 0.15 | -0.04 | [-0.13; 0.06] | 0.48 |
| Presence of Infectious Disease | |||||
| Urinary Tract Infections (UTI) | |||||
| No | 259 (87.2) | 1.17 ± 0.23 | Ref | ||
| Yes | 38 (12.8) | 1.16 ± 0.16 | -0.01 | [-0.08; 0.07] | 0.84 |
| UTI strain (n = 38) | |||||
| Stenotrophomonas maltophilia | 2 (5.3) | ||||
| Escherchia coli | 22 (57.9) | ||||
| Klebsialla pneumoniae | 11 (28.9) | ||||
| Staphylococcus epidermitis | 3 (7.9) | ||||
| Sexually Transmitted Infection (STI) | |||||
| No | 189 (63.6) | 1.18 ± 0.23 | ref | ||
| Yes | 108 (36.4) | 1.13 ± 0.22 | -0.104 | [-0.102; 0.005] | 0.07 |
| Type of STI | |||||
| Chlamydia trachomatis | |||||
| No | 235 (79.1) | 1.17 ± 0.22 | ref | ||
| Yes | 62 (20.9) | 1.12 ± 0.25 | -010 | [-0.12; 0.01] | 0.08 |
| Trichomonas vaginalis | |||||
| No | 282 (94.9) | 1.16 ± 023 | ref | ||
| Yes | 15 (5.1) | 1.17 ± 0.19 | 0.002 | [-0.11; 0.12] | 0.99 |
| Candida albicans | |||||
| No | 233 (78.5) | 1.17 ± 0.23 | ref | ||
| Yes | 64 (21.5) | 1.14 ± 0.19 | -0.51 | [-0.09; 0.03] | 0.25 |
na Sample size reduced by missing responses among covariates
Nutrition in pregnancy and leukocyte telomere length
A significant number (n = 92, 31%) of participants had anemia during pregnancy; 3.4% (n = 10) with a lower percentage having iron deficiency as indicated by ferritin or soluble transferrin receptors deficiency (Table 4). Nineteen-point two percent of participants had ferritin deficiency (n = 58) with 3.4% (n = 10) having transferrin receptor deficiency (Table 4). Continuous measures of sTfR levels were inversely associated with telomere length (β = −0.13, 95% CI: −0.03 to 0.01; p = 0.02), as were RBP levels (β = −2.19, 95% CI: −0.13 to 0.01; p = 0.03). Deficiencies in sTfR levels (> 8.3 mg/L) approached statistical significance, with a negative association between deficiency and longer leukocyte telomere length (β = −0.10, 95% CI: −0.26 to 0.17; p = 0.08; Table 4). No association was present for other markers of maternal nutritional health, including RBP deficiency, ferritin deficiency, inadequate dietary diversity, indicators of malnutrition and maternal leukocyte telomere length (Table 4).
Table 4.
Maternal nutrition and leukocyte telomere length (n=297)
| Descriptive | Simple Linear regression | ||||
|---|---|---|---|---|---|
| Total N (%) or mean ±SD) | Leukocyte telomere length mean ±SD (T/S) | β | CI | P | |
| Hemoglobin (g/dl) | 11.23 ± 1.13 | 1.16 ±0.22 | 0.04 | [-0.01; 0.03] | 0.47 |
| Anemia (<11 g/dl) | |||||
| No | 205 (69.0) | 1.15 ± 0.21 | Ref | ||
| Yes | 92 (31.0) | 1.19 ± 0.24 | 0.07 | [-02;0.09] | 0.21 |
| STFR (mg/L) (Soluble Transferrin Receptors) | 4.81 ± 1.49 | 1.16 ±0.22 | -0.13 | [-0.03; -0.01] | 0.02* |
| STFR (mg/L) Deficiency | |||||
| No deficiency (≤8.3 mg/L) | 287 (96.6) | 1.17 ± 0.22 | Ref | ||
| Deficiency (>8.3 mg/L) | 10 (3.4) | 1.04 ± 0.18 | -0.10 | [-0.26; 0.17] | 0.08 |
| Retinol Binding Protein (RBP) (μmol/L) | 1.38 ± 0.40 | 1.16 ± 0.22 | -2.19 | [-0.13; -0.01] | 0.03* |
| RBP(μmol/L) Deficiency | |||||
| No deficiency (≥0.83 μmol/L) | 239 (80.5) | 1.17 ± 0.18 | Ref | ||
| Deficiency (< 0.83 μmol/L) | 58 (19.5) | 1.16 ± 0.23 | 0.03 | [-0.05; 0.08] | 0.66 |
| Ferritin (μg/L) | 73.52 | 1.16 ± 0.22 | -0.09 | [-0.01; 0.03] | 0.12 |
| Ferritin Deficiency (<12 μg/L) | |||||
| No Deficiency (<12 μg/L) | 240 (80.8) | 1.16 ± 0.22 | Ref | ||
| Deficiency (≥12 μg/L) | 58 (19.2) | 1.17 ± 0.23 | -0.01 | [-0.07; 0.06] | 0.84 |
| Minimum Dietary Diversity for Women (MDDW) | 4.61 ± 1.68 | 1.16 ± 0.22 | -0.03 | [-0.06; 0.3] | 0.56 |
| MDDW Deficiency | |||||
| Adequate MDDW | 145 (48.8) | 1.15 ± 0.22 | Ref | ||
| Low MDDW | 152 (51.2) | 1.17 ± 0.23 | -0.03 | [-0.07; 0.04] | 0.50 |
| Mid-Upper Arm Circumference (MUAC) d(cm)(Mean +/-SD) | 25.72 ± 3.06 | 1.16 ± 0.23 | -0.09 | [-0.02; 0.002] | 0.30 |
| MUAC Indication of Malnutrition | |||||
| ≥23 cm | 236 (79.5) | 1.16 ± 0.23 | Ref | ||
| <23 cm | 58 (19.5) | 1.18 ± 0.22 | -0.04 | [-0.09; 0.04] | 0.45 |
*p < 0.05
na Sample size reduced by missing responses among covariates
Multivariable linear regression
Independent predictors of longer leukocyte telomere length included younger maternal age (β = −0.14, 95% CI: −0.01 to − 0.001; p = 0.01), no schooling vs. primary schooling (β = −0.19, 95% CI: −0.17 to − 0.041; p = 0.002), lower levels of sTfRs (β = −0.15, 95% CI: −0.04 to − 0.007; p < 0.01), and lower levels of ferritin (β = −0.13, 95% CI: −0.01 to − 0.001; p = 0.02; Table 5). Similarly, in the backward stepwise regression model, independent predictors of longer telomere length were younger age (β = −0.15, 95% CI: −0.01 to − 0.001; p < 0.01), absence of primary schooling (β = −0.21; 95% CI: −0.18 to − 0.05; p < 0.01), lower levels of sTfRs (β = −0.15, 95% CI: −0.04 to − 0.007; p < 0.01), lower levels of ferritin (β = −0.12, 95% CI: −0.01 to − 0.001; p = 0.027), and lower levels of RBP (β = −0.11, 95% CI: −0.12 to − 0.001; p = 0.04; Table 5).
Table 5.
Multiple linear regression for determinants of maternal leukocyte telomere length (n=297)
| Linear regression model full model | Adjusted model | |||||||
|---|---|---|---|---|---|---|---|---|
| Β | SE | CI | P | β | SE | CI | P | |
| Age (years) | -0.140 | 0.02 | [-0.01; -0.001] | 0.01 | -0.15 | 0.02 | [-0.01; -0.001] | 0.008** |
| Occupation | ||||||||
| Paying Job | ref | |||||||
| Housewife | -0.002 | 0.025 | [-0.05; 0.048] | 0.97 | - | - | - | - |
| Education Level | ||||||||
| Never schooled | ref | |||||||
| Primary | -0.190 | 0.033 | [-0.17; 0.041] | 0.002 | -0.21 | 0.033 | [-0.18; -0.05] | 0.001** |
| Secondary or higher | 0.122 | 0.030 | [-0.001; 0.12] | 0.045 | 0.11 | 0.109 | [-0.004; 0.11] | .070 |
| Body Mass Index (BMI) kg/m2 | -0.094 | 0.004 | [-0.014; 0.01] | 0.101 | - | - | - | - |
| Gender Based Violence (GBV) | ||||||||
| Yes | ref | |||||||
| No | -0.107 | 0.063 | [-0.25; 0.01] | 0.053 | - | - | - | - |
| Parity Nullipara(reference) | ref | - | - | - | - | |||
| Primipara | -0.020 | 0.055 | [-0.125; 0.09] | 0.75 | - | - | - | - |
| Multipara | 0.046 | 0.027 | [.0.03; 0.074] | 0.430 | - | - | - | - |
| Birth weight | 0.053 | .0001 | [-0.01; 0.001] | 0.350 | ||||
| Sexually transmitted Infections | ||||||||
| No | ref | |||||||
| Yes | -0.071 | 0.026 | [-0.84; 0.018] | 0.202 | ||||
| sTFR (mg/L) | -0.156 | 0.009 | [-0.04; 0.007] | 0.006 | -015 | 0.008 | [-0.04; -0.007] | 0.005** |
| Retinol Binding Protein (RBP) (μmol/L) | -0.093 | 0.031 | [-0.11; 0.010] | 0.098 | -0.11 | 0.03 | [-0.12; -0.001] | 0.04 |
| Ferritin (μg/L) | -0.130 | .0001 | [-0.01; 0.001] | 0.023 | -0.124 | 0.001 | [-0.01; -0.001] | 0.027* |
**p < 0.01, *p < 0.05
Discussion
This is the first study to assess association between maternal infections, nutritional health and leukocyte telomere length in pregnant women in SSA. Furthermore, it is the first study of leukocyte telomere length among women in Rwanda. We found evidence of leukocyte telomere length as a marker of biological aging in Rwandan women and also of possible associations between maternal micronutrient status and leukocyte telomere length in pregnant women in Rwanda. Other leukocyte telomere length studies with pregnant women have been conducted primarily in the European and North American context, which presents a different environmental milieu in terms of overall burden of infectious disease and maternal nutritional health [38, 39]. In contrast with these other studies, the endemicity of infectious diseases in Kigali, Rwanda, including clinical and subclinical infections such as a higher prevalence of sexually transmitted infections and endemicity of malaria, provides a different context to assess the association between maternal leukocyte telomere length and birth outcomes [40].
Maternal age, leukocyte telomere length and Rwandan women
A strong inverse association was found between maternal age and leukocyte telomere length, which is consistent with results of previous studies [4, 41, 42] demonstrating that leukocyte telomere length is a biological marker for aging in Rwandan women [41, 43]. This is the first study to our knowledge conducted of leukocyte telomere length in Rwandan women. The age range in our study was relatively narrow with the majority between 20 and 35 years of age (mean 28.16 ± 6.10) and even within this range, maternal age was highly predictive of leukocyte telomere length adjusting for other factors. Previous studies have found that Africans have longer leukocyte telomere length than Caucasians [44]; our results for reproductive age women are similar to those reported from a large population-based survey in United States for a similar age range of women and comparable to the African-Americans surveyed [45].
Maternal nutritional status
Several micronutrient markers were independently associated with shorter maternal leukocyte telomere length, after adjusting for other predictors. Specifically, we found associations between body iron stores as measured by lower numbers of soluble transferrin receptors, ferritin levels, and longer leukocyte telomere length. However, we found no associations between anemia and iron deficiency and leukocyte telomere length. Previous findings have been mixed including a study involving American adults (> 65 years) in whom high ferritin levels were associated with shorter telomere length [46] and a large study with British adults where transferrin saturation was associated with shorter leukocyte telomere length [47]. In contrast, another study found that iron consumption, as measured by dietary intake, was associated with longer telomere length in adults [48]. Importantly, the context where our study was conducted has different environmental stressors than in the previously described studies. As malaria infection is common in East Africa, including Rwanda, lower iron levels have been found to be associated with a reduced susceptibility to malaria infection in pregnant women [49]. Lower iron levels may have indirect health benefits in the context of areas with endemic malaria or other high incidence areas.
Alternatively, increased concentrations of iron can cause oxidative stress, consequently leading to telomere shortening [50]. Iron is a redox-active transitional metal with pro-oxidant properties [51]. Excessive iron during pregnancy can lead to ROS overproduction and oxidative stress [46]. Administering iron supplements to pregnant women is common as part of antenatal care services [14]; some pregnant Rwandan women may receive iron supplements to tackle iron deficiency, eventually leading to excessive iron in the body.
We also found an inverse relationship between RBP, an indirect indicator of retinol (vitamin A) levels, and leukocyte telomere length. As vitamin A is one of the antioxidants that provides a buffer against oxidative stress, it may protect against accelerated leukocyte telomere shortening [52]. Our results were unexpected and differ from those of other studies in which retinol levels were found to be positively associated with leukocyte telomere length [48]; moreover, vitamin A supplementation has been reported to be associated with longer telomere length [53]. However, these previous studies were not conducted with pregnant women in the first trimester of pregnancy. High levels of vitamin A, particularly during the first trimester, are associated with congenital malformations of the central nervous and cardiovascular systems [54], and high levels of serum retinol during pregnancy are associated with postpartum depression [55]. Elevated serum retinol levels may thus serve as an adverse risk marker of maternal and neonatal outcomes; further studies with multiple timepoints in pregnancy are warranted to assess direct effects.
Neither maternal BMI, MUAC or dietary intake measured by the MDDW index were associated with leukocyte telomere length. Our findings differ from the results of other studies that have consistently found maternal BMI levels associated with leukocyte telomere length [56, 57] as well as those that suggest that improved nutrition is associated with longer leukocyte telomere length [47]. In this study, a low percentage of women were obese (4.1%) and underweight (3.8%), with less heterogeneity in BMI compared with other population groups potentially explaining the contradictory results. Excess adipose tissue in obesity has been associated with shorter leucocyte telomere length but in North American contexts where obesity is more prevalent [58]. Additionally, our measurements were taken during early pregnancy which could have biased findings in contrast with other studies that have use pre-pregnancy BMI. Few studies measured weight during pregnancy in resource-poor settings in relation to maternal leucocyte telomere length.
Infections and inflammation
We found no association between biomarkers for inflammation and infection including sexually transmitted infections and leukocyte telomere length, although in bivariate analyses, any presence of sexually transmitted infection and infection with chlamydia trachomatis approached statistical significance for shorter leukocyte telomere length. Previous studies of infectious disease, specifically HIV in South Africa, have found shorter leukocyte telomere length with infection [59] although other studies with HIV infected individuals did not find any association, primary in the context of antiretroviral therapy immune reconstitution [38, 60]. Additional studies evaluating multiple pathogens have found that the association between shorter leukocyte telomere and infection may be pathogen specific [61]. We also assessed the association between general markers of inflammation and leukocyte telomere length as these have previously been found associated with shorter telomere length [39]. However, we did not assess duration or timing of infection and as such we may have not had sufficient information to assess associations with leukocyte telomere length. CRP levels rise sharply during the early phase of an infection, whereas AGP levels gradually increase so timing of testing may be critical to assess levels [62]. Furthermore, other studies were not conducted with pregnant women and CRP levels increase and AGP decrease with duration of gestation [21]. As such, the interplay between inflammatory status and leukocyte telomere length may not be fully understood in the context of pregnancy. Alternatively, it is possible that the cutoff points used for active and chronic infections may not be appropriate for developing countries with a high infectious disease burden [63], such as Kigali, Rwanda. Elevated CRP levels were lower than those reported in pregnant Kenyan population and AGP slightly higher, although the gestational age range for the group including women in later stages of pregnancy potentially accounting for the differences [64]. Another study with Ugandan women at similar timing of gestation had comparable elevated AGP levels but lower CRP ones [65].
Maternal sociodemographics
Maternal education level (primary schooling vs. no schooling) was inversely associated with maternal leukocyte telomere length. Previous studies involving pregnant women have reported that higher education level is associated with longer leukocyte telomere length and is a potential good proxy for socioeconomic status [66]. It remains unclear as to why primary education was associated with shorter leukocyte telomere length in our study population after adjusting for age and other risk factors. It is possible that unknown confounders are associated with higher maternal education levels that additionally adversely impact leukocyte telomere length in Rwandan women. Further studies are warranted to comprehend the relationship between education level and health outcomes in East African populations.
Maternal obstetrical history
We found no association among birth outcomes, including birth weight, low birth weight, and preterm birth, maternal reproductive history, and maternal leukocyte telomere length. Few studies have investigated these associations, but to our knowledge, none have been conducted in SSA. A study involving European mothers reported an association between shorter telomere length and intrauterine growth restriction [67], and previous studies have reported mixed results on the association between gravidity, parity, and maternal telomere length [68, 69].
Conclusions
We found associations between maternal age and micronutrients levels in early pregnancy and leukocyte telomere length in Rwandan women. Specifically, similar to other studies, we found that older maternal age was associated with shorter leukocyte telomere length even within a relatively narrow age range for women of reproductive age. We additionally found an association between markers of serum iron levels including higher ferritin levels and transferrin saturation receptor counts and shorter telomere length as well as lower retinol binding protein levels and shorter telomere length possibly suggestive of maternal health in pregnancy or alternatively specific to the environmental context of Rwanda. As the relationship between nutrition and infectious disease involves a feedback loop with infections affecting nutritional status and nutritional deficiencies impacting immune response and susceptibility to infections; further studies in this area are needed in developing countries.
Limitations
Ours is the first study to assess leukocyte telomere length in Rwanda. Additional studies are needed with more diverse population groups including men and children. Studies with pregnant women should more comprehensively explore maternal nutritional health and leukocyte telomere length in terms of nutritional assessments, including analyzing the intake of vitamin D, selenium, vitamin C, and other vitamins and minerals, in relation to maternal leukocyte telomere length outcomes. Future studies should also include multiple measurements of leukocyte telomere length during pregnancy, in addition to neonatal assessments and multiple nutritional and anthropometric maternal measurements throughout different periods of gestation.
Studies should also investigate the impact of pre-pregnancy BMI and maternal weight gain during pregnancy on maternal and neonatal outcomes including leukocyte telomere length as an outcome. Other studies should also comprehensively investigate infectious disease history including history of infection with malaria, STI history and other infectious disease burden including tuberculosis, hepatitis and dengue.
Public health implications
Nutritional intake during pregnancy is an actionable area for public health interventions in Rwandan women and children. Thus, further studies should assess the relationship between high iron levels, retinol binding protein and other markers of vitamin A status and shorter maternal leukocyte telomere length in the context of endemic infectious diseases. It is possible that the current regimens of iron and multivitamin supplementation during pregnancy do not confer the same benefits in all pregnant women, particularly those living in areas with a high incidence of malaria or a high burden of other infectious diseases.
Acknowledgements
We thank the staff members at health facilities in Gasabo for their collaboration. We are also thankful to all participants and collaborators.
Abbreviations
- AGP
α1-acid glycoprotein
- BMI
Body mass index
- CI
Confidence interval
- CRP
C-reactive protein
- HIV
Human immunodeficiency virus
- MUAC
Mid-upper arm circumference
- RBP
Retinol-binding protein
- ROS
Reactive oxygen species
- SSA
Sub-Saharan Africa
- sTfR
Soluble transferrin receptor
- WHO
World Health Organization
Authors’ contributions
EN and JW conceptualized the overall study; EN, AU, CMM, and SR, JW was the promotor of leucocyte telomeres length substudy and designed the study protocol together with EN and NS, PM, and NM revised the protocol. AU and DN contributed to data collection. EN, JW, and NS contributed to data analysis. EN, JW JLC MN and EJC drafted and edited the manuscript; JL conducted telomere length analysis and edited the manuscript. All authors reviewed, edited, contributed, and approved the final version of the manuscript.
Funding
This study was funded by the East Africa Preterm Birth Initiative, a multiyear, multi-country effort generously funded by the Bill and Melinda Gates Foundation, and the funding covered publication charges (investment ID: OPP1107312). The funder had no role in study design; data collection, analysis or interpretation; or writing the manuscript.
Further, laboratory work involving leukocyte telomere length analysis was funded by the Nutrition and Obesity Research Center (NORC) at the University of California, San Francisco (P30DK098722).
Availability of data and materials
Available with the corresponding author (etiennen70@gmail.com) and will be deposited in a public repository as soon as we gain permission to do so.
Ethics approval and consent to participate
The study protocol was approved by the Rwanda Ministry of Health and the Institutional Review Board at the College of Medicine and Health Sciences (University of Rwanda) (approval notice: No213/CMHS IRB/2017) and the Committee on Human Research (Institutional Review Board) of the University of California, San Francisco. All participants provided signed, informed consent prior to participation in the study and were allowed to terminate participation at any time point during the study’s duration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Etienne Nsereko, Email: etiennen70@gmail.com.
Janet M. Wojcicki, Email: wojcicki@gmail.com
References
- 1.Lazarides C, Epel ES, Lin J, Blackburn EH, Voelkle MC, Buss C, et al. Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: a prospective investigation. Brain Behav Immun. 2019;80(April):419–26. doi: 10.1016/j.bbi.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opresko PL, Shay JW. Telomere-associated aging disorders. Ageing Res Rev. 2017;33:52–66. 10.1016/j.arr.2016.05.009. [DOI] [PMC free article] [PubMed]
- 3.Robertson T, Batty GD, Der G, Fenton C, Shiels PG, Benzeval M. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol Rev. 2013;35(1):98–111. doi: 10.1093/epirev/mxs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi S, Raza ST, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci. 2015;7(3):161–7. doi: 10.2174/1874609808666150122153151. [DOI] [PubMed] [Google Scholar]
- 5.Berti C, Biesalski HK, Gärtner R, Lapillonne A, Pietrzik K, Poston L, et al. Micronutrients in pregnancy: current knowledge and unresolved questions. Clin Nutr. 2011;30(6):689–701. doi: 10.1016/j.clnu.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Pangrazzi L. Boosting the immune system with antioxidants: where are we now? Biochem (Lond) 2019;41:42–4. doi: 10.1042/BIO04101042. [DOI] [Google Scholar]
- 7.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2012;16(7):705–43. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marra MV, Drazba MA, Holásková I, Belden WJ. Nutrition risk is associated with leukocyte telomere length in middle-aged men and women with at least one risk factor for cardiovascular disease. Nutrients. 2019;11(3):1–16. doi: 10.3390/nu11030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entringer S, Epel ES, Blackburn EH, Buss CSB, et al. Maternal folate concentration in early pregnancy and newborn telomere length. Physiol Behav. 2017;176(1):139–48. doi: 10.1159/000381925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers KO, Ibrahimou B, Yusuf KK, Mauck DE, Salihu HM. The effect of maternal vitamin C intake on fetal telomere length. J Matern Neonatal Med. 2019;1–6. 10.1080/14767058.2019.1628940. [DOI] [PubMed]
- 11.Desyibelew HD, Dadi AF. Burden and determinants of malnutrition among pregnant women in Africa: a systematic review and meta-analysis. PLoS One. 2019;14(9):1–19. doi: 10.1371/journal.pone.0221712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan Wessells K, Ouédraogo CT, Young RR, Thierno Faye M, Brito A, Hess SY. Micronutrient status among pregnant women in zinder, niger and risk factors associated with deficiency. Nutrients. 2017;9(5). [DOI] [PMC free article] [PubMed]
- 13.Hakizimana D, Nisingizwe MP, Logan J, Wong R. Identifying risk factors of anemia among women of reproductive age in Rwanda - a cross-sectional study using secondary data from the Rwanda demographic and health survey 2014/2015. BMC Public Health. 2019;19(1):1–11. doi: 10.1186/s12889-019-8019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Statistics of Rwanda (NISR) M of, Health (MOH) [Rwanda] II . Rwanda demographic and health survey 2014-15 final report. 2016. p. 307. [Google Scholar]
- 15.Nsereko E, Uwase A, Mukabutera A, Muvunyi CM, Rulisa S, Ntirushwa D, et al. Maternal genitourinary infections and poor nutritional status increase risk of preterm birth in Gasabo District, Rwanda: a prospective, longitudinal, cohort study. BMC Pregnancy Childbirth. 2020;20(1):345. doi: 10.1186/s12884-020-03037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph Davey DL, Nyemba DC, Gomba Y, Bekker LG, Taleghani S, DiTullio DJ, et al. Prevalence and correlates of sexually transmitted infections in pregnancy in HIV-infected and- uninfected women in Cape Town, South Africa. PLoS One. 2019;14(7):1–14. doi: 10.1371/journal.pone.0218349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemu A, Moges F, Shiferaw Y, Tafess K, Kassu A, Anagaw B. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Go. BMC Res Notes. 2012;5(197):7. doi: 10.1186/1756-0500-5-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutagoma M, Balisanga H, Malamba SS, Sebuhoro D, Remera E, Riedel DJ, et al. Hepatitis B virus and HIV co-infection among pregnant women in Rwanda. BMC Infect Dis. 2017;17(1):1–7. doi: 10.1186/s12879-016-2122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.President’s Malaria Initiative. Operational plan for Rwanda. FY 2017. 2017;1–72.
- 20.USAID, CDC, USA Department of State, USA Department of Health and Human Services . Rwanda - malaria operational plan FY 2018. 2018. p. 54. [Google Scholar]
- 21.Sauder MW, Lee SE, Schulze KJ, Christian P, Wu LSF, Khatry SK, et al. Inflammation throughout pregnancy and fetal growth restriction in rural Nepal. Epidemiol Infect. 2019;147:e258. doi: 10.1017/S0950268819001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee ACC, Quaiyum MA, Mullany LC, Mitra DK, Labrique A, Ahmed P, et al. Screening and treatment of maternal genitourinary tract infections in early pregnancy to prevent preterm birth in rural Sylhet, Bangladesh: a cluster randomized trial. BMC Pregnancy Childbirth. 2015;15(1):1–14. doi: 10.1186/s12884-015-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2018;134(11):3127–32. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Miner Nutr Inf Syst World Health Organ. 2013;1–6.
- 25.Thurnham DI, Mccabe LD, Haldar S, Wieringa FT, Northrop-clewes CA, Mccabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: 2018;(August):546–55. [DOI] [PubMed]
- 26.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):10–47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jue Lin, Epel ECJ, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;3521(1):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao DSR, Pindi DKG, Rani DU, Sasikala DG, Kawle DV. Diagnosis of bacterial vaginosis: Amsel’s criteria vs Nugent’s scoring. Sch J Appl Med Sci. 2016;4(6):2027–31. [Google Scholar]
- 29.Patil M, Nagamoti J, Metgud S. Diagnosis of Trichomonas vaginalis from vaginal specimens by wet mount microscopy, in pouch TV culture system, and PCR. J Glob Infect Dis. 2012;4(1):22. doi: 10.4103/0974-777X.93756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babić M, Hukić M. Candida albicans andon-albicans species as etiological agent of vaginitis in pregnant and non-pregnant women. Bosn J Basic Med Sci. 2010;10(1):89–97. doi: 10.17305/bjbms.2010.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hislop J, Quayyum Z, Flett G, Boachie C, Fraser C, et al. Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men. Health Technol Assess (Rockv). 2010;14(29):i–125. 10.3310/hta14290. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L359134054. [DOI] [PubMed]
- 32.Bartlett JG. Laboratory diagnosis of urinary tract infections in adult patients. Infect Dis Clin Pract. 2004;12(6):360–1. [Google Scholar]
- 33.Venter SN, Lotter HS, de H D. and M. The use of the analytical profile index in the identification of activated sludge bacteria: Problems and solutions. Water SA. 2000;15(4):4738.
- 34.FAO and FHI 360. Minimum dietary diversity for women- a guide to measurement. 2016. 82 p.
- 35.Martin-Prevel Y, Arimond M, Allemand P, Wiesmann D, Ballard TJ, Deitchler M, et al. Development of a dichotomous indicator for population-level assessment of dietary diversity in women of reproductive age. Curr Dev Nutr. 2017;1(12):cdn.117.001701. doi: 10.3945/cdn.117.001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Obesity: preventing and managing the global epidemic - WHO technical report series. WHO Technical Report Series. 2000. [PubMed]
- 37.Tang AM, et al. Determining a global mid-upper arm circumference cutoff to assess malnutrition in pregnant women. Washington, DC; 2016.
- 38.Saberi S, Kalloger SE, Zhu MMT, Sattha B, Maan EJ, van Schalkwyk J, et al. Dynamics of leukocyte telomere length in pregnant women living with HIV, and HIV-negative pregnant women: a longitudinal observational study. PLoS One. 2019;14(3):1–20. doi: 10.1371/journal.pone.0212273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong JYY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One. 2014;9(1). [DOI] [PMC free article] [PubMed]
- 40.Kateera F, Nsobya SL, Tukwasibwe S, Mens PF, Hakizimana E, Grobusch MP, et al. Malaria case clinical profiles and Plasmodium falciparum parasite genetic diversity: a cross sectional survey at two sites of different malaria transmission intensities in Rwanda. Malar J. 2016;15(1):1–10. doi: 10.1186/s12936-016-1287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 42.Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol Ser A Biol Sci Med Sci. 2011;66(2):202–13. A(. [DOI] [PubMed]
- 43.Kuo CL, Pilling LC, Kuchel GA, Ferrucci L, Melzer D. Telomere length and aging-related outcomes in humans: a Mendelian randomization study in 261,000 older participants. Aging Cell. 2019;18(6):1–12. doi: 10.1111/acel.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen MEB, Hunt SC, Stone RC, Horvath K, Herbig U, Ranciaro A, et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum Mol Genet. 2016;25(11):2324–30. doi: 10.1093/hmg/ddw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katherine A, Ahrens, Lauren M, Rossen AES. Relationship between mean leucocyte telomere length and measures of allostatic load in US reproductive-aged women, NHANES 1999–2002. Paediatr Perinat Epidemiol. 2016;30(4):325–35. doi: 10.1111/ppe.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Sun Y, Xu G, Snetselaar LG, Ludewig G, Wallace RB, et al. Association between body iron status and leukocyte telomere length, a biomarker of biological aging, in a nationally representative sample of US adults. J Acad Nutr Diet. 2019;119(4):617–25. doi: 10.1016/j.jand.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin C, Baik I. Transferrin saturation concentrations associated with telomeric ageing: a population-based study. Br J Nutr. 2017;117(12):1693–701. doi: 10.1017/S0007114517001696. [DOI] [PubMed] [Google Scholar]
- 48.Mazidi M, Kengne AP, Banach M. Mineral and vitamin consumption and telomere length among adults in the United States. Polish Arch Intern Med. 2017;127 127(2 2):87–90. doi: 10.20452/pamw.3927. [DOI] [PubMed] [Google Scholar]
- 49.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Decreased susceptibility to plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis. 2008;198(2):163–6. doi: 10.1086/589512. [DOI] [PubMed] [Google Scholar]
- 50.Kepinska M, Szyller J, Milnerowicz H. The influence of oxidative stress induced by iron on telomere length. Environ Toxicol Pharmacol. 2015;40(3):931–5. 10.1016/j.etap.2015.10.002. [DOI] [PubMed]
- 51.Ellervik C, Marott JL, Tybjærg-Hansen A, Schnohr P, Nordestgaard BG. Total and cause-specific mortality by moderately and markedly increased ferritin concentrations: general population study and metaanalysis. Clin Chem. 2014;60(11):1419–28. doi: 10.1373/clinchem.2014.229013. [DOI] [PubMed] [Google Scholar]
- 52.Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22(10):895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Qun X, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89(6):1857–63. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maia SB, Souza ASR, Caminha MDFC, da Silva SL, Cruz R, de SBLC, Dos Santos CC, et al. Vitamin A and pregnancy: a narrative review. Nutrients. 2019;11(3):1–18. doi: 10.3390/nu11030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng Y, Li Y, Xia H, Wang S, Zhou J, Chen D. Retinoids, anxiety and peripartum depressive symptoms among Chinese women: a prospective cohort study. BMC Psychiatry. 2017;17(1):1–6. doi: 10.1186/s12888-017-1405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rode L, Nordestgaard BG, Weischer M, Bojesen SE. Increased body mass index, elevated C-reactive protein, and short telomere length. J Clin Endocrinol Metab. 2014;99(9):E1671–5. doi: 10.1210/jc.2014-1161. [DOI] [PubMed] [Google Scholar]
- 57.Gielen M, Hageman GJ, Antoniou EE, Nordfjall K, Mangino M, Balasubramanyam M, et al. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr. 2018;108(3):453–75. doi: 10.1093/ajcn/nqy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvestrini V, Sell C, Lorenzini A. Obesity may accelerate the aging process. Front Endocrinol (Lausanne) 2019;10(MAY):1–16. doi: 10.3389/fendo.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. Aids. 2013;27(15):2375–84. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Côte HCF, Hsieh AYY. Leukocyte telomere length in HIV infection: a marker of persistent immune aging or transient immune reconstitution? J Infect Dis. 2018;218(10):1521–2. doi: 10.1093/infdis/jiy365. [DOI] [PubMed] [Google Scholar]
- 61.Aiello AE, Jayabalasingham B, Simanek AM, Diez-Roux A, Feinstein L, Meier HCS, et al. The impact of pathogen burden on leukocyte telomere length in the multi-ethnic study of atherosclerosis. Epidemiol Infect. 2017;145(14):3076–84. doi: 10.1017/S0950268817001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gannon BM, Glesby MJ, Finkelstein JL, Raj T, Erickson D, Mehta S. A point-of-care assay for alpha-1-acid glycoprotein as a diagnostic tool for rapid, mobile-based determination of inflammation. Curr Res Biotechnol. 2019;1:41–8. doi: 10.1016/j.crbiot.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohler IV, Anglewicz P, Kohler HP, McCabe JFCB. and BS. Evaluating health and disease in Sub-Saharan Africa: minimally invasive collection of plasma in the Malawi Longitudinal Study of Families and Health (MLSFH). Genus. 2012;68(2):1–27. [PMC free article] [PubMed]
- 64.Mwangi MN, Echoka E, Knijff M, Kaduka L, Werema BG, Kinya FM, et al. Iron status of Kenyan pregnant women after adjusting for inflammation using brinda regression analysis and other correction methods. Nutrients. 2019;11(2). [DOI] [PMC free article] [PubMed]
- 65.Baingana RK, Enyaru JK, Tjalsma H, Swinkels DW, Davidsson L. The aetiology of anaemia during pregnancy: a study to evaluate the contribution of iron deficiency and common infections in pregnant Ugandan women. Public Health Nutr. 2015;18(8):1423–35. doi: 10.1017/S1368980014001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell AM, Kowalsky JM, Epel ESLJ. and LC. Child adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology. 2018;87:43–52. [DOI] [PMC free article] [PubMed]
- 67.Perales-Puchalt A, Soberón N, Monterde M, Hervas-Marin D, Foronda M, Desantes D, et al. Maternal telomere length is shorter in intrauterine growth restriction versus uncomplicated pregnancies, but not in the offspring or in IVF-conceived newborns. Reprod Biomed Online. 2019;38(4):606–12. doi: 10.1016/j.rbmo.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 68.Lane-Cordova AD, Puterman E, Gunderson EP, Chan C, Hou L, Carnethon M. Gravidity is not associated with telomere length in a biracial cohort of middle-aged women: The Coronary Artery Risk Development in Young Adults (CARDIA) study. PLoS One. 2017;12(10):1–11. doi: 10.1371/journal.pone.0186495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollack AZ, Rivers K, Ahrens KA. Parity associated with telomere length among US reproductive age women. Hum Reprod. 2018;33(4):736–44. doi: 10.1093/humrep/dey024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available with the corresponding author (etiennen70@gmail.com) and will be deposited in a public repository as soon as we gain permission to do so.