Abstract
Goblet cell hyperplasia and metaplasia and excessive mucus are prominent pathologies of chronic airway diseases such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and chronic bronchitis. Chronic infection by respiratory pathogens, including Pseudomonas aeruginosa, exacerbates cyclical proinflammatory responses and mucus hypersecretion. P. aeruginosa and its virulence factor pyocyanin contribute to these pathologies by inhibiting FOXA2, a key transcriptional regulator of mucus homeostasis, through activation of antagonistic signaling pathways EGFR-AKT/ERK1/2 and IL-4/IL-13-STAT6-SPDEF. However, FOXA2-targeted therapy has not been previously explored. Here, we examined the feasibility of repurposing the incretin mimetic Exendin-4 to restore FOXA2-mediated airway mucus homeostasis. We have found that Exendin-4 restored FOXA2 expression, attenuated mucin production in COPD and CF-diseased airway cells, and reduced mucin and P. aeruginosa burden in mouse lungs. Mechanistically, Exendin-4 activated the GLP1R-PKA-PPAR-γ-dependent phosphatases PTEN and PTP1B phosphatases, which inhibited key kinases within both EGFR and STAT6 signaling cascades. Our results may lead to the repurposing of Exendin-4 and other incretin mimetics to restore FOXA2 function and ultimately regulate excessive mucus in diseased airways.
Keywords: COPD, Cystic Fibrosis, Incretin mimetics, Exenatide, FOXA2, Mucus, Pseudomonas aeruginosa, STAT6, EGFR
Mucociliary clearance is a vital defense against inhaled irritants and microbes1–4. However, excessive mucus is detrimental to patients with bronchiectasis diseases, including COPD, CF and chronic bronchitis. These airways exhibit permanent changes to bronchi including chronic inflammation and infection, goblet cell hyperplasia and metaplasia, and mucus hypersecretion. In CF, mutated forms of CFTR channel cause dysregulation of Cl- secretion and hyperabsorption of Na+ and H2O in airways, leading to depletion of airway surface liquid, thickening of mucus, malfunctioning of mucociliary escalator, and debilitated microbial clearance5,6. In COPD, environmental pollutants and cigarette smoke induce inflammation and oxidative damage in both airways and alveolar spaces, which contribute to mucus hypersecretion and emphysema, respectively7,8. In both of these diseased lungs, accumulated mucus creates a niche environment favoring chronic microbial infection, among which, Pseudomonas aeruginosa (PA) is prevalent.
PA thrives in the mucus-rich airways by switching to the biofilm mode of growth, becoming more resistant to antibiotics and phagocytic clearance. Acquisition of PA is associated with increased episodes of acute exacerbation and excessive mucus production, especially among advanced CF and COPD patients receiving antibiotic therapy or requiring mechanical ventilation9–13. Specifically, PA expresses several virulence factors, including pyocyanin14–19, lipopolysaccharides (LPS)17,20, flagellin17,21, alginate17,22 and proteases23 that induce mucus hypersecretion. In particular, pyocyanin is overproduced by the hypervirulent epidemic PA strains24 and recovered at 0.1 mM concentrations from COPD and CF airways (27.3 and 16.4 μg/ml sputum, respectively)25,26. Significantly, pyocyanin is critical for both acute and chronic lung infection14,27, and its concentrations within sputa negatively correlate with the lung function in CF patients25. Additionally, at the concentrations found in diseased airways, pyocyanin induces bronchoconstriction28, disrupts mucociliary transport25, and reduces mucus velocity29, and mucociliary clearance30.
Previously, we have shown that PA infection and chronic exposure to pyocyanin induce goblet cell hyperplasia and metaplasia and mucus hypersecretion by inhibiting the forkhead box protein A2 (FOXA2)15–17,19, a key transcriptional regulator of the airway mucus homeostasis31,32. FOXA2 is inhibited by both IL-4/IL-13-STAT6-SPDEF and EGFR-AKT/ERK1/2 signaling14,15,19,31–34, and by ROS/RNS-mediated posttranslational modifications16. During exposure to allergens, STAT6-SPDEF activates the T-helper 2 (Th2) response and goblet cell differentiation35–37 whereas EGFR modulates cell metabolism, survival, transcription, and differentiation38–40. Interestingly, activated STAT6 and EGFR convergently inhibit the expression of FOXA2, which relieves SPDEF to upregulate the transcription of mucin genes14,15,19,31–33,40. Selective deletion of the FOXA2 gene in mouse airways causes goblet cell hyperplasia and metaplasia, excessive mucus, emphysema and neutrophilic infiltration31, pathological features similar to mouse lungs chronically exposed to pyocyanin14–17.
Exendin-4 is an analog of glucagon peptide-1 (GLP-1) belonging to a group of incretin mimetics approved by FDA for the treatment of type 2 diabetes mellitus. Exendin-4 binds to the G protein-coupled glucagon-like peptide 1 receptor (GLP1R) and activates several pathways including MAPK, B-RAF, cAMP, PKA and TORC2 to induce exocytosis, biosynthesis of insulin, as well as beta cell proliferation and neogenesis41–42. In contrast to GLP-1, Exendin-4 is resistant to degradation by dipeptidyl peptidase-4 (DPP-4). Based on an initial report that Exendin-4 increases expression and binding of FOXA2 to the promoters of its regulated genes and induces differentiation of pancreatic duct cells into endocrine cells43, we investigated the hypothesis that Exendin-4 could augment FOXA2 expression and restore airway mucus homeostasis, resulting in reduced PA burden.
Results
FOXA2 expression is depleted in the COPD airway epithelium.
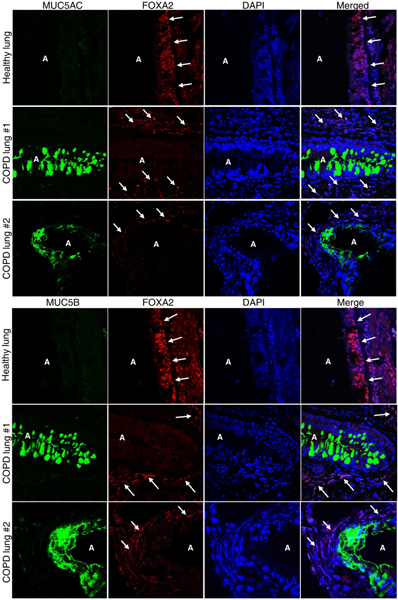
A limited number of prior studies have shown that FOXA2 expression is depleted in human airways of infants with bronchopulmonary dysplasia31 and bronchiectasis31, and in airway epithelium of asthma patients32. Here, immunohistochemical (IHC) staining revealed that FOXA2 expression was severely depleted in surface airway epithelial cells expressing abundant MUC5AC and MUC5B in COPD patients with stable disease at both Gold stage IV (Fig. 1, COPD-lung #1) and Gold stage III (Fig. 1, COPD-lung #2, Fig. S1 COPD-lung #3). In contrast, airway epithelium from control healthy lungs maintained FOXA2 expression in both surface and basal airway epithelia without significant MUC5AC and MUC5B expression. Additionally, FOXA2 expression was retained in COPD airway basal epithelial cells not expressing mucins (Fig. 1; Fig. S1).
Fig. 1.
FOXA2 expression is depleted in area of human COPD airways overexpressing mucus. Lung sections of de-identified healthy donors and COPD patients at Gold stage IV (#1) and Gold stage III (#2) with stable disease who underwent lobectomy were double-stained with primary antibodies against FOXA2 and mucus secreting cell-specific markers MUC5AC and MUC5B. Nuclei in airway cells were stained with DAPI (blue). Labeled airway sections were visualized by secondary antibodies conjugated to Alexa Fluor 594 (red, FOXA2) and to Alexa Fluor 488 (cyan-green, MUC5AC and MUC5B), respectively. Original magnification: 100x. ‘A’ indicate open airway lumens. White arrows indicate airway surface epithelial cells in healthy control lungs and airway basal epithelial cells in COPD lungs that still express FOXA2, which is absent in the surface epithelial cells expressing both MUC5AC and MUC5B in COPD lungs. Two representative healthy sections and four representative COPD sections are shown. Analysis of an additional lung sample is presented in Fig. S1.
PA infection depletes airway FOXA2 and induces mucin overexpression, and is dependent on its ability to produce pyocyanin.
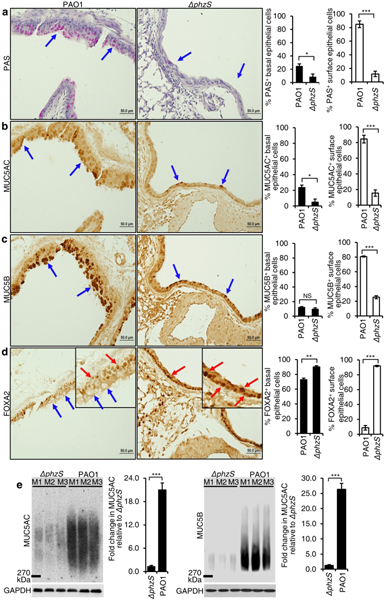
Previously, we had shown that chronic bronchitis infection by PA16,44 and chronic exposure to pyocyanin14–17 induced goblet cell hyperplasia and metaplasia and mucus hypersecretion in mouse airways. Additionally, the pyocyanin-deficient mutant ΔphzS induced less mucin expression when compared to the parental wild-type strain PAO116. However, the impact of PA infection on FOXA2 expression has not been determined. All mice infected with PAO1 (n=8) developed robust goblet cell hyperplasia and metaplasia with abundant mucin expression indicated by positive staining with Periodic acid-Schiff (PAS), MUC5AC and MUC5B (Fig. 2, a-c, blue arrows). In contrast, ΔphzS (n=8) induced low levels of mucins in only 2 mice, and was absent in the remaining 6 mice. PAO1 infection severely depleted FOXA2 expression in the airway surface epithelial goblet cells (Fig. 2d, blue arrows), which remained highly expressed in the basal epithelial cells. In contrast, FOXA2 expression was retained in both surface and basal epithelial cells of ΔphzS-infected airways (Fig. 2d, red arrows). Furthermore, elevated expression of secreted MUC5AC and MUC5B was confirmed in the bronchoalveolar lavage fluid (BALF) of PAO1-infected mice (n = 3) when compared to ΔphzS-infected mice (n = 3) (Fig. 2e). These results indicate that pyocyanin is a major inducer of goblet cell hyperplasia and metaplasia and mucus hypersecretion in PA-infected airways through inhibition of FOXA2.
Fig. 2.
Pseudomonas aeruginosa (PA) infection depletes the expression of FOXA2 in a pyocyanin-dependent manner. Six-week old C57BL6 mice (n = 8) were intranasally-infected in chronic bronchitis model with the wild-type PA strain PAO1 or the pyocyanin-deficient mutant ΔphzS, (1 × 106 CFU, Day 1, 3, 5, 7, 1-week duration). Lung sections were stained with Periodic acid-Schiff (PAS) (a, blue arrows), or by immunohistochemistry (IHC) by using antibodies against MUC5AC (b, blue arrows), MUC5B (c, blue arrows), and FOXA2 (d, blue arrows) respectively. Red arrows in d show FOXA2 expression in basal epithelial cells of PAO1-infected airways, and in both basal and surface epithelial cells of Δphz-Sinfected airways. IHC staining was visualized with the M.O.M Immunodetection Kits (FOXA2) and the VECTASTAIN ABC Kits (MUC5AC and MUC5B). a-d, % of basal and surface epithelial cells staining positive for PAS, MUC5AC, MUC5B and FOXA2 were compared by the Student’s t-test. e The expression of secreted MUC5AC and MUC5B in the mouse lungs infected by PAO1 versus ΔphzS. BALF of each mouse was individually analyzed by densitometry, and the mean value of the ΔphzS was then used as the baseline to measure mucins levels induced by the wild-type strain PAO1. All data from a-e are presented as mean ± s.e.m.; *p < 0.05, **p < 0.01, ***p < 0.001. NS: Not significant.
Exendin-4 restores the expression of FOXA2 and reduces mucin expression in pyocyanin-exposed lungs and attenuates PA burden.
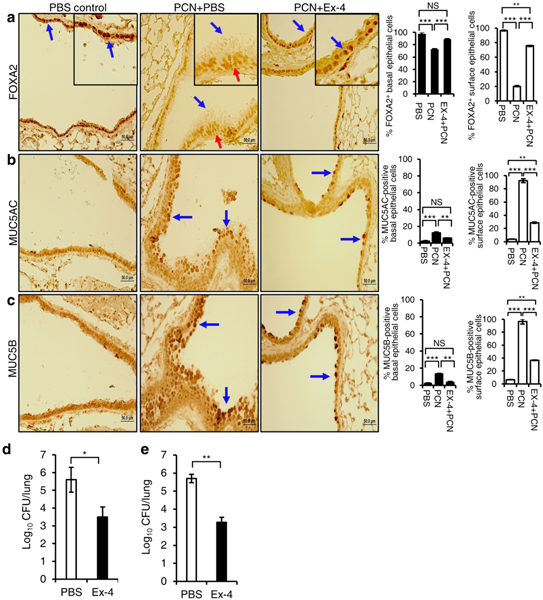
Next, we examined whether Exendin-4 could augment FOXA2 expression and restore mucus homeostasis, which could improve the mucociliary clearance of PA in a mouse model of chronic bronchitis infection16,44. The expression of FOXA2, MUC5AC and MUC5B was not altered in mouse airways exposed to sterile PBS (Fig. 3a–c, blue arrows). In contrast, mouse airways exposed to pyocyanin and subcutaneously treated with PBS showed extensive FOXA2 depletion in the surface epithelial cells (Fig. 3a, PCN+PBS, blue arrows) but not in basal epithelial cells (Fig. 3a, red arrows), with elevated MUC5AC and MUC5B expressions (Fig. 3b, c, blue arrows). Importantly, in the pyocyanin-exposed airways treated with Exendin-4, the expression of FOXA2 was restored (Fig. 3a, blue arrows), accompanied by reduced the expression of MUC5AC (Fig. 3b, blue arrows) and MUC5B (Fig. 3c, blue arrows). In addition, simultaneous treatment of pyocyanin-exposed mice with Exendin-4 reduced the bacterial burden in mouse lungs infected with PA strain PAO1 by 2.1 logs (Fig. 3d). Similarly, Exendin-4 administered post-infection also attenuated PAO1 burden by 2.4 logs in mouse lungs preexposed to 3-week pyocyanin exposure (Fig. 3e). These results indicate that restoration of FOXA2 expression by Exendin-4 attenuates excessive mucus production, which improves mucociliary clearance of PA in diseased airways.
Fig. 3.
Exendin-4 (Ex-4) restores FOXA2 expression and reduces mucins induction by pyocyanin (PCN) and improves Pseudomonas aeruginosa (PA) clearance in mouse lungs. C57BL6 mice (n = 8) were intranasally-exposed to PCN (25 μg in 50 μl, once daily) and subcutaneously treated with Ex-4 (100 μg/kg, in 50 μl, once daily) or PBS vehicle control (in 50 μl, once daily), for 3 weeks. Mice exposed to sterile PBS (50 μl daily) were used as baseline control. Lung sections were stained with antibodies against FOXA2 (a), MUC5AC (b) and MUC5B (c), respectively, and visualized by the M.O.M Immunodetection Kits (FOXA2) and the VECTASTAIN ABC Kits (MUC5AC and MUC5B). Ten representative lung sections were analyzed by using the ImageJ software. a-c The % of basal and surface epithelial cells stained positive for FOXA2, MUC5AC and MUC5B were compared by the one-way ANOVA analysis with Fisher’s exact test. d Simultaneous treatment with Ex-4 during PCN exposure improves the clearance of PA. C57BL6 mice (n=8 per cohort) were intranasally exposed to PCN (25 μg, once daily) for 3 weeks. Mouse cohorts were treated subcutaneously with Ex-4 (100 μg/kg daily, 6 hours after each PCN instillation) or PBS. At 48 hours after the completion of PCN exposure/Ex-4 treatment, mice were infected with PAO1 (1 × 106 CFU, Day 1, 3, 5). Bacterial burden was analyzed on Day 7. Data are presented as mean ± s.e.m.; *p < 0.05 by the Student’s t-test. e Ex-4 improves the clearance of bacteria when administered post-PCN exposure and post-PA infection. C57BL6 mice (n=8 per cohort) were intranasally exposed to PCN for 3 weeks as described above except no Ex-4 was administered. At the end of PCN exposure, mice were infected with 1 × 106 CFU of PAO1 on Day 1, 3, and 5. On Days 6, 7 and 8, mice were treated with Ex-4 (100 μg/kg, subcutaneous) or PBS, once daily. PA burden in the infected mice was analyzed on Day 9 as described above. Data are presented as mean ± s.e.m.; **p < 0.01 by the Student’s t-test.
Restoration of FOXA2 by Exendin-4 inhibits the induction of airway mucins by pyocyanin in diseased human primary bronchial epithelial cells.
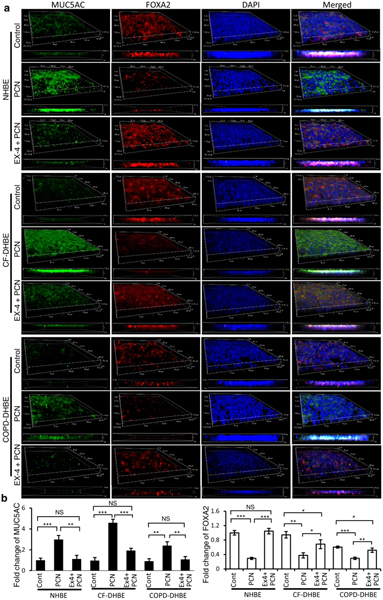
As discussed above, excessive mucus is detrimental to patients with bronchiectasis diseases. Therefore, we evaluated if Exendin-4 could restore the expression of FOXA2 to reduce mucin production in pyocyanin-exposed normal (NHBE), CF (CF-DHBE), and COPD (COPD-DHBE) primary bronchial epithelial cells cultured at the air-liquid interface (ALI), respectively (Fig. 4a, b). Unchallenged NHBE, CF-DHBE and COPD-DHBE cell expressed only basal levels of mucins. In contrast, pyocyanin increased the expression of MUC5AC and MUC5B in NHBE cells (Fig. 4a, b; Fig. S2a, b). Significantly, pyocyanin also induced MUC5AC and MUC5B expression in both CF-DHBE and COPD-DHBE cells (Fig. 4a, b; Fig. S2a, b). FOXA2 expression was depleted by 50%−70% in pyocyanin-exposed NHBE and CF-DHBE and COPD-DHBE cells, but significantly, was restored by Exendin-4 treatment. Restoration of FOXA2 was associated with significant attenuation of MUC5AC and MUC5B expression in normal and diseased airway epithelial cells (Fig. 4a, b; Fig. S2a, b).
Fig. 4.
Ex-4 restores the expression of FOXA2 to attenuate excessive mucins in pyocyanin-exposed normal (NHBE) and diseased (CF-DHBE and COPD-DHBE) primary airway epithelial cells. Two-week old, partially polarized air-liquid interface (ALI) cultures of airway cells were potentiated to differentiate into mucin secreting goblet cells by exposing to PCN (5 μg/ml) for 24 hours in presence or absence of Ex-4 (1 μg/ml). a MUC5AC and FOXA2 expression were visualized by immunofluorescence labeling using a confocal microscope, and were processed into 3D-models and Z-stack images. Experiments were performed in triplicates independently three times. Representative images from one replicate is shown. b Intensity of MUC5AC and FOXA2 expression was determined using the Z-stack images. Densitometry analyses were normalized against airway cells treated with PBS control (Cont), and mean ± s.e.m from three experiments are shown. MUC5AC and FOXA2 expression were compared by Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001. NS: Not significant. MUC5B results are presented in the Fig. S2.
Because FOXA2 is a transcriptional factor, we examined whether restoration of FOXA2 expression by Exendin-4 could inhibit the transcription of MUC5AC and MUC5B genes in pyocyanin-exposed NHBE, CF-DHBE, COPD-DHBE cells and small airway primary cells from COPD lungs (COPD-DSAE). Real-time qRT-PCR analysis indicated that pyocyanin exposure increased MUC5AC and MUC5B transcripts in both normal and diseased human airway cells. Treatment of airway cells with Exendin-4 (1 μM) significantly reduced the expression of both mucin genes (Fig. S3, a–c).
Next, we examined whether restoration of FOXA2 expression by Exendin-4 inhibited excessive mucin expression. Only basal levels of intracellular mucins (total cell lysates, Fig. S4, a–c) and secreted mucins (culture supernatants, Fig. S5, a–c) were detected in NHBE, CF-DHBE, and COPD-DHBE and COPD-DSAE cells exposed to PBS vehicle control and Exendin-4 alone. In contrast, pyocyanin induced a robust increase of intracellular and secreted MUC5AC and MUC5B, by 8.0-fold and 4.3-fold respectively in NHBE cells (Fig. S4a, Fig. S5a); by 4.9-fold and 3.4-fold in CF-DHBE cells (Fig. S4b, Fig.S5b), and by 5.5-fold and 3.4-fold increase of MUC5AC in both COPD-DHBE and COPD-DSAE cells (Fig. S4c, Fig. S5c), respectively. Importantly, Exendin-4 significantly decreased the expression of intracellular and secreted MUC5AC and MUC5B in all airway cells exposed to pyocyanin (Fig. S4, a–c; Fig. S5, a–c). These results were further validated using the immortalized human lung mucoepidermoid carcinoma NCI-H292 cell line where Exendin-4 robustly attenuated the amount of intracellular mucins induced by pyocyanin (Fig. S6a, b). Collectively, these results indicate that Exendin-4 inhibits mucin induction by pyocyanin in both normal and diseased airway cells.
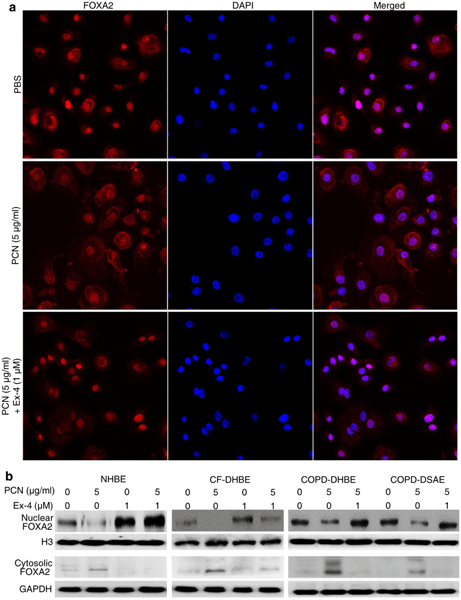
Exendin-4 restores nuclear localization and expression of FOXA2 in diseased human primary bronchial epithelial cells.
As a transcription factor, the active form of FOXA2 resides within nuclei. When FOXA2 is phosphorylated at the threonine residue T156, nuclear FOXA2 translocates to the cytoplasm for degradation45–47. In NHBE cells exposed to PBS, most FOXA2 remained in the nuclei (Fig. 5a; Fig. S7a) showing proper localization for function. In contrast, in pyocyanin-exposed NHBE cells, FOXA2 was distributed throughout the cytoplasm for degradation. However, pyocyanin-exposed NHBE cells treated with Exendin-4 showed restored nuclear localization of FOXA2 (Fig. 5a; Fig. S7a). Western blotting analysis confirmed that in PBS exposed NHBE cells, majority of FOXA2 resides within nuclear fraction, but was severely depleted in the nuclear fraction from cells exposed to pyocyanin (Fig. 5b; Fig.S7b). Importantly, Exendin-4 restored the nuclear FOXA2 expression, accompanied by reduction of cytoplasmic FOXA2 (Fig. 5b; Fig. S7b). Similarly, Exendin-4 increased the expression of nuclear FOXA2 in pyocyanin-exposed NHBE cells, CF-DHBE cells, COPD-DHBE and COPD-DSAE cells (Fig. 5b; Fig. S7c, d). Similar results were obtained in the NCI-H292 cells (Fig. S7e, f). Collectively, these results indicate that Exendin-4 effectively restores nuclear FOXA2 expression in both normal and diseased airway epithelial cells exposed to pyocyanin.
Fig. 5.
Exendin-4 (Ex-4) restores the expression of nuclear FOXA2 in primary airway epithelial cells from normal and diseased lungs. a Immunofluorescence analysis of FOXA2 in normal human primary airway epithelial (NHBE) cells exposed to pyocyanin (PCN). NHBE cells were incubated with Ex-4 for 1 hour before the addition of PCN. After 24 hours, NHBE cells were labeled with anti-FOXA2 antibody, and visualized using a secondary antibody conjugated to Alexa Fluor 594 (red). Nuclei were stained with DAPI (blue). Experiments were independently performed three times. Representative images from one experiment is shown. Quantification of fluorescence signal and statistical analysis are presented in the Fig. S7a. b Ex-4 restores the expression of FOXA2 in NHBE cells and in primary airway epithelial cells derived from cystic fibrosis (CF-DHBE), and large and small airways of chronic obstructive pulmonary disease (COPD-DHBE and COPD-DSAE) lungs. Airway cells were pretreated with Ex-4 for 1 hour prior to addition of PCN for 24 hours. Nuclear and cytoplasmic extracts were analyzed for expression of FOXA2. H3 was used as loading control. Experiments were independently performed three times. Representative Western blots from one experiment are shown. Densitometry analysis of changes in the expression of nuclear and cytoplasmic FOXA2 were compared by using Student’s t-test, and presented in the Fig. S7b–d.
Exendin-4 inhibits EGFR-AKT/ERK1/2 and STAT6-SPDEF signaling pathways that antagonize FOXA2
Previously, we have demonstrated that pyocyanin activates EGFR-MEK1/2-ERK1/215,19, EGFR-PI3K-AKT,19,48 and IL-13/IL-4-STAT6-SPDEF signaling pathways14,15,19, which convergently inhibit FOXA2 expression. Therefore, we examined whether Exendin-4 interfered with the induction of these anti-FOXA2 kinase cascades. Pyocyanin increased the levels of pEGFR, pERK1/2 and pAKT, as well as pSTAT6 and SPDEF in NHBE, CF-DHBE, and COPD-DHBE and COPD-DSAE cells while Exendin-4 treatment significantly decreased these expressions (Fig. 6). Similar results were observed in the NCI-H292 cells (Fig. S8a, b). These results indicate that Exendin-4 restores the expression of FOXA2 and mucus homeostasis by inhibiting the phosphorylation and activation of EGFR-MEK1/2-ERK1/2, EGFR-PI3K-AKT and STAT6-SPDEF anti-FOXA2 kinase cascades.
Fig. 6.
Exendin-4 (Ex-4) inactivates both EGFR and STAT6 signaling pathways that antagonize FOXA2. Cytoplasmic and nuclear proteins of NHBE, CF-DHBE and COPD-DHBE cells described in Figure 5B were probed with normal and phospho-specific antibodies. Expression of total EGFR, pEGFR, total AKT, pAKT, total ERK1/2 or pERK1/2 in pyocyanin (PCN) exposed NHBE cells in the presence or absence of Ex-4. Expression of total STAT6, p-STAT6 and SPDEF in PCN-exposed NHBE cells in the presence or absence of Ex-4. GADPH and H3 were used for loading control for cytoplasmic and nuclear proteins, respectively. Experiments were independently performed three times. Representative Western blots from one experiment are shown.
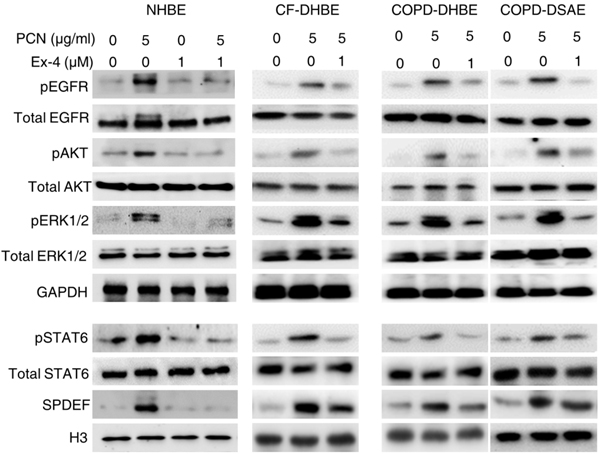
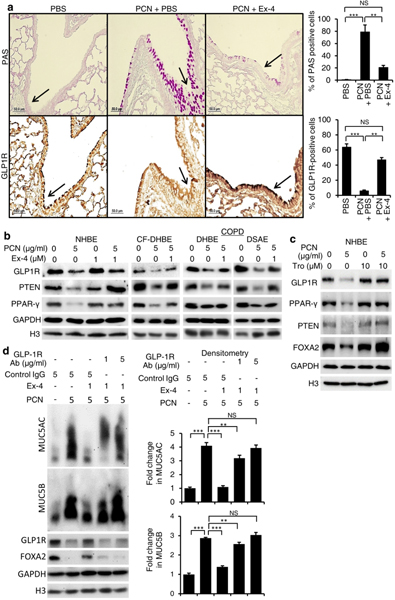
Exendin-4 restores the expression of FOXA2 through the GLP1R-PPAR-γ-PTEN/PTP1B axis
Recently, Exendin-4 was shown to reduce mortality and improve lung function in a mouse model of obstructive lung disease49. However, the effect of Exendin-4 on FOXA2 was not investigated. Because Exendin-4 induces dephosphorylation of key kinases within both EGFR and STAT6 cascades (Fig. 6; Fig. S8), we hypothesize that binding of Exendin-4 to GLP1R upregulates downstream phosphatases that dephosphorylate pEGFR, pERK1/2, pAKT and pSTAT6. Our hypothesis is supported by evidence derived from other systems showing that both Exendin-4 and GLP1 could induce the expression of PPAR-γ50,51, which positively regulates the expression of Phosphatase and tensin homolog (PTEN) and Protein tyrosine phosphatase 1B (PTP1B). PTEN dephosphorylates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) important for phosphorylation of AKT, pEGFR, pJAK-pSTAT and pERK1/2, whereas PTP1B dephosphorylates both pSTAT6 and pEGFR52–61. We examined whether Exendin-4 could augment the expression of GLP1R-PPAR-γ-PTEN/PTP1B-FOXA2 during pyocyanin challenge. IHC analysis indicated GLP1R was strongly expressed in surface epithelium of normal mouse airways (Fig. 7a, PBS, black arrow). In contrast, mouse airways exposed to pyocyanin and treated with PBS (PCN + PBS) developed goblet cell hyperplasia and metaplasia with excessive mucin production (PAS stained cells) and severe depletion of GLP1R (Fig. 7a, black arrow). Importantly, Exendin-4 restored the expression of GLP1R and inhibited excessive mucin expression in the mouse airways exposed to pyocyanin (Fig. 7a). The IHC results were confirmed by Western blotting analyses of both healthy and diseased primary airway epithelial cells where pyocyanin severely reduced the expression of GLP1R, as well as PPAR-γ and PTEN (Fig. 7b). In contrast, Exendin-4, as well as the PPAR-γ agonist, Troglitazone, restored the expression of GLP1R, PPAR-γ, PTEN and FOXA2 in both normal and diseased airway cells during pyocyanin challenge (Fig. 7b, c). Similarly, Exendin-4 restored the expression of GLP1R, PPAR-γ and PTEN in pyocyanin-exposed NCI-H292 cells (Fig. S9, a, b).
Fig. 7.
Exendin-4 (Ex-4) restores GLP1R-PPAR-γ-PTEN signaling disrupted by pyocyanin. a Mouse lung sections exposed to pyocyanin (PCN) for 3 weeks and treated with Exendin-4 or PBS vehicle control (from Fig. 3) were stained with Periodic acid-Schiff (PAS) or by immunohistochemistry (IHC) with anti-GLP1R antibody. The number of PAS and GLP1R-positive cells were compared between various treatments by using the one-way ANOVA analysis with Fisher’s exact test. **p < 0.01, ***p < 0.001. NS: Not significant. b Western blot analysis of GLP1R, PPAR-γ, PTEN and FOXA2 expression in cell lysate from NHBE, CF-DHBE and COPD-DHBE cells preexposed to Ex-4 for 1 hour before the addition of PCN (5 μg/ml). c Western blot analysis of GLP1R, PPAR-γ, PTEN and FOXA2 in cell lysate from NHBE cells pretreated with Troglitazone (Tro) for 1 hour before the addition of PCN (5 μg/ml) for 24 hours. d Competitive inhibition of GLP1R binding abolishes the protective role of Exendin-4 in NCI-H292 cells. The cells were coincubated for 2 hours with Exendine-4 together with either anti-GLP1R antibody or control mouse IgG, and then exposed to 5 μg/ml PCN for 24 hours. Cytosolic and nuclear proteins were prepared to detect the expression of MUC5AC, MUC5B, GLP-1R and FOXA2. Densitometric analysis indicated the intensity of MUC5AC and MUC5B expressions. GADPH was used as a loading control for GLP1R and PTEN. H3 was used as loading control for PPAR-γ and FOXA2. Experiments were independently performed three times. Representative Western blots from one experiment are shown. MUC5AC and MUC5B expression were compared by Student’s t-test. **p < 0.01, ***p < 0.001. NS: Not significant.
To definitively demonstrate that Exendin-4 acts through GLP1R, we performed competitive binding assay by adding GLP-1R specific antibody together with Exendin-4 in NCI-H292 cells. It was previously reported that specific binding of antibody to the extracellular domain (aa 24–145) of GLP-1R blocks the binding of GLP-1 to GLP1R62. We used a selective anti-GLP1R antibody that recognizes similar epitope (aa 94–145) to conduct competitive binding assay. Significantly, anti-GLP-1R antibody abrogated the ability of Exendin-4 to restore FOXA2 expression and to inhibit MUC5AC and MUC5B expression (Fig. 7d). Collectively, these results indicate that Exenin-4 restores FOXA2 function and attenuates excessive mucins by activating GLP-1R-PPAR-γ-PTEN signaling.
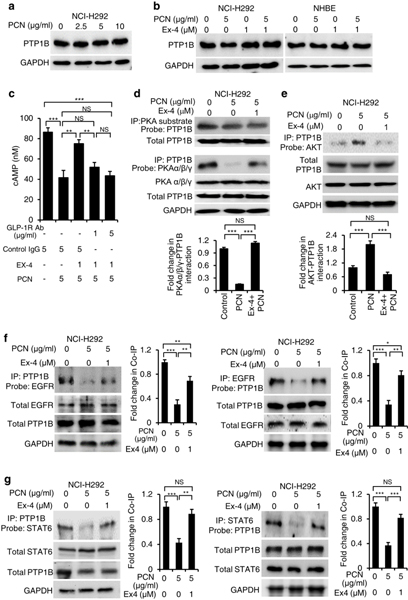
Exendin-4 increase the physical interactions between PTP1B and its target kinases
Next, we examined whether pyocyanin alters the expression of PTP1B, and if the inhibition can be rescued by Exendin-4. Western blot analyses showed that neither pyocyanin nor Exendin-4 altered the expression of PTP1B appreciably in both NCI-H292 and NHBE cells (Fig. 8a, b). Previously, it was shown in other systems that the PTP1B activity can be upregulated by cAMP-activated protein kinase A (PKA)63, but inhibited by AKT64. Because GLP1R regulates the intracellular cAMP levels and PKA activity65, 66, we examined whether Exendin-4 treatment increased the cAMP levels and PKA activity to activate PTP1B, which in turn, could dephosphorylate kinases in both EGFR and STAT6 signaling pathways. Additionally, we examined if cAMP levels and PKA induction was dependent on GLP1R, by using the antibody-dependent blocking. In NHBE cells, pyocyanin reduced cAMP levels, which was restored by Exendin-4 (Fig. 8c). Competitive blocking of GLP1R with antibody abolished the ability of Exendin-4 to restore intracellular cAMP (Fig. 8c), indicating that regulatory effects of Exendin-4 is triggered through the GLP-1R signaling (Fig. 7d; Fig. 8c). In addition, we used phospho-PKA substrate antibody to detect proteins that interacted with the catalytic subunits of PKA. Due to the requirement of large quantity proteins for co-immunoprecipitation assays, protein-protein interactions were analyzed in the NCI-H292 cells. Western blot analysis indicated that immunoprecipitation by phospho-PKA substrate antibody pulled down PTP1B (Fig. 8d). The result showed that, Exendin-4 significantly restored the binding of PKAα/β/γ catalytic subunits to PTP1B compared to pyocyanin treatment alone (Fig. 8d). In contrast, Exendin-4 downregulated the interaction between AKT and PTP1B, which was elevated by pyocyanin (Fig. 8e).
Fig. 8.
Exendin-4 (Ex-4) enhances the interaction between PTP1B-EGFR and PTP1B-STAT6. Co-immunoprecipitation (co-IP) analyses were performed using total cell lysates (2–5 mg) from NCI-H292 cells exposed to pyocyanin (PCN) with or without Ex-4 treatment. a PTP1B expression in NCI-H292 cells exposed to PCN in a dose-dependent manner. Protein expression was detected by anti-PTP1B antibody. b PTP1B expression in NCI-H292 and NHBE cells treated with PCN in the presence or absence of Ex-4. c Ex-4 restores the cAMP levels in NHBE cells exposed to PCN through GLP1R. cAMP production between various treatments were compared by Student’s t-test. Data represent mean ± s.e.m from three experiments. *p < 0.05, **p < 0.01, ***p < 0.001. NS: Not significant. d Ex-4 restores the interactions between catalytic subunits (PKAα/β/γ) of protein kinase A (PKA) with PTP1B during PCN exposure. PKA interacting proteins were pulled down using the PKA substrate and probed with antibody against PTP1B to verify the presence of the protein. e Ex-4 inhibits the interaction between AKT and PTP1B. The AKT-PTP1B complex was pulled down using the anti PTP1B antibody and probed with antibody against AKT. Total PTP1B was used as loading control. Densitometry analyses in d and e represent mean ± s.e.m from three experiments, and were compared by Student’s t-test. ***p < 0.001. NS: Not significant. (f, g) Ex-4 promotes the interaction between PTP1B-EGFR and PTP1B-STAT6. PTP1B was IP by using the anti-PTP1B antibody and analyzed by protein blotting with anti-EGFR or anti-STAT6 antibodies. Conversely, STAT6 and EGFR were IP by anti-STAT6 and anti-EGFR antibody respectively, and analyzed by Western blotting with anti-PTP1B antibody. Experiments were independently repeated three times. Representative Western blots are shown. Protein expression was examined by ImageJ and analyzed for statistical significance by Student’s t-test. Mean ± s.e.m from three experiments are shown. ***p < 0.001, **p < 0.01, *p < 0.1, NS: Not significant.
Next, we used co-immunoprecipitation to examine whether pyocyanin and Exendin-4 could modulate PTP1B-EGFR and PTP1B-STAT6 interactions in the NCI-H292 cells. Pyocyanin treatment reduced the protein-protein interaction between PTP1B-EGFR (Fig. 8f) and between PTP1B-STAT6 (Fig. 8g). Notably, Exendin-4 restored the interaction between PTP1B-EGFR (Fig. 8f) as well as PTP1B-STAT6 (Fig. 8g). Collectively, these results indicate that Exendin-4 restores FOXA2-mediated airway mucus homeostasis by augmenting the GLP1R-dependent PTEN and PTP1B to dephosphorylate key kinases within pEGFR-pAKT/pERK1/2 and pSTAT6-SPDEF signaling cascades.
The PPAR-γ agonist Troglitazone reduces the levels of phosphorylated kinases in EGFR and STAT6 pathways
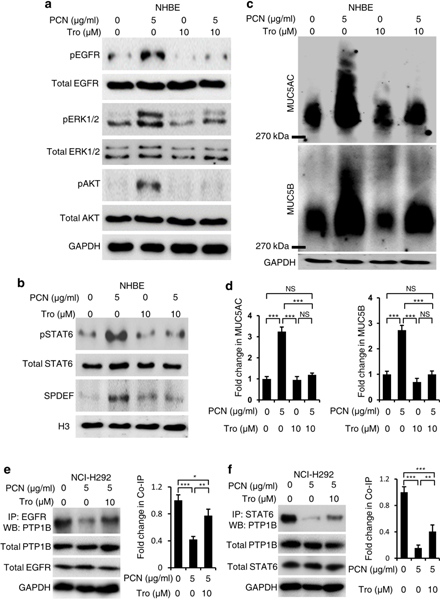
To confirm that Exendin-4-activated PTEN and PTP1B will inhibit key kinases within both EGFR and STAT6 pathways, we examined if the PPAR-γ agonist Troglitazone could modulate the levels of pEGFR, pERK1/2, pAKT and pSTAT6. Troglitazone inhibited induction of pEGFR, pERK1/2 and pAKT (Fig. 9a) and pSTAT6-SPDEF (Fig. 9b) by pyocyanin in NHBE cells. Inhibition of pEGFR, pERK1/2 and pAKT and pSTAT6-SPDEF by Troglitazone were accompanied by significant reductions in MUC5AC and MUC5B expression (Fig. 9c, d). Similar results were obtained in the NCI-H292 cells (Fig. S10, a–d). Importantly, Troglitazone also restored physical interactions between PTP1B-EGFR (Fig. 9e) and PTP1B-STAT6 (Fig. 9f) in the NCI-H292 cells. Together, these results further validate the findings that Exendin-4 activates GLP1R-dependent PTEN and PTP1B that dephosphorylate key kinases within both pro-goblet cell hyperplasia and metaplasia, EGFR, and STAT6 anti-FOXA2 cascades, leading to restoration of FOXA2 expression and mucus homeostasis.
Fig. 9.
The PPAR-γ agonist Troglitazone (Tro) restores FOXA2 expression and attenuates mucin overexpression by inhibiting EGFR and STAT6 signaling. Normal primary human bronchial epithelial (NHBE) cells were preexposed to Tro for 1 hour before the addition of pyocyanin (PCN) for 24 hours. a Western blot analysis of EGFR signaling in cell lysate of NHBE cells. Total EGFR, p-EGFR, total AKT, p-AKT, total ERK1/2 and p-ERK1/2 were probed with specific antibodies. b Western blot analysis of STAT6 signaling in cell lysate of NHBE cells probed with antibodies against STAT6, p-STAT6 and SPDEF. c Western blot analysis of MUC5AC and MUC5B in the cytoplasmic extracts of NHBE cells treated with PCN in the presence or absence of Tro. Proteins were separated in agarose-acrylamide gels. d Densitometry analyses of mucin expression shown in c normalized against airway cells treated with PBS vehicle and represent mean ± s.e.m from three experiments, and were compared by Student’s t-test. ***p < 0.001. NS: Not significant. e, f Co-immunoprecipitation (co-IP) analysis between PTP1B-EGFR and between PTP1B-STAT6 in PCN-exposed NCI-H292 cells treated with PPAR-γ agonist Troglitazone (Tro). Cells were pretreated with Tro for 1 h before exposure to PCN for 24 hours. Total cell lysate (5 mg) were used for co-IP. Protein complex were IP by anti-STAT6 and anti-EGFR antibody respectively, and analyzed by protein blotting with anti-PTP1B antibody. Experiments were independently repeated three times, and representative Western blots are shown. Densitometry of co-IP proteins were performed using the ImageJ software and normalized against airway cells treated with PBS vehicle. Mean ± s.e.m from three experiments are shown as analyzed by the Student’s t-test. ***p < 0.001, **p < 0.01, *p < 0.1, NS: Not significant.
Discussion
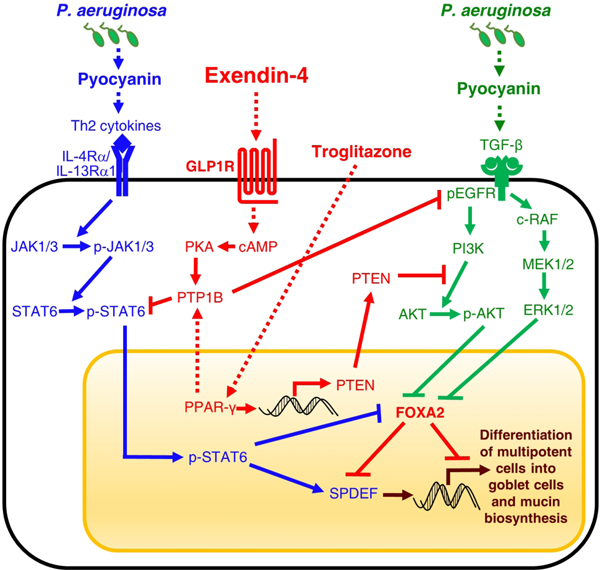
FOXA2 inactivation is an important contributor to mucus hypersecretion in diseased airways including bronchopulmonary dysplasia, bronchiectasis, asthma31,32 and COPD (Figs. 1, 4–6; Figs. S1–S5). In this study, we employ human COPD airway tissues, normal and CF and COPD diseased human primary bronchial epithelial cells, immortalized NCI-H292 cells as well as mouse models to examine the feasibility of repurposing Exendin-4, an incretin mimetic, to restore FOXA2 function and mucus homeostasis. We found that FOXA2 expression is depleted in the airway surface epithelia of COPD lung overexpressing mucus. As summarized in Figure 10, our mechanistic studies reveal that Exendin-4 restores the expression of FOXA2 and mucus homeostasis by activating GLP1R-PKA-PPAR-γ-dependent phosphatases PTEN and PTP1B, which then dephosphorylate and inhibit key kinases within pro- goblet cell hyperplasia and metaplasia signaling pathways EGFR-AKT/ERK1/2 and STAT6-SPDEF.
Fig. 10. Proposed model of restoration of FOXA2-mediated mucus homeostasis by Exendin-4 and Troglitazone.
Exendin-4 binds and activates GLP1R-dependent PKA and PPAR-γ, which in turn, activates PTEN and PTP1B phosphatases. PTEN and PTP1B dephosphorylate key kinases within both STAT6 and EGFR signaling pathways, relieving FOXA2 to inhibit SPDEF and maintain airway mucus homeostasis. Troglitazone directly induces PPAR-γ to activate PTEN and PTP1B, which inhibit STAT6 and EGFR signaling.
Despite its importance in regulating mucus homeostasis, alveolarization, Th1-Th2 immune responses in the lung31–36, and reduced expression in lungs with chronic diseases31,32, no drug is currently available to restore FOXA2 function. A previous study has shown that GLP-1 analogs, liraglutide and Exendin-4, reduce mortality and improve lung function in an acute obstructive lung disease induced mouse model49.
However, this improvement appears to be unrelated to modulation expression of lung surfactants or attenuation of lung inflammation and airway mucus, but rather, through relaxation of airway muscle cells. The authors did not examine the mucus phenotypes in detail, and, more importantly, the role of FOXA2 was not investigated. Our results indicate that Exendin-4 reactivates FOXA2 and restores mucus homeostasis. The reason underlying the discrepancy between these two studies could be caused by the acute lung obstruction model employing a combination of ovalbumin and LPS49 versus our chronic model of pyocyanin exposure. Further investigation is needed to elucidate the exact role of Exendin-4 in combating pathological challenges in different experimental disease models. Our finding that the PPAR-γ agonist Troglitazone restores FOXA2 to attenuate mucin expression is similar to the report showing that activated PPAR-γ inhibits the induction of mucin production by cigarette smoke extract 67. These published studies, together with the current results, suggest the possibility of repurposing Exendin-4 and other incretin mimetics to restore FOXA2 function in chronic lung diseases. As we have demonstrated, improved mucus status will likely lead to better mucociliary function, resulting in better clearance of microbial pathogens. Because Exendin-4 is not an antimicrobial, the selection pressure for emergence of resistance is minimal. Additionally, restoration of FOXA2-mediated mucus homeostasis will improve the penetration and efficacy of aerosolized antibiotics and other drugs in chronically-diseased airways.
Despite many of the aforementioned advantages, prolonged usage of Exendin-4 has been purported to decrease appetite, increase the risks of temporary weight loss and gastrointestinal discomfort, and is inconclusively linked to heart attack, pancreatitis, and thyroid and pancreatic cancers68–70. The appetite suppressing effect of Exendin-4 may render it unsuitable for long term administration to CF patients who have reduced ability to absorb nutrients, which warrants further study of aerosolized Exendin-4 in combination with antibiotics during episodes of exacerbation. Similarly, aerosolization of Exendin-4 with or without antibiotics during acute exacerbation may mitigate systemic toxicity in non-diabetic bronchiectasis patients. Additional FDA-approved incretin mimetics such as liraglutide, lixisenatide, or albiglutide, as well as DPP4 inhibitors (Sitagliptin, Saxagliptin, etc)71 should also be examined for their ability to augment FOXA2 expression.
The roles of GLP1R in normal and chronically-diseased airways are poorly understood. Here, we show that Exendin-4 alleviates the repression GLP1R by pyocyanin in human airway epithelial cells, and in mouse lungs. Two recent studies suggest that GLP1R repression is likely mediated through endoplasmic reticulum (ER) stress72 induced by PA virulence factors73. Our results suggest that Exendin-4 relieves the inhibitory effects of pyocyanin-induced ER stress to restore GLP1R expression, which in turn, activates downstream phosphatases to inhibit both EGFR-AKT/ERK1/2 and STAT6-SPDEF cascades. In this capacity, cAMP, PKA and PPAR-γ seem to be critical in bridging the activation of GLP1R-PKA-PPAR-γ-dependent phosphatases, including PTEN and PTP1B. The importance of PPAR-γ is supported by finding that another one of its agonists, Rosiglitazone, could inhibit cigarette smoke-induced MUC5AC through restoration of nuclear PPAR-γ and PTEN expression67. Similarly, 15-hydroxyeicosatetraenoic acid also restores the expression of PPAR-γ and PTEN to inhibit MUC5AC gene expression in airway epithelial cells induced by phorbol-12-myristate-13-acetate74. Our study, which demonstrates that both Exendin-4 and Troglitazone restore the expression of GLP1R, PPAR-γ and PTEN, and increase the interaction between PTP1B with PKA, STAT6 and EGFR in airway epithelial cells, is consistent with the aforementioned studies showing that PTEN and PTP1B dephosphorylate pSTAT6 and pEGFR to aid in mucus homeostasis through FOXA2.
A previously published study has shown that redox inactivation of PTP1B results in aggravated inflammatory responses in COPD, but oxidized PTP1B could be reactivated by glutathione (GSH), enhancing its phosphatase capacity75. In addition, GSH-deficient hepatocytes have higher levels of oxidized PTP1B with weaker phosphatase activity76. Furthermore, PPAR-γ activation increases the expression of glutamate-cystein ligase (GCL) that prevents oxidative stress in hepatic stellate cells76. GCL catalyzes the first and rate-limiting step during the de novo biosynthesis of GSH involving the ATP-dependent condensation of cysteine and glutamate to form the dipeptide gamma-glutamylcysteine (γ-GCS). Previously, we have shown that upon exposure to pyocyanin, NCI-H292 cells upregulate the expression of nuclear factor (erythroid-derived 2)-like 2 (NRF2) to confer protection against oxidative stress by inducing the expression of γ-GCSh (encoding heavy subunit of GCL), as well as NQO1 (encoding NAD(P)H dehydrogenase [quinone] 1), which detoxifies quinones and their derivatives and protects cells against redox cycling and oxidative stress48. Taken collectively, it is likely that oxidative depletion of GSH by pyocyanin also inactivates PTP1B in airway epithelial cells and reduces interaction between PTP1B and EGFR or STAT6. Therefore, restoration of PTP1B-EGFR and PTP1B-STAT6 interactions by Exendin-4 and Troglitazone is also likely to be mediated by restoration of the GSH levels through the PPAR-γ activation.
In summary, unraveling the efficacy and mechanism of Exendin-4 in restoring FOXA2 function will pave the way for using incretin mimetics as adjunctive therapies against excessive mucus and improving mucociliary clearance of pathogens. Moreover, reduced mucus will improve the penetrance and efficacy of other drugs for COPD, CF and asthma, where excessive mucus is a major contributor to morbidity and mortality.
Methods
Experimental materials and methods, including PA strains and infection, pyocyanin exposure, molecular and cellular methods were as we and others have previously published14–17,19,27,44,48 Detailed methods are provided in the online Supplementary Document.
Supplementary Material
Acknowledgements
The authors want to thank the Lung Center Tissue Bank at Temple University for providing the human lung specimens. This work was supported by the American Lung Association DeSouza Research Award (DS-192835-N), NIH (HL090699, HL142626) and the Research Board of the University of Illinois at Urbana-Champaign to GWL, and by the NIH R01 HL118171 to BK.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Fahy JV & Dickey BF Airway mucus function and dysfunction. N Engl J Med. 363, 2233–2247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J, Rubin BK & Voynow JA Mucins, Mucus, and Goblet Cells. Chest. 154, 169–176 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Symmes BA, Stefanski AL, Magin CM & Evans CM Role of mucins in lung homeostasis: regulated expression and biosynthesis in health and disease. Biochem Soc Trans. 46, 707–719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonser LR, & Erle DJ (2018). Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J Clin Med. 6(12), pii: E112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, & Mall MA Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 367, 537–550 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Hisert KB et al. Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections. Am J Respir Crit Care Med. 195, 1617–1628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polverino E et al. The overlap between bronchiectasis and chronic airways diseases: state of the art and future directions. Eur Respir J. 52(3), pii: 1800328 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Chalmers JD & Hill AT Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol. 55, 27–34 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Viniol C & Vogelmeier CF Exacerbations of COPD. Eur Respir Rev. 27(147), pii: 170103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döring G, Parameswaran IG & Murphy TF Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease. FEMS Microbiol Rev. 35, 124–146 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Murphy TF Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 15,138–142 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Riquelme SA, Ahn D & Prince A. Pseudomonas aeruginosa and Klebsiella pneumoniae adaptation to innate immune clearance mechanisms in the lung. J Innate Immun. 10(5–6), 442–454 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau GW, Hassett DJ & Britigan BE Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 13, 389–397 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Caldwell CC et al. Pseudomonas aeruginosa pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol. 175, 2473–88 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao Y et al. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell Microbiol. 14, 401–415 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Kuang Z, Xu Y, Walling BE & Lau GW Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respir Res. 14, 82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffries JL et al. Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewis X to increase binding to airway epithelial cells. Mucosal Immunol. 9, 1039–1050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rada B, Gardina P, Myers TG & Leto TL Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 4,158–171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W et al. FOXA2 depletion leads to mucus hypersecretion in canine airways with respiratory diseases. Cell Microbiol. 21, e12957 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Li JD et al. Activation of NF-κB via a Src dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci U S A. 95, 5718–5723 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben Mohamed F et al. A crucial role of Flagellin in the induction of airway mucus production by Pseudomonas aeruginosa. PLoS One. 7, e39888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishioka C, Okamoto K, Hassett DJ, De Mello D & Rubin BK Pseudomonas aeruginosa alginate is a potent secretagogue in the isolated ferret trachea. Pediatr Pulmonol. 27,174–179 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Klinger JD, Tandler B, Liedtke CM & Boat TF Proteinases of Pseudomonas aeruginosa evoke mucin release by tracheal epithelium. J Clin Invest. 74,1669–1678 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fothergill JL et al. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol. 7,45 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson R et al. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 56, 2515–2517 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter RC et al. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol. 47, 738–745 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Lau GW, Ran H, Kong F, Hassett DJ & Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun. 72, 4275–4278 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forteza R, Lauredo IT, Burch R & Abraham WM, Extracellular metabolites of Pseudomonas aeruginosa produce bronchoconstriction by different mechanisms. Am J Respir Crit Care Med. 149, 687–693 (1994). [DOI] [PubMed] [Google Scholar]
- 29.Munro NC et al. Effect of pyocyanin and 1- hydroxyphenazine on in vivo tracheal mucus velocity. J Appl Physiol. 67, 316–323 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Dormehl I, Ras G, Taylor G & Hugo N. Effect of Pseudomonas aeruginosa-derived pyocyanin and 1-hydroxyphenazine on pulmonary mucociliary clearance monitored scintigraphically in the baboon model. Nucl Med Biol. 18, 455–459 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Wan H et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 131, 953–964 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Park SW et al. Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am J Respir Crit Care Med. 180, 603–610 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G et al. Foxa2 programs Th2 cell mediated innate immunity in the developing lung. J Immunol. 184,6133–6141(2010). [DOI] [PubMed] [Google Scholar]
- 34.Tang X et al. Foxa2 regulates leukotrienes to inhibit Th2-mediated pulmonary inflammation. Am J Respir Cell Mol Biol. 49, 960–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuperman D, Schofield B, Wills-Karp M & Grusby MJ Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 187, 939–948 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park KS et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 117, 978–988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 119, 2914–2924 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohri K et al. Pseudomonas aeruginosa induces MUC5AC production via epidermal growth factor receptor. Eur Respir J. 20,1263–1270 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Perrais M, Pigny P, Copin MC, Aubert JP & Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 277,32258–32267 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Zhen G et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 36, 244–253 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drucker DJ & Nauck MA The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 368,1696–1705 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Dalle S, Burcelin R & Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell Signal. 25, 570–579 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Pineyro MA, Wang X, Doyle ME & Egan JM Exendin-4 differentiation of a human pancreatic duct cell line into endocrine cells: involvement of PDX-1 and HNF-3β transcription factors. J Cell Physiol. 192, 304–314 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Lugade AA, Bogner PN & Thanavala Y. Murine model of chronic respiratory inflammation. Adv Exp Med Biol. 780, 125–141 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Wolfrum C, Besser,,D., Luca, E. & Stoffel, M. Insulin regulates the activity of forkhead transcription factor HNF-3β/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc Natl Acad Sci USA. 100, 11624–11629 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Meyenn F et al. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab. 17, 436–447 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Pandey AK, Bhardwaj V & Datta M. Tumour necrosis factor-α attenuates insulin action on phosphoenolpyruvate carboxykinase gene expression and gluconeogenesis by altering the cellular localization of Foxa2 in HepG2 cells. FEBS J. 276, 3757–3769 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Xu Y et al. Pseudomonas aeruginosa pyocyanin activates NRF2-ARE-mediated transcriptional response via the ROS-EGFR-PI3K-AKT/MEK-ERK MAP kinase signaling in pulmonary epithelial cells. PLoS One. 8, e72528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viby NE et al. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 154, 4503–4511 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Svegliati-Baroni G et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 31, 1285–1297 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Onuma H et al. The glucagon-like peptide-1 receptor agonist enhances intrinsic peroxisome proliferator-activated receptor-γ activity in endothelial cells. Biochem Biophys Res Commun. 451, 339–344 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R & Yamada KM Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 280, 1614–1617 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Wan X & Helman LJ Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene. 22, 8205–8211 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Wu R, Sun S & Steinberg BM Requirement of STAT3 activation for differentiation of mucosal stratified squamous epithelium. Mol Med. 9, 77–84 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vivanco I et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 11, 555–569 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Vivanco I et al. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc Natl Acad Sci U S A. 107, 6459–6464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinde SR & Maddika S. PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7. Nat Commun. 7, 10689 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akasaki Y et al. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and −9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J Biol Chem. 281, 6165–6174 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Lu X et al. PTP1B is a negative regulator of interleukin-4-induced STAT6 signaling. Blood. 112, 4098–4108 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eden ER, White IJ & Futter CE Down-regulation of epidermal growth factor receptor signaling within multivesicular bodies. Biochem Soc Trans. 37(Pt 1), 173–177 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Monast CS, Furcht CM & Lazzara MJ Computational analysis of the regulation of EGFR by protein tyrosine phosphatases. Biophys J. 102(9),2012–2021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hennen S et al. Structural insight into antibody-mediated antagonism of the glucagon-like peptide-1 receptor. Sci Rep. 6:26236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao J, Malbon CC & Wang HY Insulin stimulates tyrosine phosphorylation and inactivation of protein-tyrosine phosphatase 1B in vivo. J Biol Chem. 276,29520–29525 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Ravichandran LV, Chen H, Li Y & Quon MJ Phosphorylation of PTP1B at Ser(50) by Akt impairs its ability to dephosphorylate the insulin receptor. Mol Endocrinol. 15, 1768–1780 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Zhou J, Doyle ME & Egan JM Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology. 142,1820–1827 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Qian YG et al. LINC01121 Inhibits Cell Apoptosis While Facilitating Proliferation, Migration, and Invasion Though Negative Regulation of the cAMP/PKA Signaling Pathway via GLP1R. Cell Physiol Biochem. 47,1007–1024 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Lee SY et al. Peroxisome proliferator-activated receptor-γ inhibits cigarette smoke solution-induced mucin production in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 291,L84–90 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ & Nauck MA Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes Metab. 19, 336–347 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Tseng CH, Lee KY & Tseng FH An updated review on cancer risk associated with incretin mimetics and enhancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 33, 67–124 (2015). [DOI] [PubMed] [Google Scholar]
- 70.De Heer J & Göke B. Are incretin mimetics and enhancers linked to pancreatitis and malignant transformations in pancreas? Expert Opin Drug Saf. 13, 1469–1481 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Foroutan N, Muratov S & Levine M. Safety and efficacy of dipeptidyl peptidase-4 inhibitors vs sulfonylurea in metformin-based combination therapy for type 2 diabetes mellitus: Systematic review and meta-analysis. Clin Invest Med. 39, E48–62 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Yusta B et al. GLP-1 receptor activation improves β-cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 4, 391–406 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Van’t Wout EF et al. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog. 11, e1004946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song Y-S, Kim MS, Lee DH, Oh D-K, & Yoon D-Y 15-Hydroxyeicosatetraenoic acid inhibits phorbol-12-myristate-13-Acetate-induced MUC5AC expression in NCI-H292 respiratory epithelial cells. J Microbiol Biotechnol. 25, 589–597 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Geraghty P et al. The glutathione peroxidase-1–protein tyrosine phosphatase-1B–protein phosphatase 2A Axis. A key determinant of airway inflammation and alveolar destruction. Am J Respir Cell Mol Biol. 49, 721–730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brandsch C et al. Glutathione deficiency down-regulates hepatic lipogenesis in rats. Lipids Health Dis. 9, 50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.