Abstract
Investigations into the neurophysiological underpinnings of control suggest that frontal theta activity is increased with the need for control. However, these studies typically show this link by reporting associations between increased theta and RT slowing – a process that is contemporaneous with cognitive control but does not strictly reflect the specific use of control. In this study, we assessed frontal theta responses that underpinned the switch cost in task switching – a specific index of cognitive control that does not rely exclusively on RT slowing. Here, we utilised a single-trial regression approach to assess 1) how cognitive control demands beyond simple RT slowing were linked to midfrontal theta and 2) whether midfrontal theta effects remained stable over time. In a large cohort that included a longitudinal subsample, we found that midfrontal theta was modulated by switch costs, with enhanced theta power when preparing to switch vs. repeating a task. These effects were reliable after a twoyear interval (Cronbach’s α.39–0.74). In contrast, we found that trial-by-trial modulations of midfrontal theta power predicted the size of the switch cost – so that switch trials with increased theta produced smaller switch costs. Interestingly, these relationships between theta and behaviour were less stable over time (Cronbach’s α 00.61), with participants first using both delta and theta bands to influence behaviour whereas after two years only theta associations with behaviour remained. Together, these findings suggest midfrontal theta supports the need for control beyond simple RT slowing and reveal that midfrontal theta effects remain relatively stable over time.
Keywords: Medial prefrontal cortex, Task switch, Theta, Midfrontal theta, Cognitive control, Single trial regression
1. Introduction
Goal-directed control (or cognitive control) facilitates flexible adjustments of ongoing behaviour to meet current situational demands. Such adjustments of behaviour rely on extensive frontal networks to integrate goal-appropriate information with high temporal fidelity (Cole et al., 2013; Miller and Cohen, 2001). In particular, medial prefrontal cortex (mPFC) dynamics have been linked to strategic adjustments of behaviour (Botvinick et al., 2001; van Veen and Carter, 2006). In non-invasive human electrophysiological recordings, these strategic adjustments are reflected in the dynamics of low frequency cortical rhythms. In particular, theta (4–8 Hz) oscillations over medial frontal recording sites (referred hereafter as midfrontal theta) are regularly seen in tasks requiring cognitive control and are considered a neural marker of mPFC engagement to support goal-directed control (see Cavanagh and Frank, 2014; Cohen, 2014).
Traditionally, cognitive control dynamics are explored using condition-averaged data – e.g., comparing data averaged for congruent vs. incongruent targets or data averaged for respond vs. inhibit trials (see Gratton et al., 2018). However, averaging across trials only provides evidence for general changes in functioning associated with control. For instance, many canonical cognitive control phenomena are operationalised as the difference in performance between conditions – with slower performance indicative of cognitive control employment (e.g., Gratton effect, Gratton et al., 1992; post-error slowing, Rabbitt and Rodger, 1977). However, it is likely that other processes unrelated to control may also impact on performance slowing (e.g., attentional capture due to infrequent errors; Notebaert et al., 2009). For example, Wessel (2018) argues that post-error slowing, an exemplar control phenomenon, could conceivably arise from a multi-stage sequence of processes, where first an orienting response occurs toward the source of error, followed by an inhibitory motor signal and subsequent engagement of strategic control processes. Thus, simply comparing the average of correct and error trials would fail to resolve these processes. Along similar lines, Dutilh et al. (2012) provide modelling evidence that simply computing the difference between average correct and average error trials is sensitive to additional processes (e.g., changes in global motivation levels) that can create spurious post-error slowing. In sum, can slower RT definitively reflect increased cognitive control engagement if these trials are confounded by additional processes?
While probing neural indices of cognitive control allows finer-grained dissection of cognitive processes, like RT, interpretation of neural dynamics ultimately still suffer from such process-pollution when using condition-averaged analyses to index cognitive control. To date, most evidence for a role of midfrontal theta during cognitive control is based on such condition-averaged analyses (see Cavanagh and Frank, 2014; Cavanagh et al., 2012). Therefore, it is difficult to ascertain whether increases in theta activity represent activation of specific control-related processes or simply correspond to general slowing of behaviour associated with the need for control.
In contrast to condition-averaged analyses, single-trial analyses allow neural dynamics to be assessed on a trial-by-trial basis, better reflecting the ongoing, flexible adjustments to behaviour that cognitive control facilitates. Cognitive control likely waxes and wanes across the course of an experiment, with strategic adaptations being undertaken depending on changing contextual demands. Trial-varying behaviours can thus be accounted for when jointly modelling the influence of trial-by-trial changes to behaviour and neural activity. Using single-trial analysis techniques to relate trial-by-trial changes in midfrontal time-frequency spectra with the respective trial’s RT has provided further evidence of the key role of midfrontal theta in cognitive control. Trials with increased midfrontal theta power tend to predict greater RT slowing. That is, post-error (Cavanagh et al., 2017; Munneke et al., 2015), post-conflict slowing (Cohen and Cavanagh, 2011; Cohen and Donner, 2013; van Driel et al., 2015) and post-punishment slowing (Cavanagh et al., 2010; Frank et al., 2015) are associated with increased midfrontal theta power. Similar effects have also been seen in working memory (van Driel et al., 2017; although here the authors used theta functional connectivity around midfrontal sites rather than power modulation) and online action monitoring (Cohen, 2016).
Despite the advantages of using single-trial analyses to infer mPFC involvement in cognitive control, a major limitation is still the reliance on RT slowing as an index of control. In the above studies, brain-behaviour coupling dynamics have been predominately explored with respect to peri-response control processes. Due to this, it is possible that the relationship between increases in theta and slower RT/more cautiousness reflect more basic orienting responses rather than the implementation of cognitive control (e.g., Sokolov et al., 2002; Wessel, 2018). In order to show that midfrontal theta specifically reflects a neural signature of cognitive control activation that facilitates the strategic adjustment of behaviour, it is necessary to measure it in a context where it is unadulterated by orienting and response-related processes. To this end, the current study used a cued-trials task switching paradigm that can temporally dissociate preparatory and target-driven cognitive control processes beyond mere behavioural slowing.
During task switching, participants are required to rapidly alter their categorisation goal to a stream of continuous stimuli. Performance is slower when switching between these tasks than when repeating the same task on successive trials (a phenomenon known as the switch cost; for a review see Jamadar et al., 2015). Similar to other objective measures of cognitive control, the difference between switching and repeating also includes a response speed disparity (the “switch cost”). However, when participants are given advance warning of an upcoming switch, this switch cost magnitude is reduced but typically not entirely eliminated (see Karayanidis et al., 2010; Monsell, 2003). This switch cost is a candidate for a relatively pure measure of cognitive control processes that are not related to simple orienting processes. Importantly, the reduction of the switch cost can be linked to both switch preparation processes during a cueing interval and conflict-resolution processes after target that are necessary to update new and inhibit previous task sets (see Karayanidis et al., 2010; Kiesel et al., 2010; Vandierendonck et al., 2010). Indeed, in both event-related potential (ERP) and time-frequency analyses, switch trials have differential activity compared to repeat trials in the early (Cooper et al., 2017; Karayanidis et al., 2009; Lavric et al., 2008; Tarantino et al., 2016) and late cue-target interval (Astle et al., 2008; Barceló and Cooper, 2018; Müller et al., 2009) as well as after target onset (Brydges and Barceló, 2018; Cooper et al., 2016; Karayanidis et al., 2009; Sauseng et al., 2006). In sum, during task switching, midfrontal influences on specific cognitive control processes can potentially be isolated by focussing on switch-specific processes during both cue and target intervals. Importantly, these control processes are reflected as faster RT.
Here we claim that the switch cost is a relatively pure measure of cognitive control that is not compounded by an orienting response. However, a change in task could be considered a novel event, that would elicit an orienting response. Indeed, novel stimuli are associated with morphologically similar neural responses as switch cues (e.g., Barcelo et al., 2006). Yet, when accounting for novelty, switch costs still remain. For instance, predictable switches have been shown to produce similar magnitude switch costs as unpredictable switches (Nessler et al., 2012) and while in some cases predictable vs. random switches change the magnitude of the switch cost (e.g., Tornay and Milán, 2001), the switch cost is not eliminated. Indeed, as described above, advance preparation tends to have the largest impact on switch cost rather than novelty of switch trials – suggesting an active control process that is engaged in preparation for a switch rather than responding to novelty per se.
In the current study, we aimed to explore the link between trial-by-trial fluctuations in midfrontal theta and behavioural markers of relatively isolated cognitive control processes. We expected that trials with increased theta power would result in better cognitive control, as per the influential need-for-control model (Cavanagh and Frank, 2014). Critically, we utilised a cued-trials task switching paradigm that allows quantification of cognitive control beyond RT slowing to assess such cognitive control engagement. We hypothesised that switch trials, where participants were required to change tasks, would be associated with increased midfrontal theta power when compared to repeat trials. Further, if this difference in midfrontal theta power specifically reflects cognitive control, then these differences in theta power should occur during the critical temporal intervals when switch-specific control processes occur – namely during the preparatory cueing interval and post-target. Making use of a single-trial analysis approach, we predicted that switch trials with increased theta power during both the cueing and post-target intervals would reflect trials where control engagement was high – and thus result in faster RTs. Importantly, to demonstrate that theta power was linked to strategic cognitive control, and not simply general phenomena like orienting, we included control conditions (non-informative cues) where participants were unable to prepare for a task to determine that the strategic use of a cue (rather than simply orienting to a stimulus) was key for theta-behaviour coupling.
Finally, we make use of a large sample that includes a sub-group of follow-up, longitudinal data (see 2. Material and Methods) to introduce both permutation statistical assessment and stability estimates to the time-frequency results we report in this work. As reliability estimates of time-frequency data are not routinely undertaken, it is difficult to make a priori predictions regarding expected effect sizes. Therefore, we provide these assessments in comparison to broad guidelines suggested in psychometrics (Garson, 2012).
2. Materials and methods
2.1. Participants
Two hundred and fifteen participants from the wider Newcastle, Australia region took part in the current experiment as part of the Age-ility Project (Karayanidis et al., 2016; http://www.age-ility.org.au/). Participants were recruited from the University of Newcastle, local tertiary colleges, local sporting and surf lifesaving clubs, recruitment flyers and posters placed in the community and via word of mouth. Written, informed consent was provided by all participants (with additional parental consent provided for those participants under 18 years of age). All participants were asked to abstain from caffeine and alcohol at least 2 h prior to testing and were reimbursed $20 per hour for their time. Study protocol was approved by the University of Newcastle Human Research Ethics Committee (HREC: H-2012–0157) in compliance with the Declaration of Helsinki.
Participants completed an initial testing session (Phase 1) between 2013 and 2014 and were invited to return between 2015 and 2016 to complete an identical session (Phase 2). Data from two participants were excluded as they were not engaged in the task (evidenced by very fast responses with very high error rates) and a third participant’s EEG data was incorrectly recorded. Of the remaining 213 participants, 64 of those who returned undertook the EEG experimental session. As single-trial analyses are sensitive to the number of trials, we excluded any participants who had less than 50 trials in any condition in either Phase 1 or Phase 2. This resulted in a group of 53 participants who had EEG data from both phases (mean time between Phases 1 and 2; 24.79 ± 4.44 months; range = 18.43–37.93 months) and second group of 139 participants who had appropriate data from Phase 1 and did not return for Phase 2. For these analyses, this resulted in a final cohort comprising two groups: one with two data sets across two phases of the study (i.e., identical participants, separated by an average of 24 months; Phase 1 and Phase 2) and a larger outsample with data from only Phase 1. There were no significant differences in age (t190 = 0.077, p = .939), or ratios of males:females (χ2 = 0.215, p = .746) or left:right handedness (χ2 = 0.059, p = 1) between Phase 1 and the outsample (see Table 1 for demographic information).
Table 1.
Summary information.
| Age (±SD) | Age range | Female N (%) | Right-handed N (%) | |
|---|---|---|---|---|
| Phase 1 | 21.43 ± 5.33 | 15–35 | 27 (50.94) | 52 (92.45) |
| Phase 2 | 23.45 ± 5.47 | 16–38 | 27 (50.94) | 52 (92.45) |
| Outsample | 21.37 ± 4.19 | 15–35 | 76 (54.68) | 127 (91.37) |
2.2. Task and stimuli
Participants performed a cued-trials task-switching paradigm, where they switched between three simple classification tasks: letter (vowel/consonant), digit (odd/even) and colour (hot/cold). The experimental paradigm has been described in detail elsewhere (Cooper et al., 2015, 2017).
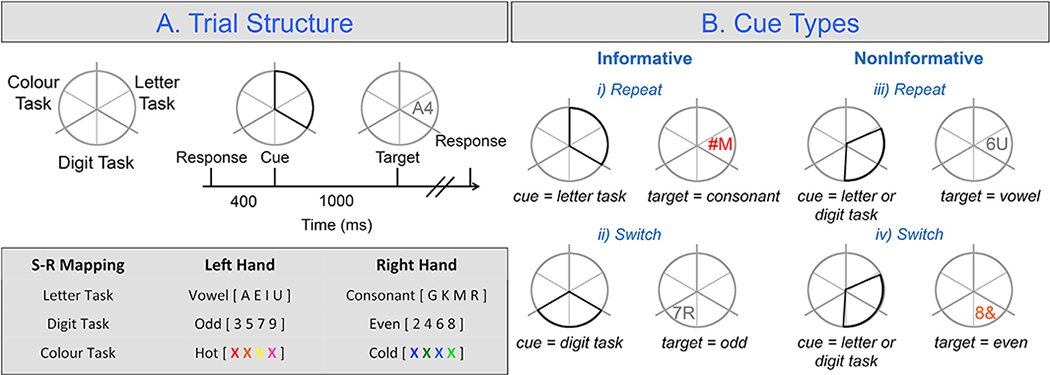
In brief, participants viewed a grey circle divided into six equal-sized segments. This circle remained on screen throughout the experiment. Pairs of adjacent sections were spatially mapped to the three classification tasks (see Fig. 1A). Participants responded to the task-relevant feature of a target presented while ignoring irrelevant features.
Fig. 1. Task switching paradigm.
A) Trial structure and example S-R mapping. Note, the trial sequence here serves as the previous trial for definitions of cue types in 1B. B) Cue types; whereby informative cues indicate the task to be prepared while noninformative do not. If the same task is presented on successive trials it is deemed a repeat trial (here letter – letter). If a different task is presented on successive trials it is deemed a switch trial (here letter – digit).
We defined a trial as a complete cue—target—response sequence. Cues were presented for 1000 ms, which were then immediately replaced by a target. Targets were removed from the screen once a response was completed or after 5000 ms had elapsed. Cues comprised a darkened outline of two adjacent segments and were presented in a pseudorandom sequence (i.e., identical cues could not be presented for more than four consecutive trials). Participants responded with button presses using their left and right index fingers (response hands counterbalanced). After participants responded to the target, the next trial’s cue was presented after a 400 ms delay.
In the current study, we focus analyses on the two classes of proactive cue types: informative cues, where task preparation was possible and non-informative cues, where task preparation was not possible (Fig. 1B). Informative repeat cues highlighted the segment associated with the task completed on the previous trial, whereas informative switch cues highlighted segments associated with a task not completed on the previous trial. In contrast, non-informative cues highlighted one segment associated with the task completed on the previous trial and an adjacent segment associated with one of the other two tasks. The task to be performed on these trials was resolved by the location of the target. Non-informative repeat trials, occurred when the target appeared in the section associated with the previous task while non-informative switch trials had targets appear in the other task segment.
Thus, for informative cues, the participant could load the new task on switch trials and be prepared to implement task rules for both switch and repeat trials during the cue-target interval (CTI), with additional switch preparation possible on informative switch trials. In contrast, non-informative cues only provided general timing information (i.e., get ready for the target to appear) and relied on reactive control processes to resolve task identity and upload the new task on switch trials, serving as control conditions.
Note, in the current paradigm, an additional cue-type exists (switch-away) which allow switch but not task preparation. With this cue in mind, each cue type is presented with equiprobability (25%). Due to this, switch trials (62.5% - i.e., switch-to [25%], switch-away [25%] and non-informative switch [12.5%]) occur more frequently than repeat trials (37.5% - i.e., repeat [25%] and non-informative repeat [12.5%]). Again, this suggests that slower RT for switch trials (i.e., the switch cost) cannot be attributed to novelty/orienting responses.
2.3. Procedure
A total of 929 task switching trials were undertaken, comprised of ten mixed-task blocks (72 trials/block), three single-task blocks (48/block) with each block containing five warm-up trials. EEG was recorded continuously at 2048 Hz using the ActiveTwo Biosemi EEG system (bandwidth [−3 dB] DC-400 Hz; low-pass response = 5th order sinc digital filter, high-pass response = fully DC coupled). The Biosemi EEG system uses a dual electrode set-up rather than typical grounds seen in other EEG. Here, a feedback loop is used to create an analogue-digital converter reference voltage using an active electrode (common mode sense; CMS) and a passive electrode (driven right leg; DRL). EEG data are recorded relative to this amplifier reference voltage and can be re-referenced offline. For technical details of the Biosemi hardware, please see https://www.biosemi.com. EEG was recorded from a 64-electrode cap system (arranged per the international 10–20 system) with additional bilateral mastoid, outer canthi-, supraorbital-, and infraorbital-ocular external recording sites.
2.4. Data analysis
For RT and EEG analyses were conducted on correct trials if they: (i) had RTs faster than 200 ms but not slower than the participant’s mean RT + 3 standard deviations (SD), (ii) were not post-error trials and (iii) were not part of the five warm-up trials included at the start of each block. Trials with high EEG noise levels were also excluded from EEG (and RT) analyses (see below). Note, for accuracy data, we employed the same RT restriction range and rejection of warm-up trials but included both correct and incorrect trials.
2.4.1. EEG analysis
EEG data were processed offline in the MATLAB (Mathworks, Navick, MA; version 2016b) programming environment using the Fieldtrip package (Oostenveld et al., 2011). First, Biosemi EEG data were re-referenced to electrode Cz to remove common-mode signals, down-sampled to 512 Hz (zero-phase anti-aliasing; low-pass 245 Hz) and were filtered (high pass: 0.1 Hz, forward phase; 50 Hz notch: zero phase). Data were visually inspected and bad channels were identified and excluded from further analyses (Phase 1, 0.91 ± 2.1 SD; Phase 2, 1.79 ± 2.62 SD; outsample, 0.85 ± 1.43 SD). For each condition, individual trial epochs were created per condition (−1000 ms to + 3500 ms with respect to cue onset) and then subjected to independent component analysis (ICA; Hyvärinen and Oja, 2000; number of components = number of electrodes). ICA components corresponding to blinks or eye movements were visually identified and removed (Phase 1, 1.38 ± 0.88 SD; Phase 2, 1.15 ± 0.5 SD; outsample, 1.42 ± 0.79 SD components removed). Those ICA components not associated with occular artefact were projected back into electrode space for subsequent analyses. Remaining data were low-pass filtered (30 Hz, zero-phase) to remove high-frequency noise such as muscular artefacts. After filtering and ocular artefact removal, any trials that still contained residual artefact were deleted (amplitude threshold ± 120 μV). At this stage of processing, we removed any participants who did not retain at least 50 trials per trial type. For Phase 1, this resulted in an average of 139.74 (±24.97 SD) informative repeat, 135.04 (±28.61 SD) informative switch and 136.32 (±26.63 SD) non--informative trials. For Phase 2, an average of 133.58 (±27.03 SD) informative repeat, 127.28 (±28.45 SD) informative switch and 129.15 (±27.8 SD) non-informative trials were retained. For the outsample, an average of 129.83 (±27.25 SD) informative repeat, 124.47(±28.33 SD) informative switch and 125.91 (±27.58 SD) non-informative trials remained. Any channels that were deemed bad in earlier processing steps were replaced with a weighted average interpolation of data from neighbouring electrodes. Finally, we applied a surface Laplacian spatial filter to EEG data (smoothing = 10−5, 50 iterations, spherical spline order = 4) to reduce volume conduction effects.
2.4.2. Time-frequency analyses
We extracted trial-averaged and single-trial power values based on time-frequency analyses performed on surface Laplacian filtered data (Cooper et al., 2015, 2017). For each trial type (informative repeat, informative switch, non-informative) single-trial time-frequency representations were extracted via complex Morlet wavelet convolution (80 logarithmically spaced frequency bins from 2 to 30 Hz with logarithmically spaced tapers ranging from 3 to 14 cycles). For averaged power values, the average of these single-trial time-frequency representations were calculated. For single-trial power values, these data remained in their single-trial representation.
All power measures (i.e., both average and single-trial data) reflected magnitude changes in time-frequency activity from a baseline period (−300 to −100 ms pre-cue interval). Note, the baseline is offset by 100 ms from cue onset to minimise post-cue signals contaminating the baseline interval. Power values were converted to normalised decibel values (10log10[power/baseline]). To focus on the hypothesised role of midfrontal theta oscillations during cognitive control, time-frequency data from electrodes F1, Fz and F2 were averaged to create a midfrontal region of interest (ROI). All further analyses were restricted to this ROI.
2.4.3. Single-trial regression
To assess the effect of trial-by-trial modulations in midfrontal power on RT, we performed regression between power and RT for each participant’s single-trial time-frequency data (Cohen and Cavanagh, 2011). We employed a univariate approach where we assessed the relationship between our signal data (time-frequency space) and our cognitive control index (RT). To quantify the relationship between time-frequency signals and RT, we performed regression to estimate parameters within the linear equation, Equation (1):
| (1) |
Here, Y is a vector containing power data, where each element represents a single-trial’s power value for one time × frequency point, INT is the intercept, bRT is the regression coefficient for RT and E is the unexplained variance term (i.e., error). The regression procedure was implemented separately for each participant and each condition. Both time-frequency data and RTs were z-scored to account for differences in the range of power and RT values. To minimise the influence of outliers on the regression, we used robust regression (iterative reweighted least squares with a bisquare weighting function). The single-trial regression procedure resulted in a time × frequency × condition matrix of standardised beta values (i.e., correlation coefficients) for each participant.
2.4.4. Condition-specific power and regression effects
To examine power and single-trial power-RT relationships that differed during task switching, we ran a one-way (informative repeat, informative switch and non-informative) ANOVA at each time-frequency pixel for power and regression maps. This process allowed us to identify differential activity across conditions in specific time × frequency regions in a data-driven way. However, as the final time-frequency maps contain 94,320 pixels, we applied a two-step procedure to correct for multiple comparisons.
For the first step, we used a permutation-based procedure to estimate α significance levels by comparing observed test statistics against an approximation of the population-level distribution of the test statistic. Specifically, time-frequency data (i.e., both power and single-trial regression) were subjected to one-way ANOVA at each time-frequency pixel. For both Phase 1 and 2, we produced F-statistic maps associated with the main effects of this ANOVA. We deem these statistical maps Forig maps. Next, we repeated the above one-way ANOVA with a random selection of 53 participants from the outsample group to retain identical degrees of freedom. Importantly, for these analyses, we randomly shuffled the data across condition labels, so as to compare the Forig maps to a null-distribution. That is, by removing the correct assignment of condition to the ANOVA design, any F-statistics reported correspond simply to chance (null) effects.
We performed 5000 random selection-with-replacement ANOVAs using the outsample to build F-statistic distributions for each time-frequency pixel (i.e., a Monte Carlo estimation procedure). From these, we estimated the population-level F-statistic by determining the F-statistic associated with the 95th percentile at each time-frequency pixel – and stored these values as a critical F (Fcrit) map for power and regression analyses.
For the second step, we created binarised matrices by identifying pixels in the Forig maps that exceeded the F values generated in each permutation. From these binarised matrices, we determined connected sets of highlighted pixels (i.e., clusters) using MATLAB’s bwconncomp function and calculated the size (in pixels) of every cluster found, as well as the maximum cluster size (critical cluster size, Clustercrit).
Ultimately, we found those time-frequency pixels belonging to clusters within the Forig > Fcrit contrast that exceeded the Clustercrit for their respective F-map. That is, significant pixels needed to exceed our estimates of the population level statistic and be part of a cluster that was larger than that expected to occur through simple autocorrelations present in time-frequency data. In essence, we undertook a modified version of the cluster-based permutation tests advocated by others (Maris and Oostenveld, 2007).
2.4.5. Phase 1 – Phase 2 electrophysiological stability assessment
Finally, we estimated the reliability of power and single-trial regression measures were over time. To do so, we computed Cronbach’s α reliability estimate in MATLAB (CronbachAlpha function; Schlegel, 2012), as denoted in Equation (2).
| (2) |
Here, K = the sum of items (i.e., the sum of each time-frequency pixel across participants), = average covariance across participants and = average variance of each item (time-frequency pixel). For improved readability, we computed Cronbach’s Alpha (Cronbach’s α) at each time-frequency pixel for power and regression matrices on the condition-averaged data. For completeness, we also report the stability assessment of behavioural data (RT and error rate).
3. Results
3.1. Behaviour
3.1.1. Phase 1 vs. Phase 2
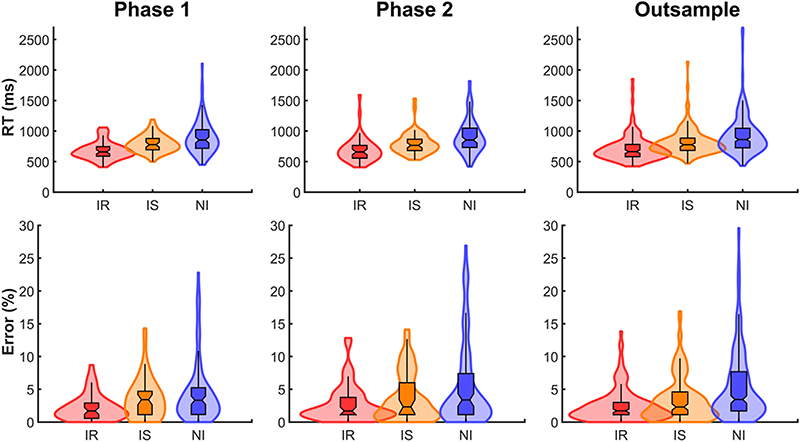
First, RT and error rates were analysed using a 3 (Condition; informative repeat, informative switch, non-informative) × 2 (Phase; Phase 1, Phase 2) repeated measures ANOVA in SPSS (version 24, IBM). For RT, there was a significant main effect of Condition (F2,104 = 25.45, p < .001, partial η2 = 0.33), where informative switch trials were slower than informative repeat (F1,52 = 5.92, p = .018, partial η2 = 0.1) and informative switch were faster than non-informative conditions (F1,52 = 37.07, p < .001, partial η2 = 0.42). There was no difference between RTs at Phase 1 and Phase 2 and no significant Condition × Phase interaction (see Fig. 2).
Fig. 2. Behavioural results for Phase 1, Phase 2 and outsample groups.
Top row, RT; bottom row, error rate. Violin plots (created using Gramm; Morel, 2018, March 4) represent the distribution of each data series, with a box plot and whisker drawn over these data (notch centre = median). IR, informative repeat; IS, informative switch; NI, noninformative.
For error rates, there was a significant main effect of Condition (F2,104 = 13.08, p < .001, partial η2 = 0.2): both informative repeat and switch trials had fewer errors than non-informative conditions (F1,52 = 13.8, p < .001, partial η2 = 0.21). There was no significant main effect of Phase but the Condition × Phase interaction was significant (F2,104 = 12.65, p < .001, partial η2 = 0.2), with a greater difference in error rate between Informative Repeat and Informative Switch trials at Phase 2 than Phase 1 (F1,52 = 18.05, p < .001, partial η2 = 0.26; see Fig. 2).
Finally, we assessed the stability of the behavioural data across phases of the study by computing Cronbach’s α for each condition separately for RT and error rate. Both RT and error rate had good (i.e., greater than 0.7) reliability (as shown in Table 2), despite significant differences in error rates over time.
Table 2.
Cronbach’s α test-retest estimates.
| Informative Repeat | Informative Switch | Noninformative | |
|---|---|---|---|
| RT | .809 | .849 | .836 |
| Error rate | .732 | .813 | .851 |
3.1.2. Outsample
As we used the outsample for permutation testing in time-frequency analyses, we first needed to ensure the larger outsample used in such analyses was comparable to the smaller Phase 1 (and 2) group. To do so, we assessed RT and error rates in a similar repeated measures ANOVA (3 Condition; informative repeat, informative switch, non-informative) × 2 (Group; Phase 1, outsample). As before, there was a significant main effect of Condition (F2,380 = 227.54, p < .001, partial η2 = 0.55), with informative switch performed slower than informative repeat (F1,190 = 145.43, p < .001, partial η2 = 0.43) and informative switch faster than non-informative (F1,190 = 28.78, p < .001, partial η2 = 0.13). No significant main effect of Group or Group × Condition interaction was found.
For error rates, there was again a significant main effect of Condition (F2,380 = 52.41, p < .001, partial η2 = 0.22), with informative switch having more errors than informative repeat (F1,190 = 67.48, p < .001, partial η2 = 0.26) but no significant difference between informative switch trials and non-informative conditions (see Fig. 2). Again, we found no significant Group effect or Group × Condition interaction.1
In sum, the task switching paradigm showed the expected effects: performance was slower and less accurate on switch than repeat trials and for non-informative than informative conditions. Interestingly, RT appeared stable over the 24 month period from Phase 1 to Phase 2 with reported effects comparable between Phase 1/2 and the outsample group.
3.2. Midfrontal dynamics
3.2.1. Midfrontal power
To characterise difference in time-frequency responses dependent on cognitive demands, we ran separate one-way (Condition: informative repeat, informative switch, non-informative) repeated-measures ANOVAs at each time-frequency pixel for Phase 1 and Phase 2 data sets. These results are presented in time-frequency space in Fig. 3 (top row). For completeness, we also present the time-frequency power spectra for all conditions and study phases separately in Appendix A.
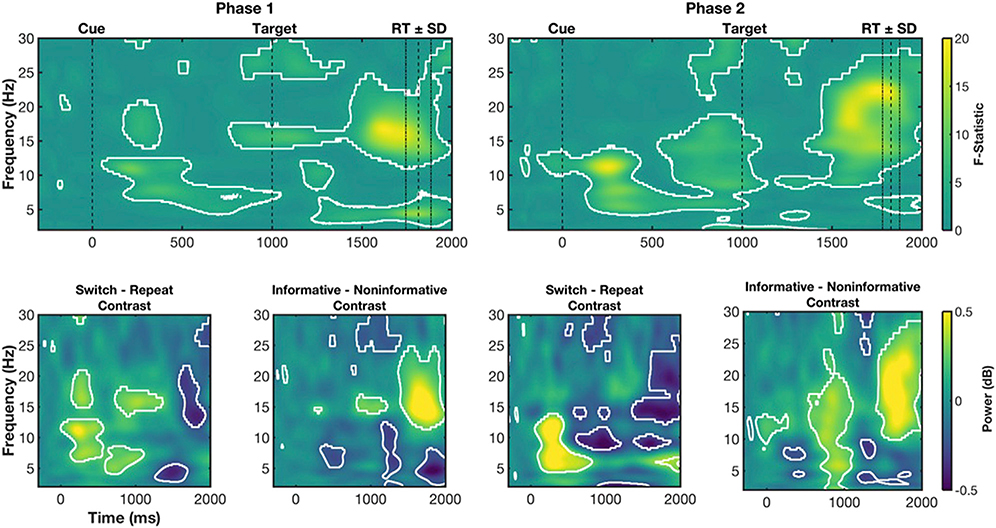
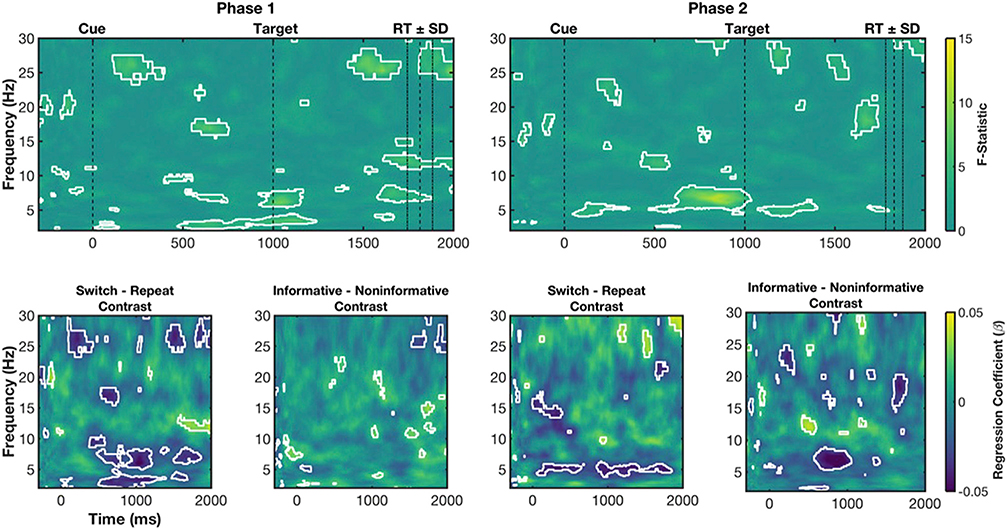
Fig. 3. Power ANOVA results.
Top row, F-statistic maps for Main Effects Trial Type for Phase 1 and Phase 2. Bottom row, contrast-maps indicating the direction of effect associated with ANOVA results for Switch – Repeat and Informative – Noninformative simple effects. For ANOVAs, white outlines indicate significant pixels associated with permutation testing. For simple effects we present uncorrected p-values from t-tests (white outlines at p < .05) for completeness but restrict interpretation to those time-frequency associated with the main effect of Condition. Cue and target onset are shown with dashed lines at 0 and 1000 ms respectively. The average RT for all conditions ±1 SD is shown to demonstrate peri-response ranges.
For Phase 1, during the CTI, sustained theta and alpha (8–13 Hz) main effects were observed (Fig. 3; top left), with simple contrasts revealing they were driven by greater power for informative switch than informative repeat trials (Fig. 3; bottom left). Additionally, in the CTI a brief beta difference was seen around 350 ms after cue, with simple contrasts revealing this effect was the result of greater beta power for informative switch than informative repeat trials.
From approximately 300 ms post-target, there was a sustained theta condition effect, with lower theta power for informative switch than repeat trials. Around target onset (1000 ms post-cue), there were two significant beta clusters. Upper beta (25–30 Hz) power was lower for informative conditions vs. non-informative conditions. In a second cluster, lower beta (~15–20 Hz) power was higher for informative vs. non-informative conditions. This lower beta effect extended into a broad peri-response process (~500 ms post-target and afterwards), likely corresponding to a peri-response difference between the informative and non-informative trial types (i.e., informative trials are performed faster than non-informative) similar to previously reported peri-response conflict (Cohen and Cavanagh, 2011; Cohen and Donner, 2013).
For Phase 2, many of these effects were replicated (Fig. 3; top right). During the CTI, there was a sustained theta/alpha effect, with greater power for informative switch than repeat conditions (Fig. 3; Switch-Repeat contrast). Likewise, after target onset, there was a broad beta condition effect from approximately 500 ms onward. However, the post-target, late sustained theta difference between conditions was not observed at Phase 2. To demonstrate this more clearly, we ran a simple conjunction analysis, where we highlight which pixels were associated with significant effects in Phase 1 and Phase 2. This analysis highlighted that common theta, alpha and beta effects during the CTI were present in Phase 1 and Phase 2, as well as the broad beta peri-response effect (see Fig. 4A).
Fig. 4. Conjunction analyses showing common significant time×frequency pixels across Phase 1 and Phase 2.
Yellow patches depict significant time × frequency pixels that were present in both Phase 1 and Phase 2 for A) power and B) regression one-way ANOVA analyses.
In sum, theta (and beta) processes were reliably influenced by differential cognitive demands across conditions. During the CTI, a sustained theta/alpha process was differentially increased for cues indicating the need to switch task and was present after 24 months in Phase 2 results. Likewise, in both phases, around target onset lower beta power was higher for informative conditions. Post-target, a sustained theta condition effect was seen but only in Phase 1. Finally, peri-response beta power was increased for informative repeat vs. informative switch (and higher also than non-informative) conditions during both phases of the study.
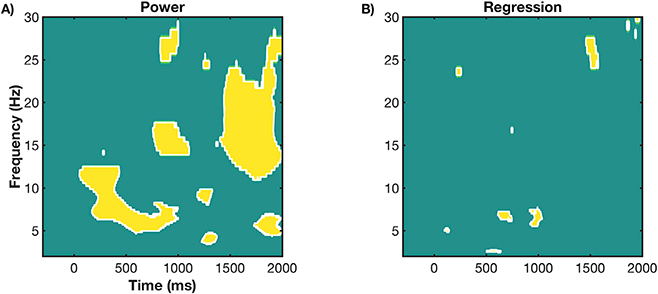
3.2.2. Midfrontal regression
Regression coefficients (beta values) were also subjected to one-way (Condition: informative repeat, informative switch, non-informative) repeated-measures ANOVAs at each time-frequency pixel for Phase 1 and Phase 2 data sets (Fig. 5). Note, that areas of significant difference between conditions were almost entirely negative regression coefficients – so that trials with increased power were associated with reduced RT (see Appendix B). First, in Phase 1, main effects for Condition were largely restricted to the CTI, with a prominent, sustained difference from approximately 500 ms post-cue to 200 ms post-target in the delta band (Fig. 5; top left). Simple contrasts revealed that main effects were driven by differences between informative switch and repeat conditions: increased delta power for switch vs. repeat trials was associated with reduced RT switch cost, suggesting greater preparation for an upcoming switch (Fig. 5; bottom left). Likewise, increased theta power around target onset produced faster RTs for informative switch conditions. From approximately 600 ms post-target, theta power again differed between conditions, however this time increased theta power was associated with longer RTs, particularly on informative repeat trials. Note, this time period is likely peri-response, where previous evidence has suggested that increased theta power around response leads to longer RTs (e.g., Cohen and Cavanagh, 2011; Cohen and Donner, 2013). Finally, sporadic condition effects for relationships between beta power and RT are also found throughout the trial (e.g., early and mid-CTI and peri-response), where switch trials with increased beta power tended to be responded to faster than repeat.
Fig. 5. Single-trial regression ANOVA results.
Top row, F-statistic maps for Main Effects Trial Type for Phase 1 and Phase 2. Bottom row, contrast-maps indicating the direction of effect associated with ANOVA results for Switch – Repeat and Informative – Noninformative simple effects. For ANOVAs, white outlines indicate significant pixels associated with permutation testing. For simple effects we present uncorrected p-values from t-tests (white outlines at p < .05) for completeness but restrict interpretation to those time-frequency associated with the main effect of Condition. Cue and target onset are shown with dashed lines at 0 and 1000 ms respectively. The average RT for all conditions ±1 SD is shown to demonstrate peri-response ranges.
For Phase 2, we again found that power during the CTI predicted RT (Fig. 5; top right). The relationship between power and RT was prominent in a series of time-frequency clusters corresponding to theta. Informative switch trials that were associated with increased early (~100–300 ms post-cue) theta power had faster RT. This relationship was stronger for informative switch than for informative repeat power-RT coupling. Likewise, we observed the same effect from target onset until approximately 450 ms post-target. Interestingly, as in Phase 1, we observed a difference in relationships between power and RT during the second half of the CTI (~500 ms to target onset) but in the theta rather than delta band (Fig. 5; top right). This effect was also driven by differences in the relationship between theta power and RT for noninformative vs. informative trial types, with stronger relationships for informative than non-informative trial types (Fig. 5; bottom right). Simple conjunction analyses, showing which pixels were significant in both Phase 1 and Phase 2, highlighted the shift from delta to theta frequencies from Phase 1 to Phase 2, with only a small group of pixels in the theta band during the late CTI consistently found in ANOVAs for both phases (see Fig. 4B). Again, as in Phase 1, sporadic relationships between beta power and RT were found throughout the trial, with informative conditions associated with stronger power-RT coupling than noninformative conditions during the CTI and peri-response and weaker power-RT relationships for switch vs. repeat post-target.
In sum, increased delta and theta power during the CTI was associated with faster RTs, particularly for informative switch conditions. While these relationships were seen during the CTI for both phases, relationships between power and RT differed between conditions in both delta and theta frequencies but only in theta time-frequency clusters during Phase 2.
3.2.3. Midfrontal reliability estimates
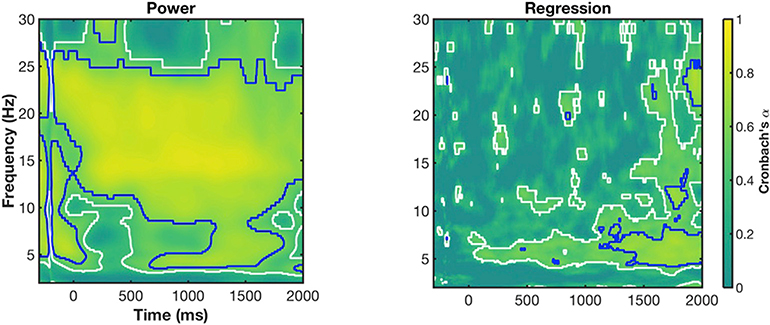
Finally, we assessed the stability of presented midfrontal dynamics (i.e., Phase 1 vs. Phase 2). To do so, we computed Cronbach’s α for each time × frequency pixel in the power and regression plots. For simplicity, we present condition-averaged power and regression stability measures. As seen in Fig. 6, generally, midfrontal power was stable across the two phases, with very high stability in the higher frequencies (>10 Hz and >0.7 Cronbach’s α). Interestingly, reliability was somewhat weaker within the theta band during the cue-target interval, consistent with theta-specific preparation changes from Phase 1 to Phase 2. On average, across the entire epoch, theta power had an average Cronbach’s α of 0.55.
Fig. 6. Reliability of power and regression values between Phase 1 and Phase 2.
Cronbach’s alpha values for condition-averaged power and regression are depicted as time-frequency maps. 0 ms; cue onset, 1000 ms; target onset. White outlines indicate alpha values > .4; blue outlines indicate alpha values > .6.
Stability estimates were lower for regression coefficients (Fig. 6B). Here, the reliability of higher frequencies was low, with the most stable effects occurring in the theta band from cue-onset until the end of the epoch (>0.4 Cronbach’s α; average Cronbach’s α for the entire epoch = 0.41). Note there was also a broad frequency effect that was stable in the latter half of the post-target period (i.e., 500 ms post-target onwards). In sum, midfrontal power responses were remarkably stable from Phase 1 to Phase 2 (a separation of 24 months). While relationships between power and behaviour were less reliable overall, power-behaviour relationships in midfrontal theta appeared the most reliable from Phase 1 to Phase 2.
4. Discussion
Increased theta power over midfrontal recording electrodes is considered to be a neural marker of cognitive control engagement. However, to date, most evidence for this line of reasoning comes from two sources: 1) condition-average analyses, where non-control influences like orienting responses may influence the interpretation that theta is associated with cognitive control and 2) peri-response analyses, that show a temporal link between RT slowing (a contemporaneous phenomenon with control) and increased theta. In this study, we overcame these limitations by assessing cognitive control using standard condition-average analysis approaches alongside single-trial analysis techniques that permit mapping of trial-by-trial changes in neural activity to trial by trial changes in performance (Cohen and Cavanagh, 2011). Further, we assessed mPFC dynamics using a cued-trials task switching paradigm, where cognitive control employment is reflected as reduced RT differences between switch vs. repeat trials and where effective use of switch-specific preparation and post-target processes leads to faster RTs.
Our results support the functional role of midfrontal theta dynamics in supporting cognitive control. Consistent with standard condition-average analyses, we found that theta power increased during both preparatory periods and post-target intervals dependent on task-demands (consistent with standard condition-average analyses). We also showed that trials, which exhibited increased theta power in both preparatory and post-target intervals tended to be responded to faster. Thus, we found that not only were trials with increased theta power associated with faster RT, but that trial-by-trial changes in theta power influenced the reduction of the switch cost. These results provide novel evidence that cognitive control is specifically associated with midfrontal theta, above and beyond other general phenomena that coincide with control. In sum, our results support the notion that midfrontal theta oscillations support context-dependent adjustments of the control system by directly affecting behavioural performance beyond RT slowing/increased cautiousness and therefore are an index of the need for cognitive control (Cavanagh et al., 2012; Cohen, 2014).
4.1. Theta underpins frontal functioning beyond general RT slowing
Theta oscillations recorded at midfrontal electrodes have been proposed to be a neural signature of strategic adjustment/organisation associated with cognitive control (e.g., Cavanagh and Frank, 2014; Cavanagh et al., 2012; Cohen, 2014). Both work from non-human primates (Tsujimoto et al., 2006; Tsujimoto et al., 2009; Womelsdorf et al., 2010) and human intracranial recordings (Cohen et al., 2008; Wang et al., 2005) suggest these oscillations likely originate from sources within mPFC. Medial PFC is widely regarded to play a critical role in goal-directed control of behaviour (e.g., see Ridderinkhof et al., 2004; Shenhav et al., 2013). In these accounts, mPFC functioning serves as a type of temporally-maintained, strategic scaffold, whereby ongoing behaviours are adapted with response to conflicts in the environment (Cavanagh and Shackman, 2015; Holroyd and Yeung, 2012).
To date, the evidence for the role of midfrontal theta supporting such frontal functions stems from associations with RT slowing during response-conflict paradigms (see Cavanagh and Shackman, 2015). When determining the specificity of this relationship, it is critical to account for the fact that RT slowing may arise from non-control related phenomena (Wessel, 2018) and that response-conflict paradigms pertain to only one set of control processes. Indeed, our work (Cooper et al., 2017) and others (Cunillera et al., 2012; Van Noordt et al., 2016; van Noordt et al., 2015) have shown that non-response-conflict paradigms are also associated with midfrontal theta. Likewise, a relationship between higher theta power and RT speeding has also been reported (Narayanan et al., 2013), wherein faster RT reflected optimal cognitive control use, although the mechanisms behind these associations were not addressed. Similarly, Valadez and Simons (2018) reported increased midfrontal theta power on single-error vs. double-error trial runs during a Flanker task. These single-error trial sequences were also associated with faster RT, again linking midfrontal theta increases with RT speeding (rather than slowing). Here, the current study utilised the reduction in the switch cost serve as an unambiguous index of control engagement. Our findings support the notion that theta oscillations reflect mPFC functioning involved in varied implementations of control.
In all conditions we observed a sustained increase in theta power during the CTI (see Appendix A). Such a finding is consistent with midfrontal power changes reflecting a general phenomenon. Yet, we also found that frequency responses were modulated by cognitive demands (Fig. 3). That is, while ongoing theta activity was present in all conditions it was specifically altered in response to current cognitive demands. We provide evidence that these demands can be proactive or reactive in nature. For example, when able to prepare for an upcoming task (i.e., informative cues) theta was differentially engaged vs. when simply preparing for the target to appear (i.e., non-informative cues). Likewise, switching between task-sets (i.e., switch cues) was associated with increased theta power vs. repeating the same task-set. Post-target, when task-identity needed be resolved (i.e., non-informative trial types) had greater power than informative trial types (when task preparation had already occurred). Together, these results point toward a neural mechanism that is modulated by specific control demands.
The condition-averaged responses reflect overlapping multiple sensorimotor and cognitive processes that are critical for cognitive control. However, the single-trial regression results revealed that theta oscillations track with behavioural performance during temporal windows where strategic adjustments of behaviour are critical for effective task performance. That is, during the late half of the CTI, after any phase-resetting driven by stimulus presentation should be resolved, we found that switch conditions with increased theta power in anticipation for the upcoming target resulted in faster (and thus more effective) responses. McKewen, Cooper, Wong, Michie and Karayanidis (under review) found that theta responses related to phase-resetting dynamics (i.e., phaselocked power) were strongest in the first half of the CTI in contrast to ongoing, non-phase-locked effects present during the entire epoch. Moreover, while every condition exhibited sustained theta activity that was associated with RT throughout the trial (see Appendix B), our ANOVA results showed that time-frequency clusters corresponding to theta bands, were differentially modulated by the type of strategic adjustment required (i.e., prepare to switch vs. prepare to repeat). Together, these findings point toward strategic mPFC dynamics where ongoing activity is modulated in response to changes in context that directly impact behaviour and manifest as cortical theta changes. Such a view, where ongoing midfrontal theta activity dynamically adjusts to control demands, is consistent with the need for control framework (Cavanagh and Frank, 2014) and with previous findings showing that theta power scales with the need for control (Cooper et al., 2016), fluctuates over short and longer timescales (Cohen, 2016) and is linked to need for adjustments in ‘real-world’ scenarios, such as in video games (Cavanagh and Castellanos, 2016).
Finally, it is important to note that theta was not the only frequency band seen to be involved in the task. Both lower and upper beta oscillations were present, at least in the standard condition-averaged approach. Beta oscillations are often seen in tasks that recruit PFC regions (Buschman et al., 2012; Engel and Fries, 2010) and have a canonical relationship with motor output – desynchronising prior to response generation and then rebounding after a response (Kilavik et al., 2013). Beta oscillations in this task appeared closely linked to these type of motor effects – for instance, showing enhanced beta power around 700 ms post-target for the informative trials (where responses have likely already occurred) compared to non-informative trials (where response preparation is likely still being finalised). Importantly, relationships between beta and RT were present in single-trial regression results but were largely found after target, where motor-related processes play a larger role in RT. Curiously, beta effects often occurred contemporaneously with theta power changes, hinting at a cross/multi-frequency dynamics in mPFC but which were unable to be explored in the current study design.
In sum, midfrontal PFC dynamics appear to closely follow the strategic changes in behaviour that cognitive control facilitates in changing contexts. While multiple frequency bands were seen during the task, theta oscillations appeared to be differentially sensitive to performance – consistent with specific cognitive control demands rather than general RT slowing.
4.2. Stability of frontal oscillations over time
Due to the longitudinal aspect of this study, we were able to explore the stability of prefrontal oscillations over a period of two years. As seen in Fig. 6 power in almost all frequency bands (i.e., ~3 Hz - ~ 25 Hz) were stable over time, although theta oscillations during the CTI appeared less stable than higher frequency bands.
However, as seen in the regression ANOVA results (Fig. 5), the stability estimates (Fig. 6) and the conjunction analyses (Fig. 4B), the relationships between these frequencies and behaviour were less stable. Indeed, it appears that during the CTI, participants in Phase 1 relied on delta and theta frequencies to influence performance whereas in Phase 2, these delta effects had disappeared and were replaced by a broader theta process (Figs. 4B and 5). Importantly, while stability estimates were lower for brain-behaviour correlations than power, theta relationships with behaviour were clearly the most stable aspect of the regression data. That is, a distinct, stable theta band was present with influencing behaviour (Fig. 5), alongside a small cluster of theta pixels that significantly differed between condition (Fig. 4B).
As theta was expected to support cognitive control, finding that theta band correlations with behaviour were specifically reliable amongst all bands provides strong evidence that midfrontal theta reflects adjustments of behaviour supported by cognitive control. Indeed, no significant difference in behavioural performance was found from Phase 1 to Phase 2, suggesting that the stability of midfrontal theta, which supports behaviour, may facilitate such behavioural stability.
In contrast, while theta was stable, the lack of delta relationships with behaviour from Phase 1 to Phase 2 hints at the possibility of additional changes across the two years. It is unclear why participants at Phase 1 used both delta and theta whereas in Phase 2, only theta influenced behaviour. These changes could simply reflect the effects of practice/training/regression to the mean or reflect maturation changes in prefrontal cortex. For now, these interpretations are speculative and require further exploration.
4.3. Conclusion
Here, we provide new insights into how medial prefrontal dynamics, identified as trial-varying theta oscillations, influence the efficacy of cognitive control. Making use of single-trial regression allowed us to reveal that trials with increased theta power resulted in better behavioural performance. We interpret these relationships as evidence that both proactive and reactive cognitive control processes utilise midfrontal theta dynamics and that fluctuations in theta power provides a mechanism for trial-by-trial changes in behavioural performance. Importantly, by using a cued-trial task switching paradigm we were able to provide strong evidence that cognitive control rather than processes that coincide with cognitive control (e.g., general RT slowing) per se uses frontal theta. Importantly, we made use of a large cohort that included a subset of longitudinal data to answer questions about the stability of these electrophysiological indices of mPFC engagement. In sum, we demonstrate further evidence that midfrontal theta serves as a functional marker of cognitive control employment and that mPFC adapts to changing contexts to support behaviour.
Supplementary Material
Acknowledgements
This work was supported by Australian Research Council Discovery Projects to FK (DP120100340, DP170100756). MM and PS are supported by Australian Postgraduate Awards. JFC is supported by NIGMS 1P20GM109089-01A1. We thank Gavin Cooper for paradigm programming and all past and present members of the Age-ility Project for assistance with data collection/entry. Thanks to two anonymous reviewers and Prof. Aina Puce for constructive comments during peer review.
Footnotes
Note, while Phase 1 and Phase 2 showed no significant differences, for completeness we ran the above ANOVAs contrasting Phase 2 with outsample. No Group differences were found for RT or error rate, although a small Group × Condition interaction term was seen for error rates – with Phase 2 making on average 1% more errors in the informative switch vs. non-informative contrast than outsample.
Declaration of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2019.01.022.
References
- Astle D, Jackson G, Swainson R, 2008. The role of spatial information in advance task-set control: an event-related potential study. Eur. J. Neurosci 28 (7), 1404–1418. [DOI] [PubMed] [Google Scholar]
- Barceló F, Cooper PS, 2018. An information theory account of late frontoparietal ERP positivities in cognitive control. Psychophysiology 55 (3), e12814. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Escera C, Corral MJ, Periáñez JA, 2006. Task switching and novelty processing activate a common neural network for cognitive control. J. Cognit. Neurosci 18 (10), 1734–1748. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD, 2001. Conflict monitoring and cognitive control. Psychol. Rev 108 (3), 624. [DOI] [PubMed] [Google Scholar]
- Brydges CR, Barceló F, 2018. Functional dissociation of latency-variable, stimulus-and response-locked target P3 sub-components in task-switching. Front. Hum. Neurosci 12, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK, 2012. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron 76 (4), 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Castellanos J, 2016. Identification of canonical neural events during continuous gameplay of an 8-bit style video game. Neuroimage 133, 1–13. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cognit. Sci 18 (8), 414–421. 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB, 2010. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage 49 (4), 3198–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Meyer A, Hajcak G, 2017. Error-specific cognitive control alterations in generalized anxiety disorder. Biol. Psychiatr.: Cognit. Neurosci. Neuroimag 2 (5), 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ, 2015. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J. Physiol. Paris 109 (1–3), 3–15. 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ, 2012. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology 49 (2), 220–238. 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, 2014. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci 37 (9), 480–490. 10.1016/j.tins.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Cohen MX, 2016. Midfrontal theta tracks action monitoring over multiple interactive time scales. Neuroimage 141, 262–272. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF, 2011. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front. Psychol 2, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Donner TH, 2013. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol 110 (12), 2752–2763. 10.1152/jn.004792013. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J, 2008. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 1238, 127–142. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS, 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci 16 (9), 1348–1355. 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PS, Darriba A, Karayanidis F, Barcelo F, 2016. Contextually sensitive power changes across multiple frequency bands underpin cognitive control. Neuroimage 132, 499–511. 10.1016/j.neuroimage.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Cooper PS, Wong AS, Fulham WR, Thienel R, Mansfield E, Michie PT, Karayanidis F, 2015. Theta frontoparietal connectivity associated with proactive and reactive cognitive control processes. Neuroimage 108, 354–363. 10.1016/j.neuroimage.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Cooper PS, Wong ASW, McKewen M, Michie PT, Karayanidis F, 2017. Frontoparietal theta oscillations during proactive control are associated with goalupdating and reduced behavioral variability. Biol. Psychol 129, 253–264. 10.1016/j.biopsycho.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Cunillera T, Fuentemilla L, Periañez J, Marco-Pallarès J, Krämer UM, Cámara E, Rodríguez-Fornells A, 2012. Brain oscillatory activity associated with task switching and feedback processing. Cognit. Affect Behav. Neurosci 12 (1), 16–33. [DOI] [PubMed] [Google Scholar]
- Dutilh G, van Ravenzwaaij D, Nieuwenhuis S, van der Maas HL, Forstmann BU, Wagenmakers EJ, 2012. How to measure post-error slowing: a confound and a simple solution. J. Math. Psychol 56 (3), 208–216. [Google Scholar]
- Engel AK, Fries P, 2010. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol 20 (2), 156–165. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Gagne C, Nyhus E, Masters S, Wiecki TV, Cavanagh JF, Badre D, 2015. fMRI and EEG predictors of dynamic decision parameters during human reinforcement learning. J. Neurosci 35 (2), 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson GD, 2012. Testing Statistical Assumptions. Statistical Associates Publishing, Asheboro, NC. [Google Scholar]
- Gratton G, Cooper P, Fabiani M, Carter CS, Karayanidis F, 2018. Dynamics of cognitive control: theoretical bases, paradigms, and a view for the future. Psychophysiology 55 (3), e13016 10.1111/psyp.13016. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E, 1992. Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen 121 (4), 480. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, 2012. Motivation of extended behaviors by anterior cingulate cortex. Trends Cognit. Sci 16 (2), 122–128. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Oja E, 2000. Independent component analysis: algorithms and applications. Neural Network. 13 (4), 411–430. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Thienel R, Karayanidis F, 2015. Task Switching Processes.
- Karayanidis F, Jamadar S, Ruge H, Phillips N, Heathcote A, Forstmann BU, 2010. Advance preparation in task-switching: converging evidence from behavioral, brain activation, and model-based approaches. Front. Psychol 1, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Keuken MC, Wong A, Rennie JL, de Hollander G, Cooper PS, Forstmann BU, 2016. The Age-ility Project (Phase 1): structural and functional imaging and electrophysiological data repository. Neuroimage 124 (Pt B), 1137–1142. 10.1016/j.neuroimage.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Mansfield EL, Galloway KL, Smith JL, Provost A, Heathcote A, 2009. Anticipatory reconfiguration elicited by fully and partially informative cues that validly predict a switch in task. Cognit. Affect Behav. Neurosci 9 (2), 202–215. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I, 2010. Control and interference in task switching—a review. Psychol. Bull 136 (5), 849. [DOI] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A, 2013. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol 245, 15–26. [DOI] [PubMed] [Google Scholar]
- Lavric A, Mizon GA, Monsell S, 2008. Neurophysiological signature of effective anticipatory task-set control: a task-switching investigation. Eur. J. Neurosci 28 (5), 1016–1029. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164 (1), 177–190. 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Monsell S, 2003. Task switching. Trends Cognit. Sci 7 (3), 134–140. [DOI] [PubMed] [Google Scholar]
- Morel P, 2018. Gramm: grammar of graphics plotting in matlab. Journal of Open Source Software 3 (23), 568. [Google Scholar]
- Müller N, Schlee W, Hartmann T, Lorenz I, Weisz N, 2009. Top-down modulation of the auditory steady-state response in a task-switch paradigm. Front. Hum. Neurosci 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munneke G-J, Nap TS, Schippers EE, Cohen MX, 2015. A statistical comparison of EEG time-and time–frequency domain representations of error processing. Brain Res. 1618, 222–230. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M, 2013. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat. Neurosci 16 (12), 1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessler D, Friedman D, Johnson R Jr., 2012. A new account of the effect of probability on task switching: ERP evidence following the manipulation of switch probability, cue informativeness and predictability. Biol. Psychol 91 (2), 245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Van Opstal F, Gevers W, Fias W, Verguts T, 2009. Posterror slowing: an orienting account. Cognition 111 (2), 275–279. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM, 2011. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci 2011, 156869 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Rodger B, 1977. What does a man do after he makes an error? An analysis of response programming. Q. J. Exp. Psychol 29 (4), 727–743. [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S, 2004. The role of the medial frontal cortex in cognitive control. Science 306 (5695), 443–447. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Freunberger R, Pecherstorfer T, Hanslmayr S, Doppelmayr M. l., 2006. Relevance of EEG alpha and theta oscillations during task switching. Exp. Brain Res 170 (3), 295–301. [DOI] [PubMed] [Google Scholar]
- Schlegel A, 2012. CronbachAlpha. https://www.mathworks.com/matlabcentral/fileexchange/38320-cronbach-s-alpha:Mathworks.
- Shenhav A, Botvinick MM, Cohen JD, 2013. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79 (2), 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN, Spinks JA, Näätänen R, Lyytinen H, 2002. The Orienting Response in Information Processing. Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Tarantino V, Mazzonetto I, Vallesi A, 2016. Electrophysiological correlates of the cognitive control processes underpinning mixing and switching costs. Brain Res. 1646, 160–173. [DOI] [PubMed] [Google Scholar]
- Tornay FJ, Milán EG, 2001. A more complete task-set reconfiguration in random than in predictable task switch. The Quarterly Journal of Experimental Psychology Section A 54 (3), 785–803. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y, 2006. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J. Neurophysiol 95 (5), 2987–3000. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y, Sasaki K, 2009. Theta oscillations in primate prefrontal and anterior cingulate cortices in forewarned reaction time tasks. J. Neurophysiol 103 (2), 827–843. [DOI] [PubMed] [Google Scholar]
- Valadez EA, Simons RF, 2018. The power of frontal midline theta and post-error slowing to predict performance recovery: evidence for compensatory mechanisms. Psychophysiology 55 (4), e13010. [DOI] [PubMed] [Google Scholar]
- van Driel J, Gunseli E, Meeter M, Olivers CN, 2017. Local and interregional alpha EEG dynamics dissociate between memory for search and memory for recognition. Neuroimage 149, 114–128. [DOI] [PubMed] [Google Scholar]
- van Driel J, Swart JC, Egner T, Ridderinkhof KR, Cohen MX, 2015. (No) time for control: frontal theta dynamics reveal the cost of temporally guided conflict anticipation. Cognit. Affect Behav. Neurosci 15 (4), 787–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noordt SJ, Campopiano A, Segalowitz SJ, 2016. A functional classification of medial frontal negativity ERPs: theta oscillations and single subject effects. Psychophysiology 53 (9), 1317–1334. [DOI] [PubMed] [Google Scholar]
- van Noordt SJ, Desjardins JA, Segalowitz SJ, 2015. Watch out! Medial frontal cortex is activated by cues signaling potential changes in response demands. Neuroimage 114, 356–370. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS, 2006. Conflict and cognitive control in the brain. Curr. Dir. Psychol. Sci 15 (5), 237–240. [Google Scholar]
- Vandierendonck A, Liefooghe B, Verbruggen F, 2010. Task switching: interplay of reconfiguration and interference control. Psychol. Bull 136 (4), 601. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E, 2005. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J. Neurosci 25 (3), 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, 2018. An adaptive orienting theory of error processing. Psychophysiology 55 (3), e13041. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S, 2010. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. Unit. States Am 107 (11), 5248–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.