Abstract
Peripheral artery disease (PAD) is caused by atherosclerotic occlusions of vessels outside the heart, particularly those of the lower extremities. Angiogenesis is one critical physiological response to vessel occlusion in PAD, but our understanding of the molecular mechanisms involved in angiogenesis is incomplete. Dual specificity phosphatase 5 (DUSP5) has been shown to play a key role in embryonic vascular development, but its role in post-ischemic angiogenesis is not known. We induced hind limb ischemia in mice and found robust upregulation of DUSP5 expression in ischemic hind limbs. Moreover, in vivo knockdown of DUSP5 resulted in impaired perfusion recovery in ischemic limbs and was associated with increased limb necrosis. In vitro studies showed upregulation of DUSP5 in human endothelial cells exposed to ischemia, and knockdown of DUSP5 in these ischemic endothelial cells resulted in impaired endothelial cell proliferation and angiogenesis, but did not alter apoptosis. Finally, we show that these effects of DUSP5 on post-ischemic angiogenesis are a result of DUSP5-dependent decrease in ERK1/2 phosphorylation and p21 protein expression. Thus, we have identified a role of DUSP5 in post-ischemic angiogenesis and implicated a DUSP5-ERK-p21 pathway that may serve as a therapeutic target for the modulation of post-ischemic angiogenesis in PAD.
Keywords: angiogenesis, critical limb ischemia (CLI), peripheral artery disease (PAD), DUSP5, ERK, p21
Introduction
Peripheral artery disease (PAD) is the result of atherosclerotic occlusion of vessels outside the heart, with the lower extremity being the most common site of disease development.1,2 PAD affects the lives of more than 200 million individuals worldwide.1,2 In fact, it is estimated that by the 8th decade of life, one in five individuals are affected by PAD.1,2 Despite the overall advances made in managing cardiovascular disease,3 PAD rates have continued to increase in a number of cohorts studied recently.4–6 Current pharmaceutical therapies to combat PAD remain meager due to an incomplete understanding of the molecular and biological mechanisms that result in PAD manifestation. As a consequence, there is a dire need to better understand these mechanisms in order to develop new therapeutic approaches to combat the disease. In experimental PAD (e.g. hind limb ischemia (HLI)), adaptive mechanisms involved in restoring tissue perfusion include arteriogenesis and angiogenesis.7 One of the key processes in angiogenesis is endothelial cell (EC) proliferation, and studies have shown that this process is mediated through the mitogen-activated protein kinase (MAPK) pathway.8–10
Dual specificity phosphatase 5 (DUSP5) is a member of the dual specificity protein phosphatase subfamily that inactivate their target kinases by dephosphorylating both the phosphoserine/threonine and phosphotyrosine residues. DUSP5 is known to dephosphorylate the mitogen activated protein (MAP) kinases ERK1/211,12 and has been shown to play an important role in embryonic vasculature development.13 Additionally, mutation in DUSP5 has been associated with formation of hemangiomas in humans.13 However, whether DUSP5 plays a role in post-ischemic angiogenesis is not known. Given the known role of DUSP5 in vascular development and regulation of the MAPK pathway, we hypothesized that DUSP5 expression may regulate post-ischemic angiogenesis and speculated that investigation of this pathway may provide some mechanistic insight into post-ischemic adaption in PAD.
Methods
Mice, experimental PAD/HLI
In all experiments, male C57BL/6 mice aged 12–14 weeks obtained from the Jackson Laboratory (Bar Harbor, ME, USA) were used. After anesthesia induction (ketamine 90 mg/kg and xylazine 10 mg/kg), experimental PAD or HLI was achieved by unilateral femoral artery ligation and excision (Figure 1), as described previously.14–17 Perfusion in the ischemic and contralateral non-ischemic limbs were measured with the use of a laser speckle contrast imaging device (PeriCam PSI NR; Perimed, Stockholm, Sweden). Perfusion was expressed as the ratio of the left (ischemic) to right (non-ischemic) hind limb and was measured on days 0 and 3, and weekly post-surgery (Figure 2).14,18,19 In mice that developed necrosis, the extent of necrosis was scored as follows: Stage 1, involving only toes; Stage 2, extending to dorsum pedis; Stage 3, extending to crus; and Stage 4, extending to thigh or complete necrosis (Figure 2).
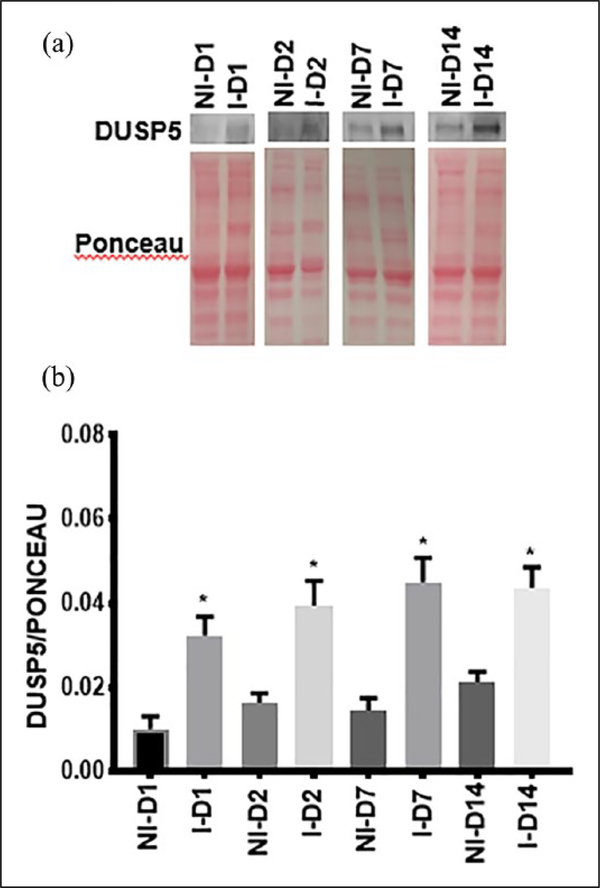
Figure 1.
DUSP5 is upregulated in ischemic mouse gastrocnemius muscle following HLI. (a) Western blot of protein samples isolated from NI and I from day 1 to day 14 post-HLI. (b) DUSP5 protein expression is upregulated from D1 of HLI. Bars represent mean ± SEM (unpaired t-test, n = 4, *p < 0.02).
D, day; DUSP5, dual specificity phosphatase 5; HLI, hind limb ischemia; I, ischemia; NI, non-ischemia.
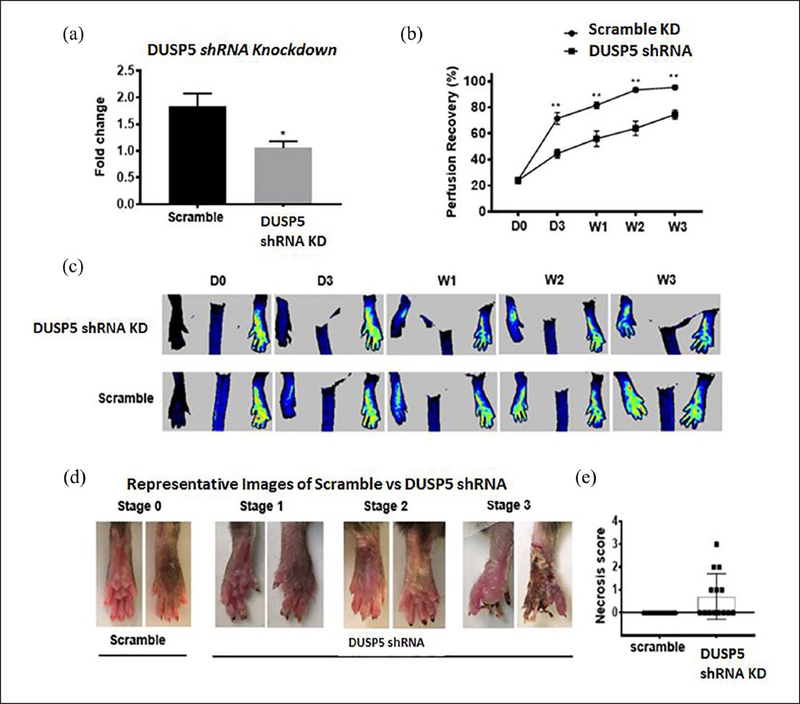
Figure 2.
Knockdown of DUSP5 in C57BL/6 mice is associated with impaired perfusion recovery and limb necrosis following HLI. (a) Gene expression of DUSP5 in control shRNA versus DUSP5 shRNA-treated day 3 post-ischemic mouse GA muscle tissue lysates. (b) Mice treated with DUSP5 shRNA showed impaired perfusion recovery compared with mice treated with control shRNA. Bars represent mean ± SEM (n = 13–14, **p < 0.01). (c) Representative perfusion images of control versus DUSP5 shRNA-treated mice post-HLI. (d) Representative images of necrosis in the control versus DUSP5 shRNA-treated mice. (e) Necrosis score in control shRNA versus DUSP5 shRNA-treated mice.
D, day; DUSP5, dual specificity phosphatase 5; GA, gastrocnemius; HLI, hind limb ischemia; KD, knockdown; shRNA, small hairpin RNA; W, week.
Knockdown experiments
In vivo DUSP5 knockdown was achieved by delivery of small hairpin RNA (shRNA) plasmid targeting DUSP5 through ultrasound-mediated bubble destruction, as previously described.18,20,21 Briefly, DUSP5 shRNA plasmid (A, B, C, D) and its scramble counterpart (S) were purchased from OriGene (Rockville, MD, USA; cat# TL518256); 200 μg of each plasmid was combined with cationic microbubbles (2 × 108) mixed in 100 μL sterile saline and infused via retro-orbital injection over 1 minute. Control animals received 800 μg of the scramble plasmid. The gastrocnemius of the left hind limb was exposed to ultrasound during the injection and for an additional 9 minutes. Ultrasound exposure was performed using a harmonic power Doppler imaging system (Acuson Sequoia C512 LCD) with transducer (Acuson 4V1) (Siemens Medical Solutions, Malvern, PA, USA) at 2 MHz, a pulsing interval of 1 second, a pulse repetition frequency of 1 Hz, and a mechanical index (MI) of 1.9. Five days post-plasmid injection, mice were subjected to HLI. DUSP5 mRNA knockdown in ischemic mouse hind limbs was determined by quantitative polymerase chain reaction (qPCR) 3 days post-HLI (Figure 2).
In vitro, DUSP5 knockdown was achieved by CRISPR/Cas9 KO Plasmid (Santa Cruz Biotechnology, Dallas, TX, USA; cat# sc-401837); in Figure 3 all other in vitro knockdown of DUSP5 was achieved by small interferring Ribinucleic Acid (siRNA) (Ambion, Austin, TX, USA; cat #104726). For siRNA transfection, Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA; cat# 13778–030) was used following the manufacturer protocol, while UltraCruz transfection reagent, and plasmid transfection medium were used for CRISPR/Cas9 (Santa Cruz Biotechnology; cat# 395739). According to the manufacturer, the CRISPR/Cas9 plasmid enables the identification and cleavage of specific genes by utilizing guide RNA (gRNA) sequences derived from the Genome-Scale CRISPR Knock-Out (GeCKO) v2 library developed in the Zhang Laboratory at the Broad Institute (Cambridge, MA, USA).22,23 Knockdown of p21 was achieved by siRNA (Ambion; cat# 107710) using Lipofectamine RNAiMAX per manufacturer protocol. Apoptosis assay and tube formation assays were assessed 24 hours after transfection.
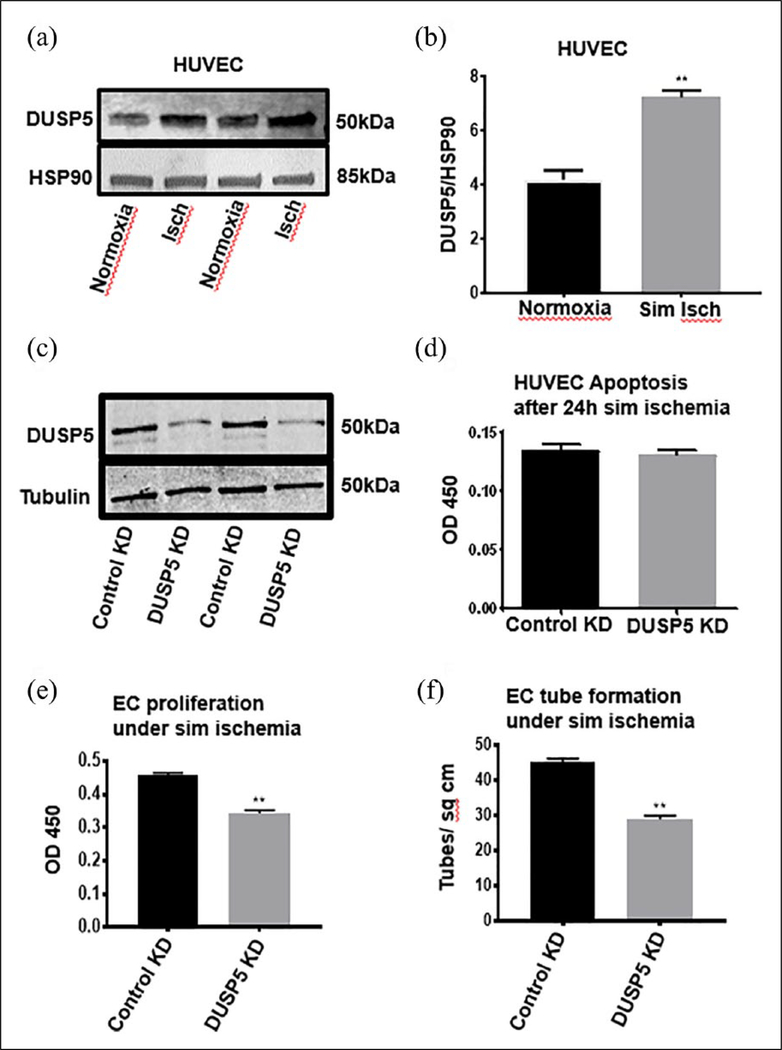
Figure 3.
DUSP5 protein expression is upregulated in ischemic endothelial cells (HUVECs). Cells were grown under normal conditions (21% oxygen and complete growth media) and simulated ischemia (2% oxygen and starvation media) for 24 hours. (a) Western blots of DUSP5 protein show expression in EC is upregulated in ischemia compared to normoxia. (b) Quantification of DUSP5 protein expression (n = 4–5, *p < 0.05); (HUVEC, **p < 0.01) (unpaired t-test). In parts (c)–(f), DUSP5 KD impairs proliferation and tube formation in ECs under simulated ischemia. (c) A representative western blot of DUSP5 KD. (d) DUSP5 KD in ECs results in no change in apoptosis compared to control KD (n = 7, *p > 0.05) (p = 0.07). (e) DUSP5 KD results in significant impairment in EC proliferation (n = 7, **p < 0.01). (f) DUSP5 KD significantly impairs EC tube formation (n = 7, **p < 0.01).
Bars represent means ± SEM.
DUSP5, dual specificity phosphatase 5; EC, endothelial cell; HUVECs, human umbilical vein endothelial cells; Isch, ischemia; KD, knockdown; sim, simulated.
Cell lines, cell culture, and simulated ischemia
C2C12 (ATCC, Manassas, VA, USA; cat# CRL-1772) and vascular smooth muscle cells (VSMC) were grown in Dulbecco’s Modified Eagle Medium (DMEM) + 10% fetal bovine serum (FBS) (Cell Applications, Inc., San Diego, CA, USA; cat# 250K-05n). Pooled human umbilical vein endothelial cells (HUVEC) were purchased from Cell Applications, Inc. (cat# 200K-05n) and grown in standard EC growth medium (Cell Applications, Inc.; cat# 211–500) with 10% FBS. Simulated ischemia was achieved, as previously described.15,16 Briefly, cells were grown under hypoxic conditions (2% oxygen; hypoxic chamber BioSpherix, Lacona, NY, USA) in EC serum starvation media (Cell Applications, Inc.; cat# 209–250) for 24 hours. Control cells (normoxia) were grown in 10% FBS EC media + 21% oxygen. To simulate ischemia in C2C12 and VSMC cells, they were grown in DMEM without serum + 2% oxygen while controls cells were grown in DMEM with 10% serum and 21% oxygen
Protein analysis
Protein expression was measured by western blot analysis using anti-DUSP5 (Santa Cruz Biotechnology; cat# sc-393801), anti-pERK1/2 (Cell Signaling Technology, Denver, MA, USA; cat# 4376), anti-ERK1/2 (Cell Signaling Technology; cat# 9102), and anti-p21 (Cell Signaling Technology; cat# 2947) primary antibodies. Equal protein loading was achieved by normalizing the band of interest to ponceau stain in the same region of the blot (Figure 1 only) or to HSP90 or tubulin (Cell Signaling Technology; cat# 9474). Western blot gel images were captured by the Odyssey Infrared Imaging System (Li-COR Biosciences, Lincoln, NE, USA). Quantification of the bands was performed by Scion Image software (Torrance, CA, USA) and Image Studio Lite, version 5.2 (Li-COR Biosciences).
Cell proliferation, apoptosis, and tube formation assays
HUVECs were either transfected with CRISPR/Cas9 DUSP5 KO Plasmid, shDUSP5, p21 siRNA or mock transfected. Post-transfection, cells were plated in a 96-well plate and were exposed to simulated ischemia for 24 hours. At the end of the incubation, apoptosis in cells was determined using a TUNEL assay (TiterTACS; Trevigen, Gaithersburg, MD, USA), as previously described.14 Cell proliferation was assessed using tetrazolium dye incorporation (BioVision, Milpitas, CA, USA), as previously described.16,18 Each experiment was repeated at least three times. EC tube formation was performed, as previously described.16,18 Briefly, HUVECs were transfected with plasmids or siRNA, as stated above. At 72 hours post-transfection, cells were plated on matrigel (Thomas Scientific, Corning, NY, USA; cat# 354234) at a cell density of 30,000 cells/well in a 96-well plate and exposed to simulated ischemia. EC tube formation was assessed 4–6 hours after plating, as previously described.16,20,24 The number of complete tubes was counted and expressed as tube number per cm2. Each experiment was repeated at least three times.
Statistical analysis
All measurements were expressed as mean ± SEM. Statistical comparisons between two groups (e.g. control vs knockdown) at a specific time point were performed with the independent Student’s t-test where appropriate. Comparisons of more than two groups at a time were performed with analysis of variance. In all cases, a p-value < 0.05 was considered statistically significant. The software used for statistical analysis was GraphPad Prism 7 (San Diego, CA, USA).
Results
DUSP5 protein levels are significantly upregulated in ischemic mouse hind limbs
We analyzed the expression levels of DUSP5 protein in the hind limbs (gastrocnemius muscle tissue) of C57BL/6 mice at different time points after performing experimental PAD (HLI). A significant increase in DUSP5 protein expression was observed, starting at day 1 (ratio of DUSP5 to ponceau in non-ischemic vs ischemic gastrocnemius: 0.010 ± 0.003 vs 0.032 ± 0.005, *p < 0.02, n = 4) post-ischemia and remained upregulated at day 14 post-HLI (ratio of DUSP5 to ponceau in non-ischemic vs ischemic gastrocnemius: 0.021 ± 0.002 vs 0.044 ± 0.005, *p < 0.02, n = 4) (Figure 1a and b).
DUSP5 knockdown in vivo impairs perfusion recovery in C57BL/6 mice and is associated with increased limb necrosis
In this set of experiments, we explored the physiologic impact of in vivo DUSP5 upregulation in ischemia by knocking down DUSP5 in C57BL/6 mice and assessing perfusion recovery following induction of HLI. Mice were treated with shRNA targeting DUSP5 to knock down DUSP5 protein expression and were subsequently subjected to HLI (see details in Methods). We observed a significant decrease in DUSP5 mRNA expression in the shRNA-treated gastrocnemius muscles 3 days post-HLI (Figure 2a). Perfusion recovery was followed over time (see Methods). By day 3 post-HLI, we could observe impaired perfusion recovery in the mice treated with DUSP5 shRNA compared to controls treated with scramble (Figure 2b and c). This difference in perfusion persisted until 3 weeks post-HLI when the experiment was completed. (Week 3: % perfusion recovery in mice treated with DUSP5 shRNA vs scramble:74.58 ± 3.44 vs 95.37 ± 1.31, n = 13–14, **p < 0.01, Figure 2b). Additionally, limb necrosis was observed in six out of the 14 DUSP5 shRNA-treated mice, while none of the control mice developed limb necrosis (Figure 2d and e). Three mice developed Stage 1 necrosis, two mice developed Stage 2 necrosis, and one mouse developed Stage 3 necrosis (please see Methods for a definition of necrosis stages.)25
Endothelial and skeletal muscle cells but not VSMCs show significant upregulation of DUSP5 protein in ischemia
To better understand what cell types in the hind limb are capable of upregulating DUSP5, we conducted in vitro experiments on pooled HUVECs, skeletal muscle cells (C2C12) and VSMCs to test whether DUSP5 protein expression changes under simulated ischemia. We analyzed DUSP5 protein expression in cells exposed to simulated ischemia compared to control cells exposed to normoxic conditions. We found a significant upregulation of DUSP5 protein in ECs exposed to 24 hours of simulated ischemia compared to normoxia (DUSP5/HSP90, normoxia vs ischemia: 4.18 ± 0.35 vs 7.23 ± 0.25, **p < 0.01, n = 4–5) and C2C12 cells (0.42 ± 0.01 vs 1.77 ± 0.10, **p < 0.01, n = 4–5). However, we observed no significant change in the DUSP5 protein expression in VSMC (0.53 ± 0.21 vs 0.61 ± 0.08, p > 0.05, n = 4) (C2C12 and VSMC data in online supplementary Figure 1).
DUSP5 knockdown in endothelial cells exposed to simulated ischemia impaired cell proliferation and in vitro angiogenesis but did not alter the level of apoptosis
To gain insight into the role of DUSP5 upregulation in ischemia, we investigated the effect of DUSP5 knockdown on EC proliferation, apoptosis and in vitro angiogenesis in simulated ischemia. DUSP5 was successfully knocked down (Figure 3c), as detailed in the Methods section. Analysis of EC proliferation showed decreased proliferation in the DUSP5 knockdown cells compared to control cells. (OD 450 control vs knockdown: 0.45 ± 0.006 vs 0.34 ± 0.001, **p < 0.01, n = 7) (Figure 3e). We observed no significant difference in EC apoptosis in DUSP5 knockdown cells compared to control cells (OD 450 control vs knockdown: 0.13 ± 0.01 vs 0.12 ± 0.02, p > 0.05, n = 7) (Figure 3d). Lastly, we analyzed the effect of DUSP5 knockdown on EC tube formation, a measure of in vitro angiogenesis. Our results showed a significant impairment in tube formation by ECs in which DUSP5 had been knocked down compared to control cells (tube/cm2: 45 ± 1.08 vs 28.75 ± 1.10, **p < 0.01, n = 7) (Figure 3f). Lastly, we assessed the effects of knocking down DUSP5 on EC apoptosis.
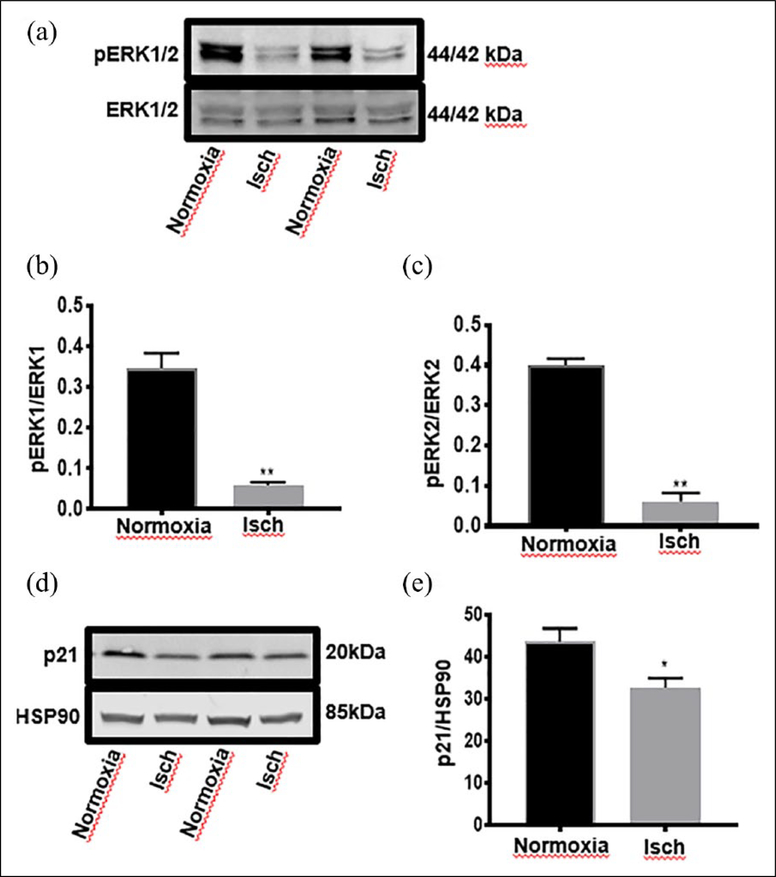
In simulated ischemia where DUSP5 is upregulated, EC ERK1/2 protein phosphorylation and p21 protein expression are decreased
Given findings from prior studies indicating that DUSP5 can dephosphorylate ERK1/2,11,12 we investigated the phosphorylation status of ERK1/2 in EC exposed to simulated ischemia where we have shown DUSP5 is upregulated. ECs were exposed to simulated ischemia for 24 hours. The 24-hour time point was chosen because this is a time point where we observed increased DUSP5 protein expression. Our results show a significant decrease in the level of ERK1 and 2 protein phosphorylation in ECs exposed to simulated ischemia compared to control ECs in normoxia (pERK1 to ERK1, normoxia vs ischemia: 0.35 ± 0.04 vs 0.06 ± 0.01; pERK2 to ERK2: 0.41 ± 0.02 vs 0.06 ± 0.02, **p < 0.01, n = 4), (Figure 4a, b and c). Given that ERK phosphorylation has been previously shown to regulate the cell cycle through p21,26,27 we sought to understand whether decreased ERK1/2 phosphorylation is associated with changes in p21 protein expression. We found a significant decrease in p21 protein expression in ischemic ECs compared to non-ischemic cells (p21/HSP90, normoxia vs ischemia: 42.7 ± 3.42 vs 32.67 ± 2.28, *p < 0.05, n = 4) (Figure 4d and e).
Figure 4.
ERK1/2 phosphorylation is significantly decreased in ECs exposed to 24 hours of simulated ischemia compared to normoxia. (a) Western blot analysis of pERK1/2 and total ERK1/2. (b) Quantification of bands in pERK1 to total ERK1 (n = 4–6, **p < 0.01); (c) Quantification of bands in pERK2/ERK2 (**p < 0.01); p21 protein expression is significantly decreased in ECs exposed to 24 hours of simulated ischemia compared to normoxia. (d) Representative western blot analysis of p21 expression. (e) Quantification of bands in p21/HSP90 (n = 4, *p < 0.05).
Isch, ischemia.
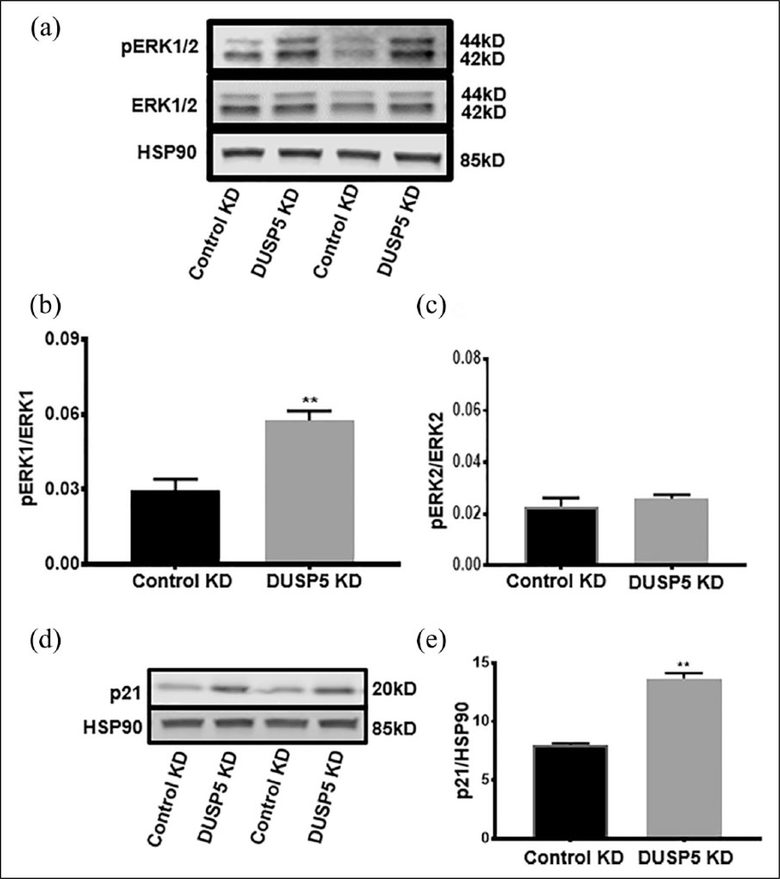
DUSP5 knockdown in ECs increased ERK phosphorylation and p21 expression following exposure to simulated ischemia
In light of the above findings, that knocking down DUSP5 impairs EC function in simulated ischemia, we sought to understand the potential mechanisms involved. Since DUSP5 has been previously implicated in the regulation of MAP kinases, we assessed ERK phosphorylation in ischemic EC transfected with DUSP5 siRNA. We also assessed p21 expression given it is downstream of ERK in the MAPK pathway and its known role in cell cycle regulation. Following EC transfection with DUSP5 siRNA, cells were exposed to simulated ischemia for 24 hours (see details in Methods) and the extent of ERK phosphorylation was assessed. We observed a significant increase in the ERK1 protein phosphorylation in the DUSP5 siRNA-treated cells compared to control siRNA-treated cells (pERK1/ERK1, control vs DUSP5 knockdown: 0.02 ± 0.004 vs 0.05 ± 0.003, **p < 0.01, n = 4) (Figure 5a and b). However, pERK2/ERK2 showed no significant difference between control vs DUSP5 knockdown (pERK2/ERK2, control vs DUSP5 knockdown: 0.022 ± 0.003 vs 0.026 ± 0.002, p = 0.4) (Figure 5c). Analysis of p21 expression showed a significant increase in p21 protein expression in DUSP5 knockdown compared to control siRNA-treated cells (p21/HSP90, control vs DUSP5 knockdown: 8.01 ± 0.13 vs 13.6 ± 0.46, **p < 0.01, n = 4) (Figure 5d and e). Taken together, these data suggest that DUSP5 may regulate EC proliferation and angiogenesis via an ERK-p21 pathway.
Figure 5.
DUSP5 KD in ECs results in a significant increase in phosphorylated ERK1 and upregulation of p21 in simulated ischemia. (a) Western blot of pERK/ERK in control vs DUSP5 KD. (b) Quantification of western blot bands. pERK1 vs ERK1 shows a significant upregulation in control compared to DUSP5 KD (n = 4, **p < 0.01). (c) pERK2/ERK2 shows no significant difference between control versus DUSP5 KD. (d) Western blot of p21 protein expression in control versus DUSP5 KD. (e) Quantification of p21/HSP90 western blot (n = 4, **p < 0.01); (p = 0.001).
DUSP5, dual specificity phosphatase 5; KD, knockdown.
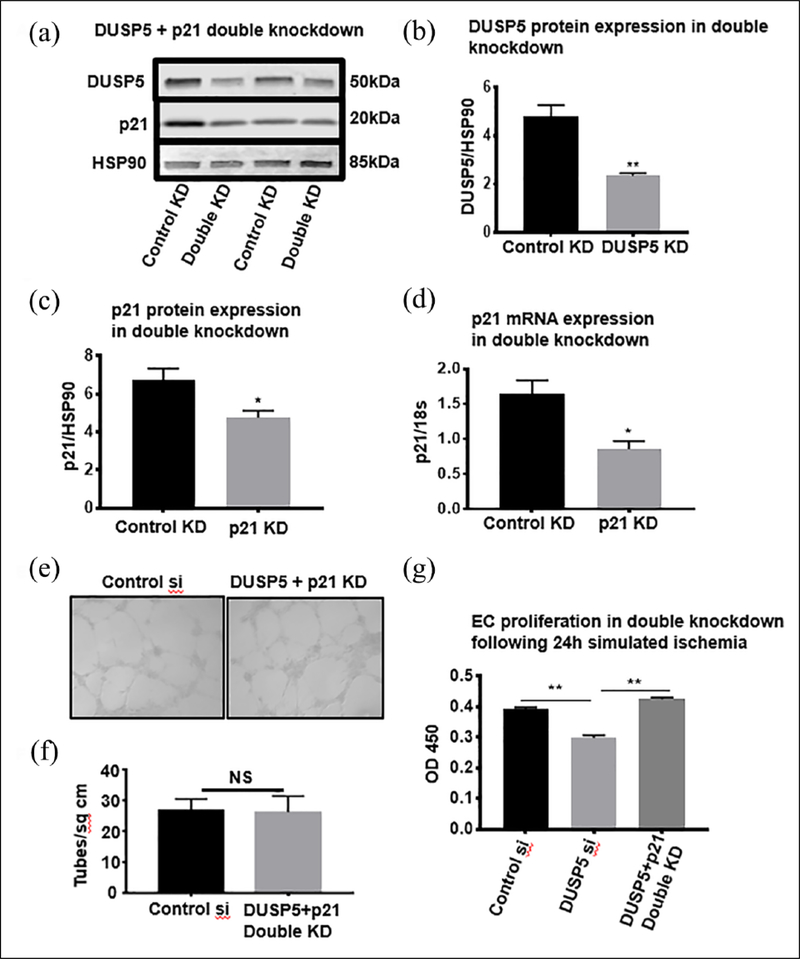
Knocking down p21 in DUSP5-deficient endothelial cells rescues cell proliferation and angiogenesis in simulated ischemia
To better understand the physiologic significance of p21 upregulation in ischemic ECs in which DUSP5 was knocked down (control knockdown vs DUSP5 knockdown, DUSP5/HSP90: 4.81 ± 0.45 vs 2.35 ± 0.10, **p < 0.01, n = 4) (Figure 6a and b), we further knocked down p21 in these cells (control knockdown vs p21 knockdown, p21/HSP90: 6.74 ± 0.60 vs 4.70 ± 0.30, *p < 0.05, n = 4 and p21/18s: 1.63 ± 0.20 vs 0.85 ± 0.11, *p < 0.02, n = 4–6) (Figure 6c and d) and analyzed EC proliferation and in vitro angiogenesis (see details in Methods). Unlike ECs in which DUSP5 only was knocked down and tube formation in simulated ischemia was impaired, DUSP5 + p21 double knockdown ECs did not show impaired tube formation in simulated ischemia when compared to control siRNA-treated cells (tube/cm2, control vs knockdown: 28.67 ± 0.91 vs 28.0 ± 1.0, NS = p = 0.6, n = 6) (Figure 6e and f). Moreover, analysis of EC proliferation showed increased proliferation in the DUSP5 + p21 double knockdown ECs compared to DUSP5 only knockdown ECs (OD 450 DUSP5 + p21 double knockdown vs DUSP5 only knockdown: 0.425 ± 0.02 vs 0.29 ± 0.02, **p < 0.01, n = 10) (Figure 6g).
Figure 6.
p21 KD recovers proliferation and tube formation in DUSP5-deficient ECs under simulated ischemia. (a) Double KD of DUSP5 + p21 in ECs was confirmed by DUSP5 and p21 protein expression in ECs (n = 4, *p < 0.05). (b) DUSP5 protein expression in double KD (n = 4, **p < 0.01). (c) p21 Protein expression in double KD. (d) p21 mRNA expression in double KD (n = 4–6, *p < 0.02, p = 0.014). (e) Representative image of EC tube formation in DUSP5 + p21 double KD. (f) Quantification of tubes/cm2 from experiment in (e) (n = 6, p > 0.05). (g) Recovery of endothelial proliferation in DUSP5 + p21 double KD (Kruskal–Wallis test, n = 8–10, **p < 0.01).
DUSP5, dual specificity phosphatase 5; ECs, endothelial cells; KD, knockdown; NS, not significant; OD, optical density.
Discussion
It is well established that reversible phosphorylation of proteins by protein tyrosine kinases (PTKs) and protein tyrosine phosphates (PTP) is a key regulatory mechanism in a myriad of physiologic processes.10,28,29 Interestingly, many more studies have focused on the understanding of PTKs in normal and pathological processes than on the role of PTPs in similar processes. This is likely due to the fact that PTKs were described many years before PTPs. Nevertheless, it is now understood that both PTKs and PTPs regulate the phosphorylated and dephosphorylated state of many proteins, and modulation of this balance is key to many physiologic processes.10,29
PAD is the result of atherosclerotic occlusion of vessels outside the heart, with the lower extremity being the most common site of development of PAD.30 Following vessel occlusion, adaptive mechanisms involved in restoring tissue perfusion include arteriogenesis and angiogenesis.31,32 Using mouse models of PAD, as well as in vitro studies, our lab and others have shown that adaption to ischemia can be modified by the underlying genetics of the mouse14,33 and the metabolic environment during ischemia.15,16 Moreover, we previously identified a genetic locus that modified outcomes following experimental PAD termed LSq-1.14,34 Following our initial identification of the LSq-1 locus, specific genes have been described within this locus that play key roles in post-ischemic adaption in the mouse following experimental PAD.35,36 Interestingly, many of these genes regulate post-ischemic angiogenesis at least in part through regulation of EC function (i.e. proliferation and apoptosis in ischemia).34,35
The MAPK pathway has been shown to be a key regulatory pathway in cell proliferation, including EC proliferation.8,10 Moreover, there is strong evidence that MAPKs are regulated by DUSPs.11,12 Interestingly, prior studies showed a prominent role for DUSP5 in embryonic vasculature development, and mutations in DUSP5 were associated with the formation of hemangiomas in humans.13 Until now, whether DUSP5 plays any role in post-ischemic blood vessel development was not known.
Our initial experiments explored whether DUSP5 expression is modulated in ischemic mouse hind limbs (Figure 1). Our data showed an upregulation of DUSP5 expression in the post-ischemic gastrocnemius muscles, thereby spurning our interest in investigating the protein’s role in post-ischemic perfusion recovery. Hence, we used shRNA to knock down DUSP5 protein expression in the hind limbs of mice and then induced HLI. We observed a significant decrease in DUSP5 protein expression and perfusion recovery starting at 3 days post-ischemia and lasting up to 3 weeks, when the study was completed. To gain insight into how DUSP5 may be regulating post-ischemic perfusion recovery, first we sought to gain insight into the cell types capable of DUSP5 upregulation in ischemia. We found, following in vitro simulated ischemia, DUSP5 upregulation occurred in skeletal muscle myoblast cell line C2C12 and primary human endothelial cells (HUVECS). Given the role of ECs in blood vessel formation, we focused the rest of our study on understanding the role of DUSP5 in the regulation of endothelial function in ischemia. Our data showed DUSP5 is upregulated in ischemic ECs, and this is associated with decreased ERK1/2 phosphorylation and decreased p21 expression. Knocking down DUSP5 in ischemic ECs resulted in increased ERK1 phosphorylation, increased p21 expression, decreased EC proliferation and in vitro angiogenesis, but no change in the extent of ERK2 phosphorylation and cell apoptosis.
Interestingly, there is strong evidence that the physiological consequence of ERK1/2 activation may be very different depending on the strength and duration of activation.37 In some settings, transient activation is key to achieving the physiological outcome, while in others sustained activation is key.37 Prior studies have shown that ERK1/2 activation plays a key role in EC proliferation, which is key to angiogenesis.8,38,39 However, Falco et al. reported that hyper-activation of ERK1/2 as a result of BAG3, mediated removal of DUSP6 in ECs was associated with increased p21 expression and cell cycle arrest.40 Our findings are consistent with theirs and other investigators that have shown similar cell cycle arrest following hyper-activation of ERK1/2.41,42 Moreover, studies have shown that some anti-angiogenic agents mediate their effect through increasing p21 expression.43 We speculate that cell cycle arrest may be a normal physiological response to hyper-activation of ERK1/2 in some settings to avoid inappropriate cell proliferation or development of malignancy. In fact, there is evidence that hyper-activation of ERK1/2 is associated with some malignancies.44 Hence, while ERK1/2 phosphorylation is a key process in angiogenesis, DUSP5 mediated de-phosphorylation of ERK1/2 following activation may be a critical step in the regulation of this process to prevent abnormal vessel formation. Consistent with this line of thought is the association of mutations in DUSP5 with hemangiomas.13 Taken together, our data suggest that DUSP5 is upregulated in ischemic ECs likely to prevent hyper-phosphorylation of ERK and p21 upregulation. As such, it prevents cell cycle arrest and allows normal EC adaptation in ischemia. Consistent with this hypothesis, we found knocking down p21 in ECs in which DUSP5 had been knocked down, restores cell proliferation and in vitro angiogenesis.
Study limitation
One limitation of this study is that we did not establish a direct link between ERK1/2 and p21 activation. Although this is beyond the scope of this current study, it would be an attractive area for future investigation.
Conclusion
We have identified a novel role for DUSP5 in post-ischemic angiogenesis. We speculate that this pathway involving DUSP5-ERK-p21 may serve as a therapeutic target for the modulation of post-ischemic angiogenesis in PAD.
Given the fact that there is currently no effective medical therapy for impaired blood flow seen in PAD, future studies to understand the mechanism regulating DUSP5 upregulation is ischemia may provide insight into how to manipulate this pathway to improve blood flow in PAD.
Supplementary Material
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Heart, Lung, and Blood Institute (R01 HL130399 to AO Dokun).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
The supplementary material is available online with the article.
References
- 1.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015; 116: 1509–1526. [DOI] [PubMed] [Google Scholar]
- 2.Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014; 60: 686–695.e2. [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality. Circ Res 2017; 120: 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens CD, Conte MS. Medical management of peripheral arterial disease: Bridging the “gap”? Circulation 2012; 126: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 5.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation 1995; 91: 1472–1479. [DOI] [PubMed] [Google Scholar]
- 6.Barrett PM, Wall CAM, Stack AG. Peripheral artery disease prevalence and mortality trends of United States dialysis population: 1995–2005. Irish J Med Sci 2010; 179: S409–S410. [Google Scholar]
- 7.Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 2002; 34: 775–787. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan R, Zabuawala T, Huang H, et al. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 2009; 4: e8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchiba M, Okajima K, Oike Y, et al. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res 2004; 95: 34–41. [DOI] [PubMed] [Google Scholar]
- 10.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev 2001; 22: 153–183. [DOI] [PubMed] [Google Scholar]
- 11.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: A gene family for control of MAP kinase function. FASEB J 2000; 14: 6–16. [PubMed] [Google Scholar]
- 12.Rushworth LK, Kidger AM, Delavaine L, et al. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci U S A 2014; 111: 18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pramanik K, Chun CZ, Garnaas MK, et al. Dusp-5 and Snrk-1 coordinately function during vascular development and disease. Blood 2009; 113: 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dokun AO, Keum S, Hazarika S, et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 2008; 117: 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dokun AO, Chen L, Lanjewar SS, et al. Glycaemic control improves perfusion recovery and VEGFR2 protein expression in diabetic mice following experimental PAD. Cardiovasc Res 2014; 101: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Okeke E, Ayalew D, et al. Modulation of miR29a improves impaired post-ischemic angiogenesis in hyperglycemia. Exp Biol Med (Maywood) 2017; 242: 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol 1999; 154: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dokun AO, Chen L, Okutsu M, et al. ADAM12: A genetic modifier of preclinical peripheral arterial disease. Am J Physiol Heart Circ Physiol 2015; 309: H790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazarika S, Farber CR, Dokun AO, et al. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation 2013; 127: 1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie A, Belcik T, Qi Y, et al. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc Imaging 2012; 5: 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tlaxca JL, Rychak JJ, Ernst PB, et al. Ultrasound-based molecular imaging and specific gene delivery to mesenteric vasculature by endothelial adhesion molecule targeted microbubbles in a mouse model of Crohn’s disease. J Control Release 2013; 165: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalem O, Sanjana NE, Hartenian E, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014; 343: 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ran FA, Hsu PD, Wright J, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013; 8: 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnaoutova I, Kleinman HK. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 2010; 5: 628–635. [DOI] [PubMed] [Google Scholar]
- 25.Dokun AO, Annex B. Genetic polymorphisms in peripheral arterial disease role of genomic methodologies In: Ginsburg GS, Willard HF (eds) Genomic and personalized medicine. Vols 1–2 Cambridge, MA: Elsevier, Academic Press, 2009. [Google Scholar]
- 26.Dangi S, Chen FM, Shapiro P. Activation of extracellular signal-regulated kinase (ERK) in G2 phase delays mitotic entry through p21CIP1. Cell Prolif 2006; 39: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang CY, Lee C, Kwon K-S. Extracellular signal-regulated kinase 2-dependent phosphorylation induces cytoplasmic localization and degradation of p21Cip1. Mol Cell Biol 2009; 29: 3379–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson KI, Brummer T, O’Brien PM, et al. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem J 2009; 418: 475–489. [DOI] [PubMed] [Google Scholar]
- 29.Alonso A, Sasin J, Bottini N, et al. Protein tyrosine phosphatases in the human genome. Cell 2004; 117: 699–711. [DOI] [PubMed] [Google Scholar]
- 30.Ouriel K Peripheral arterial disease. Lancet 2001; 358: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 31.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 2013; 10: 387–396. [DOI] [PubMed] [Google Scholar]
- 32.Jones WS, Schmit KM, Vemulapalli S, et al. Treatment strategies for patients with peripheral artery disease Comparative Effectiveness Review Number 118. AHRQ Publication No. 13-EHC090-EF. Rockville, MD: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 33.Fukino K, Sata M, Seko Y, et al. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun 2003; 310: 143–147. [DOI] [PubMed] [Google Scholar]
- 34.Okeke E, Dokun OA. Role of genetics in peripheral arterial disease outcomes: Significance of limb-salvage quantitative locus-1 genes. Exp Biol Med 2017; 243: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T, Cunningham A, Houston K, et al. Endothelial interleukin-21 receptor up-regulation in peripheral artery disease. Vasc Med 2016; 21: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClung JM, McCord TJ, Ryan TE, et al. BAG3 (Bcl-2–associated athanogene-3) coding variant in mice determines susceptibility to ischemic limb muscle myopathy by directing autophagy. Circulation 2017; 136: 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 1995; 80: 179–185. [DOI] [PubMed] [Google Scholar]
- 38.Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009; 28: 3412–3422. [DOI] [PubMed] [Google Scholar]
- 39.Miyake M, Goodison S, Gomes E, et al. Induction of endothelial proliferation and angiogenesis through activating the ERK1/2/EGF pathway mediate by CXC chemokine receptor 2 by chemokine (C-X-C motif) ligand 1. J Clin Oncol 2013; 31(suppl): 138. [Google Scholar]
- 40.Falco A, Festa M, Basile A, et al. BAG3 controls angiogenesis through regulation of ERK phosphorylation. Oncogene 2012; 31: 5153–5161. [DOI] [PubMed] [Google Scholar]
- 41.Cagnol S, Chambard J-C. ERK and cell death: Mechanisms of ERK-induced cell death – apoptosis, autophagy and senescence. FEBS J 2010; 277: 2–21. [DOI] [PubMed] [Google Scholar]
- 42.Bodart J-FL. Extracellular-regulated kinase—mitogen-activated protein kinase cascade: Unsolved issues. J Cell Biochem 2010; 109: 850–857. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Griffith EC, Sage J, et al. Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc Natl Acad Sci U S A 2000; 97: 6427–6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Yang X, Geng M, et al. Targeting ERK, an Achilles’ heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B 2018; 8: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.