Highlights
-
•
Severe disease is not common in both pregnant women and neonates.
-
•
Perinatal transmission occurs rarely and may not be linked to disease severity.
-
•
Vertical transmission has been assumed in 4 cases.
-
•
Transmission risk is low when the infection manifests during the third trimester.
-
•
Further research should provide evidence about the risk of congenital infection.
Keywords: Neonate, Pregnancy, Perinatal, Covid-19, SARS-CoV-2, Meta-analysis
Abstract
Evidence concerning coronavirus disease-19 (covid-19) in pregnancy is still scarce and scattered. This meta-analysis aims to evaluate maternal and neonatal outcomes in covid-19 pregnancies and identify factors associated with perinatal viral transmission. Medline, Scopus, CENTRAL, Web of Science and Google Scholar databases were systematically searched to 3 June 2020. Overall, 16 observational studies and 44 case reports/series were included. Fever was the most frequent maternal symptom, followed by cough and shortness of breath, while about 15 % of infected were asymptomatic. Severe disease was estimated to occur in 11 % of women in case reports/series and in 7 % (95 % CI: 4 %–10 %) in observational studies. Two maternal deaths were reported. The rate of neonatal transmission did not differ between women with and without severe disease (OR: 1.94, 95 % CI: 0.50–7.60). Preterm birth occurred in 29.7 % and 16 % (95 % CI: 11 %–21 %) in data obtained from case series and observational studies, respectively. Stillbirth occurred in 3 cases and 2 neonatal deaths were observed. Vertical transmission was suspected in 4 cases. Fever was the most common neonatal symptom (40 %), followed by shortness of breath (28 %) and vomiting (24 %), while 20 % of neonates were totally asymptomatic. In conclusion, the maternal and neonatal clinical course the infection is typically mild, presenting low mortality rates. The risk of vertical transmission is suggested to be low and may not be affected by the severity of maternal disease. Further large-scale studies are needed to clarify the risk factors associated with viral transmission and severe infection in the neonatal population.
Introduction
COVID-19 (coronavirus disease 2019) is an emerging infectious disease, first reported in Wuhan, Hubei region, Canhina, in December 2019 [1]. It is caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) which is a single-stranded RNA virus, subgenus Sarbecovirus of the genus Betacoronavirus, probably originated from bats [2]. COVID-19 rapidly triggered a global health emergency alert and spread to numerous countries, causing the WHO to announce the start of a new pandemic on 12 March 2020 [3,99]. According to the 24th April 2020 WHO bulletin, 2626321 confirmed cases and 181938 deaths have occurred globally so far [4].
Data concerning pregnant women and neonates is still scarce and scattered and evidence regarding management of pregnancy, delivery and neonates in case of suspected or confirmed COVID-19 diagnosis in either the mother or the offspring remains fragmented [5,6]. To date, the incidence of COVID-19 intended as a positive nasopharyngeal swab for SARS-CoV-2 in newborn babies is roughly 1.5 % [7] and neonatal symptoms, such as mild respiratory distress and transient thrombocytopenia, seems mainly related to late prematurity or elective C-sections due to severe maternal conditions, rather than to the neonatal infection itself [8]. Although horizontal transmission seems predominant not only in adulthood but also in infancy and childhood, the shortage of data regarding pregnancies impede to draw conclusions about vertical transmission, which seems rare but still possible [9]. The new challenge for obstetricians and neonatologists during the SARS-CoV-2 pandemic is to find a balance between implementing special measures to ensure the safety of both patients and healthcare providers and encouraging bonding and interaction between the newborn and the mother. Moreover, some questions are still to be addressed, such as the effects of SARS-CoV-2 infection during the first trimester of pregnancy or the causes underlying the apparent lower incidence and severity of COVID-19 in neonates born to affected mothers.
The purpose of the present study is to systematically accumulate current literature knowledge in the field and evaluate maternal and perinatal outcomes among pregnant women infected by SARS-CoV-2. In this line, this meta-analysis aims to shed light on the transmission pattern of the virus to neonates, as well as to clarify the disease course of COVID-19 infection in this specific population.
Materials and methods
Study design
This meta-analysis was designed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The process of study selection was performed in 3 consecutive stages. The titles or abstracts of all electronic articles were initially screened to evaluate their potential eligibility and subsequently, all articles that were presumed to meet the pre-defined eligibility criteria were retrieved as full-texts papers. At the final stage, all studies that were in accordance with the inclusion criteria and did not meet any of the exclusion criteria were included in the present review. Study selection, data collection, quality assessment and data analysis were planned to be independently conducted by two researchers (IB and AP), while any potential discrepancies were resolved by the consensus of all authors.
Eligibility criteria
Both observational studies and case reports or case series were included in the present meta-analysis. Studies were deemed eligible if they reported clinical outcomes of neonates born to mothers infected by SARS-CoV-2, as well as of infected neonates independently of maternal infection status. When the same cases were suspected to be both included in case reports and an observational study, the latter one was only included in the analysis. Deduplication of studies was performed by two researchers (IB and AP) and any disagreements were resolved through their consensus. On the other hand, studies not reporting any perinatal outcomes of infected pregnant women and those examining exclusively infants after 28 days of life were excluded from the present study. Moreover, review articles, conference proceedings/abstracts, animal and in vitro studies, as well as unpublished data from clinical registries were not included.
Literature search
The literature search was conducted using the Medline, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science databases. Google Scholar database and the full-list of all the included studies (“snowball” method11), were also screened to identify potential additional papers. Search was performed from inception to 3 June 2020 and was based on the following algorithm: “("Coronavirus"[Mesh] OR "COVID-19” [Supplementary Concept] OR "SARS Virus"[Mesh] OR sars-cov OR covid OR novel coronavirus) AND ("Pregnancy"[Mesh] OR "Infant, Newborn"[Mesh] OR pregnan* OR neonat* OR infant* OR newborn). No language restrictions were applied during literature search.
Data extraction
The following data were planned to be extracted from each of the included studies: name of first author, country, maternal age, medical history (diabetes mellitus, hypothyroidism or polycystic ovary syndrome), symptoms (fever, cough, shortness of breath, diarrhea, nausea/vomiting, myalgia, fatigue, headache, sore throat, nasal congestion, abdominal pain, chest pain), radiological signs, presence of co-infection (bacterial or influenza), laboratory tests (lymphopenia, thrombocytopenia, increased C-reactive protein, procalcitonin, ferritin, liver function tests and d-dimers), type of treatment, pregnancy outcomes (fetal distress, premature rupture of membranes-PROM, placenta previa, preeclampsia, preterm birth, cesarean section, stillbirth), maternal outcomes (admission to intensive care unit-ICU or death), neonatal outcomes (gender, gestational age, birthweight, 1-minute/5-minute Apgar score, horizontal/vertical transmission, admission to ICU, mechanical ventilation, sepsis and death).
Quality assessment
Observational studies of incidence data were evaluated by taking into account the following parameters: sample frame, representativeness and size, subjects and setting, coverage bias, classification bias, outcome measurement, statistical analysis and response rate [11]. The quality of case reports and case series was assessed by judging the potential risk of bias concerning the domains of selection, ascertainment, causality and reporting [12]. For each domain, “Major concerns”, “Some concerns” or “No concerns” of bias risk were assigned independently by two researchers, while any disagreements were resolved by discussion with all authors.
Data analysis
The pooled analysis was separately performed for case reports/series which provided individual participant data and observational studies reporting aggregate data. Regarding the analysis of case reports/series, descriptive statistics were calculated and cases were categorized depending on the transmission of SARS-CoV-2 in neonates. Subsequently, maternal characteristics and perinatal outcomes were compared between the two groups. Continuous variables were expressed as median and interquartile range, while the Mann-Whitney U test was implemented to test differences in medians [13]. The comparison of categorical variables was performed using the chi-squared or the Fisher's exact test [14]. Multivariate logistic regression analysis was performed to assess the relative importance of maternal factors (country, age, comorbidities, symptoms and pregnancy complications) in the prediction of perinatal transmission. Statistical significance was defined as p-value <0.05. Missing data were treated by pair-wise deletion [15].
Pooling of observational studies was performed by proportion meta-analysis using a random-effects (DerSimonian-Laird) model [16]. Confidence intervals (CI) were set at 95 %. Inter-study heterogeneity was quantified by calculating the inconsistency index (I2), with values >50 % denoting significant heterogeneity [17]. Publication bias was assessed by the visual inspection of funnel plots and the potential presence of asymmetry was evaluated by the Egger’s regression test [18]. The cut-off of p-value <0.10 was used to define the significance of Egger’s regression test. In case of small-study effects suspicion, the trim-fill method was implemented to provide new estimates accounting for statistically imputed missing studies [19]. Meta-regression analysis was used to evaluate the potential influence of country (China vs. other). In case of statistically significant outcomes, subgroup analysis was performed to assess the exact effects of study country. Statistical analysis was conducted in R-3.6.3 (“metafor” [20] package).
Results
Study selection
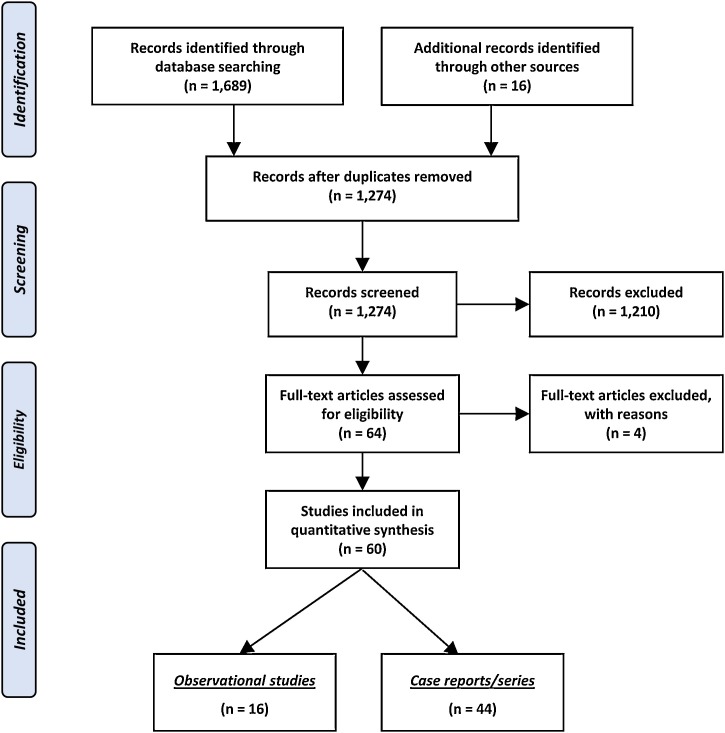
The outcomes of the literature search are schematically illustrated in Fig. 1 . Overall, 1,705 records were identified and 1,274 of them were initially screened after removal of duplicates. The majority of them were then excluded for not meeting the eligibility criteria and thus a cohort of 64 articles was retrieved as full-texts. Subsequently, 4 studies [[21], [22], [23], [24]] were excluded, as 2 studies examined infants and children but not neonates [23,24], while another 2 did not report any perinatal outcomes of pregnancies infected by SARS-CoV-2 [21,22]. As a result, the present meta-analysis was based on 16 observational studies [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]] and 44 case reports/series [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]], including a total of 920 neonates born to women with SARS-CoV-2 infection.
Fig. 1.
Search plot diagram.
Quality assessment
The outcomes of risk of bias evaluation are summarized in Appendix 1 (Suppl. Tables 1, 2). Concerning case reports/series, concerns of bias were mainly raised in the domains of ascertainment and reporting due to missing data about exposure and outcomes, while some concerns were assigned in the domain of causality in 3 studies due to inadequate description of maternal baseline characteristics and comorbidities. On the other hand, evaluation of observational studies raised concerns mainly in the domain of sample size, while moderate risk of classification bias may have arisen from studies defining SARS-CoV-2 infection based exclusively on clinical or radiological criteria.
Outcomes of case reports/series
The maternal and perinatal outcomes of pregnancies infected by SARS-Cov-2 are presented in Appendices 2–3 (Suppl. Tables 3–4). Overall, 158 cases were included in the individual participant data meta-analysis and the majority of them came from China (91.1 %). The median age of women was 30 years and the most common comorbidity was hypothyroidism (4.9 %). Fever was the most frequent symptom (69.4 %), followed by cough (35 %) and shortness of breath (10.8 %), while 13.4 % of women with the disease were asymptomatic. The vast majority of cases presented radiological signs of pneumonia (98.6 %). Co-infection with influenza virus was uncommon (1.9 %), while bacterial super-infection occurred in 5.1 % of patients. Concerning laboratory tests, lymphopenia and thrombocytopenia were found in 48.3 % and 27.8 % of cases, respectively, while the majority of women presented increased C-reactive protein (69.7 %) and procalcitonin (60.9 %). Serum ferritin and D-dimers were sparsely reported, although when tested, their values were found to be elevated in 58.3 % and 72.2 % of cases, respectively. Treatment options included the administration of hydroxychloroquine (6.3 %), azithromycin (9.9 %), antiviral agents, such as oseltamivir (20.7 %), umifenovir (22.5 %), lopinavir/ritonavir (5.4 %) and interferon (12.6 %), as well as various antimicrobial agents (38.7 %).
The majority of neonates were delivered by cesarean section (83.5 %), with preterm birth (<37 weeks) occurring in 29.7 % of cases. Other pregnancy complications included premature rupture of membranes (9.5 %), fetal distress (6.1 %), preeclampsia (5.4 %) and placenta previa (2.7 %). Moreover, 17 women (11 %) developed severe disease and were admitted to ICU, while 2 of them (1.3 %) eventually died (Table 1 ). The median gestational age at delivery was 38 weeks, while the median birthweight was estimated to be 3120 g with 7.9 % of neonates being small-for-gestational age. In addition, stillbirth occurred in 3 cases and 2 neonatal deaths were observed (Table 2 ). Both neonatal deaths occurred in SARS-CoV-2-negative neonates that were born to mothers with severe disease requiring admission to the ICU.
Table 1.
Maternal clinical characteristics and outcomes among pregnancies with and without transmission to neonates. Continuous variables are expressed as median [interquartile range].
| Maternal characteristics | All patients (N = 158) | Transmission |
p-value | |
|---|---|---|---|---|
| Yes (N = 17) | No (N = 141) | |||
| China | 144/158 (91.1 %) | 13/17 (76.5 %) | 131/141 (92.9 %) | 0.072 |
| Age (years) | 30 [[29], [30], [31], [32], [33], [34]] | 32.5 [28.25–34] | 30 [29–33.25] | 0.558 |
| History | ||||
| Diabetes mellitus | 3/122 (2.5 %) | 1/10 (10 %) | 2/112(1.8 %) | 0.228 |
| Hypothyroidism | 6/122 (4.9 %) | 4/10 (40 %) | 2/112 (1.8 %) | <0.001 |
| Polycystic ovary syndrome | 1/122 (0.8 %) | 0/10 (0 %) | 1/112 (0.9 %) | 1 |
| Symptoms | ||||
| Asymptomatic | 21/157 (13.4 %) | 1/16 (6.3 %) | 20/141 (14.2 %) | 0.698 |
| Fever | 109/157 (69.4 %) | 12/16 (75 %) | 97/141 (68.8 %) | 0.778 |
| Cough | 55/157 (35 %) | 6/16 (37.5 %) | 49/141 (34.8 %) | 0.790 |
| Shortness of breath | 17/157 (10.8 %) | 4/16 (25 %) | 13/141 (9.2 %) | 0.076 |
| Diarrhea | 12/157 (7.6 %) | 2/16 (12.5 %) | 10/141 (7.1 %) | 0.352 |
| Nausea / vomiting | 2/157 (1.3 %) | 1/16 (6.3 %) | 1/141 (0.7 %) | 0.194 |
| Myalgia | 6/157 (3.8 %) | 2/16 (12.5 %) | 4/141 (2.8 %) | 0.115 |
| Fatigue | 15/157 (9.6 %) | 2/16 (12.5 %) | 13/141 (9.2 %) | 0.652 |
| Sore throat | 11/157 (7.0 %) | 1/16 (6.3 %) | 10/141 (7.1 %) | 1 |
| Nasal congestion | 4/157 (2.8 %) | 1/16 (6.3 %) | 3/141 (2.1 %) | 0.352 |
| Headache | 4/157 (2.8 %) | 0/16 (0 %) | 4/141 (2.8 %) | 1 |
| Abdominal pain | 2/157 (1.3 %) | 0/16 (0 %) | 2/141 (1.4 %) | 1 |
| Chest pain | 3/157 (1.9 %) | 0/16 (0 %) | 3/141 (2.1 %) | 1 |
| Radiology | ||||
| Signs of pneumonia | 145/147 (98.6 %) | 16/16 (100 %) | 129/131 (98.5 %) | 1 |
| Co-infection | ||||
| Bacterial | 8/158 (5.1 %) | 1/16 (6.3 %) | 7/141 (4.9 %) | 0.587 |
| Influenza | 3/158 (1.9 %) | 0/16 (0 %) | 3/141 (2.1 %) | 1 |
| Laboratory tests | ||||
| Lymphopenia | 43/89 (48.3 %) | 5/7 (71.4 %) | 38/82 (46.3 %) | 0.256 |
| Thrombocytopenia | 10/36 (27.8 %) | 2/3 (66.7 %) | 8/33 (24.2 %) | 0.181 |
| Increased C-reactive protein | 46/66 (69.7 %) | 5/5 (100 %) | 41/61 (67.2 %) | 0.312 |
| Increased procalcitonin | 14/23 (60.9 %) | 1/2 (50 %) | 13/21 (61.9 %) | 1 |
| Increased ferritin | 7/12 (58.3 %) | 2/2 (100 %) | 5/10 (50 %) | 0.470 |
| Increased liver function tests | 19/76 (25 %) | 1/6 (16.7 %) | 18/70 (25.7 %) | 1 |
| Increased d-dimers | 26/36 (72.2 %) | 2/2 (100 %) | 24/34 (70.6 %) | 1 |
| Treatment | ||||
| Hydroxychloroquine | 7/111 (6.3 %) | 2/8 (25 %) | 5/103 (4.9 %) | 0.080 |
| Azithromycin | 11/111 (9.9 %) | 2/8 (25 %) | 9/103 (8.7 %) | 0.180 |
| Oseltamivir | 23/111 (20.7 %) | 2/8 (25 %) | 21/103 (20.4 %) | 0.669 |
| Umifenovir | 25/111 (22.5 %) | 1/8 (12.5 %) | 24/103 (23.3 %) | 0.681 |
| Lopinavir / ritonavir | 6/111 (5.4 %) | 1/8 (12.5 %) | 5/103 (4.9 %) | 0.369 |
| Interferon | 14/111 (12.6 %) | 2/8 (25 %) | 12/103 (11.7 %) | 0.265 |
| Antimicrobial | 43/111 (38.7 %) | 6/8 (75 %) | 37/103 (35.9 %) | 0.053 |
| Complications | ||||
| Fetal distress | 9/148 (6.1 %) | 0/17 (0 %) | 9/131 (6.9 %) | 0.599 |
| Premature rupture of membranes | 14/148 (9.5 %) | 1/17 (5.9 %) | 13/131 (9.9 %) | 1 |
| Placenta previa | 4/148 (2.7 %) | 0/17 (0 %) | 4/131 (3 %) | 1 |
| Preeclampsia | 8/148 (5.4 %) | 1/17 (5.9 %) | 7/131 (5.3 %) | 1 |
| Preterm birth | 47/158 (29.7 %) | 2/17 (11.8 %) | 45/141 (31.9 %) | 0.099 |
| Cesarean section | 132/158 (83.5 %) | 16/17 (94.1 %) | 116/141 (82.3 %) | 0.310 |
| Admission to ICU | 17/155 (11 %) | 3/17 (17.6 %) | 14/141 (9.9 %) | 0.398 |
| Death | 2/155 (1.3 %) | 1/16 (6.3 %) | 1/141 (0.7 %) | 0.196 |
Bold text indicates statistical significance. ICU: intensive care unit.
Table 2.
Clinical characteristics of infected and non-infected neonates. Continuous variables are expressed as median [interquartile range].
| Neonatal characteristics | All neonates (N = 158) | Transmission |
p-value | |
|---|---|---|---|---|
| Yes (N = 17) | No (N = 141) | |||
| Male gender | 52/77 (67.5 %) | 8/12 (66.7 %) | 44/65 (67.7 %) | 1 |
| Gestational age (weeks) | 38 [36–39.1] | 40 [35.35–40] | 38 [[36], [37], [38], [39]] | 0.111 |
| Birthweight (g) | 3120 [2692–3370] | 3228 [2852–3278] | 3100 [2692–3400] | 0.906 |
| Small-for-gestational age | 10/127 (7.9 %) | 2/12 (16.7 %) | 8/114 (7 %) | 0.243 |
| 1-minute Apgar score | 9 [8,9] | 8 [6.5–9] | 9 [8,9] | 0.077 |
| 5-minute Apgar score | 10 [9,10] | 9 [8.5–10] | 10 [9,10] | 0.111 |
| Stillbirth | 3/158 (1.9 %) | 1/17 (6.25 %) | 2/141 (1.4 %) | 0.291 |
| Neonatal death | 2/158 (1.3 %) | 0/17 (0 %) | 2/141 (1.4 %) | 1 |
Transmission of SARS-CoV-2 was confirmed in 17 cases. The maternal characteristics and perinatal outcomes were similar between pregnancies with and without viral transmission, with the exception of maternal hypothyroidism (Odds ratio: 36.67, 95 % CI: 5.56–241.69). Multivariate regression analysis indicated that hypothyroidism was the only factor associated with increased perinatal transmission (p-value: 0.004), while no association was found for maternal age, other comorbidities, symptoms or pregnancy complications. Importantly, the rate of neonatal transmission did not differ between women admitted to the ICU and those with the non-severe form of the disease (OR: 1.94, 95 % CI: 0.50–7.60) (Table 1). Vertical transmission was assumed to occur in 4 cases [43,68,73,74] due to positive placental/amniotic fluid SARS-CoV-2 polymerase chain reaction (PCR) testing or detection of neonatal IgM antibodies against the virus shortly after delivery. Possible vertical transmission was also suspected in 6 cases due to immediate strict neonatal isolation, although direct evidence was lacking. No neonates born to SARS-CoV-2-positive mothers were breastfed, due to concerns of potential transmission. However, the presence of SARS-CoV-2 in breast milk was tested in 27 cases and all samples were negative. Stool samples were tested in 23 neonates with SARS-CoV-2 being detected in 5 of them (21.7 %). The clinical characteristics of SARS-CoV-2-positive neonates are presented in Table 3 . Fever was the most common symptom (40 %), followed by shortness of breath (28 %) and vomiting (24 %), while 20 % of neonates were totally asymptomatic. The outcomes of complete blood count were reported for 8 cases; 2 of them presented lymphopenia and another 2 thrombocytopenia. Moreover, two neonates needed mechanical ventilation, while another one received noninvasive positive pressure ventilation. Neonatal sepsis occurred in two patients and one of them developed septic shock requiring inotropic support, although survived, was extubated and finally discharged.
Table 3.
Clinical outcomes of neonates infected by SARS-CoV-2.
| Author; Country | Gestational age (w) | Birthweight (g) | Delivery | Symptoms | Pneumonia | Lymphopenia/ Thrombocytopenia | Increased CRP/PCT | Treatment | NICU admission | Mechanical ventilation | CPAP/ NIPPV | Duration of respiratory support (days) | Sepsis | Shock | Inotropes | Neonatal death | Follow-up (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yu N.; China | 40 | 3250 | Cesarean | SOB | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 40 |
| Zamaniyan M; Iran | 30.7 | 2350 | Cesarean | Fever | No | NR/NR | NR/NR | Ampicillin, gentamicin | Yes | No | No | – | No | No | No | No | 7+ |
| Alzamora MC; Peru | 33 | 2970 | Cesarean | Cough, SOB | No | NR/NR | NR/NR | No | Yes | Yes | CPAP | 0.5 | No | No | No | No | 6+ |
| Wang S; China | 40 | 3205 | Cesarean | No | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 15 |
| Dong L; China | 37.7 | 3120 | Cesarean | No | No | No/No | No/NR | No | Yes | No | No | – | No | No | No | No | 25 |
| Zeng L; China | 40 | 3250 | Cesarean | Fever | Yes | No/No | No/Yes | NR | Yes | No | No | – | No | No | No | No | 6+ |
| 40.6 | 3360 | Cesarean | Fever | Yes | Yes/No | NR/NR | NR | No | No | No | – | No | No | No | No | 6+ | |
| 31.3 | 1580 | Cesarean | SOB | Yes | Yes/No | NR/NR | NR | Yes | No | NIPPV | 14 | Yes | No | No | No | 14+ | |
| Aghdam MK; Iran | NR | 3460 | Cesarean | Fever, cough | No | No/No | No/NR | Vancomycin, amikacin, oseltamivir | Yes | No | No | – | Yes | No | No | No | 14+ |
| Zhang ZJ; China | 40 | NR | Cesarean | SOB | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | NR |
| NR | NR | Cesarean | Fever, cough, vomiting | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 23 | |
| NR | NR | Cesarean | Fever | No | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 30 | |
| 40.1 | NR | Cesarean | No | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 16 | |
| Coronado Munoz; USA | 36 | NR | NR | Nasal congestion, SOB, feeding intolerance | Yes | No/No | Yes/Yes | Ampicillin, gentamycin, vancomycin, cefepime, hydroxychloroquine, azithromycin | Yes | Yes | No | 5 | Yes | Yes | Yes | No | 9 |
| Khan S; China | 40.6 | 3360 | Cesarean | NR | No | NR/NR | NR/NR | NR | NR | NR | NR | NR | NR | NR | NR | No | NR |
| 39.1 | 3570 | Cesarean | NR | Yes | NR/NR | NR/NR | NR | NR | NR | NR | NR | NR | NR | NR | No | NR | |
| Hu X; China | 40 | 3250 | Cesarean | No | No | No/No | No/No | No | No | No | No | – | No | No | No | No | NR |
| Salvatori G; Italy | 41.3 | 4440 | NR | No | No | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | NR |
| 39 | 3120 | NR | Cough, diarrhea | No | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 5+ | |
| Alonso Diaz C; Spain | 38.6 | 2500 | Cesarean | SOB | Yes | NR/NR | No/NR | No | Yes | No | No | – | No | No | No | No | 13 |
| Zeng LK; China | 39 | NR | NR | Fever, SOB, vomiting | Yes | No/No | No/Yes | No | No | No | No | – | No | No | No | No | 7 |
| Wang J; China | 38.6 | 3030 | Vaginal | Fever, cough, vomiting | Yes | No/Yes | No/No | Interferon | No | No | No | – | No | No | No | No | 14 |
| Xiaoyuan F; China | NR | NR | NR | Fever, vomiting | No | NR/NR | NR/NR | Antimicrobial | No | No | No | – | No | No | No | No | 16 |
| NR | NR | NR | Vomiting | No | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 16 | |
| NR | NR | NR | Diarrhea, vomiting | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 15 | |
| NR | NR | NR | Fever | Yes | NR/NR | NR/NR | Antimicrobial | No | No | No | – | No | No | No | No | 13 | |
| NR | NR | NR | Feeding intolerance | Yes | NR/NR | NR/NR | No | No | No | No | – | No | No | No | No | 7 |
NR: not reported; CRP: C-reactive protein; PCT: procalcitonin; NICU: neonatal intensive care unit; CPAP: continuous positive airway pressure; NIPPV: noninvasive positive pressure ventilation; SOB: shortness of breath.
Outcomes of observational studies
Eleven observational studies were included, comprising 762 neonates born to SARS-CoV-2-positive women (Appendix 4, Suppl. Table 5). Proportion meta-analysis indicated that the incidence of asymptomatic pregnant women was 15 % (95 % CI: 9–20 %), while fever was present in 53 % (95 % CI: 44%–62%), cough in 34 % (95 % CI: 23%–46%), shortness of breath in 18 % (95 % CI: 11%–26%) and diarrhea in 3 % (95 % CI: 2%–5%) of women. Severe disease was estimated to occur in 7 % (95 % CI: 4%–10%) of cases. The majority of neonates were delivered by cesarean section (66 %, 95 % CI: 51%–80%), while the rate of preterm birth was calculated at 16 % (95 % CI: 11%–21%) (Appendix 6, Suppl. Figs. 1–8).
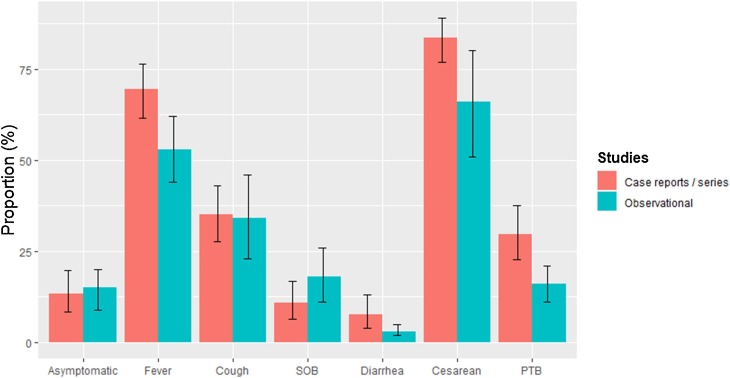
Inter-study heterogeneity was evaluated to be significant as I2 values ranged from 32 % to 91.9 %, being highest in the outcomes of cough (I2: 91.9 %) and cesarean section (I2: 88.3 %). Asymmetry of funnel plots was detected in the outcomes of asymptomatic disease (p-value = 0.036) and severe disease (p-value = 0.006) (Appendix 7, Suppl. Figs. 9–16). The adjusted estimates according to the trim-fill method indicated similar rates of asymptomatic disease (9.1 %), but significantly lower incidence of severe disease (1.8 %) after accounting for potential missing studies. No fetal or neonatal deaths were reported by observational studies, while only 6 cases of viral transmission were described by 2 studies (Suppl. Table 5). Meta-regression analysis indicated that study country exerted significant effects on the outcomes of shortness of breath, severe disease and cesarean delivery (Appendix 6, Suppl. Table 6). Specifically, the incidence of shortness of breath and severe disease was significantly lower in studies conducted in China (7.7 % vs. 28 % and 4.1 % vs. 10.3 %, respectively). Moreover, the rate of cesarean delivery was significantly higher in studies in China (88 % vs. 30 %). The outcomes of the meta-analysis of case reports/series compared to those of observational studies are depicted in Fig. 2 .
Fig. 2.
Comparison of outcomes between case reports/series and observational studies. SOB: shortness of breath; PTB: preterm birth.
Discussion
Worldwide, SARS-CoV-2 is taking its toll on health systems and has caused hospitals and healthcare providers to rearrange facilities and habits to ensure the highest degree of safety to patients and workers. The shortage of data regarding COVID-19 in the neonatal age represents a further challenge for obstetricians and neonatologists, who are called to face a rather unknown enemy. Moreover, perinatal care to the mother-neonate dyad requires special considerations due to the peculiarity of their condition, since bonding and close interaction between the mother and her baby are crucial for their well-being. Unfortunately, the paucity of supporting data impedes to draw conclusions at this point. To date, the vast majority of neonatal information is scattered and fragmented, since it is derived from case reports or small case series. To the best of our knowledge, the present meta-analysis is the largest one to date, providing pooled outcomes from 11 observational studies and 44 case reports/case series.
Maternal outcomes
The clinical course of Covid-19 is typically mild during pregnancy, with 15 % of infections being asymptomatic. The reported rates of severe disease were significantly higher in studies conducted in countries other than China, probably reflecting publication bias during the first wave of SARS-CoV-2. The most common symptoms were fever and cough, followed by shortness of breath, while gastrointestinal symptoms were rare. Radiological signs of pneumonia were almost ubiquitously present. Cesarean section rates were high, mainly due to concerns about perinatal transmission. The incidence of preterm birth was remarkable (29.7 % in case reports – 16 % in observational studies), although the risk of preeclampsia and placental previa were estimated to be low (5.4 % and 2.7 %, respectively).
Transmission
Symptomatic patients with COVID-19 are regarded as the main disseminators, but asymptomatic individuals should not be underestimated. The main transmission routes are droplets, contact, and aerosol transmission, although fecal-oral transmission should be also considered since SARS-CoV-2 RNA has been detected in fecal samples. [85] Our results confirm that horizontal spread from caregivers (primarily the mother) to the neonate is the most likely way of transmission in this population. Hence, it is recommended that in case of asymptomatic or mildly symptomatic SARS-CoV-2 positive mothers, general hygienic measures should be taken. Specifically, the neonate’s crib should be distanced from the mother’s bed by at least two meters and the mother should wear a surgical face mask when breastfeeding or looking after the neonate [86].
Vertical spread remains doubtful, in keeping with the absence of reported cases of intrauterine transmission for SARS-CoV-1 and MERS. [87] Vertical transmission was suspected in 4 cases due to positive placental/amniotic fluid SARS-CoV-2 PCR testing or detection of neonatal IgM antibodies against the virus shortly after delivery [43,68,73,74]. Alzamora et al. [68] reported a severe presentation of maternal COVID-19 during pregnancy, followed by the finding of positive RT-PCR in nasopharyngeal swab of the neonate at 16 h of life, despite immediate isolation from the mother. Moreover, Dong and coll [61] described the case of a newborn with early elevated IgM antibodies to SARS-CoV-2 born to a mother with COVID-19.
Hypothyroidism was suggested as a potential predisposing factor for perinatal transmission. Overall, six women with hypothyroidism were included and neonatal SARS-Cov-2 positivity was detected in 4 of these cases. This association remained significant in multivariate regression analysis after taking into account the effects of country, maternal age, other comorbidities, symptoms and pregnancy complications. The increased incidence of neonatal transmission may be based on the effects of thyroid hormones on placental function and maturation since subclinical hypothyroidism has been linked to increased rates of placental abruption and preterm birth [88]. In this context, thyroid hormones have been proposed to affect placental development by modulating inflammatory and apoptotic processes [89], especially by altering the placental immune profile and intrauterine trophoblast migration [90]. Nonetheless, whether the potential effects of thyroid dysfunction on placental barrier may facilitate vertical SARS-CoV-2 transmission remains to be elucidated by further studies in the field.
Nonetheless, the understanding of the mechanisms related to vertical transmission remains to be determined. One could speculate that vertical transmission is more likely in case of significant viral load in maternal samples, or critically-ill patients. However, there is currently no evidence to support any conclusion in this respect, while potential answers may be expected from the INTERCOVID study group [91].
Neonatal clinical aspects
The present findings suggest that the clinical presentation of COVID-19 in neonates may range from asymptomatic to severe respiratory distress. The most common symptoms were fever and dyspnea, although gastrointestinal manifestations, such vomiting and milk refusal may occasionally be the only ones in neonates [58]. Sporadic cases of neonatal infections (pneumonia or sepsis) requiring mechanical ventilation have been also described [62,68,92], while Zhu et al. [41] reported on a late-preterm who died on the ninth day of life due to refractory shock, multiple organ failure, and disseminated intravascular coagulation. Some laboratory findings, such as thrombocytopenia and lymphopenia, appear rather common in neonates born to COVID-19 mothers, even when transmission to the offspring is not confirmed either on serology or nasopharyngeal swab; these findings have been proposed to resemble those of certain late-acquired TORCH (Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes) infections and thus have prompted some authors to suggest the inclusion of SARS-CoV-2 among TORCH complex [93].
Delivery
Since the beginning of COVID-19 epidemic in China, in some cases, mothers with suspected or confirmed SARS-CoV-2 infections have undergone cesarean section in the absence of other obstetrical indications with the aim to reduce the odds of intrapartum transmission [63]. Notably, currently available case series show a higher than expected number of preterm deliveries and cesarean sections. According to our data, the rate of cesarean delivery was 83.5 % and 80 % in case reports and observational studies, respectively. This can be in part explained as a consequence of obstetric decision to deliver due to the severity of the maternal infection (bilateral pneumonia with respiratory insufficiency and shock) [94].
Since cesarean deliveries entail the exposure of a greater number of health care workers compared with spontaneous vaginal delivery and are linked to an increase in neonatal morbidity, the decision to deliver via cesarean should be cautiously weighted. To date, clear findings on whether spontaneous vaginal delivery favors neonatal infection are lacking. Hence, for the time being, the mode of delivery and anesthesia is best advisable as per maternal and fetal indications and spontaneous vaginal delivery should generally be preferred [94].
Neonatal care in the delivery room
Whether skin-to-skin contact increases the risk of SARS-CoV-2 transmission is yet to be clarified [95,96]. It is advisable to share decision-making with the parents before delivery regarding the potential risks and benefits of skin-to-skin care and kangaroo mother care, taking into account also risks of exposure to both the neonate and to health care providers [94]. Delayed cord clamping is unlikely to increase the odds of vertical transmission, since the fetus has exchanged the same blood supply during the entire pregnancy. Therefore, if vertical transmission was possible, it would probably have occurred before delivery. Currently, there is no evidence supporting the abolishment of delayed cord clamping, which is still recommended in all vigorous neonates for at least 60 s [94].
Breastfeeding
Despite some authors advocate for a cautious approach and recommend feeding preterm neonates on pasteurized breast milk (donor or maternal) or formula. [97] However, according to the present findings, all breast milk samples have been tested to be negative for SARS-CoV-2 and thus breast milk of a COVID-19 mother cannot be regarded as a transmission vehicle. Moreover, similarly to the 2002–2003 SARS-CoV-1 epidemic, specific SARS-CoV-2 antibodies are likely to pass via the breast milk from the COVID-19 mother to the infant within a few days after the onset of the disease, thus possibly modulating the clinical expression of the infant's infection [98,99]
Strengths and limitations of the study
The present meta-analysis gathered all the available evidence in the field, by searching 5 literature databases without applying any language restrictions. Both case reports/series and observational studies were included and separately analyzed, providing a comprehensive approach concerning the perinatal effects of SARS-CoV-2 infection. Specifically, analysis of case reports/series provided a cohort of 158 neonates with individual participant data enabling the assessment of factors potentially associated with neonatal transmission of the virus. On the other hand, the present outcomes are limited by inconsistent reporting of important clinical and laboratory characteristics leading to missing data in several variables. Moreover, inter-study heterogeneity of observational studies was estimated to be significant, reflecting potential methodological differences concerning patient selection and outcome reporting. The effects of study country was assessed by conducting meta-regression analysis; importantly, studies conducted in China reported significantly higher incidence of cesarean delivery, reflecting the remarkable variance of labor protocols among different regions. In addition, inter-study heterogeneity may be present, as different periods of evaluation may be characterized by different treatment strategies due to the rapid change of policies regarding the management of the pandemic. It should be also noted that publication bias was suspected, especially regarding the outcome of severe maternal disease, implying that its incidence may be currently overestimated by the available studies in the field.
Implications for future research
Many questions regarding the impact of the novel coronavirus SARS-CoV-2 are still to be addressed. Firstly, the possibility of vertical transmission in utero or at birth should be clearly demonstrated, since the sporadic cases of positive nasopharyngeal swabs or IgM testing in neonates might be due to PCR and ELISA assay differences. Greater reliability and reproducibility of assays used worldwide are strongly warranted. Moreover, it is not clear whether a neonate with positive IgM should be considered infected and, consequently, contagious even in case of negative nasopharyngeal swabs. Secondly, data regarding SARS-CoV-2 infection during the first trimester and possible embryotoxicity are still completely lacking. In this respect, a deeper investigation regarding the capacity of SARS-CoV-2 to infect the placenta is also needed. In the present study, hypothyroidism was identified as a potential predisposing factor for perinatal transmission; however, the sample size was small and thus further research is warranted in order to reach firm conclusions about the causality of this relationship. Lastly, preliminary evidence is reassuring about breastfeeding, however more confirmatory data are needed on breast milk and viral load. Importantly, the implementation of point-of-care immune assays and virologic assays would help early identify infected but asymptomatic women who present in labor, aiming to improve perinatal management.
Conclusions
Maternal and neonatal COVID-19 infection is linked to a variety of clinical manifestations, although asymptomatic and mild cases are most commonly seen during the third trimester. The incidence of neonatal transmission is suggested to be low and independent of maternal disease severity. Currently, most of the approaches in the management of suspected or confirmed COVID-19 mothers and their offspring are based on little evidence and experts’ opinions. Future large-scale studies are needed in order to clarify the risk of vertical transmission and identify the factors that may predispose for the development of severe neonatal infection.
Funding
None.
Ethical approval
Not required. The present study is a meta-analysis and is solely based on aggregated data of already published studies.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Conception and design: I. Bellos, A. Pandita.
Analysis and interpretation of the data: I. Bellos, R. Panza.
Drafting of the article: I. Bellos, A. Pandita.
Critical revision for important intellectual content: I. Bellos, R. Panza.
Final approval of the article: I. Bellos, A. Pandita, R. Panza.
Statistical expertise: I. Bellos.
Collection and assembly of data: I. Bellos, A. Pandita.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ejogrb.2020.11.038.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization . WHO; 2020. WHO | Pneumonia of unknown cause – China. [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London, England) 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. WHO announces COVID-19 outbreak a pandemic. [Google Scholar]
- 4.World Health Organization . 2020. COVID-19 situation reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed May 15, 2020) [Google Scholar]
- 5.Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. COVID19 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elshafeey F., Magdi R., Hindi N., Elshebiny M., Farrag N., Mahdy S. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davanzo R., Moro G., Sandri F., Agosti M., Moretti C., Mosca F. Breastfeeding and coronavirus disease-2019: Ad interim indications of the Italian Society of Neonatology endorsed by the Union of European Neonatal & Perinatal Societies. Matern Child Nutr. 2020 doi: 10.1111/mcn.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rose D.U., Piersigilli F., Ronchetti M.P., Santisi A., Bersani I., Dotta A. Novel Coronavirus disease (COVID-19) in newborns and infants: what we know so far. Ital J Pediatr. 2020;46:56. doi: 10.1186/s13052-020-0820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 12.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. 2018;23:60–63. doi: 10.1136/BMJEBM-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.J W Testing for differences with the nonparametric Mann-Whitney U test. J Wound Ostomy Cont Nurs. 1997:24. doi: 10.1016/S1071-5754(97)90044-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod. 2017;42:152. doi: 10.5395/RDE.2017.42.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402. doi: 10.4097/KJAE.2013.64.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/BMJ.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L., Lin L. The trim-and-fill method for publication bias. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000015987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 21.Zambrano L.I., Fuentes-Barahona I.C., Bejarano-Torres D.A., Bustillo C., Gonzales G., Vallecillo-Chinchilla G. A pregnant woman with COVID-19 in Central America. Travel Med Infect Dis. 2020 doi: 10.1016/J.TMAID.2020.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayor S. Covid-19: Nine in 10 pregnant women with infection when admitted for delivery are asymptomatic, small study finds. BMJ. 2020:369. doi: 10.1136/BMJ.M1485. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T., Cui X., Zhao X., Wang J., Zheng J., Zheng G. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le H.T., Nguyen L.V., Tran D.M., Do H.T., Tran H.T., Le Y.T. The first infant case of COVID-19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Heal. 2020;4:405–406. doi: 10.1016/S2352-4642(20)30091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 26.Mishra P., Pandey C.M., Singh U., Gupta A., Sahu C., Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg L.P., Hedayati S.S. Management of Traditional Cardiovascular Risk Factors in CKD: What Are the Data? Am J Kidney Dis. 2018;72:728–744. doi: 10.1053/j.ajkd.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Wang Z., Xiong G. Clinical characteristics and laboratory results of pregnant women with COVID‐19 in Wuhan, China. Int J Gynecol Obstet. 2020 doi: 10.1002/ijgo.13265. ijgo.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira A., Cruz‐Melguizo S., Adrien M., Fuentes L., Marin E., Perez‐Medina T. Clinical course of coronavirus disease‐2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savasi V.M., Parisi F., Patanè L., Ferrazzi E., Frigerio L., Pellegrino A. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003979. [DOI] [PubMed] [Google Scholar]
- 32.Lokken E.M., Walker C.L., Delaney S., Kachikis A., Kretzer N.M., Erickson A. Clinical characteristics of 46 pregnant women with a SARS-CoV-2 infection in Washington State. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lantz B. The impact of sample non-normality on ANOVA and alternative methods. Br J Math Stat Psychol. 2013;66:224–244. doi: 10.1111/j.2044-8317.2012.02047.x. [DOI] [PubMed] [Google Scholar]
- 34.Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 35.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.KDIGO Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl. 2013;3:63–72. doi: 10.1038/KISUP.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romagnani P., Remuzzi G., Glassock R., Levin A., Jager K.J., Tonelli M. Chronic kidney disease. Nat Rev Dis Prim. 2017;3:17088. doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 38.US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69:A4. doi: 10.1053/j.ajkd.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Mathew R.O., Bangalore S., Lavelle M.P., Pellikka P.A., Sidhu M.S., Boden W.E. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: a review. Kidney Int. 2017;91:797–807. doi: 10.1016/j.kint.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 40.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O’Callaghan C.A., Lasserson D.S. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z.-J., Yu X.-J., Fu T., Liu Y., Jiang Y., Yang B.X. Novel coronavirus infection in newborn babies under 28 days in China. Eur Respir J. 2020 doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Z., Wang J., Mo Y., Duan W., Xiang G., Yi M. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13:818–820. doi: 10.1016/J.JIPH.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyra J., Valente R., Rosário M., Guimarães M. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Med Port. 2020:33. doi: 10.20344/amp.13883. [DOI] [PubMed] [Google Scholar]
- 45.Lu D., Sang L., Du S., Li T., Chang Y., Yang X. Asymptomatic COVID‐19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020 doi: 10.1002/jmv.25927. jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/J.JINF.2020.02.028. n.d.;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020:1. doi: 10.1007/S11684-020-0772-Y. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao J., He X., Gong Q., Yang L., Zhou C., Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID‐19 pandemic. Int J Gynecol Obstet. 2020 doi: 10.1002/ijgo.13188. ijgo.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee D.H., Lee J., Kim E., Woo K., Park H.Y., An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J Anesthesiol. 2020 doi: 10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan S., Jun L., Nawsherwan Siddique R., Li Y., Han G. Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women. Clin Microbiol Infect. 2020 doi: 10.1016/J.CMI.2020.03.034. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karami P., Naghavi M., Feyzi A., Aghamohammadi M., Novin Ms, Mobaien A. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020 doi: 10.1016/J.TMAID.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamaniyan M., Ebadi A., Aghajanpoor Mir S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery in pregnant woman with critical COVID ‐19 pneumonia and vertical transmission. Prenat Diagn. 2020 doi: 10.1002/pd.5713. pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalafat E., Yaprak E., Cinar G., Varli B., Ozisik S., Uzun C. Lung ultrasound and computed tomographic findings in pregnant woman with COVID ‐19. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22034. uog.22034. [DOI] [PubMed] [Google Scholar]
- 55.Iqbal Sn, Overcash R., Mokhtari N., Saeed H., Gold S., Auguste T. An uncomplicated delivery in a patient with Covid-19 in the United States. N Engl J Med. 2020;382:e34. doi: 10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu X., Gao J., Luo X., Feng L., Liu W., Chen J. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) Pneumonia. Obstet Gynecol. 2020:1. doi: 10.1097/AOG.0000000000003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gidlöf S., Savchenko J., Brune T., Josefsson H. COVID‐19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13862. aogs.13862. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Wang D., Chen G., Tao X., Zeng L. SARS-CoV-2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:211–214. doi: 10.7499/j.issn.1008-8830.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yiwei Z., Zhenlang L., Xujie M., Xioale J., Wei Z. A case of neonatal coronavirus pneumonia mother’s delivery of newborn dyspnea. Chinese J Perinat Med. 2020 [Google Scholar]
- 60.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coronado Munoz A., Nawaratne U., McMann D., Ellsworth M., Meliones J., Boukas K. Late-onset neonatal sepsis in a patient with covid-19. N Engl J Med. 2020;382:e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breslin N., Baptiste C., Miller R., Fuchs K., Goffman D., Gyamfi-Bannerman C. COVID-19 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/J.AJOGMF.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020 doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamali Aghdam M., Jafari N., Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Auckl) 2020;52:427–429. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lingkong Z., Xuwei T., Wenhao Y., Jin W., Xin L., Zhisheng L. First case of neonate infected with novel coronavirus pneumonia in China. Chinese J Pediatr. 2020;58 doi: 10.3760/CMA.J.ISSN.0578-1310.2020.0009. E009–E009. [DOI] [PubMed] [Google Scholar]
- 71.Shuming H., Dongna W., Ruibin C., Deliang D., Yanping Y., Minchang H. Death of a neonate born to a critically ill mother with COVID-19: a case report. Chin J Perinat Med. 2020;23:217–220. doi: 10.3760/CMA.J.CN113903-20200228-00171. [DOI] [Google Scholar]
- 72.Li Y., Jing W., Jingjing Z., Jing C., Zhihang H. Asymptomatic COVID-19 infection in pregnant woman in the third trimester: a case report. Chin J Perinat Med. 2020;23:229–231. doi: 10.3760/CMA.J.CN113903-20200221-00143. [DOI] [Google Scholar]
- 73.Lingkong Z., Xuwei T., Wenhao Y., Jin W., Xin L., Zhisheng L. First case of neonate with COVID-19 in China. Chin J Pediatr. 2020;58:279–280. doi: 10.3760/CMA.J.CN112140-20200212-00081. [DOI] [PubMed] [Google Scholar]
- 74.Shuo C., Bo H., Danju L., Xiang L., Fan Y., Yin Z. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Chin J Pathol. 2020;49 doi: 10.3760/CMA.J.CN112151-20200225-00138. E005–E005. [DOI] [PubMed] [Google Scholar]
- 75.Yang P., Wang X., Liu P., Wei C., He B., Zheng J. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127 doi: 10.1016/J.JCV.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chao T., Lin W., Yalan L., Jing T., Qiong L., Yan C. Premature infant born to a convalescent mother with COVID-19 in mid-term pregnancy. Chin J Perinat Med. 2020;23:336–338. doi: 10.3760/CMA.J.CN113903-20200403-00311. [DOI] [Google Scholar]
- 77.Alonso Díaz C., López Maestro M., Moral Pumarega M.T., Flores Antón B., Pallás Alonso C.R. Primer caso de infección neonatal por SARS-CoV-2 en España. An Pediatría. 2020;92:237–238. doi: 10.1016/j.anpedi.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao D., Yin H., Chen J., Tang F., Peng M., Li R. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: a retrospective study. Int J Infect Dis. 2020;95:294–300. doi: 10.1016/J.IJID.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia X., Qiumei P., Hong W., Xiaopeng M., Zhihong Z., Xiang G. Vaginal delivery after 37 days of a pregnant woman diagnosed with COVID-19: a case report. Chin J Perinat Med. 2020;23:339–341. doi: 10.3760/CMA.J.CN113903-20200321-00254. [DOI] [Google Scholar]
- 80.Xiong X., Wei H., Zhang Z., Chang J., Ma X., Gao X. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID‐19. J Med Virol. 2020 doi: 10.1002/jmv.25857. jmv.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vlachodimitropoulou Koumoutsea E., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C. COVID19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020 doi: 10.1111/jth.14856. jth.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salvatori G., De Rose D.U., Concato C., Alario D., Olivini N., Dotta A. Managing COVID-19-Positive maternal–infant dyads: an italian experience. Breastfeed Med. 2020 doi: 10.1089/bfm.2020.0095. bfm.2020.0095. [DOI] [PubMed] [Google Scholar]
- 85.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020 doi: 10.1055/S-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mosca F. 2020. La gestione del neonato con infezione sospetta o accertata da SARS-CoV-2. [Google Scholar]
- 87.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses — drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casey B.M., Dashe J.S., Wells C.E., McIntire D.D., Byrd W., Leveno K.J. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 89.Chen C.Y., Chen C.P., Lin K.H. Biological functions of thyroid hormone in Placenta. Int J Mol Sci. 2015;16:4161–4179. doi: 10.3390/ijms16024161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silva J.F., Ocarino N.M., Serakides R. Maternal thyroid dysfunction affects placental profile of inflammatory mediators and the intrauterine trophoblast migration kinetics. Reproduction. 2014;147:803–816. doi: 10.1530/REP-13-0374. [DOI] [PubMed] [Google Scholar]
- 91.University of Oxford. Global study to assess the effects of Covid-19 in pregnancy launched | University of Oxford n.d. http://www.ox.ac.uk/news/2020-04-24-global-study-assess-effects-covid-19-pregnancy-launched (Accessed 14 May 2020).
- 92.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muldoon K.M., Fowler K.B., Pesch M.H., Schleiss M.R. SARS-CoV-2: is it the newest spark in the TORCH? J Clin Virol. 2020;127 doi: 10.1016/J.JCV.2020.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chandrasekharan P., Vento M., Trevisanuto D., Partridge E., Underwood M.A., Wiedeman J. Neonatal resuscitation and postresuscitation care of infants born to mothers with suspected or confirmed SARS-CoV-2 infection. Am J Perinatol. 2020 doi: 10.1055/s-0040-1709688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization . 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (Accessed 14 May 2020) [Google Scholar]
- 96.Wang L., Shi Y., Xiao T., Fu J., Feng X., Mu D. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition) Ann Transl Med. 2020;8 doi: 10.21037/atm.2020.02.20. 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trevisanuto D., Moschino L., Doglioni N., Roehr C.C., Gervasi M.T., Baraldi E. Neonatal resuscitation where the mother has a suspected or confirmed novel coronavirus (SARS-CoV-2) infection: suggestion for a pragmatic action plan. Neonatology. 2020:1–8. doi: 10.1159/000507935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davanzo R., Moro G., Sandri F., Agosti M., Moretti C., Mosca F. Breastfeeding and coronavirus disease‐2019: Ad interim indications of the Italian Society of Neonatology endorsed by the Union of European Neonatal & Perinatal Societies. Matern Child Nutr. 2020 doi: 10.1111/mcn.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vardhelli V., Pandita A., Pillai A., Badatya S.K. Perinatal COVID-19: review of current evidence and practical approach towards prevention and management. Eur J Pediatr. 2020;12:1–23. doi: 10.1007/s00431-020-03866-3. Epub ahead of print. PMID: 33184730; PMCID: PMC7660544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.