1. Introduction
COVID-19, the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has profoundly impacted healthcare throughout the world. Hematologic and oncologic care have been no less affected, with limitations posed on treatment and diagnosis, and concerns of worse outcomes in cancer patients with COVID-19 [1].
Data regarding treatment of patients with hematologic malignancies (HM) and concomitant COVID-19 is very limited [2]. Some studies in patients with cancer found that HM was a risk factor for hospitalization [4], severe disease [1,5] and mortality [1]. Separately, in large cohorts of patients with COVID-19, lymphopenia has been identified as a risk factor for poor outcomes [3]. These findings, although overall inconclusive, may raise concerns leading to delays in lymphocyte-depleting chemotherapy. Such delays can have detrimental consequences in cases of newly diagnosed, rapidly progressing HM, such as acute leukemia or aggressive lymphoma.
In this correspondence, we describe two cases of patients diagnosed simultaneously with aggressive lymphoma and likely COVID-19, both of whom received uneventful early appropriate lymphocyte-depleting chemotherapy without incident. These cases may serve as a template for other hematologist on weighing the risks and benefits of early chemotherapy in patients simultaneously diagnosed with COVID-19 and aggressive hematologic malignancies.
1.1. Case 1
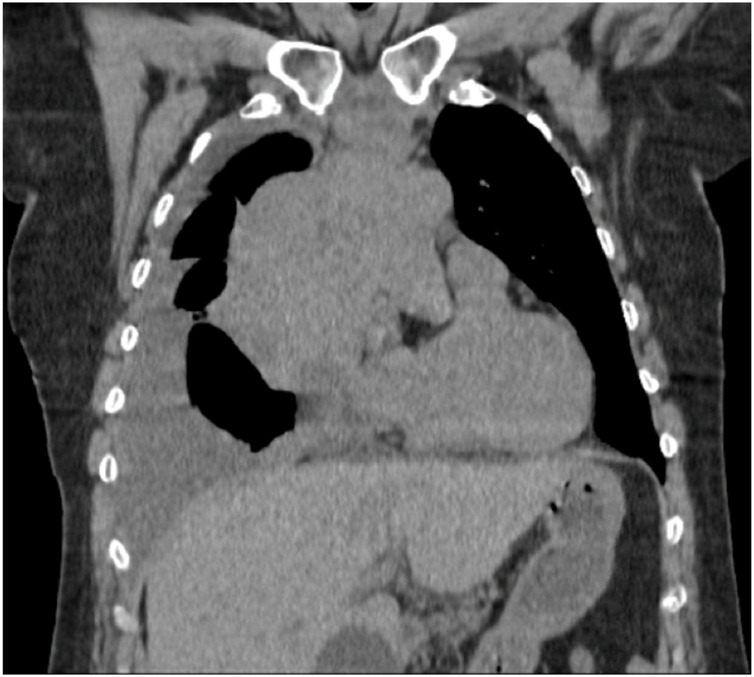
A 58-year-old previously healthy woman was diagnosed with T-lymphoblastic lymphoma (LBL) after presentation for one week of shortness of breath. Chest CT demonstrated a large mediastinal mass and right-sided pleural effusion (Fig. 1 ). After preliminary diagnosis was obtained from the pleural fluid and mediastinal mass, she was admitted to the hospital for initiation of chemotherapy given significant shortness of breath and new oxygen requirement, presumably due to her LBL. Polymerase chain reaction (PCR) from a nasopharyngeal sample, which at the time was sent for all patients admitted to the hospital, was positive for SARS-CoV-2. She was started on prednisone 1 mg/kg on day 1 of her hospitalization, for pre-phase treatment of her lymphoma, which was continued for 7 days. On day 2, she underwent bone marrow biopsy and lumbar puncture, with intrathecal infusion of 70 mg of cytarabine. Bone marrow biopsy showed less than 1 % T-lymphoblasts.
Fig. 1.
Patient 1, Chest CT on diagnosis showing mediastinal mass and pleural effusion.
The patient was enrolled in the Gilead GS US 540-5774 Study (remdesivir for moderate COVID-19). She received 200 mg of remdesivir intravenously (study day 1), followed by 100 mg daily on study days 2-10. On study day 5, repeat SARS-CoV-2 PCR was negative. With improvement in symptoms and negative PCR she was started on hyper-CVAD for treatment of her LBL. A non-asparaginase-based regimen was chosen given concerns about increased thrombotic risk with concurrent COVID infection. She briefly developed syndrome of inappropriate antidiuretic hormone secretion (SIADH), which improved with salt tabs and free water restriction. Her liver function tests and creatinine remained normal. She otherwise tolerated hyper-CVAD and remdesivir well and was discharged on hospital day 19.
1.2. Case 2
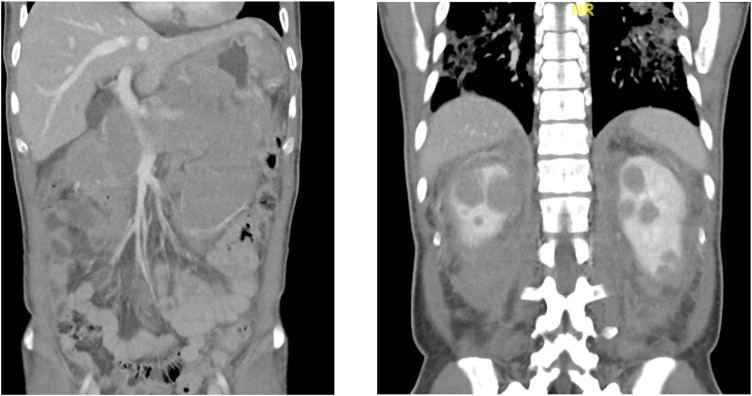
A 43-year-old previously healthy man was diagnosed with high grade B-cell lymphoma after presentation for abdominal pain. Initial imaging showed extensive soft tissue thickening involving the gastric wall and extending into the retroperitoneum involving the pancreas, bilateral adrenal glands, and pararenal spaces as well as bilateral hypoattenuating renal mass (Fig. 2 ). CT also showed bilateral airspace disease concerning for COVID-19 pneumonia (Fig. 2). SARS-CoV-2 PCR was positive. He did not provide consent for participation in the remdesivir trial.
Fig. 2.
Patient 2, CT Chest/Abdomen/Pelvis showing lymphoma and ASD concerning for COVID.
On hospital day 3, he underwent biopsy of a retroperitoneal lymph node and was started on dexamethasone 20 mg daily. On hospital day 4, he reported new right-sided ptosis and underwent brain MRI and lumbar puncture. While the MRI was normal, pathology from the biopsy and cerebrospinal fluid (CSF) were consistent with a high-grade B-cell lymphoma. He was continued on dexamethasone for 5 days and started on cyclophosphamide 200 mg/m daily for 5 days.
Final pathology noted combined morphologic and immunophenotypic features that were highly suggestive of a Burkitt-like lymphoma; however, with lack of c-myc;IgH translocation, Bcl-2 or Bcl-6 rearrangements the final diagnosis was high-grade B-cell lymphoma, NOS. Given clinical and morphologic features consistent with Burkitt’s lymphoma, he was started on R-CODOX-M/IVAC, reversed due to CNS involvement, on hospital day 10.
He tolerated the treatment well without toxicities. He had improvement in his symptoms. and was discharged on hospital day 26.
2. Discussion
The management of HM in patients infected with SARS-CoV-2 is a new and challenging area of research and clinical practice. To-date there are no large-scale studies evaluating the appropriate treatment of these patients, regarding use of chemotherapy or antiviral medications. The outcomes of patients with cancer and COVID-19 appear to be worse than those without underlying malignancies [1,8]. Patients with HM may experience even worse outcomes, although this concern is based on limited data [2,[4], [5], [6]].
While several organizations have published COVID-19 management guides for patients with cancer during the COVID-19 pandemic, most do not specifically address treatment of patients with positive SARS-CoV-2 PCR [9,10]. Our two cases highlight the possibility of treating select, especially asymptomatic patients with aggressive HM and COVID-19 with standard-of-care chemotherapy, and a potential role for remdesivir in treatment algorithms.
The FDA provided accelerated emergency use authorization of remdesivir for the treatment of hospitalized patients with severe COVID-19 patients on May 1st, 2020 [11], based on early data from two randomized-controlled trials (RCT) [12,13] and one open-label study of compassionate use [14]. The RCT included patients with cancer, however results were not stratified by comorbidity and type of cancer was not listed. In the largest RCT, remdesivir shortened the time to recovery among patients hospitalized with COVID-19 (P < 0.001) [13]. In case 1, we felt comfortable starting chemotherapy after completion of 5 days of remdesivir, since another study showed that a 5-day course is probably as effective as a 10-day course in patients who are not receiving mechanical ventilation [15].
It should be noted that our patient tolerated remdesivir co-administered with hyper-CVAD with no significant side-effects from the antiviral. In the RCT, hepatotoxicity and nephrotoxicity were not more frequent, compared to placebo [12,13]. Therefore, we believe that fear of toxicity and side-effects should not discourage providers from co-administration of remdesivir and potentially hepatotoxic or nephrotoxic chemotherapy.
3. Conclusion
In conclusion, we report two cases of minimally symptomatic patients with newly diagnosed aggressive HM and concomitant positive SARS-CoV-2 PCR. One patient received remdesivir, and they both tolerated potentially life-saving lymphocyte-depleting chemotherapy, without worsening COVID-19. We hope this correspondence can serve as fodder for further discussion and analysis of best treatment practices for patients with aggressive HM and COVID-19.
Funding
The present work was partially supported by a Brown Physicians Inc. academic assessment grant to DF. Grant number 7131868. DF has received research support by Astellas and Viracor and consultation fee from Viracor, outside of the submitted work.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He W., Chen L., Chen L., Yuan G., Fang Y., Chen W. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnson R., Bernandes M. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S., Rivera D. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. 2020;8:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda M., Martins R., Hendrie P.C., McDonnell T., Crews J., Wong T. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J. Natl. Compr. Cancer Netw. 2020:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 10.Percival M.-E.M., Lynch R.C., Halpern A.B., Shadman M., Cassaday R.D., Ujjani C. Considerations for managing patients with hematologic malignancy during the COVID-19 pandemic: the Seattle strategy. J. Clin. Oncol. 2020;16(9):571–578. doi: 10.1200/OP.20.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remdesivir D.H. FDA; 2020. Letter of Authorization. [Google Scholar]
- 12.Wang Y., Zhang D., Du G., Du R., Zhao J., Jun Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A., Zingman B., Kalil A. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020;383:992–994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 14.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman J.D., Lye D.C.B., Hui D.S., Marks K., Bruno R., Montejano R. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]