Abstract
Objectives
This scoping review aimed to map and compile the available evidence regarding the effectiveness of decontaminating N95 respirators against the novel coronavirus (SARS-CoV-2).
Data
We selected studies written in English assessing or discussing the decontamination strategies of N95 respirators against SARS-CoV-2. Two independent researchers performed the search and study screening. A descriptive analysis was carried out considering the study design of the included studies.
Sources
PubMed, SCOPUS, and Preprint platforms (bioRxiv and medRxiv).
Study selection
We included 55 reports from PubMed and SCOPUS. Nine articles were letters to the editors, 21 were in vitro studies, 16 were literature reviews, and 9 were classified as other study designs. We included 37 preprints. Two articles were letters to the editors, 24 were in vitro studies, 3 were literature reviews, and 8 were classified as other study designs. In general, vaporized hydrogen peroxide and ultraviolet irradiation were the strategies most cited and most promising. However, there is a lack of evidence and consensus related to the best method of N95 respirator decontamination.
Conclusion
The evidence regarding decontamination strategies of N95 respirators against SARS-CoV-2 remains scarce. Vaporized hydrogen peroxide and ultraviolet irradiation seem to be the current standard for N95 respirator decontamination.
Clinical significance
Vaporized hydrogen peroxide and ultraviolet irradiation appear to be the most promising methods for N95 respirator decontamination.
Keywords: Decontamination, Reuse, N95 masks, SARS-CoV 2, Coronavirus, Face mask
1. Introduction
The novel coronavirus, termed SARS-CoV-2, has produced a social disruption globally, with severe consequences for the population's general health. There are more than 47 million confirmed cases at the present moment, with a cumulative number of deaths of over 1,215,000, according to the World Health Organization (WHO) (updated data can be accessed at https://www.worldometers.info/coronavirus/). Currently, vaccines are still under trial, and there are no effective drugs to treat this disease [1]. Indeed, most of the available evidence supports the proposal that social distancing, wearing masks, and eye protection effectively prevent transmission [2]. Better hygiene (handwashing) and the use of sanitizers have also been found to reduce the spread of the disease (COVID-19) [1,3,4].
WHO has recommended using masks, and governments have established face protection policies for public spaces [4]. The resulting increase in demand and a shortage of market availability have led to price increases for masks [[5], [6], [7], [8], [9]]. Health professionals are at high risk for infection with the new coronavirus, and a lack of adequate protective equipment during critical procedures in infected patients is increasing that risk considerably [10,11]. In Brazil, for example, more nurses and nurse assistants have died due to COVID-19 than any other country [12]; most of them have been infected during their work with infected patients, and in some situations, using home-made masks.
N95 respirators are a type of respirator mask used as facial protection by healthcare providers and are specifically advocated when performing aerosol-generating procedures [7,13]. They present a hermetically sealed fit, and wearers do not breathe the surrounding air unfiltered. These respirators can filter over 95 % of pollutant particles (>0.3 μm) in the air due to a higher electrostatic charge (which blocks the particles) and have been suggested to be used to reduce the risk of SARS-CoV-2 spread [14]. These masks are intended for single use and, based on the manufacturer’s instructions, they are heat sensitive and not designed to be sterilized; however, due to their high costs and limited availability [6,13], different methods to decontaminate N95 [5,13,[15], [16], [17], [18], [19]] respirators have been discussed to allow multiple usages. Other types of N95 respirator include a mask with a valve designed for people exposed to asbestos and dust.
Decontamination methods can be classified into chemical or physical treatment, dry heat, or moist heat [7]. Such approaches need to fulfill specific criteria: elimination of harmful pathogens; minimal damage to the facemask structure; low toxicity and costs; masks should pass the fit test; the filter capacity of masks should stay the same; and no residue of the decontamination process should remain [7,13]. It is currently unknown which methods to decontaminate N95 respirators are most suitable and should be recommended to healthcare professionals worldwide.
Given the emerging importance of N95 respirator decontamination, a summary of the available decontamination methods would be highly useful. Hence, scoping reviews may be beneficial for a literature overview because they do not aim to answer a particular question, in contrast to systematic reviews. This scoping review, therefore, aimed to map, compile the evidence, and provide a literature overview of the effectiveness of different N95 respirator decontamination strategies against the novel coronavirus based on published studies and preprint material.
2. Methods
This study’s protocol is based on the framework proposed by Peters et al. (2015) [20] and is available at the following link: https://osf.io/4t936/. The reporting of this scoping review was based on the PRISMA Extension for Scoping Reviews [21].
2.1. Eligibility criteria
2.1.1. Inclusion criteria
We selected studies assessing different decontamination strategies of N95 respirators against SARS-CoV-2 or that discussed decontamination strategies; for example, letters, editorials, and literature reviews. No specifications regarding the coronavirus organisms (surrogate or not) used to test decontamination or the decontamination strategies themselves were applied.
2.1.2. Exclusion criteria
Studies discussing the use of N95 respirators that did not mention decontamination methods or discussing other types of respirators were excluded.
2.2. Information sources and search
The search was performed in two databases: Medline (PubMed) and Scopus; only articles written in English were included. The search strategy was based on MeSH terms of PubMed and specific terms of Scopus, and the last search was performed in August 2020.
The following strategies were used:
PubMed
((("Decontamination"[Mesh] OR "Decontamination" OR “Disinfection” OR “Ultraviolet-C” OR “peracetic acid”)) AND ("Masks"[Mesh] OR "Masks" OR "Respiratory Protective Devices"[Mesh] OR "Respiratory Protective Devices" OR “Device, Respiratory Protective” OR “Devices, Respiratory Protective” OR “Protective Device, Respiratory” OR “Protective Devices, Respiratory” OR “Respiratory Protective Device” OR “Respirators, Industrial” OR “Industrial Respirators” OR “Industrial Respirator” OR “Respirator, Industrial” OR “Respirators, Air-Purifying” OR “Air-Purifying Respirator” OR “Air-Purifying Respirators” OR “Respirator, Air-Purifying” OR “Respirators, Air Purifying” OR “N95″)) AND (“SARS-CoV-2″ OR “Coronavirus” OR “COVID-19″ OR “Coronaviruses”)
SCOPUS
"Decontamination" OR “Disinfection” OR “Ultraviolet-C” OR “peracetic acid” AND "Masks" OR "Respiratory Protective Devices" OR “Device, Respiratory Protective” OR “Devices, Respiratory Protective” OR “Protective Device, Respiratory” OR “Protective Devices, Respiratory” OR “Respiratory Protective Device” OR “Respirators, Industrial” OR “Industrial Respirators” OR “Industrial Respirator” OR “Respirator, Industrial” OR “Respirators, Air-Purifying” OR “Air-Purifying Respirator” OR “Air-Purifying Respirators” OR “Respirator, Air-Purifying” OR “Respirators, Air Purifying” OR “N95″ AND “SARS-CoV-2″ OR “Coronavirus” OR “COVID-19″ OR “Coronaviruses”.
An additional search was performed in two preprint platforms—bioRxiv and medRxiv—for the term "N95 AND Decontamination OR Disinfection" posted between "01 March 2020 and 10 August 2020″ and considering the inclusion criteria reported previously.
2.3. Selection
The search was undertaken using EndNote (EndNote X9, Thomson Reuters, New York, US). Two researchers independently identified relevant records by analyzing titles and abstracts for relevance according to the eligibility criteria. Retrieved records were classified as include, exclude, or uncertain. The full-text articles of the included and uncertain records were selected for further eligibility screening by the same two reviewers, again independently. Discrepancies in the screening of titles/abstracts and full-text articles were resolved through a discussion. In case of disagreement, the opinion of a third reviewer was obtained. The study selection of published studies and preprint materials was carried out separately.
2.4. Data charting and items
We created a form using Excel (Microsoft, Redmond, Washington, US), which was pilot tested by three reviewers to reach a consensus on what data to collect and how. Then, two reviewers extracted the data independently, and a third reviewer evaluated this process. The following data were collected: study design, study objective, decontamination regimens tested, organisms studied, evaluation method, and main findings. The following data were collected for studies only discussing (and not reporting on) decontamination strategies: study design, strategies discussed, and main findings.
2.5. Synthesis
The analysis was performed separately considering the published and preprint materials based on the following structure: 1) study selection analysis; 2) a descriptive analysis of the characteristics of included studies, such as study design and decontamination regimens tested or discussed and; 3) findings of the main methods tested or discussed presenting first reports describing decontamination methods, followed by results of in vitro studies/reports discussing decontamination methods’ availability and feasibility and reviews. We decided to perform a separate descriptive analysis because preprints are preliminary reports of work that have not been certified by peer review.
3. Results
3.1. Published studies
3.1.1. Literature search
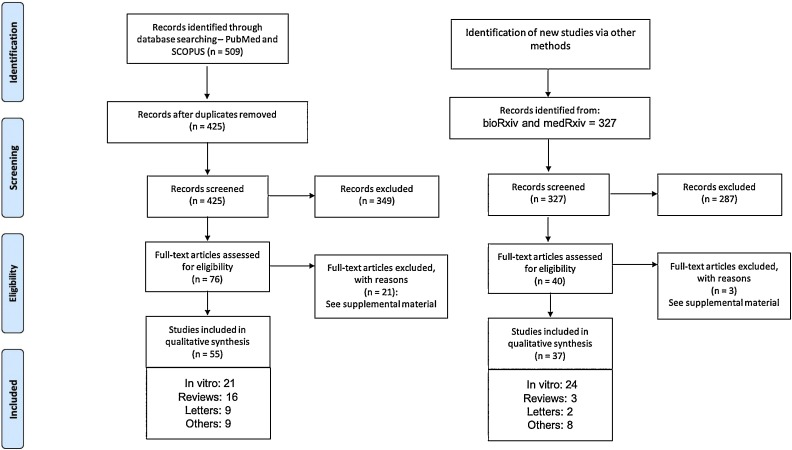
The literature search yielded 425 unique titles and abstracts (Fig. 1 ). Fifty-five articles fulfilled the eligibility criteria from which the data were extracted. Reasons for exclusion are listed in the Supplemental Material.
Fig. 1.
Flowchart of identification, screening, and assessing studies for inclusion eligibility.
3.1.2. Characteristics of included studies
Table 1, Table 2 present the characteristics of the included studies. Related to the study design of included reports, 9 articles were letters to the editors [6,7,13,16,18,[22], [23], [24], [25], [26]], 21 were in vitro studies [5,17,19,[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]], 16 were literature reviews [15,[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]], and 9 were classified as other study designs [[60], [61], [62], [63], [64], [65], [66], [67], [68]]. Considering only the 9 letters to the editors, 3 letters discussed the results of in vitro studies [6,7,13]. Details regarding published in vitro studies are presented in the Supplemental Material.
Table 1.
Characteristics of the published studies included considering in vitro studies, letters and other studies designs.
| Article | Objective | Decontamination regimens tested or discussed | Organisms tested or discussed | Organisms used as coronavirus organism | Method of evaluation | Main findings |
|---|---|---|---|---|---|---|
| IN VITRO STUDIES | ||||||
| Anderegg et al. 2020 | This research studied the effect of five cycles of heating to 85 °C for 30 min with a relative humidity of 60−85% | Heat and humidity | – | – | – | Authors found that for all of the N95 models we investigated there was no significant difference in filtration efficacy between the test groups of masks and the untreated control masks. |
| Cadnum et al. 2020 | The goal of the current study was to examine the effectiveness of UV-C light and a high-level disinfection cabinet for decontamination of N95 respirators. | Ultraviolet-C Light, Multi-purpose high-level disinfection cabinet that generates aerosolized peracetic acid and hydrogen peroxide and dry heat | Bacteriophages MS2, Bacteriophages Phi6, Bacteriophages Phi X174, Acinetobacter baumanii, Vancomycin-resistant, Enterococcus faecium, NDM1-producing Klebsiella pneumoniae, Methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Candida auris, Candida albicans, Clostridioides difficile, Bacillus subtilis | Bacteriophage Phi6 | methicillin-resistant Staphylococcus aureus MRSA test strain | The study found that UV-C reduced contamination of N95 respirators with Phi6 and MS2 bacteriophages and MRSA. However, efficacy varied with different respirator types and with different locations on the respirator. A high-level disinfection cabinet using submicron droplets of aerosolized peracetic acid and hydrogen peroxide was substantially more effective for decontamination of N95 respirators and with 3 consecutive cycles or a single extended cycle achieved >6-log10 reductions meeting criteria for disinfection. |
| Banerjee et al. 2020 | Authors proposed to combine two systems such as Warm Moist Heat standalone and Ultraviolet Germicidal Irradiation standalone to harness the combined synergistic advantages into a hybrid model called Warm Ultra Violet Hybrid Model | UV irradiation and heat treatment | – | – | – | Application wise this hybrid model may be used for medical, industrial, domestic and personal sanitization purposes. Moreover, this model is not only restricted to SARS-CoV-2 but can be used to treat any type of virus/ bacteria. |
| Bopp et al. 2020. | To examine the efficacy of autoclave-based decontamination for the reuse of single-use surgical masks and N95 filtering facepiece respirators | Moist heat autoclave | – | – | – | The specific surgical masks and N95 FFR models can withstand autoclave decontamination for up to three cycles |
| Czubryt et al. 2020 | Authors assessed potential re-use via autoclaving of N95 respirator masks worn daily in a major urban Canadian hospital | Sterilization by autoclaving | – | – | – | Reuse of N95 respirator masks is feasible in major hospitals and other healthcare facilities. Such reuse requires development of a comprehensive plan that includes communication across staffing levels, from front-line workers to hospital administration, to increase the collection, acceptance of and adherence to sterilization processes for N95 respirator masks recovery |
| Daeschler et al. 2020 | Authors investigated whether thermal disinfection at 70 °C for 60 min inactivates pathogens, including SARS-CoV-2, while maintaining critical protective properties of N95 respirators for multiple cycles of disinfection and reuse in a real-world setting | Thermal disinfection in cycles of 60 min at 70 °C, at either 0% or 50% relative humidity | Escherichia coli | SARS-CoV-2 | CFU | Thermal disinfection successfully decontaminated N95 respirators without impairing structural integrity or function. |
| Fischer et al. 2020 | Not reported | UV light, heat treatment, 70 % ethanol, vaporized hidrogen peroxide | Not reported | HCoV-19 nCoV-WA1−2020 | Cytopathogenic effect was scored and the TCID50 was calculated | NR |
| Grinshpun et al. 2020 | Authors evaluated common surgical masks and N95 respirators with respect to the changes in their performance and integrity resulting from autoclave sterilization and a 70 % ethanol treatment | Sterilization in na autoclave under 250oF, 15 psi for 30 min, fast exhaust following by drying for 30 min, for 5 times; treatment of facepieces by soaking in 70 % ethanol for two hours | – | – | – | The initial collection efficiency and the filter breathability may be compromised by sterilization in an autoclave and ethanol treatment. The effect depends on a protective device, particle size, breathing flow rate, type of treatment and other factors. Additionally, physical damages were observed in N95 respirators after autoclaving. |
| Hankenson et al. 2020 | Authors describes the intensive developmental process that was necessary to convert a multiroom animal housing facility into a regional vaporized hydrogen peroxide decontamination center in response to the impact of the coronavirus pandemic in the United States | Vaporized hydrogen peroxide | – | – | – | Authors did not have access to confirmed COVID-19 samples to test eradication of coronavirus by the hydrogen peroxide fogging system; however, the EPA data for this chemical confirm virucidal activity. |
| Ibáñez-Cervantes et al. 2020 | Authors investigated the disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria by using hydrogen peroxide plasma | Hydrogen peroxide plasma | ESKAPE bacteria (Acinetobacter baumannii and Staphylococcus aureus) | SARS-CoV-2 | Amplification of specific genes by RT-PCR and CFU | Disinfection of N95 masks by using the hydrogen peroxide plasma technology can be an alternative for their reuse in a shortage situation. |
| Jatta et al. 2020 | Authors aimed to use a readily available local resource to prolong our institutional supply of N95 respirators during a crisis capacity while maintaining the safety of frontline providers | 59 % vaporized hydrogen peroxide | – | – | – | Authors have successfully demonstrated that N95 respirator decontamination with vaporized hydrogen peroxide at 59 % hydrogen peroxide can be safely utilized to decontaminate single-use N95 respirators without significant effects on filtration efficiency or quantitative fit testing. (deixei isso p discutir caso precise): Authors believe it is important to note that decontamination methodologies should only be used as crisis capacity as these respirators were designed for single-use. Without appropriate expertise and logistics, the authors would not recommend respirator decontamination and would recommend only extended use of respirators per Centers for Disease Control and Prevention guidelines. |
| Ma et al. 2020 | The study verified a simple decontamination measure suitable to most people for reuse of MMs and N95Ms. | Steam on boiling water | Avian coronavirus of infectious bronchitis virus H120 | Vaccine strain of avian infectious bronchitis virus H120 | RT-PCR | The study observes that if a mask will be reused, it should be doffed without touching its surface, and the doffed mask should be put directly into a plastic bag or stainless steel box for steam and avoiding contamination of the surface of other items. They also presume that the masks can be used for up to seven or ten days, if they keep clean and fitted, and have not been damaged by other factors. Therefore, this study is valuable for solving the great shortage of masks in many countries for fighting the COVID-19 pandemic. It can also minimize unnecessary waste and protect the environment for discarding reusable masks. |
| Ou et al. 2020 | To evaluate the filtration performance of three commercially available and two alternative face mask and respirator materials after selected decontamination treatments | Ultraviolet germicidal irradiation, oven heating method (dry heat as 77C), steam heat treatment method; isopropanol (IPA) (soaking or spraying) | – | – | – | Both IPA soaking and spraying removed most electrostatic charges on all four electret materials (three commercial and one alternative), causing significant deterioration of filtration efficiency to unacceptable level. The other non-electret alternative material sustained its N95-grade performance after both IPA soaking and spraying treatments, demonstrating the possible application of IPA disinfection for non-electret alternative respirator/mask materials. UVGI preserved the filtration of all three commercially available respirator/mask materials after up to 10 treatments, suggesting it can be a possible decontamination method for hospital and clinic use without compromising respirator/mask performance. Between the two heat treatment methods tested, dry heat showed better compatibility with electret material by sustaining both filtration efficiency and fit (tested on commercial respirator only), although adding moisture was reported in favor of virus inactivation. Heat treatment is easily accessible method for general publics to implement at home, while it is recommended to maintain the moisture level below saturation. |
| Pascoe et al. 2020 | The study aimed to establish effective protocols for the decontamination of respirators using dry heat or microwavegenerated steam | 70oC dry heat and microwave generated steam | Staphylococcus aureus | Staphylococcus aureus | CFU | Authors found that microwave generated steam was potentially effective in decontaminating N95-type respirators, whilst dry heat was potentially effective for the reprocessing of N95-type respirators, providing possible safe reprocessing methods should the procurement of unused PPE fail. |
| Saini et al. 2020 | The study highlights the utility of vaporized hydrogen peroxide-based strategy to ensure a safe and effective disinfection of PPEs for selective reuse. | Various concentrations of hydrogen peroxide by diluting the hydrogen peroxide stock to 6, 8 and 10% with distilled water | B. stearothermophilus, saprophytic, non-virulent, recombinant laboratory strains of E. coli and M. smegmatis | Not reported | CFU | Vaporised hydrogen peroxide-based disinfection method is a suitable process to ensure a safe and effective reuse of PPEs |
| Simmons et al. 2020 | Article reports the effectiveness of a pulsed xenon ultraviolet disinfection system in reducing the load of SARSCoV- 2 on hard surfaces and N95 respirators | Pulsed Xenon Ultraviolet | The SARS-CoV-2 working stock was generated from isolate USA-WA1/2020 | The SARS-CoV-2 working stock was generated from isolate USA-WA1/2020 | Plaque assay | Authors found that Pulsed Xenon Ultraviolet significantly reduces SARS-CoV-2 on hard surfaces and N95 respirators |
| Vo et al. 2020 | The aim of the present study was to develop a test system to evaluate the effectiveness of procedures for decontamination of respirators contaminated with viral droplets | Sodium hypochlorite and UV irradiation | MS2 virus, Escherichia coli | – | The study for analazing the efficacy of decontamination(ED) for MS2 of sodium hypochlorite decontamination, were the number of viable MS2 phage was determined by a plaque assay, was calculated by determining the log reduction as follows: ED log (N°/N), where N° is the mean number of viable MS2 phage applied to the control coupons and N is the number of viable MS2 phage recovered from test coupons after decontamination. The efficacy of UV decontamination for viable MS2 was calculated as described for the efficacy of sodium hypochlorite decontamination. | The results demonstrated that the size range of the droplets was 0.5–15 μ and that the majority of the droplet particles were between 0.74 and 3.5 μ in diameter. Treatment with sodium hypochlorite (bleach) was an efficient chemical decontamination method for MS2 virus loaded onto FFRs. Treatment with low sodium hypochlorite doses (2.75–5.50 mg/liter) resulted in approximately 3- to 4-log reductions in the levels of MS2 coliphage, while treatment with higher sodium hypochlorite doses (8.25 mg/liter) resulted in no detectable MS2 virus. UV irradiation was also demonstrated to be an efficient physical decontamination treatment for MS2 virus. Treatment with low UV irradiation doses (4.32–5.76 J/cm2) resulted in 3.00- to 3.16-log reductions in the levels of MS2 coliphage, while treatment with higher UV irradiation doses (7.20 J/cm2) resulted in no detectable MS2 virus. |
| Wang et al. 2020 | Authors report a approach for the decontamination of masks using hot water at a temperature greater than 56o C for 30 min | Soaked in hot water at a temperature greater than 56o C for 30 min. The masks were then dried using an ordinary household hair dryer to recharge the masks with electrostatic charge to recover their filtration function | – | – | – | By soaking the masks in hot water at greater than 56 C for 30 min, viruses are killed and the dirt on the surface of the masks is removed. After the mask is dried with a standard hair dryer for 10 min, the static electricity of the surgical mask can be recovered to 90 % of the level of a newmask. |
| Woolverton et al. 2020 | The study tested the ability of food-grade silica bead packets to accelerate moisture removal from N95 s during 24 -h time periods. | Use of food-grade silica bead packets | – | – | – | The study does demonstrate that silica can be used to desiccate an N95, removing moisture that may be generated during the decontamination process using an autoclave or ionized/vaporized hydrogen peroxide, thus enabling the N95 to be more rapidly returned for use. |
| Xiang et al. 2020 | The study aimed to optimize the temperature of dry heat pasteurization to achieve efficient decontamination of masks | Dry heat at 60 °C and 70 °C for 1 h | Escherichia coli (ATCC25922), Staphylococcus aureus (ATCC25923), Pseudomonas aeruginosa (ATCC27853), Klebsiella pneumonia (ATCC70063), Acinetobacter baumannii (ATCC17978), Corynebacterium pseudodiphtheria (ATCC10701), and Candida albicans (ATCC10231). | H1N1 virus | Culture infective dose assay | "Dry heat at 60 °C and 70 °C for 1 h can ensure the decontamination of surgical face masks and N95 respirator while maintaining their filtering efficiency and shape for up to at least three rounds of dry heat". |
| Zulauf et al. 2020 | Authors described development and evaluation of a simple microwave steam decontamination protocol | Microwave steam | MS2 phage | MS2 phage | Plaque assay | Microwave-generated steam provides a valuable means of effective decontamination and reuse of N95 respirators. |
| LETTERS | ||||||
| Burkhart et al. 2020 | The sterilization process with the SoClean system is with activated oxygen (ozone) | Vaporized hydrogen peroxide, ethylene oxide, activated oxygen (often referred to as O3 or ozone) | – | – | – | The SoClean CPAP Sanitizer is a viable method for sterilizing against coronavirus, and therefore, reusing n95 masks or any cloth mask can be achieved with this method. |
| Carrillo et al. 2020 | Letter reporting an in vitro study assessing the use of Immediate-use steam sterilization for decontamination of N95 respirators. | Immediate-use steam sterilization (IUSS), using a Steris Amsco Evolution HC1500 PreVac Steam Sterilizer autoclave | It was tested sterelization and it was not tested specific organism | TSI PortaCount Respirator Fit Tester | The data of this study provides a valid base for the use of the IUSS method for decontamination of N95 masks to prevent the spread of the virus SARS-Cov-2 to health care workers | |
| Cheng et al. 2020 | Letter reporting an in vitro study assessing the use of Ionized H2O2 for decontamination of N95 respirators. | Ionized H2O2 (iHP) | H1N1 | H1N1 (enveloped RNA virus that has similar virological characteristics as coronaviruses) | The virus were enluted from N95 respirators for viral culture in MDCK cells. Cytophatic changes of MDCK cells were observed daily for 7 days by light microscopy and the samples were subcultured again on MDCK cells for a further seven days. It was preformed immunofluorescence staining to detect influeza A antigen. | This experiment showed that iHP could kill influenza A virus at moderate to high levels of inoculum. And the level of H2O2 on the inner surface of N95 respirators was 0.6 ppm (below the safety limit of <1 ppm) at 2 h and undetectable at 3 h. The speed of H2O2 release from N95 respirators may be variable and affected by the air current. |
| Hamzavi et al. 2020 | Letter proposing a possible repurposing of phototherapy devices, including these UVB units, to serve as a platform for UVC germicidal disinfection. | Ultraviolet germicidal irradiation (UVGI) | SARS-CoV | – | – | UVGI and repurposing phototherapy devices could be the best practical solution at this time. |
| Kobayashi et al. 2020 | Letter showing an overview of national regulatory authority recommendations. | Dry heat in a drying cabinet at 65–70 °C (Germany), vaporous hydrogen peroxide (Netherlands, Europe, and the United States), ultraviolet germicidal irradiation and moist heat (Europe and the United States) | – | – | – | The Ministry of Labor and Social Affairs of Germany described the recommended decontamination method for N95 respirators in detail (ie, dry heat at 65–70 °C in a drying cabinet for 30 min). On the other hand, up to 60% of the screened countries did not report any recommendations for extended use or reuse or decontamination of N95 respirators. |
| Li et al. 2020 [[69]] | Letter discussing an in vitro study that tested Rice Cooker-Steamer for Decontamination of Cloth and Surgical Face Masks and N95 Respirators. | Ultraviolet light treatment, hydrogen peroxide vapor, moist or dry heat, steam treatment via rice cooker steam | Clinical isolate of methicillin-resistant Staphylococcus aureus (MRSA) and thenonenveloped, single-stranded RNA virus bacteriophage MS2 | Unclear | Calculation of colony-forming units (CFU) or plaque-forming units (PFU) reduction. | The results of the study demonstrate that steam treatment using a rice cooker-steamer is effective for decontamination of face masks and N95 respirators. Investigations of moist heat are also needed as 20 min of exposure to moist heat at 65 °C has been reported to be effective with minimal adverse effects on respirator performance. |
| Narla et al. 2020 | Letter discussing the importance of the minimum dosage necessary for UVC decontamination of N95 respirators during the COVID-19 pandemic. | Ultraviolet C (UVC) | influenza A (H1N1), avian influenza A virus (H5N1), influenza A (H7N9) A/Anhui/1/2013, influenza A (H7N9) A/Shanghai/1/2013SARS-CoV-2, SARS-CoV and MERS-CoV | – | – | The study states that it should also be emphasized that there are significant limitations to UVC decontamination methods due to the variety of respirators used in healthcare facilities. Consequently, this process should only be considered as a risk mitigation effort during severe shortage of N95 respirators but is one of the most effective and best studied options available to front-line personnel. |
| Ozog et al. 2020 | Letter discussing the Importance of Fit-Testing in Decontamination of N95 1 Respirators | Ultraviolet germicidal irradiation (UVGI), hydrogen peroxide vaporization, microwave generated steaming and dry heating | The study discussed about fit-testing performance collected for the different respirator models treated with UVGI. | The data of this study strongly indicates that to protect the safety of the N95 respirator user, fit-testing after decontamination must be done each time a new model is introduced to a healthcare system. This has significant safety implications as varied decontamination methods are being used by different institutions. | ||
| Schwartz et al. 2020. | Authors shared processes of Decontamination and Reuse of N95 Respirators with Hydrogen Peroxide Vapor | Hydrogen peroxide vapor | 6-log biological indicators (Geobacillus stearothermophilus spores) | Not reported | The study talks about a quality assurance (QA) step, after complete aeration, to ensure both qualitative and quantitative degradation has not occurred, ensuring that there was no physical or erformance degradation. Also, a standardized quantitative fit testing was preformed to ensure the integrity of the respirators is maintained over many decontamination cycles. In addition, we validated the efficacy of the decontamination process by using 9 individual 6-log biological indicators (Geobacillus stearothermophilus spores). | Using hydrogen peroxide vapor is a proven method of decontamination. Authors believe that decontamination of N95 respirators with hydrogen peroxide vapor is one such solution that affords us better ability to protect our health care workers as we continue to tackle this monumental issue. |
| OTHERS | ||||||
| Garg and Garg, 2020 | Unclear | UV irradiation, vaporous hydrogen peroxide, moist heat, and microwave-generated steam | – | – | – | At present, it is unclear if these processes render the masks vulnerable and new research will address questions related to filtration efficiency and mask deformation. |
| Grossman et al. 2020 | The objective of the paper was to present a just-in-time process created for N95 respirator disinfection using VHP that allows the individual healthcare worker to retain his or her own respirator. | Vaporized hydrogen peroxide | N95 respirators used by health care workers | – | After each disinfection cycle, biologic indicators were transferred to commercially available trypticase soy broth (TSB) with a color indicator (Mesa Labs and Steris), and incubated at 56oC for at least 24 h. A negative result indicated a successful disinfection cycle. | The study shows that a reproducible and scalable process for implementing N95 respirator disinfection within a large academic hospital and healthcare system is achievable through multidisciplinary collaboration and rapid adaptation in the setting of the COVID-19 pandemic and critical N95 respirator shortages. |
| Juang and Tsai, 2020 | Unclear | Mask rotation (1 Mask Every 3–4 Days), Heat (at 70oC (158oF) for 60 M in.; Boil (for 5 M in., Steam Clean (at 125oC (257oF) for 5 M in. | – | – | – | The author present these methods and he suggest that where there are N95 respirator shortages around the world clinicians consider using one or more of these methods as a bridge until sufficient N95 masks are available. |
| le Roux and Dramowski, 2020 | The authors discusses the available PPE preservation strategies and addresses the issue of decontamination and re-use of N95 respirators as a last-resort strategy for critical shortages during the pandemic | Hydrogen peroxide vapour, UVGI (ultraviolet germicidal irradiation), Microwave (generated steam), Methods not currently endorsed owing to limited evidence (Moist heat incubation; Mask rotation; Ozone; liquid hydrogen peroxide/hydrogen peroxide plasma; dry heat; 70 % isopropyl alcohol; autoclave; soap; dry microwave irradiation; gamma irradiation; bleach; ethylene oxide) | – | – | – | Decontamination of N95 respirators should only be consider as a last resort to ensure a supply of N95 respirators for healthcare workers performing aerosol-generating procedures on patients with suspected/confirmed COVID-19. |
| Nogee and Tomassoni, 2020 | Authors propose investigating the use of ultraviolet germicidal irradiation to sterilize masks of SARS-CoV-2 for safer reuse. | Ultraviolet germicidal irradiation (UVGI), ethylene oxide and vaporized hydrogen peroxide | Influenza virus, SARS-CoV-2 and SARS-CoV | – | – | The study observes that although further work will be needed to determine dosages of UVGI to effectively sterilize SARS-CoV-2 contaminated FFRs, UVGI provides a potential avenue for greatly extending the limited FFR supply in the face of the ongoing COVID-19 pandemic in a simple, cost-effective, and rapidly deployable manner. Hospitals and healthcare facilities should consider immediate implementation of collection programs for used FFRs in anticipation of near-future sterilization and reuse programs. |
| Perkins et al. 2020 | Describe the development of a process that began in late February 2020 for selecting and implementing the use of hydrogen peroxide vapor (HPV) as viable method to reprocess N95 respirators | Hydrogen peroxide vapor (HPV) | N95 filtering facepiece respirators used by healthcare personnel | Culture and visual inspection | The data of the study presented in this article are meant to serve as an information sharing tool for other institutions who may wish to set up such processes, particularly for those who do not already have specific HPV chambers already in place. The two most important lessons learned from our experience are: (1) develop an adequate reserve of PPE for efficiently implementing the reprocessing workflow and (2) locate a suitable environment for the HPV decontamination procedure, such as an operating room, which has the pre-existing conditions required for conducting the HPV decontamination process. | |
| Prakash et al. 2020 | The article proposed the validation and eventual use of gamma irradiation, to disinfect FFRs in bulk. | Gamma irradiation | H1N1 influenza, MS2 virus and SARS-CoV-2 | SARS-CoV-2 | – | The data on re-sterilization strategies are scarce and do not address major concerns that allow for mass application. It needs to be stressed, in no uncertain terms, that re-sterilization techniques, such as gamma irradiation in this context need validation which if performed on a war footing, may just be of vital importance in these times. |
| Rowan and Laffey, 2020 | Article discussed concern regarding the shortage in supply chain of critical one-time-use personal and protective equipment. | Vaporised hydrogen peroxide, UV irradiation and High-level liquid disinfection (Actichlor+), Ethylene oxide (Eto) and pulsed UV technology (PUV) | – | – | – | The article observes that the best evidence suggest that preferred candidate methods for meeting this gap appears to be use of vaporized hydrogen peroxide (VHP) and UV irradiation technologies. |
| Zhong et al. 2020 | Authors report a plasmonic photothermal and superhydrophobic coating on N95 respirators | Deposit silver nanoparticles | – | – | – | The presence of the silver nanoparticles can provide additional protection via the silver ion’s disinfection toward microbes. |
Findings based on the article reporting.
Table 2.
Characteristics of the published reviews included.
| Article | Objective | Decontamination regimens tested or discussed | Organisms tested or discussed | Main findings |
|---|---|---|---|---|
| Boskoski et al. 2020 | The aim of this review was to summarize the protective efficacy of masks and respirators in preventing the spread of respiratory infections and to propose a proper biological decontamination process to take into consideration respirators reuse. | Autoclave, 160 °C dry heat, 70 % isopropyl alcohol, soap and water, ultraviolet germicidal irradiation (UVGI), ethylene oxide (EtO), vaporized hydrogen peroxide (VHP), microwave oven irradiation and bleach | H5N1 influenza virus, SARS-CoV and H1N1 influenza | The study suggests that the UVGI method proved to be a valid alternative to decontaminate N95 respirators, but it requires careful consideration of the type of respirator and of the biological target. |
| Carlos Rubio-Romero et al. 2020 | To consult the scientific literature to identify the main strategies for disinfecting them, and to determine the effectiveness of non-certified disposable masks | Hydrogen peroxide, chlorine dioxide, bleach, alcohol, soap solutions, ethylene oxide, ozone decontamination,etc., and physical methods, such as the use of heat with steam or with dry air, UV rays, gamma irradiation, microwave, etc. | – | The most promising methods are those that use hydrogen peroxide vapor, ultraviolet radiation, moist heat, dry heat and ozone gas. Soapy water, alcohol, bleach immersion, ethylene oxide, ionizing radiation, microwave, high temperature, autoclave or steam are not fully recommended. |
| Celina et al. 2020 | It was performed an overview on thermal responses and ongoing filtration performance of multiple face mask types | High energy irradiation (gamma-irradiation, UV), hydrogen peroxide, ethylene oxide, the use of heat to decontaminate (microwave), chemical-based sterilization (ethanol or isopropanol) | – | Authors have focused on two directions to enable the extended use of PPE face masks. One avenue that has also been recognized by others (see recent literature) is the use of thermal exposure for mask disinfection in the 75oC range, subject to confirmation of the most suitable times and temperatures by our bio-medical colleagues. Another strategy, particularly if local resources are limited and institutional large scale mask treatment approaches are not available, the subtle spraying of a mask's surface with a weak disinfectant solution might be an improvised option to enable some topical disinfection, or at least a refreshing of surfaces that are often touched and could be contaminated. |
| Garcia Godoy et al. 2020 | The purpose of the scoping review is to compile existing evidence on the use and efficacy of medical-grade and alternative forms of facial protection for healthcare workers amidst the growing global shortage. | Ultraviolet germicidal irradiation (UVGI), Microwave irradiation, microwave-generated steam, moist heat, bleach, hydrogen peroxide gas plasma, autoclave, 160 °C dry heat, 70 % isopropyl alcohol, soaking in soap and water and boiling water vapour and dry oven heating | SARS-CoV-1 | The study shows that overall, strategies involving the use of UVGI, ethylene oxide, dry oven heating and hydrogen peroxide vapor may be most promising for preservation of mask function and integrity. Decontamination with UVGI, moist heat incubation and microwave-generated steam does not appear to significantly affect N95 respirator fit or comfort. Until application of these methods has been adequately investigated in the hospital setting, their safety and effectiveness in the particular context of the SARS-CoV-2 outbreak is unknown. |
| Jinia et al. 2020 | An overview of various sterilization techniques with a particular emphasis on those that have demonstrated capability to inactivate viral population below detectability | Hydrogen peroxide (both vaporized and gas plasma), Heat, UV radiation, Gamma/X-ray irradiation | – | Sterilization processes should not compromise the quality and performance of the PPE itself. |
| Kampf et al. 2020 | Published data were reviewed to find out which temperature and exposure time is necessary for inactivation of coronaviruses. | Thermal disinfection (various temperatures) | SARS-CoV | Overall a thermal disinfection at 60 °C for 30 min, 65 °C for 15 min and 80 °C for 1 min was effective to strongly reduce coronavirus infectivity. Data do not allow to evaluate if the function of a face mask remains unchanged after heat treatment. If thermal disinfection is used for the re-use of masks all institutions should evaluate the effect on their own masks in use, as different brands of masks and different specifications (e.g. with or without cellulose) will react individually towards a combination of time and heat. Easy tests to do are "fitting" and "water-resistance”. In addition, the numbers of re-uses should be traced (mark at the side of mask per cycle) and its effects examined. |
| Katie et al. 2020 | To synthesize existing data on the effectiveness of ultraviolet germicidal irradiation for N95 FFR decontamination | Ultraviolet germicidal irradiation | – | The authors findings suggest that further work in this area (or translation to a clinical setting) should use a cumulative UV-C dose of 40,000 J/m2 or greater, and confirm appropriate mask fit following decontamination. |
| Polkinghorne and Branley, 2020 | Authors summarize previous and current research into the methods for decontamination and subsequent assessment of N95 respirators for contamination and/or filter performance | Steam, moist heat, dry heat, irradiation, bleach, ethanol, isopropanol, liquid hydrogen peroxide, benzalkonium chloride wipes, inert wipes, bleach wipes, ethylene oxide and vaporized hydrogen peroxide/hydrogen peroxide gas plasma | – | Of the methods that have been investigated to date, decontamination by steam, ultraviolet germicidal irradiation and hydrogen peroxide vapor hold the most potential as solutions that can be employed using existing health care facility infrastructure |
| Rodriguez-Martinez et al. 2020 | Summarize all of the available evidence on the different decontamination methods that might allow disposable N95 FFRs to be reused, with emphasis on decontamination from SARS-CoV-2 | Ultraviolet germicidal irradiation, ethylene oxide, vaporized hydrogen peroxide, microwave oven use, steam, bleach, heat treatment, ethanol, liquid hydrogen peroxide, autoclave, isopropyl alcohol, wipe products, tap water, soap and water and traditional electric rice cooker | – | Although all the methods for decontaminating and reusing N95 FFRs have advantages and disadvantages, ultraviolet germicidal irradiation and vaporized hydrogen peroxide seem to be the most promising methods |
| Seresirikachorn et al. 2020 | The study aims to evaluate the effectiveness of existing decontamination methods of surgical masks and N95 FFRs and provide evidence-based recommendations for selecting an appropriate decontamination method | Ultraviolet germicidal irradiation, moist heat, microwave generated steam, hydrogen peroxide vapour, microwave steam bags, bleach, steam, dry heat, ethanol or isopropyl alcohol and others | – | "The ultraviolet germicidal irradiation, moist heat, microwave generated steam, and hydrogen peroxide vapor methods were recommended as options. When these decontamination methods are used in practice, the techniques described in the literature should be strictly followed". |
| Srinivasan et al. 2020 | Authors discuss N95 FFP respirators: types, proper procedure for use, solutions for addressing the current shortage, and disadvantages | Gas plasma sterelisation with hydrogen peroxide, UV irradiation, Dry heating and others | – | The shortage of N95 respirators may be overcome by extended use and reuse - comprising rotation and decontamination by approved techniques |
| Steinberg et al. 2020. | Authors review the literature to synthesize the available evidence for N95 mask reuse, including decontamination approaches and methods to validate ongoing mask efficacy | Heat, autoclave, hydrogen peroxide vapour, hydrogen peroxide gas vapour, ionized hydrogen peroxide, ethylene oxide and ultraviolet light | – | Only two methodologies are supported to provide proper mask cleaning while maintaining physical integrity: HPV and moist heat (65–80C for 20–30 min, relative humidity of 50–85 %). |
| Su-Velez et al. 2020 | Authors explore the evidence for decontamination or sterilization of N95 respirators for health care systems seeking to conserve PPE while maintaining the health of their workforce | Time decontamination, bleach, alcohols, soap and water, vaporized hydrogen peroxide, ethylene oxide, microwave steam, microwave oven heat, dry oven heat, hot water vapor/moist heat and UVGI | – | None of these techniques are perfect in terms of balancing adequate viral decontamination with preserving mask fit and function, however, as N95 respirators are not designed for reuse. |
| Torres et al. 2020 | This article reviewed available recent evidence on different methods of filtering facepiece respirators decontamination that may potentially be applied during this pandemic or future emergencies | Ultraviolet germicidal irradiation, hydrogen peroxide vaporization, microwave-generated steaming, and dry heating | – | "UVC, hydrogen peroxide, microwave, and dry heat systems are all viable options to kill microorganisms on N95 FFRs to enable their reuse. These options are cost-effective, quick to employ, and have the potential to save many lives and valuable resources". |
| Udwadia and Raju, 2020 | Authors highlighted aspects of N95 use | Ultraviolet germicidal irradiation, vaporized hydrogen peroxide and moist heat | – | The methods cited are considered the three most promising decontamination methods. |
| Zorko et al. 2020 | Identify and synthesize data from original research evaluating interventions to decontaminate surgical masks for the purpose of reuse. | Dry heat, autoclave, ethanol, isopopanol, bleach, salt coating, quaternary ammonium agent, nanoparticle emulsion, repellant, N-halamine, | – | – |
Findings based on the article reporting.
Related to decontamination regimens tested or discussed, the use of vaporized hydrogen peroxide and ultraviolet irradiation were the regimens most cited. The use of vaporized hydrogen peroxide was in 6 letters [6,7,18,23,25,26], and two of them reported results of in vitro studies [6,7], six in vitro studies [5,32,[34], [35], [36],39], 13 reviews [15,45,[47], [48], [49],[51], [52], [53], [54], [55], [56], [57], [58]], and 6 other study designs [[60], [61], [62],64,65,67]. The use of ultraviolet irradiation was cited in 5 letters [7,18,[22], [23], [24]], and one of them discussed an in vitro study [7], 5 in vitro studies [5,30,32,37,40], 14 reviews [15,45,47,[52], [53], [54], [55], [56], [57], [58]], and 4 other study designs [62,64,65,67].
3.1.3. Vaporized hydrogen peroxide
Five studies reported the process for N95 decontamination with vaporized hydrogen peroxide. Schwartz et al. (2020) described the process implemented at Duke University (US) and demonstrated that vaporized hydrogen peroxide is an efficacious decontamination method that does not cause physical or performance degradation of the masks [25].
Perkins et al. (2020) described the process implemented at the University of New Mexico (US) and reported on the low toxicity of their methods. The authors highlighted the importance of physically assessing masks after decontamination [61]. Grossman et al. (2020) described decontamination using vaporized hydroperoxide employed by Washington University (US). They demonstrated that the entire process requires less than 24 h and showed that it is important to create a workflow to achieve effective decontamination considering pre-processing steps, decontamination, and post-processing steps [60]. Jatta et al. (2020) presented the decontamination using vaporized hydrogen peroxide (59 %) and demonstrated that this approach could be used safely without affecting mask performance [36]. Hankenson et al. (2020) described a process to develop a multiroom animal housing into a vaporized hydrogen peroxide center and found that this method can decontaminate a significant number of masks [34].
Further studies evaluated this latter strategy combined with others or discussed its availability and feasibility. Cadnum et al. (2020) performed an in vitro study and compared the use of a high-level decontamination cabinet that generates aerosolized peracetic acid and hydrogen peroxide with ultraviolet C light and dry heat at 70 °C for 30 min. They demonstrated that aerosolized peracetic acid and hydrogen peroxide are useful for the decontamination of N95 respirators [5].
Fischer et al. (2020) compared four different decontamination methods and demonstrated that vaporized hydrogen peroxide inactivated SARS-CoV-2 and preserved N95 respirator integrity [32]. Ibáñez-Cervantes et al. (2020) demonstrated that hydrogen peroxide plasma could be an alternative for N95 decontamination [35], and Saini et al. (2020) showed that a single cycle of vaporized hydrogen peroxide (7–8%) could decontaminate N95 respirators [39].
Kobayashi et al. (2020) assessed the authority recommendations in the Netherlands, the state governments in the US, the European Commission Directorate-General for Health and Consumers, and the European Medicines Agency regarding the use of vaporous hydrogen peroxide. They found that although this method seems to lead to acceptable decontamination while retaining mask integrity according to visual inspections, this type of decontamination is not available throughout all countries and institutions, and currently, no standard for its application exists [23].
Ten reviews noted that vaporous hydrogen peroxide appears to be a highly promising method for N95 respirator decontamination [45,47,[51], [52], [53],55,57,58,64].
3.1.4. Ultraviolet C light
Hamzanzi et al. (2020) presented a prototype model for N95 respirator decontamination using ultraviolet germicidal irradiation that would allow decontamination of 18–27 masks in one process [22]. Kobayashi et al. (2020) assessed the authority recommendations on ultraviolet germicidal irradiation use and found that although this method is promising, it has not been standardized by any of the authorities so far [23]. Cadnum et al. (2020) demonstrated that ultraviolet C could reduce N95 respirator contamination, but efficacy varied with different mask types and locations on the respirator [5].
Fischer et al. (2020) compared four different decontamination methods and demonstrated that ultraviolet C light inactivated SARS-CoV-2 more slowly on N95 respirators than stainless steel, most likely due to its porous nature [32]. Simmons et al. (2020) noted that a pulsed xenon ultraviolet system is promising for N95 respirator decontamination [40]. Ou et al. (2020) tested a variety of methods and showed that the use of ultraviolet C can preserve N95 respirator integrity [37]. Banerjee et al. (2020) suggested using a hybrid method, including ultraviolet C and heat treatment, that can be extended to other infections agents [28]. Narla et al. (2020) highlighted that at least 1 J/cm2 of ultraviolet C applied to all surfaces is necessary to ensure N95 respirator decontamination. However, the authors emphasized that ultraviolet C decontamination has limitations, mainly because each mask type requires a specific irradiation dose to be reliably effective [24].
In sum, eight reviews commented that ultraviolet germicidal irradiation is a highly
promising strategy for N95 decontamination [45,47,[50], [51], [52], [53],57,58].
3.1.5. Other methods
Different decontamination methods and protocols using heat have been discussed in the literature and found to be promising for N95 respirator decontamination.
The use of moist heat was cited in two letters [7,13], six in vitro studies [17,27,29,31,37,38], and two other study designs [66,67]. Regarding the in vitro studies, Anderegg et al. (2020) demonstrated that moist heat is a scalable method; all masks passed the fit testing and maintained filtration efficiency after five cycles [27]. Bopp et al. (2020) noted that N95 respirators could resist up to three cycles of moist heat [29]. Daeschler et al. (2020) tested different humidity scenarios and found that it is possible to use 50 % relative humidity for up to 10 cycles [31]. However, Pascoe et al. (2020) demonstrated that moist heat was effective only in some types of N95 respirators [38].
The use of dry heat was cited in four in vitro studies [32,37,38,43], one review [46], and one other study design [66]. Fischer et al. (2020) and Pascoe et al. (2020) demonstrated that the use of dry heat at 70 °C is effective [32,38], but Fischer et al. (2020) highlighted that this approach is not recommended for more than two rounds [32]. Ou et al. (2020) compared dry and moist heat; dry heat was a better method for maintaining filtration efficiency and fit testing, but by adding moisture, the virus inactivation was higher [37]. Xiang et al. (2020) demonstrated the successful use of dry heat at 70 °C after 1, 2, and 3 h for N95 respirator decontamination [43]. Other methods cited included the deposit of silver nanoparticles [68].
3.2. Preprints
3.2.1. Literature search
Thirty-seven studies fulfilled the eligibility criteria from which the data were extracted (Fig. 1).
3.2.2. Characteristics of included studies
Table 3, Table 4 present the characteristics of the included studies. Related to the study design of included studies, two articles were letters to the editors explaining the results of in vitro studies [70,71], 24 were in vitro studies [16,[72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94]], 3 were literature reviews [[95], [96], [97]], and 8 were classified as other study designs [[98], [99], [100], [101], [102], [103], [104], [105]]. Details regarding preprint in vitro studies are presented in the Supplemental Material.
Table 3.
Characteristics of the preprints included considering in vitro studies, letters and other studies designs.
| Objective | Decontamination regimens tested or discussed | Organisms tested or discussed | Organisms used as coronavirus organism | Method of evaluation | Main findings | |
|---|---|---|---|---|---|---|
| IN VITRO STUDIES | ||||||
| Card et al. 2020 | To outline a procedure by which PPE may be decontaminated using ultraviolet radiation in biosafety cabinets and discuss the dose ranges needed for effective decontamination of critical PPE. Furthermore, discuss the obstacles to this approach including the possibility that the UV radiation levels vary within biosafety cabinets. Determining if ultraviolet lights used in biosafety cabinets could be temporarily repurposed for ultraviolet germicidal irradiation decontamination to preserve a dwindling supply of PPE | Ultraviolet germicidal irradiation | SARS-CoV-2 | SARS-CoV-2 | Measurements of ultraviolet radiation fluence using a ultraviolet radiation meter and variance. | Authors recognize that institutions will require robust quality control processes to guarantee the efficacy of any implemented decontamination protocol. They also recognize that in certain situations such institutional resources may not be available; while we subscribe to the general principle that some degree of decontamination is preferable to re-use without decontamination. |
| Daeschler et al. 2020 | To investigate whether thermal disinfection at 70 °C for 60 min inactivates pathogens, including SARS-CoV-2, while maintaining critical N95 respirator protective properties for multiple cycles of disinfection and re-use in a real-world setting | Thermal disinfection (heat at 70 °C for 60 min) | E. coli | SARS-CoV-2 | CFU | Thermal disinfection enables large scale, low cost decontamination of existing N95 respirators using commonly sourced equipment during the COVID -19 pandemic. This process could be used in hospitals and long term care facilities and also provides a feasible approach to expand the N95 supply in low and middle income region. |
| Dave et al. 2020 (1) | To develop a cost-effective and scalable device that can sterilize any type of face covering, including N95 respirators. And to validate this system for clinical and community use by demonstrating efficacy for sterilization of surgical N95 respirators due to their widespread use and well-defined standards for minimum performance as dictated by the FDA and NIOSH | Vaporized hydrogen peroxide | P22 bacteriophage | P22 bacteriophage | Not reported | Authors suggested that vaporized hydrogen peroxide system is effective in sterilizing N95 respirators and other polypropylene masks for reuse. |
| Dave et al. 2020 (2) | Authors experimentally validated the system’s virucidal capability, impact on the filtration efficiency of N95 respirators, and effect on N95 respirator hydrophobicity | Ozone treatment system | P22 bacteriophage | P22 bacteriophage | Plaque-forming unit | Authors concluded that the ozone system will be effective in eliminating SARS-CoV-2 on various items including PPE. |
| Derr et al. 2020 | This study focused on the ability of the Curis® decontamination system to effectively achieve viral and microbial sterilization of N95 respirators by aerosolized H2O2, while preserving successful respirator fitting by medical staff after multiple cycles of decontamination | Aerosolized hydrogen peroxid | SARS-CoV-2, HSV-1, Coxsackie virus B3, Pseudomonas phi6 bacteriophage | SARS-CoV-2 | The number of infectious units, or plaque forming units (PFU) | Authors concluded that aerosolized hydrogen peroxid is a suitable decontamination process to enable reuse of N95 respirators during the COVID-19 pandemic. |
| Doshi et al. 2020 | Authors present a simple method to provide stable humidity and temperature for individual N95 masks which can be simply scaled in low resource settings | Moist heat (>50 % humidity, 65−80C temperature) | – | – | – | Authors demonstrated a highly accessible heating protocol for N95 respirators that achieves 65C and 50 % humidity for over 30 min without any advanced instrumentation or electricity. |
| Kumar et al. 2020 (1) | Authros sought to determine whether a range of different N95 masks would retain structural and functional integrity after treatment with widely available standard hospital decontamination techniques. Concurrently, we also determined the ability of each decontamination technique to effectively inactivate virus on experimentally inoculated masks. | Standard autoclaving, vaporous hydrogen peroxide exposure, peracetic acid dry fogging system, ethylene oxide gassing and low temperature hydrogen peroxide gas plasma treatment | Vesicular stomatitis virus, Indiana serotype or SARSCoV- 2 | SARSCoV- 2 | Cells were examined for determination of viral titres via observation of cytopathic effect. Titres were expressed as TCID50/mL as per the method of Reed and Muench | Authors found that one cycle of treatment with all modalities was effective in decontamination and was associated with no structural or functional deterioration. |
| Kumar et al. 2020 (2) | In this context, three types of masks, which are being used most of the countries, include N95, non-woven fabric masks (often called as surgical mask) and self-made two-ply cotton masks are tested for filtering efficiency (before and after sterilisation) with and without gamma sterilisation. Comparison of filtering efficiency of the current work and with the results available in the literature for N95 masks,and testing in accordance with two breath condition (normal and during sneezing) is highlighted. | Gamma irradiation, Hydrogen peroxide, chlorine dioxide, bleach, alcohol, soap solution and ethylene oxide, ozone decontamination, dry/steam heat treatment, UV light sterilization and electron beam | – | – | – | The gamma sterilization has shown decrease in efficiency from 99 % to about 70 % and still lesser with higher flow rate for ambient aerosols. |
| Liao et al. 2020 | To investigate multiple commonly used and easily deployable, scalable disinfection schemes on media with particle filtration efficiency of 95 % | Heat under various humidities, steam (100 °C heat based denature), 75 % alcohol 4) household diluted chlorine-based solution, ultraviolet germicidal irradiation | – | – | – | Heating (≤85 °C) under various humidities (≤100 % RH) was the most promising, nondestructive method for the preservation of filtration properties in meltblown fabrics as well as N95-grade respirators. Heating can be applied up to 50 cycles (85 °C, 30 % RH) without observation in the degradation of meltblown filtration performance. Ultraviolet (UV) irradiation was a secondary choice which was able to withstand 10 cycles of treatment and showed small degradation by 20 cycles. However, UV can also potentially impact the material strength and fit of respirators. Finally, treatments involving liquids and vapors require caution, as steam, alcohol, and household bleach may all lead to degradation of the filtration efficiency, leaving the user vulnerable to the viral aerosols. |
| Lilge et al. 2020 | Authors present a quantification of the optical absorption and light scattering coefficients for each layer of seven common filtering facepiece respirators | UVGI | – | – | – | Ultraviolet light germicidal is a reasonable approach for filtering facepiece respirators decontamination to extend a respirator’s usable lifetime when supply chains are restricted during public health emergencies. Both the investment costs and environmental impact are low |
| Ludwig-Begall et al. 2020 | Authors aim to provide information on the effects of three decontamination procedures on porcine respiratory coronavirus-contaminated masks and respirators, presenting a stable model for infectious coronavirus decontamination of these typically single-use-only products. | UV irradiation, vaporous hydrogen peroxide, and dry heat treatment | Porcine respiratory coronavirus | Porcine respiratory coronavirus | Virus titres were calculated using the Reed and Muench method | Authors describes successful validation of three decontamination methods, UV irradiation, vaporous hydrogen peroxide and dry heat treatment, in inactivating an infectious coronavirus |
| Manning et al. 2020 | To test the effectiveness of ozone on killing Pseudomonas aeruginosa on three different N95 respirators | Ozone | Pseudomonas aeruginosa | Pseudomonas aeruginosa | Kill efficacy was calculated by comparing the number of CFU/mL of the ozone-exposed respirator culture compared to the ambient airexposed controls | This study demonstrates that an ozone application achieves a high level of disinfection against Pseudomonas aeruginosa. Furthermore, conditions shown to kill these bacteria did not damage or degrade respirator filtration. |
| Massey et al. 2020 | Investigate the use of heat treatment at 75 °C as a potential method for recycling N95 respirators | Heat treatment at 75 °C (dry and humid) | Mouse hepatitis virus | Mouse hepatitis virus | Cytopathic effect for each well was recorded day 3 post-inoculation and TCID50 titer was calculated using the Spearman and Karber method | These results suggest that thermal inactivation of coronaviruses is a potentially rapid and widely deployable method to re-use N95 FFRs in emergency situations where re-using FFRs is a necessity and broad-spectrum sterilization is unavailable. |
| Meisenhelder et al. 2020 | Investigate the effects of two potential methods for decontamination; dry heat at 95 °C, and autoclave treatments | Dry heat at 95 °C and autoclave at 121 °C | – | – | – | 95 oC dry heat can be applied for 30 min for at least 5 cycles without signicant degradation of either fit or filtration |
| Nazeeri et al. 2020 | Introduce a number of methods which could be developed and validated for use in resource-limited settings. As the pandemic continues to spread in rural areas and developing nations, these would allow for local efforts to decontaminate, restore, and test medical masks. | 70 % ethanol | – | – | – | Authors replicated the drop in efficiency after 70 % ethanol treatment, but they found that the efficiency rose again after more effective drying, which we achieved with a vacuum chamber |
| Oral E et al. 2020 (1) | Explore the efficacy of vaporized hydrogen peroxide treatment of N95 respirators against surrogate viruses covering a wide range of disinfection resistance. | Vaporized hydrogen peroxide | Porcine Parvovirus (PPV), Bovine viral diarrhea virus (BVDV), Feline Calcivirus (FCV), Herpes Simplex Virus (HSV) and Influenza A Virus (InfA) | Porcine Parvovirus (PPV), Bovine viral diarrhea virus (BVDV), Feline Calcivirus (FCV), Herpes Simplex Virus (HSV) and Influenza A Virus (InfA) | Virus titer | In this study, one cycle of VHP sterilization (Steris ARD‐100®) for the 3 M 1860S N95 respirator was found to be effective in the inactivation of five different viruses with varying resistance to disinfection. |
| Oral E et al. 2020 (2) | Explore the benefits of using vaporized hydrogen peroxide treatment of N95 respirators for emergency decontamination | Vaporized hydrogen peroxide | SARS‐CoV‐2 USA‐WA1/2020 | SARS‐CoV‐2 USA‐WA1/2020 | Virus titer | One standard cycle of vaporized hydrogen peroxide (Steris LTS‐V) for one type of N95 respirator was found to be feasible in terms of preserving fit and filter efficiency |
| Oral E et al. 2020 (3) | Explore the efficacy of using moist heat as a decontamination method for an N95 respirator against various pathogens with different resistance | Moist heat | Virus: Bovine viral diarrhea virus (BVDV), Porcine Parvovirus (PPV) and Influenza A Virus (InfA) Bacteria: Staphylococcus aureus, Pseudomonas Aeruginosa and Acinetobacter Baumanii | Not reported | Virus: Virus titer; Bacteria: CFU | The obtained results showed the limits of efficacy of moist heat decontamination against various pathogens. Moist heat decontamination under the conditions studied here yielded at least a 5.3 log reduction with no residual colonies against the vegetative bacteria S. aureus, P. aeruginosa and A. Baumannii. On the other hand, the method’s efficacy against the tested viruses varied greatly; it was effective against InfA, modestly effective against BVDV, and not effective at all against PPV. |
| Ozog et al. 2020 | The objective of this study was to determine the effect of UVC on decontamination of SARS-CoV-2-innoculated N95 respirators | Ultraviolet C at a dose of 1.5 J/cm | SARS‐CoV‐2 USA‐WA1/2020 | SARS‐CoV‐2 USA‐WA1/2020 | TCID50 assay | UVC at a dose of 1.5 J/cm2 applied to both sides is effective at decontaminating SARS-CoV-2 on some N95 respirators |
| Price et al. 2020 | Assess the fit factor of filtering facepiece respirators (FFR) after two different disinfection methods (75 °C Hot Air (30 min.) for 10 cycles) and UVGI = UV 254 nm, 8 W, 30 min for 10 cycles. | Dry heat (75 °C Hot Air) and UVGI | – | – | These data suggest that UVGI methods of FFR decontamination cause fit failure in more than 40 % of the models tested to date | |
| Rockey et al. 2020 | Autros explored how temperature, humidity, and virus deposition solutions impact the inactivation of viruses deposited and dried on N95 respirator coupons. | Heat and humidity | Two bacteriophages (MS2 and phi6), a mouse coronavirus (murine hepatitis virus, MHV), and a recombinant human influenza A virus subtype H3N2 (IAV) | Two bacteriophages (MS2 and phi6), a mouse coronavirus (murine hepatitis virus, MHV), and a recombinant human influenza A virus subtype H3N2 (IAV) | Virus recovery was determined as the ratio of the control coupon virus titer to the suspended virus solution | The study demonstrated the virus inactivation efficacy of heat and humidity treatments for N95 respirator decontamination |
| Smith et al. 2020 | Investigate the effect of different decontamination methods on disposable N95 mask integrity and on eliminating the infectious potential of SARS-CoV-2 | 70 % ethanol, ultraviolet light and vaporized hydrogen peroxide | It is not clear all process used to assess SARS-CoV-2 detection and viability | Authors found that any ethanol exposure significantly altered mask integrity and the impact of 70 % ethanol on mask integrity appears time dependent. In fact, thirty minutes after 70 % ethanol application there was even a larger decline in measured integrity, even though the N95 masks felt dry to the touch. Authors did observe a decline in SARS-CoV-2 infectivity after certain decontamination strategies. | ||
| Wigginton et al. 2020 | Evaluate different N95 FFR decontamination strategies and their impact on respirator integrity and inactivating multiple microorganisms | Dry and moist heat, ethylene oxide, pulsed xenon UV, hydrogen peroxide gas plasma and vaporous hydrogen peroxide | Four viruses (MS2, phi6, influenza A virus, murine hepatitis virus), three bacteria (Escherichia coli, Staphylococcus aureus, Geobacillus stearothermophilus), and the fungus Aspergillus niger. | Murine hepatitis virus | Infectivity assays | Results suggest that either moist heat (82 oC + 62−66% RH) or vaporous hydrogen peroxide can address the hospital’s needs; however, each approach has notable limitations |
| Yim et al. 2020 | Authors reported the filtration efficiency, dipole charge density, and fiber integrity of pristine N95 and KN95 respirators before and after various decontamination methods | Dry Heat | – | – | – | Compared to the initial conditions, the filtration efficiencies of KN95 and N95 respirators increased after heat treatments; however, the filtration efficiencies stayed within a certain range after 60 min of heat treatment rather than a steady rise. |
| LETTERS | ||||||
| Kenney et al. 2020 | Authors evaluates the virucidal activity of hydrogen peroxide vapor using a BQ-50 system after inoculating N95 respirators with 3 aerosolized bacteriophages | Hydrogen peroxide vapor | T1, T7, and Pseudomonas phage phi-6 | Pseudomonas phage phi-6 | Plaque forming units | Authors found that Bioquell hydrogen peroxide vapor has high virucidal activity for N95 respirators inoculated with aerosolized virus. Use of a Bioquell machine can be scaled to permit simultaneous sterilization of a large number of used but otherwise intact respirators. Hydrogen peroxide vapor reprocessing may ease shortages and provide a higher filtration crisis alternative to non-NIOSH masks. |
| Ruzic et al. 2020 | Demonstrate that an atmospheric-pressure plasma generated by the microwave oven can decontaminate the respirator. | Microwave oven | Tulane virus in artificial saliva and Geobacillus stearothermophilus spores. | Not reported | Unclear | The plasma species generated in this manner are capable of decontamination in 30 s and anyone with a microwave oven and a few simple household items can create a N-95 respirator decontamination unit for emergency use. |
| OTHERS | ||||||
| Cramer et al. 2020 | To evaluate a recently developed technology, ionized hydrogen peroxide, specifically the SteraMist Binary Ionization Technology® from TOMI, as a method for sterilizing N95 masks and other PPE | Ionized hydrogen peroxide | Not reported | Not reported | It was measured with bacterial spores in standard biological indicator assemblies. | Authors support the use of the SteraMist Ionized hydrogen peroxide technology as a sterilization method for reuse of N95 masks, including many of the most commonly used models, following pre-treatment with an Ionized hydrogen peroxide handheld delivery device. |
| Gilbert et al. 2020 | To document procedures to build a similar type of UVGI irradiation platform with off-the-shelf components from the hardware store and UVGI bulbs sold online or from biosafety cabinets (class I, II, or III) that are ubiquitously found throughout academic research and industrial centers around the world. | Ultraviolet germicidal irradiation (UVGI) | – | – | – | The system presented is scalable and can be created for less than 50 US dollars, on site, at the point of need, and leverages resources that are currently untapped and sitting unused in public and private research facilities. |
| Huber et al. 2020 | Authors review the available literature concerning use of germicidal ultraviolet-C (UV-C) light to decontaminate N95 masks and proposed a practical method for repeated point-of-use decontamination, using commercially-available UV-C crosslinker boxes from molecular biology laboratories or a simple low-cost, custom-designed and fabricated device to expose each side of the mask to 800–1200 mJ/cm2 of UV-C. | Ultraviolet-C germicidal irradiation | – | – | – | Authos reviewed the efficacy of UV-C decontamination for N95 s, considering factors such as UV transmittance to different layers of the mask, viral sensitivity to UV-C, and potential photodegradation of masks. They also presented the Local UV Box, a practical, low-cost device for small-scale UV-C decontamination of N95 masks. This device assures that a consistent dose of UV-C is applied to the masks, enabling reliable decontamination and repeated reuse without substantial mask photodegradation. |
| John et al. 2020 | A multidisciplinary pragmatic study was conducted to evaluate the use of an ultrasonic room high-level disinfection system (HLDS) that generates aerosolized peracetic acid (PAA) and hydrogen peroxide for decontamination of large numbers of N95 respirators. | Ultrasonic room high-level disinfection system that generates aerosolized peracetic acid and hydrogen peroxide | Bacteriophage MS2 and Geobacillus stearothermophilus spores | Bacteriophage MS2 and Geobacillus stearothermophilus spores | CFU or PFU | Authors found that a ultrasonic room high-level disinfection system that generates aerosolized peracetic acid was effective for the decontamination of N95 respirators with a short cycle time. No adverse effects on filtration efficiency, structural integrity, or strap elasticity were detected after 5 treatment cycles. The ultrasonic room high-level disinfection system that generates aerosolized peracetic acid system provides a rapidly scalable solution for hospitals requiring in-hospital disinfection of N95 respirators. |
| Kayani et al. 2020 | To present the Synchronous UV Decontamination System (SUDS), a novel device for rapidly deployable, point-of-care decontamination using UV-C germicidal irradiation. | Ultraviolet germicidal irradiation (UVGI) | – | – | – | Authors designed a compact, easy-to-use device. This short decontamination time should enable care-providers to incorporate decontamination of FFR into a normal donning and doffing routine following patient encounters. |
| Lensky et al. 2020 | Authors propose a dry-heat decontamination method, using industrial dryers as the heat source. | Dry-heat | – | – | – | The data-driven protocol outlined passes the important tests of temperature stability and repeatability on a single machine. |
| Schnell et al. 2020 | To present a locally-implemented ultraviolet-C germicidal irradiation (UVGI)-based FFR decontamination pathway, utilizing a home-built UVGI array assembled entirely with previously existing components available. | Ultraviolet Germicidal Irradiation | – | – | – | Herein authors have devised a methodology that leveraged local resources and supplies to execute a local robust, data-driven, replicable UVGI-based decontamination process. |
| Su et al. 2020 | Introduce a photochromic UV-C dose quantification technique for: (1) design of UV-C treatments and (2) inprocess UV-C dose validation | UV-C decontamination | – | – | – | Authors introduce a new technique using photochromic UV-C indicators to address critical challenges hindering UV-C decontamination processes |
Findings based on the article reporting.
Table 4.
Characteristics of the preprint reviews included.
| Objective | Decontamination regimens tested or discussed | Organisms tested or discussed | Organisms used as coronavirus organism | Method of evaluation | Main findings | |
|---|---|---|---|---|---|---|
| Birgand et al. 2020 | To analyze the guidelines published by national and international societies/organizations on facemasks and respirators to prevent COVID-19 in healthcare settings | Ultraviolet germicidal irradiation, Vaporous hydrogen peroxide and Moist heat | Not reported | SARS-CoV-2 | Not reported | Authors discussed that reuse of maks was recomended in several countries, specially in period of shortage of supplies. However, some organizations as CDC and NIOSH did not recommend that FN95/99 be decontaminated or reused as standard care. Still, in times of crisis, this option may need to be considered when FFP2/3 shortages exist, recommending ultraviolet germicidal irradiation, vaporous hydrogen peroxide, and moist heat showing the most promise potential for decontamination. |
| Derraik et al. 2020 | Authors carry out a rapid review to summarize the existing evidence on SARS-CoV-2 survivorship and methods to disinfect PPE gear, particularly N95 filtering facepiece respirators | Ultraviolet germicidal irradiation and heat treatments | – | – | – | Authors proposed a protocol based on an initial storage of PPE for ≥4 days, followed by ultraviolet light, dry heat treatment, or chemical disinfection. |
| Toomey et al. 2020 | Summarise guidance and synthesize systematic review evidence on extended use, re-use or reprocessing of single-use surgical masks or filtering facepiece respirators | Microwave and heat based treatments, chemical disinfectants, ultraviolet germinicidal irradiation, disinfectant wipes, gamma irradiation and ozone decontamination | – | – | – | There is considerable discrepancy to the extent that no single reprocessing method is supported by all the guidance documents. The intervention with most support is vaporized hydrogen peroxide, though one document cautions about chemical residues and another indicates it has only been tested with some of the respirator models in common use. Similarly, ultraviolet germicidal irradiation receives both cautious support and concerns about inadequate decontamination because of incomplete penetration into deeper layers of the filter. Moist heat is cited as promising, though there are concerns when steam is microwave-generated where there may be uneven heating and where the metal nose band may generate sparks. |
Findings based on the article reporting.
Related to the decontamination regimens tested or discussed, the use of vaporized hydrogen peroxide and ultraviolet irradiation were the regimens most cited. Vaporized hydrogen peroxide was cited in one letter [70], 9 in vitro studies [74,76,78,79,81,86,87,92,93], one review [95], and two other study designs [98,101]. The use of ultraviolet irradiation was cited in 8 in vitro studies [16,72,[79], [80], [81],89,92,93], 3 reviews [[95], [96], [97]] and 5 other study designs [99,100,102,104,105].
3.2.3. Vaporized hydrogen peroxide
Seven in-vitro studies demonstrated that hydrogen peroxide is effective as a decontamination method of N95 respirators [75,76,78,81,86,87,93]. One review [95] showed that vaporous hydrogen peroxide has substantial potential for the decontamination of these respirators. Cramer et al. (2020), in a qualitative study, supported the use of SteraMist iHP technology [98], and Kenney et al. (2020) demonstrated in a letter the use of the BQ-50 system [70].
3.2.4. Ultraviolet C light
Four in vitro studies [16,72,81,89] demonstrated that ultraviolet C is effective as a decontamination method of N95 respirators. Two reviews showed that ultraviolet C light is one of the most promising decontamination [95,96]. However, Tommey et al. (2020) highlighted concerns about using this method due to incomplete penetration into deeper layers of the filter [97]. Five studies presented different devices to use ultraviolet C light as a decontamination method [99,100,102,104,105].
3.2.5. Other methods
Different decontamination methods and protocols using heat were discussed and demonstrated.
Moist heat was cited in 6 in vitro studies [73,77,80,83,91,93] and one review. Daescher et al. (2020) demonstrated that moist heat could be used even after ten cycles, maintaining mask integrity [73]. Doshi et al. (2020) presented a method using moist heat that could be scaled for low resources settings [77]. Liao et al. (2020) tested different disinfection methods; moist heat was found to be the most promising approach [80].
The use of dry heat was cited in 5 in vitro studies [81,83,84,94,103] and one review [96]. Meisenhelder et al. (2020) showed that 95 °C dry heat could be used for 30 min and five cycles, maintaining mask properties [84]. Lensky et al. (2020) proposed the use of industrial dryers to heat masks for 65 min at 80 °C and demonstrated that the masks maintained their properties after three cycles [103].
Other methods cited included ozone [74,82] and 70° ethanol [85,92]. Dave et al. (2020). and Manning et al. (2020) demonstrated that ozone is effective as a disinfection method [74,82], and Manning et al. (2020) highlighted that this method did not degrade mask properties [82]. Nazeeri et al. (2020) showed that the use of a vacuum chamber is important after 70° ethanol treatment to recover filtering efficiency [85].
4. Discussion
This study discussed the evidence about the effectiveness of decontamination strategies of N95 respirators against the novel coronavirus. Our results demonstrate a lack of evidence and consensus related to the best method for N95 respirator decontamination. However, vaporized hydrogen peroxide and ultraviolet irradiation were the regimens most cited and seemed to be the most promising methods for such decontamination.
Hydrogen peroxide vapor decontamination is common in different fields and facilities, including scientific, pharmaceutical, and medical ones. The method has low toxicity and uses the catalytic reduction of peroxide to oxygen and water [106]. However, it requires a specific room and equipment to achieve effective decontamination and, hence, is rather expensive. Ultraviolet irradiation is a decontamination method using ultraviolet light to inactivate microorganisms through RNA damage and cell function disruption [107]. This method has limitations due to different masks requiring a variety of irradiation dosages; a high dosage, in turn, could result in increased toxicity and mask structural damage. Moreover, this approach also needs specific equipment, limiting its availability.
Ideally, any decontamination method should eliminate all pathogens and maintain mask integrity and filter capacity at low toxicity and cost. Today, no one method fulfills these criteria, and the extended use of masks seems to be an appropriate, low-cost approach for overcoming the discussed availability limitations. Current recommendations consider mask-wearing periods between 4 and 40 h [23]. Notably, additional protection, such as face shields and strict adherence to hand hygiene practices is necessary, particularly if extending mask-wearing periods [108].
Outcomes such as mechanical integrity and performance of N95 respirators should be observed when assessing decontamination strategies of such respirators because decontamination may come at a price; decontaminated but ineffective masks are not useful and can even be dangerous. Ozog et al. (2020) indicated that fit testing must be performed after decontamination, and if decontamination is achieved but the masks lose their integrity, further usage should be stopped [18]. Hence, both integrity and performance should be prioritized when implementing decontamination strategies, although not all included studies concomitantly tested decontamination and subsequent mask performance.
Our study presents some limitations. First, because this was a scoping review, we did not conduct a risk of bias/quality assessment of the included studies. Second, we included only studies in English. Third, the included studies present different designs and protocols, making it difficult to compare the results, particularly because many N95 respirator brands are available on the market, different regimens were tested, and individual scenarios of wearers (such as the influence of cosmetics or sunscreen use for ultraviolet decontamination) are difficult to test. Further, many studies presented severe limitations, including small sample size and poor quantitative data reporting. Fourth, we included articles discussing decontamination methods based on opinions rather than evidence, making it difficult to provide more general conclusions and recommendations, and we included preprint studies; however, these are preliminary reports of works that have not been certified by peer review results and should, therefore, be interpreted with caution. Finally, we included different reviews that could generate an overlap of papers; however, 34 preprint reports (not considering preprint reviews) were included, but they were not part of the 16 published reviews.
The global COVID-19 pandemic is still currently accelerating. The shortage of protective equipment, particularly for healthcare workers, indicates that more investigations for safely decontaminating N95 respirators are necessary. The availability and cost-effectiveness of decontamination should also be considered in future primary studies.
5. Conclusion
The evidence supporting appropriate decontamination strategies of N95 respirators against the novel coronavirus remains scarce. Vaporized hydrogen peroxide and ultraviolet irradiation are the current standard for these respirators. However, cleaning is an essential step prior to decontamination, and decontamination methods should be followed by fit testing. Further, the extended use of masks appears to be an effective, low-cost approach to overcome the global shortage of N95 respirators.
Funding
The authors thank the funding from FAPERGS PRONEX (16.0471−4) and CAPES Print UFPel. This study was conducted in a Graduate Program supported by CAPES, Brazil (Finance Code 001).
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
RSO is funded in part by Meridional Foundation (Passo Fundo, Brazil) and LD is funded by Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). RCB is funded by National Council for Scientific and Technological Development (CNPQ, Brazil) and FFD is funded in part by National Council for Scientific and Technological Development (CNPQ, Brazil).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jdent.2020.103534.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Eikenberry S.E., Mancuso M., Iboi E., Phan T., Eikenberry K., Kuang Y., Kostelich E., Gumel A.B. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect. Dis. Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng V.C., Wong S.C., Chuang V.W., So S.Y., Chen J.H., Sridhar S., To K.K., Chan J.F., Hung I.F., Ho P.L., Yuen K.Y. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020;81:107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . 2020. Coronavirus Disease (COVID-19) Advice for the Public: When and How to Use Masks. [Google Scholar]
- 5.Cadnum J.L., Li D.F., Redmond S.N., John A.R., Pearlmutter B., Donskey C.J. Effectiveness of Ultraviolet-C light and a high-level disinfection cabinet for decontamination of N95 respirators. Pathog. Immun. 2020;5:52–67. doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng V.C.C., Wong S.C., Kwan G.S.W., Hui W.T., Yuen K.Y. Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D.T.S., Samaranayake L.P., Leung Y.Y., Neelakantan P. Facial protection in the era of COVID-19: a narrative review. Oral Dis. 2020 doi: 10.1111/odi.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oanh Ha K. 2020. The Global Mask Shortage May Get Much Worse. [Google Scholar]
- 9.WHO . 2020. Shortage of Personal Protective Equipment Endangering Health Workers Worldwide. [Google Scholar]
- 10.Mhango M., Dzobo M., Chitungo I., Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf. Health Work. 2020 doi: 10.1016/j.shaw.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veziant J., Bourdel N., Slim K. Risks of viral contamination in healthcare professionals during laparoscopy in the Covid-19 pandemic. J. Visc. Surg. 2020;157:S59–S62. doi: 10.1016/j.jviscsurg.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briso C.B., Phillips T. The Guardian; 2020. ‘My Mother Was Murdered’: How Covid-19 Stalks Brazil’s Nurses. [Google Scholar]
- 13.Carrillo I., Floyd A., Valverde C., Tingle T., Zabaneh F. Immediate use steam sterilization (IUSS) sterilizes N95 masks without mask damage. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cichowicz J., Coffey C., Fries M. National Institute for Occupational Safety and Health; 2018. A Guide to Air-Purifying Respirators; p. 176. [Google Scholar]
- 15.Boskoski I., Gallo C., Wallace M.B., Costamagna G. COVID-19 pandemic and personal protective equipment shortage: protective efficacy comparing masks and scientific methods for respirator reuse. Gastrointest. Endosc. 2020 doi: 10.1016/j.gie.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilge L., Manalac A., Weersink M., Schwiegelshohn F., Young-Schultz T., Abdalrhman A.S., Wang C., Ngan A., Gu F., Betz V., Hofmann R. 2020. Light Propagation Within N95 Filtered Face Respirators: a Simulation Study for UVC Decontamination. medRxiv. [DOI] [PubMed] [Google Scholar]
- 17.Ma Q.X., Shan H., Zhang C.M., Zhang H.L., Li G.M., Yang R.M., Chen J.M. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: experimental supports. J. Med. Virol. 2020 doi: 10.1002/jmv.25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozog D., Parks-Miller A., Kohli I., Lyons A.B., Narla S., Torres A.E., Levesque M., Lim H.W., Hamzavi I.H. The importance of fit-testing in decontamination of N95 respirators: a cautionary note. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vo E., Rengasamy S., Shaffer R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl. Environ. Microb. 2009;75:7303–7309. doi: 10.1128/AEM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters M.D., Godfrey C.M., Khalil H., McInerney P., Parker D., Soares C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Healthc. 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 21.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., Hempel S., Akl E.A., Chang C., McGowan J., Stewart L., Hartling L., Aldcroft A., Wilson M.G., Garritty C., Lewin S., Godfrey C.M., Macdonald M.T., Langlois E.V., Soares-Weiser K., Moriarty J., Clifford T., Tuncalp O., Straus S.E. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Inter. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 22.Hamzavi I.H., Lyons A.B., Kohli I., Narla S., Parks-Miller A., Gelfand J.M., Lim H.W., Ozog D. Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during COVID-19 pandemic. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi L.M., Marins B.R., Costa P.C.D.S., Perazzo H., Castro R. Extended use or reuse of N95 respirators during COVID-19 pandemic: an overview of national regulatory authorities’ recommendations. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narla S., Lyons A.B., Kohli I., Torres A.E., Parks-Miller A., Ozog D.M., Hamzavi I.H., Lim H.W. The importance of the minimum dosage necessary for UVC decontamination of N95 respirators during the COVID-19 pandemic. Photodermatol. Photoimmunol. Photomed. 2020 doi: 10.1111/phpp.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz A., Stiegel M., Greeson N., Vogel A., Thomann W., Brown M., Sempowski G.D., Alderman T.S., Condreay J.P., Burch J., Wolfe C., Smith B., Lewis S. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl. Biosaf. 2020 doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkhart C.G. Ozone disinfectants like soclean CPAP sanitizer can be used to sterilize cloth and n95 masks in the protection against COVID-19. Open Dermatol. J. 2020;14:14–15. [Google Scholar]
- 27.Anderegg L., Meisenhelder C., Ngooi C.O., Liao L., Xiao W., Chu S., Cui Y., Doyle J.M. A scalable method of applying heat and humidity for decontamination of N95 respirators during the COVID-19 crisis. PLoS One. 2020;15:e0234851. doi: 10.1371/journal.pone.0234851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee R., Roy P., Das S., Paul M.K. A Hybrid Model integrating warm heat and ultraviolet germicidal irradiation might efficiently disinfect respirators and personal protective equipment. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bopp N.E., Bouyer D.H., Gibbs C.M., Nichols J.E., Ntiforo C.A., Grimaldo M.A. Multicycle autoclave decontamination of N95 filtering facepiece respirators. Appl. Biosaf. 2020 doi: 10.1177/1535676020924171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czubryt M.P., Stecy T., Popke E., Aitken R., Jabusch K., Pound R., Lawes P., Ramjiawan B., Pierce G.N. N95 mask reuse in a major urban hospital - COVID-19 response process and procedure. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daeschler S.C., Manson N., Joachim K., Chin A.W.H., Chan K., Chen P.Z., Jones A., Tajdaran K., Mirmoeini K., Zhang J.J., Maynes J.T., Zhang L., Science M., Darbandi A., Stephens D., Gu F., Poon L.L.M., Borschel G.H. CMAJ; 2020. Effect of Moist Heat Reprocessing of N95 Respirators on SARS-CoV-2 Inactivation and Respirator Function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer R.J., Morris D.H., van Doremalen N., Sarchette S., Matson M.J., Bushmaker T., Yinda C.K., Seifert S.N., Gamble A., Williamson B.N., Judson S.D., de Wit E., Lloyd-Smith J.O., Munster V.J. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2609.201524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinshpun S.A., Yermakov M., Khodoun M. Autoclave sterilization and ethanol treatment of Re-used surgical masks and N95 respirators during COVID-19: impact on their performance and integrity. J. Hosp. Infect. 2020;105:608–614. doi: 10.1016/j.jhin.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hankenson F.C., Mauntel M., Willard J., Pittsley L., Degg W., Burnell N., Vierling A., Griffis S. Vaporized hydrogen peroxide decontamination of N95 respirators in a dedicated animal research facility for reuse during a novel coronavirus pandemic. Appl. Biosaf. 2020 doi: 10.1177/1535676020936381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibáñez-Cervantes G., Bravata-Alcántara J.C., Nájera-Cortés A.S., Meneses-Cruz S., Delgado-Balbuena L., Cruz-Cruz C., Durán-Manuel E.M., Cureño-Díaz M.A., Gómez-Zamora E., Chávez-Ocaña S., Sosa-Hernández O., Aguilar-Rojas A., Bello-López J.M. Disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria using hydrogen peroxide plasma: impact on the reutilization of disposable devices. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jatta M., Kiefer C., Patolia H., Pan J., Harb C., Marr L.C., Baffoe-Bonnie A. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou Q., Pei C., Chan Kim S., Abell E., Pui D.Y.H. Evaluation of decontamination methods for commercial and alternative respirator and mask materials – view from filtration aspect. J. Aerosol Sci. 2020;150 doi: 10.1016/j.jaerosci.2020.105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascoe M.J., Robertson A., Crayford A., Durand E., Steer J., Castelli A., Wesgate R., Evans S.L., Porch A., Maillard J.Y. Dry heat and microwave generated steam protocols for the rapid decontamination of respiratory personal protective equipment in response to COVID-19-related shortages. J. Hosp. Infect. 2020;106:10–19. doi: 10.1016/j.jhin.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saini V., Sikri K., Batra S.D., Kalra P., Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathog. 2020;12:29. doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons S., Carrion R., Alfson K., Staples H., Jinadatha C., Jarvis W., Sampathkumar P., Chemaly R.F., Khawaja F., Povroznik M., Jackson S., Kaye K.S., Rodriguez R.M., Stibich M. Deactivation of SARS-CoV-2 with Pulsed Xenon ultraviolet: implications for environmental COVID-19 control. Infect. Control Hosp. Epidemiol. 2020:1–19. doi: 10.1017/ice.2020.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D., Sun B.C., Wang J.X., Zhou Y.Y., Chen Z.W., Fang Y., Yue W.H., Liu S.M., Liu K.Y., Zeng X.F., Chu G.W., Chen J.F. Can masks be reused after hot water decontamination during the covid-19 pandemic? Engineering. 2020 doi: 10.1016/j.eng.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolverton C.J., Peeps C., Seidel J., Eng A. Use of silica beads to dry N95 respirators during storage. Appl. Biosaf. 2020 doi: 10.1177/1535676020936379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang Y., Song Q., Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am. J. Infect. Control. 2020;48:880–882. doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zulauf K.E., Green A.B., Nguyen Ba A.N., Jagdish T., Reif D., Seeley R., Dale A., Kirby J.E. Microwave-generated steam decontamination of N95 respirators utilizing universally accessible materials. mBio. 2020;11 doi: 10.1128/mBio.00997-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia Godoy L.R., Jones A.E., Anderson T.N., Fisher C.L., Seeley K.M.L., Beeson E.A., Zane H.K., Peterson J.W., Sullivan P.D. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kampf b G., Voss A., Scheithauer S. Inactivation of coronaviruses by heat. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlos Rubio-Romero J., Del Carmen Pardo-Ferreira M., Antonio Torrecilla García J., Calero-Castro S. Disposable masks: disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020;129 doi: 10.1016/j.ssci.2020.104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Celina M.C., Martinez E., Omana M.A., Sanchez A., Wiemann D., Tezak M., Dargaville T.R. Extended use of face masks during the COVID-19 pandemic - Thermal conditioning and spray-on surface disinfection. Polym. Degrad. Stabil. 2020;179 doi: 10.1016/j.polymdegradstab.2020.109251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jinia A.J., Sunbul N.B., Meert C.A., Miller C.A., Clarke S.D., Kearfott K.J., Matuszak M.M., Matuszak M.M., Pozzi S.A. Review of sterilization techniques for medical and personal protective equipment contaminated with SARS-CoV-2. IEEE. 2020;8:111347–111354. doi: 10.1109/ACCESS.2020.3002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katie O., Gertsman S., Sampson M., Webster R., Tsampalieros A., Ng R., Gibson J., Lobos A.T., Acharya N., Agarwal A., Boggs S., Chamberlain G., Staykov E., Sikora L., McNally J.D. Decontaminating N95 and SN95 masks with Ultraviolet Germicidal Irradiation (UVGI) does not impair mask efficacy and safety: a systematic review. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polkinghorne A., Branley J. Evidence for decontamination of single-use filtering facepiece respirators. J. Hosp. Infect. 2020;105:663–669. doi: 10.1016/j.jhin.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Martinez C.E., Sossa-Briceño M.P., Cortés J.A. Decontamination and reuse of N95 filtering facemask respirators: a systematic review of the literature. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seresirikachorn K., Phoophiboon V., Chobarporn T., Tiankanon K., Aeumjaturapat S., Chusakul S., Snidvongs K. Decontamination and reuse of surgical masks and N95 filtering facepiece respirators during COVID-19 pandemic: a systematic review. Infect. Control Hosp. Epidemiol. 2020:1–39. doi: 10.1017/ice.2020.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivasan S. N95 filtering facepiece respirators during the COVID-19 pandemic: basics, types, and shortage solutions. Malays. Orthop. J. 2020;14:1–7. doi: 10.5704/MOJ.2007.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinberg B.E., Aoyama K., McVey M., Levin D., Siddiqui A., Munshey F., Goldenberg N.M., Faraoni D., Maynes J.T. Efficacy and safety of decontamination for N95 respirator reuse: a systematic literature search and narrative synthesis. Can. J. Anaesth. 2020:1–10. doi: 10.1007/s12630-020-01770-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su-Velez B.M., Maxim T., Long J.L., St John M.A., Holliday M.A. Decontamination methods for reuse of filtering facepiece respirators. JAMA Otolaryngol. Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres A.E., Lyons A.B., Narla S., Kohli I., Parks-Miller A., Ozog D., Hamzavi I.H., Lim H.W. Ultraviolet-C and other methods of decontamination of filtering facepiece N-95 respirators during the COVID-19 pandemic. Photochem. Photobiol. Sci. 2020 doi: 10.1039/d0pp00131g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Udwadia Z.F., Raju R.S. The N-95 mask: invaluable ally in the battle against the COVID-19 pandemic. Lung India. 2020;37:323–328. doi: 10.4103/lungindia.lungindia_339_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zorko D.J., Gertsman S., O’Hearn K., Timmerman N., Ambu-Ali N., Dinh T., Sampson M., Sikora L., McNally J.D., Choong K. Decontamination interventions for the reuse of surgical mask personal protective equipment: a systematic review. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grossman J., Pierce A., Mody J., Gagne J., Sykora C., Sayood S., Cook S., Shomer N., Liang S.Y., Eckhouse S. Institution of a novel process for N95 respirator disinfection with vaporized hydrogen peroxide in the setting of the COVID-19 pandemic at a large academic medical center. J. Am. Coll. Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perkins D.J., Villescas S., Wu T.H., Muller T., Bradfute S., Hurwitz I., Cheng Q., Wilcox H., Weiss M., Bartlett C., Langsjoen J., Seidenberg P. COVID-19 global pandemic planning: decontamination and reuse processes for N95 respirators. Exp. Biol. Med. 2020 doi: 10.1177/1535370220925768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nogee D., Tomassoni A. Concise communication: Covid-19 and the N95 respirator shortage: closing the gap. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prakash A., Rao H.B., Nair P., Talwar S., Kumar V.A., Talwar D. Sterilization of N95 respirators: The time for action is upon us! Lung India. 2020;37(3):260–262. doi: 10.4103/lungindia.lungindia_191_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowan N.J., Laffey J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – case study from the Republic of Ireland. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garg M., Garg S. Novel coronavirus (COVID-19): N95 mask hysteria. Int. J. Crit. Illn. Inj. Sci. 2020;10:53–55. doi: 10.4103/IJCIIS.IJCIIS_78_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juang P.S.C., Tsai P. N95 Respirator Cleaning and Reuse Methods Proposed by the Inventor of the N95 Mask Material. J. Emerg. Med. 2020;58(5):817–820. doi: 10.1016/j.jemermed.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.le Roux C., Dramowski A. Personal protective equipment (PPE) in a pandemic: approaches to PPE preservation for South African healthcare facilities. S. Afr. Med. J. 2020;110:466–468. doi: 10.7196/SAMJ.2020.v110i6.14831. [DOI] [PubMed] [Google Scholar]
- 68.Zhong H., Zhu Z., You P., Lin J., Cheung C.F., Lu V.L., Yan F., Chan C.Y., Li G. ACS. Nano; 2020. Plasmonic and Superhydrophobic Self-Decontaminating N95 Respirators. [DOI] [PubMed] [Google Scholar]
- 69.Li D.F., Cadnum J.L., Redmond S.N., Jones L.D., Pearlmutter B., Haq M.F., Donskey C.J. Steam treatment for rapid decontamination of N95 respirators and medical face masks. Am. J. Infect. Control. 2020;48:855–857. doi: 10.1016/j.ajic.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kenney P., Chan B.K., Kortright K., Cintron M., Havill N., Russi M., Epright J., Lee L., Balcezak T., Martinello R. 2020. Hydrogen Peroxide Vapor Sterilization of N95 Respirators for Reuse. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruzic D.N., Oh C., Puthussery J.V., Verma V., Nguyen T.H. 2020. Converting Microwave Ovens Into Plasma-generating Decontamination Units for N-95 Respirators. medRxiv. [Google Scholar]
- 72.Card K.J., Crozier D., Dhawan A., Dinh M., Dolson E., Farrokhian N., Gopalakrishnan V., Ho E., Jagdish T., King E.S., Krishnan N., Kuzmin G., Maltas J., Mo J., Pelesko J., Scarborough J.A., Scott J.G., Sedor G., Tian E., Weaver D.T. 2020. UV Sterilization of Personal Protective Equipment With Idle Laboratory Biosafety Cabinets During the Covid-19 Pandemic. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daeschler S.C., Manson N., Joachim K., Chin A.W.H., Chan K., Chen P.Z., Tajdaran K., Mirmoeini K., Zhang J.J., Maynes J.T., Science M., Darbandi A., Stephens D., Poon L.L.M., Gu F., Borschel G.H. 2020. Reprocessing N95 Respirators During the COVID-19 Pandemic: Moist Heat Inactivates SARS-CoV-2 and Maintains N95 Filtration. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dave N., Pascavis K.S., Patterson J.M., Kozicki M., Wallace D.W., Chowdhury A., Abbaszadegan M., Alum A., Herckes P., Zhang Z., Chang J., Ewell C., Smith T., Naufel M. 2020. Characterization of a Novel, Low-cost, Scalable Ozone Gas System for Sterilization of N95 Respirators and Other COVID-19 Related Use Cases. medRxiv. [Google Scholar]
- 75.Dave N., Pascavis K.S., Patterson J.M., Wallace D.W., Chowdhury A., Abbaszadegan M., Alum A., Herckes P., Zhang Z., Kozicki M., Forzani E., Mora S.J., Chang J., Ewell C., Smith T., Naufel M. 2020. Characterization of a Novel, Low-cost, Scalable Vaporized Hydrogen Peroxide System for Sterilization of N95 Respirators and Other COVID-19 Related Personal Protective Equipment. medRxiv. [Google Scholar]
- 76.Derr T.H., James M.A., Kuny C.V., Kandel P.P., Beckman M.D., Hockett K.L., Bates M.A., Szpara M.L. 2020. Aerosolized Hydrogen Peroxide Decontamination of N95 Respirators, With Fit-testing and Virologic Confirmation of Suitability for Re-Use During the COVID-19 Pandemic. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doshi S., Banavar S.P., Flaum E., Kumar S., Chen T., Prakash M. 2020. Applying Heat and Humidity Using Stove Boiled Water for Decontamination of N95 Respirators in Low Resource Settings. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K., Vazquez-Grande G., Rush B., Lother S., Zarychanski R., Krishnan J. 2020. N95 Mask Decontamination Using Standard Hospital Sterilization Technologies. medRxiv. [Google Scholar]
- 79.Kumar A., Saneetha D.N., Yuvaraj R., Menaka M., Subramanian V., Venkatraman B. 2020. Effect of Gamma Sterilization on Filtering Efficiency of Various Respiratory Face-masks. medRxiv. [Google Scholar]
- 80.Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., Chu S., Cui Y. 2020. Can N95 Respirators Be Reused After Disinfection? And for How Many Times? medRxiv. [DOI] [PubMed] [Google Scholar]
- 81.Ludwig-Begall L.F., Wielick C., Dams L., Nauwynck H., Demeuldre P.-F., Napp A., Laperre J., Haubruge E., Thiry E. 2020. Decontamination of Face Masks and Filtering Facepiece Respirators Via Ultraviolet Germicidal Irradiation, Hydrogen Peroxide Vaporisation, and Use of Dry Heat Inactivates an Infectious SARS-CoV-2 Surrogate Virus. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manning E.P., Stephens M.D., Patel S., Dufresne S., Silver B., Gerbarg P., Gerbarg Z., Dela Cruz C., Sharma L. 2020. Disinfection of N95 Respirators With Ozone. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massey T., Borucki M., Paik S., Fuhrer K., Bora M., Kane S., Haque R., Baxamusa S.H. 2020. Quantitative Form and Fit of N95 Filtering Facepiece Respirators Are Retained and Coronavirus Surrogate Is Inactivated After Heat Treatments. medRxiv. [Google Scholar]
- 84.Meisenhelder C., Anderegg L., Preecha A., Ngooi C.O., Liao L., Xiao W., Chu S., Cui Y., Doyle J.M. 2020. Effect of Dry Heat and Autoclave Decontamination Cycles on N95 FFRs. medRxiv. [Google Scholar]
- 85.Nazeeri A.I., Hilburn I.A., Wu D.-A., Mohammed K.A., Badal D.Y., Chan M.H.W., Kirschvink J.L. 2020. Ethanol-Drying Regeneration of N95 Respirators. medRxiv. [Google Scholar]
- 86.Oral E., Wannomae K.K., Connolly R.L., Gardecki J.A., Leung H.M., Muratoglu O.K., Durkin J., Jones R., Collins C., Gjore J., Budzilowicz A., Jaber T. 2020. Vaporized H2O2 Decontamination Against Surrogate Viruses for the Reuse of N95 Respirators in the COVID‐19 Emergency. medRxiv. [Google Scholar]
- 87.Oral E., Wannomae K.K., Connolly R.L., Gardecki J.A., Leung H.M., Muratoglu O.K., Griffiths A., Honko A.N., Avena L.E., McKay L.G., Flynn N., Storm N., Downs S.N., Jones R., Emmal B. 2020. Vapor H2O2 Sterilization As a Decontamination Method for the Reuse of N95 Respirators in the COVID‐19 Emergency. medRxiv. [Google Scholar]
- 88.Oral E., Wannomae K.K., Gil D., Connolly R.L., Gardecki J., Leung H.M., Muratoglu O.K., Tsurumi A., Rahme L.G., Jaber T., Collins C., Budzilowicz A., Gjore J. 2020. Efficacy of Moist Heat Decontamination Against Various Pathogens for the Reuse of N95 Respirators in the COVID-19 Emergency. medRxiv. [Google Scholar]
- 89.Ozog D.M., Sexton J.Z., Narla S., Pretto-Kernahan C.D., Mirabelli C., Lim H.W., Hamzavi I.H., Tibbetts R.J., Mi Q.-S. 2020. The Effect of Ultraviolet C Radiation Against SARS-CoV-2 Inoculated N95 Respirators. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Price A.D., Cui Y., Liao L., Xiao W., Yu X., Wang H., Zhao M., Wang Q., Chu S., Chu L.F. 2020. Is the Fit of N95 Facial Masks Effected by Disinfection? A Study of Heat and UV Disinfection Methods Using the OSHA Protocol Fit Test. medRxiv. [Google Scholar]
- 91.Rockey N., Arts P.J., Li L., Harrison K.R., Langenfeld K., Fitzsimmons W.J., Lauring A.S., Love N.G., Kaye K.S., Raskin L., Roberts W.W., Hegarty B., Wigginton K.R. 2020. Humidity and Deposition Solution Play a Critical Role in Virus Inactivation by Heat Treatment on N95 Respirators. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith J.S., Hanseler H., Welle J., Rattray R., Campbell M., Brotherton T., Moudgil T., Pack T.F., Wegmann K., Jensen S., Jin J.S., Bifulco C.B., Prahl S.A., Fox B.A., Stucky N.L. 2020. Effect of Various Decontamination Procedures on Disposable N95 Mask Integrity and SARS-CoV-2 Infectivity. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wigginton K.R., Arts P.J., Clack H., Fitzsimmons W.J., Gamba M., Harrison K.R., LeBar W., Lauring A.S., Li L., Roberts W.W., Rockey N., Torreblanca J., Young C., Anderegg L.C., Cohn A., Doyle J.M., Meisenhelder C.O., Raskin L., Love N.G., Kaye K.S. 2020. Validation of N95 Filtering Facepiece Respirator Decontamination Methods Available at a Large University Hospital. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yim W., Cheng D., Patel S., Kui R., Meng Y.S., Jokerst J.V. 2020. Assessment of N95 and K95 Respirator Decontamination: Fiber Integrity, Filtration Efficiency, and Dipole Charge Density. medRxiv. [Google Scholar]
- 95.Birgand G., Mutters N.T., Otter J., Eichel V.M., Lepelletier D., Morgan D.J., Lucet J.C. 2020. Analysis of National and International Guidelines on Respiratory Protection Equipment for COVID-19 in Healthcare Settings. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Derraik J.G.B., Anderson W.A., Connelly E.A., Anderson Y.C. 2020. Rapid Evidence Summary on SARS-CoV-2 Survivorship and Disinfection, and a Reusable PPE Protocol Using a Double-hit Process. medRxiv. [Google Scholar]
- 97.Toomey E., Conway Y., Burton C., Smith S., Smalle M., Chan X.-H., Adisesh A., Tanveer S., Ross L., Thomson I., Devane D., Greenhalgh T. 2020. Extended Use or Re-use of Single-use Surgical Masks and Filtering Facepiece Respirators: a Rapid Evidence Review. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cramer A., Plana D., Yang H.L., Carmack M., Tian E., Sinha M.S., Krikorian D., Turner D., Mo J., Li J., Gupta R., Manning H., Bourgeois F.T., Yu S.H., Sorger P., LeBoeuf N.R. 2020. Analysis of SteraMist Ionized Hydrogen Peroxide Technology in the Sterilization of N95 Respirators and Other PPE: a Quality Improvement Study. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilbert R.M., Donzanti M.J., Minahan D.J., Shirazi J., Hatem C.L., Hayward-Piatkovskyi B., Dang A.M., Nelson K.M., Bothi K.L., Gleghorn J.P. 2020. Healthcare Worker Mask Reuse in a Global Pandemic: Using Idle Resources to Create an Inexpensive, Scalable, and Accessible UV System for N95 Sterilization. medRxiv. [Google Scholar]
- 100.Huber T., Goldman O., Epstein A.E., Stella G., Sakmar T.P. 2020. Principles and Practice of SARS-CoV-2 Decontamination of N95 Masks With UV-C. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.John A.R., Raju S., Cadnum J.L., Lee K., McClellan P., Akkus O., Miller S.K., Jennings W., Buehler J.A., Li D.F., Redmond S.N., Braskie M., Hoyen C.K., Donskey C.J. 2020. Scalable In-hospital Decontamination of N95 Filtering Facepiece Respirator With a Peracetic Acid Room Disinfection System. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kayani B.J., Weaver D.T., Gopalakrishnan V., King E.S., Dolson E., Krishnan N., Pelesko J., Scott M.J., Hitomi M., Scott J.G., Charnas I. 2020. UV-C Tower for Point-of-care Decontamination of Filtering Facepiece Respirators. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lensky Y.D., Mazenc E., Ranard D., Vilim M., Prakash M., Brooks B., Bradley A., Engelschenschilt A., Plutz J., Zellmer T. 2020. Heat-based Decontamination of N95 Masks Using a Commercial Laundry Dryer. medRxiv. [Google Scholar]
- 104.Schnell E., Harriff M.J., Yates J.E., Karamooz E., Pfeiffer C.D., McCarthy J., Trapp C.L., Frazier S.K., Dodier J.E., Smith S.M. 2020. Homegrown Ultraviolet Germicidal Irradiation for Hospital-based N95 Decontamination During the COVID-19 Pandemic. medRxiv. [Google Scholar]
- 105.Su A., Grist S.M., Geldert A., Gopal A., Herr A.E. 2020. UV-C Decontamination for N95 Emergency Reuse: Quantitative Dose Validation With Photochromic Indicators. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Battelle Memorial Institute; 2016. Final Report for the Bioquell Hydrogen Peroxide Vapor (HPV) Decontamination for Reuse of N95 Respirators. [Google Scholar]
- 107.Bedell K., Buchaklian A.H., Perlman S. Efficacy of an automated multiple emitter whole-room Ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infect. Control Hosp. Epidemiol. 2016;37:598–599. doi: 10.1017/ice.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.National Institute for Occupational Safety and Health . 2020. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.