Abstract
The majority of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected individuals remain paucisymptomatic, contrasting with a minority of infected individuals in danger of death. Here, we speculate that the robust disease resistance of most individuals is due to a swift production of type I interferon (IFNα/β), presumably sufficient to lower the viremia. A minority of infected individuals with a preexisting chronic inflammatory state fail to mount this early efficient response, leading to a delayed harmful inflammatory response. To improve the epidemiological scenario, we propose combining: (i) the development of efficient antivirals administered early enough to assist in the production of endogenous IFNα/β; (ii) potentiating early IFN responses; (iii) administering anti-inflammatory treatments when needed, but not too early to interfere with endogenous antiviral responses.
Keywords: COVID-19, SARS-CoV-2, antiviral molecules, immunomodulators, IFNα/β, cytokine storm
Highlights
Although the coronavirus disease 2019 (COVID-19) pandemic is exceptional, lessons may be learned from previous outbreaks (coronavirus, dengue, influenza viruses), especially when considering drug design and cytokine storms.
We propose that efficient treatments for COVID-19 patients should combine antivirals and immunomodulators. This combination and, especially the use of immunomodulators, might be adapted according to the disease stage.
Among the repurposed antiviral drugs currently being tested against COVID-19, none shows high potency.
We posit that the innate type 1 interferon (IFNα/β)-dependent antiviral immune response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection should be amplified. To this end, we propose two putative approaches: the inhibition of transforming growth factor (TGFβ) signaling, and perhaps, the administration of 1,8-cineole.
We suggest that an early diagnosis during COVID-19 is essential when aiming to purposely combine antivirals with the use of an immunomodulator (e.g., a drug to potentiate IFNα/β), ideally early in the disease course to lower the risk of cytokine storm manifestation. When the disease becomes severe, the new combination should prioritize targeting of the cytokine storm.
The SARS-CoV-2 Crisis and Its Associated Immunological Outcome
Zoonotic RNA viruses provide an infinite reservoir of potentially dangerous emerging viruses, impossible to eradicate from the wild. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mediates a respiratory infection that is difficult to deal with during an asymptomatic and contagious incubation period. An average basic reproduction number (R0) (see Glossary) of ~3 (i.e., one infected person infecting an average of three other persons) ensures vigorous spread of the SARS-CoV-2-associated disease coronavirus disease 2019 (COVID-19) in the absence of containment measures, enough to overwhelm most clinical/hospital settings, witnessing a fatality:case ratio (FCR) ranging from 0.03 to 30% across patients aged <17 to >85 years old, respectively [1]. Past problematic pandemics comparable with the present SARS-CoV-2 crisis include the ‘1918’ Influenza, the 1957–1958 Asian Influenza, and the 1968 Hong Kong Influenza. The 1918 Influenza (an H1N1 pandemic) had an estimated 2% fatality case rate, which led to an astonishing number of more than 40 million fatal cases [2].
The fact that most SARS-CoV-2-infected individuals remain pauci- or asymptomatic suggests that an efficient immune response can be mounted against this virus. A first concern would therefore be to increase the probability of occurrence of this spontaneous response. When this first immune response is defective and the disease worsens, the major hurdle becomes the cytokine storm, which may lead to acute respiratory distress syndrome (ARDS). Endeavors to prevent tipping into this phase are another key issue. This opinion article examines these various questions (Figure 1 ), from virology to immunology. We analyze the scientific basis by which antivirals are being considered and tested in clinical settings to treat COVID-19. We mention two molecules capable of increasing the spontaneous antiviral response and propose several approaches that might be used to dampen the virus-induced cytokine storm. The conclusion will bear on how these virological and immunological approaches should be combined, for present and future pandemics.
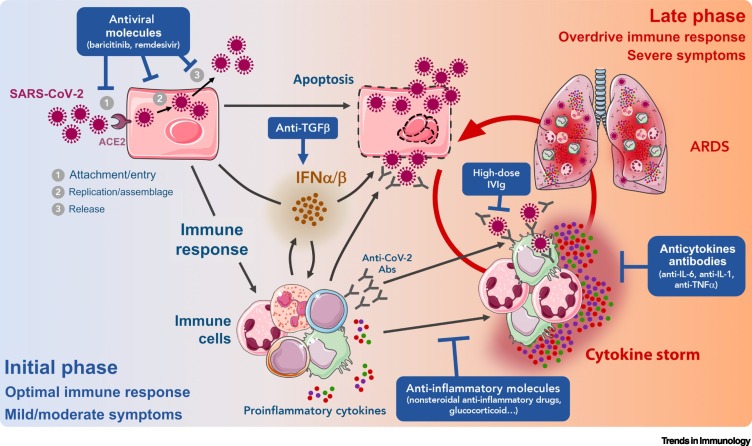
Figure 1.
Hypothetical Model of the Two Phases of Coronavirus Disease 2019 (COVID-19) and the Steps at Which Various Treatments Are Likely to Be Efficient (Blue Rectangles).
During the initial phase, infection of epithelial cells by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces weak production of interferon (IFN) α/β by these cells and the initiation of a limited antiviral immune response, leading to apoptosis of infected cells, the production of proinflammatory molecules, and the recruitment of immune cells. At this time, the viral load might be reduced by antivirals combined with an antiviral IFNα/β response enhanced by immunomodulator treatments [e.g., potentially transforming growth factor β (TGFβ) blockade]. Later, in some patients, an excessive inflammatory/immune response might give rise to a cytokine storm and/or acute respiratory distress syndrome (ARDS). This deleterious hyperinflammatory immune response might be dampened by anti-inflammatory/immunosuppressive treatments. Abbreviations: Abs, antibodies; ACE2, angiotensin-converting enzyme 2; IVIg, intravenous immunoglobulin therapy; IL, interleukin; TNF, tumor necrosis factor.
Looking for Efficient Anti-COVID-19 Antivirals
The birth of ‘successful’ antiviral therapies can be traced back to the fight against HIV, which became deeply influential for the hepatitis C virus (HCV) field and provided a clear start for antiviral discoveries against non-chronic RNA viruses [3., 4., 5., 6., 7., 8.]. All antiviral molecules target at least one part of the virus life cycle (Figure 2 ) and are classified as early (entry) or late (replication) inhibitors.
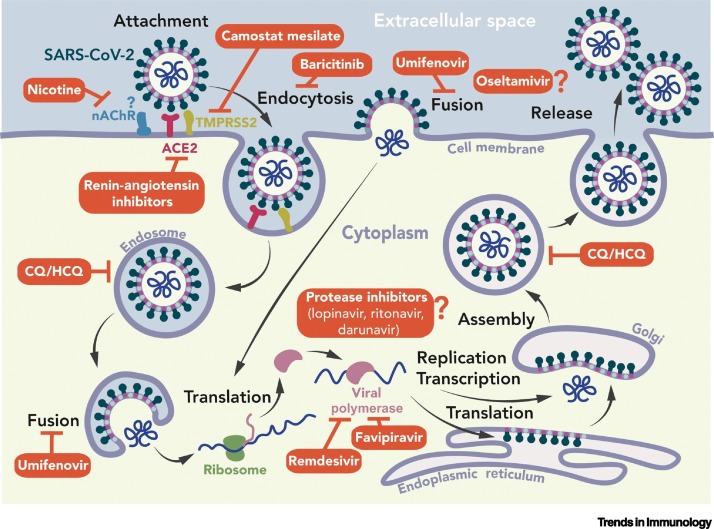
Figure 2.
Coronavirus Life Cycle and Target Sites of Potential Antiviral Agents.
The spike (S) protein binds to its main receptor, the cellular angiotensin-converting enzyme 2 (ACE2), and the virion enters through endocytosis and/or direct fusion of cell and viral membranes. The S protein is cleaved by various cellular proteases (e.g., TMPRSS2) into two subunits, S1 and S2 (‘priming process’) and at a S2′ site upstream of the fusion peptide [13]. The viral genome is translated into two polyproteins, which are cleaved by two viral proteases, chymotrypsin-like (3CLpro) and papain-like (PLpro), to generate a large replication and transcription complex orchestrating genome replication and the synthesis of mRNAs. New viral genomes recruit viral structural proteins to generate new virions released by exocytosis [6]. Red, potential inhibitors pointing towards their demonstrated targets. A question mark indicates that the target is putative, as discussed in the text. Abbreviations: CQ, chloroquine; HCQ, hydroxychloroquine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Antiviral drugs having a viral target are called direct acting antivirals (DAAs), while those having an antiviral effect through host cell proteins are called indirect acting antivirals (IAAs). Since the mutation rate of cellular genes is lower than that of viruses, drug resistance is less likely to occur with IAAs [9]. Examples are the pegylated type I interferons (IFNs) against HCV and the CCR5 antagonist maraviroc, targeting HIV-1 entry, although neither is now a first-line therapeutic choice for HCV- or HIV-1-infected patients. However, for COVID-19, the efficacy of type I IFN needs to be evaluated, and a drug targeting SARS-CoV-2 entry is being tested. Baricitinib, targeting the Janus kinase involved in the endocytosis of angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor, is a potential candidate [10] and has shown some activity against COVID-19 in a recent clinical trial (NCT04401579i). Accordingly, the TMPRSS2 protease, which also influences ACE2 endocytosis [11], might also constitute a potential target.
Based on HIV-1 and HCV research, effort should be targeted to DAAs, which generally target the most conserved viral enzymes: they remain efficacious within a given viral family and might be tested as broad-spectrum antivirals, even for future outbreaks. There is, for example, a high probability that a nucleoside analog active against SARS-CoV might also work against SARS-CoV-2 and Middle East respiratory syndrome coronavirus (MERS-CoV), given that their RNA-dependent RNA polymerases are structurally and functionally conserved [12]. However, this may not apply to the spike protein of SARS-CoV-2, be it for vaccine or antiviral design, given that its receptor binding domain sequence differs significantly between coronavirus strains [12,13].
Efficient antivirals might be discovered by proper drug repositioning (i.e., using a drug that is already efficient against another disease), as illustrated by favipiravir. Favipiravir is a purine-base analog that is converted in the cell into a 5′-triphosphate nucleoside analog. Once incorporated into viral RNA, it selectively alters the genetic makeup of RNA viruses [14]. Favipiravir was initially designed and approved against influenza virus [15]. However, a similar favipiravir mode of action has been recently described for SARS-CoV-2 [16]. Thus, it has now been repurposed against SARS-CoV-2 and is currently being tested in clinical trials for COVID-19 (NCT04358549ii, NCT04373733iii). Nevertheless, its efficacy against COVID-19 remains unknown. Remdesivir, a purine nucleotide analog initially developed against Ebola virus, showed good and moderate efficacy in vitro and in vivo, respectively, and is currently the sole FDA-approved drug in the treatment of COVID-19 [17]. However, the efficacy of remdesivir remains a controversial issue [18].
Unfortunately, drug repositioning is sometimes attempted without a scientific basis. In Box 1 are examples of DAAs currently repurposed in clinical trials for COVID-19 without solid scientific bases. In Box 2 , we discuss the case of hydroxychloroquine (HCQ), a highly controversial antiviral molecule.
Box 1. Illegitimate Drug Repositioning.
The first illegitimate drug repositioning for COVID-19 is the neuraminidase inhibitor oseltamivir, initially used against influenza viruses. This inhibitor was designed using the crystal structure of the influenza A N9 neuraminidase [88]. It is the first successful example of structure-based drug design against a virus pathogen. However, since there is no neuraminidase in SARS-CoVs, one wonders what is motivating the repositioning of this drug against COVID-19. Perhaps public ignorance and confusion are sustaining this illegitimate path. The second example is that of ritonavir or lopinavir. These drugs are HIV protease inhibitors, tested as early as 2004 and found to have an ‘apparent favorable effect’ in the original publication [89]. Later, the effects of these drugs were found to be nonexistent [90], yet they are still being tested in numerous clinical trials that have mobilized research efforts based on a result that is already known to be disappointing (https://clinicaltrials.gov/ct2/results?cond=COVID-19). The HIV protease is an aspartate protease, whose structure, substrate specificity, and mechanism are totally different from those of the Cys–His main protease chymotrypsin-like (3CLpro) of the SARS viruses [91].
Alt-text: Box 1
Box 2. The Case of HCQ.
HCQ has a clinical recognized efficacy as an antimalarial agent [92,93] and as an anti-inflammatory/immunosuppressor drug useful in certain inflammatory diseases [94]. In addition, certain HCQ antiviral effects against HIV-1 were demonstrated in vitro 30 years ago [95]. However, its in vitro activity does not translate to comparable concentrations in vivo. This is the case for several viruses in mouse, ferret, hamster, and guinea pig models, for influenza [96], Nipah [97], and Ebola [98], and in humans for chikungunya [99] and dengue [100].
The direct antiviral effects of HCQ observed in vitro are likely to be linked to the alkalinization of acid compartments of infected cells. This can interfere with the entry of the virus into the cell (since endocytosis is slowed by such alkalinization) and/or at a later stage of viral replication [98]. However, in vivo any potential antiviral effects of HCQ (if an effective concentration is large enough), if at all, are likely to be masked by its immunosuppressive properties, although this remains to be tested. This might explain why HCQ, while efficient in vitro against the Vero cell line infected with SARS-Cov-2, is totally inefficient in preventing infection, or in treating SARS-CoV-2-infected macaques [101]. HCQ has also been reported to be an efficient putative treatment against COVID-19 in a few clinical trials without control groups [102], findings that so far have not been confirmed in trials with control groups [103., 104., 105., 106.]. Therefore, any use of HCQ as a putative treatment/aid in COVID-19 patients remains completely unsubstantiated.
Alt-text: Box 2
In summary, despite our urgent need, only a few sound candidate antivirals have been identified. They include bariticinib, expected to block the entry of SARS-CoV-2 in ACE2-expressing cells, and favipiravir and remdesivir, which target viral replication.
The Natural Antiviral Immune Response and Its Reinforcement
All viruses trigger an antiviral response that relies on the immediate production of IFNβ in the host. The binding of IFNβ to its receptor IFNAR then triggers the production of IFNα. Both IFNβ and IFNα bind the receptor IFNAR, with different affinities [19]. Both IFNs trigger the expression of hundreds of interferon-stimulated genes (ISGs) [20,21]. All cell types are able to produce IFNα, but plasmacytoid dendritic cells (pDCs) can rapidly produce large amounts of this cytokine [22]. If the production of IFNα/β occurs immediately and is intense enough, the infection can be stopped. Although this remains to be demonstrated, this is probably what happens for SARS-CoV-2-infected individuals who remain asymptomatic or paucisymptomatic, as in almost all children.
However, the virus-induced IFNα/β response may be weak, due to aging, comorbidities, and anti-IFN mechanisms that most viruses have developed throughout millions of years of coevolution with vertebrates [23,24]. In such situations, the virus replicates and this triggers a second inflammatory/immune response, which may become explosive and potentially result in a cytokine storm and ARDS.
All coronaviruses (for a review see [25]) have developed multiple mechanisms for blocking IFNβ production or signaling in infected cells [26., 27., 28.]. During the replication process of RNA viruses, double-stranded RNA (dsRNA) can be detected by receptors such as Toll like-receptor 3 (TLR3) or retinoic acid-inducible gene-I (RIG-I)-like, and activate the IFNα/β response. However, coronaviruses hide their dsRNA replication/transcription intermediates within double-membrane vesicles that prevent detection by TLR3 [29,30] or RIG-I [31,32]. Numerous non-structural proteins (NSPs) (1, 3, 13, and 15), accessory open reading frame (ORF) proteins (3b, 4ba, and 6), and M and N proteins from various coronaviruses (MERS, SARS-CoV) have also been shown to prevent IFNα/β induction in human cell lines [3., 4., 5., 6., 7., 8.].
Another mechanism likely to occur but never reported so far, is the involvement of transforming growth factor beta (TGFβ) in coronavirus-induced inhibition of IFNα/β. SARS-CoV can prevent the phosphorylation and nuclear translocation of IRF3, a key transcription factor for IFNβ induction, by a mechanism involving the viral protease papain-like protease ( PLpro) in human promonocyte cells [33]. PLpro can significantly increase the expression of TGFβ in vitro in the same cells [33]. Also, higher serum concentrations of TGFβ were measured in early-stage SARS-CoV patients compared with age-matched normal controls [34]. The same difference in serum TGFβ was observed between severe and mild SARS-CoV-2-infected patients [35]. Moreover, TGFβ can be an effective blocker of IRF3 phosphorylation, fully preventing its nuclear translocation and IFNβ signaling [36,37] particularly in myeloid cells, as shown in a mouse tumor model of breast cancer. In addition, in human THP-1 macrophages, TGFβ inhibits the production of proinflammatory cytokines, including IFNβ [38]. There is a wealth of data underlining the immunosuppressive action of TGFβ in cancer [36] as well as during viral infections [39], suggesting that relieving this suppression might increase the efficacy of the immune response.
To allow efficient IFNα/β production, one could aim not only at alleviating a TGFβ-dependent brake, but also at potentiating its production. One such possibility could hypothetically be offered by 1,8-cineole, a small molecule capable of amplifying an immune response dependent on the IRF3/IFNβ pathway [40], as demonstrated in healthy human tissue maintained for several days in culture, in response to poly (I:C) stimulation. In this work, performed with biopsy slices of nasal mucosa isolated during nasal surgery, cineole accelerated the poly (I:C)-induced nuclear translocation of IRF3 [40]. What remains to be established is whether this potentiation of IRF3 activation translates, as expected, into faster production of IFNα/β. Such a possibility must be examined, given that efficient ways of potentiating IFNα/β production are not available so far.
Taking these findings together, a swift and vigorous IFNα/β increase is necessary to inhibit viral replication. We speculate that potentiating its production with 1,8-cineole or with the blockade of TGFβ signaling deserves to be rigorously tested. In addition, whether the administration of exogenous IFNα, either inhaled or injected subcutaneously when symptoms become clear, might be beneficial to the host is another direction to be investigated, without forgetting the well-known adverse effects of IFNα/β injections [41].
Cytokine Storm and ARDS
One main complication of unresolved viral infections can include a cytokine storm that occurs when many leucocytes, mainly macrophages, become overactivated and secrete proinflammatory cytokines. The system then triggers an uncontrolled positive feedback, with these cytokines activating more leukocytes. If not properly treated, it may rapidly result in ARDS, multiorgan failure, and potentially death [42].
The role of macrophages in the cytokine storm is central [42]. The release by these cells of exuberant proinflammatory cytokines and chemokines can follow massive epithelial and endothelial cell apoptosis and vascular leakage caused by early and rapid viral replication [42]. A key process triggering the cytokine storm is pyroptosis, or proinflammatory programmed cell death [43], affecting mostly macrophages but also lymphocytes; during pyroptosis, the inflammasome of murine macrophages and African green monkey kidney-derived Vero E6 cells have been respectively activated via viroporin 3a and E protein, two SARS-CoV proteins [44,45]. In addition, in SARS-CoV-infected Chinese macaques, binding of SARS-CoV-IgG complexes to monocyte/macrophage Fc receptors promoted inflammasome activation, the subsequent production of a large amount of proinflammatory cytokines in the lungs, and frequent fatal acute lung injury [46].
Twenty years ago, the concept of ‘inflammaging’ was proposed. It helps to explain the weakness of the immune system in the elderly [47]. One specific aspect of it underlines the importance of interleukin (IL)-6. The plasma concentrations of IL-6 are low or undetectable in most young individuals and begin increasing in healthy individuals at approximately 50–60 years of age [47]. In the elderly, the plasma concentration of IL-6 is elevated [48], but not that of tumor necrosis factor alpha (TNFα) or IL-1β [49,50]. We posit that inflammaging might potentially contribute to explaining the predominant susceptibility of the elderly to COVID-19, at least in part [51]. Specifically, several aging-related characteristics have been correlated with most COVID-19 fatalities, generally comprising individuals older than 70 years, with a median age of COVID-19-induced death of 80 years in Italy [52]. These age-related features concern, namely: (i) the presence of subclinical systemic inflammation without overt disease; (ii) a blunted acquired immune system and IFNα/β response, as shown by comparing young and old macaques infected with SARS-CoV [53]; and (iii) a dramatic reduction of ACE2 expression, demonstrated in old versus young rats relative to uninfected controls [54]. An aging-dependent reduction in anti-inflammatory ACE2 activity is likely to worsen SARS-CoV-2 infection outcomes [52]. These possibilities remain conjectural at this point and the contribution of inflammaging to COVID-19 disease severity will have to be robustly assessed.
Taking these findings together, an excessive and prolonged inflammatory response leading to ARDS may underlie the main danger for SARS-CoV-2-infected patients. A major factor favoring its occurrence may be inflammaging, accompanied by elevated and persistent serum proinflammatory IL-6 in aged individuals, and by low expression of ACE2 in the lung of aged animals compared with healthy controls. A variety of approaches to treat cytokine storm are discussed in Box 3 .
Box 3. Treating the Cytokine Storm.
The main treatments used to limit the consequences of cytokine storms include the administration of anti-inflammatory molecules such as glucocorticoids, antibodies neutralizing proinflammatory cytokines, and high-dose IVIg.
Anti-inflammatory Molecules
Since inflammation is an essential component in the establishment of an effective antiviral immune response, anti-inflammatory drugs may constitute an aggravating factor in the initial stages of viral infection [107]. It is thus reasonable to propose that their use should be strictly restricted to the late stage of the cytokine storm. During the pandemics of SARS-CoV and MERS-CoV outbreaks, corticosteroids were not routinely recommended. However, in the case of SARS-CoV-2, the use of dexamethasone significantly reduced mortality in patients with severe COVID-19 and is now considered an FDA- and European Medicines Agency (EMA)-approved treatment for COVID-19 [85]. An interesting alternative to corticosteroids might be colchicine, a safe and low-cost drug, which can inhibit the inflammasome [108] and is already used to treat several inflammatory diseases such as atherosclerosis [109] as well as having shown some antiviral effects against Flaviviridae [110]. Accordingly, colchicine is currently being tested in several clinical trials related to COVID-19 and results are eagerly awaited (NCT04322565iv, NCT04326790v, NCT04322682vi), the latter foreseeing the inclusion of 6000 participants.
Neutralizing Antibodies against Proinflammatory Cytokines
Anti-IL-6, anti-TNFα, and anti-IL-1 antibodies have been successfully used to treat several autoimmune inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, ankylosing spondylitis, and gout [111]. The serum concentration of these cytokines can be found to be abnormally high in patients with severe COVID-19 and are therefore being considered as potential therapeutic targets [112., 113., 114.]. Moreover, the cytokine storm that can be triggered in certain cancer patients on the adoptive transfer of chimeric antigen receptor (CAR) T cells can be controlled in certain situations with anti-IL-6/IL-6R neutralizing antibodies [115,116]. Preliminary studies have described that treatment with anti-IL-6R might reduce the risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia [117].
High-Dose IVIg
High-dose IVIg therapy has successfully been used to treat inflammatory autoimmune diseases such as thrombocytopenia purpura [118] or Kawasaki’s disease [119]. Such therapy in humans has achieved good tolerance and variable clinical benefits in respiratory syncytial virus (RSV) [120], SARS-CoV [121], and dengue virus infections [122]. Its most likely mechanism of action includes inhibition of myeloid cells mediated by the FcγRIIB receptor (CD32b), since the effect of IVIg was reported to be completely lost in FcγR–deficient mice [123]. Consequently, IVIg has begun to be used for severe cases of COVID-19, in some cases successfully [124].
These three ways of treating cytokine storms (anti-inflammatory molecules, neutralizing monoclonal antibodies, and IVIg) – among others – await further consideration in future studies.
Alt-text: Box 3
Kinetics of the Antiviral Immune Response, a Key Issue
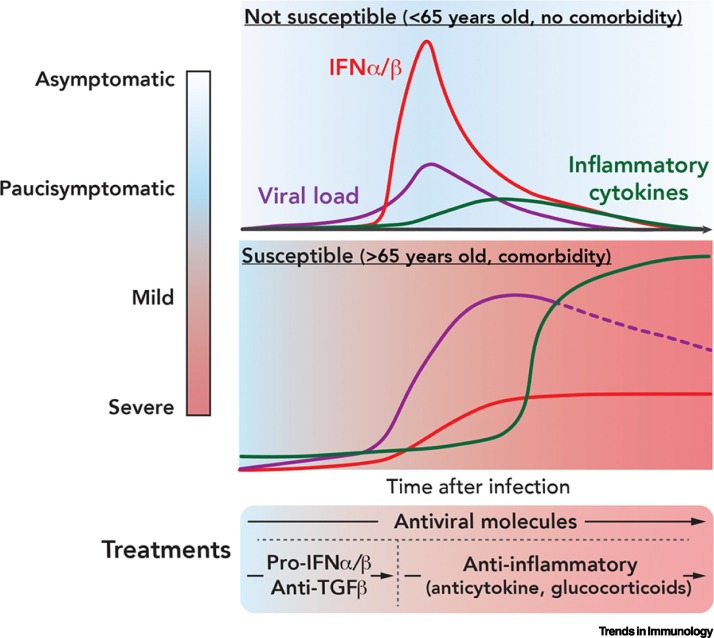
Figure 3 illustrates hypothetical kinetics of viral load and cytokine production in young versus aged individuals infected with SARS-CoV-2. In this model, resistance to the virus would be conditioned by the ability to mount a fast IFNα/β response (top panel) despite the anti-IFN arsenal of the virus, thus allowing a status of low viremia. This speculation is based on a large amount of data on the key antiviral role of IFNα/β. In susceptible individuals, a slowly growing IFNα/β response might be unable to control the viremia, presumably allowing a strong inflammatory response to follow (second panel).
Figure 3.
Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load Following Infection, in Parallel with the Interferon (IFN) α/β Response and the Evolution of Inflammatory Cytokines.
Top: Illustrates the case of most individuals in the population who remain asymptomatic or paucisymptomatic. In these individuals, efficient antiviral immune responses – characterized by significant production of IFNα/β and limited production of inflammatory cytokines – can lead to virus eradication [1]. Bottom: Illustrates the case of patients more severely affected by the virus. These patients show ineffective/delayed production of IFNα/β, uncontrolled viral load, and subsequent overproduction of inflammatory cytokines (cytokine storm) [1]. In the latter case, we propose that antivirals should be administered to patients as soon as possible, and maintained, whereas immunomodulators should be given when the disease worsens because of harmful inflammation. Abbreviation: TGFβ, transforming growth factor β.
The link between the kinetics of IFNα/β responses and viremia has been established with a mouse-adapted strain of SARS-CoV [55]. In mice infected with a lethal dose of SARS-CoV, an early IFNα/β response was clearly beneficial, whereas the inhibition of this early response combined with a late IFN response was absolutely deleterious, as shown by lung immunopathology, vascular leakage, and suboptimal T cell responses [55]. The efficient response depended on the ability of pDCs to mount initial IFNα/β production in these mice [55]. The lack of beneficial effect of IFNα when administered too late, at the ARDS stage, has also been reported in patients infected with MERS-CoV [56].
Similarly, humans that are unable to mount a robust IFNα/β response [e.g., due to signal transducer and activator of transcription 1 (STAT1) or (tyrosine kinase 2) TYK2 deficiency] are overly sensitive to a virus such as herpes simplex virus (HSV-1) [57]. At least 10% of 987 patients with life-threatening COVID-19 pneumonia have been reported to harbor neutralizing IgG autoantibodies against type I IFNs at the onset of the critical disease. These auto-autoantibodies, which neutralize the ability of the corresponding type I IFNs to block SARS-CoV-2 infection in vitro, were totally absent in 663 individuals with asymptomatic or mild SARS-CoV-2 infection [58].
If an immune response remains sustained instead of being transient, it may progressively lose its efficacy and be replaced by a harmful inflammatory state. The loss of efficacy results in part from the ‘exhaustion’ of T cells subjected to chronic stimulation [59]. The inflammatory state might be a consequence of excessive cell death due to large tissue damage [60]. Such a hypothesis is based on experimental models and on human findings, as illustrated below.
Epstein–Barr virus (EBV) is an example of a viral infection that is properly controlled in most humans. First, EBV infection leads to a transient intense immune response that lowers viremia and is accompanied by signs of transient inflammation, including fever [61]. This response is usually not followed by persistent inflammation, but in a second phase, viremia is kept under control due to the dynamic process of expansion and contraction of memory CD4+ and CD8+ T cell populations [62,63]. Thus, the virus is not eradicated, but it is symbiotic with the host. However, in immunocompromised individuals who are unable to mount an intense antiviral response, this virus may become dangerous [64] and can be associated with several malignancies (e.g., endemic Burkitt’s lymphoma, nasopharyngeal carcinoma, Hodgkin’s lymphoma) [65].
An example of an inappropriate antiviral immune response is that occurring upon HIV-1 infection. In this case, the initial antiviral response is usually unable to lower viremia enough to avoid progressive weakening of the adaptive response (in part because activated CD4+ T cells are directly targeted by this virus) and an ensuing chronic inflammatory state. According to our hypothesis, if the initial attack against HIV-1 infection were to be stronger, it might presumably dampen the development of this harmful inflammation. Accordingly, although not a direct demonstration of this hypothesis, in a study of HIV-1-uninfected young women at high risk of infection who later became infected during follow up, an early HIV-1-induced cytokine storm was minimized in the subgroup who were given antiretroviral therapy as soon as viral RNA could be detected and not just after serological tests indicated positivity for the virus [66]. This suggests that an early efficient antiviral treatment might be a solid option to minimize the cytokine storm.
Furthermore, the importance of a biphasic immune response is illustrated by monkeys infected with simian immunodeficiency virus (SIV). The natural hosts, African green monkeys, develop a strong but transient IFNα/β response following infection, allowing them to control SIV without developing chronic pathology. By contrast, rhesus monkeys have shown a sustained IFNα/β response to SIV infection and progressively develop chronic pathology that is similar to AIDS [67,68]. This suggests that a strong early rise in IFNα/β may be key in preventing a delayed, chronic, deleterious IFNα/β increase.
In another example, bats can be persistently infected with many viruses but rarely display clinical symptoms [69]. A viral infection usually triggers a rapid and significant – but transient – IFNα/β response without evidence of subsequent persistent inflammation [70]. In vitro, macrophages obtained from bats or mice respond differently to stimulation from a TLR3 ligand [70]. Compared with murine macrophages, bat macrophages initially produce more IFNα/β and more IL-10 [70]. If the same is true in vivo, which we still do not know, it might lead to an optimal, swift, intense, and transient antiviral response. Of note, bats have developed an efficient antioxidant arsenal [71]. Thus, one might speculate that these different characteristics could help virus-infected bats to better control viremia compared with other mammals and without developing chronic inflammation.
A better understanding of the kinetics of antiviral immune responses against different viruses (highlighting successful outcomes) may better inform the development of new putative antiviral treatments.
Summary of Rational Treatments against COVID-19
In our view, a rational treatment against SARS-CoV-2 should take into account the disease kinetics summarized in Figure 1, Figure 3. We argue that some of the most important points include: (i) a SARS-CoV-2 infection that starts with an asymptomatic but contagious phase in which (ii) both the kinetics and the amplitude of the IFNα/β response are likely to differ between susceptible and non-susceptible individuals; (iii) there is a preexisting, basal amount of inflammatory cytokines in susceptible patients (potentially due to inflammaging or to comorbidities; e.g., obesity, diabetes); and (iv) the roughly 2-week delay between infection and the surge of inflammatory cytokines might correspond to the delay necessary to produce immunoglobulins (Figure 3).
Importance of Early Diagnosis and DAAs
To reduce or impair viral replication, a safe DAA might constitute an interesting option, prophylactically centered around index cases and administered as early as possible following infection. A drug capable of curtailing viremia at the beginning of the infection – even modestly – might save a series of complications (e.g., cytokine storms or hemorrhagic fevers, depending on the virus) and lower the FCR [72,73]. To do this, the whole chain of diagnostics must be brought to a higher level of understanding and efficiency; for example, by providing education about being tested (in the general population via family doctors) and offering accurate/cost-effective tests. We posit that only if accurate diagnostics are optimized in the management of infected patients can morbidity and mortality be reduced in a viral pandemic. Thus, we argue that the identification and detection of early markers of infection, as well as normalized sampling for qPCR protocols, need to become a standard routine.
Amplifying the IFNα/β-Dependent Spontaneous Antiviral Response
The natural antiviral response, IFNα dependent, is decisive for blocking virus propagation in the infected tissue. We propose that a first option might be to treat virally infected individuals with IFNα/β when a paucisymptomatic disease begins to worsen. Associated with ribavirin, subcutaneous IFNα injections have increased the probability of clearance of HCV [74] and the survival of MERS-infected patients [75]. Moreover, compared with controls, a twofold reduction in 28-day mortality of severe COVID-19 patients receiving intravenous IFNα injections was recently reported [76]. A second option may be to unleash the endogenous IFNα/β response that has been blunted by the virus. Specifically, since TGFβ is a potent immunosuppressor, and as SARS-CoV can trigger a PLpro-dependent increase of TGFβ in human promonocytic cells [33], we argue that there may be a strong rationale for unblocking the IFNα/β response with inhibitors of the TGFβ pathway, although this possibility remains to be rigorously investigated. Accordingly, several TGFβ inhibitors have been tested in ongoing clinical trials; for example, along with chemotherapy in combination with galunisertib in patients with carcinosarcoma of the uterus or ovary (NCT03206177vii) or with TEW-7197, in patients with pancreatic ductal adenocarcinoma (NCT03666832viii). Concerning PLpro, a careful and significant amount of work will be necessary to examine whether the in vitro antiviral efficacy of PLpro inhibitors [77,78] can translate in vivo, first in animal models and only then in possible future clinical trials.
An anti-TGFβ approach might also be important for another reason: a chronic inflammatory condition is likely to activate anti-inflammatory mechanisms, including increased TGFβ concentrations that are susceptible to block IFNα/β signaling. This hypothesis is consistent with the finding that increased serum TGFβ concentrations have been measured in patients with asthma [79] or diabetes [80].
We hypothesize that 1,8-cineole might be another molecule of interest that could be tested with the goal of increasing the antiviral IFNα/β response in virally infected patients. In ex vivo cultivated human nasal mucosa [40], 1,8-cineole inhibits NF-κB activation, a key pathway in inflammation, in human mucosa and in human cell lines [40,81]. NF-κB inhibition might contribute to the in vivo anti-inflammatory properties of 1,8-cineole in murine models of inflammation [82] and influenza virus-induced murine pneumonia [83], as well as in a double-blind placebo-controlled trial of asthma treatments [84]. If proven useful, this molecule (already available as an oral capsule) might be potentially administered when the first clear symptoms appear, but this possibility also remains to be robustly tested.
Blocking the Cytokine Storm
We suggest that the best approach to preventing the occurrence of a cytokine storm in virally infected individuals is via an efficient early antiviral treatment. A similar observation was made in the case of hyperacute HIV-1 infection [66]. We propose that the main treatments to be considered on severe exacerbation of patient symptoms include the following. (i) Administering anti-inflammatory molecules. The use of the corticosteroid dexamethasone can reduce the mortality of patients with severe COVID-19 [85]. Colchicine is already being used to safely treat certain chronic inflammatory diseases. Another possible approach might be the injection of anakinra, which is a natural antagonist to the receptor for the inflammatory molecule IL-1. In a retrospective cohort study, anakinra administration reduced the need for invasive mechanical ventilation, as well as mortality among patients with severe forms of COVID-19, relative to controlsix [86]. (ii) Administering neutralizing antibodies against proinflammatory cytokines. At present, the most widely tested antibody is anti-IL-6R, but anti-TNFα and anti-IL-1β might certainly deserve consideration. (iii) Administering high-dose intravenous immunoglobulin therapy (IVIg) as a potentially promising treatment. Note that the rationale for this approach (i.e., as an anti-inflammatory agent) is entirely distinct from that of administering serum from convalescent patients, in which improving the antiviral response of the patient is the major goal; unfortunately, this approach does not seem to be efficient against SARS-CoV-2 [87]. (iv) We argue that maintaining an antiviral treatment when the inflammatory symptoms increase might be important in limiting virus-induced inflammation.
Concluding Remarks
In this opinion article, we have provided arguments in favor of the presented hypothesis to explain the paradox between the low morbidity of SARS-CoV-2 observed in a majority of individuals and the high morbidity observed in a minority of the global population. The key arguments for our reasoning are the importance of IFNα/β and its release kinetics, the presumed deleterious effects of inflammaging and other inflammatory conditions, and the importance of early antiviral treatments, which underscores the continued efforts in the discovery of efficient candidate antivirals (see Outstanding Questions). Thus, we argue that research efforts should not be placed exclusively on vaccination, but also on antiviral discovery. HIV-1 or HCV infections constitute examples of vaccination attempts that have not been successful in the past, contrary to progress in antiviral discovery research. Anticipating other pandemics due to emerging viruses in the future, we posit that if efficient and early diagnostics are made available, a combination of antivirals and immunomodulators with appropriate treatment kinetics might significantly lessen the burden of an epidemic/pandemic.
Outstanding Questions.
Which basic viral properties or targets (e.g., SARS-CoV-2) are most likely to allow the development of broad-spectrum antiviral molecules?
Inflammaging in the elderly might in some cases partially explain their propensity for developing excessive inflammatory responses. Which preventive treatments might help dampen such responses?
Early COVID-19 diagnostics are key in deciding efficient combinations of antivirals and immunomodulatory treatments. Which tools might allow the improvement of such early diagnostics?
Alt-text: Outstanding Questions
Acknowledgments
Acknowledgments
We thank Cecile Peltekian, Carine Cormary-Feuillet, Guillaume Hoeffel, and Ivan and Philippe Ascher for critical reading of the manuscript and for correcting the English language. B.C. is supported by a grant from the Fondation pour la Recherche Médicale (FRM - Aide aux Equipes, France). Due to the lack of space, several meaningful papers could not be quoted and we apologize to the authors for this.
Author Contributions
All authors contributed equally to the writing of the review. All artwork was done by V.F.
Glossary
- Acute respiratory distress syndrome (ARDS)
clinical manifestations of severe lung damage and respiratory failure.
- Angiotensin-converting enzyme 2 (ACE2)
converts angiotensin I to angiotensin; expressed in lung, heart, intestine, and kidney. ACE2 is the cellular receptor of SARS-CoV and SARS-CoV-2.
- Basic reproduction number (R0)
indicator of the contagiousness of infectious agents. One infected person infects an average of R0 other persons.
- Cineole
1,8-cineole (eucalyptol) is a small monoterpene (152 Da), highly lipophilic, present in the essential oil of eucalyptus or rosemary, with well-established antibacterial effects.
- Coronaviruses
enveloped, positive-stranded RNA viruses associated with respiratory and enteric diseases of a broad range of vertebrate hosts. Among seven human coronaviruses, only SARS-CoV, MERS, and SARS-CoV-2 are highly pathogenic.
- Coronavirus disease 2019 (COVID-19)
associated with SARS-CoV-2 infection.
- Cytokine storm
or cytokine release syndrome (CRS); excessive and uncontrolled release of proinflammatory cytokines mediating systemic inflammation and multiple organ failure, with high inflammatory parameters.
- Dengue virus
RNA virus of the Flaviviridae family, responsible for a mosquito-borne tropical disease (dengue fever). In some cases, the disease may evolve towards dengue hemorrhagic fever or dengue shock syndrome.
- Direct acting antivirals (DAAs)
antiviral molecules targeting viral proteins.
- Exhaustion
state of T cells that have lost most of their efficacy and effector function after prolonged, chronic stimulation.
- Fatality:case ratio (FCR)
proportion of disease-induced deaths compared with the total number of people diagnosed with the disease.
- Favipiravir
purine-base analog showing broad-spectrum antiviral activity against influenza virus infections.
- Indirect acting antivirals (IAAs)
molecules targeting host cell components required for virus replication, thus producing an indirect antiviral effect.
- Inflammaging
used to describe changes observed in the elderly, appearing as chronic low-grade inflammation associated with a weakening of immune system efficacy.
- Inflammasome
cytoplasmic complex regulating caspase activation, converting ILs from inactive to active forms.
- Interferon beta (IFNβ)
natural and potent antiviral molecule secreted by most cells of the organism. Binds to its receptor IFNAR, triggering the expression of IFNα, which binds to the same receptor with a lower affinity than IFNβ.
- Papain-like protease (PLpro)
a deubiquitinating CoV enzyme, also regulating several host cell genes, including TGFB.
- Poly (I:C)
TLR3 ligand mimicking the presence of viral RNA in the cell.
- Remdesivir
broad-spectrum antiviral nucleotide adenosine analog prodrug; active against coronaviruses.
- Transforming growth factor beta (TGFβ)
multifunctional cytokine secreted by various cell types, in particular macrophages; key for tissue repair and fibrosis, with immunosuppressive properties; potent blocker of IFNβ induction.
Resources
ihttps://clinicaltrials.gov/ct2/show/NCT04401579iihttps://clinicaltrials.gov/ct2/show/NCT04358549iiihttps://clinicaltrials.gov/ct2/show/NCT04373733ivhttps://clinicaltrials.gov/ct2/show/NCT04322565vhttps://clinicaltrials.gov/ct2/show/NCT04326790vihttps://clinicaltrials.gov/ct2/show/NCT04322682viihttps://clinicaltrials.gov/ct2/show/NCT03206177viiihttps://clinicaltrials.gov/ct2/show/NCT03666832ixhttps://clinicaltrials.gov/ct2/show/NCT04365530References
- 1.Wiersinga W.J. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Taubenberger J.K., Morens D.M. 1918 Influenza: the mother of all pandemics. Emerg. Infect. Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon D.E. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wathelet M.G. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81:11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopecky-Bromberg S.A. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y. Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets. Sci. Rep. 2015;5:17554. doi: 10.1038/srep17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu K.-L. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3·TANK·TBK1/IKKϵ complex. J. Biol. Chem. 2009;284:16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji X., Li Z. Medicinal chemistry strategies toward host targeting antiviral agents. Med. Res. Rev. 2020;40:1519–1557. doi: 10.1002/med.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson P. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon A. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antivir. Res. 2020;178:104793. doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta Y. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuta Y. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon A. Rapid incorporation of favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat. Commun. 2020;11:4682. doi: 10.1038/s41467-020-18463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang C. A promising antiviral candidate drug for the COVID-19 pandemic: a mini-review of remdesivir. Eur. J. Med. Chem. 2020;201:112527. doi: 10.1016/j.ejmech.2020.112527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Solidarity Trial Consortium, W.S. trial et al Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. medRxiv. 2020 doi: 10.1101/2020.10.15.20209817. Published online October 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaitin D.A. Inquiring into the differential action of interferons (IFNs): an IFN-α2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-β. Mol. Cell. Biol. 2006;26:1888–1897. doi: 10.1128/MCB.26.5.1888-1897.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight E., Korant B.D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc. Natl. Acad. Sci. U. S. A. 1979;76:1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larner A.C. Transcriptional induction of two genes in human cells by beta interferon. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6733–6737. doi: 10.1073/pnas.81.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lande R., Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. N. Y. Acad. Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 23.García-Sastre A. Ten strategies of interferon evasion by viruses. Cell Host Microbe. 2017;22:176–184. doi: 10.1016/j.chom.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz-Jordan J.L. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2:264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zielecki F. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J. Virol. 2013;87:5300–5304. doi: 10.1128/JVI.03496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco-Melo D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knoops K. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oudshoorn D. Expression and cleavage of Middle East respiratory syndrome coronavirus nsp3–4 polyprotein induce the formation of double-membrane vesicles that mimic those associated with coronaviral RNA replication. mBio. 2017;8 doi: 10.1128/mBio.01658-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y. The severe acute respiratory syndrome coronavirus nucleocapsid inhibits type I interferon production by interfering with TRIM25-mediated RIG-I ubiquitination. J. Virol. 2017;91 doi: 10.1128/JVI.02143-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siu K.-L. Middle East respiratory syndrome coronavirus 4a protein is a double-stranded RNA-binding protein that suppresses PACT-induced activation of RIG-I and MDA5 in the innate antiviral response. J. Virol. 2014;88:4866–4876. doi: 10.1128/JVI.03649-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Li S.-W. Correlation between TGF-β1 expression and proteomic profiling induced by severe acute respiratory syndrome coronavirus papain-like protease. Proteomics. 2012;12:3193–3205. doi: 10.1002/pmic.201200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C.-H. Altered p38 mitogen-activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. J. Immunol. 2004;172:7841–7847. doi: 10.4049/jimmunol.172.12.7841. [DOI] [PubMed] [Google Scholar]
- 35.Agrati C. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerin M.V. TGFβ blocks IFNα/β release and tumor rejection in spontaneous mammary tumors. Nat. Commun. 2019;10:4131. doi: 10.1038/s41467-019-11998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerin M.V. Preclinical murine tumor models: a structural and functional perspective. Elife. 2020;9 doi: 10.7554/eLife.50740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotz M., Seth P. TGF beta and HIV infection. Ann. N. Y. Acad. Sci. 1993;685:501–511. doi: 10.1111/j.1749-6632.1993.tb35912.x. [DOI] [PubMed] [Google Scholar]
- 40.Müller J. 1,8-Cineole potentiates IRF3-mediated antiviral response in human stem cells and in an ex vivo model of rhinosinusitis. Clin. Sci. 2016;130:1339–1352. doi: 10.1042/CS20160218. [DOI] [PubMed] [Google Scholar]
- 41.Calvaruso V. Pegylated-interferon-α2a in clinical practice: how to manage patients suffering from side effects. Expert Opin. Drug Saf. 2011;10:429–435. doi: 10.1517/14740338.2011.559161. [DOI] [PubMed] [Google Scholar]
- 42.Tisoncik J.R. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 44.Nieto-Torres J.L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen I.-Y. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franceschi C. Inflamm-aging. an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 48.Harris T.B. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 49.Fagiolo U. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 50.Di Iorio A. Serum IL-1β levels in health and disease: a population-based study. “The InCHIANTI study”. Cytokine. 2003;22:198–205. doi: 10.1016/s1043-4666(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 51.The OpenSAFELY Collaborative et al OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020 doi: 10.1101/2020.05.06.20092999. Published online May 7, 2020. [DOI] [Google Scholar]
- 52.Bonafè M. Inflamm-aging: why older men are the most susceptible to SARS-Cov-2 complicated outcomes. Preprints. 2020 doi: 10.20944/preprints202004.0143.v1. Published online April 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smits S.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xudong X. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Channappanavar R. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Tawfiq J.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int. J. Infect. Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jouanguy E. Human primary immunodeficiencies of type I interferons. Biochimie. 2007;89:878–883. doi: 10.1016/j.biochi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Bastard P. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zajac A.J. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horwitz M.S. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J. Clin. Invest. 2002;109:79–87. doi: 10.1172/JCI11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadinoto V. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood. 2008;111:1420–1427. doi: 10.1182/blood-2007-06-093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bickham K. EBNA1-specific CD4+ T cells in healthy carriers of Epstein–Barr virus are primarily Th1 in function. J. Clin. Invest. 2001;107:121–130. doi: 10.1172/JCI10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crough T. Contemporaneous fluctuations in T cell responses to persistent herpes virus infections. Eur. J. Immunol. 2005;35:139–149. doi: 10.1002/eji.200425548. [DOI] [PubMed] [Google Scholar]
- 64.van Baarle D. Dysfunctional Epstein–Barr virus (EBV)-specific CD8+ T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2001;98:146–155. doi: 10.1182/blood.v98.1.146. [DOI] [PubMed] [Google Scholar]
- 65.Kieff E. Epstein–Barr virus and its replication. In: Fields B.N., editor. Fields Virology. Lippincott–Raven; 1996. pp. 2343–2396. [Google Scholar]
- 66.Muema D.M. Association between the cytokine storm, immune cell dynamics, and viral replicative capacity in hyperacute HIV infection. BMC Med. 2020;18:81. doi: 10.1186/s12916-020-01529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacquelin B. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris L.D. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J. Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sulkin S.E., Allen R. Virus infections in bats. Monogr. Virol. 1974;8:1–103. [PubMed] [Google Scholar]
- 70.Kacprzyk J. A potent anti-inflammatory response in bat macrophages may be linked to extended longevity and viral tolerance. Acta Chiropterolog. 2017;19:219–228. [Google Scholar]
- 71.Brook C.E., Dobson A.P. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015;23:172–180. doi: 10.1016/j.tim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srikiatkhachorn A. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017;39:563–574. doi: 10.1007/s00281-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuczera D. Highlights for dengue immunopathogenesis: antibody-dependent enhancement, cytokine storm, and beyond. J. Interf. Cytokine Res. 2018;38:69–80. doi: 10.1089/jir.2017.0037. [DOI] [PubMed] [Google Scholar]
- 74.McHutchison J.G. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 75.Omrani A.S. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davoudi-Monfared E. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng K.-W. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antivir. Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li G., Clercq E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 79.Karagiannidis C. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-β-mediated airway remodeling in asthma. J. Allergy Clin. Immunol. 2006;117:111–118. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 80.Vasanthakumar R. Serum IL-9, IL-17, and TGF-β levels in subjects with diabetic kidney disease (CURES-134) Cytokine. 2015;72:109–112. doi: 10.1016/j.cyto.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greiner J.F.-W. 1,8-Cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim. Biophys. Acta. 2013;1833:2866–2878. doi: 10.1016/j.bbamcr.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Linghu K.-G. 1,8-Cineole ameliorates LPS-induced vascular endothelium dysfunction in mice via PPAR-γ dependent regulation of NF-κB. Front. Pharmacol. 2019;10:178. doi: 10.3389/fphar.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y. 1,8-Cineol protect against influenza-virus-induced pneumonia in mice. Inflammation. 2016;39:1582–1593. doi: 10.1007/s10753-016-0394-3. [DOI] [PubMed] [Google Scholar]
- 84.Juergens U.R. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir. Med. 2003;97:250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- 85.RECOVERY Collaborative Group et al Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. Published online July 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huet T. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agarwal A. Convalescent plasma in the management of moderate Covid-19 in adults in India: open label Phase II multicentre randomised controlled trial (PLACID trial) BMJ. 2020;371 doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bossart-Whitaker P. Three-dimensional structure of influenza A N9 neuraminidase and its complex with the inhibitor 2-deoxy 2,3-dehydro-N-acetyl neuraminic acid. J. Mol. Biol. 1993;232:1069–1083. doi: 10.1006/jmbi.1993.1461. [DOI] [PubMed] [Google Scholar]
- 89.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao B. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steuber H., Hilgenfeld R. Recent advances in targeting viral proteases for the discovery of novel antivirals. Curr. Top. Med. Chem. 2010;10:323–345. doi: 10.2174/156802610790725470. [DOI] [PubMed] [Google Scholar]
- 92.Loughlin E.H. The treatment of Plasmodium falciparum malaria with a single dose antimalarial; a preliminary report of the use of hydroxychloroquine, 7-chloro-4(4-(N-ethyl-N-B-hydroxyethylamino)-1-methylbutylamino)-quinoline diphosphate. Antibiot. Chemother. (Northfield) 1952;2:171–174. [PubMed] [Google Scholar]
- 93.Hoekenga M.T. The treatment of malaria with hydroxychloroquine. Am. J. Trop. Med. Hyg. 1955;4:221–223. doi: 10.4269/ajtmh.1955.4.221. [DOI] [PubMed] [Google Scholar]
- 94.Akhavan P.S. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J. Rheumatol. 2013;40:831–841. doi: 10.3899/jrheum.120572. [DOI] [PubMed] [Google Scholar]
- 95.Tsai W.P. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retrovir. 1990;6:481–489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- 96.Vigerust D.J., McCullers J.A. Chloroquine is effective against influenza A virus in vitro but not in vivo. Influenza Other Respir. Viruses. 2007;1:189–192. doi: 10.1111/j.1750-2659.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Freiberg A.N. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J. Gen. Virol. 2010;91:765–772. doi: 10.1099/vir.0.017269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dowall S.D. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J. Gen. Virol. 2015;96:3484–3492. doi: 10.1099/jgv.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Lamballerie X. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8:837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 100.Tricou V. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maisonnasse P. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 102.Million M. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Horby P. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 doi: 10.1101/2020.07.15.20151852. Published online July 15, 2020. [DOI] [Google Scholar]
- 104.Tang W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenberg E.S. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skipper C.P. Hydroxychloroquine in nonhospitalized adults with early COVID-19. Ann. Intern. Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russell C.D. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Misawa T. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 109.Martínez G.J. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262–271. doi: 10.1016/j.atherosclerosis.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 110.Perricone C. The anti-viral facet of anti-rheumatic drugs: lessons from COVID-19. J. Autoimmun. 2020;111:102468. doi: 10.1016/j.jaut.2020.102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Möller B., Villiger P.M. Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. Springer Semin. Immunopathol. 2006;27:391–408. doi: 10.1007/s00281-006-0012-9. [DOI] [PubMed] [Google Scholar]
- 112.Giamarellos-Bourboulis E.J. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hadjadj J. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. medRxiv. 2020 doi: 10.1101/2020.04.19.20068015. Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carvelli J. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 2020 doi: 10.1038/s41586-020-2600-6. Published online July 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Norelli M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 116.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 117.Guaraldi G. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Imbach P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 119.Newburger J.W. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 120.Khanna N. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin. Infect. Dis. 2008;46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 121.Wang J.-T. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg. Infect. Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kumar P. Intravenous immunoglobulin responsive persistent thrombocytopenia after dengue haemorrhagic fever. J. Clin. Diagn. Res. 2016;10:OD10–OD11. doi: 10.7860/JCDR/2016/17770.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Samuelsson A. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 124.Mohtadi N. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: a case series. Virology. 2020;548:1–5. doi: 10.1016/j.virol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]