Abstract
Severe infection with severe acute respiratory syndrome coronavirus (SARS-CoV)-2 is characterized by massive cytokine release and T cell loss. The exaggerated host immune response, incapable of viral clearance, instead aggravates respiratory distress, as well as cardiac, and/or damage to other organs. The mortality pattern of SARS-CoV-2 infection, higher in older versus younger adults and almost absent in children, is possibly caused by the effects of age and pre-existing comorbidities on innate and adaptive immunity. Here, we speculate that the abnormal and excessive immune response to SARS-CoV-2 infection partly depends on T cell immunological memory, which is more pronounced in adults compared with children, and may significantly contribute to immunopathology and massive collateral damage in coronavirus disease 2019 (COVID-19) patients.
Highlights
T cell-mediated immunity is crucial for the effective clearance of severe acute respiratory syndrome coronavirus (SARS-CoV)-2 infection.
In severe and critical coronavirus disease 2019 (COVID-19) patients, peripheral blood T cells are significantly decreased and show a phenotype of hyperactivation/exhaustion relative to controls.
Older age, characterized by high basal proinflammatory status coupled with a progressive inability to mount proper immune responses, is a major risk factor for severe and critical SARS-CoV-2 infection.
Children are nearly always asymptomatic in SARS-CoV2 infection; this clinical observation may be linked to a higher proportion of naïve T cells in circulation than adults and, consequently, a presumed reduced susceptibility to hyperinflammatory collateral damage.
The COVID-19 Pandemic
SARS-CoV-2, a novel single strand RNA virus belonging to the same family as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), was identified as the cause of an outbreak of pneumonia cases starting in late December, 2019, in the city of Wuhan, China [1., 2., 3.]. Typical clinical symptoms of patients with COVID-19 are fatigue, fever, dry cough, and dyspnea (see Glossary), and the disease is mostly spread by airborne transmission, although other possible routes exist [3]. On March 11, 2020, the World Health Organization declared a COVID-19 pandemic, with alarming levels of spread and severity [4]. In the following weeks, the numbers of affected world regions and infected individuals further climbed, reaching 190 countries, with almost 49 000 000 confirmed cases and more than 1 200 000 global deaths as on November 6, 2020, according to the Coronavirus Resource Center at Johns Hopkins Universityi. Approximately 80% of SARS-CoV-2 infections are mild or asymptomatic, while the remaining cases show severe (15%, requiring oxygen) and critical (5%, requiring ventilation) pneumonia. Organ dysfunction (shock, acute cardiac and kidney injury), acute respiratory distress syndrome (ARDS), and death can occur in severe or critical cases [5., 6., 7.]. Interstitial pneumonia is frequently associated with the massive release of cytokines, the so-called cytokine storm, now recognized as a major COVID-19 pathogenic factor potentially leading to fatal outcomes [5., 6., 7.].
The rapid spread of SARS-CoV-2 is paralleled by an unprecedented global effort to accelerate the research on disease pathology and develop efficient candidate antiviral drugs and vaccines. Nonetheless, the biological mechanisms underlying the different responses to SARS-CoV-2 infection are still elusive: why do most infected people exhibit mild symptoms or are asymptomatic, while others have severe or critical outcomes? Studies to date indicate that COVID-19 pathogenesis may be dependent on an aberrant host immune response, characterized by overactive cells that are unable to efficaciously neutralize the virus, but our limited knowledge on this phenomenon has hampered our efforts to identify effective candidate therapeutic drugs. Hence, there is an urgent need to untangle the different components of the immune response (both innate and adaptive) to SARS-CoV-2 and unveil their role in COVID-19 pathogenesis.
Here, we discuss the dynamics of SARS-CoV-2 T cell immunity in controlling the key balance between immune activation and its regulation, suggesting possible pathogenic mechanisms. In particular, we propose that the mortality pattern of SARS-CoV-2 infection, higher in older versus younger adults and almost absent in children, might be associated with host T cell immunological memory and innate trained immunity, both of which appear to be significantly more pronounced in older individuals.
Key Role of T Cells in the Successful Immune Responses against SARS-CoV-2 Infection
Current estimates show that approximately 80% of COVID-19 cases are mild-to-moderate, with patients fully recovering from infection [5., 6., 7.]. In previous studies, the humoral response to SARS-CoV-2 infection seemed to be ubiquitous among infected individuals and the magnitude of the anti-SARS-CoV-2 IgG titers strongly correlated with the breadth of circulating virus-specific CD4+ and CD8+ T cell responses (Box 1 ) [8., 9., 10., 11.]. Notwithstanding, most convalescent plasma samples have not contained high concentrations of neutralizing activity, and rare antibodies toward specific viral proteins bearing potent antiviral activity have been found in all analyzed subjects recovering from COVID-19 [12]. Exposure to SARS-CoV-2 within households has induced virus-specific interferon (IFN)-γ producing T cells without seroconversion, suggesting that cellular responses might be more sensitive indicators of SARS-CoV-2 exposure than antibodies, although this remains to be fully demonstrated [13]. One study reported a population of polyfunctional SARS-CoV-2-specific T cells with a stem-like memory phenotype in the circulation of antibody-seronegative convalescent individuals presenting asymptomatic and mild COVID-19 [14]; this suggested that in the absence of antibodies, a robust and broad T cell response might be sufficient to provide immune protection against SARS-CoV-2. Thus, the effective cooperation between T cells and antibody responses during the clinical course of COVID-19 might represent key future research for candidate vaccine design.
Box 1. T Cell Subsets and Related Functions.
CD8+ Cytotoxic T Lymphocytes (CTLs)
CTLs recognize class I MHC-associated peptides and, upon antigen-dependent stimulation, kill virus-infected cells by secreting granzymes and perforins [118]. Perforin creates cell membrane pores, allowing intracellular delivery of granzymes; this leads to cleavage and activation of caspases that induce apoptotic death [118].
CD4+ T Helper (Th) Cells
These cells orchestrate adaptive immunity by producing cytokines and chemokines that enhance cytotoxic CD8+ T cell responses and are indispensably required for B cell-dependent antibody production and plasma cell generation [119,120]. These cells respond to class II MHC-associated antigen stimulation and differentiate into functionally distinct subpopulations of effector cells, characterized by specific transcription factors, cytokine fingerprints, and pathogenic targets [121].
Th1 cells: defined by the master regulator T-bet, produce high concentrations of interleukin (IL)-2 and interferon (IFN)-γ and direct immunity toward intracellular bacteria and viruses. IFN-γ is a potent activator of macrophages, stimulating phagocyte-mediated ingestion and killing of microbes [122].
Th2 cells: differentiation of these is driven by Gata3, stimulating phagocyte-independent, eosinophil-mediated immunity, necessary to combat helminthic parasites. They produce IL-4, IL-5, and IL-13, stimulating the production of IgE antibodies, activate eosinophils, and promote the expulsion of parasites from mucosal tissues [122].
Th17 cells: promoted by the expression of Rorγt, these secrete IL-17, IL-22, and other cytokines and chemokines that recruit neutrophils and monocytes. They intervene in the defense against extracellular bacterial and fungal infections, but also contribute to inflammation in autoimmune and other immune-mediated disease [122].
T regulatory (Treg) cells: express CD25 and the transcription factor FOXP3 and play a key role in the anti-inflammatory/immunosuppressive control of the immune response, sustaining immunological homeostasis. Treg cells act by inhibiting the action of the proinflammatory counterpart Th1 and Th17, via production of IL-10, IL-35 and, transforming growth factor (TGF)-β [122,123].
T follicular helper cells: these are driven by Bcl-6. Inside B cell follicles of secondary lymphoid organs, they mostly secrete IL-4 and IL-21 and directly help the development of humoral immunity [122].
Alt-text: Box 1
Peripheral Lymphopenia as a Consistent Associated Factor of SARS-CoV-2 Infection and Severity
In COVID-19 patients, the total blood lymphocyte count, and in particular that of T cells, is lower than in healthy controls [15]. Furthermore, lymphopenia is more accentuated in symptomatic compared with asymptomatic individuals, as well as among symptomatic COVID-19 patients with pneumonia, compared with those without pneumonia [16., 17., 18., 19., 20., 21.]. In severe cases, both CD4+ and CD8+ T cell blood counts are further decreased compared with moderate cases [22., 23., 24., 25., 26., 27., 28.].
Notably, a lower lymphocyte count was found to be a clinical predictor of mortality due to SARS-CoV-2 infection [29,30]. In elderly patients (median age 71 years) with COVID-19, while ARDS was a strong predictor of death, high numbers of lymphocytes in circulation were predictive of a better outcome [31]. Finally, the transfusion of convalescent plasma derived from recent COVID-19-recovered donors with high neutralizing antibody titers has led to significant improvement in clinical symptoms, including increased lymphocyte counts in prospectively enrolled severe COVID-19 patients [32]. Of note, while lymphopenia has not been observed in low pathogenic coronavirus infections, it has been associated with severe illness and poor survival in human subjects infected with SARS-CoV-1 [33., 34., 35., 36.]. The consistent absence of angiotensin-converting enzyme II expression (ACE2, the cellular receptor for both SARS-CoV-1 and SARS-CoV-2) in immune cells suggests that direct viral infection is an unlikely cause of T cell loss, although this warrants robust investigation [3,37,38]. Moreover, while direct viral entry into lymphocytes has been sporadically observed for both SARS coronaviruses, it seems that the virus is not able to replicate in this cell type, leaving the biological relevance of this abortive infection uncertain [39].
In conclusion, we posit that a simple lymphocyte enumeration in circulation might become useful in identifying patients at highest risk of developing an overly harmful response to SARS-CoV-2 infection.
The Vicious Cycle of the Cytokine Storm and T Lymphocyte Loss in Severe SARS-CoV-2 Infection
A growing number of clinical observations show how decreases in blood lymphocyte count and lymphocyte percentage are frequently associated with an increase in the number of neutrophils, not only in the blood but also in the bronchoalveolar lavage fluid (BALF) of COVID-19 patients [18,40., 41., 42.] (Figure 1 ). Two meta-analyses draw superimposable conclusions: that subjects with a more severe COVID-19 progression, compared with nonsevere, harbor more neutrophils, less lymphocytes, higher neutrophil-to-lymphocyte ratios (NLR) and low lymphocyte-to-C-reactive protein ratios (LCR), proposing the potential use of these parameters in routine laboratory blood tests as putative reliable biomarkers of poor prognosis in COVID-19 patients [43,44].
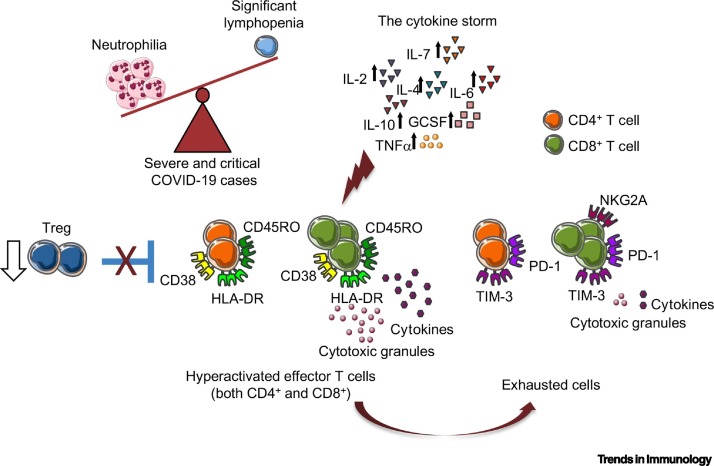
Figure 1.
Aberrant and Ineffective Immune Response in Severe Coronavirus Disease 2019 (COVID-19) Patients.
In severely ill COVID-19 patients, an increased neutrophil-to-lymphocyte ratio and elevated concentrations of several cytokines are consistently registered. Activation (HLA-DR, CD45RO, and CD38) and exhaustion [programmed cell death marker 1 (PD-1), receptor mucin domain-containing protein-3 (TIM-3) and NKG2A] markers on T cells can point to a hyperactivated/exhausted/not functional state. In addition, the number of regulatory CD4+CD25+ T cells (Tregs) is significantly lower in these patients compared with controls. Abbreviations: GCSF, granulocyte-colony stimulating factor; IL, interleukin; TNF, tumor necrosis factor.
While minimal concentrations of proinflammatory cytokines and chemokines were found in patients recovering from COVID-19, even in the symptomatic phase [8], COVID-19 patients with ARDS suffered from a cytokine storm, characterized by higher plasma concentrations of interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-8, IL-10, granulocyte-colony stimulating factor (GCSF), monocyte chemoattractant protein (MCP)-1, and tumor necrosis factor (TNF)-α, relative to patients without ARDS; this has directly implicated the excessive release of cytokines in COVID-19 pathology [6,19,45,46]. In particular, the abnormal circulating cytokine concentrations observed in severe COVID-19 patients have been proposed to negatively impact on T cell proliferation and/or survival [45,47., 48., 49., 50.]. Autoptic analyses of patients who succumbed to COVID-19 unveiled extensive splenic atrophy and a significant extent of lymphocyte death in lymph follicles and paracortical areas of lymph nodes, potentially mediated, among other mechanisms, by macrophage-derived IL-6 directly promoting lymphocyte necrosis [51]. Recently, through the examination of human postmortem thoracic lymph nodes and spleens, aberrant extrafollicular TNF-α amounts were proposed to contribute to a specific block in BCL-6+ T follicular helper cell differentiation (Box 1), as well as in the dramatic loss of germinal centers in acute SARS-CoV-2 infection [52]. The vicious cycle of cytokine storm and T lymphocyte loss makes cytokines promising putative therapeutic targets for COVID-19. Accordingly, the intravenous administration of tocilizumab (a specific blocker of the IL-6 pathway) to severe COVID-19 patients, has been accompanied by an increase in the absolute lymphocyte blood count within the first day [49]; this has suggested that in immune-dysregulated patients, increased circulating cytokines might sharpen defective lymphocyte functions, thus contributing to an ineffective antiviral response. Along the same lines, subcutaneous administration of anakinra (a recombinant IL-1 receptor antagonist) to hospitalized patients with severe COVID-19-related bilateral pneumonia significantly decreased the need for mechanical ventilation and contributed to reduced mortality compared with controls [53].
Since T cells are known to dampen overactive innate immune responses during viral infections [54,55], it is reasonable to speculate that T cell loss might exacerbate certain pathological inflammatory responses during SARS-CoV-2 infection, sustaining a dysregulated T cell-cytokine loop, although this warrants rigorous investigation. On the one hand, pathogenic T cells themselves can contribute to systemic concentrations of proinflammatory cytokines [56]. On the other hand, an overt inflammatory response might also play an immunosuppressive role. Recent work revealed the occurrence of neutrophil precursors and dysfunctional mature neutrophils with immunosuppressive properties [e.g., expressing the immune checkpoint molecule programmed death-ligand 1 (PD-L1)] in the blood of severe COVID-19 patients, presumably further decreasing T cell numbers in circulation [57]. Collectively, these studies suggest a picture of a feed-forward loop that might comprise the proinflammatory actions of innate immune cells, the effect of cytokines in the immune milieu, and T cell loss.
A Pattern of Treg Cell Decline and Effector T Cell Hyperactivation/Exhaustion in Severe SARS-CoV-2 Infection
Several studies show that, while numerically decreased, both CD4+ and CD8+ T cells from severe COVID-19 patients present a dysregulated status of activation and function, characterized by high concentrations of inflammatory genes, such as those encoding IL-2R, IL-6, JUN, FOS, perforin, granzymes, as well as the coexpression of HLA-DR, CD38, and CD45RO activation markers [21,45,58,59] (Figure 1). This activation status seems to be unspecific. Indeed, the majority of CD8+ T cells belong to expanded clones in patients with moderate SARS-CoV-2 infection, in which a higher amplification index has been reported and CD8+ T cells preferentially expresses tissue-residence genes, such as those encoding CXCR-6, and XCL1; by contrast, CD8+ T cells from patients with severe/critical infection are less expanded, more proliferative, and more phenotypically heterogeneous [42]. Regarding function, in parallel with excessive blood CD8+ T cell activation, CD4+ T cells from severe COVID-19 cases have demonstrated a higher release in cytotoxic granules and a marked decrease in the secretion of functional molecules such as IFN-γ and TNF-α compared with CD4+ T cells from a group of mild COVID-19 patients [58,60]. Moreover, a transcriptomics study showed substantially reduced adaptive immunity gene expression (MHC class II and T cell activation genes, e.g., coding for IL-23A and CD74) in peripheral blood bulk T cells from severe COVID-19 patients [61]. In another study, blood CD8+ T cells isolated from severely ill subjects were not only drastically reduced, but also showed a decreased percentage of positivity for CD107a, IFN-γ, IL-2, and lower expression of granzyme B relative to mild COVID-19 patients [62]. Unlike subjects showing mild symptoms, no correlation was found between SARS-CoV-2-reactive IFN-γ producing blood CD8+ T cell counts and SARS-CoV-2-specific antibody titers in severe COVID-19 patients, suggesting a lack of coordination between cellular and humoral immunity in these subjects [63]. Moreover, a skewing of CD8+ T cells toward a terminally differentiated/senescent phenotype, also displaying reduced antiviral cytokine production capability, was reported in COVID-19 patients who required intensive care [64].
The apparently conflicting observations of both hyperactivation and functional impairment of T cell compartments remain enigmatic, but might potentially be reconciled when considering that persistent stimulation and long-term activation are known to induce both CD4+ and CD8+ T cell exhaustion [65., 66., 67.]. Accordingly, protein expression of three exhaustion markers, that is, the CD28 family member programmed cell death marker 1 (PD-1), the receptor mucin domain-containing protein-3 (TIM-3), and the ITIM-bearing receptor NKG2A [68., 69., 70.] were increased in both peripheral CD4+ and CD8+ T cells from severe COVID-19 patients, a finding that was paralleled by the decreased expression of co-stimulatory molecule CD28 in both subsets [21,45,62,71] (Figure 1).
From another angle, effector T cell hyperactivation (and subsequent exhaustion) might be functionally linked to the reported significant reduction of circulating regulatory T (Treg) cells in patients with severe compared with moderate COVID-19 [19,21,26] (Box 1 and Figure 1). In particular, the proportion of both blood naïve (CD45RA+) and induced (CD45RO+) Treg cells declined in severely ill patients, suggesting an important impairment in the immunoregulatory arm of the T cell-mediated response [26]. An increased proportion of blood T follicular helper cells responding to SARS-CoV-2 correlated with reduced numbers of circulating SARS-CoV-2-reactive Treg cells by large-scale single-cell transcriptomic analysis in COVID-19 patients [72]. Since Treg cells are known to play an important role in limiting the host antiviral response and the consequent tissue immunopathology [73,74], their reduction might have a relevant impact on fueling systemic inflammation in severe COVID-19 patients, a hypothesis that deserves to be fully explored.
The quality (T cell subset differentiation), beyond magnitude and regulation, of the immune response might also be crucial [75,76]. The strongest T cell responses to SARS-CoV-2 are directed against the spike (S) surface glycoprotein, and SARS-CoV-2-specific T cells predominantly produce effector and T helper (Th)1 (Box 1) cytokines [6,11,77], a response that is effective in keeping the infection under control via macrophages and cytotoxic T cells. However, in severe COVID-19 patients, the signals of a Th2 immune response in peripheral blood have been reported, as evidenced by a high proportion of basophils and degranulated eosinophils and increased concentration of Th2 cytokines (such as IL-4 and IL-10) relative to controls (Box 1) [6,77,78]. In addition, higher numbers of proinflammatory CCR6 + Th17 cells in peripheral blood have also been reported in severe relative to mild cases of COVID-19 and, thus, Th17 cell-related proinflammatory cytokine IL-17 has been proposed as an immunologically plausible druggable target that might help prevent ARDS in these patients, although this remains to be further tested (Box 1) [58,71,77,79].
In summary, T cell dysregulation in COVID19 appears to depend on both a quantitative and qualitative modification that might render T cells overreactive and exhausted: on the one hand, able to fuel inflammation, and on the other hand, underperforming in their antiviral function.
Role of T Cell Immunosenescence in Age- and Sex-Related SARS-CoV-2 Mortality
Epidemiological reports describing the mortality pattern of COVID-19 patients clearly indicate that age, male-sex, and the number of pre-existing comorbidities are key risk factors for a high case-fatality rate of SARS-CoV-2 infection [6,7]. These conditions are characterized by a higher basal proinflammatory status coupled with a progressive inability by the immune system to mount proper responses, that might be referred to as ‘immunosenescence’ in aging individuals [80] (Box 2 ). Accordingly, SARS-CoV-2 has caused more severe interstitial pneumonia in old compared with young macaques, paralleled by more active viral replication (in nasopharyngeal swabs) in the former, demonstrating age-dependent, impaired viral clearance [81].
Box 2. Immunosenescence.
Immunosenescence has been described as a complex age-dependent remodeling of the immune system, with many alterations affecting T cells and in particular: (i) reduction in the CD4+/CD8+ T cell ratio; (ii) impaired development of CD4+ T follicular helper cells and, consequently, an altered development of plasma cells and memory B cells; (iii) shrinkage of the antigen-recognition repertoire of T cell receptor (TCR) diversity; (iv) impaired proliferation in response to antigenic stimulation; (v) decreased cytotoxic activity of natural killer T cells (NKTs); (vi) accrual and clonal expansion of memory and effector T cells; (vii) defective immune defense against viruses, characterized by inefficacious cytotoxic CD8+ T cells; and (viii) increased circulating concentrations of soluble proinflammatory cytokines. This age-associated immune decline may result in an inefficient immune response to novel antigens and an inability to develop proper immunity against infections and upon vaccination. However, the immunosenescence-dependent higher inflammatory status/overactive cytokine secretion might predispose old/frail patients to harmful responses against novel antigens coupled with inefficient viral clearance [80,124,125]. Recent work utilized a systems immunology approach and confirmed that human aging is characterized by decreased T cell functions and a parallel increase in cytotoxic and monocyte cell functions [83].
Alt-text: Box 2
An age-dependent reduction in peripheral blood T cell numbers was indeed observed in COVID-19 patients, with the lowest count reported in patients older than 60 years, suggesting that a decline in both CD4+ and CD8+ T cell numbers might be a potential cause for increased susceptibility to SARS-CoV-2 infection in elderly patients [45]. Furthermore, weakened adaptive responses and elevated systemic inflammation might dramatically compromise antiviral responses and might be key drivers of SARS-CoV-2-induced mortality [82], although further robust studies are warranted to address these possibilities.
An additional insight regarding sex-dependent differences in terms of immune-related aging comes from chromatin accessibility and RNA-seq analyses showing that older women (>65 years) have higher genomic activity compared with age-matched older men, as evidenced from transcription factor expression in adaptive immune cells; by contrast, the older men comparatively exhibited a greater age-related CD4+ and CD8+ T cell decline and higher concentrations of circulating proinflammatory IL-6 and IL-18 cytokines [83]. This sexual-dimorphism in human immune system aging might be key in helping to explain some of the reported sex-specific differences in COVID-19 clinical characteristics, in which relative to females, age and comorbidities are associated with greater susceptibility to disease in male patients, when considering prognosis [84].
Beyond age and gender, several additional risk factors can contribute to the development of a chronic, low-grade inflammatory status. Among these, obesity and the resulting adipose tissue dysfunction have clearly emerged as a major fuel of both local and systemic inflammatory responses [85,86]. Indeed, generally, a number of triggers linked to overnutrition (e.g., glucose, cholesterol derivatives, and free fatty acids) can promote the activation of multiple inflammatory pathways, including signaling via IKK complex-NF-kB and JNK-AP1, or inducing reticulum stress by stimulating pattern recognition receptors (PRRs) in both adipocytes and adipose tissue-resident immune cells [87]. The local inflammatory response is mirrored in the plasma of obese patients, characterized by high circulating concentrations of multiple proinflammatory mediators, such as IL-6, IL-8, MCP-1, TNFα, and high sensitivity C reactive protein (hs-CRP) [88,89]; these conditions can also foster or precipitate premature immunosenescence ex vivo, as evidenced by decreased expression of CD28 in human peripheral CD8+ T cells [90]. Obese subjects are more prone to suffer from selected infectious diseases (such as surgical-site infections) and pancreatitis, and skin infections harbor an increased risk of poor prognosis when infected [91]. Although the underlying mechanisms are still a matter of investigation, a recent meta-analysis suggested that obesity, as a comorbidity, might increase the risk for hospitalization, intensive care unit admission, and death among patients with COVID-19 [92].
Overall, although warranting further and rigorous investigation, these observations suggest that a deeper understanding of immune remodeling during aging in the two sexes and in dysmetabolic conditions might provide crucial information that could influence the development of targeted drugs to limit SARS-CoV-2-induced mortality.
Naïve versus Memory T Cell Frequencies: Protection from Severe SARS-CoV-2 Infection in Children
In contrast to infected adults, most children experience a milder COVID-19 clinical course [93,94]. Pediatric cases classified as severe and critical (respectively, 2.5% and 0.6%) are significantly less frequent than in adults [95., 96., 97., 98., 99.]. Age disparities observed in severe cases might be due to a lower susceptibility of children to infection, a lower propensity to showing clinical symptoms, or both. A mathematical model based on epidemiological data from China, Italy, Japan, Singapore, Canada, and South Korea, estimated that susceptibility to infection in subjects younger than 20 years might be half that of adults, with clinical symptoms manifesting from 21% of infections in 10- to 19-year-olds, to 69% in individuals aged over 70 years [100].
Since SARS-CoV-2 has emerged as a novel human virus, individuals remain naïve and should be equally susceptible to being infected, while the immunological milieu that is in contact with the virus presumably significantly differs in children compared with adults. During the immune trajectory from the neonatal age into adulthood, the clonal composition of the expressed T cell receptor (TCR) repertoire changes from a highly diversified set of antigen-specific TCRs, to a less diverse and more oligoclonal collection of TCR molecules, possibly as a result of immune responses to past infections [101]. Moreover, at birth, nearly all T lymphocytes express CD45RA, a typical marker of naïve cells [102]. A study analyzing the immune dynamics of healthy individuals from 5 to 96 years showed that peripheral blood naïve CD4+ and CD8+ T cell numbers decrease linearly with age; circulating memory cells outnumber naïve cells around 35 years of age and subjects aged 65 years or older are characterized by an extremely reduced naïve T cell repertoire (Figure 2 , Key Figure) [103]. These different cellular frequencies have been associated with lower CD4+ and CD8+ T cell activation and multifunctional populations of healthy pediatric T cells upon in vitro stimulation, as well as less severe toxic shock syndrome-associated morbidity in children compared with adults [104]. Furthermore, early-life naïve CD4+ T cells isolated from healthy fetal tissues (18–22 gestational weeks) tend to differentiate toward a Foxp3+CD25+ Treg cell phenotype and persist for an extended period of time (compared with healthy adult naïve CD4+ T cells), shaping a stable anti-inflammatory profile [105].
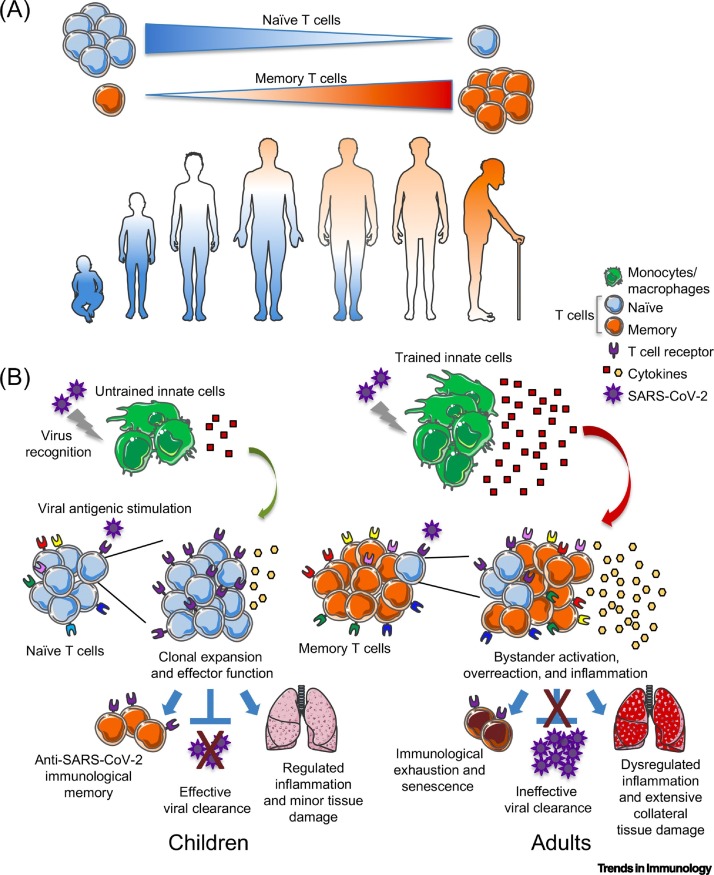
Figure 2.
Key Figure. Model of Memory versus Naïve T Cell Proportions as Potential Contributors to Severe Coronavirus Disease 2019 (COVID-19) in Adults.
We hypothesize that the higher proportion of memory versus naïve T cells in adults compared with children (A) may contribute to the extensive collateral tissue damage and cytokine storm observed in severely ill COVID-19 patients. (B) Substantially increased overactivation might be fueled by excessive trained innate immunity leading to a dysregulated release of cytokines by innate immune cells (primarily monocytes/macrophages) upon severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in adults; this phenomenon might be associated with bystander memory T cell activation, inefficient clonal expansion of SARS-CoV-2-specific T cells, and low viral clearance. In children, trained immunity would be presumably lower and naïve T cells predominant, with a progressive antiviral response reflecting clonal T cell expansion and efficient viral clearance. These phenomena might contribute to a regulated response that could lead to only minor tissue damage, not compromising the clinical response of infected children. This model remains hypothetical.
On the one hand, the accumulation of immunological memory upon entering adult life provides higher protection from infections and, indeed, young adults suffer fewer infections than children. On the other hand, immunological memory can also be detrimental for the host. As a remarkable example, it has been hypothesized that many young adults who succumbed to the ‘1918’ H1N1 influenza virus infection had developed a deadly illness because of a triggered excessive inflammatory cellular response and the recruitment of an overwhelming number of crossreactive CD8+ T cells, possibly having been infected with another strain of influenza virus in their infancy [106]. From another angle, mice studies have demonstrated that immunological memory is acquired throughout life by infections and/or vaccinations and by commensalism, including in the skin, respiratory tract, and gut microbiome; subsequently, primed CD8+ cytotoxic T cells may ‘kick in’ in response to unrelated viral infections through crossreactivity, hence augmenting unspecific inflammation [107]. In addition, while T cell proliferation in vivo is presumed to reflect TCR-mediated recognition of specific exogenous antigens, memory CD4+ and CD8+ T cells expressing the memory marker CD44, can also be activated via a bystander effect, a recognized phenomenon that induces their proliferation via cytokines instead of TCR triggering [108,109]. For instance, bystander T cells are not pathogen-specific and have been reported to contribute to a protective immune response via rapid production of IFN-γ during acute hepatitis A viral infections [108]. However, memory (but not naïve) CD4+ T cells have produced IL-17A in the absence of TCR engagement and increased the expression of pathogenic Th17 signature genes, such as those encoding CCR6 and granulocyte-macrophage colony-stimulating factor (GM-CSF), in a mouse model of multiple sclerosis, strongly suggesting a pathogenic function of bystander-activated T cells in autoimmunity and host tissue injury [109]. Thus, we hypothesize that this T cell-mediated bystander effect might be significantly more pronounced in adults than in children upon SARS-CoV-2 infection, given the higher proportion of memory (compared with naïve) T cells in the former subjects, potentially contributing to a massive T cell-derived cytokine release in severely ill adult patients (Figure 2). We also reason that another potential contributing factor to tissue injury and disease severity in COVID-19 patients might be the effects of trained immunity (Box 3 ) on both local and systemic inflammation [110]. Of note, although warranting further investigation, the Bacillus Calmette-Guerin (BCG) vaccine has been speculated to confer protection from nonrelated pathogenic viruses, including SARS-CoV-2, through trained immunity: upon challenge with pathogen-associated molecular patterns (PAMPs), innate immune cells can display an enhanced response that promotes antiviral host defense [111,112]. Thus, in children infected with SARS-CoV-2, boosting innate antimicrobial mechanisms via trained immunity (such as upon BCG vaccination) might be a potentially beneficial mechanism leading to more efficient inhibition of viral replication and subsequent reduced inflammation. Conversely, an initially amplified, but defective, antiviral innate immune response in some adult or elder individuals might result in high viral replication and activation of an inefficient systemic inflammation [112,113], although this hypothesis remains to be tested. Notwithstanding, trained immunity has indeed been implicated in the persistence of nonresolving inflammation in certain immune-mediated pathologies, such as cardiovascular diseases, as evidenced from animal models and ex vivo experiments [114]. Consistent with this hypothesis, a prominent proinflammatory monocyte/macrophage activation profile has been described in patients with severe COVID-19 infection [42,56,115., 116., 117.]. In conclusion, we speculate that the abnormal and excessive immune reaction observed in severe cases of SARS-CoV-2 infection might potentially arise from a pathogenic synergy between T cell-related bystander effects and more pronounced trained innate immunity effects in adults compared with children, reflecting dysregulated adaptive and innate immunity, as well as tissue-damaging inflammation (Figure 2). However, this hypothesis remains to be rigorously explored.
Box 3. Trained Immunity.
Trained immunity is a process that leads to enhanced responsiveness of previously activated innate immune cells, mostly myeloid, to subsequent triggers, hence defined as an ‘innate immune memory’ [110]. The molecular mechanisms implicated in this enhanced innate cell responsiveness are believed to pertain to significant modulation of chromatin organization: innate cell stimulation is accompanied by specific changes in DNA methylation status, unfolding of chromatin, and facilitation of gene expression [126]. This DNA rearrangement is only partially removed after cessation of the stimulus, allowing a quicker and enhanced expression of proinflammatory factors after secondary challenge with a novel stimulus [126]. In addition to systemic phenomena, the induction of trained immunity may also take place at the mucosal level, where long-lived alveolar macrophages display the classical trained phenotype (defense-ready epigenetic signature, high metabolic rate, and increased release of chemokines upon re-stimulation) after a respiratory viral infection, ensuring innate memory independently from monocytes and bone marrow progenitors [127]. As in the case of adaptive memory, trained immunity confers broad benefits for host defense [128].
Alt-text: Box 3
Concluding Remarks
Progression of COVID-19 may be represented by three phases: acute viral replication, hyperactive immunity, and then either recovery, or organ dysfunction and potentially death. The key is what decides the outcome between appropriate immunity and immunopathology. Here, we have summarized how T cell-mediated immunity, crucial in the successful clearance of SARS-CoV-2, may instead be dramatically impaired in severe cases of COVID-19, with T cells being both ‘victims’ and active participants of the systemic cytokine storm. In particular, we have evoked trained immunity and bystander T cell activation as potentially powerful biases in the excessive innate and adaptive immune responses to SARS-CoV-2 infection, as revealed in adults but not in children. We posit that these putative mechanisms certainly merit future research efforts.
Deeper investigations into innate, humoral, and T cell-mediated immunity (see Outstanding Questions) during the critical first weeks upon SARS-CoV2 infection, when patients either die or recover, should contribute to providing a roadmap to entangle the molecular mechanisms of COVID19 pathogenesis and host response and better inform future vaccine design and candidate therapeutics.
Outstanding Questions.
Are T cell-mediated responses and antibody production equally essential for effective SARS-CoV-2 viral clearance? Is T cell memory to SARS-CoV-2 protective toward a subsequent SARS-CoV-2 infection and, if so, how long does it last?
What is the clinical significance of the (im)balance of Th1, Th2, Th17, and Treg cell responses in COVID-19 pathogenesis? Could this imbalance be used as a potential benchmark for the selection of patients requiring immunosuppressing therapy and in which time frame?
What are the key molecular and cellular determinants that shift the balance from a proper T cell response to hyperinflammation and a cytokine storm during COVID-19? What is the functional link between these determinants and the emerging risk factors (i.e., age, sex, hypertension, diabetes, and cardiovascular diseases)?
Could the proportion of naïve versus memory T cells be used as a potential prognostic biomarker of COVID-19 disease course, especially when considering the possible development of a cytokine storm and hyperinflammatory reaction?
Alt-text: Outstanding Questions
Acknowledgments
The authors want to truly thank the clinicians, nurses, and all healthcare professionals who have worked and are working tirelessly on the frontlines of the COVID-19 pandemic. We also want to acknowledge the precious dissemination about SARS-CoV-2 related biology carried by TWiV (This Week in Virology, twiv@microbe.tv). Schematic figures were created with images adapted from Smart Servier Medical Art (http://www.servier.fr/servier-medical-art). This work has been supported by the Italian Ministry of Health Ricerca Corrente - IRCCS MultiMedica. P.d.C. is also funded by Fondazione Italiana Sclerosi Multipla (FISM n. 2018/R/4) and G.M by FISM (n. 2016/R/18 and 2018/S/5) and Progetti di Rilevante Interesse Nazionale (PRIN, 2017 K55HLC 001).
Glossary
- Acute respiratory distress syndrome (ARDS)
life-threatening condition caused by significant injury to the lungs, in which not enough oxygen reaches the bloodstream. Pneumonia is among the most common causes of ARDS.
- Amplification index
parameter indicating the rate at which a cell is dividing.
- Anakinra
human IL-1 receptor antagonist used to treat rheumatoid arthritis; usually administered by subcutaneous injection.
- Bacillus Calmette-Guerin (BCG) vaccine
obtained from Mycobacterium bovis isolates; used as a tuberculosis vaccine.
- Bronchoalveolar lavage fluid (BALF)
fluid collected in a minimally invasive procedure. Can be used as diagnostic in the evaluation of diseases affecting the lower respiratory tract.
- Bystander effect
unrelated (heterologous) polyclonal T cells expand and release cytokines (e.g., during infections and vaccinations), potentially leading to massive, dysregulated, and unspecific inflammation.
- CCR6+Th17 cells
exhibit pathogenic activity in autoimmune diseases.
- C-reactive protein
blood circulating protein produced by the liver, usually present in low quantities but significantly elevated in cases of acute inflammation.
- Cytokine storm
severe or life-threatening condition, potentially leading to multiple organ failure; can be caused by infection, autoimmunity, other immune-mediated diseases, and certain types of immunotherapy; excessive and harmful dysregulated release of cytokines into the bloodstream from immune cells. Symptoms include fever, redness, swelling, and severe fatigue.
- Dyspnea
shortness of breath, described as an intense tightening in the chest, or a feeling of air hunger and suffocation.
- Humoral response
arm of the adaptive immune response functioning to neutralize and eliminate extracellular microbes and toxins; mediated by antibodies released by plasma cells.
- IgG
an antibody isotype, secreted as a monomer; bears a key role in antibody-dependent cell-mediated cytotoxicity, complement activation, opsonization, and feedback inhibition of B cells.
- Interstitial pneumonia
affects the walls of lung alveoli and the spaces around blood vessels and small airways (interstitial space); results in abnormal accumulation of inflammatory cells in lung tissue, causing cough and dyspnea.
- Low pathogenic coronaviruses
include HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU, which infect upper airways, causing seasonal mild-to-moderate cold-like respiratory illnesses in healthy individuals.
- Pathogen-associated molecular patterns (PAMPs)
microbial molecules sharing a number of general ‘patterns’, or structures, recognized by PRRs, such as TLRs. Include bacterial lipoproteins, bacterial and viral genomes, phosphorylcholine, and other lipids common to microbial membranes.
- Pattern recognition receptors (PRRs)
membrane proteins mainly expressed by innate immune cells; activated by a range of molecules conserved among different pathogens (PAMPs).
- Splenic atrophy
loss of T lymphocytes (in periarteriolar lymphatic sheaths) and/or B cells (in follicles, germinal centers, and marginal zones). Depending on the severity, can result in decreased cellularity or follicle number.
- Stem-like memory phenotype
characteristic of a rare subset of memory lymphocytes endowed with multipotent capability to self-renew and reconstitute the entire spectrum of memory and effector T cell subsets.
- T cell exhaustion
progressive acquisition of a distinct transcriptional/epigenetic profile and loss of effector functions and proliferative potential, thus preventing optimal control of an immune response (e.g., infection).
- Terminally differentiated/senescent phenotype
cell state of complete differentiation associated with impaired function; in T cells, refers to the inability to produce a required response and to proliferate after antigen exposure; characterized by typical transcriptional changes and high expression of inhibitory receptors.
- TLRs
Toll like receptors; cell surface and endosomal receptors expressed by many cell types, functioning in pattern recognition for a plethora of pathogen-associated molecules, including lipopolysaccharides and microbial nucleic acids. TLRs activate specific signal transduction pathways that promote an inflammatory response (e.g., to infections).
- Tocilizumab
humanized monoclonal antibody directed against the interleukin-6 receptor (IL-6R); used as an immunosuppressive drug, mainly for the treatment of rheumatoid arthritis.
Resources
ihttps://gisanddata.maps.arcgis.comReferences
- 1.Lu R. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368 doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 5.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thevarajan I. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.X. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 10.Ni L. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grifoni A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbiani D.F. Convergent antibody responses to SARS-CoV-2 infection in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallais F. Intrafamilial exposure to SARS-CoV-2 induces cellular immune response without seroconversion. medRxiv. 2020 doi: 10.1101/2020.06.21.20132449. Published online June 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekine T. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortaz E. The immune response and immunopathology of COVID-19. Front. Immunol. 2020;11:2037. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng H. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J. Infect. 2020;81:e33–e39. doi: 10.1016/j.jinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir. Res. 2020;21:74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R. Clinical characteristics of 225 patients with COVID-19 in a tertiary hospital near Wuhan, China. J. Clin. Virol. 2020;127:104363. doi: 10.1016/j.jcv.2020.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R. Decreased T cell populations contribute to the increased severity of COVID-19. Clin. Chim. Acta. 2020;508:110–114. doi: 10.1016/j.cca.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J. Infect. Dis. 2020;222:198–202. doi: 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin C. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang W. Lymphocyte subset counts in COVID-19 patients: a meta-analysis. Cytometry A. 2020;97:772–776. doi: 10.1002/cyto.a.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo M. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan K. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falsey A.R. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J. Infect. Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vabret A. An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin. Infect. Dis. 2003;36:985–989. doi: 10.1086/374222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Z. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong R.S. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamming I. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou X. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu J. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia X.Y. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J. Clin. Virol. 2020;127:104360. doi: 10.1016/j.jcv.2020.104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao M. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 43.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. Published online April 3, 2020. http://doi/org/10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng F. Can we predict the severity of COVID-19 with a routine blood test? Pol. Arch. Intern. Med. 2020;130:400–406. doi: 10.20452/pamw.15331. [DOI] [PubMed] [Google Scholar]
- 45.Diao B. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S. Clinical and pathological investigation of severe COVID-19 patients. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urra J.M. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. 2020;217:108486. doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giamarellos-Bourboulis E.J. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1003. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bordoni V. An inflammatory profile correlates with decreased frequency of cytotoxic cells in COVID-19. Clin. Infect. Dis. 2020;71:2272–2275. doi: 10.1093/cid/ciaa577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020 doi: 10.1101/2020.03.27.20045427. Published online March 31, 2020. [DOI] [Google Scholar]
- 52.Kaneko N. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huet T. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K.D. Adaptive immune cells temper initial innate responses. Nat. Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palm N.W., Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat. Med. 2007;13:1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Y. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulte-Schrepping J. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song J.W. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11:3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng H.Y. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ong E.Z. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27:879–882. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng M. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gimenez E. SARS-CoV-2-reactive interferon-gamma-producing CD8+ T cells in patients hospitalized with coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.26213. Published online June 24, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazzoni A. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 2020;130:4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng C.T. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe. 2013;13:652–664. doi: 10.1016/j.chom.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi J.S. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 68.Jones R.B. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barber D.L. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 70.Andre P. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–1743. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Biasi S. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meckiff B.J. Single-cell transcriptomic analysis of SARS-CoV-2 reactive CD4+ T cells. SSRN. 2020 doi: 10.2139/ssrn.3641939. July 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belkaid Y., Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu. Rev. Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 74.Suvas S. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 75.Li C.K. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang J.L. Th2 predominance and CD8+ memory T cell depletion in patients with severe acute respiratory syndrome. Microbes Infect. 2005;7:427–436. doi: 10.1016/j.micinf.2004.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiskopf D. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roncati L. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020;99:1419–1420. doi: 10.1007/s00277-020-04066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pacha O. COVID-19: a case for inhibiting IL-17? Nat. Rev. Immunol. 2020;20:345–346. doi: 10.1038/s41577-020-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fulop T. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu P. Age-related rhesus macaque models of COVID-19. Anim. Model Exp. Med. 2020;3:93–97. doi: 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin L. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marquez E.J. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng Y. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 86.Prattichizzo F. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res. Rev. 2018;41:1–17. doi: 10.1016/j.arr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Lee Y.S. An integrated view of immunometabolism. Cell. 2018;172:22–40. doi: 10.1016/j.cell.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohamed-Ali V. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 89.Prattichizzo F. Prevalence of residual inflammatory risk and associated clinical variables in patients with type 2 diabetes. Diabetes Obes. Metab. 2020;22:1696–1700. doi: 10.1111/dom.14081. [DOI] [PubMed] [Google Scholar]
- 90.Parisi M.M. Immunosenescence induced by plasma from individuals with obesity caused cell signaling dysfunction and inflammation. Obesity (Silver Spring) 2017;25:1523–1531. doi: 10.1002/oby.21888. [DOI] [PubMed] [Google Scholar]
- 91.Huttunen R., Syrjänen J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu X. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai K. Clinical analysis of 25 novel coronavirus infections in children. Pediatr. Infect. Dis. J. 2020;39:e100–e103. doi: 10.1097/INF.0000000000002740. [DOI] [PubMed] [Google Scholar]
- 95.Dong Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 96.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States. MMWR Morb. Wkly. Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Onder G. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 98.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 99.Feldstein L.R. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies N.G. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 101.Wedderburn L.R. The developing human immune system: T-cell receptor repertoire of children and young adults shows a wide discrepancy in the frequency of persistent oligoclonal T-cell expansions. Immunology. 2001;102:301–309. doi: 10.1046/j.1365-2567.2001.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shearer W.T. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Saule P. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech. Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 104.Rudolph M.E. Differences between pediatric and adult T cell responses to in vitro staphylococcal enterotoxin B stimulation. Front. Immunol. 2018;9:498. doi: 10.3389/fimmu.2018.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mold J.E. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McAuley J.L. Host immunological factors enhancing mortality of young adults during the 1918 influenza pandemic. Front. Immunol. 2015;6:419. doi: 10.3389/fimmu.2015.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Selin L.K. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J. Exp. Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim T.-S., Shin E.-C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 2019;51:154. doi: 10.1038/s12276-019-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee H.G. Pathogenic function of bystander-activated memory-like CD4(+) T cells in autoimmune encephalomyelitis. Nat. Commun. 2019;10:709. doi: 10.1038/s41467-019-08482-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Netea M.G., van der Meer J.W. Trained immunity: an ancient way of remembering. Cell Host Microbe. 2017;21:297–300. doi: 10.1016/j.chom.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Ozdemir C. Is BCG vaccination affecting the spread and severity of COVID-19? Allergy. 2020;75:1824–1827. doi: 10.1111/all.14344. [DOI] [PubMed] [Google Scholar]
- 112.O’Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riksen N.P. Trained immunity and atherosclerotic cardiovascular disease. Curr. Opin. Lipidol. 2019;30:395–400. doi: 10.1097/MOL.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 115.Zhou Y. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hadjadj J. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. Front. Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swain S.L. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitchison N.A. T-cell-B-cell cooperation. Nat. Rev. Immunol. 2004;4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 121.Kleinewietfeld M., Hafler D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bluestone J.A. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohkura N. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 124.Pangrazzi L., Weinberger B. T cells, aging and senescence. Exp. Gerontol. 2020;134:110887. doi: 10.1016/j.exger.2020.110887. [DOI] [PubMed] [Google Scholar]
- 125.Olivieri F. Cellular senescence and inflammaging in age-related diseases. Mediat. Inflamm. 2018;2018:9076485. doi: 10.1155/2018/9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Netea M.G. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao Y. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175:1634–1650. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 128.Ciarlo E. Trained immunity confers broad-spectrum protection against bacterial infections. J. Infect. Dis. 2019;222:1869–1881. doi: 10.1093/infdis/jiz692. [DOI] [PMC free article] [PubMed] [Google Scholar]