Abstract
Objective
During the most aggressive phase of the COVID-19 outbreak in Italy, the Regional Authority of Lombardy identified a number of hospitals, named Hubs, chosen to serve the whole region for highly specialised cases, including vascular surgery. This study reports the experience of the four Hubs for Vascular Surgery in Lombardy and provides a comparison of in hospital mortality and major adverse events (MAEs) according to COVID-19 testing.
Methods
Data from all patients who were referred to the Vascular Surgery Department of Hubs from 9 March to 28 April 2020 were collected prospectively and analysed. A positive COVID-19 polymerase chain reaction swab test, or symptoms (fever > 37.5 °C, upper respiratory tract symptoms, chest pain, and contact/travel history) associated with interstitial pneumonia on chest computed tomography scan were considered diagnostic of COVID-19 disease. Patient characteristics, operative variables, and in hospital outcomes were compared according to COVID-19 testing. A multivariable model was used to identify independent predictors of in hospital death and MAEs.
Results
Among 305 included patients, 64 (21%) tested positive for COVID-19 (COVID group) and 241 (79%) did not (non-COVID group). COVID patients presented more frequently with acute limb ischaemia than non-COVID patients (64% vs. 23%; p < .001) and had a significantly higher in hospital mortality (25% vs. 6%; p < .001). Clinical success, MAEs, re-interventions, and pulmonary and renal complications were significantly worse in COVID patients. Independent risk factors for in hospital death were COVID (OR 4.1), medical treatment (OR 7.2), and emergency setting (OR 13.6). COVID (OR 3.4), obesity class V (OR 13.5), and emergency setting (OR 4.0) were independent risk factors for development of MAEs.
Conclusion
During the COVID-19 pandemic in Lombardy, acute limb ischaemia was the most frequent vascular disease requiring surgical treatment. COVID-19 was associated with a fourfold increased risk of death and a threefold increased risk of major adverse events.
Keywords: Covid-19, Limb ischaemia, Lombardy, Pandemics, SARS-CoV-2, Vascular surgery
What this paper adds.
This study reports the experience of four centres dedicated to vascular surgery during the first seven weeks of the COVID-19 outbreak in Lombardy, Italy. Acute limb ischaemia was the most frequent condition requiring surgical treatment. Patients with associated COVID-19 had a fourfold increased risk of death and a threefold increased risk of major adverse events.
Introduction
On 9 January 2020, the Chinese Centre for Disease Control and Prevention reported the identification of a novel coronavirus, later named SARS-CoV-2, causing a severe acute respiratory syndrome, the so called Coronavirus Disease 2019 (COVID-19).1 Spreading through small droplet transmission, it rapidly diffused in mainland China and worldwide, becoming a pandemic. As of 1 June 2020, more than 6.2 million cases have been reported across 188 countries and territories, resulting in more than 372 000 deaths.2
Italy was the first Western country to experience the COVID-19 epidemic, and it remains one of the countries that is suffering the most. Within Italy, the first and most affected region was Lombardy, a northern district with 10 million inhabitants. After the first case was confirmed during the night of 20 February in the town of Codogno, COVID cases rose to over 530 by 28 February and 5 830 by 8 March, clearly making Lombardy the epicentre of the Italian epidemic.
On 9 March 2020, concurrently with the imposition of a national quarantine, the Regional Authority of Lombardy remodelled the hospital system in order to allocate appropriate resources to treat COVID-19 patients, increasing the number of intensive care unit (ICU) beds, and identifying “Hub” hospitals where specific highly specialised medical activities should be concentrated to ensure the delivery of non-deferrable care.3
The aim of this study is to report the combined experience of Hub hospitals for Vascular Surgery in Lombardy during the first seven weeks after total lockdown due to the COVID-19 epidemic, and to compare in hospital mortality and major adverse events (MAEs) according to COVID-19 testing.
Materials and Methods
Hospital Hub/Spoke system model in Lombardy
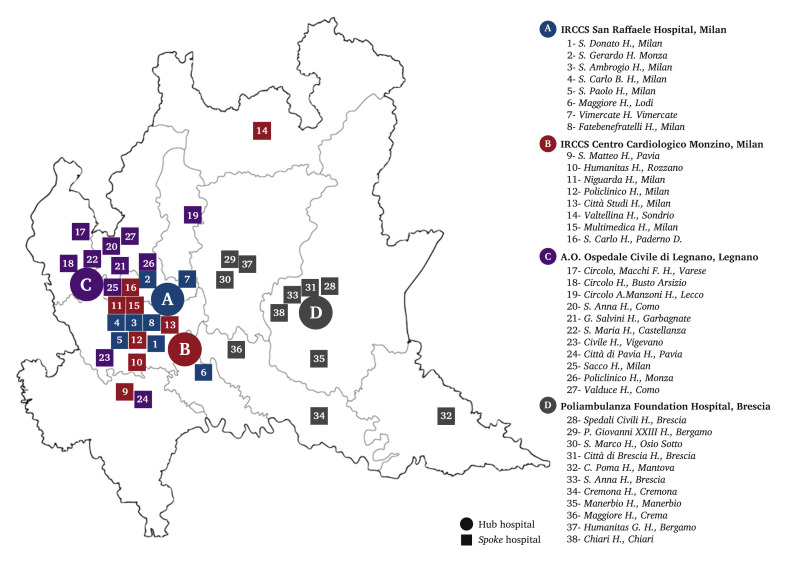
Four hospitals were identified as vascular surgery Hubs (Fig. 1 ), each of them associated with a number of afferent centres (Spokes), mainly redirected to the care of COVID-19 patients.
Figure 1.
Map of Lombardy, Italy, showing identified Hub hospitals for vascular surgery in Lombardy region during COVID-19 epidemic, and referring Spoke hospitals.
Hubs were required to guarantee:
-
•
24/7 evaluation of patients presenting with known or suspected vascular disease, referred from the territorial emergency net or from Spoke hospitals.
-
•
Medical or surgical treatment of all patients presenting with urgent/emergency vascular disease or deemed not able to wait for more than 60 days (Table 1 ).
-
•
At least two surgical teams available 24/7 (each consisting of two surgeons, one anaesthetist, and two nurses), with at least one on duty. Collaboration with personnel coming from Spoke hospitals was permitted.
-
•
A dedicated independent pathway for patients with suspected or confirmed COVID-19 separated from negative patients.
Table 1.
Indications for urgent/emergency or elective vascular surgery during pandemic COVID-19 infection in the Lombardy region, Italy, from 9 March to 28 April 2020
| Urgent/emergency diseases | Patients suffering from serious diseases that involve immediate risk to life or the function of vital organs or limbs, for whom urgent/emergency surgery is indicated |
| Elective diseases | Patients suffering from serious diseases that do not imply an immediate risk to life or to the function of vital organs or limbs, but who require surgical interventions that cannot be postponed for a period of more than two months |
| Thoracic aortic aneurysms with a diameter >6 cm, or rapidly growing, or symptomatic, or in the presence of connective tissue disease on a genetic basis | |
| Abdominal aortic aneurysms with a diameter >5.5 cm, or rapidly growing, or symptomatic, or in the presence of genetically based connective tissue disease | |
| Iliac aneurysms with a diameter >4 cm, or rapidly growing, or symptomatic, or in the presence of connective tissue disease on a genetic basis | |
| Symptomatic peripheral/visceral aneurysms or with a diameter >4 cm | |
| Acute or subacute dissections of the descending thoracic aorta (type B according to Stanford) | |
| Symptomatic carotid or supra-aortic stenosis, of any degree | |
| Asymptomatic carotid or supra-aortic trunk stenosis >80% (NASCET) or with PSV > 3 m/s | |
| Arteriopathy of the limbs with critical ischaemia | |
| Femoro-iliocaval deep vein thrombosis at risk of pulmonary embolism |
PSV = peak systolic velocity; NASCET = The North American Symptomatic Carotid Endarterectomy Trial.
All patients referred to Hub hospitals were tested for SARS-CoV-2 with nasopharyngeal swab and chest Xray. A chest computed tomography (CT) scan was performed in selected cases. COVID-19 was considered confirmed in patients presenting a positive real time reverse transcription polymerase chain reaction swab test, and in those presenting a history and/or symptoms suspicious of COVID-19 (fever > 37.5 °C, upper respiratory tract symptoms, chest pain, and contact/travel history) associated with interstitial pneumonia on chest CT scan.
For suspected or confirmed COVID-19 in a patient requiring surgical treatment, a dedicated negative pressure operating theatre was used, equipped with full personal protective equipment. In the post-operative period, COVID-19 patients were admitted to a COVID-19 dedicated ICU or ward.
Data from all patients who were referred to vascular Hubs in Lombardy from 9 March 2020 to 28 April 2020 were prospectively collected into a computerised database.
The study was conducted according to the principles of the Declaration of Helsinki, and employed anonymised data obtained from review of patients’ charts. Patients gave their consent for the anonymous data collection on the standard consent sheet provided by all involved institutions. Data collection was carried out in accordance with the Italian law on privacy (Art. 20–21, DL 196/2003) published in the Official Journal no. 190 of 14 August 2004, which explicitly exempts the need for ethics approval for the use of anonymous data. Pre-operative assessment of cardiac, pulmonary, and kidney status was conducted according to the European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms.4 Pre-operative demographics and risk factors were reported following the International Consortium of Vascular Registries Consensus Recommendations for Peripheral Revascularisation Registry Data Collection.5 Age, obesity, and pre-operative renal status were stratified according to the Society for Vascular Surgery (SVS) recommended reporting standards.6 , 7
The indications for admission were categorised into six groups: acute limb ischaemia (ALI, including upper and lower limb symptomatic disease developed in the last 14 days, due to related arterial occlusive disease confirmed by imaging studies), chronic limb threatening ischaemia (CLTI, lower limb symptomatic disease with known history of chronic ischaemic disease or developed in more than 14 days, with the exclusion of limbs already presenting with irreversible necrosis or gangrene), limb necrosis/gangrene, aortic disease (aneurysms, dissections, penetrating ulcers, and fistulae), cerebrovascular disease (including symptomatic and asymptomatic carotid stenosis), and other (all other vascular disease not falling into the other groups).
ALI cases were classified according to the Rutherford classification,7 and CLTI cases according to Rutherford categories7 and the wound, ischaemia, and foot infection (WIfI) classification system.8
Treatment was categorised into five groups: open surgery, endovascular surgery, hybrid (mix of open and endovascular surgical techniques), primary major limb amputation, and medical therapy. Timing of surgery was defined as elective, urgent or emergency according to the SVS recommended reporting standards.6
Global in hospital mortality was reported considering both patients submitted to any kind of surgery, and those submitted to medical therapy. Clinical success was defined, adapted from the SVS reporting standards for endovascular aortic aneurysm repair,9 as technical successful conclusion of the intervention (e.g., successful revascularisation for limb ischaemia) without death or MAEs. Primary/secondary clinical success was defined as clinical success with/without the need for an additional or secondary surgical or endovascular procedure, respectively.
Post-operative complications were defined according to the SVS reporting standards.9 A peri-operative complication was referred as MAE when graded >1, or in case of secondary major amputation.
Statistical analysis
Categorical variables were expressed as frequencies and percentages. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test; those with normal distribution were expressed as mean and standard deviation, those with non-normal distribution as median and interquartile range.
Patients were divided in two groups according to COVID-19 testing: the COVID group patients who tested positive on admission or during hospitalisation, and the non-COVID group patients who always tested negative. Differences between groups were tested using the Fisher exact test for dichotomous variables, and the Student t test or the Mann–Whitney test for continuous variables, as appropriate. All tests were two tailed, and p ≤ .05 was considered to be statistically significant.
A logistic regression model using stepwise selection was used to identify independent predictors of in hospital death and MAEs. Data were entered into the model if they had a univariable p ≤ .05. Collinearity and overfitting were assessed using a stepwise regression model and Pearson correlation test. In the multivariable model, results were expressed as odds ratio (OR) with 95% confidence interval (CI).
Statistical analysis was performed using Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA) version 14.0 for Windows.
Results
In the study period, 305 consecutive patients (69.8% males; mean age 73.3 ± 11.1 years) were referred to Hubs (19 patients to Centro Cardiologico Monzino Scientific Institute, 95 to Poliambulanza Foundation Hospital, 56 to Ospedale Civile di Legnano, and 135 to San Raffaele Scientific Institute).
COVID-19 was diagnosed in 64 patients (21%), on admission in 57 and during subsequent hospitalisation in seven. Chest CT scan was performed in 64 patients (21.0%), presenting signs of interstitial pneumonia in 22 cases. Dyspnoea was present in 38 COVID patients (59.4%). Respiratory support was provided by oxygen mask in 43 patients (67.2%), non-invasive ventilation in 24 (37.5%), and tracheal intubation in nine (14.1%). Mean fraction of inspired oxygen (FiO2) was 46.1% ± 24.6% and mean measured arterial partial pressure of oxygen (PaO2) was 80.0 mmHg ± 36.2 mmHg.
Demographics and pre-operative risk factors are summarised in Table 2 .
Table 2.
Demographics and pre-operative risk factors of all patients referred to the Vascular Surgery Hubs in the Lombardy region, Italy, from 9 March to 28 April 2020 confirmed to be affected or non-affected by COVID on admission or during hospitalisation
| Variable | All patients (n = 305) | COVID (n = 64) | Non-COVID (n = 241) | p |
|---|---|---|---|---|
| Male gender | 213 (69.8) | 51 (79.7) | 162 (67.2) | .066 |
| Age – y∗ | 73.3 ± 11.1 | 72.5 ± 11.2 | 73.6 ± 11.2 | .49 |
| Age class | ||||
| Class 1, <55 years | 19 (6.2) | 5 (7.8) | 14 (5.8) | .56 |
| Class 2, 55–69 years | 80 (26.2) | 18 (28.1) | 62 (25.7) | .75 |
| Class 3, 70–79 years | 107 (35.1) | 19 (29.7) | 88 (36.5) | .38 |
| Class 4, ≥80 years | 99 (32.5) | 22 (34.4) | 77 (32.0) | .76 |
| Body mass index – kg/m2† | 25 (22–29) | 26 (23–29) | 25 (22–29) | .60 |
| Hypertension | 246 (80.7) | 48 (75.0) | 198 (82.2) | .21 |
| Tobacco use | 175 (57.4) | 33 (51.6) | 142 (58.9) | .32 |
| Diabetes | 98 (32.1) | 18 (28.1) | 80 (33.2) | .55 |
| Hyperlipaemia | 192 (62.9) | 36 (56.2) | 156 (64.7) | .24 |
| Atrial fibrillation | 59 (19.3) | 15 (23.4) | 44 (18.3) | .37 |
| Renal status‡ | ||||
| Normal | 177 (58.0) | 36 (56.2) | 141 (58.5) | .78 |
| Class 1 | 89 (29.2) | 22 (34.4) | 67 (27.8) | .35 |
| Class 2 | 32 (10.5) | 6 (9.4) | 26 (10.8) | 1.0 |
| Class 3 | 7 (2.3) | 0 (0) | 7 (2.9) | .35 |
| Chronic obstructive pulmonary disease | 28 (9.2) | 7 (10.9) | 21 (8.7) | .63 |
| Coronary artery disease | 104 (34.1) | 15 (23.4) | 89 (36.9) | .053 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range).
Parametric test (Student t test) was used for comparison.
Non-parametric test (Mann-Whitney U test) was used for comparison.
Renal status (refers to stable levels, not transient drops, or elevations in response to intravenous medication, hydration, or contrast media): Normal = no known renal disease, normal serum creatinine level; Class 1 = moderately elevated creatinine level, as high as 2.4 mg/dL; Class 2 = creatinine level, 2.5 to 5.9 mg/dL; Class 3 = creatinine level greater than 6.0 mg/dL, or on dialysis or with kidney transplant (Defined according to the Society for Vascular Surgery (SVS) recommended reporting standards).
Ninety-seven patients presented with ALI, involving one lower limb in 81 patients, one upper limb in eight, both lower limbs in six (four of those were COVID patients), and concomitant upper and lower limbs in two (both COVID patients). According to the Rutherford classification, ALI class was IIA in 26 cases, IIB in 55, and III in 16. Possible causes of ALI were identified in 53/97 patients, including cardiac embolism in 23, bypass graft failure in 13, pre-occlusive atherosclerotic plaque in 11, vascular trauma in three, systemic sepsis in two, and popliteal aneurysm in one patient. No clear causes of ALI were found in 25/41 COVID (61.0%), and in 19/56 non-COVID patients (33.9%).
Seventy-one patients presented with Rutherford category three (three cases), four (25 cases), and five (43 cases) CLTI. Twenty-nine patients presented with CLTI and already established irreversible lower limb necrosis (Rutherford category 6). Including all CLTI cases, the risk of amputation at one year according to the WIfI classification was considered very low in 11, low in 14, medium in 23, and high in 52 patients.
Aortic disease was the indication for admission in 45 patients (14.7%), including 21 cases of abdominal aortic or iliac aneurysm (ruptured in seven cases), seven cases of endoleak after previous endovascular repair of abdominal aortic aneurysm (AAA) (ruptured in three cases), eight cases of thoraco-abdominal aortic aneurysm (ruptured in one case), four cases of acute type B aortic dissection, two cases of penetrating ulcer of the descending thoracic aorta, one case of descending thoracic aortic aneurysm, one case of abdominal aortic graft infection, and one case of aortoduodenal fistula.
A carotid stenosis was the indication for admission in 36 patients (11.8%), symptomatic in 21 cases, and asymptomatic but meeting treatment criteria (Table 1) in 15. Other indications for admission (27 patients, 8.9%) included femoral pseudo-aneurysm in four cases; popliteal aneurysm, malignant neoplasm of the neck, cardiac failure requiring placement of a cardiac assist device, in three cases each; vascular access for haemodialysis, deep venous thrombosis, peripheral vascular graft infection, renal artery stenosis, in two cases each; aneurysm of the superficial temporal artery, superior mesenteric artery thrombosis, spontaneous haematoma of the thigh, renal artery aneurysm, thoracic outlet syndrome, and caval leiomyosarcoma in one case each.
ALI was the most common indication to admission in COVID patients, significantly more than in non-COVID patients (64.1% vs. 23.2%, respectively; p < .001). Conversely, aortic and cerebrovascular disease were more common in the non-COVID group. Indications for admission and presentation characteristics are summarised in Table 3 .
Table 3.
Characteristics of vascular disease, presentation, treatment and general mortality of all patients referred to the Vascular Surgery Hubs in the Lombardy region, Italy, from 9 March to 28 April 2020 confirmed to be affected or non-affected by COVID on admission or during hospitalisation
| Variable | All patients (n = 305) | COVID (n = 64) | Non-COVID (n = 241) | p |
|---|---|---|---|---|
| Vascular disease | ||||
| Acute limb ischaemia | 97 (31.8) | 41 (64.1) | 56 (23.2) | <.001 |
| Chronic limb ischaemia | 71 (23.3) | 12 (18.7) | 59 (24.5) | .41 |
| Limb necrosis/gangrene | 29 (9.5) | 3 (4.7) | 26 (10.8) | .16 |
| Aortic disease | 45 (14.7) | 1 (1.6) | 44 (18.3) | <.001 |
| Cerebrovascular disease | 36 (11.8) | 2 (3.1) | 34 (14.1) | .015 |
| Other | 27 (8.9) | 5 (7.8) | 22 (9.1) | 1.0 |
| Patient presentation | ||||
| Coming from home | 143 (46.9) | 19 (29.7) | 124 (51.4) | .002 |
| Referred by another department | 28 (9.2) | 20 (31.2) | 8 (3.3) | <.001 |
| Referred by another hospital (Spoke) | 88 (28.8) | 19 (29.7) | 69 (28.6) | .88 |
| Referred by another hospital (non-Spoke) | 46 (15.1) | 6 (9.4) | 40 (16.6) | .17 |
| Time from symptoms to treatment – h∗ | 50.8 ± 77.1 | 79.2 ± 103.7 | 28.2 ± 33.7 | <.001 |
| Pre-operative haemoglobin – g/dL∗ | 12.4 ± 2.3 | 12.5 ± 2.1 | 12.4 ± 2.3 | .75 |
| Pre-operative platelet count – ×109∗ | 241.6 ± 106.0 | 243.7 ± 88.6 | 240.9 ± 110.8 | .85 |
| Treatment | ||||
| Open surgery | 158 (51.8) | 36 (56.2) | 122 (50.6) | .48 |
| Endovascular surgery | 70 (22.9) | 6 (9.4) | 64 (26.6) | .003 |
| Hybrid surgery | 39 (12.8) | 10 (15.6) | 29 (12.0) | .53 |
| Primary major limb amputation | 17 (5.6) | 3 (4.7) | 14 (5.8) | 1.0 |
| Medical therapy | 21 (6.9) | 9 (14.1) | 12 (5.0) | .022 |
| Global in hospital mortality | 31 (10.2) | 16 (25.0) | 15 (6.2) | <.001 |
| In patients undergoing surgery | 23 (8.1) | 11 (20.0) | 12 (5.2) | .001 |
| In patients treated medically | 8 (38.1) | 5 (55.5) | 3 (25.0) | .20 |
Data are presented as n (%) or mean ± standard deviation.
Parametric test (Student t-test) was used for comparison.
Overall, 21 patients (6.9%) were treated medically, and 284 (93.1%) surgically (Table 3). Reasons for choosing medical therapy alone were high surgical risk, related to SARS-CoV-2 infection in seven patients and to general comorbidities or advanced age in six; vascular disease severity too low to justify urgent intervention in six patients; and patient refusal in two cases. COVID patients underwent medical treatment alone more frequently than non-COVID patients (14.1% vs. 5.0%; p = .022), whereas the latter underwent endovascular interventions more frequently than the former (26.6% vs. 9.4%; p = .003).
Global in hospital mortality was 10.2% (31 patients), and was higher in patients treated medically (38.1% vs. 8.1%; p < .001).
Cause of death was multi-organ failure in 10 cases (five COVID), respiratory failure in nine (all COVID patients), cardiac failure in four (one COVID), aortic rupture in two (both non-COVID patients treated medically for general comorbidities), cerebral ischaemia in two (both non-COVID), sepsis in one (non-COVID), and aortic thrombosis with bowel ischaemia in one case (COVID). In two non-COVID patients the cause of death was unknown.
Patients with COVID had a significantly higher mortality (25.0% vs. 6.2%; p < .001). When stratified by type of vascular disease, type of intervention, and timing (Table 4 ), in hospital mortality was significantly higher in COVID patients, especially in cases of ALI or CLTI, in open surgical intervention cases, and in the elective setting.
Table 4.
Comparison of in hospital mortality in patients affected or non-affected by COVID, according to vascular disease, type of intervention, and timing in the Lombardy region, Italy, from 9 March to 28 April 2020
| COVID group (n = 64) |
Non-COVID group (n = 241) |
p∗ | |||
|---|---|---|---|---|---|
| Patients – n | In hospital deaths | Patients – n | In hospital deaths | ||
| Vascular disease | |||||
| Acute limb ischaemia | 41 | 12 (29.3) | 56 | 5 (8.9) | .014 |
| Chronic limb ischaemia | 12 | 4 (33.3) | 59 | 1 (1.7) | .002 |
| Limb necrosis/gangrene | 3 | 0 (0) | 26 | 2 (7.7) | 1.0 |
| Aortic disease | 1 | 0 (0) | 44 | 6 (13.6) | 1.0 |
| Cerebrovascular disease | 2 | 0 (0) | 34 | 1 (2.9) | 1.0 |
| Other | 5 | 0 (0) | 22 | 0 (0) | 1.0 |
| Type of intervention | |||||
| Open surgery | 36 | 10 (27.8) | 122 | 8 (6.6) | .001 |
| Endovascular surgery | 6 | 0 (0) | 64 | 1 (1.6) | 1.0 |
| Hybrid surgery | 10 | 1 (10.0) | 29 | 1 (3.4) | .45 |
| Primary major limb amputation | 3 | 0 (0) | 14 | 2 (14.3) | 1.0 |
| Medical therapy | 9 | 5 (55.5) | 12 | 3 (25.0) | .20 |
| Timing | |||||
| Elective | 20 | 4 (20.0) | 107 | 3 (2.8) | .012 |
| Urgent | 1 | 0 (0) | 58 | 2 (3.4) | 1.0 |
| Emergent | 43 | 12 (27.9) | 76 | 10 (13.2) | .054 |
Data are presented as n (%) unless stated otherwise.
Comparison of in hospital mortality between COVID and non-COVID groups (Fisher's exact test).
Operative details and in hospital outcomes of patients submitted to surgical interventions are reported in Table 5 . Only one patient, an 85 year old man with a ruptured AAA (non-COVID group), died intra-operatively. Peri-operative mortality was 15.2% (14/92) for ALI, 3.2% (2/63) for CLTI, 6.9% (2/29) for limb necrosis/gangrene, 9.8% (4/41) for aortic disease, and 2.9% (1/35) for cerebrovascular disease. Peri-operative pulmonary (grades 2 and 3) and renal (grade 2) complications occurred more frequently in COVID patients (Table 5).
Table 5.
Operative details and in hospital outcomes of patients affected or non-affected by COVID submitted to surgical interventions in the Lombardy region, Italy, from 9 March to 28 April 2020
| Variable | All patients (n = 284) | COVID (n = 55) | Non-COVID (n = 229) | p |
|---|---|---|---|---|
| Timing of surgery | ||||
| Elective | 113 (39.8) | 15 (27.3) | 98 (42.8) | .046 |
| Urgent | 58 (20.4) | 1 (1.8) | 57 (24.9) | <.001 |
| Emergent | 113 (39.8) | 39 (70.1) | 74 (32.3) | <.001 |
| Duration of intervention – min∗ | 122.4 ± 88.1 | 112.4 ± 79.8 | 124.3 ± 89.7 | .37 |
| Intra-operative death | 1 (0.3) | 0 (0) | 1 (0.4) | 1.0 |
| Need of ICU | 23 (8.1) | 6 (10.1) | 17 (7.4) | .41 |
| Post-operative drugs regimen† | ||||
| Single antiplatelet | 101 (35.7) | 8 (14.5) | 93 (40.8) | <.001 |
| Double antiplatelet | 45 (15.9) | 4 (7.3) | 41 (18.0) | .063 |
| IV unfractionated heparin | 26 (9.2) | 13 (23.6) | 13 (5.7) | <.001 |
| Low molecular weight heparin | 111 (39.2) | 30 (54.5) | 81 (35.5) | .013 |
| Peri-operative (in hospital) mortality | 23 (8.1) | 11 (20.0) | 12 (5.2) | .001 |
| Primary clinical success | 223 (78.5) | 32 (58.2) | 191 (83.4) | <.001 |
| Early re-intervention | 29 (10.2) | 12 (21.8) | 17 (7.4) | .005 |
| Secondary clinical success | 228 (80.3) | 34 (61.8) | 194 (84.7) | <.001 |
| Patients experiencing one or more MAE | 58 (20.4) | 21 (38.2) | 37 (16.2) | <.001 |
| Neurological complications | 10 (3.5) | 1 (1.8) | 9 (3.9) | .69 |
| TIA | 3 (1.1) | 0 (0) | 3 (1.3) | 1.0 |
| Stroke | 5 (1.8) | 1 (1.8) | 4 (1.7) | 1.0 |
| Spinal cord ischaemia | 2 (0.7) | 0 (0) | 2 (0.9) | 1.0 |
| Cardiac complications | 12 (4.2) | 1 (1.8) | 11 (4.8) | .47 |
| 1. Little or no haemodynamic consequences | 2 (0.7) | 0 (0) | 2 (0.9) | 1.0 |
| 2. Symptomatic, IV medications, PTCA | 8 (2.8) | 1 (1.8) | 7 (3.1) | 1.0 |
| 3. Severe haemodynamic dysfunction, resuscitation, cardiac arrest, or fatal outcomes | 2 (0.7) | 0 (0) | 2 (0.9) | 1.0 |
| Pulmonary complications | 31 (10.9) | 17 (30.1) | 14 (6.1) | <.001 |
| 1. Prompt recovery with medical treatment | 7 (2.5) | 2 (3.6) | 5 (2.2) | .62 |
| 2. Prolonged hospitalisation or IV antibiotics | 11 (3.9) | 5 (9.1) | 6 (2.6) | .041 |
| 3. Prolonged intubation, tracheostomy, deterioration in pulmonary function, O2 dependence or fatal outcome | 13 (4.6) | 10 (18.2) | 3 (1.3) | <.001 |
| Renal complications | 24 (8.4) | 9 (16.4) | 15 (6.5) | .029 |
| 1. No dialysis, AKIN>2 | 12 (4.2) | 4 (7.3) | 8 (3.5) | .26 |
| 2. Temporary dialysis, prolonged hospitalisation, permanent impairment | 8 (2.8) | 4 (7.3) | 4 (1.7) | .048 |
| 3. Permanent dialysis, transplant or fatal outcome | 4 (1.4) | 1 (1.8) | 3 (1.3) | .58 |
| Bowel ischaemia | 4 (1.4) | 1 (1.8) | 3 (1.3) | .58 |
| 1. Recovered with IV or parenteral nutrition | 2 (0.7) | 1 (1.8) | 1 (0.4) | .35 |
| 2. Bowel resection or fatal outcome | 2 (0.7) | 0 (0) | 2 (0.9) | 1.0 |
| Secondary limb amputation | 23 (8.1) | 6 (10.9) | 17 (7.4) | .41 |
| 1. Major (thigh, leg) | 6 (2.1) | 3 (5.4) | 3 (1.3) | .089 |
| 2. Minor (forefoot, fingers/toes) | 17 (6.0) | 3 (5.4) | 14 (6.1) | 1.00 |
| Discharge from hospital | 218 (76.8) | 31 (56.4) | 187 (81.7) | <.001 |
| 1. Patient's home | 169 (59.5) | 22 (40.0) | 147 (64.2) | .001 |
| 2. Rehabilitation/facility | 49 (17.3) | 9 (16.4) | 40 (17.5) | 1.0 |
| Length of stay – days‡ | 4 (3–6) | 4 (3–8) | 4 (3–6) | .50 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range). ICU = intensive care unit; IV = intravenous; MAE = major adverse event; TIA = transient ischaemic attack; PTCA = percutaneous transluminal coronary angioplasty; AKIN = Acute Kidney Injury Network grading.
Parametric test (Student t test) was used for comparison.
The patient who died intra-operatively is not considered.
Non-parametric test (Mann–Whitney U Test) was used for comparison.
On multivariable analysis of predictive factors for in hospital death and MAEs (Table 6 ), COVID, medical treatment, and emergency setting were independently associated with the risk of in hospital death, with an OR of 4.1, 7.2, and 13.6, respectively. COVID, obesity class V, and emergency setting were independently associated with the risk of MAEs, with an OR of 3.4, 13.5, and 4.0, respectively.
Table 6.
Uni- and multivariable analysis results, showing significant predictors of in hospital death and peri-operative major adverse events in vascular surgical patients treated in the Lombardy region, Italy, from 9 March to 28 April 2020
| Outcome | Risk factor | Univariable analysis |
Multivariable analysis |
|
|---|---|---|---|---|
| p | OR (95% CI) | p | ||
| Death | ||||
| COVID | <.001 | 4.128 (1.563–10.903) | .004 | |
| Age class (SVS) | .008 | |||
| Acute ischaemia | .004 | |||
| Type of intervention | ||||
| Open | NS | |||
| Endo | .006 | |||
| Hybrid | NS | |||
| BMT | <.001 | 7.203 (1.891–27.437) | .004 | |
| Setting | ||||
| Elective | .024 | |||
| Urgent | ||||
| Emergent | <.001 | 13.573 (2.715–67.866) | .001 | |
| MAEs | ||||
| COVID | <.001 | 3.425 (1.353–8.671) | .009 | |
| Obesity class 5 SVS | .004 | 13.554 (1.136–161.777) | .039 | |
| Acute ischaemia | .001 | |||
| Referral from non-Spoke hospital | .025 | |||
| Setting | ||||
| Elective | <.001 | |||
| Urgent | NS | |||
| Emergent | <.001 | 4.050 (1.113–14.738) | .034 | |
| Need of ICU | <.001 | |||
OR = odds ratio; CI = confidence interval; COVID = patients confirmed to be affected by COVID-19 on admission or during hospitalisation; SVS = Society for Vascular Surgery; BMT = best medical therapy; MAE = major adverse event; ICU = intensive care unit; NS = non-significant.
Discussion
Rapid spread of the COVID-19 pandemic imposed a need to find a model to guarantee the treatment of positive patients in tertiary hospitals, but maintaining standard quality of treatment for other pathologies.10 , 11 How regional authorities in Lombardy decided to create the cardiovascular Hub/Spoke system that would serve the whole region for non-elective and elective cases has been reported previosly.12
The first finding from this extensive experience is that ALI was the most common vascular disease requiring hospital admission and surgical therapy, especially in COVID patients. Previously reported worldwide experiences emphasised the link between SARS-CoV-2 infection and microvascular inflammation,13 distal vasculitis, and the prothrombotic state.14 These data seemed to be related to the inflammatory cytokine storm (interleukin-6 and interleukin1-beta) contributing to the pro-coagulative and pro-adhesive state of dysfunctional endothelium.15 Moreover, abnormal coagulation parameters are usually associated with poor prognosis in COVID-19 patients:16 , 17 this may be the cause of the findings of Bellosta et al.,18 who first reported the increase in the incidence and extension of native peripheral arterial occlusion in COVID-19 patients.
The results are consistent with those previously published; in particular, it was found that COVID patients presenting with ALI had the highest peri-operative mortality, even higher than patients presenting with aortic disease in the same time frame.
ALI was not the most common vascular disease observed in other regions of Italy during COVID-19 pandemic. Recently, Pini et al. reported the results of vascular surgical activity in the Bologna metropolitan area between 8 March and 8 April 2020.19 Among 34 patients treated in the acute/emergency setting, only two were operated on for ALI (one being positive to SARS-CoV-2), a number even lower than the same period in 2019. However, in that series, COVID-19 affected only 3.9% of patients (6/152), whereas in another experience COVID-19 was present in 21% of patients (64/305). This probably reflects the different epidemiology of SARS-CoV-2 in the Emilia-Romagna region compared with Lombardy, where the outbreak was particularly violent, and the different role of the Hub hospitals, mainly dedicated to urgent/emergency surgery rather than to elective surgery.
A recent Editorial, based on a number of case reports suggesting a potential increased rate of thrombo-embolic events in COVID-19 patients, including fulminant strokes in young fit patients, suggested that routine administration of therapeutic doses of low molecular weight heparin after vascular surgery in COVID-19 patients in general and specifically for those requiring intensive care treatment should be considered.20 This is consistent with the approach chosen by physicians in the current report, who preferred the use of anticoagulants (unfractionated and low molecular weight heparin) rather than antiplatelet drugs in COVID-19 patients (Table 5).
In the exceptional conditions in which patients have been evaluated, identifying which vascular patients should be included or excluded from urgent care (Table 1) is both difficult and dangerous, but a regional consensus about indications for hospital admission and treatment was obtained. Further international discussion is needed about these criteria, and about safe protocols that may guarantee COVID free hospital access to the vascular patient.
A large number of patients who presented with vascular diseases associated with COVID-19 pulmonary and/or systemic complications were considered unfit for surgery, and medical therapy was indicated due to poor general condition (14% of the cohort). Moreover, among patients undergoing medical therapy, an in hospital mortality of 55% was recorded, the highest recorded in the subgroups of this study. This finding was confirmed on multivariable analysis, in which medical therapy was associated with a sevenfold increased risk of death.
In this view, the recent ESVS guidelines on the management of ALI,21 published in January 2020, just before the pandemic outbreak, stated that ALI often affects very frail patients and that decision making between surgical and medical approaches may be difficult. In particular, in patients suffering from associated threatening diseases (such as full blown COVID-19) thrombosis may be a terminal event, sometimes being referred to as agonal thrombosis. These considerations adapt well in the particular, dramatic situation that occurred during the COVID-19 emergency, and probably underlie the high number of patients treated conservatively, and who are dead as a consequence.
In the same guidelines, CT angiography is recommended as the first line imaging modality for patients presenting with ALI (recommendation 6, class I, level B). If in normal times this seems to be adequate, during the COVID-19 outbreak CT scanners were extremely busy screening patients for pneumonia, so probably other diagnostic modalities (e.g., Doppler ultrasound and digital subtraction angiography) should be preferred. Interestingly, it was also found that the delay from the onset of symptoms to definitive treatment was significantly higher in COVID-19 patients. A significant number of these were in ICUs with severe pneumonia, mostly intubated and sedated, and this may have contributed to late diagnosis in patients not complaining of lower limb pain.
Regarding peri-operative death and MAEs, multivariable analysis identified emergency presentation and COVID-19 disease as independent risk factors for both outcomes, with ORs ranging from 3.4 to 13.6. These findings are of paramount importance in order to understand the role of SARS-CoV-2 infection as a negative prognostic factor, not only for pulmonary expression, but also for vascular complications. Clarifying the impact of COVID on surgical patients could lead to the creation of decision making tools to allocate intensive care resources better, should the surges in COVID prevalence saturate the capacity of healthcare systems.
The number of urgent/emergency aortic cases referred to Lombardy Hub hospitals was lower than expected. Global AAA prevalence was reported to be 2 275 cases/100 000 inhabitants in 2010;22 a significant decline in incidence of ruptured AAA over the last three decades has been reported from many countries in Europe and the United States.21 The analysis of the nationwide Swedish Vascular Registry (Swedvasc) published in 2017 revealed that the incidence of ruptured AAA decreased from 9.2/100 000 in the 1994–1999 period, to 6.9/100 000 in the 2010–2014 period.23 In 2017, 1 934 patients were treated for AAA in Lombardy, 164 of them for rupture.24 Thus, assuming that in normal conditions around 14 ruptured AAAs are treated each month in Lombardy, a 60% reduction was experienced compared with the estimated number. Actually, a limitation of the Hub/Spoke model may be the management of haemorrhagic emergencies (such as ruptured aneurysms) in haemodynamically unstable patients. Most of these patients require emergency treatment at the nearest hospital (more probably a Spoke), thus making referral to another centre quite impossible. For this reason, these cases may have been kept of these statistics, partially explaining the unexpected decrease in aortic emergencies recorded in Hub centres during the pandemic. A second reason for this decrease may be the environmental situation in which patients, having to face quarantine, renounced already scheduled visits or refused to go to hospital for fear of SARS-CoV-2 infection. In this scenario, an important aspect to discuss is also that there are probably unknown patients who died at home from aneurysm rupture or other unrecognised vascular emergencies.
Likewise, the 2020 ESVS guidelines on vascular graft infection25 report a 0.16%–0.19% yearly incidence of aortic graft and 2.5% of prosthetic lower limb bypass infection. This would mean that around 25–30 patients would have been expected to present with this complication in the study period, but only four were treated in the Hubs. As this drop in graft infection rates is unlikely explained by a decreased incidence, alternative explanations are the conservative therapy that many could have received. Moreover, at the peak of the pandemic period, people with unexplained fever without overt signs of vascular graft infection could have been isolated from the community and labelled as “suspect COVID” rather than given the work up necessary to diagnose vascular graft infection.
In conclusion, vascular surgery activity during the COVID-19 pandemic in Lombardy was characterised by a high number of ALI cases and high in hospital mortality, especially in patients selected for medical treatment. COVID-19 was associated with a fourfold increased risk of mortality and a threefold increased risk of MAEs. In the immediate future, international collaborative studies (e.g., the COvid-19 Vascular sERvice [COVER] Study initiative26) will be extremely important to assess the changes in practice and the outcomes of vascular surgery during the COVID-19 pandemic, and to propose specific therapeutic protocols in these patients.
Conflict of interest
None.
Funding statement
None.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins university (JHU). ArcGIS. Johns Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 [Accessed 1 June 2020].
- 3.Regional Authority of Lombardy (Regione Lombardia). DELIBERAZIONE N° XI/2906 Seduta del 08/03/2020. Available at: https://www.regione.lombardia.it/wps/wcm/connect/5e0deec4-caca-409c-825b-25f781d8756c/DGR+2906+8+marzo+2020.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-5e0deec4-caca-409c-825b-25f781d8756c-n2.vCsc. [Accessed 28 October 2020].
- 4.Wanhainen A., Verzini F., Van Herzeele I., Allaire E., Bown M., Cohnert T. Editor's Choice – European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Behrendt C.A., Bertges D., Eldrup N., Beck A.W., Mani K., Venermo M. International Consortium of vascular Registries consensus recommendations for peripheral revascularisation registry data collection. Eur J Vasc Endovasc Surg. 2018;56:217–237. doi: 10.1016/j.ejvs.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Chaikof E.L., Fillinger M.F., Matsumura J.S., Rutherford R.B., White G.H., Blankensteijn J.D. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford R.B., Baker J.D., Ernst C., Johnston K.W., Porter J.M., Ahn S. Recommended standards for reports dealing with lower extremity ischaemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 8.Mills J.L., Sr., Conte M.S., Armstrong D.G., Pomposelli F.B., Schanzer A., Sidawy A.N. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischaemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–234. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Chaikof E.L., Blankensteijn J.D., Harris P.L., White G.H., Zarins C.K., Bernhard V.M. Ad hoc committee for standardized reporting practices in vascular surgery of the society for vascular surgery/American association for vascular surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 10.Zangrillo A., Beretta L., Silvani P., Colombo S., Scandroglio A.M., Dell'Acqua A. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020;22:91–94. doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross S.W., Lauer C.W., Miles W.S., Green J.M., Christmas A.B., May A.K. Maximising the calm before the storm: tiered surgical response plan for novel coronavirus (COVID-19) J Am Coll Surg. 2020;230:1080–1091. doi: 10.1016/j.jamcollsurg.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melissano G., Mascia D., Baccellieri D., Kahlberg A., Bertoglio L., Rinaldi E. Pattern of vascular disease in Lombardy, Italy, during the first month of the COVID-19 outbreak. J Vasc Surg. 2020;72:4–5. doi: 10.1016/j.jvs.2020.04.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 15.Nencioni A., Trzeciak S., Shapiro N.I. The microcirculation as a diagnostic and therapeutic target in sepsis. Intern Emerg Med. 2009;4:413–418. doi: 10.1007/s11739-009-0297-5. [DOI] [PubMed] [Google Scholar]
- 16.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020 doi: 10.1016/j.jvs.2020.04.483. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pini R., Faggioli G., Vacirca A., Gallitto E., Mascoli C., Attard L. Is it possible to safely maintain a regular vascular practice during the COVID-19 pandemic? Eur J Vasc Endovasc Surg. 2020;60:127–134. doi: 10.1016/j.ejvs.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eilenberg W., Busch A., Wagenhäuser M., Giannoukas A., Wanhainen A., Neumayer C. Vascular surgery in unreal times. Eur J Vasc Endovasc Surg. 2020;60:167–168. doi: 10.1016/j.ejvs.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Björck M., Earnshaw J.J., Acosta S., Bastos Gonçalves F., Cochennec F., Debus E.S. Editor's Choice – European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Sampson U.K.A., Norman P.E., Fowkes F.G.R., Aboyans V., Song Y., Harrell F.E., Jr. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart. 2014;9:159–170. doi: 10.1016/j.gheart.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Lilja F., Mani K., Wanhainen A. Editor's Choice – trend-break in abdominal aortic aneurysm repair with decreasing surgical workload. Eur J Vasc Endovasc Surg. 2017;53:811–819. doi: 10.1016/j.ejvs.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Italian National Authority for healthcare, National Agency for Regional Health Services (AGENAS), National Program for Outcomes (PNE). Available at: https://pne.agenas.it. [Accessed 28 October 2020].
- 25.Chakfé N., Diener H., Lejay A., Assadian O., Berard X., Caillon J. Editor's Choice – European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc Endovasc Surg. 2020;59:339–384. doi: 10.1016/j.ejvs.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 26.The Vascular And Endovascular Research Network Vern Executive Committee The COvid-19 vascular sERvice (COVER) study: an international vascular and endovascular research Network (VERN) collaborative study assessing the provision, practice, and outcomes of vascular surgery during the COVID-19 pandemic. Eur J Vasc Endovasc Surg. 2020;60:156–157. doi: 10.1016/j.ejvs.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]