Abstract
Spinel ferrite magnetic nanoparticles have attracted considerable attention because of their high and flexible magnetic properties and biocompatibility. In this work, a set of magnetic nanoparticles of cobalt ferrite doped with zinc was synthesized via the eco-friendly sol-gel auto-combustion method. Obtained particles displayed a room-temperature ferromagnetic behavior with tuned by chemical composition values of saturation magnetization and coercivity. The maximal values of saturation magnetization ~74 Am2/kg were found in cobalt ferrite nanoparticles with a 15–35% molar fraction of cobalt replaced by zinc ions. At the same time, the coercivity exhibited a gradually diminishing trend from ~140 to ~5 mT whereas the concentration of zinc was increased from 0 to 100%. Consequently, nanoparticles produced by the proposed method possess highly adjustable magnetic properties to satisfy the requirement of a wide range of possible applications. Further prepared nanoparticles were tested with bacterial culture to display the influence of chemical composition and magnetic structure on nanoparticles-bacterial cell interaction.
Keywords: magnetic nanoparticles, cobalt ferrite, zinc ferrite, magnetic properties, antimicrobial
1. Introduction
Magnetic spinel ferrites nanoparticles with general chemical formula MFe2O4 (where M = Co2+, Ni2+, Mn2+, Zn2+…) offer great magnetic property tunability by varying their size and chemical composition [1,2,3]. This kind of material is very attractive for biomedical applications such as magnetic resonance imaging (MRI) contrast agents and mediators of heating in magnetic fluid hyperthermia [4,5,6,7,8]. The possibility to control the movement of magnetic nanoparticles (MNPs) by applying a magnetic field gradient opens the doors for targeted drug- [9], genes- [10], or peptides-delivery, for instance, of antimicrobial or antifungal peptides [11]. The use of doped ferrites for antimicrobial or antifungal delivery for water purification is also within the scope of interest because of the possible effect on the dynamics of bacterial growth of some metals ions (silver, zinc, and copper) incorporated with MNPs and, because of the possibility to easily filter them by using a magnetic field [11,12,13]. Additionally, perspectives of the use of magnetic nanoparticles in water purification are reinforced by their effectiveness to absorb heavy metals [14,15,16].
Chemical engineering of spinel ferrites results in the modification of their structural and magnetic properties [17,18,19,20]. Magnetic spinel ferrites consist of two antiferromagnetically ordered sublattices: the tetrahedrally and octahedrally coordinated A- and B-sites. According to Néel’s model [1,21], in ferrimagnetic spinels, the net magnetic moment can be ascribed to the non-equality of magnetic moments of two sublattices (i.e., μ = μa − μb). The distribution of divalent ions in octahedral sites represents inversion degree γ. Magnetic properties of spinel ferrite nanoparticles (i.e., saturation magnetization MS and coercivity force HC), are strongly correlated with both chemical composition and value of inversion degree γ.
Recently, quite high MS values have been observed in Zn-doped spinel ferrites (i.e., ZnxFexFe2O4 [4,22,23,24], ZnxCo1 − xFe2O4 [5,25,26,27], ZnxNi1 − xFe2O4 [28], ZnxMn1 − xFe2O4 [4,26] MNPs where MS increase while x (i.e., Zinc content) is less than ~0.5. For example, the Cheon team observed an extremely high MS value of 161 and 175 Am2/kg for Zn0.4Fe2.6O4 and Zn0.4Mn0.6Fe2O4 respectively [4]. For Zn2+xFe2+(1 − x)Fe3+2O4 ferrites an increase of magnetic moment from 4.0 μB/f.u. (Bohr magnetons per formula units) to 8.3 μB/f.u., according to the (4 + 6x) μB/f.u. rule, was confirmed through density functional numerical calculations [24]. This rule was valid for Zn concentration 0 < x < 0.75 where magnetization reached maximal value and start to decrease. That was explained by the replacement of Fe3+ with magnetic moment 5 μB/f.u. in A-site by non-magnetic Zn2+ cations. The Co2+ in the structure of spinel ferrite makes material magnetically harder keeping the relatively high value of saturation magnetization [29,30,31]. Mameli and colleagues found that the MS at 5 K reaches a value of 157 Am2/kg for Zn0.46Co0.54Fe2.02O4 synthesized by the high-temperature decomposition method [24]. Barrera et al. synthesized a set of ZnxCo1 − xFe2O4 MNPs with x in the range of 0.08−0.56 using a sol-gel auto-combustion method (SGAC) [27]. In their work, authors observed that starting from inverse spinel at x = 0.08 future increase of zinc content to x ~ 0.4 leads to the formation of mixed ferrite where Zn2+ forces Co2+ migrate to A-site and Fe3+ to B-site. It was highlighted that non-equilibrium cation distribution is strongly related to the method of synthesis and, together with spin canting, leads to a non-monotonous change of saturation magnetization.
Recently several methods were employed in order to obtain Zn and Co co-doped ferrites MNPs such as high-temperature decomposition [5,25], SGAC [27], polyol [32,33], co-precipitation [26,34]. Among them, the SGAC has several advantages: (i) low-cost and eco-friendly reagents; (ii) fast time reaction; (iii) high crystallinity of the particles. The SGAC method utilizing citric acid as a raw material can be considered a green synthesis method [35]. Keeping relatively good magnetic performance of the samples this method indeed does not consume hazardous organic solvents and does not require the use of any specific washing procedure with, for example, acetone as in high-temperature decomposition or polyol methods. Moreover, due to the very high yield of the reaction, no waste excepting nitrogen oxides is present at the end of the process. The applicability in magnetorheological and multiferroic composites of MNPs obtained with this method was discovered [36]. Recently we obtained a set of cobalt ferrite MNPs doped with nickel x = 0, 0.25, 0.5, 0.75 and 1 with gradually changed coercivity and non-monotonically changing saturation magnetization [37].
In this work, we used SGAC for the synthesis of cobalt-zinc ferrites MNPs with x in the range 0.15–0.75 to study the evolution of MS and HC. The structural and magnetic properties were studied and compared with previously reported work of Ni-doped cobalt ferrite MNPs [37]. Further, we give new insights about the influence of obtained MNPs on bacterial growth in correlation with their magnetic structure. Although the importance of the magnetic structure of spinel MNPs is well investigated in catalysis [38,39], there is a lack of information about its influence on biological activity. Thus, all samples have been investigated using wild-type bacterial Escherichia coli (E.coli) K-12 MG1655 [40,41].
2. Materials and Methods
2.1. Nanoparticles Synthesis
Samples of MNPs were prepared by the SGAC method described in detail elsewhere [37,42]. The metal salts Fe(NO3)3·9H2O (Carlo Erba Reagenti SpA, Cornaredo, Italy), Co(NO3)2·6H2O (Scharlab S.L., Barcelona, Spain) and Zn(NO3)2·6H2O (Merck KGaA, Darmstadt, Germany) were used without future purification. The 1-molar aqueous solutions of metal salts in distilled water (DW) were prepared with different a molar ratio (i.e., ZnxCo1-xFe2O4 with x = 0, 0.15, 0.25, 0,35, 0.5, 0.75 and 1). Then, the preliminary prepared 1-molar solution of the same volume of the citric acid (Scharlab S.L., Barcelona, Spain) in DW was added to mixtures of metal salts under magnetic stirring. The pH-level was adjusted to the value of 7 by dropwise adding of 30% ammonia solution (Carlo Erba Reagenti SpA, Cornaredo, Italy). The obtained sol was dried for about 60 min at 150 °C to form a gel; then the temperature was increased up to 300 °C to induce the self-combustion reaction. Obtained powders were collected and grind with an agate mortar (C. Giese Achat-Laborbedarfs, Idar-Oberstein, Germany).
2.2. Structural and Magnetic Properties
The X-ray diffraction (XRD) studies were performed with a PW 1830 powder diffractometer (Philips, Eindhoven, The Netherlands) using Co Kα source (λ = 1.78919 Å) in the 2θ geometry in the range of 30–80 degrees. The morphology of the samples was investigated with a JEM-2100 transmission electron microscope (TEM, JEOL, Tokyo, Japan) operating at 30 kV. The magnetic properties were measured with a vibrating sample magnetometer (7400 System, Lake Shore Cryotronics Inc., Weterville, OH, USA) in the field range up to 1.0 T at room temperature (~300 K). The powder samples were fixed with diamagnetic glue in plastic holders to prevent any movement of the powder during the measurements.
2.3. Antimicrobial Activity
All experiments were done using E.coli K-12 MG1655 bacterial cells (taken from laboratory collection of IKBFU, Kaliningrad, Russia). First, antimicrobial activity was tested with the disk diffusion method in order to qualitatively define the parameters of the experiment (see Figure S1 and description below). Then quantitative analysis was performed by the optical density (OD600) measurement method on a UV/vis spectrophotometer (SmartSpec Plus, Bio-Rad, Hercules, CA, USA) detecting absorbance at 600 nm wavelength every half hour. Bacterial cells were grown aerobically at 37 °C under constant shaking (~120 rpm) in 5 mL of liquid (without adding of agar) hand-made Lysogeny Broth (LB) medium, containing 1% tryptone, 0.5% yeast extract and 1% sodium chloride. In all experimental samples was added 0.5 mg of nanoparticles. To assess the changes in the optical properties of the LB medium after the addition of nanoparticles, its optical density was detected at a wavelength of 600 nm. It was found that the increase of optical density occurred no more than 10% compared with the control. Obtained OD600 data values were analyzed using a two-way analysis of variance (ANOVA, GraphPad Prism 7.04 software, Graph Pad Software Inc., San Diego, CA, USA). All OD600 data discussed in the Biology section below are statistically significant (p-value meanings were ranked by asterisks **** (p ≤ 0.0001), *** (p ≤ 0.001), ** (p ≤ 0.01).
3. Results and Discussion
3.1. Structural and Morphological Properties
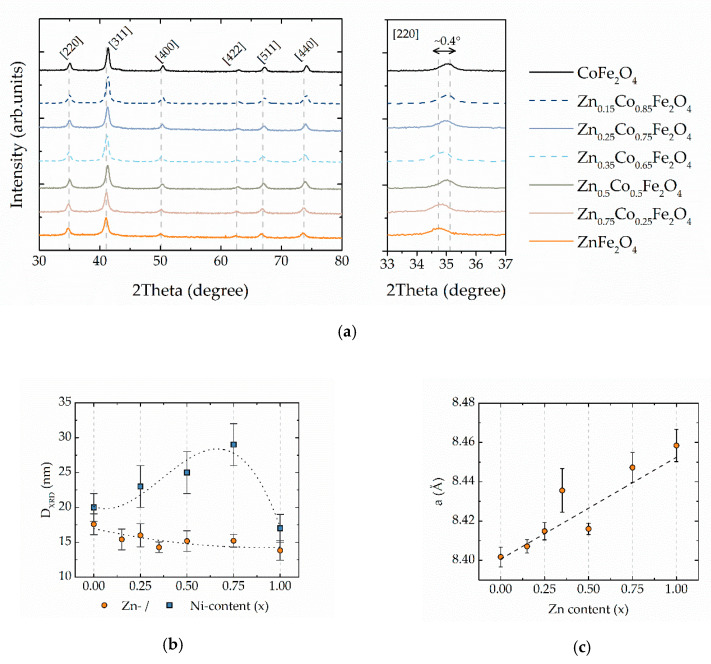
The spinel ferrite structure was confirmed by XRD patterns (Figure 1a) showing for all the samples the presence of spinel cubic structure (card № 00-154-0973 for cobalt ferrite or card № 00-154-0936 for zinc ferrite). Any other phases have been not detected, in contrast to nickel-doped cobalt ferrite MNPs prepared with the same SGAC method where at a high nickel content (x = 0.75 and 1) a small amount of metallic nickel and hematite (α-Fe2O3) has been observed [37]. The size of crystallites (DXRD) was calculated by using the Scherrer formula [43]:
| DXRD = K λ/B cos θ, | (1) |
where K is a crystallite-shape factor (0.94 for spherical particles), B is the full width at the half maximum estimated after fitting of peaks with the Voigt function and θ is the position of corresponding peaks. The DXRD value is slowly decreased over the concentration of zinc (Figure 1b, Table 1). A similar decrease was observed in ref. [27] in zinc-cobalt ferrite MNPs in the range of x = 0.08−0.56. It is worth to underline, that in the case of cobalt-nickel ferrite MNPs obtained with the same method, the smaller size of crystallites was in pure cobalt and nickel ferrite but remarkably higher for mixed Ni-Co ferrite nanoparticles [37,44]. Similarly, the position of main reflections is continuously increasing with the increase of zinc content despite the similar ionic radii of Zn2+ (~0.82 Å [45]) and Co2+ (~0.82 Å [45]) ions: this can be ascribed to the migration of the smaller Fe3+ (~0.67 Å [45]) ions to octahedral sites since zinc ions prefer to occupy the tetrahedral position [25]. The fact that bigger Zn2+ occupies the smaller tetrahedral sites is confirmed by the expansion of the lattice parameter a (Figure 1c). The lattice parameter of cubic structure (a) was calculated by the equation , where dhkl is the interplanar spacing of a plane with Miller indices h, k and l. The linear dependence of a versus x agrees with Vegard’s law predicting that the lattice parameter of a chemically homogenous mixture will be the approximately weighted mean of two constituents [46].
Figure 1.
(a) diffraction patterns for all samples, in the left panel is a zoomed region for [220] reflection showing the shift of peak position; the Miller indexes for corresponding peaks are reported; (b) evolution of grain size (DXRD) calculated with Scherrer formula through zinc content compared with the values obtained for Ni-Co ferrite MNPs in ref. [37]. Error bars are the standard deviation of DXRD calculated for 5 most intensive peaks; (c) lattice parameter (a) as a function of zinc content.
Table 1.
The crystallite size (DXRD), room temperature saturation magnetization (MS), coercivity field (μ0HC), reduced remanent magnetization (MR/MS) and effective magnetic anisotropy (Keff).
| Sample Composition | DXRD, nm | MS,** Am2/kg | MR/MS, a.u. | μ0HC, mT | Keff, ×104 J/m3 |
|---|---|---|---|---|---|
| CoFe2O4 | 18(2) * | 69(2) | 0.42(2) | 140(4) | 14(2) |
| Zn0.15Co0.85Fe2O4 | 15(2) | 71(2) | 0.35(1) | 76(3) | 12(2) |
| Zn0.25Co0.75Fe2O4 | 16(2) | 74(2) | 0.34(1) | 54(2) | 9.5(4) |
| Zn0.35Co0.65Fe2O4 | 14(1) | 65(2) | 0.27(1) | 37(2) | 7.5(3) |
| Zn0.50Co0.50Fe2O4 | 15(2) | 52(2) | 0.22(1) | 21(1) | 3.3(1) |
| Zn0.75Co0.25Fe2O4 | 15(1) | 32(1) | 0.09(1) | 5.5(2) | 0.72(4) |
| ZnFe2O4 | 14(1) | 10(1) | 0.08(1) | 4.8(2) | 0.064(4) |
* In parenthesis the systematical error in the last digit is presented; ** MS values are after approximation with LAS, the error is fittings error.
The TEM-images indicate the presence of non-regular shape of particles with relatively broad distribution (i.e., in the range 10–30 nm) and shape edges confirming the crystalline nature of particles. Examples of typical TEM-images for CoFe2O4 are presented in the SI (Figure S2).
3.2. Chemical Control. of Magnetic Properties
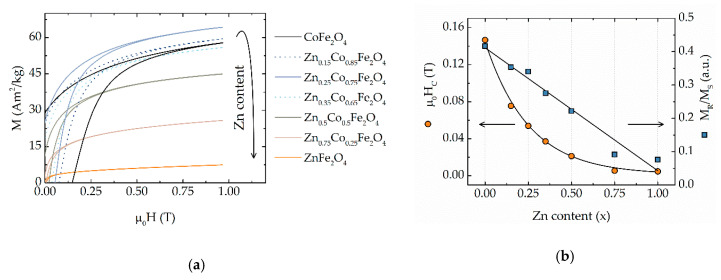
Preliminary magnetic characterization has been performed for all the samples at 300 K. All investigated samples exhibited hysteretic behavior (Figure 2a) with the values of saturation magnetization (MS), coercivity field (HC) and reduced remanent magnetization (MR/MS) reported in Table 1. Since almost all M-H curves were not saturated, the Law of Approach to Saturation (LAS) was used to estimate MS values [47,48]:
| M = MS·(1 − a/H − b/H2), | (2) |
where a and b are free parameters of the fitting. MS first increases with the increase of Zn-content, up to x = 0.25. After this value, MS starts to decrease in good agreement with literature data and may be explained by the cation distribution [5,25,26,27]. The maximal value of MS of ~74 Am2/kg was for x = 0.25, which is higher than reported for samples prepared with a similar method, probably because we used a higher reaction temperature, leading to more crystalline particles. For Ni-doped ferrite, we observed maximal value MS of ~69 Am2/kg for Ni0.25Co0.75Fe2O4 [37]. The coercivity field (HC) and reduced remanence (MR/MS) are decreasing with the increase of zinc concentration (Figure 2b).
Figure 2.
(a) The first quarter of M-H hysteresis cycles recorded at 300 K of CoFe2O4 nanoparticles doped with Zn. The arrow indicates the trend of MS; (b) Dependence of coercivity HC and reduced remanence MR/MS versus Zn-content.
MS values calculated with Equation (3) were converted into the magnetic moment per formula unit in Bohr magnetons to better understand processes associated with filling sublattices in the structure of ferrites. The following equation was used to do that converting [27,45]:
| nB = MS·Mw/NA·μB, | (3) |
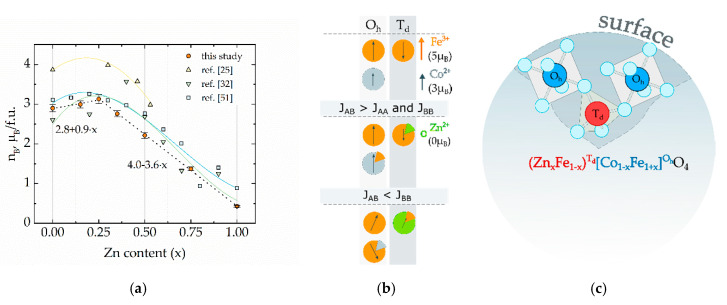
where Mw is the molecular weight, NA is Avogadro constant (6.022 × 1023 mol−1) and μB is Bohr magneton (9.274 × 10−24 J/T). Figure 3a shows the values of MS in μB units extracted from M-H curves recorded at 300 K. In the region of 0 < x < 0.25 increase of magnetization is due to a partial migration of Fe3+ cations to B-sites. In fact, for low zinc concentration, Zn2+ push Fe3+ from A to B sites (Figure 3b). If exchange interactions between two different sites (exchange integral JAB) are stronger than intra-lattice interactions (JAA and JBB), the net magnetization may be expressed as (3 + 7x) × μB, because one uncompensated magnetic moment of Co2+ (3μB) is replaced by two uncompensated Fe3+ (5 μB) in the octahedral position [4,49,50]. With an increase of concentration of zinc, A-B interaction is weakening. In an extremal case x = 1, JAB and JAA are equal to 0 because the tetrahedral positions are filled with non-magnetic Zn2+ ions and B-B interaction leads to the establishment of antiferromagnetic ordering among magnetic moment in the octahedrally coordinated lattice. On basis of this simple model, a general rule of zinc dependence of magnetization can be ascribed as m = (μI + δμ`x) × μB, where δ is delta equal to 1 if JAB >> JAA + JBB and equal to −1 if JAB << JAA + JBB, μI is the initial magnetic moment of undoped ferrite and μ` is a concentration-depended magnetic moment resulting due to recombination of cations between lattices and disruption of ideal ferrimagnetic ordering.
Figure 3.
(a) Dependence of net magnetic moment per formula unit for cobalt ferrite nanoparticles doped with zinc from this research compared with reference systems [25,32,51]; (b) diagram of the evolution of the magnetic structure of cobalt ferrite when is doped with zinc; (c) illustration of spinel structure of co-doped Zn-Co ferrite: in nanoferrites the octahedral (Oh) positions of spinel preferably allocated on the surface rather than tetrahedral (Td) positions.
The linear fitting of region 0 < x < 0.25 gives the value (2.8 + 0.9x) × μB. The value of magnetization per formula unit in the extremal case x = 0, pure cobalt ferrite, is very close to the theoretical value of 3 μB (only spin contribution to the magnetic moment it is taken into account) for a totally inverted spinel (γ = 1). In our case coefficient before x is much lower than estimated which indicates the Zn2+ occupy only partially tetrahedral sites while some amount of Zn2+ can occupy octahedral positions. This value is also affected due to antiferromagnetic order between magnetic cations in B sites since the B-B super-exchange interactions become more significant because of the breaking of the dominant A-B interactions. Spin canting arises when in B-sites concentration of non-magnetic ions becomes too high and can be explained in the frame of the Yafet-Kittel model [27,32,34]. Gómez-Polo and colleagues observed a divergence of the calculated value of magnetic moment by measured value of inversion degree by neutron diffraction which was enhanced when the concentration of zinc increased [34]. In our case trend becomes more pronounced in the region of 0.25 < x < 1 because of the change of the slope in the linear fitting of this region (4 − 3.6 x) × μB. This non-monotonical trend of magnetization suggests that the filling of the spinel positions is also non-monotonical. The observed trend well agrees with literature data (adopted values from the literature of room temperature MS are presented in Figure 3a). Mameli et al. observed maximal value MS for ~7-nm particles prepared with thermal decomposition method for concentration of zinc of 0.46 at 5K, however at room temperature, the maxim of MS-x dependence shifted to the lower x values [25]. Ben Tahar et al. observed a maximum for x = 0.4 in ~5-nm particles prepared with the polyol method [32]. Andersen et al. found the maximum value of MS at x = 0.2 for ~14-nm particles prepared with the hydrothermal method [51]. A small difference in the absolute value of magnetic moment and position of maximum related to the fact, that particles were prepared with different methods and have different sizes and cation distribution.
The surface of spinel ferrites is preferentially formed by ions located in the octahedral sites. (Figure 3c). Thus, the chemical, catalytical and biological activity of mixed ferrites will mainly depend on structural properties (level of inversion degree) rather than on their chemical composition [38,39]. While the importance of the magnetic microstructure on the catalytical properties of nanoferrites is well known, their influence on biological activity is still relatively not well understood.
The introduction of zinc in ferrite structure significantly decreases Curie (or Néel) temperature of ferrites MNPs which leads to the decrease of magnetization at room temperature. Previously it was demonstrated that the Curie temperature of cobalt ferrite MNPs was decreasing from 713 to 453 K when the concentration of zinc changed from x = 0 to 0.5 [52]. That makes this material potentially useful in, for example, the self-controlled hyperthermia [26,53] or any other applications where MNPs with high saturation magnetization and tunable Curie temperature are requested [54,55].
In contrast to this non-monotonically behavior of MS, the HC trends to decreasing continuously as presented in Figure 2b as expected for the reduction of Co2+. It is interesting to underline the difference in comparison with a similar system of cobalt ferrite MNPs doped with nickel [37]. In the case of zinc, the HC decreases much faster showing the downward trend, while for nickel-cobalt ferrite MNPs, the upward trend was observed.
To better understand the role of magnetic anisotropy the constant of effective magnetic anisotropy (Keff) was calculated following [56]:
| Keff = MS·μ0HA/2, | (4) |
where μ0HA is anisotropy field can be estimated by the closure field of hysteresis (Hirr). This assumption was first suggested by Kodama et al. for NiFe2O4 MNPs [57] and it has been used for other similar nanoparticle systems [58,59]. Equation (4) is valid for non-interacting MNPs with uniaxial anisotropy. Despite, the magnetocrystalline anisotropy of cobalt ferrite is cubic type, in the case of nanoparticle system the effective anisotropy frequently turns on uniaxial type because of the contribution of such factors of shape, surface, and interparticle interactions [47]. Keff calculated using equation 4 was maximal for CoFe2O4 14 × 104 J/m3 (the bulk 30 × 104 J/m3 [60]), and fast drop until reaching a value in three-orders of magnitude lower for ZnFe2O4. The obtained MR/MS and Keff values are lower than expected probably due to the influence of temperature and interparticle interactions leading to the magnetization relaxation for particles with the lower anisotropy. Also, it is interesting to note a divergence in the behavior of coercivity and closure field, the Hirr/HC increased in two times for Zn-doped cobalt ferrites comparing with the pure cobalt ferrite MNPs. That confirms the crucial role of magnetocrystalline (core) anisotropy in cobalt ferrite samples and arising a stronger surface anisotropy in magnetically softer mixed Zn/Co ferrites MNPs.
3.3. Biology
Since the nanoparticles are iron-based, their presence should affect the bacteria iron metabolism system. Fe-containing MNPs may affect bacterial cells by reactive oxygen species production and induction of membrane disruption [61,62]. Thus, the data on the different effects of nanoparticles on wild-type cells and bacteria transformed with the plasmid can indirectly indicate the possibility of their penetration into the cell. The effect of iron ions on cell growth is a complicated parameter since iron is participating in many important biochemical processes, even if its overdose can cause a strong cytotoxic effect. To protect genomes from damage, E.coli uses the DNA-binding protein of starved cells (Dps), which combines ferroxidase activity and the ability to bind DNA. Both activities depend on the integrity of this multi-subunit protein, which has an inner cavity for iron oxides. It was shown by X-ray absorption near edge structure (XANES) and Mössbauer spectroscopy presence of both trivalent and divalent iron ions in the Dps protein [40,41]. Thus, if iron-based MNPs penetrate the cell, then they should affect the metabolic processes involving Dps.
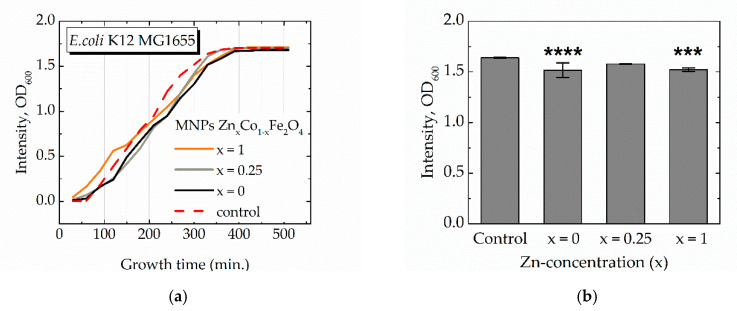
Measured values of the OD600 signal are proportional to the cell’s population after the incubation time [63,64]. In the presence of CoFe2O4 and ZnFe2O4 MNPs, E.coli demonstrate a decreased OD600 of the bacterial medium compared with the control (non-treated) samples, resulting in the lag of bacterial growth inhibition in different timepoints (Figure 4a). The values of OD600 at 330 min, the time selected as moments when bacterial strains reached sub-maximum population, demonstrate that pure CoFe2O4 and ZnFe2O4 MNPs have a more pronounced lag effect than mixed Zn-Co ferrite MNPs with the concentration of zinc x = 0.25 (Figure 4b). In Figure 4, results for selected samples only are presented, more data confirming the same trend are in Figure S3. Cells growth of E.coli K-12 MG1655 was inhibited after MNPs (x = 1) from 240 to 330 min (p-value ≤ 0.001) and MNPs (x = 0) from 120 to 360 min (p-value ≤ 0.01). Therefore, it can be concluded that in the case of different Zn concentrations, a slowdown in the increase in OD is observed in the period from 200 to 400 min. Results cannot be attributed to the inhibition of bacterial growth that occurs during toxic exposure, since the achievement of the stationary growth phase occurs simultaneously for control cells and cells grown in the presence of nanoparticles. This bimodality can be explained by the triggering of special compensatory or protective mechanisms in bacterial cells. Such mechanisms can be implemented by post-translational modification [65], special chaperone proteins [66], or the triggering of special proteins of stresses [67].
Figure 4.
(a) Optical density (OD600) of E.coli K-12 MG1655 in LB medium as a function of cultivation time in presence of MNPs; (b) saturated value of OD600 after 330 min of cultivation (more data are in Figure S3).
The observed structural properties of the materials correlate with the non-monotonical effect of MNPs on bacterial growth. The toxicity of both Zn2+ and Co2+ ions, when they are incorporated in spinel structure, expected to be different, because of the occupation of different positions in spinel structure: octahedral positions are mostly located on the surface of particles and thus should have a stronger effect on biological and chemical properties. Nevertheless, zinc ferrite with Zn2+ mainly in tetrahedral positions and cobalt ferrite with Co2+ mainly in octahedral positions in our experiments showed similar behavior when bacteria were reached sub-maximum population. While the MNPs with the intermediate concentrations x = 0.25 do not exhibit a significant effect. This fact agrees with the non-monotonical evolution of the magnetic structure. Namely, at Zn concentration of around x = 0.25, Fe3+ ions partially migrate to octahedral positions lowering the toxic effect of Co2+ ions at the same positions. However, at high zinc concentration, Zn2+ ions start also occupy octahedral positions due to the non-thermodynamically equilibrium state of nanoparticles involving an increase of toxicity.
4. Conclusions
Zn-Co ferrite MNPs synthesized by the green synthesis method revealed tunable magnetic properties, which can be adjusted via their chemical composition. The increase of zinc content reduces magnetocrystalline anisotropy and has a non-monotonical effect on the saturation magnetization value with a maximal of ~74 Am2/kg for x = 0.25 zinc-concentration. This can be considered in a strong correlation with change of the magnetic structure of spinel structure: at small Zn-concentration (<25%), Zn2+ ions can occupy the tetrahedral sites pushing the Fe3+ with a high magnetic moment into the octahedral site. At a higher concentration of zinc, the ferrimagnetic order between two lattices is disturbed that leads to dropping in saturation magnetization and magnetic anisotropy. Here we should stress that magnetic properties correlate with microstructural and magnetic structural properties (first of all, cation distribution) which in their turn are in strong dependence on the synthesis method (in general, with the kinetic of reaction). Zn-Co ferrite MNPs induced the lag in E.coli growth, which was found in correlation with the magnetic and structural properties of spinel ferrites structure.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/21/5014/s1, Figure S1. Antimicrobial susceptibility testing of E. coli, with experimental composition time 24h, using 6 mm filter-paper disks (a) containing Zn25Co75Fe2O4 MNPs and combination of different types of treatment (b). I-V - disks containing 16 mg/mL, 8 mg/mL, 4 mg/mL, 2 mg/mL, 1 mg/mL MNPs solution accordingly (5 μL of solution per disc), ab – disks containing 5 μL of antibiotic Kanamycin solution (50 μg/mL, used as positive control), E – empty segment, C - disks saturated with 5 μL distilled water, 1 – empty well used as a control, 2 – drop of 5 μL 16 mg/mL MNPs solution, 3 – well filled with 16 mg/mL MNPs solution, 4 – disk submerged in 16 mg/mL MNPs solution and placed on Lysogeny broth agar surface, 5 - 5 μL of 1 mg/mL MNPs solution covered by empty disk, 6 – disk saturated with 5 μL of 1 mg/mL MNPs. Red arrows show zones of inhibition surrounding disks containing antibiotics. Figure S2. Bright-field TEM image of CoFe2O4 nanoparticles prepared with sol-gel auto-combustion method: (a) low magnification and (b) higher magnification of the different areas of the same sample. Figure S3. (a) Optical density (OD600) of E.coli K-12 MG1655 in LB medium as a function of cultivation time in presence of MNPs; (b) saturated value of OD600 after 330 min of cultivation.
Author Contributions
Conceptualization, A.O., K.L., V.R. and D.P.; methodology, K.L., V.R., D.P. and S.A.; validation, K.L., V.R., D.P. and S.A.; formal analysis, K.L., and D.P.; investigation, A.O., S.P., M.A., A.G., M.B., S.S., L.A., A.K. and B.F.; resources, B.F., K.L., V.R., D.P. and S.A.; data curation, A.O. and S.P.; writing—original draft preparation, A.O., S.A. and S.P.; writing—review and editing, K.L., V.R. and D.P.; visualization, A.O.; supervision, K.L., V.R., D.P. and S.A.; project administration, V.R. and D.P.; funding acquisition, V.R. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 5 top 100 Russian Academic Excellence Project at the Immanuel Kant Baltic Federal University. Partial support from The Russian Foundation for Basic Research (RFBR) is acknowledged according to the research project №18-32-20219.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Da Silva F.G., Depeyrot J., Campos A.F.C., Aquino R., Fiorani D., Peddis D. Structural and Magnetic Properties of Spinel Ferrite Nanoparticles. J. Nanosci. Nanotechnol. 2019;19:4888–4902. doi: 10.1166/jnn.2019.16877. [DOI] [PubMed] [Google Scholar]
- 2.Lee J.-H., Huh Y.-M., Jun Y., Seo J., Jang J., Song H.-T., Kim S., Cho E.-J., Yoon H.-G., Suh J.-S., et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 3.Socoliuc V., Peddis D., Petrenko V.I., Avdeev M.V., Susan-resiga D., Szabó T., Turcu R., Tombácz E., Vékás L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magetochemistry. 2019;6:2. doi: 10.3390/magnetochemistry6010002. [DOI] [Google Scholar]
- 4.Jang J., Nah H., Lee J.-H., Moon S.H., Kim M.G., Cheon J. Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant-Controlled Magnetic Nanoparticles. Angew. Chemie Int. Ed. 2009;48:1234–1238. doi: 10.1002/anie.200805149. [DOI] [PubMed] [Google Scholar]
- 5.Albino M., Fantechi E., Innocenti C., López-Ortega A., Bonanni V., Campo G., Pineider F., Gurioli M., Arosio P., Orlando T., et al. Role of Zn 2+ Substitution on the Magnetic, Hyperthermic, and Relaxometric Properties of Cobalt Ferrite Nanoparticles. J. Phys. Chem. C. 2019;123:6148–6157. doi: 10.1021/acs.jpcc.8b10998. [DOI] [Google Scholar]
- 6.Kozenkova E., Levada K., Efremova M.V., Omelyanchik A., Nalench Y.A., Garanina A.S., Pshenichnikov S., Zhukov D.G., Lunov O., Lunova M., et al. Multifunctional Fe3O4-Au Nanoparticles for the MRI Diagnosis and Potential Treatment of Liver Cancer. Nanomaterials. 2020;10:1646. doi: 10.3390/nano10091646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvador M., Moyano A., Martínez-García J.C., Blanco-López M.C., Rivas M. Synthesis of Superparamagnetic Iron Oxide Nanoparticles: SWOT Analysis Towards Their Conjugation to Biomolecules for Molecular Recognition Applications. J. Nanosci. Nanotechnol. 2019;19:4839–4856. doi: 10.1166/jnn.2019.16931. [DOI] [PubMed] [Google Scholar]
- 8.Levada K., Omelyanchik A., Rodionova V., Weiskirchen R., Bartneck M. Magnetic-Assisted Treatment of Liver Fibrosis. Cells. 2019;8:1279. doi: 10.3390/cells8101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W., Cheng C.A., Zink J.I. Spatial, Temporal, and Dose Control of Drug Delivery using Noninvasive Magnetic Stimulation. ACS Nano. 2019;13:1292–1308. doi: 10.1021/acsnano.8b06655. [DOI] [PubMed] [Google Scholar]
- 10.Mulens V., Morales M., del P., Barber D.F. Development of Magnetic Nanoparticles for Cancer Gene Therapy: A Comprehensive Review. ISRN Nanomater. 2013;2013:1–14. doi: 10.1155/2013/646284. [DOI] [Google Scholar]
- 11.Franco O., Lopez-Abarrategui C., Figueroa-Espi V., Lugo-Alvarez M., Pereira C., Garay H., Barbosa J., Jimenez-Hernandez L., Estevez-Hernandez O., Reguera-Ruiz E., et al. The intrinsic antimicrobial activity of citric acid-coated manganese ferrite nanoparticles is enhanced after conjugation with the antifungal peptide Cm-p5. Int. J. Nanomed. 2016;11:3849–3857. doi: 10.2147/IJN.S107561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanpo N., Wen C., Berndt C.C., Wang J. Antibacterial properties of spinel ferrite nanoparticles. In: Méndez-Vilas A., editor. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. Formatex Research Center; Badajoz, Spain: 2013. pp. 239–250. [Google Scholar]
- 13.Hola K., Markova Z., Zoppellaro G., Tucek J., Zboril R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015;33:1162–1176. doi: 10.1016/j.biotechadv.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Jovanović S., Kumrić K., Bajuk-Bogdanović D., Jaňcar B., Spreitzer M., Trtićc-Petrović T., Suvorov D. Cobalt Ferrite Nanospheres as a Potential Magnetic Adsorbent for Chromium(VI) Ions. J. Nanosci. Nanotechnol. 2019;19:5027–5034. doi: 10.1166/jnn.2019.16803. [DOI] [PubMed] [Google Scholar]
- 15.Campos A.F.C., de Oliveira H.A.L., da Silva F.N., da Silva F.G., Coppola P., Aquino R., Mezzi A., Depeyrot J. Core-Shell Bimagnetic Nanoadsorbents for Hexavalent Chromium Removal from Aqueous Solutions. J. Hazard. Mater. 2019;362:82–91. doi: 10.1016/j.jhazmat.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Baragaño D., Forján R., Welte L., Gallego J.L.R. Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-58852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moustafa A.M., Salah L.M., Salerno M., Abdellatif M.H. Symmetry in magnetic and vibrational spectra of multi-element spinel ferrite. J. Magn. Magn. Mater. 2020;513:167267. doi: 10.1016/j.jmmm.2020.167267. [DOI] [Google Scholar]
- 18.Abdellatif M.H., El-Komy G., Azab A.A., Moustafa A.M., Salerno M. Crystal field deformation by Ce3+ doping in spinel Mn-Cr ferrite. J. Magn. Magn. Mater. 2020;502:166517. doi: 10.1016/j.jmmm.2020.166517. [DOI] [Google Scholar]
- 19.Abdellatif M.H., El-Komy G.M., Azab A.A., Salerno M. Crystal field distortion of La3+ ion-doped Mn-Cr ferrite. J. Magn. Magn. Mater. 2018;447:15–20. doi: 10.1016/j.jmmm.2017.09.040. [DOI] [Google Scholar]
- 20.Abdellatif M.H., Azab A.A., Salerno M. Effect of rare earth doping on the vibrational spectra of spinel Mn-Cr ferrite. Mater. Res. Bull. 2018;97:260–264. doi: 10.1016/j.materresbull.2017.09.012. [DOI] [Google Scholar]
- 21.Cullity B.D., Graham C.D. Introduction to magnetic materials. Mater. Today. 2009;12:45. [Google Scholar]
- 22.Liu X., Liu J., Zhang S., Nan Z., Shi Q. Structural, Magnetic, and Thermodynamic Evolutions of Zn-Doped Fe3O4 Nanoparticles Synthesized Using a One-Step Solvothermal Method. J. Phys. Chem. C. 2016;120:1328–1341. doi: 10.1021/acs.jpcc.5b10618. [DOI] [Google Scholar]
- 23.Petrova E., Kotsikau D., Pankov V. Structural characterization and magnetic properties of sol-gel derived ZnxFe3-xO4 nanoparticles. J. Magn. Magn. Mater. 2015;378:429–435. doi: 10.1016/j.jmmm.2014.11.076. [DOI] [Google Scholar]
- 24.Cheng Y.H., Li L.Y., Wang W.H., Liu H., Ren S.W., Cui X.Y., Zheng R.K. Tunable electrical and magnetic properties of half-metallic ZnxFe3−xO4 from first principles. Phys. Chem. Chem. Phys. 2011;13:21243. doi: 10.1039/c1cp22463h. [DOI] [PubMed] [Google Scholar]
- 25.Mameli V., Musinu A., Ardu A., Ennas G., Peddis D., Niznansky D., Sangregorio C., Innocenti C., Thanh N.T.K., Cannas C. Studying the effect of Zn-substitution on the magnetic and hyperthermic properties of cobalt ferrite nanoparticles. Nanoscale. 2016;8:10124–10137. doi: 10.1039/C6NR01303A. [DOI] [PubMed] [Google Scholar]
- 26.Pilati V., Cabreira Gomes R., Gomide G., Coppola P., Silva F.G., Paula F.L.O., Perzynski R., Goya G.F., Aquino R., Depeyrot J. Core/Shell Nanoparticles of Non-Stoichiometric Zn-Mn and Zn-Co Ferrites as Thermosensitive Heat Sources for Magnetic Fluid Hyperthermia. J. Phys. Chem. C. 2018;122:3028–3038. doi: 10.1021/acs.jpcc.7b11014. [DOI] [Google Scholar]
- 27.Barrera G., Coisson M., Celegato F., Raghuvanshi S., Mazaleyrat F., Kane S.N.N., Tiberto P. Cation distribution effect on static and dynamic magnetic properties of Co1-xZnxFe2O4 ferrite powders. J. Magn. Magn. Mater. 2018;456:372–380. doi: 10.1016/j.jmmm.2018.02.072. [DOI] [Google Scholar]
- 28.Tovstolytkin A.I., Kulyk M.M., Kalita V.M., Ryabchenko S.M., Zamorskyi V.O., Fedorchuk O.P., Solopan S.O., Belous A.G. Nickel-zinc spinel nanoferrites: Magnetic characterization and prospects of the use in self-controlled magnetic hyperthermia. J. Magn. Magn. Mater. 2019;473:422–427. doi: 10.1016/j.jmmm.2018.10.075. [DOI] [Google Scholar]
- 29.Viñas S.L., Simeonidis K., Li Z.-A., Ma Z., Myrovali E., Makridis A., Sakellari D., Angelakeris M., Wiedwald U., Spasova M., et al. Tuning the magnetism of ferrite nanoparticles. J. Magn. Magn. Mater. 2016;415:20–23. doi: 10.1016/j.jmmm.2016.02.098. [DOI] [Google Scholar]
- 30.López-Ortega A., Lottini E., Fernández C.D.J., Sangregorio C. Exploring the Magnetic Properties of Cobalt-Ferrite Nanoparticles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015;27:4048–4056. doi: 10.1021/acs.chemmater.5b01034. [DOI] [Google Scholar]
- 31.Jovanović S., Spreitzer M., Otoničar M., Jeon J.-H., Suvorov D. pH control of magnetic properties in precipitation-hydrothermal-derived CoFe2O4. J. Alloy. Compd. 2014;589:271–277. doi: 10.1016/j.jallcom.2013.11.217. [DOI] [Google Scholar]
- 32.Tahar L.B., Basti H., Herbst F., Smiri L.S., Quisefit J.P., Yaacoub N., Grenèche J.M., Ammar S. Co 1-xZn xFe 2O 4 (0 ≤ x ≤ 1) nanocrystalline solid solution prepared by the polyol method: Characterization and magnetic properties. Mater. Res. Bull. 2012;47:2590–2598. doi: 10.1016/j.materresbull.2012.04.080. [DOI] [Google Scholar]
- 33.Hanini A., Massoudi M.E., Gavard J., Kacem K., Ammar S., Souilem O. Nanotoxicological study of polyol-made cobalt-zinc ferrite nanoparticles in rabbit. Environ. Toxicol. Pharmacol. 2016;45:321–327. doi: 10.1016/j.etap.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Gómez-Polo C., Recarte V., Cervera L., Beato-López J.J., López-García J., Rodríguez-Velamazán J.A., Ugarte M.D., Mendonça E.C., Duque J.G.S. Tailoring the structural and magnetic properties of Co-Zn nanosized ferrites for hyperthermia applications. J. Magn. Magn. Mater. 2018;465:211–219. doi: 10.1016/j.jmmm.2018.05.051. [DOI] [Google Scholar]
- 35.Ben-Arfa B.A.E., Miranda Salvado I.M., Ferreira J.M.F., Pullar R.C. Clove and cinnamon: Novel anti–oxidant fuels for preparing magnetic iron oxide particles by the sol–gel auto–ignition method. J. Alloy. Compd. 2019;786:71–76. doi: 10.1016/j.jallcom.2019.01.306. [DOI] [Google Scholar]
- 36.Makarova L.A., Alekhina Y.A., Omelyanchik A.S., Peddis D., Spiridonov V.V., Rodionova V.V., Perov N.S. Magnetorheological foams for multiferroic applications. J. Magn. Magn. Mater. 2019;485:413–418. doi: 10.1016/j.jmmm.2019.04.001. [DOI] [Google Scholar]
- 37.Omelyanchik A., Singh G., Volochaev M., Sokolov A., Rodionova V., Peddis D. Tunable magnetic properties of Ni-doped CoFe2O4 nanoparticles prepared by the sol–gel citrate self-combustion method. J. Magn. Magn. Mater. 2019;476:387–391. doi: 10.1016/j.jmmm.2018.12.064. [DOI] [Google Scholar]
- 38.Jacobs J.P., Maltha A., Reintjes J.G.H., Drimal J., Ponec V., Brongersma H.H. The surface of catalytically active spinels. J. Catal. 1994;147:294–300. doi: 10.1006/jcat.1994.1140. [DOI] [Google Scholar]
- 39.Vozniuk O., Tabanelli T., Tanchoux N., Millet J.M.M., Albonetti S., Di Renzo F., Cavani F. Mixed-oxide catalysts with spinel structure for the valorization of biomass: The chemical-loop reforming of bioethanol. Catalysts. 2018;8:332. doi: 10.3390/catal8080332. [DOI] [Google Scholar]
- 40.Antipov S., Turishchev S., Purtov Y., Shvyreva U., Sinelnikov A., Semov Y., Preobrazhenskaya E., Berezhnoy A., Shusharina N., Novolokina N., et al. The oligomeric form of the Escherichia coli dps protein depends on the availability of iron ions. Molecules. 2017;22:1904. doi: 10.3390/molecules22111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turishchev S.Y., Antipov S.S., Novolokina N.V., Chuvenkova O.A., Melekhov V.V., Ovsyannikov R., Senkovskii B.V., Timchenko A.A., Ozoline O.N., Domashevskaya E.P. A soft X-ray synchrotron study of the charge state of iron ions in the ferrihydrite core of the ferritin Dps protein in Escherichia coli. Biophys. Russ. Fed. 2016;61:705–710. doi: 10.1134/S0006350916050286. [DOI] [Google Scholar]
- 42.Cannas C., Falqui A., Musinu A., Peddis D., Piccaluga G. CoFe2O4 nanocrystalline powders prepared by citrate-gel methods: Synthesis, structure and magnetic properties. J. Nanoparticle Res. 2006;8:255–267. doi: 10.1007/s11051-005-9028-7. [DOI] [Google Scholar]
- 43.Holzwarth U., Gibson N. The Scherrer equation versus the ‘ Debye—Scherrer equation. Nat. Nanotechnol. 2011;6:534. doi: 10.1038/nnano.2011.145. [DOI] [PubMed] [Google Scholar]
- 44.Yang C., Hu Y., Li X., Li J., Wang L., Li H. Tuning of magnetic properties and hyperfine interaction by the substitution of Ni2+ for cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2019;30:19647–19653. doi: 10.1007/s10854-019-02338-1. [DOI] [Google Scholar]
- 45.Smit J., Wijn H.P.J. Ferrites. Philips’ Technical Library; Eindhoven, The Netherlands: 1959. p. 384. [Google Scholar]
- 46.Vegard L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921;5:17–26. doi: 10.1007/BF01349680. [DOI] [Google Scholar]
- 47.Muscas G., Jovanović S., Vukomanović M., Spreitzer M., Peddis D. Zn-doped cobalt ferrite: Tuning the interactions by chemical composition. J. Alloy. Compd. 2019;796:203–209. doi: 10.1016/j.jallcom.2019.04.308. [DOI] [Google Scholar]
- 48.Morrish A.H. The Physical Principles of Magnetism. Volume 1. Wiley-IEEE Press; Hoboken, NJ, USA: 1965. [Google Scholar]
- 49.Cobos M.A., de la Presa P., Llorente I., Alonso J.M., García-Escorial A., Marín P., Hernando A., Jiménez J.A. Magnetic Phase Diagram of Nanostructured Zinc Ferrite as a Function of Inversion Degree δ. J. Phys. Chem. C. 2019;123:17472–17482. doi: 10.1021/acs.jpcc.9b02180. [DOI] [Google Scholar]
- 50.Concas G., Spano G., Cannas C., Musinu A., Peddis D., Piccaluga G. Inversion degree and saturation magnetization of different nanocrystalline cobalt ferrites. J. Magn. Magn. Mater. 2009;321:1893–1897. doi: 10.1016/j.jmmm.2008.12.001. [DOI] [Google Scholar]
- 51.Andersen H.L., Granados-Miralles C., Saura-Múzquiz M., Stingaciu M., Larsen J., Søndergaard-Pedersen F., Ahlburg J.V., Keller L., Frandsen C., Christensen M. Enhanced intrinsic saturation magnetization of Zn x Co 1-x Fe 2 O 4 nanocrystallites with metastable spinel inversion. Mater. Chem. Front. 2019;3:668–679. doi: 10.1039/C9QM00012G. [DOI] [Google Scholar]
- 52.Yaseneva P., Bowker M., Hutchings G. Structural and magnetic properties of Zn-substituted cobalt ferrites prepared by co-precipitation method. Phys. Chem. Chem. Phys. 2011;13:18609–18614. doi: 10.1039/c1cp21516g. [DOI] [PubMed] [Google Scholar]
- 53.Apostolov A., Apostolova I., Wesselinowa J. Specific absorption rate in Zn-doted ferrites for self-controlled magnetic hyperthermia. Eur. Phys. J. B. 2019;92:58. doi: 10.1140/epjb/e2019-90567-2. [DOI] [Google Scholar]
- 54.Chaudhary V., Wang Z., Ray A., Sridhar I., Ramanujan R.V. Self pumping magnetic cooling. J. Phys. D. Appl. Phys. 2017;50:03LT03. doi: 10.1088/1361-6463/aa4f92. [DOI] [Google Scholar]
- 55.Skokov K.P., Gutfleisch O. Viewpoint on the letter ‘Self pumping magnetic cooling’ by V Chaudhary et al (2017 J. Phys. D: Appl. Phys. 50 03LT03) J. Phys. D. Appl. Phys. 2017;50:131001. doi: 10.1088/1361-6463/aa60ab. [DOI] [Google Scholar]
- 56.Mørup S., Hansen M.F., Frandsen C. Comprehensive Nanoscience and Nanotechnology. Elsevier; Amsterdam, The Netherlands: 2019. 1.04 Magnetic Nanoparticles; pp. 89–140. [Google Scholar]
- 57.Kodama R.H., Berkowitz A.E., McNiff E.J., Foner S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 1996;77:394–397. doi: 10.1103/PhysRevLett.77.394. [DOI] [PubMed] [Google Scholar]
- 58.Muscas G., Yaacoub N., Concas G., Sayed F., Sayed Hassan R., Greneche J.M., Cannas C., Musinu A., Foglietti V., Casciardi S., et al. Evolution of the magnetic structure with chemical composition in spinel iron oxide nanoparticles. Nanoscale. 2015;7:13576–13585. doi: 10.1039/C5NR02723C. [DOI] [PubMed] [Google Scholar]
- 59.Peddis D., Cannas C., Musinu A., Ardu A., Orru F., Fiorani D., Laureti S., Rinaldi D., Muscas G., Concas G., et al. Beyond the Effect of Particle Size: Influence of CoFe2O4 Nanoparticle Arrangements on Magnetic Properties. Chem. Mater. 2013;25:2005–2013. doi: 10.1021/cm303352r. [DOI] [Google Scholar]
- 60.Sharifi I., Shokrollahi H., Amiri S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012;324:903–915. doi: 10.1016/j.jmmm.2011.10.017. [DOI] [Google Scholar]
- 61.Frtús A., Smolková B., Uzhytchak M., Lunova M., Jirsa M., Kubinová Š., Dejneka A., Lunov O. Analyzing the mechanisms of iron oxide nanoparticles interactions with cells: A road from failure to success in clinical applications. J. Control. Release. 2020;328:59–77. doi: 10.1016/j.jconrel.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 62.Rodrigues G.R., López-Abarrategui C., de la Serna Gómez I., Dias S.C., Otero-González A.J., Franco O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019;555:356–367. doi: 10.1016/j.ijpharm.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 63.Sutton S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011;17:46–49. [Google Scholar]
- 64.Stevenson K., McVey A.F., Clark I.B.N., Swain P.S., Pilizota T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016;6:38828. doi: 10.1038/srep38828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mamonova I.A., Babushkina I.V., Norkin I.A., Gladkova E.V., Matasov M.D., Puchin’yan D.M. Biological activity of metal nanoparticles and their oxides and their effect on bacterial cells. Nanotechnol. Russ. 2015;10:128–134. doi: 10.1134/S1995078015010139. [DOI] [Google Scholar]
- 66.Makumire S., Revaprasadu N., Shonhai A. DnaK Protein Alleviates Toxicity Induced by Citrate-Coated Gold Nanoparticles in Escherichia coli. PLoS ONE. 2015;10:e0121243. doi: 10.1371/journal.pone.0121243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.