Abstract
Objectives:
The first objective of this study was to compare the predicted audiometric thresholds obtained by auditory steady state response (ASSR) and auditory brainstem response (ABR) in infants and toddlers when both techniques use optimal stimuli and detection algorithms. This information will aid in determining the basis for large discrepancies in ABR and ASSR measures found in past studies. The hypothesis was that advancements in ASSR response detection would improve (lower) thresholds and decrease discrepancies between the thresholds produced by the two techniques. The second objective was to determine and compare test times required by the two techniques to predict thresholds for both ears at the 4 basic audiometric frequencies of 500, 1000, 2000, and 4000 Hz.
Design:
A multicenter clinical study was implemented at three university-based children’s hospital audiology departments. Participants were 102 infants and toddlers referred to the centers for electrophysiologic testing for audiometric purposes. The test battery included wideband tympanometry, distortion-product otoacoustic emissions, and threshold measurements at four frequencies in both ears using ABR and ASSR (randomized) as implemented on the Interacoustics Eclipse systems with “Next-Generation” ASSR detection and FMP analysis for ABR. Both methods utilized narrow band CE-Chirp stimuli. Testers were trained on a specialized test battery designed to minimize test time for both techniques. Testing with both techniques was performed in one session. Thresholds were evaluated and confirmed by the first author and correction factors were applied. Test times were documented in system software.
Results:
Corrected thresholds for ABR and ASSR were compared by regression, by the Bland–Altman technique and by matched pairs t tests. Thresholds were significantly lower for ASSR than ABR. The ABR–ASSR discrepancy at 500 Hz was 14.39 dB, at 1000 Hz was 10.12 dB, at 2000 Hz was 3.73 dB, and at 4000 Hz was 3.67 dB. The average test time for ASSR of 19.93 min (for 8 thresholds) was found to be significantly lower (p < 0.001) than the ABR test time of 32.15 min. One half of the subjects were found to have normal hearing. ASSR thresholds plotted in dB nHL for normal-hearing children in this study were found to be the lowest yet described except for one study which used the same technology.
Conclusions:
This study found a reversal of previous findings with up to 14 dB lower thresholds found when using the ASSR technique with “Next-Generation” detection as compared with ABR using an automated detection (FMP). The test time for an audiogram prediction was significantly lower when using ASSR than ABR but was excellent by clinical standards for both techniques. ASSRs improved threshold performance was attributed to advancements in response detection including utilization of information at multiple harmonics of the modulation frequency. The stimulation paradigm which utilized narrow band CE-Chirps also contributed to the low absolute levels of the thresholds in nHL found with both techniques.
Keywords: Auditory brainstem response, Auditory steady state response, Children, Hearing loss
INTRODUCTION
The Need for Fast Electrophysiologic Assessments
The importance of early identification of hearing loss is well established but the ultimate goal of these programs is early intervention. Substantial progress has been made in the technology used and in the implementation of newborn hearing screening (NHS) programs worldwide. Some aspects of follow-up on failed screenings, however, are not performing as expected. Families whose infants require the diagnostic audiology test battery may experience delays in receiving the final information for a variety of reasons. A limited number of clinics have expertise in auditory electrophysiology for children and wait times for appointments can be long. Auditory assessments with electrophysiology require a very quiet patient and the amount of naptime available for testing is reduced as the child’s age increases. By 6 months, most clinics resort to anesthesia or sedation for electrophysiologic testing which comes with health risks, increased parental anxiety, and increased costs. Ideally, all necessary information required to either confirm normal hearing or to establish frequency-specific thresholds for each ear for initial hearing aid fitting should be obtained at the first audiology appointment after referral from newborn screening. It is also necessary to obtain some estimate of conductive component, if present.
The need for second appointments can trigger a cascade of events that delay diagnosis and the fitting of amplification and habilitation. Multiple audiology sessions decrease the number of available appointments and increase wait times for appointments at busy audiology clinics. The potential for missed appointments goes up dramatically and contributes to loss-to-follow-up. As families wait for appointments, infants may reach the age at which they need anesthesia for testing and all the scheduling considerations and risks that entails. Most important, the child with a hearing loss may be delayed in receiving the amplification that is so sorely needed.
Holte et al. (2012) evaluated the factors that lead to follow-up delays after NHS in a study of 193 infants with hearing loss who had failed screenings. Of the families who waited more than 3 months after the first diagnostic (electrophysiologic) test for confirmation of hearing loss, the most common reason given by parents was “multiple retesting.” According to Yoshinaga-Itano, “the number one reason why children with hearing loss are delayed in fitting of amplification is the need for multiple auditory brainstem response appointments” (C. Yoshinaga-Itano, personnal communication, April 3, 2017).
Electrophysiologic testing for audiometry requires children to be asleep. The amount of time available for testing of infants is dependent on how long they will remain in natural sleep. Janssen et al. (2010) found the average “natural sleep” time for infants (median age 4 months) was 48.8 min with 20% sleeping 33.1 min or less. The average time for a sedated evaluation was 58.2 min and yet, only 80% of those evaluations completed 6 thresholds which was the minimum that they considered adequate for fitting of amplification. Eighty percent of the naturally-sleeping infants completed only four thresholds. Of 188 children seen for audiometric ABR evaluation in their clinic, at least 20% did not receive a full evaluation (3 frequencies per ear). Based on the sedated evaluation time, a minimally-adequate ABR evaluation takes almost 60 min and yet the average natural sleep time is 48.8 min.
ABR is known as the measure of choice for predicting the audiogram in infants and toddlers (JCIH 2007). This is due in large part to results published by Stapells et al. (1995) who demonstrated excellent agreement between ABR and behavioral thresholds for 0.5, 2, and 4 kHz. Although the accuracy of ABR threshold prediction has been considered good, the test times for a full assessment with ABR continue to exceed the natural sleep time of many children.
In the 1990s, a new technique for evaluating thresholds, now known as the auditory steady state response (ASSR), was commercially developed. For a complete review, see Korczak et al. (2012). Excitement surrounding this new technique had much to do with the possibility reducing test time by evaluating four frequencies in each ear simultaneously.
Van Maanen and Stapells (2009) used ASSR with simultaneous four-frequency stimulation bilaterally to evaluate a group of normally-hearing infants previously tested by ABR. They reported ASSR test times as the time spent making an assessment (all frequencies and ears) at a single stimulus level. The average time per level was 6.3 min with a SD of 3.10 min. They also state that one to six intensities were recorded per infant. By interpolation, an average of 3.5 intensities at 6.3 min would be 22.05 min for the ASSR assessment in the normally-hearing infants.
Venail et al. (2015) evaluated children with hearing loss using “Next Generation” ASSR and reported an average test time of 22.90 min for 4 thresholds in each ear. Mueller et al. (2012) reported ASSR test times for adults with and without hearing loss, also using “Next Generation” ASSR. They reported an average test time of 18.6 min overall with normally-hearing subjects being tested somewhat more quickly (16.1 min). Vander Werff (2009) reported test times from adult subjects, both normally hearing and with hearing loss while comparing analysis techniques both with simultaneous binaural simulation. The average test times for 4-frequency thresholds in both ears were 46.1 and 43.6 min for the 2 techniques but test times were faster (38 to 39.2 min) for normally-hearing subjects.
With the possible exception of the Vander Werff (2009) study, the reported test times for ASSR using simultaneous, binaural testing of multiple frequencies appears to produce test times that are in the 20- to 25-min time frame which should make a full assessment possible during most natural sleep time durations (33 to 48 min). While the test times for ASSR are very encouraging, the accuracy of the threshold measures produced by ASSR has been questioned, especially when compared with ABR measures.
Early reports on the accuracy of threshold predictions using ASSR with children found that the threshold levels were not well correlated to behavioral thresholds and too high above normal-hearing thresholds to be useful (JCIH 2007). For example, Rance et al. (2006) found some 4000 Hz thresholds by ASSR in normally-hearing infants as high as 50 dB nHL, while ABR in the same infants demonstrated thresholds below 20 dB nHL. More recently, Attias et al. (2014) found correlations with r values as low as 0.63 for ASSR thresholds compared with audiometric thresholds in adults which is well below the general standard. For comparison, Stapells et al. (1995) found ABR thresholds correlated to behavioral thresholds in infants with r = 0.94 to 0.97.
Previous Studies Comparing ABR and ASSR
Some investigations have compared ABR thresholds generated with clicks to ASSR thresholds elicited by frequency-specific stimuli in both adults and children with a range of fair results (Cone-Wesson et al. 2002a; Firszt et al. 2004; Luts et al. 2004; Swanepoel & Ebrahim 2009; Vander Werff 2009; Rodrigues et al. 2010; Venail et al. 2015). However, the present study will compare the two tests with like-frequency stimuli and with children as participants and therefore the following discussion will focus only on those studies also using frequency-specific stimuli in children. These are summarized in Table 1.
TABLE 1.
Previous studies comparing frequency-specific thresholds obtained by ABR and ASSR in children
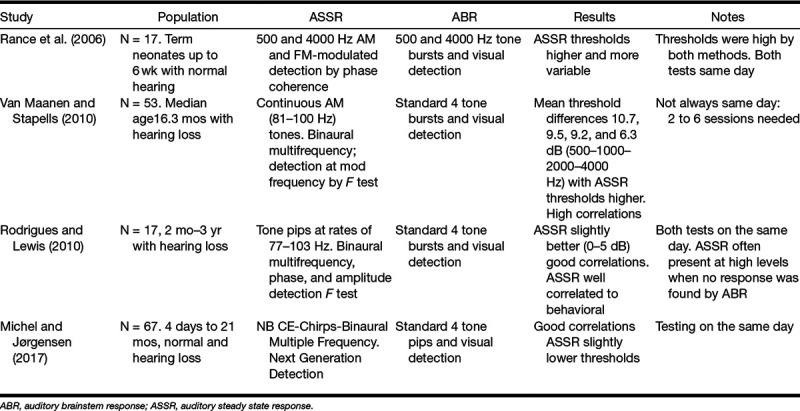
Table 1 indicates that Rance et al. (2006) and Van Maanen & Stapells (2010) found that ASSR thresholds were consistently elevated relative to ABR while better agreement between the two technologies was found for Rodriguez et al. (2010) and Michel and Jørgensen (2017). The studies summarized in Table 1 discuss overall findings in children but discrepancies between ABR thresholds and ASSR thresholds seem to be greatest in normally-hearing subjects.
Rance et al. (2005) compared ASSR thresholds to behavioral thresholds in 575 children, 285 with normal hearing and the remainder with hearing loss. The average ASSR threshold in the normally-hearing group was elevated relative to behavioral by an average of 27.85 dB for the 4 standard audiometric frequencies. For the whole group, behavioral and ASSR thresholds were correlated (r ≥ 0.96) but the regression equations showed a somewhat nonlinear relationship (slopes of 0.73 to 0.81) and corrections of 22.9 to 33 dB indicating that predictions for normal hearing were the poorest. They summarized these findings stating “ASSR testing (as carried out in this study) cannot reliably differentiate between normal ears and those with mildly elevated hearing levels” (Rance et al. 2005, p. 298).
The difficulties experienced when using the early ASSR systems are likely due to automatic detection schemes that lacked sufficient sensitivity to find low-amplitude responses. The stimulus and detection paradigms used for ASSR are complex. Segments of electroencephalography (EEG) are analyzed for evidence of the amplitude and phase of the stimulus modulation frequency. Some detection algorithms look for “coherence” of the phase across segments. Others assess the amplitude of the target frequency component relative to surrounding frequencies in the FFT and test for statistical differences with an F test. Some systems test for evidence of a response with both phase and amplitude measurements. Simultaneous tests are run for all modulation frequencies in the multifrequency paradigm.
New Advances in ASSR Detection
A new detection algorithm for the ASSR has been described (Stürzebecher et al. 1999; Cebulla et al. 2006). They proposed a specialized “q-sample” statistical assessment that evaluates both the phase and amplitude at multiple harmonics of the modulation frequency, rather than looking only at the first harmonic as in the past. The multiharmonic (q-sample) technique improved detection rates and reduced time to detection by ASSR (Cebulla et al. 2006). Bonferroni corrections for multiple statistical tests were replaced by a more appropriate corrected critical test value developed with a simulation procedure (Stürzebecher et al. 2005). The Eclipse ASSR system implemented a table lookup for individual ASSR tests further improving detection rates and decreasing test time (Stürzebecher & Cebulla 2013; Cebulla & Stürzebecher 2015). This detection scheme including the assessment at multiple modulation harmonics and new approach to critical test values for ASSR has been referred to as “Next Generation.”
Automated Detection of ABR
Excellent statistically-based detection schemes have been described for use with ABR but are generally not utilized in clinical audiology. As previously stated, ABR frequency-specific thresholds are the clinical standard for audiogram prediction in infants and toddlers and are used universally for follow-up of failed NHSs.
Elberling and Don (1984) and Don et al. (1984) described a technique to determine the overall quality of an ABR including an estimate of the averaged background noise. The technique produced a value known as the FSP which was calculated in real time during the averaging. The formula for calculation is
VAR(S) is a calculation of the amplitude variance of the averaged response in a 10 msec window surrounding the area of expected ABR. This value will include the RMS of the response and the residual background noise. The VAR(SP) is the variance of a single point within the window over a series of sweeps. Herein lies the difference between FSP and FMP. The FSP (SP for single point) used one point in each sweep in the noise calculation and the FMP uses 5 spaced points (MP = multiple points) from each sweep. A total of 250 points go into the noise calculation but this happens more quickly (50 sweeps for FMP and 250 for FSP) with FMP. The value of FMP is updated every 50 sweeps and, if a response is present, grows as the noise is reduced with averaging. The FMP (or FSP) as a ratio of variances has an F distribution with 5 and 250 degrees of freedom (Elberling & Don 1984) which allows the determination of confidence levels for FMP values. Clinically, an FSP or FMP of 2.25 is the 95% confidence level of a true response presence and correlates best with the “visual response detection” of a trained clinician (Sininger 1993).
The use of FMP has several important advantages in a clinical setting. Quantifying the level of confidence in a response will improve consistency in test results across users and clinical sites. The use of this statistical method also eliminates uncertainty regarding the number of sweeps which need to be averaged for any given measurement. The amount of averaging needed to achieve an adequate signal to noise ratio (SNR) is based on the amplitude of the response and the overall noise in the recording, neither of which is known to the tester beforehand. The FSP/FMP technique allows the user to stop averaging when an adequate SNR is achieved. Large amplitude responses require less averaging for a given background noise than do small ones and averages containing high levels of noise require more averaging than those with low levels for a given amplitude response. It is possible to monitor the background noise in the average or the VAR(SP) in itself during the average. When a response is absent, the recording can be stopped when the background noise has been reduced sufficiently to have allowed detection of a small response if it were present. Regardless of whether a response is detected or absent, the FMP technique will optimize the averaging time based on the characteristics of the recording and will ultimately reduce test time and improve detection of small responses (Don & Elberling 1996).
Advances in Stimuli for Electrophysiologic Testing
On the stimulus side, significant strides have been made in the use of chirp stimuli for electrophysiology. Dau et al. (2000) developed a chirp stimulus for use in electrophysiologic testing. This stimulus had the spectrum of a traditional click, but the order of energy presentation was dispersed from low frequency to high frequency with timing of frequency components based on estimates of the cochlear traveling wave delay. Theoretically, all areas of the basilar membrane are activated simultaneously by the chirp producing a larger neural response due to improved synchronization.
Stürzebecher et al. (2001) developed a wideband chirp for electrophysiologic testing and screening and later Stürzebecher et al. (2006) created octave band chirps built by the addition of individual cosines with the phase of each component adjusted to compensate for cochlear delay based on the model of de Boer (1980). The cosigns are spaced around the center frequency of intended stimulus, in this case 500, 1000, 2000, and 4000 Hz. The frequency spacing of the cosigns determines the modulation so if spaced at 90 Hz, for example, there is an automatic 90 Hz modulation.
The ASSR “Next Generation” detection algorithm is designed to detect amplitude and phase at up to 12 harmonics of the modulation. At 500 Hz, there was a potential overlap between the frequencies of the stimulus cosigns and the frequencies of the harmonics of the modulation. This would have led to a possible “stimulus artifact” detection problem. Consequently, the whole series of cosigns was shifted by ½ of the modulation rate (for example 45 Hz). This eliminated the overlap avoiding detection of stimulus. In addition, if the offset is ½ of the modulation rate, a phase coupling across two periods of the modulation results in an alternating stimulus.
Elberling et al. (2007) developed chirp stimuli using delay models based on data from Manny Don’s lab. Elberling and Don (2008) demonstrated a near doubling of ABR amplitude with these chirps compared with standard click stimuli. This stimulus is now termed the CE-Chirp for Claus Elberling, its primary developer.
Narrow band (NB) CE-Chirps are used for audiometric measures. These have the same center frequencies as standard tonebursts such as those used by Stapells et al. (1995) and are approximately one octave in spectral width. These stimuli use the same formula discussed above for traveling wave compensation within the spectrum of the narrow band and frequency offset to allow for multiharmonic detection schemes with ASSR.
Are chirps of value for electrophysiologic testing? Studies using adult subjects (Dau et al. 2000; Elberling & Don 2008, 2010; Cebulla & Elberling 2010; Petoe et al. 2010; Kristensen & Elberling 2012) have shown that the chirp will enhance electrophysiologic response amplitudes relative to traditional stimuli. Several clinical studies of children have proven that, compared with traditional tone bursts, NB CE-Chirps produce larger amplitude responses, reduce test time, and even reduce the expected threshold and lower correction factors from ABR measures to predicted hearing thresholds in infants and children (Ferm et al. 2013; Rodrigues et al. 2013; Ferm & Lightfoot 2015).
Purpose
The purpose of this study was twofold. The first was a comparison of actual test times for ABR and ASSR with each test optimized by the use of NB Chirps and advanced detection techniques. The second was to evaluate any discrepancies between threshold predictions by ASSR and ABR techniques. Will improvements in response detection allow ASSR to produce thresholds at the same level as ABR? Children were evaluated at the same appointment with testing protocols counterbalanced. A variety of clinics with experienced pediatric audiology staff performed the evaluations on actual patients.
MATERIALS AND METHODS
The study was conducted by the Audiology Departments at three, university-affiliated hospitals including Cincinnati Children’s Hospital Medical Center, Children’s Hospital of Colorado in Aurora, and University of University of North Carolina Medical Center in Chapel Hill. This study was reviewed and approved by the respective Institutional Review Boards of each hospital and all testing was performed after the informed consent of a parent or guardian.
Participants
One hundred and two children were studied including 58 females and 44 males. The mean age of the children was 12.55 months. The range was 0.7 to 80 months of age. The distribution of the children’s ages is shown in Figure 1 demonstrating that the majority (59%) were 5 months of age or younger at the time of test. All children who were referred to audiology for an audiologic evaluation that required electrophysiologic technology were eligible for the study. Reasons for referral are shown in Figure 2 with “failed NHS” being the most common reason given in 41 cases.
Fig. 1.
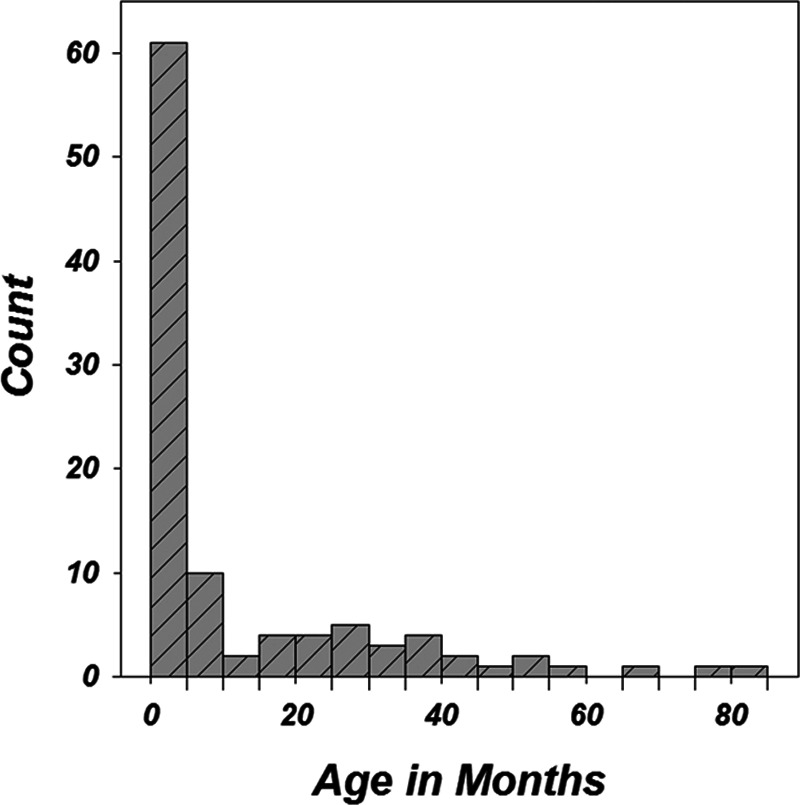
Distribution of participant ages. Sixty-one subjects of 102 (59%) were ≤5 mos of age.
Fig. 2.

A breakdown of reasons for referral to audiology among the children evaluated in this study. The major reason for testing was follow-up to failed newborn hearing screening which occurred in 41 of 102 participants.
Test Measures
The test protocol included some audiology procedures designed to augment clinical conclusions and expedite the testing sessions. The protocol was as follows:
Wideband tympanometry was performed using the Interacoustics Titan Suite. The test utilized medium pump speed, pressure range from +200 to −400 daPa and a click stimulus used to elicit the response. The wideband response was plotted in three dimensions. Pressure by frequency by absorbance plots were displayed and NB (800 to 2000 Hz) and single frequency tympanograms (226 and 1000 Hz) were extracted and displayed along with normative data based on the child’s age. Absorbance was also plotted by frequency overlaid on age-specific normative areas.
Distortion-product otoacoustic emissions (DPOAEs) were tested using the Interacoustics Titan with the same probe used for wideband tympanometry. DPOAE SNR was measured with f2 of 2000, 3000, 4000, 5000, 6000, and 8000 Hz, f2/f1 = 1.22 and stimulus levels of 65 and 55 (L1 and L2) dB SPL. Criteria for a response at each frequency included a minimum DP level of −10 dB, SNR of 6 dB, and residual noise of −20 dB SPL.
An ABR threshold using a wideband CE-Chirp was established in each ear (see ABR threshold procedure below).
Random participant numbers were assigned to each subject. Each number was randomly assigned to a test order starting either with ASSR or ABR. The threshold searches for all stimuli by either method started with a stimulus level at or just above the broad-band CE-Chirp threshold as a time-saving measure.
Electrophysiology
The stimuli used for threshold detection with ABR and ASSR were octave band CE-Chirps with center frequencies of 0.5, 1.0, 2.0, and 4.0 kHz (Stürzebecher et al. 2006; Elberling & Don 2010; Elberling et al. 2010). Alternating-polarity CE-Chirps were level specific as described by Kristensen and Elberling (2012), calibrated in nHL and presented via ER-3A transducers (Etymotic Research Inc., Elk Grove Village, IL) coupled to the ear canals of the children with flexible, pediatric ear tips. Both ABR and ASSR were performed within the same session using the same electrodes and preamplifier. Surface recording electrodes were placed at the midline of the forehead at the hairline (noninverting) referenced to an electrode on the mastoid or earlobe ipsilateral to the ear of stimulation. A ground electrode was placed on the forehead. Interelectrode impedances were less than 5000 ohms. All testing was performed by licensed audiologists with several years of experience with pediatric electrophysiologic testing. For both techniques, testers were instructed that threshold searches should begin with levels near the broad-band chirp ABR threshold. The exact level for the start of the threshold search was at the tester’s discretion. A 5-dB step size (only near threshold) was encouraged. The minimum test level used was 20 dB nHL for 500 Hz and 10 dB nHL for all other frequencies. The maximum level tested was between 80 and 100 dB nHL at the discretion of the tester.
Auditory Brainstem Response
Thresholds for each stimulus and ear were measured individually with the order of testing at the discretion of the audiologist. Stimuli were presented at 39.7/sec. The Eclipse system adjusts the stimulus onset relative to the recording window by frequency allowing for an equivalent expected latency for each frequency and a standard 20 msec recording window regardless of stimulus (Ferm et al. 2013). The EEG was filtered from 100 to 1500 Hz, 12 dB/octave. Bayesian weighting (Elberling & Wahlgreen 1985) was utilized to reduce the impact of variable noise along with an artifact rejection set to ±40 μV. Stopping of averaging on a criterion based on SNR will optimize the use of test time and reduce threshold levels (Sininger 1993; Don & Elberling 1996). FMP, a statistical measure proportional to the SNR of the recording was used for objective ABR response detection. FMP is calculated using 5 points/sweep for noise estimation but is otherwise identical to the FSP (Elberling & Don 1984). FMP was calculated every 50 sweeps along with an estimate of the residual background noise (Don & Elberling 1994). The criteria for threshold was the lowest stimulus level producing an evoked potential recording achieving an FMP of 2.25 (95% response confidence) or greater. If the FMP condition was not achieved, the recording was stopped when the residual noise reached a value of 20 nV or lower or when 6000 sweeps had finished. The audiologist could extend the maximum number of sweeps if deemed necessary. ABRs to any given stimulus level were not repeated unless deemed necessary by the audiologist. Rather, response confidence was determined by the FMP value. If there was a discrepancy between the FMP and visual detection of wave V, the average to a given level could be repeated.
Auditory Steady State Response
Thresholds for each of the four stimuli and both ears were evaluated simultaneously. In the event of unilateral or significantly asymmetric hearing loss, each ear was assessed independently. Each stimulus/ear combination had a unique modulation frequency near 90 Hz. The starting level for all frequencies was set at or no more than 20 dB above the broad-band CE-Chirp ABR threshold in that ear.
The detection algorithm for the ASSR used on the Eclipse system is as described in the background section. Response detection is evaluated using 12 harmonics of the modulation frequency for each stimulus and detection incorporates both the amplitude and the phase of the response at these harmonics. The ASSR procedure tests for statistical significance of a response at regular time intervals as described by Stürzebecher and Cebulla (2013).
The stopping rule was set to the 95% confidence limit and the growth of the detector over time was plotted along with the background noise. Noise rejection was set to 40 nV. Eight growth curves (four frequencies per ear) were displayed simultaneously. If not stopped by background noise criterion or response detection, the average stopped automatically in 6 min. This time could be extended by the user. In addition, this study utilized a noise-stopping rule that had two conditions. If the background noise was ≤15 nV and the detection indicator was at 50% or less, the audiologist would stop the recording. When a response was detected, the growth curve turned from red to green and the averaging stopped. If appropriate, a new average was started by clicking in the box and setting a new level. This process happened independently of the sweeps that were running simultaneously at other frequencies although there are some limitations on the range of levels that could be presented simultaneously to prevent stimulus interactions.
ASSR stimuli consisted of NB CE-Chirps. The stimuli themselves are comprised of a series of cosines centered around the desired stimulus frequency in which the spectral separation of components is the modulation rate. Compensation for travel time in the cochlea is accomplished by phase corrections in the individual cosine components of the stimulus.
Every effort was made to reduce test time for both techniques. Specifically, the following steps were taken during threshold searches:
Testing started at a level at or just above the wideband CE-Chirp threshold.
Using a statistical criterion for stopping optimized test time by stopping the averaging process as soon as a response was detected, as early as 800 sweeps for ABR or less than a minute for ASSR. In addition, a small response (ABR or ASSR) requires more sweeps than may be routinely used. Finally, if the noise was reduced by averaging to a point where a small response should be detected and yet the statistical criterion had not been met, the response would stop earlier than 6000 sweeps.
If response criterion was met early, for example after 800 to 1000 sweeps (ABR) or within 1 min (ASSR), the assumption was that the stimulus was well above threshold. Therefore, a large step size was recommended, for example dropping 20 dB or more and conversely, when a given stimulus level achieved response late in the process, a small decrease in level was used for the next test.
Audiologists were instructed not to mark any latencies or amplitudes until after the testing was completed, so as not to bias the ABR test time measurement.
All test data were stored in patient files in the OtoAccess program. The “client” information included the test site, tester, child’s subject number, age, sex, reason for testing, and the sleep state. Three sleep states were specified as anesthesia, natural sleep quiet, or natural sleep fussy. After all testing was completed, the file was stripped of any personal identifying information and encrypted. The files were sent by e-mail to the primary investigator who analyzed the information. Extracted data were stored in the REDCap data management system at the University of Colorado, Denver.
Data Analysis
ABRs were analyzed by the principal investigator. The thresholds were designated as the response from the lowest stimulus level that reached the 95th percentile FMP criterion of 2.25 or greater. An exception to this rule was sometimes noted when a response was stopped due to low background noise level. The noise criterion was set at ≤20 nV and if this was achieved before a response noted on FMP, the average was stopped and would be regarded as below threshold. However, in many cases, evidence of a response was seen in these cases (when stopped by 20 nV background noise) appearing as a small wave V with appropriate latency. It became clear that a lower noise-stopping criteria of 15 nV should have been used. When a clear response (small wave V at an appropriate latency for the series) was seen on an average that was stopped at 20 nV criteria, it was assumed to be the threshold and was noted as such.
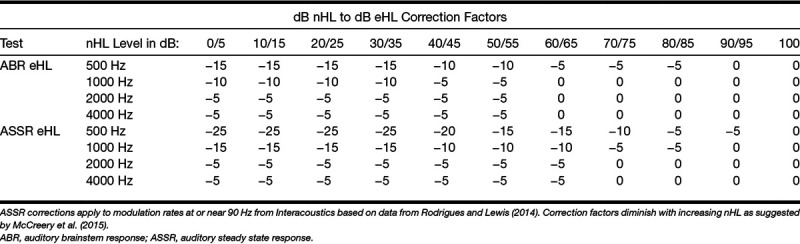
Correction factors (Table 2) were applied to the dial reading threshold values to correct from dB nHL to dB eHL. ASSR correction factors were obtained from Interacoustics and were based on data from Rodrigues and Lewis (2014) and ABR threshold corrections were also recommended by Interacoustics based on data from the United Kingdom (Stevens et al. 2013). For both tests, the acceptable correction factors were based on the dial level and were diminished as nHL level increased as suggested by McCreery et al. (2015). Unless otherwise specified, data presented have been corrected based in Table 2.
TABLE 2.
Correction factors applied to thresholds at dial reading for children tested with ER-3A insert transducers
Test session time was automatically calculated and stored by the acquisition software for the ASSR procedure. For ABR, the test time was calculated based on procedure time stamps that are stored with the session. Test time recorded for each participant was the time needed to achieve eight thresholds, four in each ear. When fewer than eight threshold measures were completed, the projected test time was based on the time needed for those that were completed. For example, if only 6 thresholds were obtained in 18 min, the projected time was 24 min.
Paired t tests were used to compare matched pairs of data from ABR and ASSR for individual subjects such as thresholds for a particular frequency or test times. In addition, ASSR (as the dependent variable) was regressed on ABR (as the independent variable) for each threshold. The Bland–Altman test was applied to assess whether the tests were clinically equivalent. This method focuses on the mean and SD of the differences between the values provided by two measurements. The Bland–Altman technique plots the difference scores as a function of the mean score with 2 SDs of the mean difference illustrated (Bland & Altman 1995). Analyses of variance are used for evaluation of the effects of sleep state and degree of hearing loss on test times and amount of averaging needed.
RESULTS
Participants
Of the 102 enrolled participants, 35 were evaluated under anesthesia and 67 were tested while in natural sleep. Of those in natural sleep, 53 were considered “quiet” and 14 were “fussy.” Fifty of the children were evaluated with ABR before ASSR and 52 had the ASSR test first.
All 8 thresholds were achieved in 83% of the ABR tests and 87% of the ASSR evaluations. The average number of thresholds per subject completed for ABR was 7.43 (SD = 1.51) and for ASSR was 7.36 (SD = 1.98). Both tests were competed on the same day for 82 subjects.
Preliminary Measures
Wideband tympanometry was completed in the right ear of 80 children and the left ear in 79. Results were categorized based on whether the pattern of absorbance by frequency at peak pressure fell primarily within the normal range. A breakdown of wideband tympanometric results can be found in Figure 3. DPOAE measurements were conducted on the right ear of 91 children and the left ear of 89. Figure 4 displays the results in terms of numbers of frequencies (DP points) which met response criteria (“passed”) for the left and right ears.
Fig. 3.
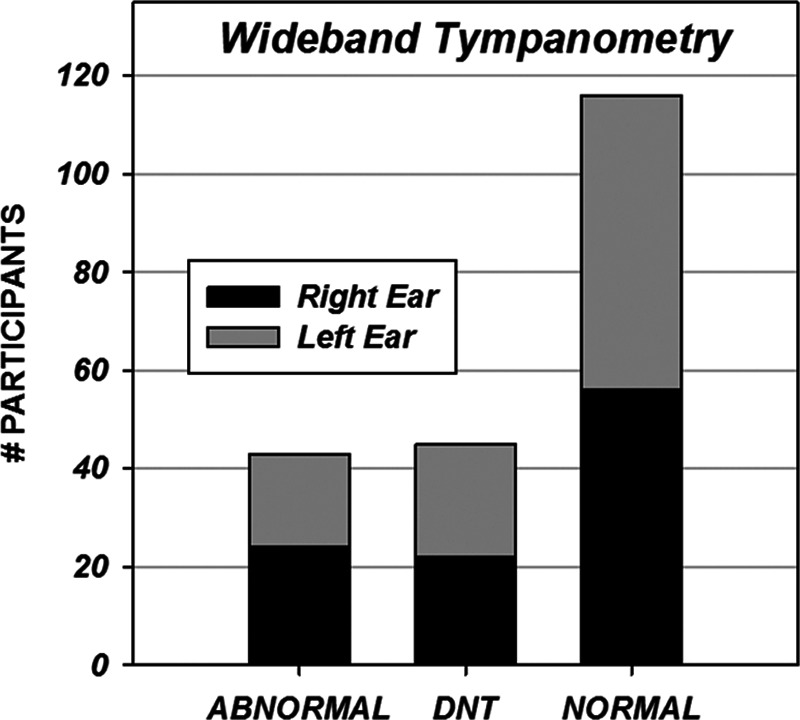
Wideband tympanometry results by left and right ears.
Fig. 4.
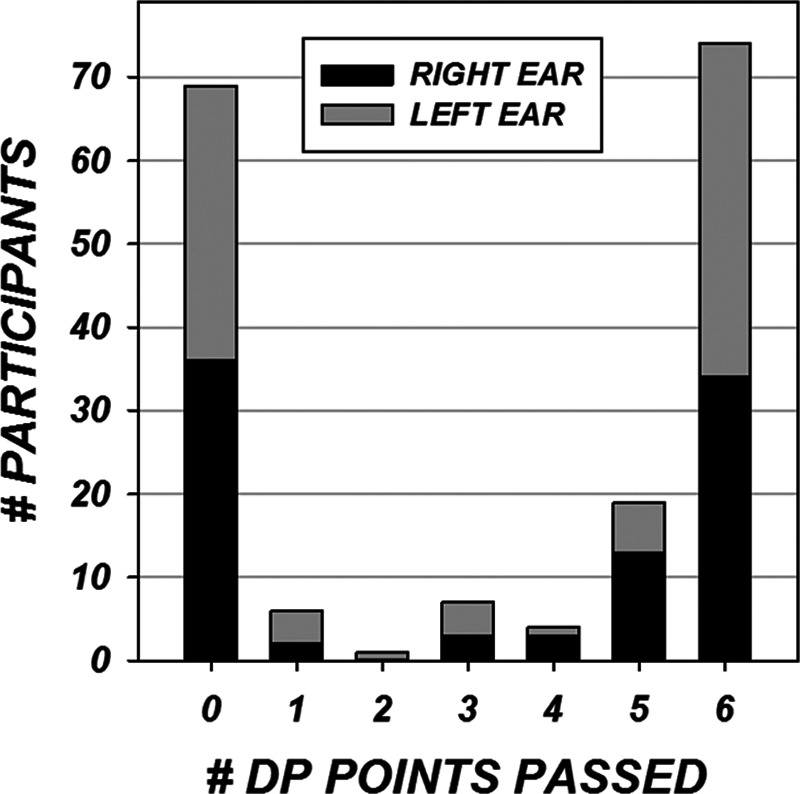
DPOAEs were evaluated with f2 of 2000, 3000, 4000, 5000, 6000, and 8000 Hz. Criteria for “pass” at each frequency was a minimum DPOAE amplitude of −10 dB and a signal to noise ratio of 6 dB or greater. DPOAEs were evaluated in the right ear on 91 subjects and in the left ear on 89 subjects. The bars represent the number of points passed by numbers of subjects in each ear.
Threshold Comparisons
Individual ASSR thresholds by frequency were plotted against ABR thresholds in Figure 5. These plots represent all data including those points with floor effects brought on by not testing thresholds below 10 dB at 1000, 2000, and 4000 Hz or 20 dB at 500 Hz and ceiling effects due to limiting test levels to a maximum of 80 to 100 dB. In these cases, the minimum or maximum test values are shown as thresholds in Figure 5, although technically they are not. These are termed “all” data. When these values are omitted, the data set will be termed “center” with floor and ceiling “thresholds” omitted.
Fig. 5.
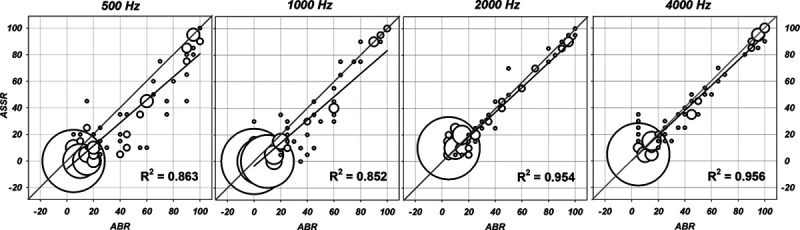
Regression bubble plots of auditory brainstem response (ABR) by auditory steady state response (ASSR) thresholds in dB eHL for all stimulus frequencies. The size of the symbol is scaled to represent the number of subjects at a given intersection. The actual regression line is shown in black and the perfect fit diagonal is in gray. The R2 from the regression equation is shown in the lower right.
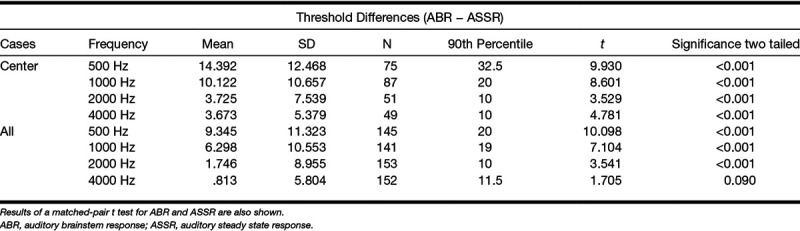
Regression equation values for data plotted in Figure 5 are shown in Table 3. Regression with “all” and “center” values are shown separately. This change reveals a small effect on the equation values. In general, the R2 values do not change substantially after removal of ceiling and floor thresholds. Figure 5 demonstrates that ABR thresholds overall are higher than ASSR. Statistics on the difference scores for ABR and ASSR thresholds are given in Table 4. The mean differences are largest at 500 Hz, and smallest at 4000 Hz.
TABLE 3.
The format of the regression equations is Y = aX + b, where Y = ASSR threshold, X = ABR threshold, a = slope and b = mean shift in dB
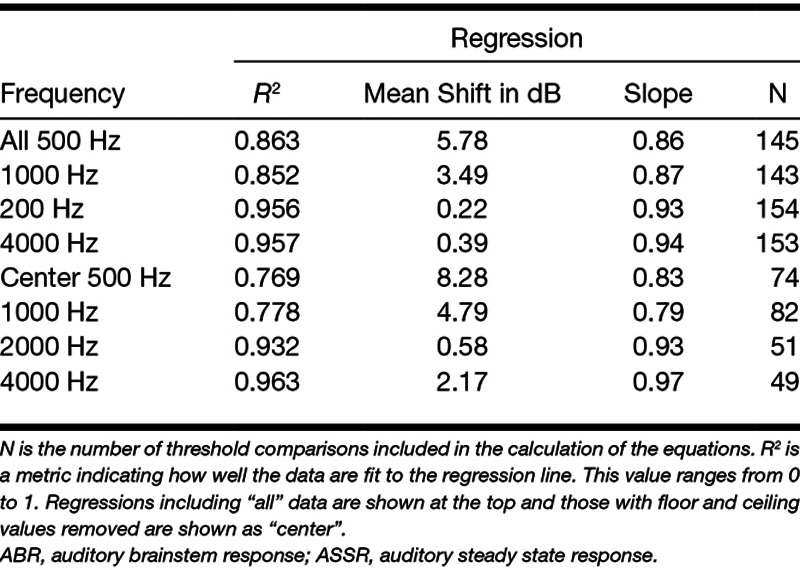
TABLE 4.
Statistical analysis of difference scores for corrected thresholds (ABR − ASSR) when floor and ceiling thresholds are eliminated (center) and for the entire data set (all)
Bland–Altman plots in Figure 6 illustrate the range of the difference scores relative to the average of the ABR and ASSR thresholds. These plots demonstrate the amount of data falling within and without ±1.96 SDs of the mean difference scores.
Fig. 6.
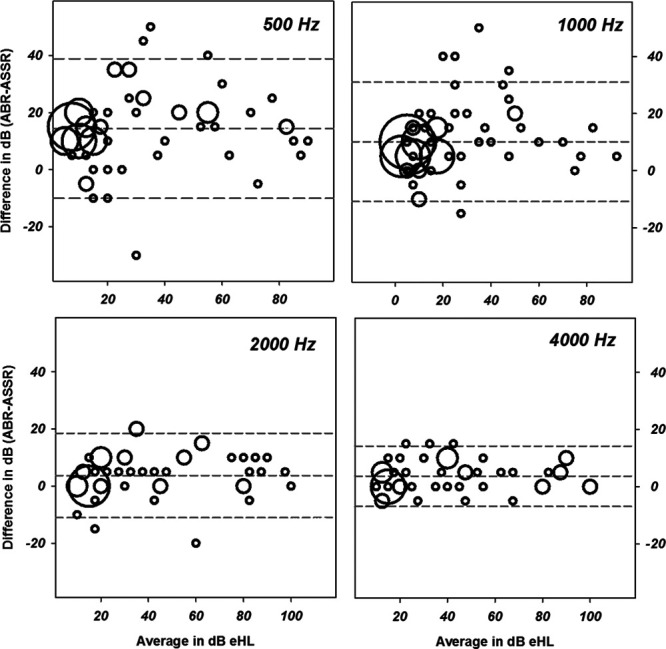
Bland–Altman plots using center data comparing differences scores (auditory brainstem response [ABR] by minus auditory steady state response [ASSR]) by stimulus frequency. The average of the two measures is on the x axis and the difference scores are plotted on the y. The mean difference and the mean ± 1.96 SDs are shown in the gray dashed lines. The size of the symbol is proportional to the number of data points represented with minimum size indicating one data point.
Time Analysis
Figure 7 displays individual paired projected test times for ABR by ASSR with regression. The bivariate mean projected test times with SDs are also shown. Based on the 82 comparisons, average projected test time for the ABR is 32.15 (SD = 18.23) min and for ASSR is 19.93 (SD = 8.73) min. The difference, 12.51 min, is statistically significant by paired t test (t(81) = 6.22; p < 0.001). When all data are considered including times for tests when both were not completed, the average ABR time based on 89 measures is 32.38 min with a SD of 18.23 and average ASSR time based on 86 measures is 19.71 min with a SD of 8.73. This represents a time difference of 12.67 min and a 39%-time decrease (ASSR compared with ABR). The inset at the upper left of Figure 7 shows the distribution of time differences (ABR time − ASSR time) which range from −23 to 71.89 min.
Fig. 7.
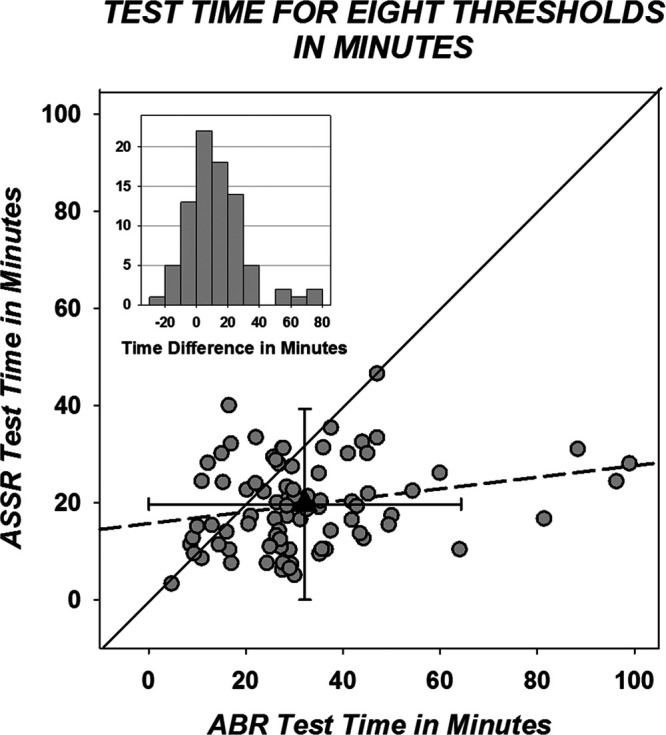
Projected test time in minutes (see text) for 82 individual participants with data from both methods. Mean time is plotted with a triangle with error bars of 1 SD; mean/SD time for auditory brainstem response (ABR) is 32.15/18.15 min and for auditory steady state response (ASSR) is 19.63/8.92 min. The diagonal is in black and the regression line is dashed. The regression equation is ABRtime = 0.119 × ASSRtime + 15.652. The R2 for the regression is 0.057. Inset, The distribution of the test time difference scores (ABR test time − ASSR test time).
Figure 8 demonstrates changes in test times based on the degree of hearing loss. For both ABR and ASSR, the fastest test times are found when the hearing is in the normal range. The ASSR takes progressively longer with increasing hearing loss but the ABR test times are longest when the loss is mild to moderate.
Fig. 8.
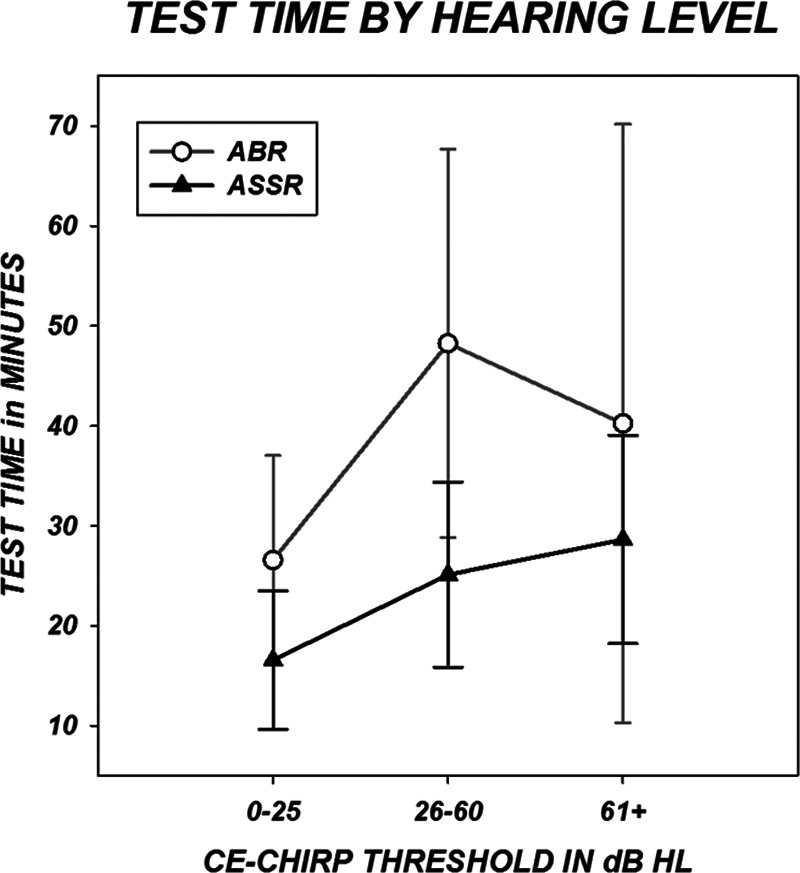
Test time is plotted by hearing level. Three hearing groups were created based on the average of the broad-band chirp thresholds for the left and right ear. Symbols represent group means and error bars indicate SDs.
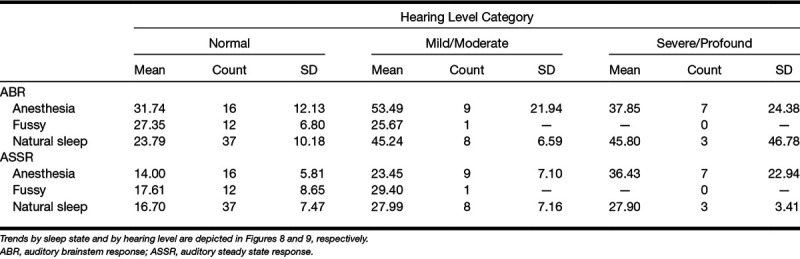
Figure 9 shows test time changes with sleep state. The largest difference in test time for the two techniques is for children under anesthesia. Analyses of variance for both ABR and ASSR test times were conducted investigating sleep state and degree of hearing loss (as indexed by the broad-band chirp threshold) as factors. Both found significant overall effects with F(31,7) = 7.185, p = 0.001 for ABR and F(31,7) = 1.864, p = 0.028 for ASSR. In both analyses, degree of hearing loss demonstrated a significant effect on test time (p < 0.00 for ABR and p = 0.032 for ASSR) but sleep state was not significant for either analysis nor was there any interaction between degree of loss and sleep. Table 5 gives the average test times for ASSR and ABR broken down by both hearing loss category and sleep state.
Fig. 9.
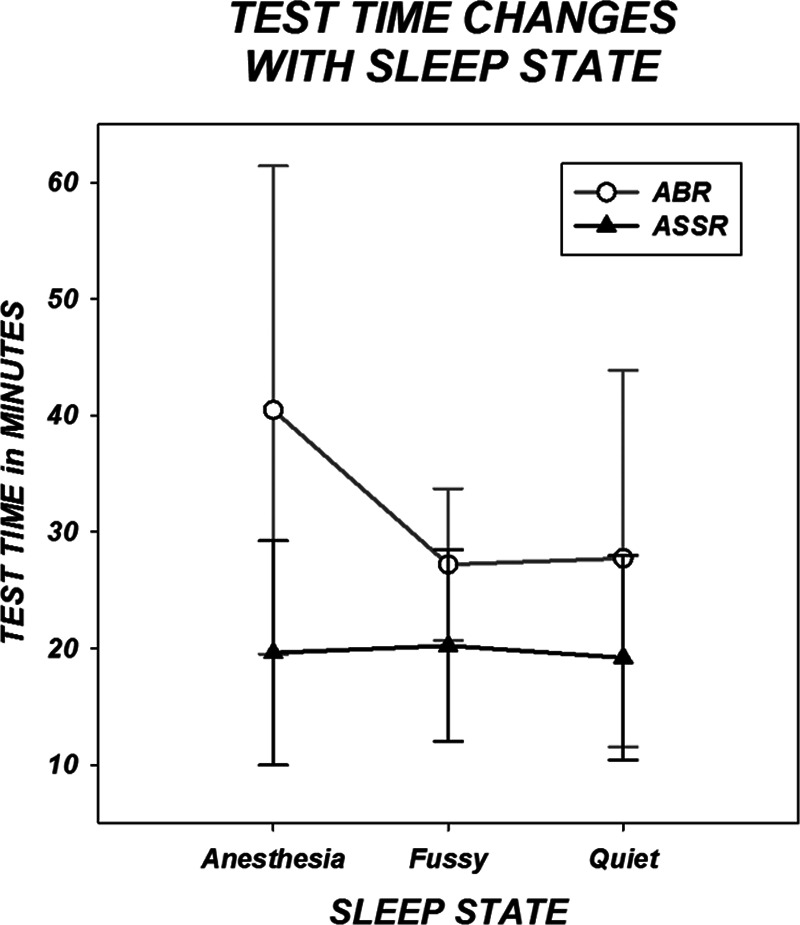
Test time is plotted by sleep state as characterized by the tester. Symbols represent group means and error bars indicate SDs.
TABLE 5.
Average test times by hearing level, by sleep state (anesthesia, fussy, natural sleep) and by test (ABR or ASSR)
The only time information that was saved for ASSR was the time for the entire evaluation. For ABR, the number of sweeps for each run at threshold (lowest stimulus level at a single frequency) were recorded. The mean number of sweeps required for the ABR run at threshold, and therefore meeting the prescribed FMP value, was 2181 with a SD of 1361.49. The range of number of sweeps required to reach threshold was from 800 sweeps, which is the minimum allowed by the ABR system, to just under 8000.
The amount of averaging needed to achieve ABR thresholds was also analyzed by hearing threshold level. As an estimate of the average hearing level, the threshold obtained by the broad-band CE-Chirp was used. The data are shown in Figure 10 and indicated that the threshold responses required more averaging as the degree of hearing loss increased. This trend was significant with F(19,47) = 1.818, p = 0.049.
Fig. 10.
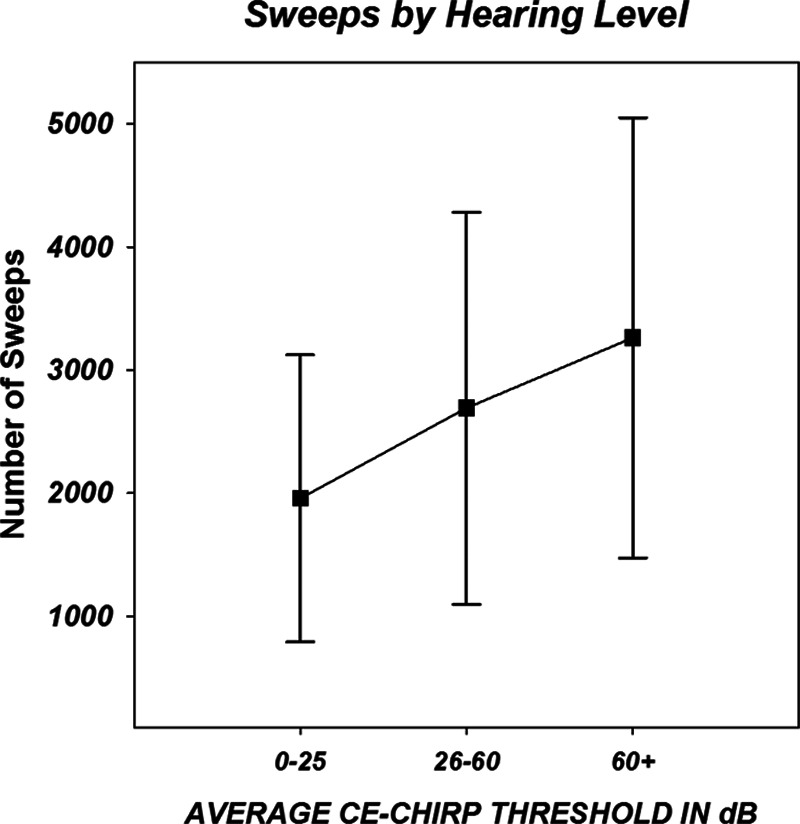
Three hearing groups were created based on their average broad-band chirp thresholds for the left and right ears. Symbols indicate the mean (across frequencies) number of sweeps needed to stop recording at auditory brainstem response (ABR) threshold level for each group. Error bars indicate 1 SD. The number of ABR sweeps required to achieve threshold increases significantly with the degree of hearing loss (see text).
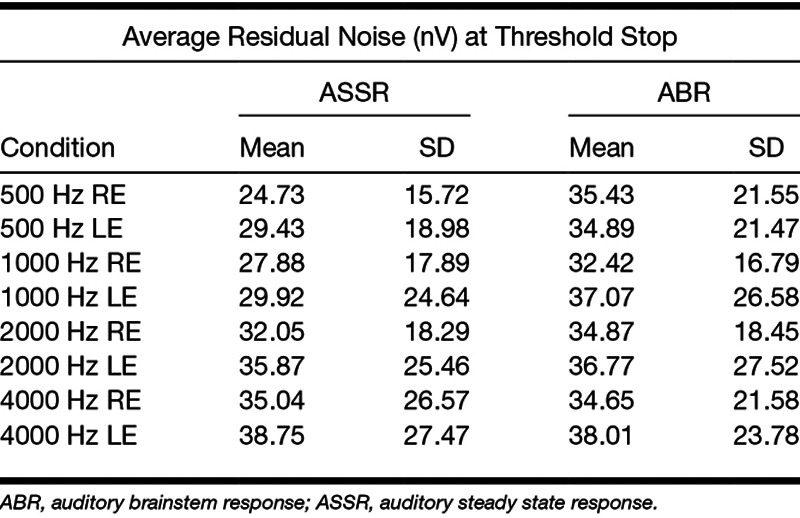
The average residual noise at threshold was computed for both ASSR and ABR techniques at each frequency by ear. These are shown in Table 6.
TABLE 6.
Average background noise in nV for the threshold average for ASSR and ABR
Normally-Hearing Group
Of the 93 children with valid tests by ABR or ASSR, 47 (51%) were found to have average thresholds of 12 dB eHL or less in both ears by one or both methods without discrepancies between the 2. Of the 41 children originally referred because of newborn hearing fails, 20 (49%) were determined to have normal hearing. Most of the children with normal hearing had 5 or more points passing on the DPOAE and were judged normal on tympanometry. Overall, of the 47 children with normal hearing, 25 (53%) were considered normal on measures of DPOAE and tympanometry in both ears and had average broad-band CE-Chirp thresholds of 20 dB or less. Otoacoustic emissions outcomes were also evaluated by overall hearing level as estimated by average ASSR thresholds. DPOAEs could be absent with any level of hearing while most children with present DPOAEs were found to have average thresholds of 20 dB or under. The maximum average hearing level for a child with 5 DPOAE points present was 36.25 dB. Sixty-two ears demonstrated both 6 points present on DPOAE and normal tympanometry. These were found to have average hearing levels of 14 dB or less.
The average thresholds in the normally-hearing group are 4.01 and 6.32 dB eHL for ASSR and ABR, respectively. The mean difference of 2.31 dB is statistically significant by paired t test (p < 0.001). The relationship between age and threshold in the normally-hearing group showed a slow decline (improvement) in thresholds with age from 0.7 to 53 months. The regression equation for ABR was y = 6.47 − 0.01 × x and for ASSR was y = 4.43 − 0.05x, where x is average threshold and y is age in months.
When evaluating electrophysiologic threshold measures, an excellent way to compare data across studies is to isolate the thresholds produced by normally-hearing subjects in each study. Our normally-hearing children revealed ASSR thresholds (SDs) of 25.0 (6.9), 16.0 (7.3), 7.8 (5.7), and 6.3 (6.8) dB nHL for 500, 1000, 2000, and 4000 Hz, respectively. By ABR, normally-hearing children revealed thresholds (SDs) of 20.19 (4.8), 13.1 (3.8), 10.1 (1.5), and 10 (0) dB nHL for 5000, 1000, 2000, and 4000 Hz, respectively.
Figure 11 shows threshold results for normally-hearing infants and children using ASSR from ours and 13 prior studies that utilized a wide variety of stimulus, recording and detection parameters. Results from the normally-hearing participants in the present study demonstrated lower ASSR thresholds than all other studies except for Rodrigues and Lewis (2014) who used the same “Next Generation” technology. The ABR thresholds produced by normally-hearing participants in this study are in good agreements with a meta-analysis by Stapells (2000) at 500 Hz and are 2 to 3 dB lower at 1000 to 4000 Hz.
Fig. 11.
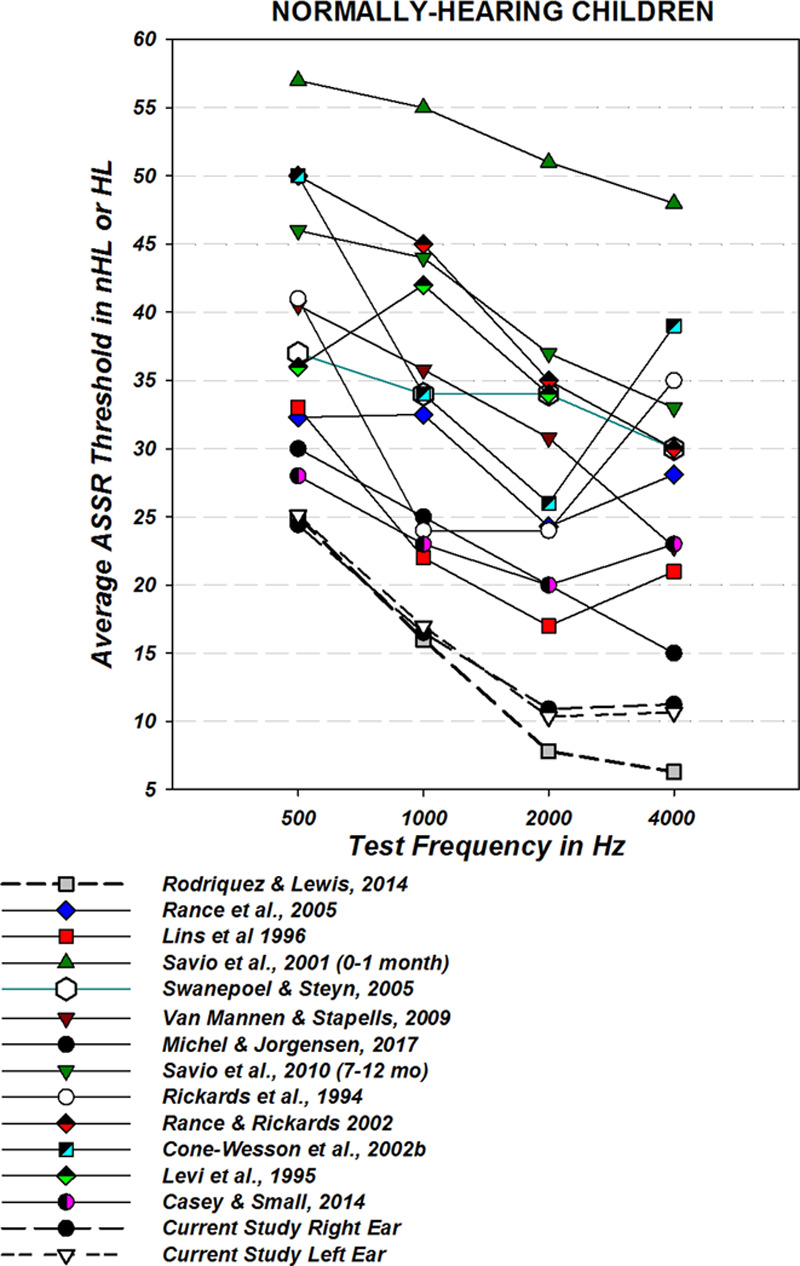
Auditory steady state response (ASSR) thresholds from infants and children with normal hearing from this study (left and right ears) compared with normally-hearing neonates from Rodrigues and Lewis (2014), who used the same methodology, and other earlier studies using a variety of technologies. Because of differences in stimuli, some are reported in HL, some have been converted to HL from SPL (John et al. 2004) and some are reported in nHL.
DISCUSSION
Comparison of Thresholds
The first experimental question of this study was “When using NB CE-Chirp stimuli for both, does Second Generation ASSR detection technology (as implemented in the Interacoustics Eclipse) reveal frequency-specific threshold predictions that are equivalent to those found by ABR (using the FMP automated detection method)?” Every effort was made to avoid any bias toward either technique including the randomization of test order.
Table 3 and Figure 5 indicate that the thresholds found by the two techniques were highly correlated with r2 values ranging from 0.769 to 0.963. Regression slopes range from 0.79 to 0.97 indicating that the relationship between the two measures were reasonably consistent across levels. However, regression shifts (Table 3) and threshold difference scores (Table 4) indicated that ASSR thresholds are consistently lower than ABR thresholds. The differences were largest at 500 Hz and progressively lower with frequency. Statistically, the threshold values were found to be different for all comparisons except 4000 Hz when all data are included. However, statistical significance in this regard is not the most relevant factor. The actual question is whether a clinician would feel that one of these tests could substitute for the other reliably. To address this question, the Bland and Altman (1995) method was applied.
The Bland–Altman method recommends that for equivalent techniques, 95% of data points should fall within 2 SDs of the mean difference score on their plots. Evaluation of the Bland–Altman plots in Figure 6 shows that more than 5% of the data points are outside the ±1.96 SD lines for the data at 500, 1000, and 2000 Hz but not for 4000 Hz.
The results from these analyses indicated that ASSR and ABR thresholds as evaluated in this study are equivalent only at 4000 Hz. Both paired t tests and the Bland–Altman analysis indicate that we cannot consider the two techniques to reveal the “equivalent” thresholds for 500, 1000, or 2000 Hz. At these frequencies, the ASSR revealed lower thresholds than the ABR. Lower thresholds in this context would be considered closer to “real or behavioral” thresholds and more sensitive.
The finding from this study that threshold estimations are lower by ASSR than by ABR is opposite to some previous findings in children (Rance et al. 2006; Van Maanen & Stapells 2010). The largest discrepancy between ABR and ASSR for Van Maanen and Stapells (2010) was at 500 Hz with ABR thresholds lower by an average of 10.7 dB. In contrast, this study found ASSR thresholds at 500 Hz to be more sensitive by an average of 9.35 dB. Therefore, the difference between studies in sensitivity of ASSR thresholds re ABR at 500 Hz is 20 dB which is substantial.
The correction factors at 500 Hz used in this study are 10 dB greater for ASSR than for ABR for thresholds of 45 dB or less. These have been recommended by the manufacturer and ASSR corrections are based on published data (Rodrigues et al. 2010) However, correction factors are complicated and dependent on many factors, particularly for ABR where protocols and detection routines are not standardized. Corrections for ABR are based on data from the United Kingdom (Stevens et al. 2013) where the protocols are slightly different. It is possible that correction factors contributed slightly to the discrepancies between ABR and ASSR found in this study.
The 20 dB increase in ASSR sensitivity at 500 Hz must be attributed to a combination of amplitude advantage afforded by the NB CE-Chirp and next-generation improvements in response detection. Five hundred Hertz has always been a difficult frequency for ASSR detection and was the focus of the study by Stürzebecher et al. (2006). This study showed the systematic improvement in detection of the 500 Hz stimulus by (1) changing from standard amplitude modulation stimulus to a 7-cosine series with modulation rate determined by the frequency spacing. The detection improved further when (2) a phase correction was applied to the cosines which imposed the appropriate cochlear delay time and improved even more when (3) a simple frequency offset was applied to the series, eliminating overlap of the cosines with the modulation harmonics used for detection. Later, Stürzebecher and Cebulla (2013) found that the table lookup for determining critical test values showed the greatest benefit in detection at 500 Hz. Certainly, the improved performance of the “Next Generation” ASSR in detection of the 500 Hz threshold can be attributed to the attention paid to this goal.
This study differs from previous comparisons of ABR and ASSR in that it employed an objective criterion, FMP, for response detection of ABR. Visual detection was also considered in the final decisions regarding threshold by ABR but very few discrepancies were noted. Consequently, ABR thresholds from this study should be in close agreement with other studies or slightly lower due to the increased averaging time allowed. As noted, the ABR thresholds achieved in this study are in agreement or very slightly better than those of the meta-analysis of Stapells (2000). This finding would lend credibility to the FMP as it agrees favorably with good “visual detection” used in the studies of the meta-analysis.
The FMP was particularly useful in determining when to stop averaging. However, if there was a discrepancy between the yes-no decision of a response by visual and FMP methods, the first author would decide. For example, the original noise-stopping rule terminated an average when the background noise reached 20 nV. This led to the stopping of several near threshold runs before the FMP criterion was reached, even though a small but clear response was present. In those cases, if a clear response was noted, the threshold was adjusted to the stopped level rather than one step above. A noise-stopping rule of 15 nV is now recommended. This discrepancy only happened near threshold where the amplitude of the response was small causing a slow rise in the FMP curve. In three other cases, electrical interference distorted waveforms and FMP values were artificially inflated and did not agree with visual detection. These cases were excluded from the analysis. After an external isolation transformer was installed, the interference did not reoccur.
Another reason why the FMP protocol should have produced accurate thresholds is that testing continued, when necessary, for longer periods of time than are generally used in ABR studies. The maximum number of sweeps was set to 6000 and this could be extended by the tester if it appeared that a pass by FMP was imminent. Typical protocols continue to 2000 sweeps and some to 3000 or 4000 which may not be adequate to resolve a very small response at threshold (Sininger 1993). This is apparent from the number of sweeps needed to achieve an adequate response at threshold being 2181 on average with a range from 800 to 8000.
Test Time
The second experimental question was whether there was a significant time advantage for ASSR when compared with ABR. The data indicate that, on average, the full audiogram (4 frequencies per ear) could be estimated in 19.71 min for ASSR and 32.38 min for ABR. This represents a 13.28 min and 41% time decrease for ASSR over ABR. These differences are statistically significant (p < 0.00) but also clinically significant. It is important to note that the largest time savings of ASSR over ABR was found for children under anesthesia where ABR average time was 40.47 min while ASSR was 19.62 which represents a 52% decrease in test time. Given the concerns with and costs of anesthesia, any decrease in test time is important. It is not certain why test time for ASSR did not change from natural sleep to anesthesia conditions. Because the test time for ASSR is under 20 min, it is possible that the natural sleeping child has ample time to be fully asleep and quiet for the entire test, much like under anesthesia.
Van Maanen and Stapells (2009) used ASSR with simultaneous four-frequency stimulation bilaterally to evaluate a group of normally-hearing infants previously tested by ABR. They reported ASSR test times as the time spent making an assessment (all frequencies and ears) at a single stimulus level. The average time per level was 6.3 min with a SD of 3.10. They also state that one to six intensities were recorded per infant. By interpolation, an average of 3.5 intensities at 6.3 min would be 22.05 min for the ASSR assessment in the normally-hearing infants which is slightly longer but in the same range of the 15.31 min (SD = 6.71) found in this study. In another study that utilized the same equipment as the current one, Venail et al. (2015) evaluated children with hearing loss and reported an average test time of 22.90 min, very close to the average 19.71 min found here. Vander Werff (2009) reported test times from adult subjects, both normally hearing and with hearing loss while comparing analysis techniques both with simultaneous binaural simulation. The average test times for 4-frequency thresholds in both ears were 46.1 and 43.6 min for the 2 techniques but test times were faster for normally-hearing subjects. Mueller et al. (2012) reported ASSR test times for adults with and without hearing loss using and an Eclipse system and found an average of 18.6 min overall with normally-hearing subjects being tested somewhat more quickly (16.1) min.
Overall, the test times for this study are well in line with most and faster than some. The Eclipse system has a feature that allows independence of frequency and levels during testing while other systems will not change stimulus level until all frequencies are ready to do so. The latter would increase the testing time.
The automated protocols of ASSR should lead to greater consistency across labs and clinics on any given model of equipment. The short test times reported here were expected as a benefit of the protocol used in this study. Testing started with an “estimate” of overall threshold levels obtained by ABR with a broad-band CE-Chirp. ASSR threshold searches for all frequencies then started with levels at or just above this threshold. The protocol also called for elimination of the 10-dB step in favor of intelligent bracketing using time-to-response as an indicator of sensation level; an ASSR that registers a response in less than a minute is likely well above threshold whereas those that require 5 min or so many be close to threshold. The protocol also called for DPOAEs and multifrequency tympanometry before electrophysiological testing. Testers expected a child with normal tympanometry and present DPOAEs and a 10 or 20 dB broad-band chirp threshold to have excellent hearing and moderate or high level thresholds were avoided during testing.
Regardless of test times being longer for ABR than ASSR, the times reported for ABR are considered excellent by most clinical standards and are clearly lower than many reported in the literature. Janssen et al. (2010) found the mean test time for an ABR protocol nearly equivalent to the one used here, to be 54.6 min compared with our finding of 32.38 min. It should be noted that the test times from this study are prorated to estimate the time needed to complete eight thresholds making the time differences even more dramatic. All 8 thresholds were obtained by ABR in 83% of subjects and 90% of subjects had 6 or more thresholds completed. For ASSR, all 8 thresholds were completed in 87% subjects and 91% had 6 or more thresholds completed.
Data from Janssen et al. (2010) on test times can be assumed to be average sleep times. The average time in natural sleep for infants was 48.4 min. The combined average times for ABR and ASSR in the present study was 52.08 min which helps to explain why both tests could be completed in 1 session for 82 subjects. It should not be necessary in a clinical setting to use both frequency-specific ABR and ASSR, but it is encouraging to know either test is estimated to take well under the expected natural sleep time.
The amplitude advantage of the NB CE-Chirp over traditional tone bursts will produce a larger response SNR that can meet a specified criterion in a shorter amount of time. While Ferm et al. (2013) employed averaging using a fixed number of sweeps (3000), they did find that the resulting FMP for NB CE-Chirp ABRs was more than twice that of the tone pip responses and acknowledged the time savings that this could afford when stopping on an SNR rule as FMP does.
The same protocol features that are mentioned for ASSR also reduced test time for the ABR. In addition, as is traditional with the ASSR, ABR averages were not repeated unless they were highly questionable. Rather than needing to see replication, the testers relied on the statistical FMP along with visual recognition of a response, for verification. Split-half averages could be viewed as well. This feature of the protocol has the potential to cut testing time in half.
Finally, the use of a statistical detection criterion has the distinct advantage of determining the number of sweeps needed to achieve a response, or a nonresponse for the exact conditions (ABR amplitude and background noise) being tested. Compared with traditional fixed sweep protocols, less averaging time is needed for suprathreshold testing and more time will be spent averaging near threshold. This has the added advantage of lowering the threshold of the response which could be lost from insufficient averaging in a fixed sweep protocol.
Test times reported here include only the actual electrophysiologic testing. Preliminary testing including wideband tympanometry, DPOAEs, and Broad-Band CE-Chirp thresholds was performed on most subjects as well. Only the chirp threshold, however, required the child to be asleep. The test time did not include any waveform marking for latency or amplitude which was done after the program timer was off. If a full audiogram can be predicted in less than 20 min, and the average sleep time is 48 min, the additional sleep time may be available for other valuable tests such as real ear to coupler measures or even for parent counseling. Most important is the very realistic expectation that the audiogram prediction can be achieved in one visit thus avoiding the chain reaction discussed in the introduction.
This study confirms what others have found that children with normal hearing can be tested in less time than their counterparts with hearing loss. This is shown in Figure 8 for both techniques. Figure 10 shows how the number of sweeps needed to reach threshold by ABR increases with hearing loss.
Normally-Hearing Subset
Half of the children evaluated including 49% of those referred by NHS were found to have normal hearing in both ears. This has been seen in previous studies (Janssen et al. 2010) and is consistent with clinical reports. The protocol for this study did not include searching to true threshold but was tested at levels below those used to define normal hearing in other places, for example Canada. We tested down to 20 dB nHL at 500 but correction factors would yield threshold predictions of 5 dB eHL for ABR and 0 dB eHL for ASSR at that level. No thresholds were corrected below 0 eHL. Corrected thresholds for 1000 Hz at 10 dB were 0 dB eHL for both techniques and 5 dB eHL for 2000 and 4000 Hz for both techniques. These excellent thresholds were predicted on average in 24.62 min for ABR and 15.31 min by ASSR. While these are not “true” thresholds, they are very close to 0 dB and stopping represented a trade-off regarding test time. Establishing normal responses that are close to threshold is certainly preferable to a “screening” type measure where the threshold is essentially unknown. Children who need follow-up in the future will have a true baseline on which to judge any changes in hearing, mild asymmetries can be revealed and without time restrictions there seems to be no good reason not to test at low levels. This, of course, is a clinical decision but may be viewed more positively if time constraints are reduced.
Having the DPOAE and tympanometry information ahead of time was valuable in terms of planning the full electrophysiologic assessment. Absent DPOAEs at all frequencies was not found to be a good predictor of hearing levels but present ones were. Also, in the decision regarding whether to use test time for bone conduction measures, the wideband tympanometry can be very helpful. Bone conduction was often omitted in the test battery of this study. It was employed for 11 cases and tested with a wideband CE-Chirp by ABR. Nine cases had confirmed sensorineural hearing loss by bone conduction, one was clearly conductive and one was inconclusive. This study had time constraints due to the need to test both technologies in the experimental protocol. In a clinical test battery, where either ABR or ASSR (but not both) is used, there should be adequate time for a complete assessment of bone conduction when thresholds are elevated.
Figure 11 reveals the wide variations in ASSR results from the past. The spread of values for “normal” thresholds predicted by ASSR is as much as 40 dB or more with stimuli calibrated in nHL. These results certainly have contributed to a lack of confidence in the ASSR technique. There appears to be a lowering of the thresholds with time, based on the dates of the studies, which must relate to improvements in technique particularly for response detection. This figure should reassure users of ASSR that a lack of sensitivity seen in some implementations of the technique are not inherent in the ASSR strategy, but simply represent detection technology that was not fully developed.
The results from this study (right and left ears) and Rodrigues and Lewis (2014) are clearly lower than found in other studies, nearly identical and both used the “Next Generation” detection and NB CE-Chirp stimuli with the Eclipse system. The reason for slightly higher thresholds found in this study relative to Rodrigues and Lewis relates to the 10 dB nHL stopping rule for 2000 and 4000 Hz while Rodrigues and Lewis sought true thresholds. The thresholds for 500 and 1000 are nearly identical.
One other study, Michel and Jørgensen (2017) used the same technology as this study for a group of children with hearing loss and normal hearing and yet, their normally-hearing group demonstrated higher thresholds than this study or Rodrigues and Lewis (2014; see Fig. 19). For their normal group of infants <12 weeks of age, they found thresholds of 30, 25, 20, and 15 dB nHL for 500, 1000, 2000, and 4000 Hz, respectively. Older normally-hearing children had slightly higher thresholds in their study. Careful reading of the Michel and Jørgensen (2017) study finds that assessments of the normally-hearing group were stopped before reaching threshold as they were “found to have normal hearing.” This is the only possible explanation for the differences as other methods were consistent with this study.
There are many possible reasons why this study found lower thresholds for normally-hearing children than others and certainly, whether threshold is truly sought, or testing stops at suprathreshold levels was a factor. The acoustic environment of the testing, the type of stimulus used, and the age of subjects can all influence thresholds obtained with ASSR along with many other factors. The NB CE-Chirp undoubtedly contributed to low thresholds. The sensitivity of the detection algorithm and control of maximum allowable noise levels, however, may have had the largest influence. The features of what we call “Next Generation” detection, including the assessment at 12 modulation harmonics, rather than one, and the use of both phase and amplitude information, rather than one or the other as well as the careful calculation of appropriate test criterion all must contribute to the speed, accuracy, and sensitivity of this ASSR system.
The ABR thresholds from normally-hearing infants in this study are in line with previous studies. Our thresholds ranged from 10 to 20.1 dB nHL before corrections. Stapells et al. (1995) found thresholds of 13.2 to 15.9 dB nHL and Sininger et al. (1997) had a range from 6 to 16 dB n HL for normally-hearing infants. The agreement among these studies is good although the latter two included threshold searches to 0 dB while the present study stopped at low, but suprathreshold levels.
The consistency of automated detection as implemented with any given ASSR technology is a factor that should be considered when deciding whether to utilize ABR or ASSR. Clinicians can expect to have the accuracy and test time results seen in this study when utilizing the same technology for testing, given that good testing technique and environment are maintained. However, unless the nontraditional protocol for ABR was adopted, it is not clear that an audiologist could expect the good ABR results that are presented here. In addition to improved accuracy and speed, the use of ASSR will make testing more consistent across clinics and testers.
In conclusion, this study demonstrated that both ABR with automated detection and Next Generation ASSR, each using NB CE-Chirp stimuli, give consistent predictions of audiometric thresholds in a time frame that is reasonable for testing nonsedated infants and toddlers. ASSR as executed in this study will produce lower (better) thresholds in considerably less time and should be considered an excellent choice for electrophysiologic audiometric testing.
ACKNOWLEDGMENTS
The authors acknowledge funding for this study from the Oticon Foundation of Denmark. The authors thank the conscientious audiology staff members who provided feedback on protocols and patient data, including Sue Windmill, AuD, Carrie Wingo, AuD, Kelly Baroch, AuD and Gayle Riemer, MA in Cincinnati, Emily Spitzer, BS, Lauren Okulski, AuD, Shana Jacobs, AuD. Lauren Johnson, AuD, Mallory Baker, AuD, and Sarah Martino, AuD in Chapel Hill and Erica Schicke, AuD and Tamara Scott, AuD in Denver. Study coordinators were invaluable in enrolling subjects and managing information. Amanda Ruiz served as study coordinator in Denver and Morgan Bamberger, MS, was coordinator in Cincinnati. Jane Gralla, University of Colorado, provide invaluable advice on statistical analyses.
Footnotes
This research was funded by the Oticon Foundation in a grant to the first author. The REDcap data system was provided by NIH/NCRRCTSI Grant number UL1TR001082. Y.S.S. and L.L.H. have received speaking honoraria from Interacoustics Company.
The authors have no conflicts of interest to disclose.
REFERENCES
- Attias J., Karawani H., Shemesh R., et al. Predicting hearing thresholds in occupational noise-induced hearing loss by auditory steady state responses. Ear Hear, 2014). 35, 330–338.. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet, 1995). 346, 1085–1087.. [DOI] [PubMed] [Google Scholar]
- Casey K. A., Small S. A. Comparisons of auditory steady state response and behavioral air conduction and bone conduction thresholds for infants and adults with normal hearing. Ear Hear, 2014). 35, 423–439.. [DOI] [PubMed] [Google Scholar]
- Cebulla M., Elberling C. Auditory brain stem responses evoked by different chirps based on different delay models. J Am Acad Audiol, 2010). 21, 452–460.. [DOI] [PubMed] [Google Scholar]
- Cebulla M., Stürzebecher E. Automated auditory response detection: Further improvement of the statistical test strategy by using progressive test steps of iteration. Int J Audiol, 2015). 54, 568–572.. [DOI] [PubMed] [Google Scholar]
- Cebulla M., Stürzebecher E., Elberling C. Objective detection of auditory steady-state responses: Comparison of one-sample and q-sample tests. J Am Acad Audiol, 2006). 17, 93–103.. [DOI] [PubMed] [Google Scholar]
- Cone-Wesson B., Dowell R. C., Tomlin D., et al. The auditory steady-state response: Comparisons with the auditory brainstem response. J Am Acad Audiol, 2002a). 13, 173–187.. [PubMed] [Google Scholar]
- Cone-Wesson B., Rickards F., Poulis C., et al. The auditory steady-state response: Clinical observations and applications in infants and children. J Am Acad Audiol, 2002b). 13, 270–282.. [PubMed] [Google Scholar]
- Dau T., Wegner O., Mellert V., et al. Auditory brainstem responses with optimized chirp signals compensating basilar-membrane dispersion. J Acoust Soc Am, 2000). 107, 1530–1540.. [DOI] [PubMed] [Google Scholar]
- de Boer E. A cylindrical cochlea model: The bridge between two and three dimensions. Hear Res, 1980). 3, 109–131.. [DOI] [PubMed] [Google Scholar]
- Don M., Elberling C. Evaluating residual background noise in human auditory brain-stem responses. J Acoust Soc Am, 1994). 96(5 Pt 1), 2746–2757.. [DOI] [PubMed] [Google Scholar]
- Don M., Elberling C. Use of quantitative measures of auditory brain-stem response peak amplitude and residual background noise in the decision to stop averaging. J Acoust Soc Am, 1996). 99, 491–499.. [DOI] [PubMed] [Google Scholar]
- Don M., Elberling C., Waring M. Objective detection of averaged auditory brainstem responses. Scand Audiol, 1984). 13, 219–228.. [DOI] [PubMed] [Google Scholar]
- Elberling C., Don M. Quality estimation of averaged auditory brainstem responses. Scand Audiol, 1984). 13, 187–197.. [DOI] [PubMed] [Google Scholar]
- Elberling C., Don M. Auditory brainstem responses to a chirp stimulus designed from derived-band latencies in normal-hearing subjects. J Acoust Soc Am, 2008). 124, 3022–3037.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberling C., Don M. A direct approach for the design of chirp stimuli used for the recording of auditory brainstem responses. J Acoust Soc Am, 2010). 128, 2955–2964.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberling C., Wahlgreen O. Estimation of auditory brainstem response, ABR, by means of Bayesian inference. Scand Audiol, 1985). 14, 89–96.. [DOI] [PubMed] [Google Scholar]
- Elberling C., Callo J., Don M. Evaluating auditory brainstem responses to different chirp stimuli at three levels of stimulation. J Acoust Soc Am, 2010). 128, 215–223.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberling C., Don M., Cebulla M., et al. Auditory steady-state responses to chirp stimuli based on cochlear traveling wave delay. J Acoust Soc Am, 2007). 122, 2772–2785.. [DOI] [PubMed] [Google Scholar]
- Ferm I., Lightfoot G. Further comparisons of ABR response amplitudes, test time, and estimation of hearing threshold using frequency-specific chirp and tone pip stimuli in newborns: Findings at 0.5 and 2 kHz. Int J Audiol, 2015). 54, 745–750.. [DOI] [PubMed] [Google Scholar]
- Ferm I., Lightfoot G., Stevens J. Comparison of ABR response amplitude, test time, and estimation of hearing threshold using frequency specific chirp and tone pip stimuli in newborns. Int J Audiol, 2013). 52, 419–423.. [DOI] [PubMed] [Google Scholar]
- Firszt J. B., Gaggl W., Runge-Samuelson C. L., et al. Auditory sensitivity in children using the auditory steady-state response. Arch Otolaryngol Head Neck Surg, 2004). 130, 536–540.. [DOI] [PubMed] [Google Scholar]
- Holte L., Walker E., Oleson J., et al. Factors influencing follow-up to newborn hearing screening for infants who are hard of hearing. Am J Audiol, 2012). 21, 163–174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R. M., Usher L., Stapells D. R. The British Columbia’s Children’s Hospital tone-evoked auditory brainstem response protocol: How long do infants sleep and how much information can be obtained in one appointment? Ear Hear, 2010). 31, 722–724.. [DOI] [PubMed] [Google Scholar]
- JCIH. (Year 2007 Position Statement: Priniciples and Guidelines for Early Hearing Detection and Intervention Programs. Pediatrics, 2007). 120, 23. [DOI] [PubMed] [Google Scholar]
- John M. S., Brown D. K., Muir P. J., Picton T. W. Recording auditory steady-state responses in young infants. Ear Hear, 2004). 25, 539–553.. [DOI] [PubMed] [Google Scholar]
- Korczak P., Smart J., Delgado R., et al. Auditory steady-state responses. J Am Acad Audiol, 2012). 23, 146–170.. [DOI] [PubMed] [Google Scholar]
- Kristensen S. G., Elberling C. Auditory brainstem responses to level-specific chirps in normal-hearing adults. J Am Acad Audiol, 2012). 23, 712–721.. [DOI] [PubMed] [Google Scholar]
- Levi E. C., Folsom R. C., Dobie R. A. Coherence analysis of envelope-following responses (EFRs) and frequency-following responses (FFRs) in infants and adults. Hear Res, 1995). 89, 21–27.. [DOI] [PubMed] [Google Scholar]
- Lins O. G., Picton T. W., Boucher B. L., et al. Frequency-specific audiometry using steady-state responses. Ear Hear, 1996). 17, 81–96.. [DOI] [PubMed] [Google Scholar]
- Luts H., Desloovere C., Kumar A., et al. Objective assessment of frequency-specific hearing thresholds in babies. Int J Pediatr Otorhinolaryngol, 2004). 68, 915–926.. [DOI] [PubMed] [Google Scholar]
- McCreery R. W., Kaminski J., Beauchaine K., et al. The impact of degree of hearing loss on auditory brainstem response predictions of behavioral thresholds. Ear Hear, 2015). 36, 309–319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Jørgensen K. F. Comparison of threshold estimation in infants with hearing loss or normal hearing using auditory steady-state response evoked by narrow band CE-chirps and auditory brainstem response evoked by tone pips. Int J Audiol, 2017). 56, 99–105.. [DOI] [PubMed] [Google Scholar]
- Muhler R., Mentzel K., Verhey J. Fast hearing-threshold estimation using multiple auditory steady-state responses with narrow-band chirps and adaptive stimulus patterns. Sci World J, 2012). 2012, 192178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoe M. A., Bradley A. P., Wilson W. J. On chirp stimuli and neural synchrony in the suprathreshold auditory brainstem response. J Acoust Soc Am, 2010). 128, 235–246.. [DOI] [PubMed] [Google Scholar]
- Rance G., Rickards F. Prediction of hearing threshold in infants using auditory steady-state evoked potentials. J Am Acad Audiol, 2002). 13, 236–245.. [PubMed] [Google Scholar]
- Rance G., Tomlin D., Rickards F. W. Comparison of auditory steady-state responses and tone-burst auditory brainstem responses in normal babies. Ear Hear, 2006). 27, 751–762.. [DOI] [PubMed] [Google Scholar]
- Rance G., Roper R., Symons L., et al. Hearing threshold estimation in infants using auditory steady-state responses. J Am Acad Audiol, 2005). 16, 291–300.. [DOI] [PubMed] [Google Scholar]
- Rickards F. W., Tan L. E., Cohen L. T., et al. Auditory steady-state evoked potential in newborns. Br J Audiol, 1994). 28, 327–337.. [DOI] [PubMed] [Google Scholar]
- Rodrigues G. R., Lewis D. R. Establishing auditory steady-state response thresholds to narrow band CE-chirps(®) in full-term neonates. Int J Pediatr Otorhinolaryngol, 2014). 78, 238–243.. [DOI] [PubMed] [Google Scholar]
- Rodrigues G. R. I., Lewis D. R., Fichino S. N. Steady-state auditory evoked responses in audiological diagnosis in children: A comparison with brainstem evoked auditory responses. Braz J Otorhinolaryngol, 2010). 76, 96–101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. R. I., Ramos N., Lewis D. R. Comparing auditory brainstem responses (ABRs) to toneburst and narrow band CE-chirp in young infants. Int J Pediatr Otorhinolaryngol, 2013). 77, 1555–1560.. [DOI] [PubMed] [Google Scholar]
- Savio G., Cárdenas J., Pérez Abalo M., et al. The low and high frequency auditory steady state responses mature at different rates. Audiol Neurootol, 2001). 6, 279–287.. [DOI] [PubMed] [Google Scholar]
- Sininger Y. S. Auditory brain stem response for objective measures of hearing. Ear Hear, 1993). 14, 23–30.. [DOI] [PubMed] [Google Scholar]
- Sininger Y. S., Abdala C., Cone-Wesson B. Auditory threshold sensitivity of the human neonate as measured by the auditory brainstem response. Hear Res, 1997). 104, 27–38.. [DOI] [PubMed] [Google Scholar]
- Stapells D. R. Threshold estimation by the tone-evoked auditory brainstem response: A literature meta-analysis. J Speech Language Pathol Audiol, 2000). 24, 9. [Google Scholar]
- Stapells D. R., Gravel J. S., Martin B. A. Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear, 1995). 16, 361–371.. [DOI] [PubMed] [Google Scholar]
- Stevens J., Sutton G., Wood S. Guidelines for the early audiological assessment and management of babies referred from the Newborn Hearing Screening Programme Version 3.1. National Health Service, Screening Programmes:Newborn Hearing, 2013). 44. [Google Scholar]
- Stürzebecher E., Cebulla M. Automated auditory response detection: Improvement of the statistical test strategy. Int J Audiol, 2013). 52, 861–864.. [DOI] [PubMed] [Google Scholar]
- Stürzebecher E., Cebulla M., Elberling C. Automated auditory response detection: Statistical problems with repeated testing. Int J Audiol, 2005). 44, 110–117.. [DOI] [PubMed] [Google Scholar]
- Stürzebecher E., Cebulla M., Pschirrer U. Efficient stimuli for recording of the amplitude modulation following response. Audiology, 2001). 40, 63–68.. [PubMed] [Google Scholar]
- Stürzebecher E., Cebulla M., Wernecke K. Objective response detection in the frequency domain: Comparison of several q-sample tests. Audiol Neurootol, 1999). 4, 2–11.. [DOI] [PubMed] [Google Scholar]
- Stürzebecher E., Cebulla M., Elberling C., et al. New efficient stimuli for evoking frequency-specific auditory steady-state responses. J Am Acad Audiol, 2006). 17, 448–461.. [DOI] [PubMed] [Google Scholar]
- Swanepoel D., Ebrahim S. Auditory steady-state response and auditory brainstem response thresholds in children. Eur Arch Otorhinolaryngol, 2009). 266, 213–219.. [DOI] [PubMed] [Google Scholar]
- Swanepoel d. e. W., Steyn K. Short report: Establishing normal hearing for infants with the auditory steady-state response. S Afr J Commun Disord, 2005). 52, 36–39.. [PubMed] [Google Scholar]
- Van Maanen A., Stapells D. R. Normal multiple auditory steady-state response thresholds to air-conducted stimuli in infants. J Am Acad Audiol, 2009). 20, 196–207.. [DOI] [PubMed] [Google Scholar]
- Van Maanen A., Stapells D. R. Multiple-ASSR thresholds in infants and young children with hearing loss. J Am Acad Audiol, 2010). 21, 535–545.. [DOI] [PubMed] [Google Scholar]
- Vander Werff K. R. Accuracy and time efficiency of two ASSR analysis methods using clinical test protocols. J Am Acad Audiol, 2009). 20, 433–452.. [DOI] [PubMed] [Google Scholar]
- Venail F., Artaud J. P., Blanchet C., et al. Refining the audiological assessment in children using narrow-band CE-Chirp-evoked auditory steady state responses. Int J Audiol, 2015). 54, 106–113.. [DOI] [PubMed] [Google Scholar]


