Highlights
-
•
In the early stages of COVID-19 respiratory failure, a trial of non-invasive ventilation CPAP mode under close monitoring seems reasonably safe.
-
•
CPAP failure patients had a lower DTF, experienced more ICU days and longer in-hospital length of stay.
-
•
In the context of critically ill patients with COVID-19 respiratory failure admitted to ICU, DTF could be a predictor of CPAP failure.
-
•
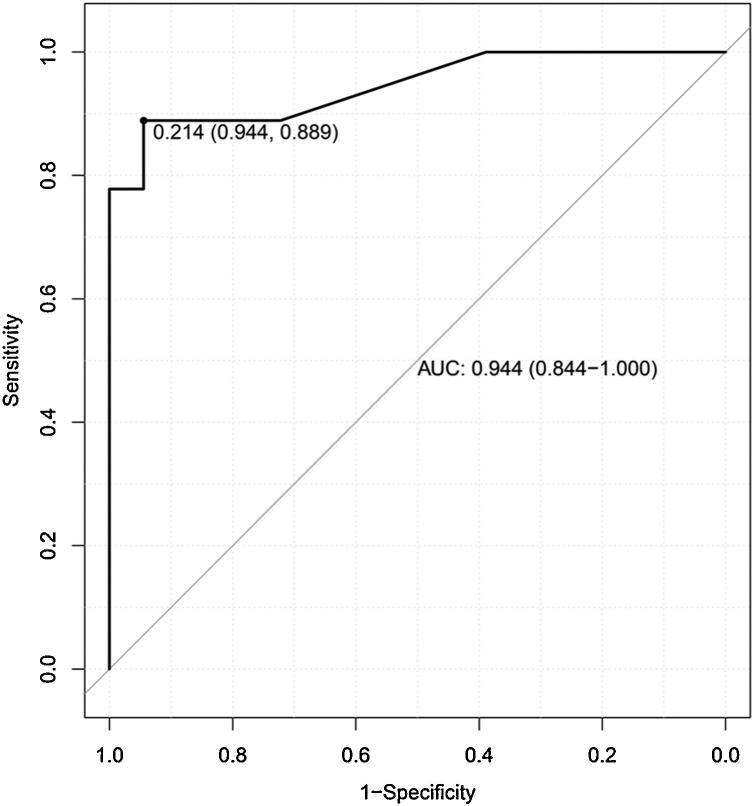
The DTF best threshold value was 21% (AUC: 0.944, CI: 0.844-0.999; 94 % sensitivity and 78 % specificity).
Keywords: Covid-19, Non-invasive ventilation, ICU, Respiratory failure, Prediction, Diaphragmatic thickening fraction
Abstract
Background
In a variable number of Covid-19 patients with acute respiratory failure, non-invasive breathing support strategies cannot provide adequate oxygenation, thus making invasive mechanical ventilation necessary. Factors predicting this unfavorable outcome are unknown, but we hypothesized that diaphragmatic weakness may contribute.
Methods
We prospectively analyzed the data of 27 consecutive patients admitted to the general Intensive Care Unit (ICU) from March 19, 2020, to April 20, 2020 and submitted to continuous positive airway pressure (CPAP) before considering invasive ventilation. Diaphragmatic thickening fraction (DTF) inferred by ultrasound was determined before applying CPAP.
Results
Eighteen patients recovered with CPAP, whereas nine required invasive mechanical ventilation with longer stay in ICU (p < 0.001) and hospital (p = 0.003). At univariate logistic regression analysis, CPAP failure was significantly associated with low DTF [β: -0.396; OR: 0.673; p < 0.001] and high respiratory rate [β: 0.452; OR: 1.572; p < 0.001] but only DTF reached statistical significance at multivariate analysis [β: -0.384; OR: 0.681; p < 0.001]. The DTF best threshold predicting CPAP failure was 21.4 % (AUC: 0.944; sensitivity: 94.4 %, specificity: 88.9 %).
Conclusions
In critically ill patients with Covid-19 respiratory failure admitted to ICU, a reduced DTF could be a potential predictor of CPAP failure and requirement of invasive ventilation.
1. Background
In the early stages of Covid-19 respiratory failure, a trial of non-invasive breathing support with high flow nasal oxygen, continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV) under close monitoring seems reasonably safe. (Vitacca et al., 2020) In some of the earlier studies on Covid-19 the use of NIV was reported without details on methods and results. (Bhatraju et al., 2020; Grasselli et al., 2020; Yang et al., 2020)
More recently, the systematic use of CPAP to avoid or delay oro-tracheal intubation has been reported in three studies. (Aliberti et al., 2020; Brusasco et al., 2020; Oranger et al., 2020) The percentage of CPAP failure range from 17 % to 50 %. Reasons for these different findings maybe the criteria for intubation and patient severity. However, at variance with acute respiratory distress syndrome associated with conditions other than Covid-19 pneumonia(Maiolo et al., 2018), neither arterial hypoxemia nor lung density resulted to be significant predictors of CPAP failure.(Brusasco et al., 2020) In planning this study we reasoned that the rapid increase in dyspnea and deterioration in gas exchange in critically ills Covid-19 patients may be associated with an inadequacy of diaphragmatic function to cope with an increased inspiratory elastic load.
Indeed, diaphragmatic dysfunction has ben reported in a large proportion of critically ill patients at the time of ICU admission (Demoule et al., 2013) and possibly associated with early multi-organ dysfunction (Ricoy et al., 2019; Vetrugno et al., 2019) and alteration of mitochondrial function. (Duan and Bai, 2020)
Ultrasound has been used for the assessment of diaphragm function in different clinical situations including respiratory failure requiring non-invasive ventilation.(Cammarota et al., 2019; Marchioni et al., 2019; Ricoy et al., 2019) In particular the diaphragmatic thickening fraction (DTF) has been shown to correlate with pressure-generating capacity of the muscle (Ueki et al., 1995), work of breathing and respiratory effort. (Umbrello et al., 2015; Vivier et al., 2012)
Although the ultrasound evaluation of the diaphragmatic function has been proved to be a feasible and reproducible method (Garofalo et al., 2019), no studies have yet been conducted in Covid-19 respiratory failure. Therefore, we plan this study to evaluate whether measurement of DTF may be a non-invasive method clinically useful for identifying patients at risk of CPAP and need of invasive mechanical ventilation (IMV).
2. Methods
The local ethics committee approved the study, waiving written consent owing to the observational design. (CEAVNO 230,320). We prospectively studied Covid-19 patients admitted to the Covid-19 Intensive Care Unit in Pisa from March 19, 2020, to April 20, 2020 and submitted to CPAP due to acute respiratory failure. Inclusion criteria were: laboratory-confirmed Covid-19 infection with radiologically confirmed interstitial pneumonia. According to the initial management strategy established by our institution, all patients with early Covid-19 were submitted to CPAP and no patients underwent directly IMV unless admitted with the tracheal tube already in place. No patients were excluded from the study. The following clinical data were prospectively collected: age, sex, heart rate, respiratory rate, arterial pressure, body temperature, and gas exchange at intensive care unit (ICU) admission before starting CPAP, history of chronic obstructive lung disease, cardiovascular disease, diabetes, chronic kidney disease, or cerebrovascular disease.
2.1. Study protocol
Initial treatment strategy in the ward was initially determined based on the ratio between the arterial oxygen pressure (PaO2) and the fraction of inspired oxygen (FiO2) while breathing room air, respiratory rate (RR), or presence of dyspnea. Patients with pulse oxygen saturation (SpO2) <95 % or the ratio of arterial oxygen pressure to inspired oxygen fraction (PaO2/FiO2) <300 were given oxygen support via Ventimask. Indication to CPAP was posed in cases with one or more of these criteria: PaO2/FiO2 ratio <200 mmHg, PaO2 <60 mmHg, RR > 30/min, and worsening dyspnea at rest and/or during minimal efforts. No patient have been previously submitted to any type of non-invasive mechanical support because at the time of the inclusion in the study CPAP was managed only in negative pressure rooms inside ICU due to safety issues in order to minimize Covid-19 spread. Criteria for starting IMV were the following: persistence of severe respiratory failure with PaO2/FiO2 ratio <200 mmHg, respiratory rate still >30 breaths/min, PaO2 <60 mmHg despite increasing FiO2 and/or clinical signs of respiratory exhaustion. (Brusasco et al., 2020)
A CPAP helmet interface was applied in all patients. A CPAP flow generator with a monitor (Dimar, Mirandola, Italy) was used. The CPAP flow-generator system, connected to high-pressure O2 wall port, can provide airflow outputs up to 150 L/min and on-line monitoring of FIO2, airflow output, PEEP, RR, cardiac rate, SpO2 and the temperature inside the helmet. The circuits downstream of Venturi flow generators had anti-bacterial and anti-viral filters added to the outflow port immediately before the PEEP valve to minimize contamination of room air. Filters added to the Venturi inlet served to reduce noise and patient discomfort, as well as to purify the air entering the system. Airflow was set to the highest possible value (150 L/min), PEEP to 10 cmH2O, and FiO2 to between 40 and 70 %, depending on PaO2. All patients were in semi-supine or sitting positions during CPAP.
All patients underwent diaphragm ultrasound at baseline before applying CPAP to measure DTF in the zone of apposition of the diaphragm to the rib cage. The linear probe was placed above the right 10th rib on the mid-axillary line. The inferior border of the costo-phrenic sinus was identified as the zone of transition from the artifactual representation of normal lung to the visualization of the diaphragm and liver. In this area, the diaphragm appears as a three-layer structure: a non-echogenic middle layer bordered by two echogenic layers, i.e., peritoneum and diaphragmatic pleurae. Measurements were repeated at least once until consistently within 10 % of variation; the mean of three measurements was used for analysis. The diaphragmatic thickening fraction (DTF) was calculated as follows:
| DTF = (End inspiratory thickness – End expiratory thickness) / End − expiratory thickness x 100. |
Ultrasound measurements were made by an intensivist (FC) not involved in the clinical management of the patients, and the measurements were not disclosed to the operators involved in the clinical management (i.e., the decision whether to intubate the patient or not). Patients were followed-up until hospital discharge for the following events: intubation, ICU discharge, or death.
2.2. Statistical analysis
The results are expressed as mean ± standard deviation, median, interquartile range (IQR) or percentage (%). Mann Whitney test or Fisher's exact test were used to evaluating differences between groups. Cumulative probability for lack of adverse outcomes in patients with or without low baseline diaphragm muscle mass was calculated with the Kaplan-Meier product-limit estimator. The end of follow-up for censored/uncensored patients corresponded to hospital discharge/occurrence of complications. The log-rank (Mantel-Cox) test was applied to evaluate the difference in probability for lack of complications after grouping for low baseline diaphragm muscle mass or not. Logistic regression was used to identify variables potentially associated with adverse outcomes, and those with significant P values at univariate analysis were entered into a multivariate logistic regression model. The Hosmer-Lemeshow omnibus test was used for goodness-of-fit evaluation of each logistic regression model. Only converged regression models that passed the goodness-of-fit test were reported. Regression coefficient (β) and odds ratio (OR) with the corresponding 95 % confidence interval (CI) were assumed as outputs of the logistic regression models. The Akaike information criterion (AIC) was also calculated (Akaike, 1974). For the multivariate logistic regression model, both sensitivity/specificity and the receiver operating characteristic (ROC) curve were evaluated. The accuracy of the DTF for adverse event occurrence was assessed using the ROC curves. A test's false positive rate (x-axis) is plotted in a ROC curve versus its sensitivity (y-axis). For each ROC curve, the area under the curve (AUC) with the corresponding 95 % CI was provided. The AUC is the conventional summary measure for the accuracy of a ROC curve, ranging from 0.5 (chance) to 1.0 (full accuracy). An optimal DTF cut-point that maximizes sensibility and specificity on the ROC curve was also calculated. Statistical significance was assumed with a two-tailed P value <0.05. Statistical analysis was performed by using the R environment (version 3.6.3, R Foundation for Statistical Computing. Vienna, Austria) with the pROC package (Robin et al., 2011) and SPSS (version 20.0; SPSS Inc, Chicago, Ill).
3. Results
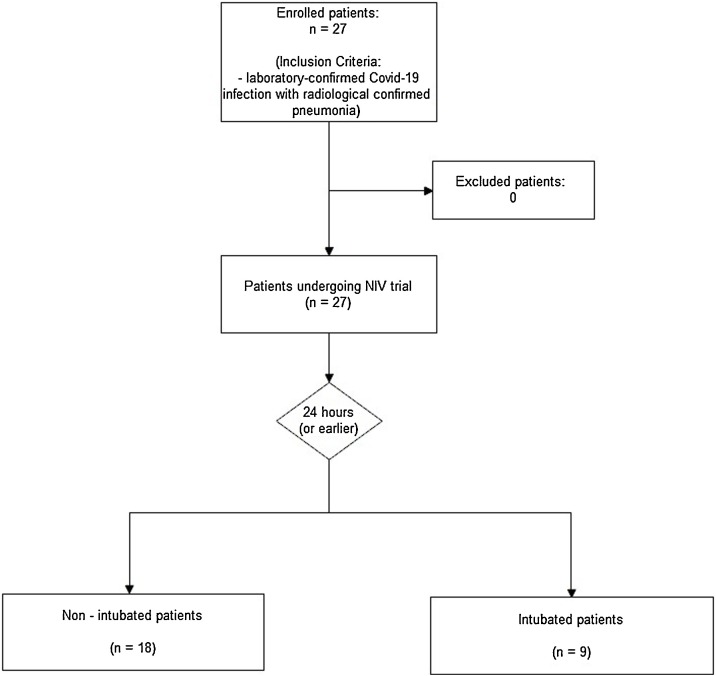
Over the study period (Fig. 1 ), we treated 27 consecutive patients in the ICU (23 males). The demographic characteristic of the study population and their comorbidity are showed in Table 1 . The median age was 66 years (IQR 57–73). The median lung attenuation on computer tomography was -692 HU (-751 to -652), indicating moderate-to-severe lung involvement, which was not significantly different between CPAP responders (R) and those who did not (NR) and required to be intubated (-703 vs.−663 HU, p = 0.145). Median length of CPAP support was 3 days (2–6) without statistically significant differences between R and NR [3 (2–9) vs 3 (2–5); p = 0.381]. Twenty-four patients (85 %) successfully recovered and were discharged with a median ICU length of stay of 9 (3–12) days. Nine patients (33 %) worsened their PaO2/FiO2 from 196 mmHg (165–242) vs to 90 mmHg (86–106) mmHg; p < 0.001] despite an initial improvement in gas exchange and experienced a late CPAP failure eventually requiring IMV. Finally 3 of them died in ICU. The median time span between hospital admittance and DTF exam was 2 days (0–6), and the time spam between DTF assessment and oro-tracheal intubation was 3 days (2–5). Patients requiring IMV had a significantly lower DTF at ICU admission [12 % (range 25−31) vs. 27 % (range 25−31), p < 0.001] as well as lower end-expiratory diaphragmatic thickness (1.9 ± 0.2 mm vs 2.2 ± 0.2; p = 0.013) but not a statistically significant difference in PaO2/FiO2 ratio [195 (167–269) vs 195 (168–246) mmHg; p = 0.82]. Patients requiring IMV experienced also more ICU days compared with those who did not (25 vs. 5 days, p < 0.001) and longer in-hospital length of stay (39 vs. 25 days, p = 0.003).
Fig. 1.
Study design: All critically ill patients with Covid-19 respiratory failure from the ED and admitted to ICU were treated for at least 24 h with non-invasive ventilation. At the end of this period they were re-evaluated, and a weaning trial was carried out. Based on the clinical decision of the doctors, the patient was intubated and subjected to invasive ventilation or continued with CPAP. At the same time, another operator not involved in the clinical management of the patient performed ultrasound measurements of the diaphragmatic functionality.
Table 1.
Clinical characteristics, laboratory data and imaging findings at intensive care unit admission. Data are median with interquartile range (IQR) or absolute numbers and percentage (%). COPD, chronic obstructive pulmonary disease; Bpm, beats per minute; PaO2/FiO2, ratio of arterial oxygen pressure to inspired oxygen fraction breathing room air; CT, chest tomography; HU, Hounsfield Units; US, ultrasound; DTF, diaphragmatic thickening fraction.
| Characteristics | All patients (27) | CPAP success (18) | CPAP failure (9) | p |
|---|---|---|---|---|
| Age (years) | 66 (57−73) | 61 (57−68) | 71 (50−74) | 0.433 |
| Male sex | 23 (85 %) | 16 (89 %) | 7 (78 %) | 0.582 |
| SAPS | 21 (18−26) | 21 (17−24) | 23 (20−30) | 0.275 |
| RASS | 0 | 0 | 0 | – |
| KMS | 2 | 2 | 2 | – |
| Coexisting disorders | ||||
| Cardiovascular disease | 8 (29) | 5 (28) | 3 (33) | 0.550 |
| Hypertension | 14 (50) | 7 (39) | 6 (67) | 0.236 |
| COPD | 3 (11) | 3 (17) | 0 (0) | 0.529 |
| Diabetes | 3 (11) | 2 (11) | 1 (11) | 0.750 |
| Chronic kidney disease | 2 (7) | 1 (6) | 1 (11) | 0.564 |
| Solid cancer | 2 (7) | 2 (11) | 0 (0) | 0.538 |
| Vital signs | ||||
| Temperature (°C) | 36.6 (36−37) | 36.8 (36−37) | 36 (36−37) | 0.433 |
| SpO2 (%) | 98(97−100) | 98 (97−99) | 99 (96−100) | 0.403 |
| Heart rate (bpm) | 74 (66−91) | 73 (65−89) | 84 (66−100) | 0.463 |
| Respiratory rate (breath/min) | 24 (20−26) | 22 (20−24) | 28 (24−29) | 0.004 |
| Systolic blood pressure (mmHg) | 137 (124−149) | 135 (123−148) | 147 (124−151) | 0.403 |
| Diastolic blood pressure (mmHg) | 70 (61−83) | 71 (61−84) | 63 (58−82) | 0.433 |
| PaO2/FiO2 ratio (mmHg) | 195 (168−246) | 195 (167−269) | 196 (165−243) | 0.820 |
| P(A-a)O2 | 215 (196−243) | 217(196−244) | 205 (185−311) | 0.887 |
| pH | 7.45 (7.33−7.47) | 7.46 (7.43−7.47) | 7.41 (7.33−7.46) | 0.043 |
| PaO2 (mmHg) | 96 (81−117) | 94 (72−117) | 98 (92−117) | 0.298 |
| PaCO2(mmHg) | 38 (36−43) | 37 (35−39) | 49 (39−65) | 0.007 |
| HCO3 (mEq/L) | 25 (24−29) | 25 (24.6−27.8) | 27 (22−32) | 0.900 |
| BE | 3 (1−5) | 3.4 (1.1−5.1) | 1.4 (-6 – 6) | 0.403 |
| Lactate (mmol/L) | 1.1 (0.7−1.4) | 1.1 (0.8−1.4) | 1.1 (0.7−1.9) | 0.703 |
| Quantitative CT | ||||
| Mean lung density (HU) | −692 (-652; -751) | −703 (-665; -757) | −663 (-592; -734) | 0.145 |
| Diaphragmatic US | ||||
| DTF (%) | 27 (17−30) | 27 (25−31) | 12 (10−18) | <0.001 |
Data are median with interquartile range (IQR) or absolute numbers and percentage (%). COPD, chronic obstructive pulmonary disease; Bpm, beats per minute; PaO2/FiO2, ratio of arterial oxygen pressure to inspired oxygen fraction breathing room air; P(A-a)O2, alveolo-arterial oxygen gradient; CT, chest tomography; HU, Hounsfield Units; US, ultrasound; DTF, diaphragmatic thickening fraction.
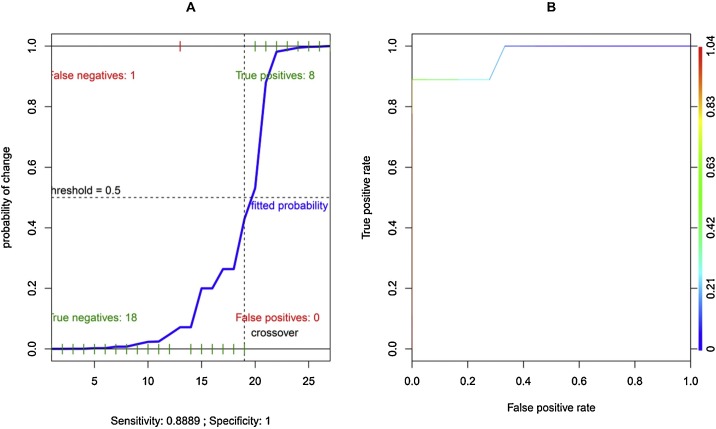
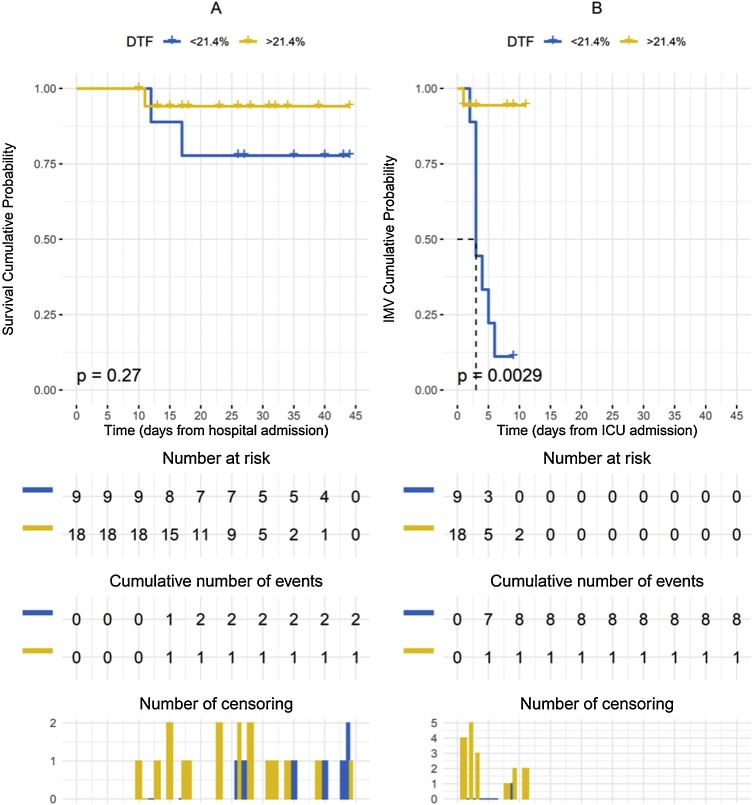
In the univariate logistic regression models performed for CPAP failure, only DTF [β: -0.396; OR: 0.673; p < 0.001] and respiratory rate [β: 0.452; OR: 1.572; p < 0.001] resulted statistically significant (Table 2 ). In the subsequent multivariate model, only DTF reached statistical significance [β: -0.384; OR: 0.681; p < 0.001]. The multivariate model presented an AIC = 16.532. By adjusting multivariate model for patient age and sex, a critical AIC increase occurred (AIC: 698.12). No statistical significance occurred for correlation analysis performed by entering DTF and RR (r = -0.324; 95 % CI from -0.627 to -0.0629; p = 0.098). The global performance of the original unadjusted multivariate model and the corresponding ROC curve (AUC: 0.966) are showed in Fig. 2 . The accuracy of the DTF for adverse event occurrence returned 21.4 % as the best threshold value (AUC: 0.944; sensitivity: 94.4 %, specificity: 88.9 %) (Fig. 3 ). Comparison of the Kaplan–Meier curves by stratifying patients for DTF higher or lower than 21.4 % returned no significant difference (p = 0.270) for survival (Fig. 4 A), while a significant difference (p = 0.028) for cumulative probability of IMV was found in favor of patients with DTF > 21.4 % (Fig. 4B).
Table 2.
Univariate and multivariate logistic regression of potential predictive parameters at intensive care unit admission for non-invasive ventilation failure (only converged models are reported). β indicates regression coefficients; CI, confidence intervals; OR, odds ratio; PaO2/FiO2, ratio of arterial oxygen pressure to inspired oxygen fraction breathing room air; CT, chest tomography; HU, Hounsfield Units; DTF, diaphragmatic thickening fraction.
| Parameter | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| β | OR | 95 %CI | p | β | OR | 95 %CI | p | |
| Age (years) | 0.021 | 1.021 | 0.947 - 1.111 | 0.598 | Not entered | |||
| Sex (F) | −0.827 | 0.438 | 0.044 - 4.247 | 0.454 | Not entered | |||
| SAPS | 0.072 | 1.075 | 0.958 - 1.227 | 0.222 | Not entered | |||
| Temperature | −0.904 | 0.405 | 0.088 - 1.554 | 0.320 | Not entered | |||
| SpO2 | −0.000 | 1.000 | 0.996 - 1.002 | 0.728 | Not entered | |||
| Heart rate | 0.022 | 1.002 | 0.975 - 1.076 | 0.362 | Not entered | |||
| Respiratory rate | 0.452 | 1.572 | 1.128 - 2.498 | <0.001 | 0.588 | 1.800 | 1.032 - 10.999 | 0.105 |
| Systolic blood pressure | 0.011 | 1.011 | 0.964 - 1.066 | 0.649 | Not entered | |||
| Diastolic blood pressure | −0.023 | 0.977 | 0.913 - 1.038 | 0.460 | Not entered | |||
| PaO2/FiO2 ratio | −0.004 | 0.996 | 0.980 - 1.012 | 0.641 | Not entered | |||
| PaO2 | 0.019 | 1.019 | 0.979 - 1.064 | 0.353 | Not entered | |||
| HCO3 | 0.045 | 1.046 | 0.842 - 1.303 | 0.679 | Not entered | |||
| Lactate | 0.765 | 2.148 | 0.681 - 11.696 | 0.197 | Not entered | |||
| CT Mean lung density | 0.009 | 1.009 | 0.998 - 1.024 | 0.113 | Not entered | |||
| DTF | −0.396 | 0.673 | 0.447 - 0.837 | <0.001 | −0.384 | 0.681 | 0.350 - 0.875 | <0.001 |
B, regression coefficients; CI, confidence intervals; OR, odds ratio; PaO2/FiO2, ratio of arterial oxygen pressure to inspired oxygen fraction breathing room air; CT, chest tomography; DTF, diaphragmatic thickening fraction.
Fig. 2.
A: Global performance of the multivariate logistic regression model for non-invasive ventilation failure (independent variables: diaphragmatic thickening fraction and respiratory rate). B: ROC curve of the same multivariate logistic regression model.
Fig. 3.
Receiver operator characteristic (ROC) curve for diaphragm thickening fraction (DTF) related to continuous positive airway pressure success/failure (AUC: area under the curve with the 95 % confidence interval). The best DTF value (0.214) that maximize sensibility (0.944) and specificity (0.889) is reported.
Fig. 4.
A: Comparison of the Kaplan–Meier curves for cumulative probability of survival in patients with a diaphragmatic thickening fraction (DTF) higher or lower than 21.4 %, by censoring patients at hospital discharge (p = 0.270). B: Comparison of the Kaplan–Meier curves for cumulative probability of adverse outcomes (IMV) in patients with DTF higher or lower than 21.4 %, by censoring patients at intensive care unit (ICU) discharge (p = 0.003).
4. Discussion
Through our study, we were able to identify DTF as a predictor of failure during a CPAP in patients with Covid-19 related acute respiratory failure. The DTF is a parameter that inversely correlates with the success of a CPAP, i.e., the lower the values, the more likely the CPAP failure. Furthermore, the AUC found for DTF is greater than 90 %. The DTF is related to the patient's ability to support the respiratory effort. A reduced DTF correlates with the need for invasive ventilatory support, as an indication of muscle exhaustion. A correlation between reduced peripheral muscle strength and the reduced respiratory reserve has been demonstrated in chronic interstitial disease models.(Holland, 2010; Kabitz et al., 2006) Furthermore, the lung aeration appears to correlate with diaphragmatic contractility. Xia et al., have shown that the increased workload imposed on the diaphragm by reducing the aerated parenchyma, determines a greater thickening capacity.(Xia et al., 2019) However, several studies have shown that oxidative mechanisms, mitochondrial impairment, and cytokine action (including interleukin 6 and tumor-necrosis factor α) contribute together determining the activation of catabolic processes of the diaphragm.(Duan and Bai, 2020) From the data in our possession, we cannot correlate the degree of aeration or the plasma cytokine levels to support the respiratory muscle load. Further studies in this direction would be required. However, the muscle deconditioning phenomena affecting the diaphragm of patients with Covid-19 related acute respiratory failure would not appear to be different from those that occur during other forms of respiratory failure. In this aspect, Covid-19 patients do not differ much from patients with non Covid-19 respiratory failure.
A recent meta-analysis has verified that the best predictor of weaning failure from invasive ventilation is the diaphragm thickening fraction (sensitivity = 0.76 and specificity = 0.86).(Medrinal et al., 2020) Besides, diaphragmatic dysfunction, defined as a reduced maximum thickening fraction during the maximum inspiratory pressure, correlates with the failure of NIV in COPD patients.(Marchioni et al., 2018) Since the 90 s is known that the force produced by the diaphragmatic contraction correlates with the ability to support the ventilatory effort (Spicer et al., 1997), but only in recent years ultrasound techniques have been used to evaluate the diaphragmatic function.(Tuinman et al., 2020) The sonographic techniques used to evaluate the diaphragmatic activity are reproducible and, although there is no standardization of the method, the literature has shown that there is no substantial bias whatever the technique used.(Kalın and Gürsel, 2019) In the present study we didn’t explore the ability of ultrasound measured diaphragmatic displacement but only DTF. We choose DTF instead of ultrasonographic diaphragmatic displacement because of the complex interplay between the rib cage, abdomen and diaphragmatic excursion, especially when resistive loads are applied, being diaphragm motion affected by the abdominal contents and pressure that limit diaphragm displacement.(Houston et al., 1994) Moreover, diaphragmatic displacements are influenced by the body position of the subject, being greater in the supine position than in the sitting position for the same inspiratory volume.(Wait et al., 1989) However, the combination of diaphragmatic displacement and rapid shallow breathing index has been showed to be effective in the weaning context (Spadaro et al., 2016), therefore deserves to be further investigated also in this setting.
Ultrasound evaluation in Covid-19 patients has already been shown to be useful for the diagnosis of lung involvement by SARS-CoV2 and monitoring clinical progress (Vetrugno et al., 2020) as previously reported in other clinical contexts.(Vezzani et al., 2014) The advantages of the method reside in evaluating the patient directly at the bedside, reducing the operators' exposure, and not subjecting the patient to ionizing radiation. The same advantages are applicable in the evaluation of the diaphragmatic activity. The method allows to quickly and early evaluate the patient's ability to support the respiratory effort, theoretically before the metabolic alterations related to respiratory fatigue (i.e., respiratory acidosis) are established. Initially, the organism tends to compensate the respiratory failure by increasing the respiratory rate. On the one hand, these mechanisms determine a temporary compensation of the gas exchange deficit, on the other, they establish an inefficient ventilation mode.(Yan et al., 1993)
This hypothesis seems to be confirmed by our data showing that CPAP failure had higher baseline respiratory rate and PaCO2 and lower pH, despite SO2 and PaO2/FiO2 ratio being insignificantly different from patient with CPAP success. These findings can be explained by an increase in death space associated with rapid shallow breathing due to the inability to increase minute ventilation by increasing tidal volume in CPAP failure possibly related to the reduced DTF.
We acknowledge that some of patients of the CPAP failure group having hypercapnia would have benefited from non-invasive ventilation with inspiratory pressure support or even immediate IMV. Nevertheless, this does not invalidate the conclusion that requirement of IMV was associated with reduced DTF, whether alveolar hypoventilation was already present or not.
Several authors have placed the attention on the fact that the type of respiratory failure induced by Covid-19 cannot be substantially superimposed on the classic forms (i.e., ARDS), even if they fall within the classic definitions (i.e., Berlin definition).(Tobin et al., 2020) At least initially, the pathological process induced by SARS-CoV2 determines hypoxemia – often of severe degree – not associated with muscle fatigue, and therefore, without causing so-called respiratory pump failure. Therefore, the early use of intubation has been debated, and this approach is not widely shared.(Oranger et al., 2020) If this approach should be confirmed in large cases, the discrimination of patients who, after the first phase of non-invasive ventilatory support, require invasive support, compared to those who can continue with a non-invasive strategy, becomes crucial.
Dyspnea is difficult to be graded by a scale because unpleasant breathing can be recognized only by a patient and it is purely a subjective symptom and patients vary widely in behavioral responses to discomfort thus leading the risk to overestimate or underestimate the symptom.(Tobin, 1990) Usually, the decision whether to resort to invasive ventilation or not is established on the basis not only of the blood gas analysis values but also on the so-called "clinical impression" or "gestalt": a patient who activates the accessory respiratory muscles, which presents the activation of the sympathetic system (tachycardic, sweaty, etc.), is a patient who will have to undergo intubation. However, this approach is not sensitive enough and became overt in a phase in which the compensation mechanisms are already outdated.(Al-Rajhi et al., 2018)
Ultrasound evaluation in Covid-19 patients has already been shown to be useful for the diagnosis of lung involvement by SARS-CoV2 and monitoring clinical progress.(Guarracino et al., 2020; Vetrugno et al., 2020) The advantages of the method reside in evaluating the patient directly at the bedside, reducing the operators' exposure, and not subjecting the patient to ionizing radiation. The same advantages are applicable in the evaluation of the diaphragmatic activity. The method allows to quickly and early evaluate the patient's ability to support the respiratory effort, theoretically before the metabolic alterations related to respiratory fatigue (i.e., respiratory acidosis) are established. Experimental studies have shown that the reduction of muscle fibers (and their possible replacement with lipid-rich myocytes) represents an early process that can be evident after 6 h of mechanical ventilation.(Corpeno et al., 2014) Therefore, as we found, diaphragmatic impairment is an early event that predicts the best time to start an invasive ventilation strategy.
4.1. Study limitations
Although we have found statistical outputs that support the potential role of DTF to predict CPAP failure in critically ill Covid-19 patients, our study has several limitations. The most important is clearly represented by the low number of enrolled patients, with a marked prevalence of male patients. In addition, only the original non-adjusted multivariate regression model returned reliable results, while the evaluation of adjusted models for patients’ age and sex produced outputs with high AIC values and information loss. On the other hand, the lower reliability of adjusted models may reflect both the very limited number of female patients (n = 4; 15 %) and the relatively small IQR for patient’s age.
Second, we only measure the DTF, which must be related to shortening and in term to displacement. As displacement depends on the balance between the force generated by the muscle, geometry factors and the load the muscle has to work against, our data do not allowed to make any conclusion on force generating capacity. Third, parameters and settings of ultrasound device could possibly affect DTF measurements, which makes our cutoff values not generalizable. Fourth, in our ICU the non-invasive management strategy was made by CPAP and our results cannot be generalized to other non-invasive ventilation modes. Thus, our results are to be taken as a proof-of-concept for a larger study.
5. Conclusions
In critically ill Covid-19 patients admitted to ICU for acute respiratory failure due to Covid-19 and treated with CPAP, low DTF represents a promising predictor of CPAP failure.
Funding
No fund was used for this study.
Consent for publication
The local ethics committee approved the study, waiving written consent owing to the observational design and the urgent need for patient care and data collection. (CEAVNO 230,320).
Availability of data and material
Data are available following a reasoned request.
Authors' contributions
FC designed the study, collected the data, performed the statistical analysis, drafted the second draft, and supervised the final draft; LV designed the study, and supervised the final draft; DO designed the study, performed the statistical analysis, drafted the first draft; TB supervised the final draft; AS supervised the final draft; EB collected the data; GS performed the statistical analysis, drafted the second draft and supervised the final draft; AI supervised the final draft; GB performed the statistical analysis; FF supervised the final draft.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
None.
References
- Akaike H. A new look at the statistical model identification. IEEE T Automat Contr. 1974;19:716–723. [Google Scholar]
- Aliberti S., Radovanovic D., Billi F., Sotgiu G., Costanzo M., Pilocane T., Saderi L., Gramegna A., Rovellini A., Perotto L., Monzani V., Santus P., Blasi F. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicenter, cohort study. Eur. Respir. J. 2020 doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rajhi A., Murad A., Li P.Z., Shahin J. Outcomes and predictors of failure of non-invasive ventilation in patients with community acquired pneumonia in the ED. Am. J. Emerg. Med. 2018;36:347–351. doi: 10.1016/j.ajem.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., Kritek P.A., West T.E., Luks A., Gerbino A., Dale C.R., Goldman J.D., O’Mahony S., Mikacenic C. Covid-19 in critically ill patients in the Seattle region - case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusasco C., Corradi F., Di Domenico A., Raggi F., Timossi G., Santori G., Brusasco V., Galliera CPAP-Covid-19 study group Continuous positive airway pressure in Covid-19 patients with moderate-to-severe respiratory failure. Eur. Respir. J. 2020 doi: 10.1183/13993003.02524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G., Sguazzotti I., Zanoni M., Messina A., Colombo D., Vignazia G.L., Vetrugno L., Garofalo E., Bruni A., Navalesi P., Avanzi G.C., Della Corte F., Volpicelli G., Vaschetto R. Diaphragmatic ultrasound assessment in subjects with acute hypercapnic respiratory failure admitted to the emergency department. Respir. Care. 2019;64:1469–1477. doi: 10.4187/respcare.06803. [DOI] [PubMed] [Google Scholar]
- Corpeno R., Dworkin B., Cacciani N., Salah H., Bergman H.-M., Ravara B., Vitadello M., Gorza L., Gustafson A.-M., Hedström Y., Petersson J., Feng H.-Z., Jin J.-P., Iwamoto H., Yagi N., Artemenko K., Bergquist J., Larsson L. Time course analysis of mechanical ventilation-induced diaphragm contractile muscle dysfunction in the rat. J. Physiol. (Lond.) 2014;592:3859–3880. doi: 10.1113/jphysiol.2014.277962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoule A., Jung B., Prodanovic H., Molinari N., Chanques G., Coirault C., Matecki S., Duguet A., Similowski T., Jaber S. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am. J. Respir. Crit. Care Med. 2013;188:213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- Duan H., Bai H. Is Mitochondrial Oxidative Stress the Key Contributor to Diaphragm Atrophy and Dysfunction in Critically Ill Patients? Crit. Care Res. Pract. 2020;2020:8672939. doi: 10.1155/2020/8672939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo E., Bruni A., Pelaia C., Landoni G., Zangrillo A., Antonelli M., Conti G., Biasucci D.G., Mercurio G., Cortegiani A., Giarratano A., Vetrugno L., Bove T., Forfori F., Corradi F., Vaschetto R., Cammarota G., Astuto M., Murabito P., Bellini V., Zambon M., Longhini F., Navalesi P., Bignami E. Comparisons of two diaphragm ultrasound-teaching programs: a multicenter randomized controlled educational study. Ultrasound J. 2019;11:21. doi: 10.1186/s13089-019-0137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A., COVID-19 Lombardy ICU Network, Nailescu A., Corona A., Zangrillo A., Protti A., Albertin A., Forastieri Molinari A., Lombardo A., Pezzi A., Benini A., Scandroglio A.M., Malara A., Castelli A., Coluccello A., Micucci A., Pesenti A., Sala A., Alborghetti A., Antonini B., Capra C., Troiano C., Roscitano C., Radrizzani D., Chiumello D., Coppini D., Guzzon D., Costantini E., Malpetti E., Zoia E., Catena E., Agosteo E., Barbara E., Beretta E., Boselli E., Storti E., Harizay F., Della Mura F., Lorini F.L., Donato Sigurtà F., Marino F., Mojoli F., Rasulo F., Grasselli G., Casella G., De Filippi G., Castelli G., Aldegheri G., Gallioli G., Lotti G., Albano G., Landoni G., Marino G., Vitale G., Battista Perego G., Evasi G., Citerio G., Foti G., Natalini G., Merli G., Sforzini I., Bianciardi L., Carnevale L., Grazioli L., Cabrini L., Guatteri L., Salvi L., Dei Poli M., Galletti M., Gemma M., Ranucci M., Riccio M., Borelli M., Zambon M., Subert M., Cecconi M., Mazzoni M.G., Raimondi M., Panigada M., Belliato M., Bronzini N., Latronico N., Petrucci N., Belgiorno N., Tagliabue P., Cortellazzi P., Gnesin P., Grosso P., Gritti P., Perazzo P., Severgnini P., Ruggeri P., Sebastiano P., Covello R.D., Fernandez-Olmos R., Fumagalli R., Keim R., Rona R., Valsecchi R., Cattaneo S., Colombo S., Cirri S., Bonazzi S., Greco S., Muttini S., Langer T., Alaimo V., Viola U. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarracino F., Vetrugno L., Forfori F., Corradi F., Orso D., Bertini P., Ortalda A., Federici N., Copetti R., Bove T. Lung, heart, vascular, and diaphragm ultrasound examination of COVID-19 patients: a comprehensive approach. J. Cardiothorac. Vasc. Anesth. 2020 doi: 10.1053/j.jvca.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A.E. Exercise limitation in interstitial lung disease - mechanisms, significance and therapeutic options. Chron. Respir. Dis. 2010;7:101–111. doi: 10.1177/1479972309354689. [DOI] [PubMed] [Google Scholar]
- Houston J.G., Angus R.M., Cowan M.D., McMillan N.C., Thomson N.C. Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax. 1994;49:500–503. doi: 10.1136/thx.49.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabitz H.-J., Lang F., Walterspacher S., Sorichter S., Müller-Quernheim J., Windisch W. Impact of impaired inspiratory muscle strength on dyspnea and walking capacity in sarcoidosis. Chest. 2006;130:1496–1502. doi: 10.1378/chest.130.5.1496. [DOI] [PubMed] [Google Scholar]
- Kalın B.S., Gürsel G. Does it make difference to measure diaphragm function with M mode (MM) or B mode (BM)? J. Clin. Monit. Comput. 2019 doi: 10.1007/s10877-019-00432-7. [DOI] [PubMed] [Google Scholar]
- Maiolo G., Collino F., Vasques F., Rapetti F., Tonetti T., Romitti F., Cressoni M., Chiumello D., Moerer O., Herrmann P., Friede T., Quintel M., Gattinoni L. Reclassifying acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2018;197:1586–1595. doi: 10.1164/rccm.201709-1804OC. [DOI] [PubMed] [Google Scholar]
- Marchioni A., Castaniere I., Tonelli R., Fantini R., Fontana M., Tabbì L., Viani A., Giaroni F., Ruggieri V., Cerri S., Clini E. Ultrasound-assessed diaphragmatic impairment is a predictor of outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease undergoing noninvasive ventilation. Crit Care. 2018;22:109. doi: 10.1186/s13054-018-2033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioni A., Tonelli R., Fantini R., Tabbì L., Castaniere I., Livrieri F., Bedogni S., Ruggieri V., Pisani L., Nava S., Clini E. Respiratory mechanics and diaphragmatic dysfunction in COPD patients who failed non-invasive mechanical ventilation. Int. J. Chron. Obstruct. Pulmon. Dis. 2019;14:2575–2585. doi: 10.2147/COPD.S219125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrinal C., Combret Y., Hilfiker R., Prieur G., Aroichane N., Gravier F.-E., Bonnevie T., Contal O., Lamia B. ICU outcomes can be predicted by non invasive muscle evaluation: a meta-analysis. Eur. Respir. J. 2020 doi: 10.1183/13993003.02482-2019. [DOI] [PubMed] [Google Scholar]
- Oranger M., Gonzalez-Bermejo J., Dacosta-Noble P., Llontop C., Guerder A., Trosini-Desert V., Faure M., Raux M., Decavele M., Demoule A., Morélot-Panzini C., Similowski T. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur. Respir. J. 2020 doi: 10.1183/13993003.01692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoy J., Rodríguez-Núñez N., Álvarez-Dobaño J.M., Toubes M.E., Riveiro V., Valdés L. Diaphragmatic dysfunction. Pulmonology. 2019;25:223–235. doi: 10.1016/j.pulmoe.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro S., Grasso S., Mauri T., Dalla Corte F., Alvisi V., Ragazzi R., Cricca V., Biondi G., Di Mussi R., Marangoni E., Volta C.A. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit Care. 2016;20:305. doi: 10.1186/s13054-016-1479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer M., Hughes P., Green M. A non-invasive system to evaluate diaphragmatic strength in ventilated patients. Physiol. Meas. 1997;18:355–361. doi: 10.1088/0967-3334/18/4/008. [DOI] [PubMed] [Google Scholar]
- Tobin M.J. Dyspnea. Pathophysiologic basis, clinical presentation, and management. Arch. Intern. Med. 1990;150:1604–1613. doi: 10.1001/archinte.150.8.1604. [DOI] [PubMed] [Google Scholar]
- Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinman P.R., Jonkman A.H., Dres M., Shi Z.-H., Goligher E.C., Goffi A., de Korte C., Demoule A., Heunks L. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020;46:594–605. doi: 10.1007/s00134-019-05892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki J., De Bruin P.F., Pride N.B. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax. 1995;50:1157–1161. doi: 10.1136/thx.50.11.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbrello M., Formenti P., Longhi D., Galimberti A., Piva I., Pezzi A., Mistraletti G., Marini J.J., Iapichino G. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161. doi: 10.1186/s13054-015-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrugno L., Guadagnin G.M., Barbariol F., Langiano N., Zangrillo A., Bove T. Ultrasound imaging for diaphragm dysfunction: a narrative literature review. J. Cardiothorac. Vasc. Anesth. 2019;33:2525–2536. doi: 10.1053/j.jvca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Vetrugno L., Bove T., Orso D., Barbariol F., Bassi F., Boero E., Ferrari G., Kong R. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A., Manca T., Brusasco C., Santori G., Valentino M., Nicolini F., Molardi A., Gherli T., Corradi F. Diagnostic value of chest ultrasound after cardiac surgery: a comparison with chest X-ray and auscultation. J. Cardiothorac. Vasc. Anesth. 2014;28:1527–1532. doi: 10.1053/j.jvca.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Vitacca M., Nava S., Santus P., Harari S. Early consensus management for non-ICU ARF SARS-CoV-2 emergency in Italy: from ward to trenches. Eur. Respir. J. 2020 doi: 10.1183/13993003.00632-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Mekontso Dessap A., Dimassi S., Vargas F., Lyazidi A., Thille A.W., Brochard L. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012;38:796–803. doi: 10.1007/s00134-012-2547-7. [DOI] [PubMed] [Google Scholar]
- Wait J.L., Nahormek P.A., Yost W.T., Rochester D.P. Diaphragmatic thickness-lung volume relationship in vivo. J. Appl. Physiol. 1989;67:1560–1568. doi: 10.1152/jappl.1989.67.4.1560. [DOI] [PubMed] [Google Scholar]
- Xia J., Qian C.-Y., Yang L., Li M.-J., Liu X.-X., Yang T., Lu Q. Influence of lung aeration on diaphragmatic contractility during a spontaneous breathing trial: an ultrasound study. J. Intensive Care. 2019;7:54. doi: 10.1186/s40560-019-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Sliwinski P., Gauthier A.P., Lichros I., Zakynthinos S., Macklem P.T. Effect of global inspiratory muscle fatigue on ventilatory and respiratory muscle responses to CO2. J. Appl. Physiol. 1993;75:1371–1377. doi: 10.1152/jappl.1993.75.3.1371. [DOI] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available following a reasoned request.