Abstract
Hepatocellular carcinoma (HCC) is frequently associated with macrovascular invasion of the portal vein or hepatic veins in advanced stages. The accurate diagnosis of macrovascular invasion and the differentiation from bland non-tumoral thrombus has significant clinical and management implications, since it narrows the therapeutic options and it represents a mandatory contraindication for liver resection or transplantation. The imaging diagnosis remains particularly challenging since the imaging features of HCC with macrovascular invasion may be subtle, especially in lesions showing infiltrative appearance. However, each radiologic imaging modality may provide findings suggesting the presence of tumor thrombus rather than bland thrombus. The purpose of this paper is to review the current guidelines and imaging appearance of HCC with macrovascular invasion. Knowledge of the most common imaging features of HCC with macrovascular invasion may improve the diagnostic confidence of tumor thrombus in clinical practice and help to guide patients’ management.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the third leading cause of cancer death worldwide (1). HCC most commonly arises in the background of cirrhosis secondary to viral hepatitis, nonalcoholic steatohepatitis, or alcohol abuse. The prognosis of patients with HCC largely depends on the stage of the disease at the time of the diagnosis. According to the Barcelona Clinic Liver Cancer (BCLC) staging criteria, HCC patients should be classified as advanced stage once macrovascular invasion has manifested (2). Macrovascular invasion is associated with an extremely poor prognosis, with a median survival of 6 to 8 months (3). Moreover, its diagnosis significantly narrows the therapeutic options in HCC patients, due to the high rate of tumor recurrence, and it represents one of the most common tumor-related contraindications for locoregional treatments with curative intent (3).
The diagnosis of HCC in cirrhotic or high-risk patients is reached noninvasively with different contrast-enhanced imaging modalities when demonstrating the typical HCC landmarks in lesions larger than 1 cm, including arterial phase hyperenhancement (APHE), washout on portal venous or delayed phases, and the presence of enhancing capsule (3, 4). Although each imaging modality also provides suggestive or conclusive imaging features to rule out the presence of macrovascular invasion, the diagnosis remains challenging in clinical practice. Histopathologic characterization of thrombus is considered the reference standard for the diagnosis of macrovascular invasion, but it is usually not feasible in routine clinical practice since it is an invasive procedure not immune to complications or sampling errors (3).
Macrovascular invasion often coexists with advanced HCC or lesions with infiltrative appearance. However, the latter refers to the macroscopic growth pattern characterized by tumor nodular spreading throughout cirrhotic parenchyma with permeative growth (Fig. 1), regardless of the presence of tumor thrombus (5). The presence of infiltrative HCC makes the diagnosis of macrovascular invasion challenging since discrete enhancing HCC nodules are difficult to distinguish from background cirrhosis and it may not be visualized in up to 40% of patients (5, 6). Moreover, macrovascular invasion is not pathognomonic for HCC. Other non-HCC hepatic malignancies (i.e., intrahepatic cholangiocarcinoma, combined hepatocellular-cholangiocarcinoma) may occasionally manifest with tumor thrombus (7). Prior studies have reported macrovascular invasion in 10%–24% of non-HCC primary hepatic malignancies, including intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma (8, 9). Cirrhotic patients also tend to develop nontumoral portal vein thrombosis in up to 16% of cases, especially in Child-Pugh B-C stages, which may resolve with anticoagulant therapy (3). Therefore, the accurate imaging diagnosis of HCC with macrovascular invasion has important implications for patients’ treatment and management.
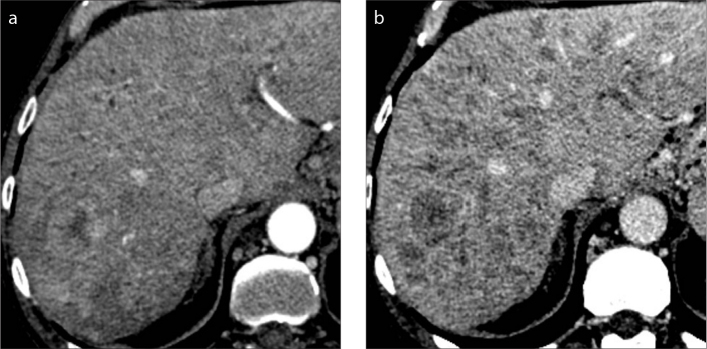
Figure 1. a, b.
A 61-year-old woman with hepatitis C virus (HCV)-related cirrhosis and infiltrative HCC. Contrast-enhanced CT on hepatic arterial (a) and portal venous (b) phases show innumerable tumor nodules with permeative growth spreading through the liver parenchyma with mild heterogeneous enhancement and washout on portal venous phase.
This article aims to review the current guidelines and multimodality imaging features of HCC with macrovascular invasion and to provide pearls for a noninvasive imaging diagnosis.
Macrovascular invasion in current HCC guidelines
Even though several scientific organizations have developed different definitions and diagnostic criteria for the diagnosis of HCC, the presence of macrovascular invasion, alternatively termed as “tumor thrombus” or “tumor in vein” (TIV), is considered to be one of the worst prognostic factors in the management of HCC (3, 4, 7, 10). According to the clinical practical guidelines recently released by the European Association for the Study of the Liver (EASL) (3), macrovascular invasion of the main portal vein or hepatic veins represents an absolute contraindication for liver resection or transplantation due to the low survival and high post-transplant recurrence. The two imaging findings recommended by EASL for the diagnosis of macrovascular invasion on contrast-enhanced imaging include the presence of arterial phase hyperenhancement and restricted diffusion within the portal thrombus (3). Similarly, the Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines (10) mention the presence of vascular infiltration as a mandatory contraindication for liver transplantation.
The LI-RADS perspective
The Liver Imaging Reporting and Data System (LI-RADS), released by the American College of Radiology and endorsed by the American Association for the Study of Liver Diseases (AASLD) practice guidance, aims to standardize the interpretation of liver lesions based on the probability of being HCC in high-risk patients (4, 7). The classification of lesions with macrovascular invasion has been significantly redefined and evolved among the subsequent LI-RADS versions (11). Initially, HCC with the presence of TIV was categorized as LR-5V in the LI-RADS v2013 and v2014. The LI-RADS v2017 included a major update for the classification of HCC with macrovascular invasion, renaming LR-5V as LR-TIV, since also other non-HCC primary hepatic malignancies may manifest with macrovascular invasion (Fig. 2). The LR-TIV is defined as “unequivocal enhancing soft tissue in vein, regardless of visualization of parenchymal mass” (Table) (7). Other imaging features that suggest the presence of TIV but do not establish its diagnosis according to LI-RADS algorithm are: occluded vein with ill-defined walls, occluded vein with restricted diffusion, occluded or obscured vein in contiguity with malignant parenchymal mass or heterogeneous vein enhancement not attributable to artifacts (7). Finally, in the latest CT/MRI LI-RADS v2018 algorithm the LR-TIV was sub-classified in three categories including: “LR-TIV, definitely due to HCC”, if in continuity with a LR-5 observation; “LR-TIV, may be due to non-HCC malignancy”, if adjacent to a targetoid mass; otherwise “LR-TIV, probably due to HCC” (Table) (7). A recent study performed by Ludwig et al. (12) demonstrated that the combination of LR-TIV definitely due to HCC and LR-5 had a sensitivity of 57%–67% and a specificity of 85%–90% as predictor of HCC. On the contrary, the categorization as “LR-TIV may be due to non-HCC malignancy” combined with LR-M showed a sensitivity of 76%–87% and a specificity of 75%–89% as predictor of non-HCC malignancies.
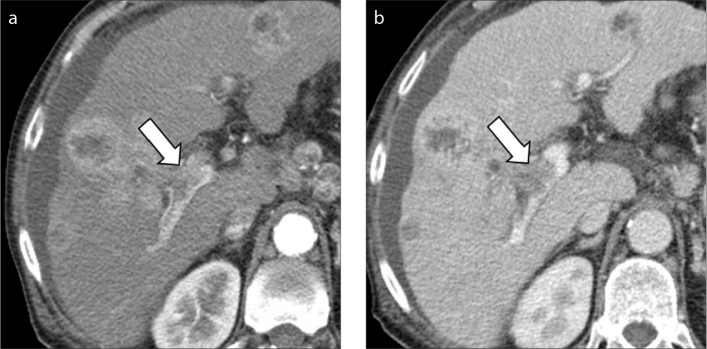
Figure 2. a, b.
A 70-year-old man with cirrhosis and multifocal intrahepatic cholangiocarcinoma. Contrast-enhanced CT on hepatic arterial (a) and portal venous (b) phases demonstrate a hepatic mass with rim arterial phase hyperenhancement and macrovascular invasion in the main portal vein (arrows).
Table.
Liver imaging reporting and data system (LI-RAD) v2018 (7) criteria for tumor in vein
Tumor in vein (LR-TIV):
|
Other imaging features that suggest the presence of TIV but do not establish its diagnosis:
|
Reporting:
|
Ultrasonography
Ultrasonography (US) is the most common imaging modality for HCC surveillance in high-risk or cirrhotic patients. At US surveillance, the main portal vein should always be scrutinized since portal vein thrombosis may be incidentally found even in asymptomatic patients with cirrhosis. In case of bland thrombus, US images show the presence of hyperechoic material with variable echogenicity within the lumen of hepatic vessels, distention of the portal vein and absence of flow at color- or pulsed-Doppler. However, the ability to characterize venous thrombi is low and conventional US does not allow a definitive diagnosis to rule out the presence of malignant thrombus since the thrombus echogenicity is not specific. Findings suggestive of tumor thrombus (Fig. 3) rather than bland thrombus are the new onset of portal vein thrombosis at subsequent US examinations, the presence of an adjacent liver mass with direct intravascular extension and color signal within the thrombus at Doppler evaluation (13, 14). However, color Doppler has demonstrated significantly lower sensitivity (20%–87%) for the characterization of tumor thrombus compared to contrast-enhanced imaging modalities (14–16). In contrast, the presence of collateral vessels with cavernous transformation of the portal vein is pathognomonic of long standing thrombosis and may be suggestive of bland thrombus since the low survival of patients with macrovascular invasion does not give enough time for cavernous transformation (13).
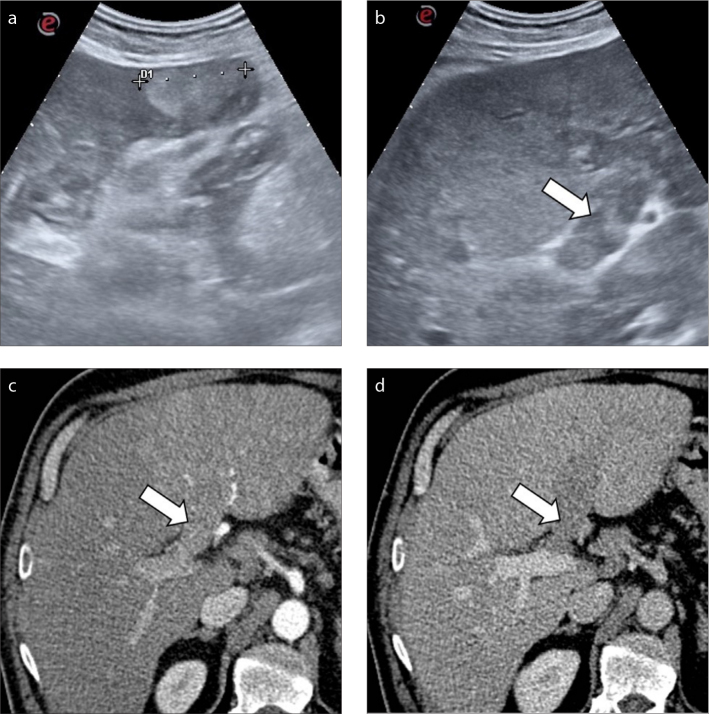
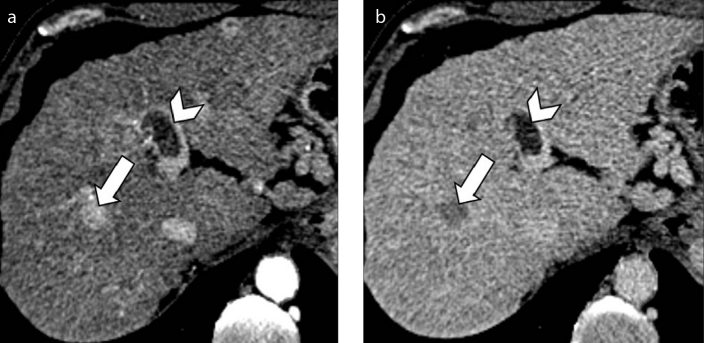
Figure 3. a–d.
A 47-year-old man with alcohol-related cirrhosis. Conventional ultrasound examination depicts the presence of a hyperechoic lesion in the left hepatic lobe (a) and a heterogeneously hyperechoic thrombus (b, arrow) expanding the left portal vein. Subsequent contrast-enhanced CT (c, d) confirmed the presence of a tumor thrombus in the left hepatic vein (arrows).
When using the LI-RADS US algorithm for HCC screening (17), the presence of a new thrombus in vein, whether considered bland or TIV, should be categorized as LI-RADS US-3 positive and a further multiphasic contrast-enhanced diagnostic exam should be recommended for characterization.
Contrast-enhanced ultrasonography
Contrast-enhanced ultrasonography (CEUS) allows to characterize liver lesions in cirrhotic patients according to the vascular pattern after the intravenous administration of a sulphur-hexafluoride microbubbles-based contrast agent (18). CEUS may be performed in case of visible nodule detected during the US screening in high-risk patients or as second step in patients with main contraindications or inconclusive contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) (3). According to the WFUMB-EFSUMB practice guidelines (19), CEUS is listed among the possible imaging modalities for the differential diagnosis of portal venous thrombosis. CEUS has shown significantly higher sensitivity for the differentiation of portal vein tumor thrombus from bland thrombus compared to ultrasound with color Doppler (14, 15, 20). The sensitivity and specificity for the diagnosis of portal vein tumor thrombus have been reported as 88%–100% and 66%–100%, respectively (13, 16, 20, 21). The main advantage of CEUS is the real-time imaging examination, which may allow to detect arterial phase hyperenhancement of the thrombus even if missed or equivocal on multiphasic contrast-enhanced CT or MRI (20). Moreover, CEUS is fast, well tolerated even in patients with renal failure, and can be performed in the same session when the thrombus is detected. However, CEUS allows the evaluation of only one or few lesions after contrast injection, limiting the applicability in patients with multiple hepatic findings, and shares the same limitations of US, including large body habitus, poor acoustic window and movement artifacts in poorly cooperative patients (18).
The arrival time of the contrast agent in the portal vein compared to the hepatic artery is the most important feature to differentiate malignant thrombosis from bland thrombus. Normally, the microbubbles of contrast agent reach the portal vein about 10 seconds after the opacification of the hepatic artery (22). Bland portal vein thrombi are typically avascular and do not demonstrate enhancement on early arterial phase. In contrast, in patients with macrovascular invasion there is an early (13–15 seconds after contrast injection) and almost simultaneous enhancement of the hepatic artery and portal vein thrombus, which is suggestive for malignant thrombosis (Fig. 4) (22). Washout on the extended portal venous phase may also be observed (23). Other findings that may be observed using CEUS are the presence of formed small arterial vessels within the thrombus or portal vein expansion (16).
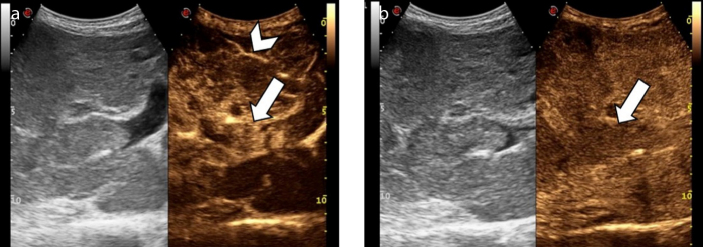
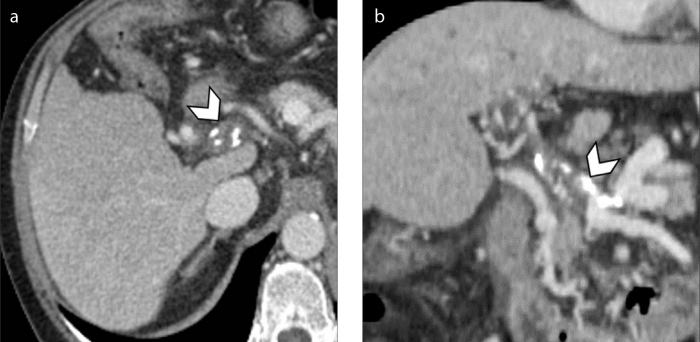
Figure 4. a, b.
An 84-year-old man with hepatitis B virus (HBV)-related cirrhosis and tumor thrombus. Contrast-enhanced ultrasound image (a) at 13 seconds after the intravenous administration of contrast agent demonstrates the presence of an enhancing thrombus within the main portal vein (arrow), simultaneously with the intra-hepatic artery (arrowhead). Image acquired at 180 seconds (b) shows washout of the tumor thrombus (arrow).
CT imaging
Multiphasic contrast-enhanced CT is performed as a noninvasive imaging modality for HCC diagnosis. Portal vein thrombosis is frequently encountered in cirrhotic patients undergoing contrast-enhanced CT for HCC diagnosis, therefore the radiologist should carefully scrutinize the presence of unequivocal findings of malignant thrombosis rather than bland non-neoplastic thrombus.
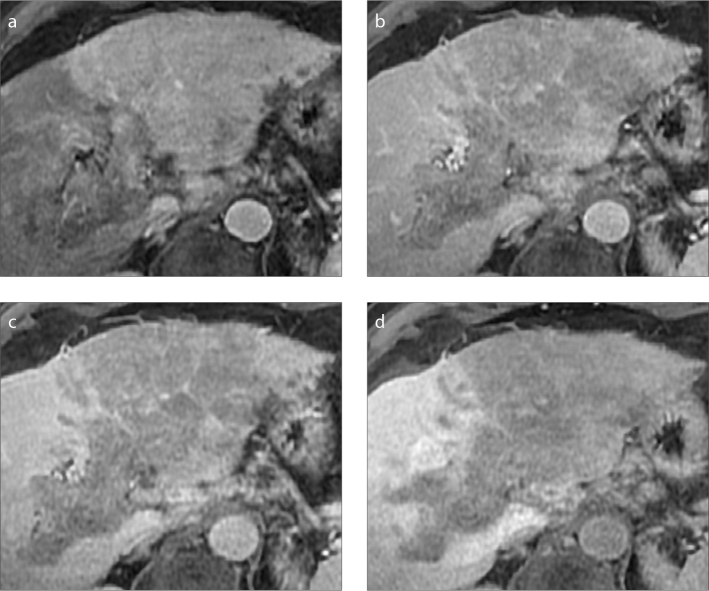
In cirrhotic patients bland thrombosis may coexist with HCC lesions, making the differential diagnosis particularly challenging (Fig. 5) (24). Bland portal vein thrombosis manifests as hypodense filling defect within the main portal vein or its branches, without contrast enhancement in any phase, and shows no continuity with concomitant HCC lesions (25, 26). Of note, radiologists should be aware that bland thrombosis may appear as hyperdense luminal defect on unenhanced CT with vessels expansion in the acute phase (25, 26). Chronic thrombosis may develop calcifications within the thrombus or the vascular wall (Fig. 6), which should not be mistaken for enhancing tissue (27). Another common pitfall is the presence of contrast mixing artifacts during the hepatic arterial phase which may be misinterpreted as enhancing tissue (27). However, these artifacts completely resolve in the portal venous and delayed phases.
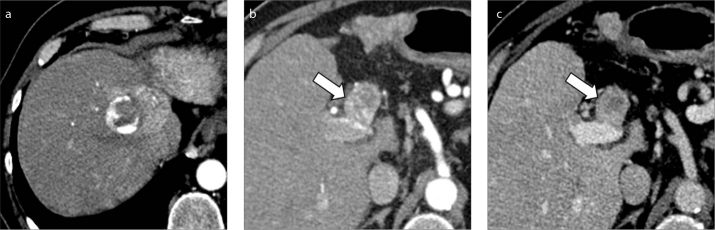
Figure 5. a, b.
A 52-year-old man with HCV-related cirrhosis and multifocal HCC. Contrast-enhanced CT depicts a 1.5 cm HCC with arterial phase hyperenhancement (a, arrow) and washout (b, arrow) on delayed phase. In the same images, a coexistent nonenhancing bland thrombus (arrowheads) is seen in the left portal branch, not in contact with the HCC lesion.
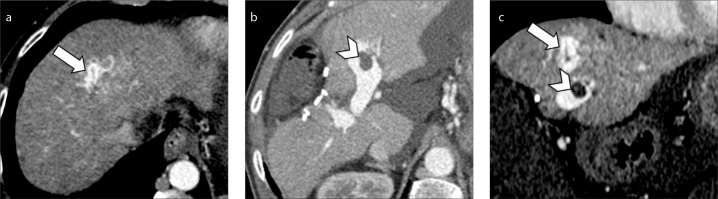
Figure 6. a, b.
Axial (a) and coronal (b) contrast-enhanced CT on portal venous phase of a 60-year-old man with HCV-related cirrhosis shows chronic thrombosis of the main portal vein with wall calcification (arrowheads) and associated cavernous transformation of the portal vein at the level of hepatic hilum.
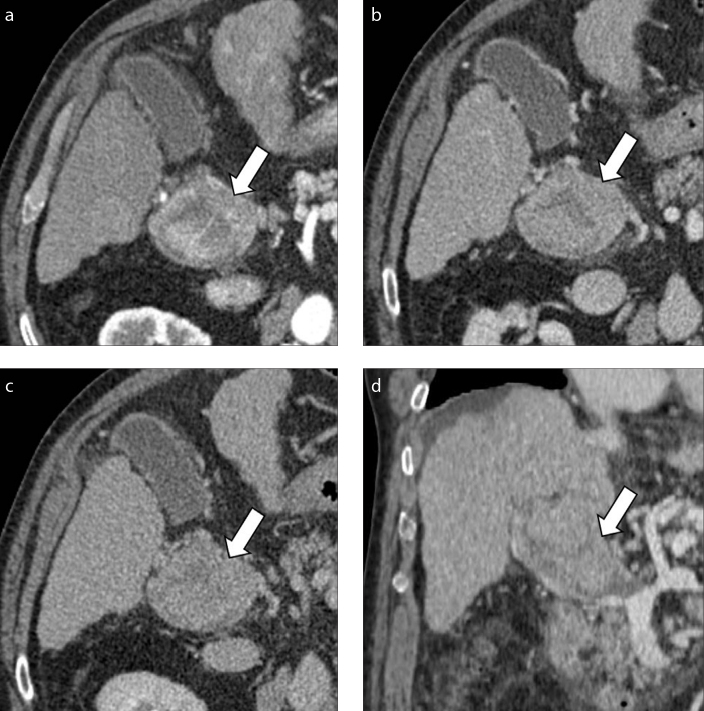
HCCs with macrovascular invasion typically manifest as tumor thrombus adjacent to a liver lesion showing the typical landmarks of HCC. Macrovascular invasion usually tends to be associated with larger HCC lesions (i.e., >5 cm) (Fig. 7) compared to bland thrombus (6, 28). CT imaging characteristics suggesting the presence of tumor thrombosis are the increased diameter of the portal vein and unequivocal enhancing soft tissue within the vein on arterial phase, with subsequent washout on portal venous and delayed phases (Fig. 8). Unequivocal arterial phase hyperenhancing tissue has demonstrated the highest sensitivity (76%–100%) and specificity (87%–91%) as standalone feature on CT images (28, 29), and it is the only criterion for definitive diagnosis of TIV according to LI-RADS algorithm (7). When combining the enhancing thrombus with portal vein diameter larger than 23 mm, the study performed by Tublin et al. (30) has demonstrated a sensitivity and specificity for the diagnosis of tumor thrombus on contrast-enhanced CT of 86% and 100%, respectively. CT is also helpful in detecting macrovascular invasion into the hepatic veins, although this is less frequently encountered.
Figure 7. a–d.
A 75-year-old man with HCV-related cirrhosis and HCC with macrovascular invasion. Contrast-enhanced hepatic arterial (a), portal venous (b) and delayed (c) phase CT images show macrovascular invasion (arrows) of the portal vein adjacent to a 12 cm HCC lesion (arrowheads). Coronal image (d) of the same patient demonstrates enhancing soft tissue (arrow) within the main portal vein.
Figure 8. a–d.
A 73-year-old woman with nonalcoholic steatohepatitis (NASH)-related cirrhosis and tumor thrombus. Contrast-enhanced CT shows increased diameter of the main portal vein caliber with unequivocal enhancing soft tissue within the vein (a, arrow) and subsequent washout (arrows) during portal venous (b) and delayed (c) phases consistent with tumor thrombus. Coronal image on portal venous phase (d) shows the extension of the macrovascular invasion involving the main portal vein (arrow) and portal confluence.
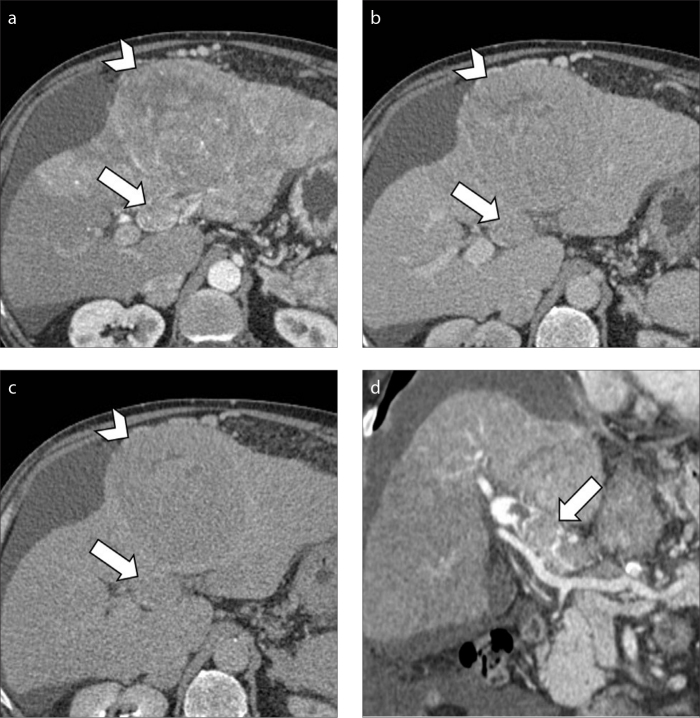
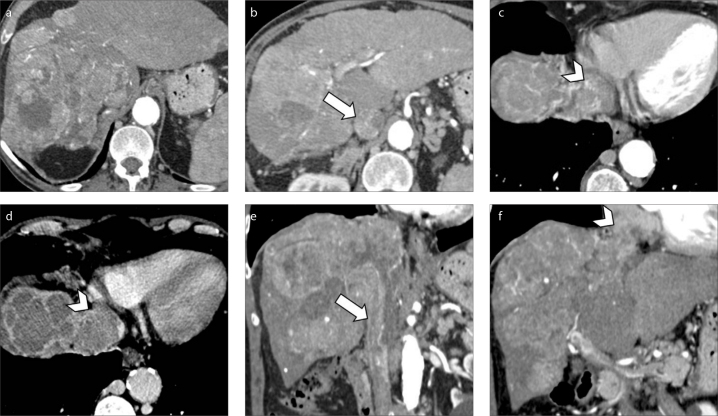
CT represents the gold standard modality to evaluate the extension of tumor thrombus which can range from segmental portal vein invasion to occlusion of the main portal vein trunk. Radiologists should also meticulously scrutinize the involvement of extra-hepatic portal system and superior mesenteric vein in case of portal vein invasion, or the inferior vena cava and right atrium in case of hepatic vein invasion (Fig. 9). Of note, large progressed HCC lesions may also demonstrate macrovascular invasion in both portal vein branches and hepatic veins or inferior vena cava (Fig. 10). When evaluating the extension of macrovascular invasion, radiologists should also be aware that tumor thrombosis may also coexist with bland thrombus in the same patient (Fig. 11). A correct differential diagnosis is important for precise tumor staging since a recent study performed by Mähringer-Kunz et al. (31) have reported how involvement of main trunk or contralateral portal vein branch to the primary involved lobe carries worse overall survival in HCC patients.
Figure 9. a–f.
A 73-year-old man with cirrhosis and HCC with macrovascular invasion. Contrast-enhanced CT image (a) demonstrates a massive HCC involving the whole right hepatic lobe. The HCC is extending into the right hepatic vein along with inferior vena cava (b, arrow) and right atrium (c, arrowhead). Portal venous phase (d) depicts tumor thrombus involving the vast majority of the right atrium (arrowhead). Coronal images (e, f) show the massive macrovascular tumor invasion of the inferior vena cava (arrow) and right atrium (arrowhead).
Figure 10. a–c.
An 80-year-old man with HBV-related cirrhosis and HCC. Contrast-enhanced CT on hepatic arterial (a), portal venous (b) and delayed (c) phases demonstrate a 4.5 cm HCC in the caudate lobe with macrovascular invasion on both right portal vein branch (arrows) and inferior vena cava (arrowheads).
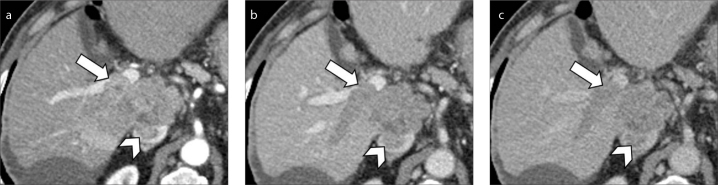
Figure 11. a–c.
A 75-year-old man with HCV-related cirrhosis, history of treated HCC and co-existence of bland and tumor thrombi. Contrast-enhanced CT on hepatic arterial phase shows enhancing tumor thrombus (a, arrow) in the upper branch of the left portal vein and bland non-enhancing thrombus (b, arrowhead) in the left portal vein. Coronal images (c) better demonstrate the co-existence of tumor (arrow) and bland (arrowhead) thrombus in the same patient.
Finally, macrovascular invasion may manifest even in HCC treated with locoregional therapies (Fig. 12). In this setting, the new onset of tumor thrombosis is an unequivocal sign of tumor viability, even if an enhancing nodule is not visualized in the treated area.
Figure 12. a–c.
A 79-year-old man with HCV-related cirrhosis and history of HCC treated with transarterial chemoembolization (TACE). Contrast-enhanced CT on hepatic arterial phase (a) shows treated HCC with TACE with adjacent residual enhancing tumor. CT images on hepatic arterial (b) and portal venous (c) phases at the level of portal vein bifurcation demonstrate macrovascular invasion of the left portal vein (arrows), not present at prior examinations (not shown).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) has high sensitivity for the noninvasive diagnosis of HCC, allowing the investigation of vascular changes (i.e., APHE and washout) as well as the detection of several ancillary findings (i.e., intralesional fat, T2 hyperintensity, restricted diffusion, hepatobiliary phase hypointensity). MRI is a highly accurate modality to diagnose portal vein thrombosis as well as HCC with infiltrative appearance (32). Bland thrombus usually appears as a non-enhancing filling defect on post-contrast images, and it may show hyperintensity on T1-weighted images in acute phase.
Several MRI findings are associated with the diagnosis of malignant tumor thrombus (Fig. 13). Macrovascular invasion usually demonstrates hypointensity on T1-weighted images, presence of arterial phase hyperenhancement within the thrombus and subsequent washout on portal venous and delayed phases (32). On T2-weighted images the macrovascular invasion may demonstrate moderate-to-high T2 hyperintensity (Fig. 14).
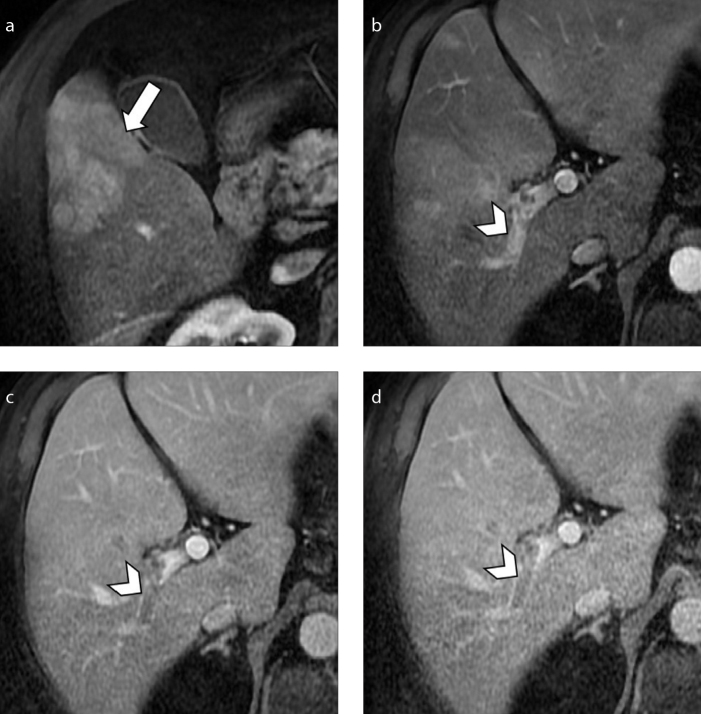
Figure 13. a–d.
A 70-year-old man with NASH-related cirrhosis and HCC. MRI on hepatic arterial phase (a) shows a 6.8 cm arterial phase hyperenhancing HCC (arrow). The lesion invades the right portal vein, which demonstrates enhancing tumor thrombus (b, arrowhead) with subsequent washout on portal venous (c) and delayed (d) phases (arrowheads).
Figure 14. a, b.
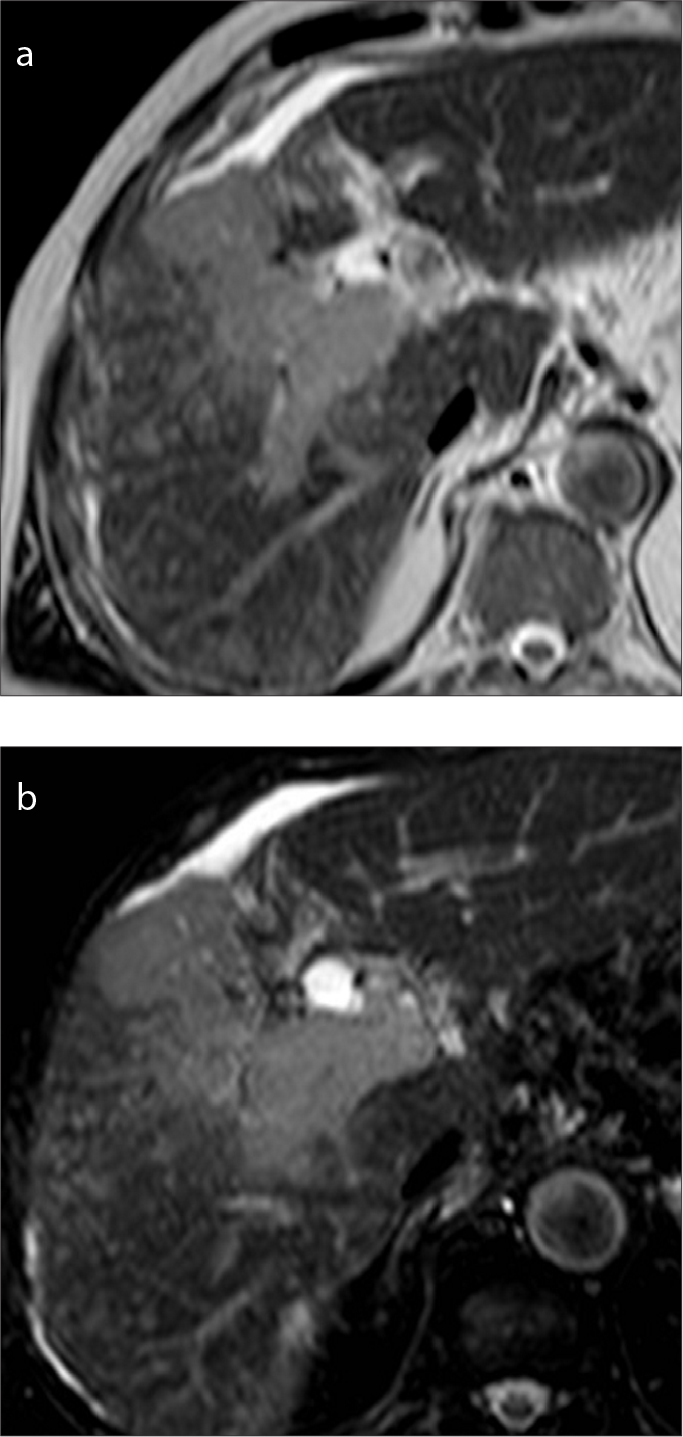
A 71-year-old man with HBV-related cirrhosis and HCC with macrovascular invasion on the right portal vein. The tumor thrombus shows mild-to-moderate hyperintensity on T2-weighted (a) and SPIR images (b).
Diffusion-weighted images may show restricted diffusion (Fig. 15) in case of macrovascular invasion due to the increased cellularity within the thrombus. Prior studies (32–34) have assessed the potential of restricted diffusion with quantification of apparent diffusion coefficient (ADC) values, obtaining discordant results for the differentiation of portal vein tumor thrombus from bland thrombus. Catalano et al. (33) reported lower ADC values and ADC ratios in tumor thrombi compared to bland portal vein thrombi. In contrast, Sandrasegaran et al. (32) and Ahn et al. (34) did not find any significant differences in ADC values between bland and tumor thrombi. When using the LI-RADS algorithm, restricted diffusion is considered among the additional imaging features suggesting the presence of TIV, but cannot establish the diagnosis without the presence of unequivocal enhancing tumor thrombus. However, DWI may be useful to better delineate the tumor extension by increasing its conspicuity in case of hypovascular infiltrative HCC (35, 36). Indeed, HCC with macrovascular invasion may be extremely subtle on MRI due to less conspicuous arterial phase hyperenhancement, especially in lesions with infiltrative appearance blending into background cirrhotic parenchyma.
Figure 15. a–d.
An 82-year-old man with cirrhosis and history of HCC. MRI images on hepatic arterial (a) and portal venous (b) phases show a large HCC with infiltrative imaging appearance and macrovascular invasion (arrows) in the right portal vein. DWI image at b= 800 s/mm2 (c) demonstrates diffusion restriction of the liver mass and hypointensity on ADC map (d).
The administration of hepatobiliary contrast agents may be helpful in the identification of an infiltrative parenchymal mass which typically demonstrates hypointensity on hepatobiliary phase (Fig. 16). Gadoxetic acid-enhanced MRI, in particular, has shown excellent sensitivity (81%–93%) and accuracy (92%–95%) in differentiating portal vein tumor thrombus from bland thrombus in a large retrospective study (37).
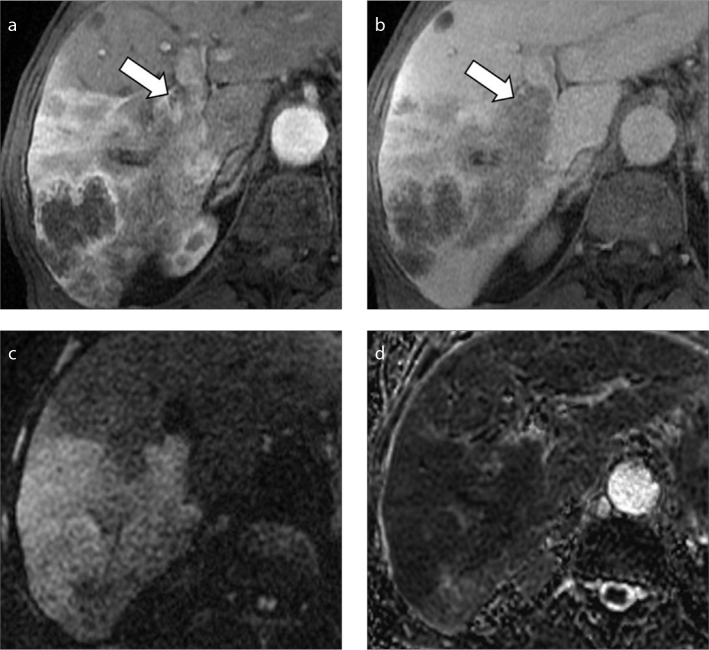
Figure 16. a–d.
A 70-year-old woman with cryptogenic cirrhosis and HCC with infiltrative appearance. Gadoxetic acid-enhanced MRI shows a large infiltrative HCC with macrovascular invasion of the left portal vein demonstrating mild arterial phase hyperenhancement (a), washout on portal venous phase (b), and hypointensity on 3 minutes transitional (c) and 20 minutes (d) hepatobiliary phases.
Other MRI features associated with the presence of macrovascular invasion are a distance less than 2 cm from the lesion, the presence of an HCC larger than 5 cm and portal vein caliber higher than 1.8 cm, due to the mass effect of growing tumor thrombus (32).
PET-CT
Although positron emission tomography-computed tomography (PET-CT) is currently not recommended as primary imaging modality for HCC diagnosis due to its low sensitivity for the detection of smaller or well-differentiated lesions, PET-CT with 18F-fluorodeoxyglucose (18F-FDG) may provide prognostic information for more aggressive and poorly differentiated HCC (3, 38). Moreover, 18F-FDG PET-CT may be required to stage patients with advanced HCC, especially for the detection and evaluation of extrahepatic metastasis (10).
Only a few studies have investigated the potential of PET-CT for the differential diagnosis of bland from tumor thrombus in patients with HCC demonstrating a higher FDG uptake of the tumor thrombus compared with the bland thrombus (39–42). A recent study from Wu et al. (42) reported a sensitivity of 62% and a specificity of 92% for the differential diagnosis of bland from tumor thrombus using 18F-FDG PET-CT, with a mean SUVmax of 4.3 for the tumor thrombus. Moreover, FDG uptake of the tumor thrombus has been demonstrated to be a prognostic factor for overall survival in patients with HCC and macrovascular invasion and may be adopted for risk stratification of these patients (43).
Radiomics
Radiomics is the new frontier of advanced imaging analysis, which is emerging as a promising tool for radiologic diagnosis in several research studies with potential future applications in clinical practice. Radiomics extracts and analyzes quantitative imaging features that reflect the lesion’s heterogeneity, providing additional information otherwise undetectable by human eyes. Recently published studies have explored the potential of radiomics and texture analysis in liver imaging for the staging of hepatic fibrosis, differential diagnosis of focal liver lesions, and prediction of survival or treatment response of HCC (44, 45). Regarding portal vein thrombosis, a study performed by Canellas et al. (46) demonstrated an excellent diagnostic performance of CT-based texture analysis for the differentiation of bland from tumor thrombus, which correctly classified 96% of the thrombi. Recently, radiomics has also provided new insights for the noninvasive diagnosis of microvascular invasion in HCC (47, 48), which is one of the few established prognostic factors in HCC. Indeed, unlike macrovascular invasion, microvascular invasion cannot currently be detected at imaging and it is largely diagnosed postoperatively from pathologic assessment of the tumor specimen.
Treatment
Macrovascular invasion represents an absolute contraindication for locoregional treatments and significantly limits the therapeutic options. Patients with macrovascular invasion may be candidates for systemic treatment with anti-angiogenic drugs. Particularly sorafenib, a multi-tyrosine kinase inhibitor that suppresses tumor angiogenesis, has demonstrated to increase the overall survival in patients with advanced stage HCC and it is now considered the standard treatment option in patients with HCC complicated with tumor thrombus (3, 4). As second line therapy, regorafenib, a similar multi-kinase inhibitor, is recommended in patients who progressed after first-line treatment with sorafenib (3).
Conclusion
Macrovascular invasion may be frequently encountered in patients with advanced HCC. Imaging plays a crucial role for the differentiation between bland and tumor thrombi as well as in suggesting the correct underlying etiology. Knowledge of the imaging appearance on diagnostic modalities, each one with their strengths and limitations, may help to improve the diagnostic performance in patients with advanced HCC and guide the clinician towards the most appropriate management.
Main points.
Hepatocellular carcinoma with macrovascular invasion is associated with an extremely poor prognosis, and its recognition represents one of the major tumor-related contraindications for locoregional treatments with curative intent.
Imaging features of hepatocellular carcinoma with macrovascular invasion may be subtle on different contrast-enhanced imaging modalities, especially in lesions showing infiltrative appearance.
Unequivocal enhancing soft tissue within the vein, restricted diffusion of the thrombus and vessel expansion are the most characteristic imaging findings suggesting the presence of tumor thrombus.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds AR, Furlan A, Fetzer DT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015;35:371–386. doi: 10.1148/rg.352140114. [DOI] [PubMed] [Google Scholar]
- 6.Kneuertz PJ, Demirjian A, Firoozmand A, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012;19:2897–2907. doi: 10.1245/s10434-012-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Radiology. CT/MRI Liver imaging reporting and data system v2018 Core. [Accessed on October 2019]. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
- 8.Fraum TJ, Tsai R, Rohe E, et al. Differentiation of hepatocellular carcinoma from other hepatic malignancies in patients at risk: diagnostic performance of the Liver Imaging Reporting and Data System Version 2014. Radiology. 2018;286:158–172. doi: 10.1148/radiol.2017170114. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Lee SS, Yu E, et al. Combined hepatocellular-cholangiocarcinoma: Gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J Magn Reson Imaging. 2017;46:267–280. doi: 10.1002/jmri.25568. [DOI] [PubMed] [Google Scholar]
- 10.Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsayes KM, Kielar AZ, Chernyak V, et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocel Carcinoma. 2019;6:49–69. doi: 10.2147/JHC.S186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig DR, Fraum TJ, Cannella R, et al. Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom Radiol. 2019;44:2116–2132. doi: 10.1007/s00261-019-01948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danila M, Sporea I, Popescu A, Şirli R. Portal vein thrombosis in liver cirrhosis - the added value of contrast enhanced ultrasonography. Med Ultrason. 2016;18:218–233. doi: 10.11152/mu.2013.2066.182.pvt. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Rosa L, Ravetta V, et al. Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am J Roentgenol. 2006;186:763–773. doi: 10.2214/AJR.04.1218. [DOI] [PubMed] [Google Scholar]
- 15.Tarantino L, Francica G, Sordelli I, et al. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging. 2006;31:537–544. doi: 10.1007/s00261-005-0150-x. [DOI] [PubMed] [Google Scholar]
- 16.Raza SA, Jang HJ, Kim TK. Differentiating malignant from benign thrombosis in hepatocellular carcinoma: contrast-enhanced ultrasound. Abdom Imaging. 2014;39:153–161. doi: 10.1007/s00261-013-0034-4. [DOI] [PubMed] [Google Scholar]
- 17.American College of Radiology. Ultrasound LI-RADS® v2017. [Accessed on October 2019]. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/Ultrasound-LI-RADS-v2017.
- 18.Bartolotta TV, Taibbi A, Midiri M, Lagalla R. Contrast-enhanced ultrasound of hepatocellular carcinoma: where do we stand? Ultrasonography. 2019;38:200–214. doi: 10.14366/usg.18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Rossi S, Ghittoni G, Ravetta V, et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol. 2008;18:1749–1756. doi: 10.1007/s00330-008-0931-z. [DOI] [PubMed] [Google Scholar]
- 21.Song ZZ, Huang M, Jiang TA, et al. Diagnosis of portal vein thrombosis discontinued with liver tumors in patients with liver cirrhosis and tumors by contrast-enhanced US: a pilot study. Eur J Radiol. 2010;75:185–188. doi: 10.1016/j.ejrad.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 22.American College of Radiology. CEUS LI-RADS® v2017. [Accessed on October 2019]. https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/CEUS-LI-RADS-2017-Core.pdf?la=en.
- 23.Dănilă M, Sporea I, Popescu A, Sirli R, Sendroiu M. The value of contrast enhanced ultrasound in the evaluation of the nature of portal vein thrombosis. Med Ultrason. 2011;13:102–107. doi: 10.1016/j.ultrasmedbio.2011.05.205. [DOI] [PubMed] [Google Scholar]
- 24.Cannella R, Fowler KJ, Borhani AA, Minervini MI, Heller M, Furlan A. Common pitfalls when using the Liver Imaging Reporting and Data System (LI-RADS): lessons learned from a multi-year experience. Abdom Radiol. 2019;44:43–53. doi: 10.1007/s00261-018-1720-z. [DOI] [PubMed] [Google Scholar]
- 25.Carneiro C, Brito J, Bilreiro C, et al. All about portal vein: a pictorial display to anatomy, variants and physiopathology. Insights Imaging. 2019;10:38. doi: 10.1186/s13244-019-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzubaidi S, Patel I, Saini A, et al. Current concepts in portal vein thrombosis: etiology, clinical presentation and management. Abdom Radiol. 2019;44:3453–3462. doi: 10.1007/s00261-019-02174-1. [DOI] [PubMed] [Google Scholar]
- 27.Sereni CP, Rodgers SK, Kirby CL, Goykhman I. Portal vein thrombus and infiltrative HCC: a pictoral review. Abdom Radiol. 2017;42:159–170. doi: 10.1007/s00261-016-0855-z. [DOI] [PubMed] [Google Scholar]
- 28.Sherman CB, Behr S, Dodge JL, Roberts JP, Yao FY, Mehta N. Distinguishing tumor from bland portal vein thrombus in liver transplant candidates with hepatocellular carcinoma: the A-VENA criteria. Liver Transpl. 2019;25:207–216. doi: 10.1002/lt.25345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorrentino P, Tarantino L, D’Angelo S, et al. Validation of an extension of the international non-invasive criteria for the diagnosis of hepatocellular carcinoma to the characterization of macroscopic portal vein thrombosis. J Gastroenterol Hepatol. 2011;26:669–677. doi: 10.1111/j.1440-1746.2010.06564.x. [DOI] [PubMed] [Google Scholar]
- 30.Tublin ME, Dodd GD, 3rd, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997;168:719–723. doi: 10.2214/ajr.168.3.9057522. [DOI] [PubMed] [Google Scholar]
- 31.Mähringer-Kunz A, Steinle V, Düber C, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int. 2019;39:324–331. doi: 10.1111/liv.13988. [DOI] [PubMed] [Google Scholar]
- 32.Sandrasegaran K, Tahir B, Nutakki K, et al. Usefulness of conventional MRI sequences and diffusion-weighted imaging in differentiating malignant from benign portal vein thrombus in cirrhotic patients. AJR Am J Roentgenol. 2013;201:1211–1219. doi: 10.2214/AJR.12.10171. [DOI] [PubMed] [Google Scholar]
- 33.Catalano OA, Choy G, Zhu A, Hahn PF, Sahani DV. Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology. 2010;254:154–162. doi: 10.1148/radiol.09090304. [DOI] [PubMed] [Google Scholar]
- 34.Ahn JH, Yu JS, Cho ES, Chung JJ, Kim JH, Kim KW. Diffusion-weighted MRI of malignant versus benign portal vein thrombosis. Korean J Radiol. 2016;17:533–540. doi: 10.3348/kjr.2016.17.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenkrantz AB, Lee L, Matza BW, Kim S. Infiltrative hepatocellular carcinoma: comparison of MRI sequences for lesion conspicuity. Clin Radiol. 2012;67:e105–111. doi: 10.1016/j.crad.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Lim S, Kim YK, Park HJ, Lee WJ, Choi D, Park MJ. Infiltrative hepatocellular carcinoma on gadoxetic acid-enhanced and diffusion-weighted MRI at 3.0T. J Magn Reson Imaging. 2014;39:1238–1245. doi: 10.1002/jmri.24265. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Lee JM, Yoon JH, et al. Portal vein thrombosis in patients with hepatocellular carcinoma: diagnostic accuracy of gadoxetic acid-enhanced MR imaging. Radiology. 2016;279:773–783. doi: 10.1148/radiol.2015150124. [DOI] [PubMed] [Google Scholar]
- 38.Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912–1921. doi: 10.2967/jnumed.108.055087. [DOI] [PubMed] [Google Scholar]
- 39.Sun L, Guan YS, Pan WM, et al. Highly metabolic thrombus of the portal vein: 18F fluorodeoxyglucose positron emission tomography/computer tomography demonstration and clinical significance in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1212–1217. doi: 10.3748/wjg.14.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P1, Kumar R, Jeph S, et al. 18F-FDG PET-CT in the diagnosis of tumor thrombus: can it be differentiated from benign thrombus? Nucl Med Commun. 2011;32:782–788. doi: 10.1097/MNM.0b013e32834774c8. [DOI] [PubMed] [Google Scholar]
- 41.Hu S, Zhang J, Cheng C, Liu Q, Sun G, Zuo C. The role of 18F-FDG PET/CT in differentiating malignant from benign portal vein thrombosis. Abdom Imaging. 2014;39:1221–1227. doi: 10.1007/s00261-014-0170-5. [DOI] [PubMed] [Google Scholar]
- 42.Wu B, Zhang Y, Tan H, Shi H. Value of 18F-FDG PET/CT in the diagnosis of portal vein tumor thrombus in patients with hepatocellular carcinoma. Abdom Radiol. 2019;44:2430–2435. doi: 10.1007/s00261-019-01997-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee JW, Hwang SH, Kim DY, Han KH, Yun M. Prognostic value of FDG uptake of portal vein tumor thrombosis in patients with locally advanced hepatocellular carcinoma. Clin Nucl Med. 2017;42:e35–e40. doi: 10.1097/RLU.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 44.Park HJ, Lee SS, Park B, et al. Radiomics analysis of gadoxetic acid-enhanced MRI for staging liver fibrosis. Radiology. 2019;290:380–387. doi: 10.1148/radiol.2018181197. [DOI] [PubMed] [Google Scholar]
- 45.Mulé S, Thiefin G, Costentin C, et al. Advanced hepatocellular carcinoma: pretreatment contrast-enhanced CT texture parameters as predictive biomarkers of survival in patients treated with sorafenib. Radiology. 2018;288:445–455. doi: 10.1148/radiol.2018171320. [DOI] [PubMed] [Google Scholar]
- 46.Canellas R, Mehrkhani F, Patino M, Kambadakone A, Sahani D. Characterization of portal vein thrombosis (neoplastic versus bland) on CT images using software-based texture analysis and thrombus density (Hounsfield Units) AJR Am J Roentgenol. 2016;207:W81–W87. doi: 10.2214/AJR.15.15928. [DOI] [PubMed] [Google Scholar]
- 47.Xu X, Zhang HL, Liu QP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133–1144. doi: 10.1016/j.jhep.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol. 2019;44:539–548. doi: 10.1007/s00261-018-1768-9. [DOI] [PubMed] [Google Scholar]