Abstract
There are many therapeutic options for primary hepatocellular carcinoma (HCC), but very limited options for unresectable HCC with a single lesion larger than 5 cm (Barcelona Clinic Liver Cancer [BCLC] stage A) or with 2–3 nodules beyond 5 cm (BCLC stage B). Transcatheter arterial chemoembolization (TACE) is considered the first-line treatment for these patients, and combination therapy has also been tried. However, the effectiveness of microwave ablation (MWA) combined with TACE in the treatment of the above tumors remains to be further confirmed. Therefore, this meta-analysis aimed to compare the effectiveness of combination therapy and TACE monotherapy on these patients. PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure, and the Wan Fang electronic databases were retrieved to search for studies comparing combination therapy and TACE monotherapy, published between the earliest available date and August 20, 2019. A total of 20 articles (reporting 1736 patients) were included. Meta-analysis showed that, compared to TACE alone, TACE + MWA resulted in significantly higher 1-, 2-, and 3-year overall survival (OS) (1-year OS rate: RR = 1.36, 95% CI 1.28–1.44, P < 0.001; 2-year OS rate: RR = 1.56, 95% CI 1.40–1.74, P < 0.001 and 3-year OS rate: RR = 2.07, 95% CI: 1.67–2.57, P < 0.001). Complete response, partial response, and objective response rates were significantly higher in TACE + MWA than those in TACE alone (P < 0.001). Meanwhile, publication bias and sensitivity analysis were performed and did not show statistical significance.
According to the latest clinical practice guideline for hepatocellular carcinoma (HCC) issued by the European Association for the Study of the Liver (EASL) (2018), for HCC patients with a single tumor larger than 5 cm that cannot be surgically removed, or multiple nodules with a maximum nodule diameter more than 3 cm, transcatheter arterial chemoembolization (TACE) is recommended as the first-choice treatment (1). However, the overall survival (OS) of these patients is much lower than that of patients with early stage HCC. It has been reported that TACE combined with radiofrequency ablation (RFA) for multiple nodules larger than 3 cm can achieve a better tumor control rate and longer OS compared with TACE or RFA monotherapy (2–4), which provides a new therapeutic approach for such patients. However, owing to the recent increased use of microwave ablation (MWA) therapy, meta-analyses have indicated similar effectiveness between MWA and RFA, with one study showing possible superiority of MWA in larger HCCs (5–7). So, TACE combined with MWA may provide a better alternative for the treatment of large HCCs, but whether the combination treatment can achieve these results is still uncertain.
This meta-analysis aimed to compare the effectiveness of TACE + MWA with TACE alone for unresectable Barcelona Clinic Liver Cancer [BCLC] stage A or B HCC with maximum nodule diameter beyond 5 cm.
Methods
Search strategy
PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure, and the Wan Fang databases were systematically searched to identify studies published from the earliest available date to August 20, 2019. The terms “carcinoma, hepatocellular” or “liver cell carcinoma” or “liver cancer,” “microwave ablation” or “MWA,” and “transcatheter arterial chemoembolization” or “TACE,” and their combinations were used. Only randomized controlled trials (RCTs) and cohort studies were included. Language restrictions were not imposed.
Study selection
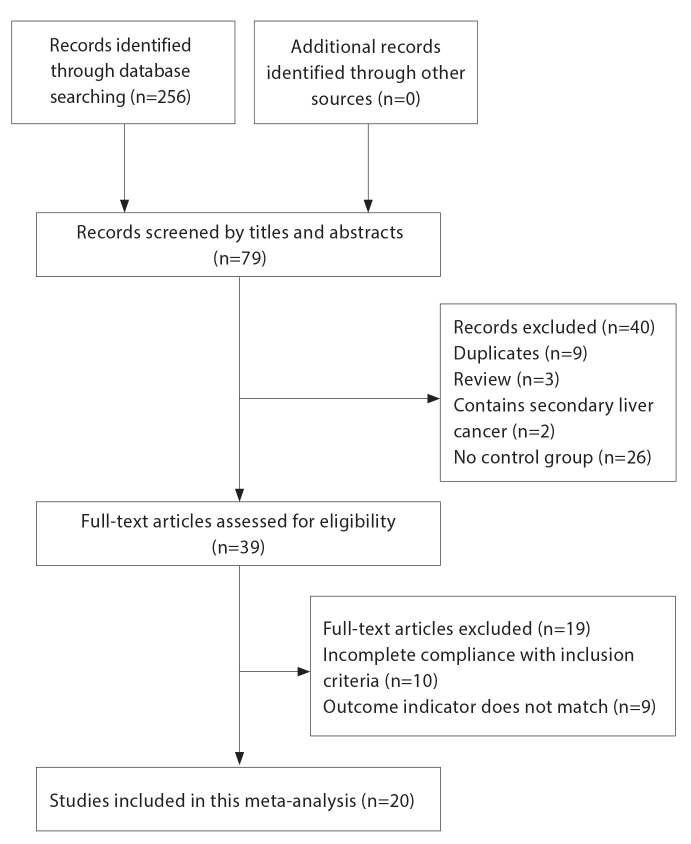
The studies that fulfilled the following criteria were included in the analysis: 1) Patients diagnosed with single HCC larger than 5 cm (BCLC stage A) or with BCLC stage B HCC (maximum nodule diameter >5 cm) (8, 9); 2) Patients treated with TACE alone or TACE + MWA performed within 1 month after TACE; 3) RCTs or cohort studies; and 4) Treatment response reported according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (10) and OS of patients reported. Fig. 1 shows the flowchart for inclusion of studies.
Figure 1.
Flowchart of article selection process.
Data extraction
Two reviewers independently completed the data extraction. When the extracted results were inconsistent, a discussion or a judgment from a third reviewer was performed. The following information was extracted: first author’s name, country, year of publication, study type, average age of patients, type of treatment, BCLC stage, tumor size, outcome indicators according to mRECIST standards—complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)—objective response rate (ORR), 1-, 2-, and 3-year OS rates and complications.
Statistical analysis
All included studies were analyzed using Stata version 15.0 software. The Q test combined with the I2 test was first calculated to evaluate overall heterogeneity of the studies for each meta-analysis. If the heterogeneity of the included studies was acceptable (I2 < 50% or P > 0.05), a fixed-effects model was considered. If the heterogeneity was large (I2 ≥ 50% or P < 0.05), a random-effects model was considered. Complications could not be pooled and were analyzed descriptively. In this study, the estimation of pooled RR with 95% confidence interval (CI) was calculated. Each included RCT was evaluated according to the Cochrane Collaboration’s tool for assessing risk of bias (11). The included cohort studies were evaluated according to the Newcastle-Ottawa scale (12). In addition, the Egger and Begg tests were used to assess publication bias. Finally, sensitivity analysis was performed by removing studies one by one to evaluate every study’s effect on the overall result.
Results
A total of 256 articles were found, of which 20 studies (13–32) consisting of 13 RCTs and 7 cohort studies were included in this meta-analysis. All 20 studies were Asian studies; 2 studies were written in English and 18 studies were written in Chinese. In total 1736 patients were enrolled, including 924 treated with TACE and 812 treated with TACE + MWA (Table 1). All included TACE treatments were conventional (cTACE).
Table 1.
Studies included in the meta-analysis and characteristics of patients
| Study | Year | Country | BCLC stage | Therapy | Age (years) | Sex (M/F) | No of pts | CR | PR | SD | PD | 1-year OS | 2-year OS | 3-year OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zheng et al. (13) | 2018 | China | A–B | TACE+MWA | 53.3±8.2 | 79/13 | 92 | 54 | 12 | 9 | 17 | 79 | 55 | 30 |
| TACE | 54.6±10.5 | 143/23 | 166 | 21 | 49 | 22 | 74 | 98 | 67 | 19 | ||||
|
| ||||||||||||||
| An et al. (23) | 2018 | China | A | TACE+MWA | 55 (47–65) | 25/12 | 37 | 11 | 18 | 7 | 1 | 23 | 10 | NA |
| TACE | 56 (49–67) | 22/13 | 35 | 5 | 17 | 10 | 3 | 12 | 3 | NA | ||||
|
| ||||||||||||||
| Zhang et al. (24) | 2017 | China | A–B | TACE+MWA | 55.6±4.6 | 19/11 | 30 | 10 | 12 | 7 | 1 | 24 | 19 | NA |
| TACE | 55.9±4.2 | 18/12 | 30 | 2 | 10 | 12 | 6 | 18 | 12 | NA | ||||
|
| ||||||||||||||
| Huo et al. (25) | 2017 | China | B | TACE+MWA | 58.1±7.8 | 32/4 | 36 | 20 | 10 | 2 | 4 | 29 | 11 | NA |
| TACE | 56.4±7.6 | 29/3 | 32 | 8 | 8 | 4 | 12 | 18 | 5 | NA | ||||
|
| ||||||||||||||
| Dong et al. (31) | 2017 | China | B | TACE+MWA | 54.9±12.3 | 28/3 | 31 | 18 | 8 | 4 | 1 | 30 | 25 | NA |
| TACE | 59.8±8.9 | 27/4 | 31 | 1 | 7 | 11 | 12 | 19 | 15 | NA | ||||
|
| ||||||||||||||
| An et al. (26) | 2017 | China | A | TACE+MWA | 51.3±2.9 | 40/9 | 49 | 10 | 25 | NA | NA | 29 | 23 | NA |
| TACE | 50.3±2.6 | 39/10 | 49 | 6 | 15 | NA | NA | 18 | 13 | NA | ||||
|
| ||||||||||||||
| Zhou et al. (20) | 2016 | China | B | TACE+MWA | 52.3±4.2 | 29/25 | 54 | 16 | 32 | 4 | 2 | 51 | 42 | 36 |
| TACE | 50.5±3.1 | 32/22 | 54 | 10 | 12 | 17 | 15 | 38 | 28 | 21 | ||||
|
| ||||||||||||||
| Yan et al. (21) | 2016 | China | A–B | TACE+MWA | 61.4±4.2 | 27/14 | 41 | NA | NA | NA | NA | 29 | 21 | 13 |
| TACE | 58.7±3.4 | 19/13 | 32 | NA | NA | NA | NA | 19 | 12 | 6 | ||||
|
| ||||||||||||||
| Liu et al. (29) | 2016 | China | B | TACE+MWA | 58.7±7.1 | 43/19 | 62 | 19 | 37 | 4 | 2 | 59 | 48 | NA |
| TACE | 58.3±7.3 | 45/17 | 62 | 11 | 14 | 21 | 16 | 43 | 32 | NA | ||||
|
| ||||||||||||||
| He et al. (28) | 2016 | China | A–B | TACE+MWA | 53 (32–73) | 36/6 | 32 | 6 | 12 | 5 | 5 | 26 | 18 | NA |
| TACE | 52 (40–69) | 22/6 | 28 | 2 | 12 | 6 | 8 | 18 | 12 | NA | ||||
|
| ||||||||||||||
| Guo et al. (19) | 2015 | China | B | TACE+MWA | 48 (35–67) | 26/16 | 42 | 19 | 11 | 7 | 5 | 31 | 14 | NA |
| TACE | 50 (39–73) | 24/18 | 42 | 10 | 8 | 9 | 15 | 24 | 4 | NA | ||||
|
| ||||||||||||||
| Chang et al. (18) | 2015 | China | B | TACE+MWA | 59.4±10.5 | 30/3 | 33 | 18 | 14 | 0 | 1 | 21 | 5 | 1 |
| TACE | 57.3±10.9 | 28/6 | 34 | 7 | 15 | 9 | 3 | 9 | 2 | 0 | ||||
|
| ||||||||||||||
| Zhao et al. (22) | 2009 | China | A–B | TACE+MWA | 51 (20–79) | 18/9 | 28 | 5 | 14 | NA | NA | 26 | 17 | NA |
| TACE | 52 (27–80) | 46/9 | 55 | 3 | 19 | NA | NA | 46 | 21 | NA | ||||
|
| ||||||||||||||
| Liu et al. (29) | 2018 | China | B | TACE+MWA | 55.7±5.1 | 30/23 | 53 | 11 | 14 | 16 | 12 | NA | NA | NA |
| TACE | 55.7±5.1 | 26/27 | 53 | 5 | 10 | 15 | 23 | NA | NA | NA | ||||
|
| ||||||||||||||
| Zhang et al. (15) | 2013 | China | B | TACE+MWA | 52.2±2.3 | 20/2 | 22 | 1 | 6 | 1 | 14 | NA | NA | NA |
| TACE | 48.3±2.2 | 18/0 | 18 | 0 | 3 | 0 | 15 | NA | NA | NA | ||||
|
| ||||||||||||||
| Shu et al. (17) | 2014 | China | B | TACE+MWA | 61.2±11.4 | 15/9 | 24 | 7 | 10 | 4 | 3 | 23 | 20 | 16 |
| TACE | 60.3±8.9 | 15/11 | 26 | 2 | 8 | 10 | 6 | 18 | 13 | 9 | ||||
|
| ||||||||||||||
| Hu et al. (16) | 2013 | China | B | TACE+MWA | 43.2±5.1 | 23/25 | 48 | 13 | 20 | 10 | 5 | NA | NA | NA |
| TACE | 44.3±4.9 | 22/26 | 48 | 8 | 15 | 14 | 9 | NA | NA | NA | ||||
|
| ||||||||||||||
| Tao et al. (32) | 2016 | China | A–B | TACE+MWA | 46.5±6.5 | 15/10 | 25 | 8 | 10 | 5 | 2 | 23 | 20 | 16 |
| TACE | 46.7±6.5 | 17/8 | 25 | 2 | 7 | 8 | 8 | 17 | 13 | 9 | ||||
|
| ||||||||||||||
| Huang et al. (30) | 2015 | China | A | TACE+MWA | 62.3±3.5 | 20/4 | 24 | NA | NA | NA | NA | 11 | NA | 5 |
| TACE | 61.2±3.1 | 18/6 | 24 | NA | NA | NA | NA | 10 | NA | 3 | ||||
|
| ||||||||||||||
| Xu et al. (14) | 2013 | China | B | TACE+MWA | 54.5±13.0 | 48/8 | 56 | NA | NA | NA | NA | 49 | NA | 28 |
| TACE | 53.1±14.8 | 73/7 | 80 | NA | NA | NA | NA | 50 | NA | 14 | ||||
Age is presented as mean ± standard deviation or median (range).
BCLC, Barcelona Clinic Liver Cancer staging; M/F, male/female; pts, patients; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; OS, overall survival; TACE, transarterial chemoembolization; MWA, microwave ablation; NA, not applicable.
The quality evaluation of the RCTs and the case-control studies is shown in Tables 2 and 3, respectively. All RCTs were low risk and the 7 case-control studies scored 5–7 points.
Table 2.
Methodological quality assessment of randomized controlled trials: the Cochrane collaboration’s tool for assessing risk of bias*
| Study (year) | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias |
|---|---|---|---|---|---|---|
| An et al. (2018) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Zhang et al. (2017) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| An et al. (2017) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Zhou et al. (2016) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Liu et al. (2016) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| He et al. (2016) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Guo et al. (2015) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Liu et al. (2018) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Shu et al. (2014) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Hu et al. (2013) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Tao et al. (2016) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| Huang et al. (2015) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Yan et al. (2016) | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
Results of the articles were divided into low risk, unclear risk, and high risk.
Table 3.
Methodological quality assessment of cohort studies: the Newcastle-Ottawa Scale
| First author (year) | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Selection of exposure | Outcome of interest was Ffter start of study | Control for important factor | Assessment of outcome | Sufficient follow-up | Adequacy of follow-up of cohorts |
|---|---|---|---|---|---|---|---|---|
| Zheng et al. (2018) | ★ | ✰ | ★ | ★ | ✰✰ | ✰ | ★ | ★ |
| Huo et al. (2017) | ★ | ★ | ★ | ★ | ✰✰ | ✰ | ★ | ★ |
| Dong et al. (2017) | ★ | ★ | ★ | ★ | ✰★ | ✰ | ★ | ★ |
| Chang et al. (2015) | ★ | ★ | ★ | ★ | ✰✰ | ✰ | ★ | ★ |
| Zhao et al. (2009) | ★ | ★ | ★ | ★ | ✰★ | ✰ | ★ | ★ |
| Zhang et al. (2013) | ★ | ★ | ★ | ★ | ✰★ | ✰ | ★ | ★ |
| Xu et al. (2013) | ★ | ★ | ★ | ★ | ✰★ | ✰ | ★ | ★ |
Article is given a point for meeting the corresponding criterion.
Indicates no point.
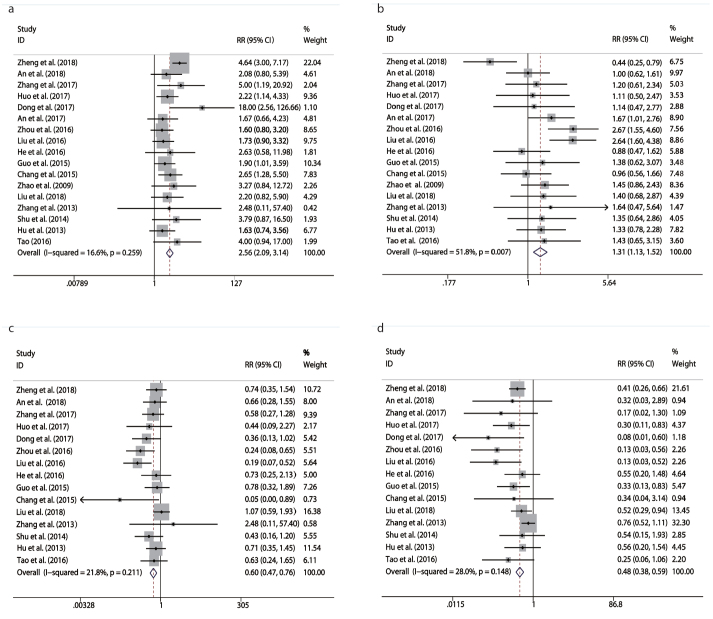
CR and PR were reported in 17 studies. A fixed-effects model was used for CR and a random-effect model was used for PR, based on the results of heterogeneity evaluation (PCR = 0.259, ICR2 = 16.6%; PPR = 0.007, IPR2 = 51.8%). The CR and PR rates of TACE + MWA combination were significantly higher than those of TACE alone (RRCR = 2.56, 95% CI 2.09–3.14, P < 0.001; RRPR = 1.31, 95% CI 1.13–1.52, P < 0.001) (Table 4, Fig. 2a, 2b).
Table 4.
Meta-analysis of efficacy in combined group and control group
| Outcomes | BCLC stage | No of studies | RR (95% CI) | Z | P | I2 (%) | Phetero |
|---|---|---|---|---|---|---|---|
| CR | Overall data | 17 | 2.56 (2.09, 3.14) | 9.03 | 0.000 | 16.6 | 0.259 |
| BCLC A | 2 | 1.86 (0.96, 3.61) | 1.83 | 0.068 | 0.0 | 0.744 | |
| BCLC B | 10 | 2.07 (1.59, 2.69) | 5.42 | 0.000 | 0.0 | 0.623 | |
|
| |||||||
| PR | Overall data | 17 | 1.31 (1.13, 1.52) | 3.5 | 0.000 | 51.8 | 0.007 |
| BCLC A | 2 | 1.27 (0.90, 1.80) | 1.37 | 0.170 | 51.9 | 0.149 | |
| BCLC B | 10 | 1.57 (1.27, 1.94) | 423 | 0.000 | 29.2 | 0.176 | |
|
| |||||||
| SD | Overall data | 16 | 0.60 (0.47, 0.76) | 4.21 | 0.000 | 21.8 | 0.211 |
| BCLC A | 1 | 0.66 (0.28, 1.55) | 0.95 | 0.341 | * | ||
| BCLC B | 10 | 056 (0.41, 0.76) | 3.72 | 0.000 | 47.6 | 0.046 | |
|
| |||||||
| PD | Overall data | 16 | 0.48 (0.38, 0.59) | 6.78 | 0.000 | 28 | 0.148 |
| BCLC A | 1 | 0.32 (0.03, 2.89) | 1.02 | 0.307 | |||
| BCLC B | 10 | 0.51 (0.40, 0.66) | 5.08 | 0.000 | 46.4 | 0.058 | |
|
| |||||||
| ORR | Overall data | 17 | 1.69 (1.54,1.85) | 11.33 | 0.000 | 13.4 | 0.296 |
| BCLC A | 2 | 1.40 (1.11, 1.78) | 2.82 | 0.005 | 29.1 | 0.235 | |
| BCLC B | 10 | 1.79 (1.58, 2.20) | 9.29 | 0.000 | 15.4 | 0.301 | |
|
| |||||||
| 1-year OS | Overall data | 17 | 1.36 (1.28,1.44) | 9.87 | 0.000 | 0.0 | 0.622 |
| BCLC A | 3 | 1.50 (1.12, 2.00) | 2.74 | 0.006 | 0.0 | 0.762 | |
| BCLC B | 8 | 1.40 (1.29, 1.53) | 7.69 | 0.000 | 0.0 | 0.622 | |
|
| |||||||
| 2-year OS | Overall data | 15 | 1.56 (1.40,1.74) | 7.81 | 0.000 | 0.0 | 0.981 |
| BCLC A | 2 | 1.92 (1.17, 3.18) | 2.56 | 0.011 | 0.0 | 0.483 | |
| BCLC B | 7 | 1.60 (1.36, 1.88) | 5.76 | 0.000 | 0.0 | 0.779 | |
|
| |||||||
| 3-year OS | Overall data | 8 | 2.07 (1.67,2.57) | 6.63 | 0.000 | 0.0 | 0.728 |
| BCLC A | 1 | 1.67 (0.45, 6.21) | 0.76 | 0.000 | |||
| BCLC B | 4 | 2.02 (1.53, 2.66) | 4.96 | 0.446 | 0.0 | 0.501 | |
BCLC, Barcelona Clinic Liver Cancer stage; RR, risk ratio; 95% CI, 95% confidence interval; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; OS, overall survival
Due to the nature of statistical analysis, the value was absent.
Figure 2. a–d.
Meta-analysis of results in treatment response between TACE + MWA and TACE alone: (a), comparison of complete response (CR) rate; (b), comparison of partial response (PR) rate; (c), comparison of stable disease (SD) rate; (d), comparison of progressive disease (PD) rate.
Sixteen studies reported data for SD and PD, and heterogeneity evaluation among these studies showed no significance (PSD = 0.211, ISD2 = 21.8%; PPD = 0.148, IPD2 = 28%). Thus, the results were pooled by the fixed-effects model. Meta-analysis showed that the SD and PD rates of TACE + MWA combination of were significantly lower than those of TACE alone (RRSD = 0.6, 95% CI 0.47–0.76, P < 0.0001; RRPD = 0.48, 95% CI 0.38–0.59, P < 0.0001) (Table 4, Fig. 2c, 2d).
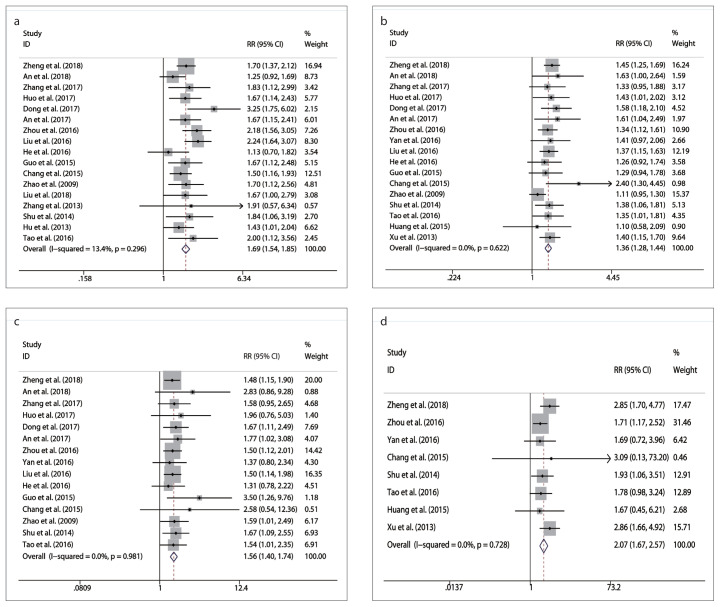
ORR rates were reported in 17 studies. A fixed-effects model was applied for pooling the results, because no significant heterogeneity was found among these studies (P = 0.296, I2 = 13.4%). The results showed that the ORR rate of the combination therapy was significantly higher than that of TACE alone (RR = 1.69, 95% CI 1.54–1.85, P < 0.001) (Table 4, Fig. 3a).
Figure 3. a–d.
Meta-analysis of results in objective response (ORR) rate and 1-, 2-, and 3-year overall survival (OS) rates: (a), comparison of ORR; (b), comparison of 1-year OS rate; (c), comparison of 2-year OS rate; (d), comparison of 3-year OS rate.
Seventeen, 15, and 8 studies reported data for 1-, 2-, and 3-year OS, respectively. According to the results of heterogeneity evaluation among these studies (P1-year = 0.622, I1-year2 = 0.0%; P2-year = 0.981, I2-year2 = 0.0% and P3-year = 0.728, I3-year2 = 0.0) a fixed-effects model was applied. Meta-analysis showed that the 1-, 2-, and 3-year OS rates of the combination therapy were significantly higher than those of TACE alone (RR1-year = 1.36, 95% CI 1.28–1.44, P < 0.001; RR2-year = 1.56, 95% CI 1.40–1.74, P < 0.001 and RR3-year = 2.07, 95% CI 1.67–2.57, P < 0.001) (Table 4, Fig. 3b–3d).
Studies were divided into subgroups according to BCLC stage A and BCLC stage B to show the comparison of efficacy between TACE + MWA combination therapy and TACE alone in different disease stages (Table 4). In patients with BCLC stage A, the combined therapy had a lower RR value in ORR but a higher RR value in 1- and 2-year OS.
No uniform standard exists for reporting complications among studies, and only descriptive analysis was performed in this meta-analysis. Complications occurred in all 20 included studies. Minor complications included nausea, vomiting, fever, and transient increase of reactive hydrothorax and transaminase, which regressed or disappeared within a short time after support treatment. Ten studies reported major complications, including liver abscess, subcapsular hemorrhage, gastrointestinal bleeding, cholecystitis and biliary stricture (Table 5). Huo et al. (25) reported a patient who died of postoperative sepsis related to hepatic abscess.
Table 5.
Comparison of major complications
| Study (year) | TACE | n (%) | TACE+MWA | n (%) | P |
|---|---|---|---|---|---|
| Zheng et al. (2018) | 4 liver abscess, 2 upper GI bleeding | 6 (3.6) | 1subcapsular hemorrhage, 1 liver abscess | 2 (2.2) | 0.791 |
| An et al. (2018) | NA | 1 (0) | 5 needle tract bleeding | 5 (14.2) | 0.024 |
| Zhang et al. (2017) | NA | 0 (0) | NA | 0 (0) | NA |
| Huo et al. (2017) | 1 death after liver abscess | 1 (3.1) | NA | 0 (0) | 0.471 |
| Dong et al. (2017) | NA | 0 (0) | NA | 0 (0) | NA |
| An et al. (2017) | 1subcapsular hemorrhage | 1 (2.0) | 2 subcapsular hemorrhage | 2 (4.1) | 1.000 |
| Zhou et al. (2016) | 4 jaundice | 4 (7.4) | 3 infection | 3 (5.6) | 1.000 |
| Yan et al. (2016) | 1 upper GI bleeding, 2 cholecystitis | 3 (9.3) | 1 needle track implantation | 1 (2.4) | 0.313 |
| Liu et al. (2016) | NA | 0 (0) | NA | 0 (0) | NA |
| He et al. (2016) | NA | 0 (0) | NA | 0 (0) | NA |
| Guo et al. (2015) | NA | 0 (0) | NA | 0 (0) | NA |
| Chang et al. (2015) | NA | 0 (0) | NA | 0 (0) | NA |
| Zhao et al. (2009) | NA | 0 (0) | NA | 0 (0) | NA |
| Liu et al. (2018) | 3 upper GI bleeding | 3 (5.7) | 1 upper GI bleeding, 2 local biliary obstruction | 3 (5.7) | 1.000 |
| Zhang et al. (2013) | NA | 0 (0) | NA | 0 (0) | NA |
| Shu et al. (2014) | 2 jaundice | 2 (8.3) | 1 local biliary obstruction | 1 (3.8) | 0.602 |
| Hu et al. (2013) | NA | 0 (0) | NA | 0 (0) | NA |
| Tao et al. (2016) | NA | 0 (0) | 1 local biliary obstruction | 1 (4.0) | 1.000 |
| Huang et al. (2015) | NA | 0 (0) | 2 subcapsular hemorrhage | 2 (8.0) | 0.489 |
| Xu et al. (2013) | NA | 0 (0) | NA | 0 (0) | NA |
TACE, transarterial chemoembolization; MWA, microwave ablation; NA, not available; GI, gastrointestinal.
The Egger and Begg tests showed no obvious publication bias (Table 6). Sensitivity analysis showed a stable outcome.
Table 6.
Results of Begg and Egger tests for publication bias
| Begg test | Egger test | |||
|---|---|---|---|---|
|
|
|
|||
| Z | P | t | P | |
| CR | 2.02 | 0.044 | 0.27 | 0.79 |
|
| ||||
| PR | 0.29 | 0.773 | −0.32 | 0.752 |
|
| ||||
| SD | 1.48 | 0.138 | −1.87 | 0.084 |
|
| ||||
| PD | 1.88 | 0.06 | −3.85 | 0.002 |
|
| ||||
| ORR | 1.77 | 0.077 | 0.9 | 0.384 |
|
| ||||
| 1-year OS | 0.62 | 0.537 | 1.63 | 0.124 |
|
| ||||
| 2-year OS | 2.47 | 0.013 | 3.65 | 0.003 |
|
| ||||
| 3-year OS | −0.12 | 1 | 0.11 | 0.915 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; OS, overall survival.
Discussion
For BCLC stage A and B patients with tumors larger than 5 cm who are not suitable for surgical resection, TACE is usually the preferred treatment. However, the median survival of patients treated with TACE alone is only 16 months, which is much lower than that of patients treated with early surgical resection or RFA (1). Moreover, for large HCCs, pure ablation is often restricted by the limited ablation range and the higher residual recurrence rate (33–35). Thus, TACE combined with ablation was designed for clinical practice. In small HCCs, the efficacy of MWA is similar to that of RFA. However, faster heating, larger ablation range, and shorter ablation time of MWA provide potential advantages in the treatment of HCC, especially in large HCCs (6, 36). Our meta-analysis data showed that TACE combined with MWA was significantly superior to TACE alone for the treatment of large HCC in both treatment response and 1-, 2-, and 3-year OS.
MWA delivered after TACE to treat tumors larger than 5 cm can promote the efficacy of treatment and play a joint role in inhibiting and killing tumors. First, TACE blocks tumor nourishing arteries and further promotes the tumoricidal effect of chemotherapeutic drugs. Second, MWA reaches a large ablation range with its high thermal efficiency and triggers an immune tumoricidal effect secondary to tumor antigen exposure after MWA (37). Moreover, the combination therapy may increase the mutual therapeutic effects as follows: 1) After TACE, the tumors can be clearly visualized on the monitoring equipment, which helps accurate tumor location during the MWA procedure; 2) Local microperfusion of tumors decreases significantly after TACE, reducing the possible perfusion-mediated tissue cooling effect and increasing the ablation range (38); 3) The deposition of lipiodol after TACE causes stronger heat conduction and tumor local edema, which relatively increases the water content, both increasing the microwave heating rate and enlarging the ablation range (39); and 4) TACE can control microscopic vascular invasion and satellites around the HCC, reducing the local recurrence rate.
Gu et al. (40) reported that TACE combined with local ablation was superior to monotherapy in the treatment of HCC, with 1-, 2, and 3-year OS lower than our results. This may be due to the heterogeneity of ablation methods in their study, but was more likely due to the superiority of MWA in the treatment of large HCCs (5–7, 41). The ablation zone of RFA is restricted by a self-limiting process (water vapor, desiccation, and charring increasing impedance). Compared to RFA, microwave energy can produce effectively larger ablation zones (42). Thus, MWA has a higher complete ablation rate and a better prognosis for the patients.
In this study, all TACE treatments were conventional. No drug-eluting bead TACE (DEB-TACE) was included. Wen et al. (43) reported that DEB-TACE had better efficacy in CR rate and longer progression-free survival, similar to the results reported by Liu et al. (44). However, there was no significant difference in OS between these two treatments, although DEB-TACE had a lower rate of postoperative adverse reactions (44–46). Whereas, considering the combination with MWA, conventional TACE may have a more obvious advantage due to the visibility and positioning effect of lipiodol.
TACE + MWA seemingly increases treatment times, but patients do not receive interventional therapy for a long time after complete ablation, which helps to improve the quality of life and reduce the financial burden. Although MWA treatment was generally continued for 1 month after TACE, the major complications did not change significantly (47).
This study had several limitations. The included RCTs lack detailed implementation details about blinding and randomized allocation methods, increasing the risk of related bias. In addition, the included articles were all from Asian regions and whether the results can be extended to a wider population range needs to be confirmed by larger controlled studies. Moreover, considering few studies that included patients with BCLC stage A alone, the comparison of the efficacy of combination therapy for tumors larger than 5 cm between BCLC stage A and B requires further clinical research. Finally, owing to the vague or inconsistent evaluation indexes of each study, the complications could not be quantified.
In conclusion, we found that TACE combined with MWA achieved higher treatment response rate and prolonged OS in patients with early- and intermediate-stage HCCs larger than 5 cm compared with TACE alone.
Main points.
Early- and intermediate-stage hepatocellular carcinomas larger than 5 cm were included.
Microwave ablation (MWA) plus transcatheter arterial chemoembolization (TACE) was compared with TACE alone.
20 studies were included in this meta-analysis.
TACE + MWA achieved higher treatment response and prolonged survival compared with TACE alone.
Footnotes
Financial disclosure
This research was funded by the National Natural Science Foundation of China (No. 81771944). Liaoning Innovative Talent Support Plan; Shenyang City Innovative Talent Support Plan (RC170048).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 3.Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 4.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 5.Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hypothermia. 2016;32:339–344. doi: 10.3109/02656736.2015.1127434. [DOI] [PubMed] [Google Scholar]
- 6.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J BUON. 2019;24:163–170. [PubMed] [Google Scholar]
- 8.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Zheng L, Li HL, Guo CY, Luo SX. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018;19:237–246. doi: 10.3348/kjr.2018.19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456–463. doi: 10.1111/jgh.12088. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JL, Fan WJ, Zhang L, et al. Transcatheter arterial chemoembolization combined with percutaneous microwave tissue coagulation for the treatment of massive liver cancer. Guangdong Med J. 2013;34:212–214. [Google Scholar]
- 16.Hu AX, Yang F, Chu LS. Clinical observation of 48 cases of advanced liver cancer treated by transcatheter arterial chemoembolization combined with microwave ablation. Nat Medical Frontiers China. 2013;8:59. +9. [Google Scholar]
- 17.Shu Y, Wang HY, Tao LM, et al. Curative effect of transcatheter hepatic arterial chemoembolization combined with microwave ablation for large liver cancer. Practice J Cancer. 2014;29:996–998. [Google Scholar]
- 18.Chang P, Zhang HY, Xiao M. Efficacy of transcatheter arterial chemoembolization alone or combination with microwave ablation in treatment of primary large liver cancer: a comparative analysis. J Clin Hepatol. 2015;31:880–885. [Google Scholar]
- 19.Guo HQ, Yan P, Zou CY, Li RF, Yang P. TACE combined with microwave ablation for the treatment of large-sized hepatic carcinoma: a preliminary study. J Pract Radiol. 2015;31:1692–1694. [Google Scholar]
- 20.Zhou P, Wang JZ, Liu JP, Wang YG, Liu DQ, Su L. Clinical observation of hepatic arterial chemoembolization combined with microwave ablation for large primary hepatocellular carcinoma. Chin J Clin Rational Drug Use. 2016;9:114–115. [Google Scholar]
- 21.Yan H, Zhao ZY. Therapeutic effect of microwave ablation combined with transcatheter arterial chemoembolization (TACE) in the treatment of advanced massive liver cancer. China Medical Device Information. 2016;22:95–98. [Google Scholar]
- 22.Zhao C, Ma YL, Kang P, et al. Clinical review of transcatheter hepatic arterial chemoembolization combined with microwave ablation in patients with advanced liver cancer. Clin J Oncol Prev Treat. 2009;1:218–220. [Google Scholar]
- 23.An JL, Han XY, Sha JF, et al. Clinical study of transcatheter arterial chemoembolization sequentially combined with microwave ablation in the treatment of single primary hepatocellular carcinoma with diameter greater than 5 cm. J Hepatopancreatobiliary Surg. 2018;30:191–196. [Google Scholar]
- 24.Zhang H. The efficacy and superiority of hepatic artery chemoembolization combined with microwave ablation in the treatment of liver cancer. J China Prescription Drug. 2017;15:141–142. [Google Scholar]
- 25.Huo XH, Zhang H, Li ZX. Short-term efficacy and safety evaluation of transcatheter artical chemoembolization in combination with microwave ablation for large hepatic cancer. J Medical Imaging. 2017;27:677–681. [Google Scholar]
- 26.An XY. Study on the efficacy of TACE combined with microwave ablation in patients with giant liver cancer. J Clinical Medical. 2017;4:420–421. [Google Scholar]
- 27.Liu J, Zou GP, Fan H. Clinical efficacy observation of hepatic artery chemoembolization combined with microwave ablation for primary liver cancer. China Higher Medical Education. 2016:135–136. [Google Scholar]
- 28.He ZJ, Liu YJ, Wen J, Zhang ZY, He WQ, Chen H. Clinical application of hepatic arterial chemoembolization combined with percutaneous microwave coagulation therapy for primary massive liver cancer. J Clin Res. 2016;33:1348–1350. [Google Scholar]
- 29.Liu YL. Clinical curative effect observation of microwave ablation combined with transcatheter hepatic artery chemoembolization in the treatment of advanced liver cancer. Medical J Chinese People’s Health. 2018;30:10–11. [Google Scholar]
- 30.Huang SM, Chen SK, Zhang T, Li JB. Clinical analysis of hepatic artery embolization chemotherapy combined with microwave ablation for giant liver cancer. Chongqing Medicine. 2015;44:5149–5152. [Google Scholar]
- 31.Dong J, Chen QF, Xia JG, et al. TACE combined with microwave ablation versus pure TACE for hepatocellular carcinomas larger than five cm in diameter: a propensity matching analysis. J Intervent Radiol. 2017;26:894–898. [Google Scholar]
- 32.Tao HY, Qu ZY, Wei GM, Sheng J, Wang WL, Wan LX. Short-term and long-term efficacy of transcatheter arterial chemoembolization (TACE) combined with microwave ablation (MWA) in the treatment of primary liver cancer. Chinese J Hospital Pharmacy. 2016:281–282. [Google Scholar]
- 33.Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol. 2011;2011 doi: 10.4061/2011/104685. 104685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Won HJ, Shin YM, et al. Medium-sized (3.1–5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624–1629. doi: 10.1245/s10434-011-1673-8. [DOI] [PubMed] [Google Scholar]
- 36.Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983–1990. doi: 10.1007/s00330-012-2442-1. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Liang P. Immunotherapy for hepatocellular carcinoma following thermal ablation. J BUON. 2014;19:867–871. [PubMed] [Google Scholar]
- 38.Lin ZY, Li GL, Chen J, Chen ZW, Chen YP, Lin SZ. Effect of heat sink on the recurrence of small malignant hepatic tumors after radiofrequency ablation. J Cancer Res Therapeutics. 2016;12:C153–158. doi: 10.4103/jcrt.JCRT_959_16. [DOI] [PubMed] [Google Scholar]
- 39.Ni JY, Sun HL, Chen YT, et al. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17483–17490. doi: 10.3748/wjg.v20.i46.17483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu L, Liu H, Fan L, et al. Treatment outcomes of transcatheter arterial chemoembolization combined with local ablative therapy versus monotherapy in hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:199–210. doi: 10.1007/s00432-013-1528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Ni CF. Current status of the combination therapy of transarterial chemoembolization and local ablation for hepatocellular carcinoma. Abdom Radiol. 2019;44:2268–2275. doi: 10.1007/s00261-019-01943-2. [DOI] [PubMed] [Google Scholar]
- 42.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen P, Chen SD, Wang JR, Zeng YH. Comparison of treatment response and survival profiles between drug-eluting bead transarterial chemoembolization and conventional transarterial chemoembolization in chinese hepatocellular carcinoma patients: a prospective cohort study. Oncol Res. 2019;27:583–592. doi: 10.3727/096504018X15368325811545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu YS, Lin CY, Chuang MT, et al. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterology. 2018;18:124. doi: 10.1186/s12876-018-0848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Brit J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Digestive Dis. 2016;17:510–517. doi: 10.1111/1751-2980.12380. [DOI] [PubMed] [Google Scholar]
- 47.Kim W, Cho SK, Shin SW, Hyun D, Lee MW, Rhim H. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol. 2019;44:2283–2292. doi: 10.1007/s00261-019-01952-1. [DOI] [PubMed] [Google Scholar]